Mental Disorders in Young Adults from Families with the Presenilin-1 Gene Mutation E280A in the Preclinical Stage of Alzheimer’s Disease
Abstract
Background:
There are forms of Alzheimer’s disease (AD) that have an autosomal dominant inheritance pattern; one of them is caused by the E280A mutation in the gene that codes for Presenilin-1 (PSEN1). Studying families of people with this mutation allows the evaluation of characteristics of the subjects before cognitive decline begins.
Objective:
To determine whether having the mutation E280A in PSEN1 increases the risk of presenting mental disorders in adults under 30 years old who are in the preclinical stage of AD and may be eligible for primary prevention studies of AD.
Methods:
A psychiatric evaluation was made to 120 people belonging to families with a history of early onset AD. Of these, 62 carried the E280A mutation in PSEN1. The occurrence of mental disorders between carriers and non-carriers of the mutation was compared.
Results:
No statistically significant differences were found in the frequency of any mental disorder between the group of carriers and non-carriers of the mutation (Hazard Ratio: 0.80, 95% CI 0.49 to 1.31); nor were differences observed when evaluating specific disorders.
Conclusion:
The E280A mutation does not increase the risk of mental disorders before the age of 30 in the relatives of people affected by familial AD. Studies with larger sample sizes are required to assess the risk of low incidence mental disorders.
INTRODUCTION
Alzheimer’s disease (AD) is the leading cause of dementia worldwide [1, 2]; it has a great impact on public health, among other reasons, due to the emotional burden and the economic cost to society, caregivers, and those who suffer it [3]. It is defined as a neuropsychiatric disease with a neuropathological component of neuronal loss, gliosis, abnormal accumulation of amyloid-β (Aβ) in the form of extracellular plaques, and intraneuronal accumulations of hyperphosphorylated tau protein in the form of neurofibrillary tangles and dystrophic neurites [4–6].
The clinical component is a spectrum of progressive and irreversible cognitive impairment that, in general, initially compromises the episodic memory and eventually leads to dementia [1, 2]. Dementia due to AD can be diagnosed as definitive, possible, or probable according to the criteria of the National Institute of Aging and the Alzheimer’s Association [7]. The diagnosis of dementia is equivalent to the Major Neurocognitive Disorder that is used in the Diagnostic and Statistical Manual of Mental Disorders Fifth edition (DSM-5) [8].
There are two variants of AD according to its genetic transmission: familial that corresponds to less than 5% of cases and is characterized by Mendelian inheritance patterns and the sporadic one that does not have a clear inheritance pattern [5, 9]. Familial AD can be caused by mutations in the genes that code for the Amyloid Precursor Protein (APP), Presenilin 1 (PSEN1), and Presenilin 2 (PSEN2) [2]. The most common causes are mutations in PSEN1 [9–11], of which 241 have been described in the database Alzforum (www.alzforum.org/mutations). PSEN1 and PSEN2 are part of the gamma secretase responsible for the abnormal cleavage of AβPP and produce the accumulation of Aβ [2, 12, 13].
The symptomatic phase prior to dementia is called mild cognitive impairment (MCI) characterized by a decrease in cognitive performance that is not normal for age and educational level. Level of performance on cognitive tests in MCI has been estimated at 1.5 standard deviations below the mean [14]. The phase prior to cognitive impairment is called preclinical and in it have been described neuropsychological and neuropathological alterations [1, 14, 15]. Research is being conducted in this preclinical phase directed at the discovery of reliable predictive markers that allow early diagnosis, disease monitoring, and response to treatment [16, 17].
The presence of neuropsychiatric symptoms other than cognitive impairment such as apathy, depression, agitation, and hallucinations, has been proposed as a marker for AD, before the development of dementia [18–20]. It has also been considered that personality structure can influence how a person experiences AD and is a cause of neuropsychiatric symptoms or that people with AD could have a unified behavior profile, an “Alzheimer’s personality” that would make them respond in a specific way to cognitive deterioration [21, 22]. However, most of the studies that describe this type of neuropsychiatric symptoms as possible markers, do not allow the clarification if these occur in phases in which there is already cognitive impairment that could not be considered preclinical.
There are differences in pathology and clinical expression between the familial and non-familial AD [23]. To identify AD markers and investigate other phenomena that occur in the preclinical phases, studies in autosomal dominant AD can be very useful because the penetrance is almost complete and could allow their results to be extrapolated to all forms of AD [23]. A study published in 2015 compared the frequency of psychiatric symptoms between 271 people with different mutations associated with familial AD at different phases and 106 people non-carriers of mutations [24]. In those without cognitive impairment, which could be considered as the preclinical phase, carriers of the mutation had a lower frequency of depressive symptoms than non-carriers and did not find any differences in other type of symptoms. Among those with MCI and dementia, there was a greater frequency of depression, apathy, disinhibition, irritability, and sleep disturbances compared to non-carriers of mutations. However, in this study the symptoms were measured cross-sectionally and mental disorders were not identified. It would be important to determine if in the preclinical phases there is a higher incidence of mental disorders associated with the presence of a mutation.
The Neurosciences Group of Antioquia (NGA) described and continues doing the follow-up of families with AD caused by the E280A mutation in PSEN1, a mutation characterized by a substitution of glutamic acid by alanine at codon 280 [12, 25, 26]. This constitutes the world’s largest group of families at risk of early-onset AD, with an average of 32 years for asymptomatic cognitive decline [9, 27]. The objective of the present study is to determine in adults under 30 years old belonging to these families, and possible candidates to be eligible for studies of primary prevention, if the carriers of the E280A mutation in PSEN1 have an increased risk of presenting mental disorders in comparison with non-carriers of that mutation.
METHODS
A historical cohort design was made taking as the starting point for the follow-up the birth of each of the participants and the end, the date of the evaluation. This study followed the ethical guidelines of the Helsinki Declaration (2013 Review) and Resolution 8430 of 1993 of the Ministry of Health of the Republic of Colombia that oversees the rights of people who participate in medical research. Before collecting information, this study was approved by the Bioethics Committee of the Faculty of Medicine of the University of Antioquia.
Participants
Individuals between 18 and 29 years with a family history of autosomal dominant Alzheimer’s disease (ADAD) by the E280A mutation in PSEN1, who have been genotyped for this mutation were included. Those with a history of brain injuries, moderate or severe mental retardation, and those who could not attend the evaluations due to living in other departments of Colombia or outside the country were excluded from the study. The sample size was calculated using the formula for survival analysis with two independent groups with censorship, a Type I error of 0.05, a Type II error of 0.20, a risk rate for the exposed group of 0.025, a risk rate for the control group of 0.01, a proportion of subjects control group of 0.5, and a time to the event of 29 years. The risk rates were taken from the frequencies throughout the life of minor depression in adults according to the last National Survey of Mental Health of Colombia of 2015 [28].
Procedures
The information was collected between December 2016 and January 2018. Personnel different from the evaluators identified potential participants from the database of the NGA Information System. For ethical reasons, at no time was the genetic status of a subject revealed to those who conducted the interviews or to the participants. The individuals were invited to participate by telephone, and they were evaluated at their home or in an office of the Research Headquarters of the University of Antioquia. Informed consent was read and signed and immediately after, a psychiatrist or a resident of psychiatry applied the Diagnostic Interview for Genetic Studies (DIGS) 3.0 and the Zarit scale to evaluate the caregiver’s burden. The evaluation of each individual took between one and two hours. Before starting data collection, the researchers responsible for applying the tools were trained.
The diagnosis of each mental disorder was made based on the information from DIGS and with the criteria of the Tenth Edition of the International Classification of Diseases (ICD-10). Best Diagnostic Estimation procedure was done consistent with the assignment of the diagnosis based on the independent review of the DIGS by two expert psychiatrists (different from those who did the interview). If a diagnostic consensus was not reached, a third psychiatrist was called.
Instruments
The Diagnostic Interview for Genetic Studies (DIGS) is a semi-structured interview that was designed as a tool for the evaluation of subjects participating in genetic studies of psychiatric disorders [29]. It has sections for the diagnosis of affective, psychotic, substance use, obsessive-compulsive, eating and panic disorders; in addition, phobias, pathological gambling, suicidal behavior, and dissocial personality. It was validated in Colombia by Palacio et al. [30], who demonstrated that it was understandable and useful in practice with high inter-rater and test-retest reliability.
The Zarit Scale is a psychometric test of objective qualification composed of 22 items, which assesses the presence of caregiver burden [31]. A total rating of 46 to 56 indicates a mild to moderate burden and greater than 56 severe burden. This scale was validated in Colombia by Barreto et al. [32].
Statistical analysis
The data distribution was evaluated using the Shapiro-Wilk test and the visual inspection of the normality graphs. Then, the population was described using frequencies and percentages for qualitative variables, medians, interquartile ranges, minimum and maximum for the quantitative variables because of the lack of normal distribution.
Survival analysis was used to calculate the risk of each disorder. The event time was the age of onset of each disease and the age at the time of the interview for those without history of mental disease. To adjust for possible confusing variables (sex, age, education, living together with a family member with dementia and caregiver burden), Cox proportional risk analysis was used and hazard ratio (HR) were estimated with their respective 95% Confidence Intervals (95% CI).
We evaluated whether there were differences between the group of carriers and non-carriers in the presence of several psychiatric comorbidities. A variable was generated with three categories: 0 = Without psychiatric disorder, 1 = Only one psychiatric disorder, 2 = Two or more psychiatric disorders. This was the dependent variable of a polytomous logistic regression, and the absence of psychiatric disorders was considered as comparison category. The independent variables were: carrier status, sex, age, education, living together with a family member with dementia and caregiver burden. Odds ratio (OR) were calculated with their respective 95% CI.
All statistical tests had a level of significance of 0.05 and were performed with the statistical software Stata 14.0.
RESULTS
The NGA database had 445 potential participants that met the inclusion criteria. Of these, 95 living in other departments or countries were excluded. It was only possible the communication with 154 potential participants, 32 refused to participate in the study, and 122 were finally interviewed, of which two were excluded for a moderate mental retardation (Fig. 1).
Fig.1
Participants selection.
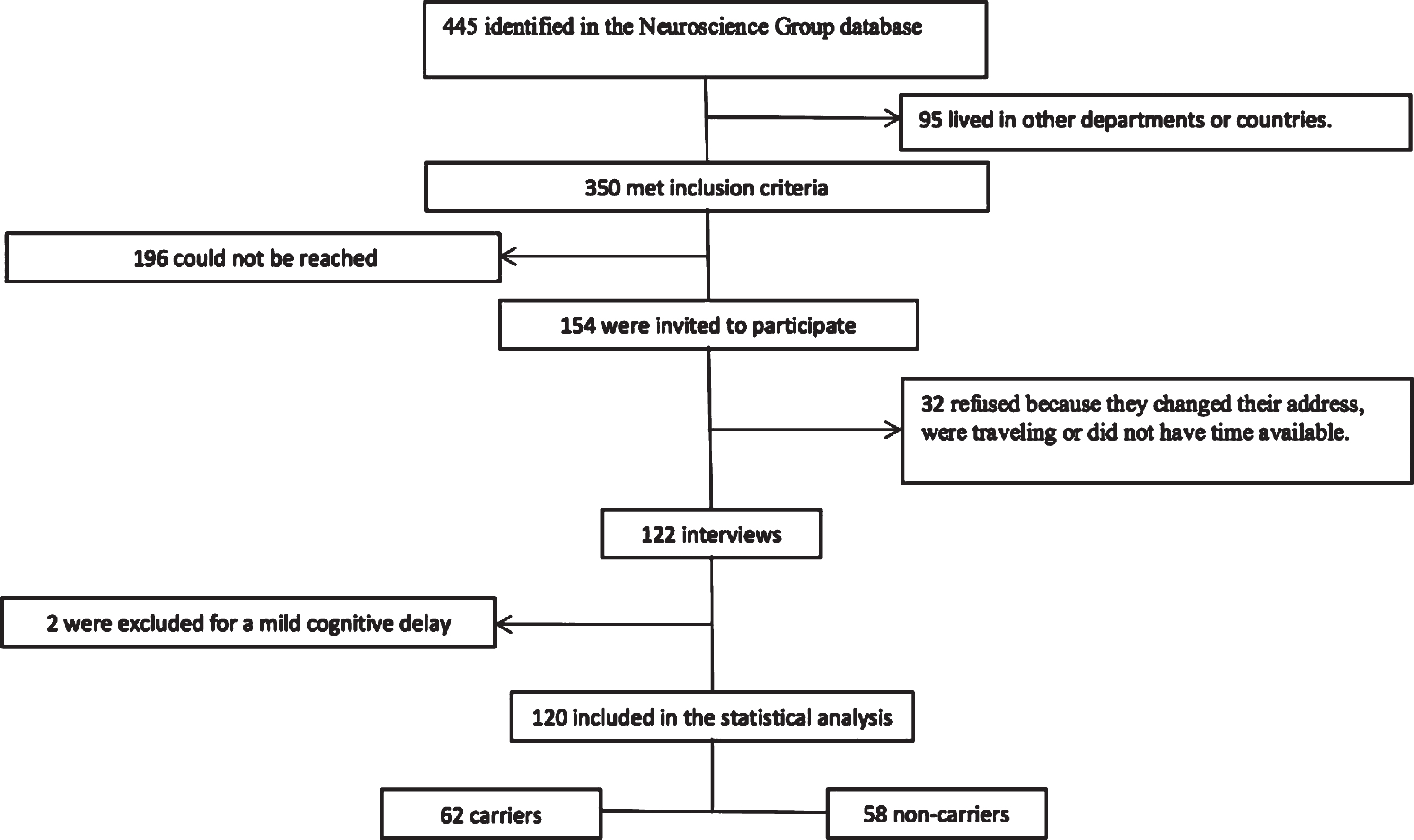
Of the 120 participants, 62 belonged to the group of carriers of the mutation, with a median age of 24 years (minimum 18 and maximum 29) and education of 11.5 years (minimum 0 and maximum 18). The group of non-carriers had a median age of 25 years (minimum 18 and maximum 29 and schooling of 12 years (minimum 2 and maximum 17). There was a greater frequency of cohabitants affected by AD among the carriers; other features were similar between the groups (Table 1).
Table 1
Demographic characteristics of the participants
| Characteristics | Carriers n = 62 | Non-Carriers n = 58 | ||
| Frequency | % | Frequency | % | |
| Male gender | 37 | 59.7 | 34 | 58.6 |
| Single marital status | 41 | 66.1 | 37 | 63.8 |
| Religious Practice | 55 | 88.7 | 42 | 73.7 |
| Occupation | ||||
| - Employee | 41 | 66.1 | 36 | 62.1 |
| - Without formal work* | 12 | 19.4 | 13 | 24.4 |
| - Student | 9 | 14.5 | 9 | 15.5 |
| Urban origin | 56 | 90.3 | 49 | 84.5 |
| Disabling physical illness | 6 | 9.7 | 4 | 6.9 |
| Cohabitation with family member with AD | 23 | 37.1 | 7 | 12.1 |
| Median | Min-Max | Median | Min-Max | |
| Age | 24 | 18 – 29 | 25 | 18 – 29 |
| Education (y) | 11.5 | 0 – 18 | 12 | 2 – 17 |
| Caregiver burden (Zarit scale score) | 0 | 0 – 73 | 0 | 0 – 44 |
Without formal work, Housewife or unemployed; Min, minimum; Max, maximum.
Frequency of mental disorders
The frequency of mental disorders in the group of carriers and non-carriers of the mutation was similar (63% and 64%, respectively), without statistically significant differences in the risk of developing them even after adjusting for possible confusing variables (HR: 0.82, 95% CI: 0.51–1.33, p = 0.42) (Table 2).
Table 2
Mental disorders in adults under 30 years old carriers and non-carriers of the E280A mutation of PS1
| Disorder | Carriers n = 62 n (%) | Non-Carriers n = 58 n (%) | HR (95CI%) | HRA. (95% CI) | p |
| Any mental disorder | 39 (63.0) | 37 (64.0) | 0.91 (0.58–1.42) | 0.80 (0.49–1.31) | 0.389 |
| Affective disorders* | 21 (33.9) | 13 (22.4) | 1.68 (0.84–3.36) | 1.59 (0.76–3.29) | 0.211 |
| - Bipolar affective disorder | 2 (3.2) | 2 (3.4) | 1.00 (0.14–7.07) | 0.37 (0.03–4.34) | 0.429 |
| - Unipolar depressive disorder** | 16 (25.8) | 11 (19.0) | 1.46 (0.68–3.14) | 1.35 (0.60–3.04) | 0.462 |
| Anxiety disorder*** | 11 (18.7) | 15 (25.9) | 0.64 (0.30–1.40) | 0.49 (0.21–1.17) | 0.108 |
| - Panic disorder | 1 (1.6) | 2 (3.4) | 0.47 (0.04–5.16) | 0.34 (0.02–5.36) | 0.449 |
| - Any phobia**** | 9 (14.5) | 11 (19.0) | 0.72 (0.30–1.75) | 0.66 (0.25–1.69) | 0.388 |
| Personality disorder | 7 (11.3) | 7 (12.1) | 0.95 (0.33–2.70) | 0.84 (0.27–2.63) | 0.771 |
| - Impulsive personality traits | 7 (11.3) | 6 (10.3) | 1.11 (0.37–3.30) | 1.10 (0.33–3.60) | 0.871 |
| -Impulsive control disorder | 3 (4.8) | 3 (5.2) | 0.94 (0.19–4.66) | 1.08 (0.21–5.42) | 0.926 |
| Substance use disorders | 12 (19.4) | 13 (22.6) | 0.92 (0.42–2.01) | 0.77 (0.32–1.82) | 0.552 |
| - Alcohol use disorder | 10 (16.1) | 9 (15.5) | 1.17 (0.47–2.88) | 0.75 (0.27–2.05) | 0.580 |
| - Drug use disorder | 9 (14.5) | 7 (12.1) | 1.23 (0.46–3.29) | 1.34 (0.46–3.87) | 0.587 |
| - Tobacco dependence | 8 (12.9) | 11 (19.0) | 0.66 (0.27–1.65) | 0.75 (0.27–2.07) | 0.580 |
| Pathological gambling | 4 (6.5) | 2 (3.4) | 1.95 (0.36–0.68) | 1.87 (0.28–12.14) | 0.511 |
| Suicide attempt | 6 (9.7) | 5 (8.6) | 1.15 (0.35–3.76) | 1.02 (0.27–3.76) | 0.972 |
HR, hazard ratio; HRA. hazard ratio adjusted for age, sex, education, living with a patient with Alzheimer’s disease and caregiver burden. *Affective disorders include bipolar affective disorder, unipolar depressive disorder and others. **Depressive disorder includes major depressive episode (single episode) and recurrent depressive disorder. ***Anxiety disorders include panic disorder, phobias, and others. ****Any phobia: Includes social phobia, specific, and agoraphobia.
When grouping the disorders into affective, anxious, personality, and alcohol and drug use, no statistically significant differences were found in the risk between carriers and non-carriers of the mutation (Table 2). However, there is a tendency of lower risk of anxiety disorders among carriers of the mutation compared to non-carriers (HR: 0.51, 95% CI: 0.22 – 1.17, p = 0.11). Also, this tendency of a lower presence of anxiety disorders can be observed in the Anxiety Disorder survival curve (Fig. 4).
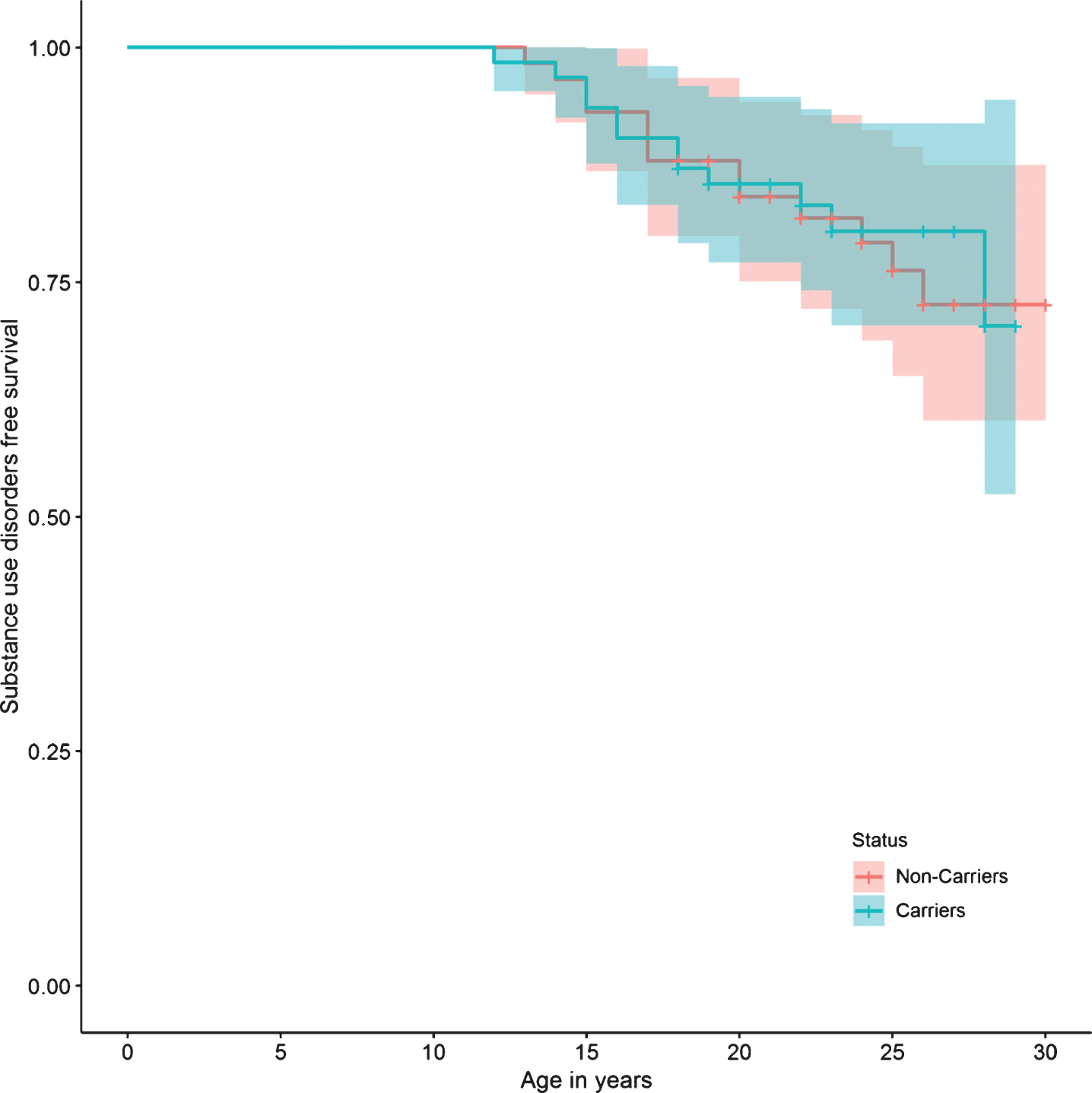
Regarding specific mental disorders, no statistically significant differences were found between the two groups in the risk of bipolar affective disorder, unipolar depressive disorder, panic disorder, phobias, impulse control disorder, alcohol and drug use disorders, tobacco dependence, and pathological gambling. There were also no differences in the risk of presenting suicide attempts, or in personality disorders (Table 2). The survival curves do not show a significant difference in the presence of any mental, affective, personality, or substance use disorders between carriers and non-carriers (Figs. 2, 3, 5, and 6).
Fig.2
Any mental disorder survival curve.
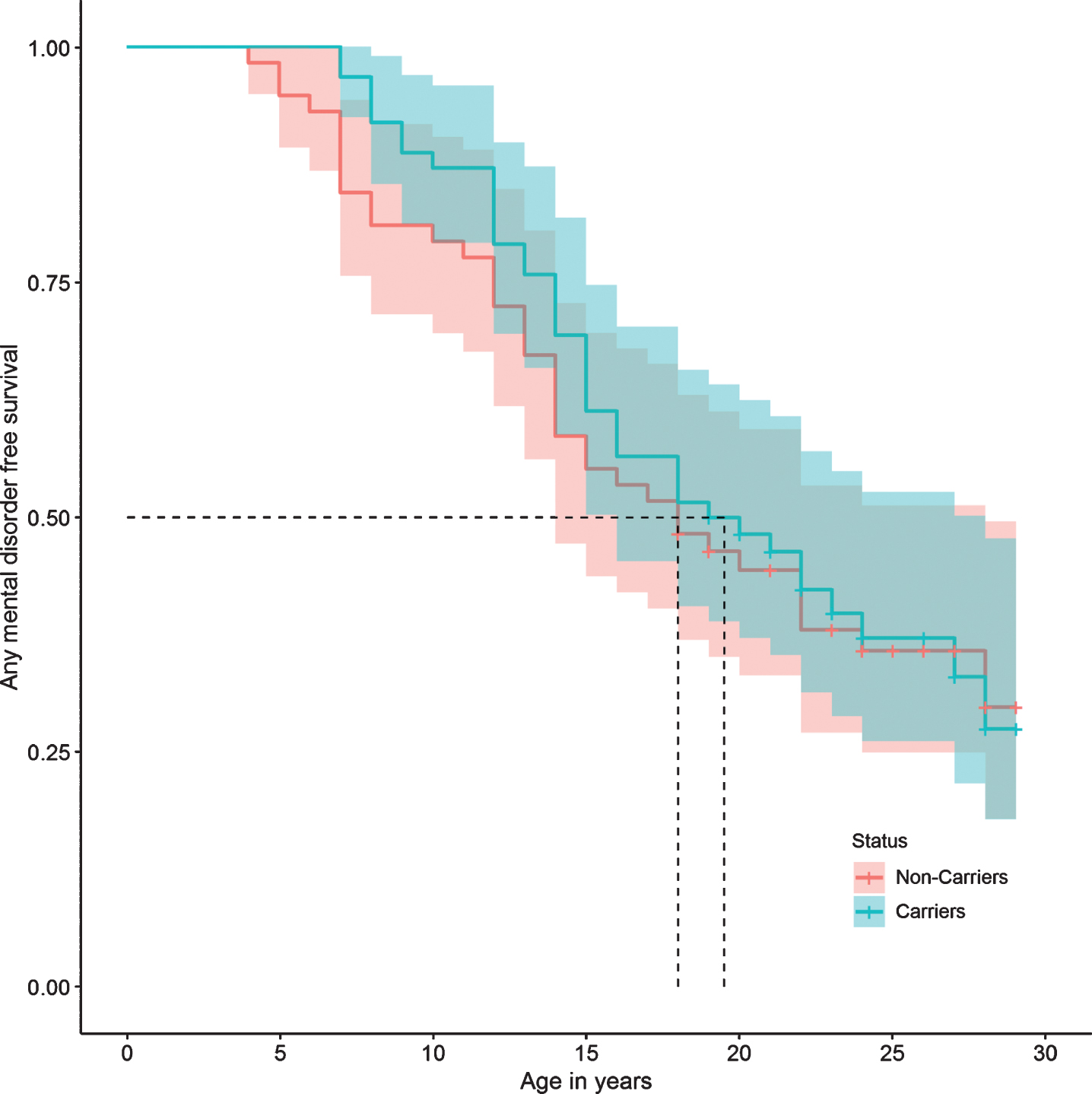
Fig.3
Affective disorder survival curve.
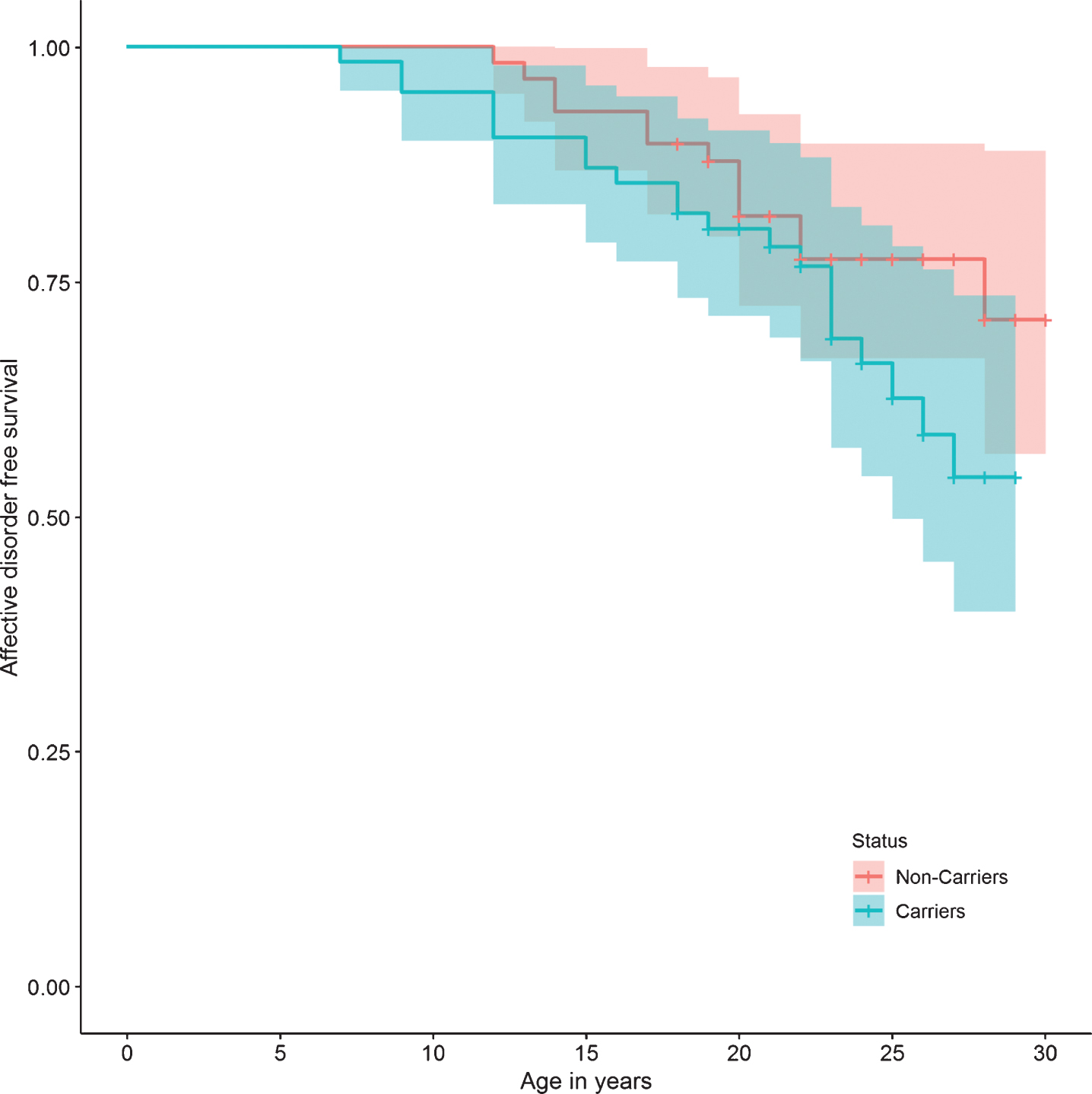
Fig.4
Anxiety disorder survival curve.
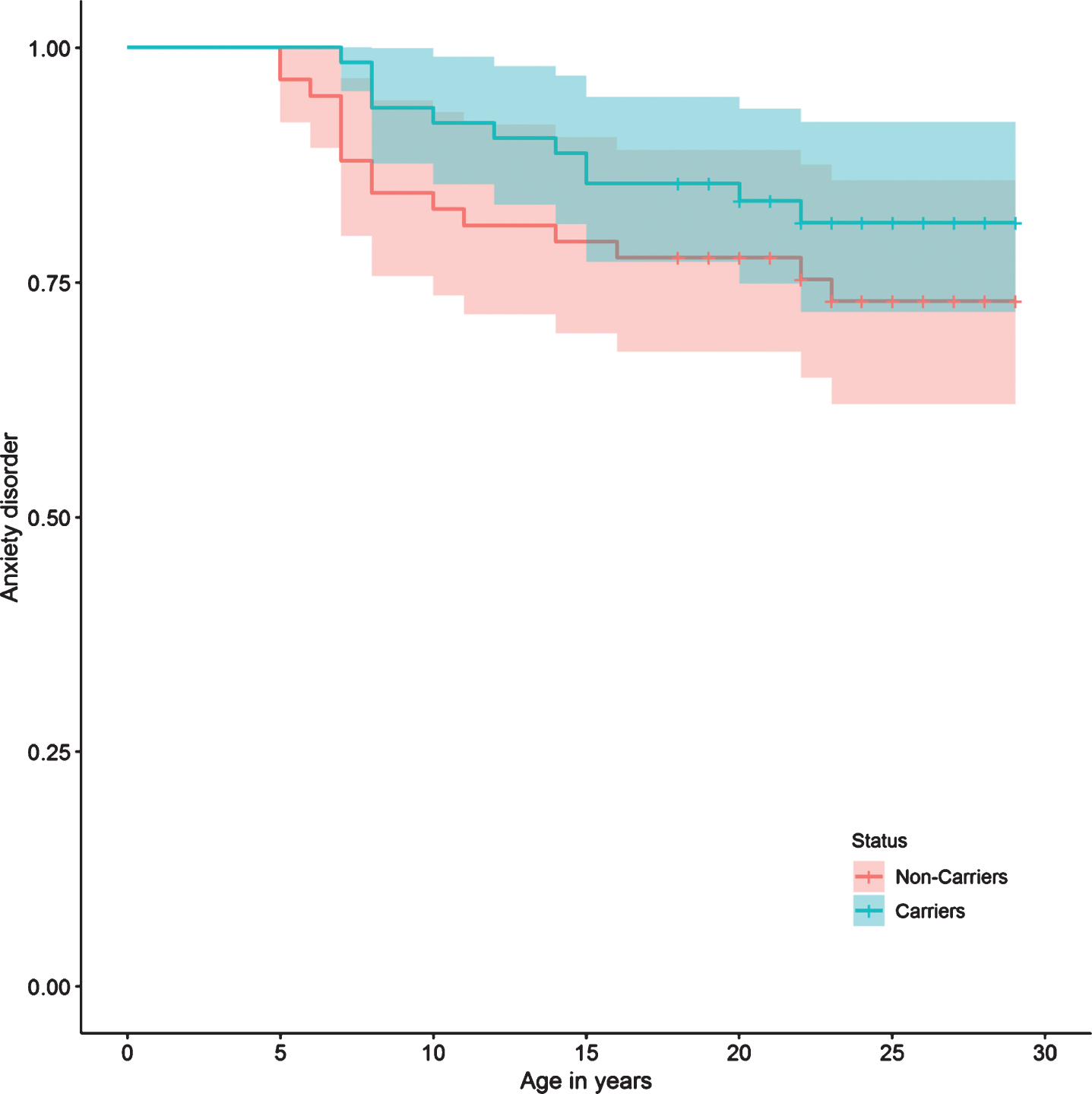
Fig.5
Personality disorder survival curve.
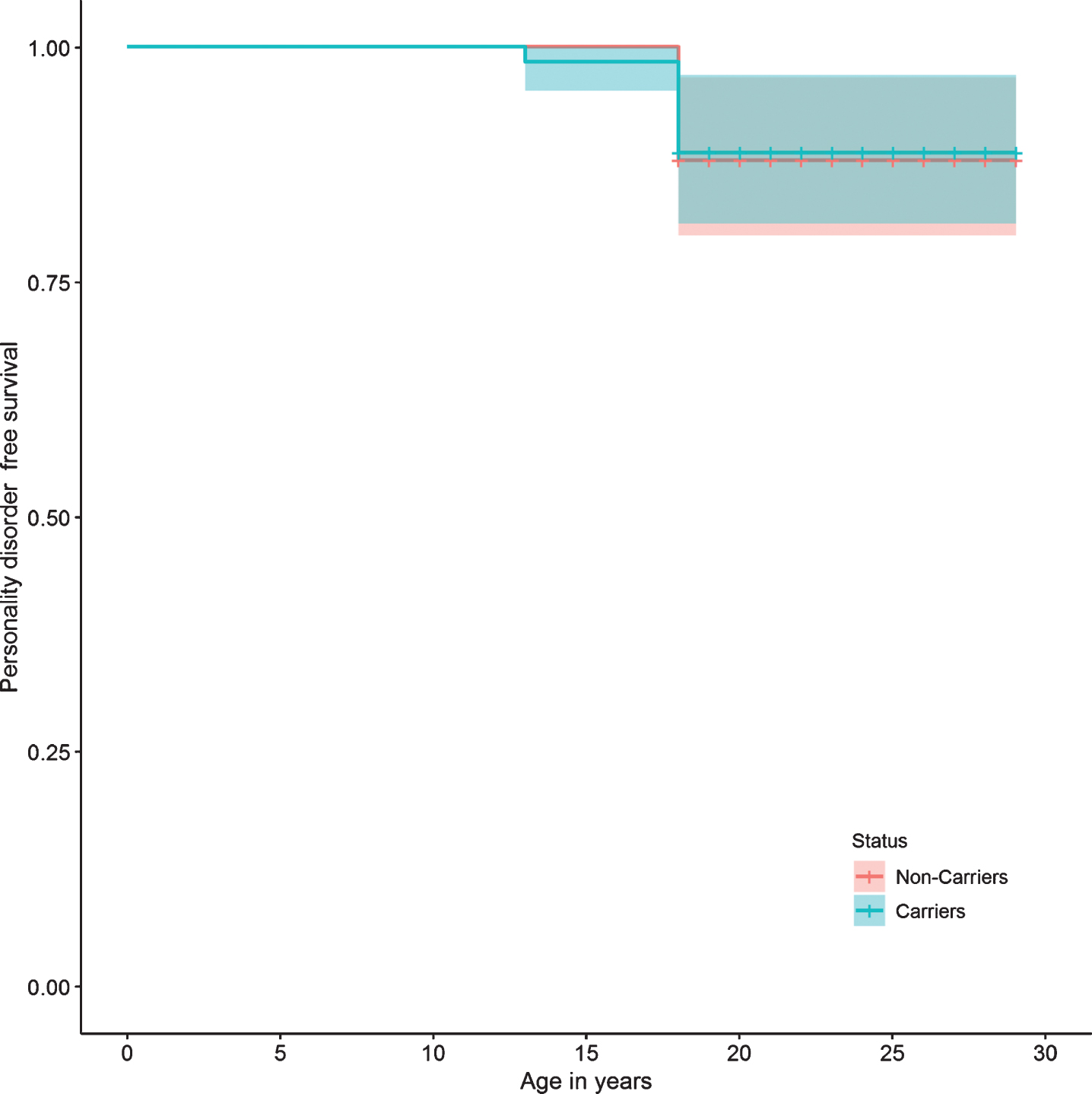
Fig.6
Substance use disorder survival curve.
No patients were found diagnosed with schizophrenia, delusional disorder, cyclothymia, or eating disorders in either of the two groups.
No differences were found between carriers and non-carriers in the possibility of having a single psychiatric disorder (OR = 1.28, 95% CI = 0.51 – 3.24, p = 0.59) or several (OR = 0.83, 95% CI = 0.32 – 2.14, p = 0.70) compared to the absence of psychiatric disorders.
DISCUSSION
The current study shows that in relatives younger than 30 years of patients with AD, those who are carriers of the E280A mutation in PSEN1 do not have a higher risk of mental disorders than those who do not have the mutation. The similarity in the risk of mental disorders between the two groups may be explained by the fact that the psychiatric manifestations in the preclinical stage represent a component that is not directly associated with the mutation and that could be determined mainly by the environment or variants in different genes.
The findings of the present study agree with those reported by Ringman et al. [24], who evaluated neuropsychiatric symptoms in families of mutation carriers in the APP, PSEN1, and PSEN2 genes. They included 97 mutation carriers without cognitive impairment, 25 with MCI, and 33 with dementia, which compared with 106 non-carriers. They applied the Neuropsychiatric Symptom Inventory (NPI), the 15-item Geriatric Depression Scale, and the Clinical Dementia Rating (CDR). They did not find a higher frequency of psychiatric symptoms in the mutation carriers without cognitive deterioration than in the non-carriers; on the contrary, the depressive symptoms were more common among non-carriers (5% versus 17%, p = 0.014), and the possibility of experiencing at least one behavioral sign in carriers without deterioration was lower than in non-carriers (Odds Ratio: 0.50, 95% CI: 0.26–0.98). The authors suggested that psychiatric symptoms related to the mutation occur when a threshold of neurodegeneration is reached, which supports the higher frequency of depressive and behavioral symptoms in those with MCI and dementia compared to non-carriers. [33]. It is important to highlight that the findings on that study are not completely comparable with ours because they evaluated symptoms in a transversal way, and not psychiatric disorders throughout life. Nevertheless, it is interesting how carriers showed a tendency of lower risk of anxiety disorders compared to non-carriers. There are several areas involved in fear and anxiety circuitries: thalamus, amygdala, dorsal anterior cingulate cortex, hypothalamus, hippocampus, and medial prefrontal cortex [34]. Asymptomatic PSEN1 E280A mutation carriers have hyperactivation in some of those areas like hippocampus and cingulate gyrus [35]; maybe this abnormal activation could be related to a lower risk of having an anxiety disorder, but another study with a bigger sample size and neuroimages would be necessary to prove this hypothesis.
Other studies have suggested that psychiatric symptoms and disorders, such as depression, are more frequent in carriers of PSEN1 mutations before cognitive decline and are related to having an early onset age [36]. However, these studies, due to their retrospective nature, have not been able to differentiate whether these symptoms occurred in preclinical stages or if there was already some degree of cognitive deterioration. There are other studies on neuropsychiatric symptoms in familial AD patients who already have cognitive impairment, and have shown that these symptoms occur in more than half of patients and may be more common in carriers of mutations in the PSEN1 gene than in carriers of mutations in other genes [37–39].
With regards to the preclinical phase, although the current study shows no difference in the risk of psychiatric disorders between young carriers and non-carriers of the E280A mutation in PSEN1, there are reasons to believe that clinical manifestations may already exist at this stage. In PSEN1 E280A mutation carriers between 9 to 17 years, Quiroz et al. founded abnormalities as elevated plasma concentrations of Aβ, higher volume of grey matter in the temporal and parietal regions, and lower activation of posterior parietal regions during a memory-encoding task in functional magnetic resonance compared to those without the mutation [27]. It is possible that there are differences in the risk of specific mental disorders that were not detected in the present study because some are not included in the diagnostic tool used. For instance, the DIGS does not have sections to evaluate attention deficit and hyperactivity disorder, nor does it include personality disorders other than dissocial; these diagnostics also are better evaluated through interviews with a second informant [40–43]. Another possibility is that there were psychiatric manifestations caused by the mutation in the preclinical phase before the age of 30, which consisted of symptoms that do not meet diagnostic criteria for disorders defined in the ICD-10.
In the present investigation, it was decided to evaluate the presence of mental disorders and not only of symptoms, due to the clinical implication in terms of dysfunction and need for treatment. Furthermore, the symptoms would have to be evaluated prospectively and by examining the context, since some may be normal responses to stressful or vital situations, and others may be prodromal manifestations of clinically relevant alterations. Therefore, it is complicated to evaluate subclinical neuropsychiatric symptoms as early markers of AD.
It is important to highlight the very high frequency of mental disorders in these families. Indeed, both in carriers and non-carriers of the mutation, the frequency is higher than in the general population according to the latest National Mental Health Survey of Colombia, in which it is reported a life-long prevalence of 9.1% for mental disorders, 3.9% for anxiety disorders, and 6.7% for affective disorders [28]. It is possible that in the members of these families there would actually be a greater frequency of mental disorders which could be explained by environmental factors such as knowing that they belong to a family with a genetic risk of a disabling disease, witnessing the cognitive impairment of close relatives, the family environment altered by cohabitation with a person with dementia, or the caregiver burden that has been associated with depressive and anxious symptoms [44, 45]. Another explanation could be that the frequency of mental disorders may not be greater than that of the general population, but there is a selection bias that the individuals who agreed to attend the evaluations felt the need for psychiatric assessment. However, the people who refused the evaluation were relatively few and the main reason was that they did not live in the urban area. It can also be thought that the difference in frequency estimates has been observed due to the use of different diagnostic tools, since the DIGS applied by trained psychiatrists was used in the present study and the Composite International Diagnostic Interview was used in the National Mental Health Survey. Therefore, it is possible that the frequencies of the present study cannot be compared with those reported in the general population. In fact, in other studies that have used DIGS, frequencies of mental disorders similar to those found in the current study have been reported [46].
A strength of this study is that the semi-structured DIGS interview has been used, standardized and validated for the diagnosis and detailed assessment of affective, psychotic, and substance use disorders. In addition, caregiver burden was considered as a variable of confusion and people belonging to families with the same mutation were taken. As limitations we recognize: 1) DIGS is not a suitable tool for diagnosing certain psychiatric disorders that may be important, such as attention deficit disorder, generalized anxiety disorder, and personality disorders; not only because of the lack of specific questions, but also because of the need for additional informants; 2) it was already mentioned that it is difficult to establish if there were differences between those who accepted and did not accept to participate in the study; 3) the sample size was low to estimate whether there were differences in the risk of less frequent mental disorders such as bulimia, anorexia nervosa, obsessive-compulsive disorder, dysthymia, and psychotic disorders; 4) it was not considered if individuals suspected to be carriers of the mutation and how this would modify the report of the symptoms.
Conclusion
Having the E280A mutation in the PSEN1 gene does not increase the risk of mental disorders, specifically affective, anxiety, and substance use, before the age of 30 in those who belong to the families of affected by AD autosomal dominant. This can be reassuring because having found otherwise could imply greater stigma in presenting these disorders and belonging to risk families. The frequency of mental disorders in these families is relatively high, so it is important to provide members with access to mental health care services. It is necessary to conduct studies with methods that facilitate the diagnosis of disorders that could not be evaluated in the present study, such as attention deficit hyperactivity disorder and personality disorders, with larger sample sizes to evaluate infrequent psychiatric disorders, and with designs that allow the evaluation of subclinical psychiatric disorders, such as apathy, demotivation, impulsivity, among others. Finally, the result of this study is of much interest to design primary prevention studies for AD since the population participating in this study, were young adults over 18 and under 30 years in a stage free of the neurocognitive symptoms of AD.
AUTHOR DISCLOSURES
A. Villalba, J. García, R. Cuastumal and D.C. Aguirre have nothing to disclosure. C. Ramos reports the following (pertinent for the last two years): grant and contract support from the NIA, Genentech/Roche, and an anonymous foundation to develop the Alzheimer’s Prevention Initiative Autosomal Dominant Alzheimer’s Disease - API ADAD - Registry since 2015 and she helps to conduct the API ADAD Trial in Colombia. D. Aguillón reports the following (pertinent for the last two years): grant and contract support from the National Institute on Aging - NIA -, Genentech/Roche, and an anonymous foundation to develop the API ADAD Registry since 2017 and he helps to conduct the API ADAD Trial in Colombia. L. Madrigal receives grant and contract support from the NIA, Genentech/Roche, and an anonymous foundation to develop the API ADAD Registry since 2013 and she helps to conduct the API ADAD Trial in Colombia. F. Lopera receives grand and contract support from the NIA, Genentech/Roche, and an anonymous foundation to develop the API ADAD Registry since 2013 and he is the principal investigator of the API ADAD Trial in Colombia.
ACKNOWLEDGMENTS
The authors thank every family with PSEN1 E280A mutation from Colombia.
The study was supported by “Programa de Sostenibilidad 2018-2019 del CODI al GNA” and by Neurosciences Group of Antioquia.
REFERENCES
[1] | Dubois B , Feldman HH , Jacova C , Cummings JL , DeKosky ST , Barberger-Gateau P , Delacourte A , Frisoni G , Fox NC , Galasko D , Gauthier S , Hampel H , Jicha GA , Meguro K , O’Brien J , Pasquier F , Robert P , Rossor M , Salloway S , Sarazin M , de Souza LC , Stern Y , Visser PJ , Scheltens P ((2010) ) Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol 9: , 1118–1127. |
[2] | Ballard C , Gauthier S , Corbett A , Brayne C , Aarsland D , Jones E ((2011) ) Alzheimer’s disease. Lancet 377: , 1019–1031. |
[3] | Wimo A , Guerchet M , Ali G-C , Wu Y-T , Prina AM , Winblad B , Jönsson L , Liu Z , Prince M ((2017) ) The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13: , 1–7. |
[4] | Zou Z , Liu C , Che C , Huang H ((2014) ) Clinical genetics of Alzheimer’s disease. BioMed Res Int 2014: , 1–10. |
[5] | Querfurth HW , Laferla FM ((2010) ) Alzheimer’s disease. N Engl J Med 362: , 329–344. |
[6] | Selkoe DJ , Hardy J ((2016) ) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8: , 595–608. |
[7] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[8] | American Psychiatric Association ((2013) ), Diagnostic and Statistical Manual of Mental Disorders. Washington, DC. |
[9] | Sepulveda-Falla D , Glatzel M , Lopera F ((2012) ) Phenotypic profile of early-onset familial Alzheimer’s disease caused by presenilin-1 E280A mutation. J Alzheimers Dis 32: , 1–12. |
[10] | Ringman JM , Goate A , Masters CL , Cairns NJ , Danek A , Graff-Radford N , Ghetti B , Morris JC ((2014) ) Genetic heterogeneity in Alzheimer disease and implications for treatment strategies. Curr Neurol Neurosci Rep 14: , 499. |
[11] | Giri M , Zhang M , Lü Y ((2016) ) Genes associated with Alzheimer’s disease: An overview and current status. Clin Interv Aging 11: , 665–681. |
[12] | Lemere CA , Lopera F , Kosik KS , Lendon CL , Ossa J , Saido TC , Yamaguchi H , Ruiz A , Martinez A , Madrigal L , Hincapie L , Arango JC , Anthony DC , Koo EH , Goate a M , Selkoe DJ ((1996) ) The E280A presenilin 1 Alzheimer mutation produces increased A beta 42 deposition and severe cerebellar pathology. Nat Med 2: , 1146–1150. |
[13] | Naj AC , Schellenberg GD , Alzheimer’s Disease Genetics Consortium (ADGC) ((2017) ) Genomic variants, genes, and pathways of Alzheimer’s disease: An overview. Am J Med Genet Part B Neuropsychiatr Genet 174: , 5–26. |
[14] | Acosta-Baena N , Sepulveda-Falla D , Lopera-Gómez CM , Jaramillo-Elorza MC , Moreno S , Aguirre-Acevedo DC , Saldarriaga A , Lopera F ((2011) ) Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: A retrospective cohort study. Lancet Neurol 10: , 213–220. |
[15] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimes disease. Alzheimers Dement 7: , 270–279. |
[16] | El Kadmiri N , Said N , Slassi I , El Moutawakil B , Nadifi S ((2018) ) Biomarkers for Alzheimer disease: Classical and novel candidates’ review. Neuroscience 370: , 181–190. |
[17] | Talwar P , Sinha J , Grover S , Rawat C , Kushwaha S , Agarwal R , Taneja V , Kukreti R ((2016) ) Dissecting complex and multifactorial nature of Alzheimer’s disease pathogenesis: A clinical, genomic, and systems biology perspective. Mol Neurobiol 53: , 4833–64. |
[18] | Rosenberg PB , Nowrangi MA , Lyketsos CG ((2015) ) Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol Asp Med 43-44: , 25–37. |
[19] | Canevelli M , Adali N , Voisin T , Soto ME , Bruno G , Cesari M , Vellas B ((2013) ) Behavioral and psychological subsyndromes in Alzheimer’s disease using the Neuropsychiatric Inventory. Int J Geriatr Psychiatry 28: , 795–803. |
[20] | Pocnet C , Rossier J , Antonietti JP , von Gunten A ((2013) ) Personality traits and behavioral and psychological symptoms in patients at an early stage of Alzheimer’s disease. Int J Geriatr Psychiatry 28: , 276–283. |
[21] | Pocnet C , Rossier J , Antonietti JP , Von Gunten A ((2011) ) Personality changes in patients with beginning Alzheimer disease. Can J Psychiatry 56: , 408–417. |
[22] | Robins Wahlin T-B , Byrne GJ ((2011) ) Personality changes in Alzheimer’s disease: A systematic review. Int J Geriatr Psychiatry 26: , 1019–1029. |
[23] | Schindler SE , Fagan AM ((2015) ) Autosomal dominant Alzheimer disease: A unique resource to study CSF biomarker changes in preclinical AD. Front Neurol 6: , 142. |
[24] | Ringman JM , Liang L-J , Zhou Y , Vangala S , Teng E , Kremen S , Wharton D , Goate A , Marcus DS , Farlow M , Ghetti B , McDade E , Masters CL , Mayeux RP , Rossor M , Salloway S , Schofield PR , Cummings JL , Buckles V , Bateman R , Morris JC , Dominantly Inherited Alzheimer Network ((2015) ) Early behavioural changes in familial Alzheimer’s disease in the Dominantly Inherited Alzheimer Network. Brain 138: , 1036–1045. |
[25] | Lopera F , Ardilla A , Martínez A , Madrigal L , Arango-Viana JC , Lemere CA , Arango-Lasprilla JC , Hincapíe L , Arcos-Burgos M , Ossa JE , Behrens IM , Norton J , Lendon C , Goate AM , Ruiz-Linares A , Rosselli M , Kosik KS ((1997) ) Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA 277: , 793–799. |
[26] | Lendon CL , Martinez A , Behrens IM , Kosik KS , Madrigal L , Norton J , Neuman R , Myers A , Busfield F , Wragg M , Arcos M , Arango Viana JC , Ossa J , Ruiz A , Goate AM , Lopera F ((1997) ) E280A PS-1 mutation causes Alzheimer’s disease but age of onset is not modified by ApoE alleles. Hum Mutat 10: , 186–195. |
[27] | Quiroz YT , Schultz AP , Chen K , Protas HD , Brickhouse M , Fleisher AS , Langbaum JB , Thiyyagura P , Fagan AM , Shah AR , Muniz M , Arboleda-Velasquez JF , Munoz C , Garcia G , Acosta-Baena N , Giraldo M , Tirado V , Ramírez DL , Tariot PN , Dickerson BC , Sperling RA , Lopera F , Reiman EM ((2015) ) Brain imaging and blood biomarker abnormalities in children with autosomal dominant Alzheimer disease: A cross-sectional study. JAMA Neurol 72: , 912–919. |
[28] | Ministerio de Salud y Protección Social de Colombia, Colciencias, Pontificia Universidad Javeriana, Datos P y TS. Encuesta Nacional de Salud Mental 2015. |
[29] | Nurnberger JI , Blehar MC , Kaufmann CA , York-Cooler C , Simpson SG , Harkavy-Friedman J , Severe JB , Malaspina D , Reich T ((1994) ) Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry 51: , 849–59; discussion 863-4. |
[30] | Palacio CA , Garcia J , Arbelaez MP , Sanchez R , Aguirre B , Garces IC , Montoya GJ , Gomez J , Agudelo A , Lopez CA , Calle JJ , Cardeno CA , Cano JF , Lopez MC , Montoya P , Herrera CP , Gonzalez N , Gonzalez A , Bedoya G , Ruiz A , Ospina J ((2004) ) [Validation of the Diagnostic Interview for Genetic Studies (DIGS) in Colombia]. Biomed Rev Inst Nac Salud 24: , 56–62. |
[31] | Vélez Lopera JM , Berbesí Fernández D , Cardona Arango D , Segura Cardona A , Ordóñez Molina J ((2012) ) Validación de escalas abreviadas de zarit para la medición de síndrome del cuidador primario del adulto mayor en Medellín. Aten Primaria 44: , 411–416. |
[32] | Barreto-Osorio RV , Campos MS , Carrillo-González GM , Coral-Ibarra R , Chaparro-Díaz L , Duran Parra M , Rosales-Jiménez R , Tamara-Ortiz V ((2015) ) Entrevista Percepción de Carga del Cuidado de Zarit: Pruebas psicométricas para Colombia. Aquichan 15: , 368–380. |
[33] | Aguirre-Acevedo DC , Lopera F , Henao E , Tirado V , Muñoz C , Giraldo M , Bangdiwala SI , Reiman EM , Tariot PN , Langbaum JB , Quiroz YT , Jaimes F ((2016) ) Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease: A retrospective cohort study. JAMA Neurol 73: , 431–438. |
[34] | Liberzon I , Duval E , Javanbakht A ((2015) ) Neural circuits in anxiety and stress disorders: A∼focused review. Ther Clin Risk Manag 11: , 115–126. |
[35] | Quiroz YT , Budson AE , Celone K , Ruiz A , Newmark R , Castrillón G , Lopera F , Stern CE ((2010) ) Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann Neurol 68: , 865–875. |
[36] | Mejía S , Giraldo M , Pineda D , Ardila A , Lopera F ((2003) ) Nongenetic factors as modifiers of the age of onset of familial Alzheimer’s disease. Int Psychogeriatr 15: , 337–349. |
[37] | Tang M , Ryman DC , McDade E , Jasielec MS , Buckles VD , Cairns NJ , Fagan AM , Goate A , Marcus DS , Xiong C , Allegri RF , Chhatwal JP , Danek A , Farlow MR , Fox NC , Ghetti B , Graff-Radford NR , Laske C , Martins RN , Masters CL , Mayeux RP , Ringman JM , Rossor MN , Salloway SP , Schofield PR , Morris JC , Bateman RJ , Dominantly Inherited Alzheimer Network (DIAN) ((2016) ) Neurological manifestations of autosomal dominant familial Alzheimer’s disease: A comparison of the published literature with the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS). Lancet Neurol 15: , 1317–1325. |
[38] | Shea Y-F , Chu L-W , Chan AO-K , Ha J , Li Y , Song Y-Q ((2016) ) A systematic review of familial Alzheimer’s disease: Differences in presentation of clinical features among three mutated genes and potential ethnic differences. J Formos Med Assoc Taiwan Yi Zhi 115: , 67–75. |
[39] | Ryan NS , Nicholas JM , Weston PSJ , Liang Y , Lashley T , Guerreiro R , Adamson G , Kenny J , Beck J , Chavez-Gutierrez L , de Strooper B , Revesz T , Holton J , Mead S , Rossor MN , Fox NC ((2016) ) Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer’s disease: A case series. Lancet Neurol 15: , 1326–1335. |
[40] | Lieberman L , Liu H , Huggins AA , Katz AC , Zvolensky MJ , Shankman SA ((2016) ) Comparing the validity of informant and self-reports of personality using laboratory indices of emotional responding as criterion variables. Psychophysiology 53: , 1386–1397. |
[41] | Kelley SE , Edens JF , Morey LC ((2017) ) Convergence of self-reports and informant reports on the personality assessment screener. Assessment 24: , 999–1007. |
[42] | De Los Reyes A , Augenstein TM , Wang M , Thomas SA , Drabick DAG , Burgers DE , Rabinowitz J ((2015) ) The validity of the multi-informant approach to assessing child and adolescent mental health. Psychol Bull 141: , 858–900. |
[43] | Bied A , Biederman J , Faraone S ((2017) ) Parent-based diagnosis of ADHD is as accurate as a teacher-based diagnosis of ADHD. Postgrad Med 129: , 375–381. |
[44] | Bianchi R , Schonfeld IS , Laurent E ((2015) ) Burnout-depression overlap: A review. Clin Psychol Rev 36: , 28–41. |
[45] | Bianchi R , Brisson R ((2017) ) Burnout and depression: Causal attributions and construct overlap. J Health Psychol, 1359105317740415. |
[46] | Palacio-Ortiz JD , Peña-Quintero CE , Gómez-Valero MA , Bustamante-Gómez PA , Arroyave-Sierra PH , Vargas-Upeguí CD , López-Jaramillo C ((2017) ) Trastornos psiquiátricos a través de la vida: Un estudio de comparación de hijos de padres con trastorno afectivo bipolar tipo I frente a hijos de padres controles de la comunidad. Rev Colomb Psiquiatr 46: , 129–139. |



