Effects of Self-Identification as a Caregiver on Expectations of Public Stigma of Alzheimer’s Disease
Abstract
Background:
As a result of caring for a person with dementia, caregivers of persons with Alzheimer’s disease (AD) may be uniquely aware of public stigma for persons with AD.
Objective:
The purpose of this study was to compare self-identified caregivers and non-caregivers’ expectations of public stigma experienced by persons living with dementia.
Methods:
Analysis of data from a survey of 910 adults (median age = 49 years) who read a vignette about a man with mild stage dementia. Multivariable ordered logistic regression was used to examine how AD caregiver status associated with responses on a modified Family Stigma in Alzheimer’s Disease Scale (FS-ADS).
Results:
9%(n = 82) of respondents self-identified as a current or former primary caregiver of a person with AD, about the same as the national estimate of informal caregivers (8.8%). Compared to non-caregivers, AD caregivers were more likely to report stronger reactions on all seven domains of the FS-ADS (all p < 0.05). As compared to AD caregivers with less factual knowledge about caregiving, AD caregivers with more knowledge expected the person with dementia to experience less social distance (p < 0.05). In addition, female AD caregivers reported fewer negative aesthetic attributions than male AD caregivers (p < 0.05).
Conclusion:
Compared to non-caregivers, respondents who self-identified as an AD caregiver gave responses that suggest they perceived more stigma of dementia among members of the public. Their reactions were attenuated by AD knowledge and being female. The findings have key implications for interventions to reduce AD stigma.
INTRODUCTION
Stigma is understood as a set of prejudicial attitudes, stereotypes, discriminatory behaviors, and biased social structures reported by a person about another person or group of persons. Stigma in Alz-heimer’s disease (AD)—whether because of negative perceptions, expectations, or experiences—is a common and serious problem [1]. Because of it, individuals often fear a formal diagnosis of dementia. They may avoid seeking help for themselves or for their family members. Delays in help seeking can worsen the health of the person with AD [2] and add to the physical, emotional, and social costs for caregivers [3]. It is, therefore, essential to discover ways to reduce or mitigate stigma of AD. One way to do this is to study caregivers’ perspectives on stigma in AD.
Their close social and, often, physical proximity and shared experiences with persons with AD may provide a unique and important perspective. Caregivers are likely to have witnessed “public stigma”—that is, endorsement of prejudice and discrimination toward those with dementia. They may incorpor-ate public stigma into their own views and behaviors. AD caregivers may also experience “spillover sti-gma” [4–6], also called “stigma by association,” which occurs when a person is stigmatized by virtue of their association with a stigmatized group [7]. Spillover stigma can have deleterious effects on how caregivers feel about themselves and may distort their views of and relationship with the person with dementia in their care [8]. This may have negative effects on caregiver well-being even after accounting for the burden and stress associated with providing direct care [9, 10]. Understanding differences in stigma reactions between AD caregivers and members of the public who do not identify as AD caregivers may garner new information about mechanisms of AD stigma and possible opportunities to intervene.
The purpose of this study was to investigate whether self-identified AD caregivers reacted differently than others to a person in a vignette described as having symptoms of mild stage dementia. We conducted a secondary analysis of data from a survey of U.S. adults reflective of the general population. Drawing on the results of prior studies [1, 11], we hypothesized that self-identification as a current or former AD caregiver would be associated with lower stigmatizing personal reactions but worse expectations for how others would treat the person in the vignette.
Understanding how caregivers view individuals with dementia and the expectations they have for how others will treat those individuals can inform effo-rts to reduce stigma both among caregivers and non- caregivers, better support caregivers who anticipate negative reactions to their loved one and help in-form public policies to protect caregivers and persons with dementia against consequences of stigma, such as discrimination.
MATERIALS AND METHODS
Study design
This is a secondary analysis of data collected from a survey of the general public [12]. The survey was distributed between September 5–13, 2013 via an online panel provider to a randomly selected sample of American adults able to provide informed consent and read English. Recruitment materials and consent documents described the study as being about “health beliefs;” AD was not mentioned so as not to bias responses. The survey completion rate was 58%[12]. Respondents read a vignette and then answered survey questions. Respondents also provided standard demographic information. Respondents’ characteristics were similar to those of the general U.S. population [1].
Vignette
The vignette (see the Supplementary Material) described a man, Mr. Andrews, with cognitive impairment corresponding to mild stage dementia (i.e., Stage 1) on the Clinical Dementia Rating (CDR) Scale [13]. Mr. Andrews’s symptoms were described as observable impairments in memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. Mr. Andrews was referred to as “he,” but no other demographic characteristics were provided.
Nine variations of the vignette were developed, which varied Mr. Andrew’s diagnosis (i.e., AD, tra-umatic brain injury (TBI), or no diagnosis) and prognosis (i.e., worsen, improve, or remain unchanged). Each respondent was exposed to one of these nine variations. After reading the vignette, all respondents were given two opportunities to correctly complete a comprehension question. Those who failed on the second attempt were excluded (n = 30).
Questionnaire
Stigmatizing attributions were assessed using a version of the Family Stigma in Alzheimer’s Disease Scale (FS-ADS) [4] that was adapted for understandability and relevance in the context of the original study [12]. Modifications included exclusion of two items related to perceptions of danger and inclusion of vignette text and directions to the reader, such as “To what extent do you think that OTHER PEOPLE feel the following emotions about Mr. Andrews” (see [12] for further details). The FS-ADS contains 44 items that measure seven domains of stigma. For three domains, respondents were asked about the ex-tent to which they personally worried the person in the vignette, Mr. Andrews, would encounter (1) structural discrimination and the extent to which they expected the person in the vignette to exhibit (2) negative severity attributions (e.g., physical symptoms and cognitive problems), and (3) show negative aesthetic qualities (e.g., poor hygiene). For the remaining four domains, respondents were asked how they expected others to react to the person in the vignette. In particular, they were asked about the extent to which they expected others to feel (4) antipathy, (5) support, or (6) pity and (7) social distance from Mr. Andrews. Responses were recorded on a 5-point rating scale from “not at all” to “a very great extent”. Higher scores indicated stronger endorsement. Data on the internal consistency of the adapted FS-ADS and its 7 domains has been published elsewhere [12].
A shortened Alzheimer’s Disease Knowledge Scale (mADKS) was used to assess factual knowledge about AD dementia [14]. In a prior study [12], the mADKS showed invariability with respect to the FS-ADS however the ADKS domain specific to caregiving was included in this study given its relevance to the topic. The domain contains five true-or-false items that assess fact-based knowledge of AD caregiving (e.g., “When people with AD begin to have difficulty taking care of themselves, caregivers should take over right away.”).
Respondents were asked “Do you, or have you, considered yourself the primary caregiver of a person with Alzheimer’s?” They selected “yes” or “no”. No definition of “primary caregiver” was provided. We focus on self-identification as it demarcates individuals who view that being an AD caregiver is an aspect of their personal identity. A similar question was asked about primary caregiving for a person with TBI. Individuals who self-identified as a current or former TBI caregiver were excluded from the analyses (n = 34). All respondents were asked how much time they had personally spent with persons with AD dementia; response options ranged in frequency and intensity from “rarely or never” to “every day for many hours.” A fuller description of the study methods is published elsewhere [1, 11, 12].
Statistical analysis
Multivariable ordered logistic regression models were used to examine the relationship between FS-ADS domains and demographic characteristics and fact-based knowledge about AD caregiving (i.e., from the mADKS). Models statistically controlled for the vignette character’s study-assigned diagnosis (i.e., AD, TBI, or no diagnosis) and prognosis (i.e., worsen, improve, or remain unchanged). All coefficients were exponentiated to derive adjusted odds ratios (AOR), which provide an estimate of the average probability of higher scores compared to relatively lower ones.
In analyses of interaction effects, we examined the difference (ratio) in the odds ratios (DOR) between those who did and did not self-identify as AD caregivers. Models met the proportional odds assumption. In analyses that adjusted for multiple comparisons, all independent variables were screened for multicollinearity and interactions with study prognostic and diagnostic category (p > 10.0). All statistical tests had at least 80%statistical power to detect a minimum difference of 10%in most cases and 15%in the most constrained. These estimates are informed by minimum effect sizes observed in the parent study [1, 11] and assume a standard deviation twice the mean effect [15].
All statistical tests were two-sided. p-values<0.05 were considered statistically significant. Confidence intervals (95%CI) that do not include “1” within the range of possible values suggest the difference between the groups is statistically significant at p < 0.05. Statistical analyses were performed using Stata 14 (College Station, TX). All procedures involving human subjects were approved by the University of Pennsylvania Institutional Review Board (IRB).
RESULTS
Respondent characteristics
In this sample of 910 adults, 9%(n = 82; 95%CI 7.2 to 11.1) of respondents self-identified as a current or former AD caregiver. This group’s median age was 51.5 years (interquartile range [IQR] = 29), about half (47.6%) were female, most (67.1%) identified as White (non-Latino), and over half (62.2%) had less than a 4-year college degree (Table 1). These characteristics were similar to respondents who did not identify as AD caregivers (all p > 0.05). Most self-identified caregivers (93.9%) reported having at least weekly contact with a friend or family member with AD (Table 1), which was significantly higher than non-caregivers (22.6%; p < 0.001).
Table 1
Characteristics of respondents by Alzheimer’s disease (AD) caregiver status (N = 910)
| Respondent Characteristic | AD Caregivers (N = 82) | Non-AD Caregivers (N = 828) | p |
| Age, median (IQR) | 51.5 (29) | 48.59 (29) | 0.88 |
| 65 + years old, %(n) | 17.6 (13) | 16.9 (140) | 0.93 |
| Females, %(n) | 47.6 (43) | 51.3 (403) | 0.01 |
| White, Non-Latino, %(n) | 67.1 (55) | 77.8 (644) | 0.09 |
| Education, %(n) | 0.92 | ||
| High School/GED or Less | 22.0 (18) | 24.8 (205) | |
| Some College or 2-year Degree | 40.2 (33) | 40.1 (332) | |
| 4-year College Degree or beyonda | 37.8 (31) | 35.1 (291) | |
| Shortened version Alzheimer’s Disease | 3 (5) | 3 (5) | 0.73 |
| Knowledge Scale (mADKS) Caregiving | |||
| Domain,b median (IQR) | |||
| At least weekly contact with person | 93.9 (77) | 22.6 (187) | <0.001 |
| with AD,c %(n) |
Column percentages may not total 100 due to rounding. Respondents were asked to select “yes” or “no” in response to the question: “Do you, or have you, considered yourself the primary caregiver of a person with Alzheimer’s?” a 4-year college, master’s, doctorate, or professional degrees. b Caregiving domain comprised of 5 fact-based items. Maximum possible score = 5. c Respondents were asked how much time they personally spent with a person with AD dementia; response options ranged in frequency and intensity from “rarely or never” to “every day for many hours.”
Between-group comparisons adjusted for experimental conditions
In analyses that adjusted for respondents’ gender and the experimental conditions of diagnosis and prognosis, we examined how reactions to the person in the vignette, described as having cognitive impairment consistent with mild stage dementia, differed between those who self-identified as current or former AD caregivers (n = 82) and those who did not self-identify as current or former AD caregivers (n = 828; Table 2).
Table 2
Comparisons of reactions of Alzheimer’s disease (AD) caregivers versus non-AD caregivers in the general public (N = 910)
| Family Stigma in Alzheimer’s Disease Scale (FS-ADS) Domain | AD Caregivers versus Non-AD Caregivers (N = 910) | |
| AOR (95%CI) | ||
| Report on self | Structural | 1.58 * |
| Discrimination | (1.06 to 2.36) | |
| Negative Severity | 2.47 *** | |
| Attributions | (1.64 to 3.72) | |
| Negative Aesthetic | 1.95 ** | |
| Attributions | (1.28 to 2.96) | |
| Report on expectations of others | Antipathy | 3.11 *** |
| (2.05 to 4.71) | ||
| Support | 1.48 * | |
| (0.99 to 2.20) | ||
| Pity | 1.57 * | |
| (1.05 to 2.34) | ||
| Social Distance | 2.20 *** | |
| (1.45 to 3.34) |
AOR, Adjusted odds ratio from ordered logistic regression. Analyses are adjusted for gender and experimental conditions of prognosis and diagnosis. * p≤0.05 ** p≤0.01 *** p≤0.001.
Self-identified AD caregivers’ reactions to Mr. Andrews showed they were 2.51 (95%CI 1.65 to 3.82) times as likely as non-AD caregivers to report more severe symptoms (negative severity attributions) and 1.91 (95%CI 1.26 to 2.91) times as likely to report a poorer physical appearance (negative aesthetic attributions). They were also 1.72 (95%CI 1.13 to 2.61) times as likely as non-AD caregivers to worry more that the person with cognitive impairment would encounter structural discrimination.
When anticipating how others would react, AD caregivers were 2.71 (95%CI 1.77 to 4.14) times as likely as non-AD caregivers to report that other people would feel more antipathy and 2.37 (95%CI 1.55 to 3.64) times as likely to expect that Mr. Andrews would experience more social distance. AD caregivers were also more likely to expect others to express more support (AOR = 1.51, 95%CI 1.01 to 2.27) and pity (AOR = 1.67, 95%CI 1.11 to 2.51) toward Mr. Andrews than non-AD caregivers.
The influence of demographic characteristics on Family Stigma of Alzheimer’s Disease Scale (FS-ADS) scores
In separate multivariable models of the sample of 910 adults that statistically adjusted for study experimental conditions, we assessed the influence of respondent demographic characteristics of age and gender and knowledge of AD caregiving. Adjusting for these covariates, statistically independent associations remained between AD caregiver status and higher endorsement on each of the seven FS-ADS domains (all p < 0.05; Table 3).
Table 3
Adjusted odds ratios (AORs) from multivariable models comparing Alzheimer’s disease (AD) caregivers to non-AD caregivers (N = 910)
| Report on self | ||||
| Model Covariates | Structural Discrimination | Negative Severity Attributions | Negative Aesthetic Attributions | |
| AOR (95%CI) | AOR (95%CI) | AOR (95%CI) | ||
| AD caregiver versus non-AD caregiver | 1.67 * (1.10 to 2.54) | 2.43 *** (1.60 to 3.69) | 1.95 ** (1.29 to 2.96) | |
| Age (decades) | 1.06 (0.98 to 1.14) | 0.96 (0.90 to 1.04) | 0.98 (0.91 to 1.05) | |
| Femalea | 1.07 (0.84 to 1.35) | 1.17 (0.92 to 1.49) | 0.73 ** (0.58 to 0.93) | |
| Alzheimer’s Disease Knowledge Scale (ADKS) Caregiving Domain | 1.05 (0.95 to 1.17) | 0.95 (0.86 to 1.05) | 0.96 (0.87 to 1.06) | |
| Report on expectations of others | ||||
| Model Covariates | Antipathy | Support | Pity | Social Distance |
| AOR (95%CI) | AOR (95%CI) | AOR (95%CI) | AOR (95%CI) | |
| AD caregiver versus non-AD caregiver | 2.80 *** (1.84 to 4.28) | 1.52 * (1.01 to 2.29) | 1.69 * (1.13 to 2.55) | 2.34 *** (1.53 to 3.56) |
| Age (decades) | 0.90 ** (0.84 to 0.97) | 0.89 *** (0.82 to 0.95) | 0.89 ** (0.83 to 0.96) | 1.02 (0.95 to 1.10) |
| Femalea | 0.79 * (0.62 to 1.00) | 1.11 (0.87 to 1.40) | 1.28 * (1.01 to 1.62) | 0.90 (0.71 to 1.15) |
| Alzheimer’s Disease Knowledge Scale (ADKS) Caregiving Domain | 0.90 * (0.82 to 1.00) | 1.10 (0.99 to 1.22) | 1.02 (0.92 to 1.14) | 0.86 ** (0.78 to 0.96) |
AOR, Adjusted odds ratio from ordered logistic regression statistically controlling for study condition. Although a shortened version of the ADKS was used in this study, all items on the caregiving domain are consistent with the original ADKS. a Male is reference category. * p≤0.05 ** p≤0.01 *** p≤0.001.
In interaction analyses, we assessed how each covariate that was independently associated with FS-ADS responses, shown in Table 3, modified AD caregivers’ reactions to a person with mild stage de-mentia (Table 4). An analysis of the interaction between gender and self-reported AD caregiver st-atus found that self-identified female AD caregiv-ers believed Mr. Andrews was less affected by a poor physical appearance as compared to self-ide-ntified male AD caregivers (negative aesthetic attributions; DOR = 0.30, 95%CI 0.13 to 0.70). In add-ition, self-identified female AD caregivers reported weaker feelings of antipathy toward Mr. Andrews than male caregivers (negative aesthetic attributions; DOR = 0.40, 95%CI 0.17 to 0.91).
Table 4
Difference in adjusted odds ratios (DORs) from multivariable models comparing influence of covariate in Alzheimer’s disease (AD) caregivers and non-AD caregivers (N = 910)
| Report on expectations of others | Report on self | ||||
| Model Interaction terms | Antipathy | Support | Pity | Social Distance | Negative Aesthetic Attributions |
| DOR (95%CI) | DOR (95%CI) | DOR (95%CI) | DOR (95%CI) | DOR (95%CI) | |
| Age (decades)× | 0.99 | 0.82 | –0.08 | ||
| caregiver statusa | (0.77 to 1.27) | (0.64 to 1.05) | (–0.33 to 0.16) | ||
| Femaleb× | 0.40 * | 0.07 | 0.30 ** | ||
| caregiver status | (0.17 to 0.91) | (–0.73 to 0.87) | (0.13 to 0.70) | ||
| Alzheimer’s Disease | 0.85 | 0.59 ** | |||
| Knowledge Scale (ADKS) | (0.57 to 1.28) | (0.39 to 0.88) | |||
| Caregiving Domain× | |||||
| caregiver status | |||||
DOR, Difference in odds ratios from ordered logistic regression statistically controlling for study condition. Although a shortened version of the ADKS was used in this study, all items on the caregiving domain are consistent with the original ADKS. aCaregiver status coded as self-identified Alzheimer’s disease (AD) caregiver versus non-AD caregiver (ref.) bMale is reference category. * p≤0.05 ** p≤0.01 *** p≤0.001.
As compared to self-identified AD caregivers who had less factual knowledge about caregiving, those with greater factual knowledge expected Mr. Andrews would experience less social distance (DOR = 0.59, 95%CI 0.39 to 0.88). As a follow up to this analysis, we conducted a 3-way interaction analysis of self-reported AD caregiving status, educational attainment, and factual knowledge about caregiving. In this 3-way interaction analysis, the interaction between caregiver status and knowledge of caregiving remained statistically significant (p < 0.05). No other statistically significant interactions were observed (all p > 0.05).
Influence of intensity of contact with persons with Alzheimer’s disease dementia on FS-ADS Scores
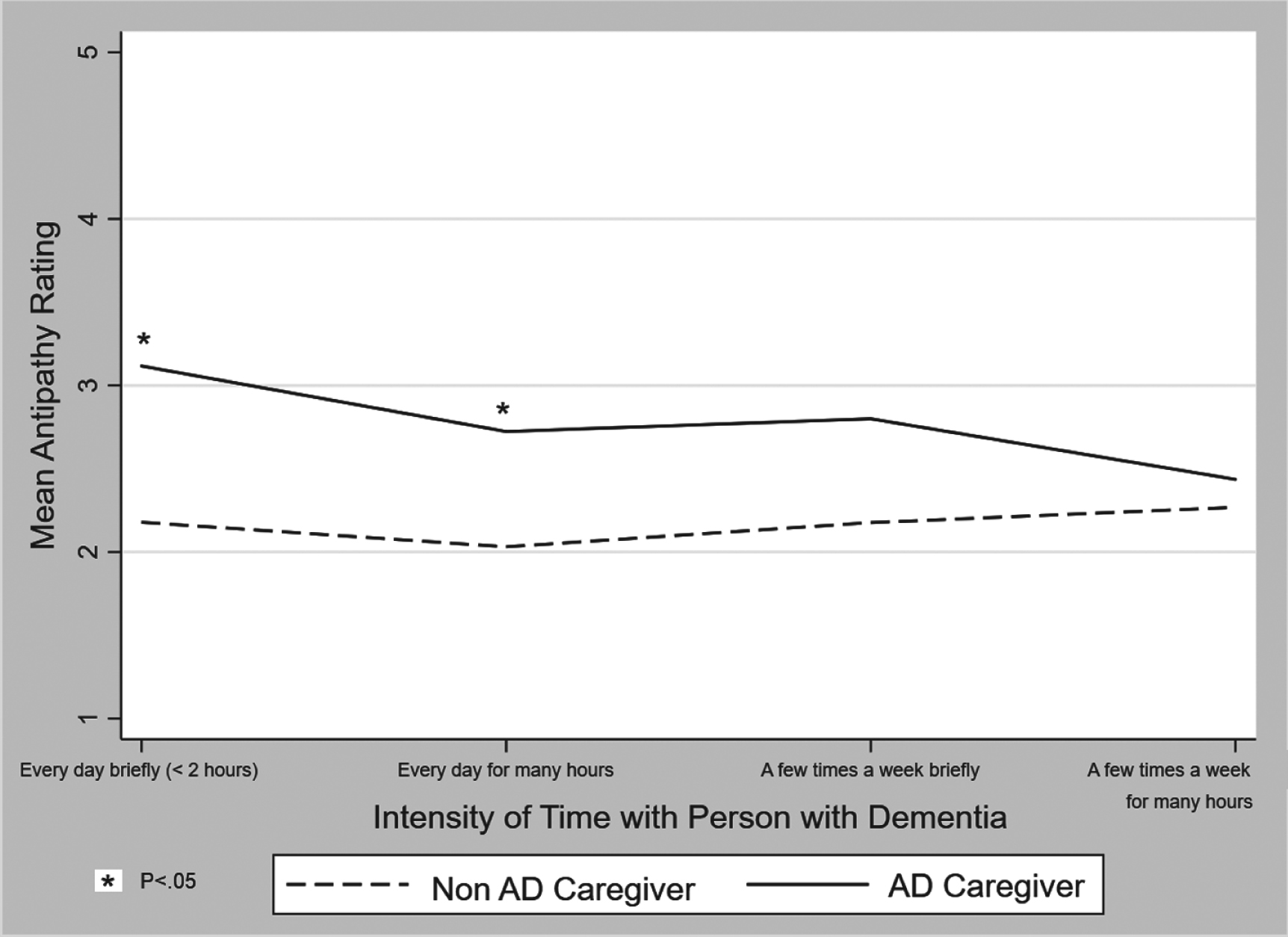
We examined whether self-identifying as an AD caregiver may have an effect on stigma that’s unique from reactions associated with the intensity of contact with persons with AD. To do this, we tested the main study comparisons in respondents who reported more than weekly contact with a friend or family me-mber with AD (n = 273). In multivariable analyses of this subsample that controlled for respondent gender and experimental condition, self-identified AD caregivers endorsed stronger negative severity attributions (AOR = 3.27, 95%CI 1.98 to 3.39), negative aesthetic attributions (AOR = 1.91, 95%CI 1.16 to 3.13), and greater worries about social distance (AOR= 2.60, 95%CI 1.57 to 4.29). Although differences based on caregiver status did not reach statistical significance at p < 0.05 in the model assessing antipathy, a trend was observed whereby differences in subgroups defined by the greatest intensity of contact with a friend or family member with AD did reach statistical significance (Fig. 1).
Fig. 1
Mean score on Family Stigma of Alzheimer’s Disease (FS-ADS) Antipathy Scale by Alzheimer’s disease (AD) caregiver status in subsample of respondents who reported at least weekly contact with a friend or family member with Alzheimer’s disease (n = 273).
DISCUSSION
The purpose of this study was to investigate whether self-identified primary caregivers for a person with AD reacted differently than non-caregivers to a vignette description of a person with mild stage dementia. Nine percent of survey respondents self-identified as a current or former primary AD caregiver; this appears consistent with national estimates that 8.8%of adults nationally have served or do serve as an informal caregiver for someone with dementia [16, 17]. But, about 48%of AD caregivers in our sample also identified as women, whereas 52.3%of non-caregivers did. This suggests that, while the overall percentage of AD caregivers in our sample was consistent with national estimates, respondents may not be demographically representative of AD caregivers nationally, as most caregivers of persons with dementia are women [18].
Three domains of the FS-ADS asked what the respondent personally believed and four domains asked how they expected others to react. We hypothesized that respondents who self-identified as AD caregivers—who were presumably more likely than others to have first-hand experiences with persons with dementia and AD stigma—would report weaker stigmatizing reactions than non-caregivers. We expected this to be the case because close contact with a person with dementia would lead AD caregivers to respond in ways that may be less consistent with stereotypes of those with dementia. We also hypothesized that caregivers’ first-hand experiences with others’ stigmatizing reactions would lead caregivers to have worse expectations for how others would treat the person in the vignette as compared to respondents who did not identify as AD caregivers. Our results run counter to our first hypothesis but support our second.
As compared to non-caregivers, AD caregivers endorsed greater stigma toward the person with dementia in the vignette and expected that others would treat the person with dementia worse. Put differently, we found that self-identified AD caregivers reported stronger reactions across all seven FS-ADS domains.
Our finding raises two questions: As compared to non-caregivers, why do caregivers personally report greater stigma toward a person with mild stage dementia and why do they expect others to act in more stigmatizing ways toward a person with mild stage dementia? Given the consistency of our findings across all domains of the FS-ADS, it is plausible that, while our questions were framed to ask about the respondent’s reactions and how the respondents expected others to react, caregivers did not make this distinction between self and other. Prior research on social desirability bias shows that survey respondents tend to project their own beliefs and evaluations to answer indirect questions that ask about “others” rather than directly about them personally [19].
If this is the case, we must ask why AD caregivers report greater stigma toward a person with mild stage dementia than non-caregivers. One hypothesis is that AD caregivers’ survey responses were informed by their own caregiving experiences taking care of persons with perhaps more severe dementia. Caregivers in our study may have been reporting on what they experienced and observed in interactions with the general public in their role as an AD caregiver rather than responding to the vignette in isolation. Another interpretation is that as a result of stigma that spills over from a person with dementia to a caregiver [20], the caregiver may project greater stigmatizing reactions onto the person with dementia. To our knowledge, current frameworks do not address this type of cyclical exchange between caregivers and persons with dementia.
There’s one exception to our finding that AD caregivers’ responses were consistent with prior studies on public stigma of severe dementia. We found AD caregivers were around 2.5 times as likely as non-AD caregivers to more strongly report antipathy (i.e., others feeling disgust and repulsion). In prior work, we showed that feelings of antipathy are not a prominent component of public stigma of AD [1, 11]. Antipathy has, however, been shown to be correlated with stigma by association [21]. Thus, a possible interpretation of the finding in this study is that caregivers’ responses may have been impacted by the stigma they personally experienced in their role caring for a person with dementia. AD caregivers’ reports may also be affected by caregiver burden, which was not assessed in the present study.
Our findings may help inform understanding of the effects of AD stigma and help inform scholarship on how to measure stigma and design interventions to reduce it. While patterns in our findings are consistent with prior studies of public stigma [11, 12, 19], reactions of self-identified caregivers were stronger in magnitude. For example, self-identified AD caregivers were around 2.5 times as likely as non-AD caregivers to more strongly report social distance (i.e., friends and loved ones avoiding interactions with the person with dementia) and to more strongly report negative severity attributions (i.e., that the person displays symptoms like incontinence and disturbing others). In light of prior studies [11, 12, 19], our findings suggest that how individuals enact stigma (i.e., members of general public reacting to vignette) and experience stigma (i.e., caregivers reacting to same vignette on same measure but informed by direct personal experiences) may similar in kind but different in magnitude of effect. In other words, when informed by personal experience, AD stigma is much worse. This may operate as a form of confirmatory bias [22].
This finding is in conflict with the common strategy to decrease stigma through personal contact with members of the stigmatized group [23, 24]. In fact, we found that stigmatizing reactions were stronger in AD caregivers even though most (93.9%) described having at least weekly contact with a friend or family member with AD. In some cases, like antipathy (Fig. 1), stigmatizing reactions appeared to worsen with intensity of contact. Results from our interaction analyses also underscore this contradiction. In a prior study in the general public [11], we found that women reported lower negative aesthetic attributions (p < 0.05). That gender effect was also observed in the current study; women AD caregivers believed Mr. Andrews was less affected by a poor physical appearance as compared to male AD caregivers (negative aesthetic attributions; DOR = 0.37, 95%CI 0.16 to 0.85). This suggests the shared experience of identifying as an AD caregiver was not sufficient to washout the gender effects in the general public.
A possible explanation for the contradiction between our findings and the commonly promulgated belief that personal contact reduces stigma is that, in some cases, interacting with a person with dementia may confirm rather than refuted personally held negative stereotypes [25]. An alternative explanation may be that the strategy of increased personal contact may not be as effective an intervention for AD stigma as it is for other types of stigma. Studies supporting increased personal contact have centered on stigma of mental illness; those results may not generalize to stigma of dementia [7, 24]. Moreover, these studies have often focused on length of exposure as measured in college samples and health care providers. If the time spent with a person with dementia involves caregiving, the length of exposure alone may oversimplify the complex mechanisms that lead to stigma. Our findings therefore raise a question: how to implement recommendations to limit stigma by increasing personal contact with persons living with dementia. Our results suggest personal contact in persons who self-identify as a caregiver can lead to negative feelings, attitudes, and beliefs.
Limitations of this study include the vignette described a specific patient, Mr. Andrews, with symptoms characteristic of mild dementia. Public stigma may differ based on an AD patient’s characteristics such as gender, race/ethnicity, or severity of sym-ptoms. How patient characteristics mitigate or compound stigmatizing attributions warrants further research. Second, although the sample for this study was drawn from a large national panel that has been shown not to differ from the U.S. general population on basic demographic characteristics [1], the number of self-reported AD caregivers is relatively small. It is unlikely to reflect the full range of diversity and experience of AD caregivers. Further studies in population-based samples would be valuable.
Respondents who self-identified as a current or former AD caregiver reported both more stigmatizing attitudes themselves and expected others to hold more stigmatizing attitudes; put differently, caregivers were significantly more likely to have higher responses across all seven domains on the FS-ADS. The findings from this study underscore the complexity of AD stigma. Further research is warranted to understand its mechanisms, with the goal that by advancing our understanding we will also be able to advance methods to reduce or mitigate stigma. In particular, research that may help elucidate how stigma reports among caregivers do or do not link to practical behaviors. Understanding this may help inform clinical interventions to enhance both caregiver and patient.
CONFLICT OF INTEREST
The authors have no conflicts to disclose.
ACKNOWLEDGMENTS
This work was supported by grants from the Al-zheimer’s Association (AARF-17-528934), the University of Pennsylvania Alzheimer’s Disease Center (NIA P30 AG 010124). This publication is the result of work conducted by the CDC Healthy Brain Research Network. The CDC Healthy Brain Research Network is a Prevention Research Centers program funded by the CDC Healthy Aging Program-Healthy Brain Initiative. Efforts were supported in part by cooperative agreement from CDC’s Prevention Research Centers Program (U48 DP 005053). The views of this publication are those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention. The Institutional Review Board of the University of Pennsylvania approved all procedures involving human subjects.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-200206.
REFERENCES
[1] | Stites SD , Rubright JD , Karlawish J ((2018) ) What features of stigma do the public most commonly attribute to Alzheimer’s disease dementia? Results of a survey of the U.S. general public. Alzheimers Dement 14: , 925–932. |
[2] | Drennan VM , Cole L , Iliffe S ((2011) ) A taboo within a stigma? a qualitative study of managing incontinence with people with dementia living at home. BMC Geriatr 11: , 75. |
[3] | MacRae H ((2008) ) Managing courtesy stigma: The case of Alzheimer’s disease. Sociol Health Illn 21: , 54–70. |
[4] | Werner P , Goldstein D , Heinik J ((2011) ) Development and validity of the family stigma in Alzheimer’s Disease Scale (FS-ADS):. Alzheimer Dis Assoc Disord 25: , 42–48. |
[5] | Garand L , Lingler JH , Conner KO , Dew MA ((2009) ) Diagnostic labels, stigma, and participation in research related to dementia and mild cognitive impairment. Res Gerontol Nurs 2: , 112–121. |
[6] | Werner P , Mittelman MS , Goldstein D , Heinik J ((2012) ) Family stigma and caregiver burden in Alzheimer’s disease. Gerontologist 52: , 89–97. |
[7] | Mukadam N , Livingston G ((2012) ) Reducing the stigma associated with dementia: Approaches and goals. Aging Health 8: , 377–386. |
[8] | Behuniak SM ((2011) ) The living dead? The construction of people with Alzheimer’s disease as zombies. Ageing Soc 31: , 70–92. |
[9] | Park M , Sung M , Kim SK , Kim S , Lee DY ((2015) ) Multidimensional determinants of family caregiver burden in Alzheimer’s disease. Int Psychogeriatr 27: , 1355–1364. |
[10] | Heller T , Scott HM , Janicki MP ((2018) ) Caregiving, intellectual disability, and dementia: Report of the Summit Workgroup on Caregiving and Intellectual and Developmental Disabilities. Alzheimers Dement Transl Res Clin Interv 4: , 272–282. |
[11] | Stites SD , Johnson R , Harkins K , Sankar P , Xie D , Karlawish J ((2018) ) Identifiable characteristics and potentially malleable beliefs predict stigmatizing attributions toward persons with Alzheimer’s disease dementia: Results of a survey of the U.S. General Public. Health Commun 33: , 264–273. |
[12] | Johnson R , Harkins K , Cary M , Sankar P , Karlawish J ((2015) ) The relative contributions of disease label and disease prognosis to Alzheimer’s stigma: A vignette-based experiment. Soc Sci Med 143: , 117–127. |
[13] | Hughes CP , Berg L , Danziger WL , Coben LA , Martin RL ((1982) ) A new clinical scale for the staging of dementia. Br J Psychiatry J Ment Sci 140: , 566–572. |
[14] | Carpenter BD , Balsis S , Otilingam PG , Hanson PK , Gatz M ((2009) ) The Alzheimer’s Disease Knowledge Scale: Development and psychometric properties. Gerontologist 49: , 236–247. |
[15] | Whitehead J ((1993) ) Sample size calculations for ordered categorical data. Stat Med 12: , 2257–2271. |
[16] | Hurd MD , Martorell P , Delavande A , Mullen KJ , Langa KM ((2013) ) Monetary costs of dementia in the United States. N Engl J Med 368: , 1326–1334. |
[17] | National Alliance for Caregiving (2017) Dementia Caregiving in the U.S. |
[18] | Winblad B , Amouyel P , Andrieu S , Ballard C , Brayne C , Brodaty H , Cedazo-Minguez A , Dubois B , Edvardsson D , Feldman H , Fratiglioni L , Frisoni GB , Gauthier S , Georges J , Graff C , Iqbal K , Jessen F , Johansson G , Jönsson L , Kivipelto M , Knapp M , Mangialasche F , Melis R , Nordberg A , Rikkert MO , Qiu C , Sakmar TP , Scheltens P , Schneider LS , Sperling R , Tjernberg LO , Waldemar G , Wimo A , Zetterberg H ((2016) ) Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol 15: , 455–532. |
[19] | Fisher RJ ((1993) ) Social desirability bias and the validity of indirect questioning. J Consum Res 20: , 303. |
[20] | Link BG , Phelan JC ((2001) ) Conceptualizing stigma. Annu Rev Sociol 27: , 363–385. |
[21] | Werner P , Heinik J ((2008) ) Stigma by association and Alzheimer’s disease. Aging Ment Health 12: , 92–99. |
[22] | Haselton MG , Nettle D , Murray DR ((2015) ) The evolution of cognitive bias. In The Handbook of Evolutionary Psychology, Buss DM, ed. John Wiley & Sons, Inc., Hoboken, NJ, USA, pp. 1–20. |
[23] | Corrigan PW , Penn DL ((1999) ) Lessons from social psychology on discrediting psychiatric stigma. Am Psychol 54: , 765–776. |
[24] | Committee on the Science of Changing Behavioral Health Social Norms; Board on Behavioral, Cognitive, and Sensory Sciences; Division of Behavioral and Social Sciences and Education; National Academies of Sciences, Engineering, and Medicine ((2016) ) Approaches to reducing stigma. In: Ending Discrimination Against People with Mental and Substance Use Disorders: The Evidence for Stigma Change, National Academies Press (US), Washington, DC. |
[25] | Richeson JA , Shelton JN ((2006) ) A Social Psychological Perspective on the Stigmatization of Older Adults, National Academies Press (US). |




