The Universal Patient Language: A set of resources and tools to design patient communications that support better health literacy
Abstract
Recently, pharmaceutical companies have increased their focus on engaging with patients, following trends toward patient centricity and delivering services around individual drugs. Meanwhile, the U.S. Food and Drug Administration has released draft guidance on disclosing risk information in consumer-directed materials that recommends against verbatim presentations of the risk-related sections within prescribing information, as this may be of limited value to consumers who lack medical or scientific training.
In this context, Bristol-Myers Squibb set out to build an organizational capability to communicate complex health topics to patients called the Universal Patient LanguageTM, or UPL. In this report, the authors explore health literacy considerations within the pharmaceutical industry; introduce the UPL; explain how human-centered service design methods were deployed to build the UPL; and present two UPL case studies.
1.Introduction
Historically, the U.S. pharmaceutical industry did not focus on communicating with patients; the industry status quo emphasized prescribers as key customers [1]. Since the industry’s primary audience usually was highly trained medical professionals, a concern for health literacy was not normative. In fact, the U.S. Food and Drug Administration (FDA) once noted that drug advertisements aimed at patients typically re-used information originally written for health care providers [2].
More recently, pharmaceutical companies have expanded their focus to include patient communications, following a broad trend toward patient centricity [3]. Simultaneously, there has been an increased emphasis on going ‘beyond the pill’ as the industry looks at delivering services around individual drugs [4]. Meanwhile, the FDA has released draft guidance for the U.S. pharmaceutical industry on disclosing risk information in consumer-directed materials. The FDA’s 2015 draft guidance states (‘PI’ refers to prescribing information or a drug label):
FDA strongly recommends against the use of the traditional approach to fulfill the brief summary requirement in consumer-directed advertisements, an approach in which risk-related sections of the PI are presented verbatim, often in small font. Because the target audience of the PI is health care providers, it is written in highly technical medical terminology, which is potentially of limited value to consumers who may not have the medical or scientific background to understand this information [5].
Within this context, Bristol-Myers Squibb (BMS) set out to build an organizational capability to communicate complex health topics to patients—going above and beyond industry standards. This capability came to be known as the Universal Patient LanguageTM, or UPL. In this report, the authors will:
explore health literacy considerations within the pharmaceutical industry;
introduce the components of the UPL;
explain how human-centered service design methods of co-creation, prototyping, and systems thinking were deployed to build the UPL; and
present two UPL case studies and their outcomes.
2.Health literacy, patient communications, and the pharmaceutical industry
Like all pharmaceutical companies, BMS regularly produces communications about complex healthcare topics for lay audiences. Given modern advances in medical science, these communications may seem complex, especially for audiences who lack specialist medical training. Broad, society-wide trends toward patient-centered care and the availability of information online means patients often attempt to parse this information independently, in addition to consulting with their healthcare teams. Unfortunately, patients with limited levels of health literacy may struggle with comprehension, and low health literacy levels are associated with poor health outcomes. Only 12% of adults in the U.S. have proficient health literacy levels, and even patients with high levels of health literacy sometimes find health information overwhelming [6,7].
2.1.What BMS learned in co-design sessions
In co-design sessions focused on clinical trials, BMS learned prospective study participants sometimes conflated a clinical trial treatment with an approved one and might be unaware that a study drug’s efficacy could be an open question. Thus, BMS realized the company needed to be more mindful of health literacy when considering what information should be included in informed consent forms. Before BMS could tackle explaining more sophisticated topics like trial design or the risks of participating in a specific trial, the organization needed to explain the concept of a clinical trial more generally.
Similarly, in co-design sessions focused on drug safety information, BMS asked patients what they understood from traditional presentations of the data, which typically list common side effects with no information about how frequently side effects were observed in clinical trials. While many patients assumed side effects were seen in more than half of clinical trial participants, in most cases the actual incidence was substantially lower—under 10%. As a result, BMS learned, by including natural frequencies or percentages relating to side effects (when available), a revised disclosure could help patients better understand risks. Of course, in providing percentages BMS assumed a certain level of reader numeracy, so BMS needed to know how to present numerical data in a way that would be more easily understood by patients and their caregivers.
2.2.Regulatory considerations
As a manufacturer of medicines, BMS also is heavily regulated regarding its public communications in the U.S. For instance, when communicating about approved drugs, BMS has a responsibility to stay within the approved prescribing information (PI), sometimes referred to as the label [8]. Labels are sophisticated, negotiated documents based on the results of complex trials. Although they are not written for the layperson, labels nonetheless capture information that patients want and need to know, such as the risks and benefits of a therapy [9]. BMS’s challenge is to give patients the general background information to help them understand its products while clarifying the relatively narrow indications outlined in the drug’s label.
When considering communications in research and development, it is important to recognize a clinical trial often is not a static, centrally controlled process. For example, clinical trials conducted by BMS will update informed consent forms throughout the study as new risks are uncovered or new samples need to be collected. Meanwhile, a BMS trial might be recruiting and obtaining consent from participants at hundreds of sites around the world, each with a separate ethical review process, required language, standards, and constraints. Hence, for BMS as a manufacturer, being mindful of health literacy in clinical trial communications is a matter of creating documents that are easier to understand and collaborating with other study sites to offer tools and templates that may help others improve and create consistency across their communications materials.
While informed consent and drug safety information provide only two examples, overall, BMS must be mindful of health literacy whenever it communicates with patients and caregivers about its medicine—whether while a drug is still in development, through promotional materials on a product website, over the phone in a patient support program, or in a printed brochure providing education on a broader disease state. Consequently, BMS decided to invest in an organizational capability to help employees communicate about complex topics to patients. The project was dubbed the Universal Patient LanguageTM, or UPL.
3.The Universal Patient LanguageTM
BMS’s Universal Patient Language (UPL) is a set of tools and resources that provide guidance on communicating with patients. The UPL is not designed for use by patients—rather, the target users are people who create patient communications, whether they are marketers, clinical scientists, or others who produce information for patients. The UPL has three broad components: principles, tools, and stewardship (Fig. 1).
Fig. 1.
Components of the UPL.

3.1.Principles
The principles provide overarching guidance, distilling the UPL down to seven foundational imperatives to create patient communications:
Enable Patient Learning: Equip patients with the knowledge they need to understand complex topics.
Share Qualified, Quantified Data: Present complete, relevant, and unbiased data in context.
Design for Digital First: Consider how patients want to navigate and engage with information.
Demonstrate Empathy for Patients and Caregivers: Acknowledge the experiences of patients and their caregivers and establish an emotional connection.
Use Plain Language: Explain complex topics in a straightforward and accurate way.
Communicate Visually: Visualize complex information to make it more digestible.
Format Materials for Understanding: Design layouts that are purposeful and can be easily navigated.
3.2.Tools
The UPL’s tools are used to apply the guiding principles in the tactical creation of communications. The toolset is extensive and diverse, encompassing guidance documents with tips and suggestions to apply UPL principles; build assets that can be used directly to make materials; and provide assessment tools to help people evaluate their communications. Some tools are quite specific to the work BMS does, such as templates for informed consent forms and drug safety information. Others are more generally applicable to anyone who creates patient communications. The toolset includes:
A graphic assets library that provides a starting collection of visuals that can be reused to support and strengthen patient understanding
The UPL rules, which provide detailed guidance on how each of the principles can be applied in practice
A style guide that offers detailed guidance on a UPL “look and feel,” covering everything from size of type and layout to the style of visuals and writing tone
A collection of thought starters that outlines key challenges and evidence regarding how to explain diverse healthcare topics such as biological processes, data, and even health-related financial information
A reflection guide that provides questions that can be used to self-assess how well a finished communication aligns with UPL principles
A patient impact measurement framework that provides initial guidance on testing the effectiveness of a communication created by following the UPL.
3.3.Stewardship
Of course, in any large organization, it would be insufficient to simply create the aforementioned resources. BMS invested significant effort in creating supporting materials to help its employees find, use, and update the UPL. Several UPL training courses have been delivered internally at BMS for different audiences, from legal and regulatory teams, to marketers, to the company’s advertising agency partners. The learning objectives of each course are tailored to different audiences at BMS according to role. There also is a digital repository for the UPL itself, and ‘case files’ showcase both final deliverables and interim work products from internal UPL projects.
In sum, the UPL is a rich repository of resources related to patient communications, ranging from the high level (principles), to the tactical (images that can be copied and pasted), to the pedagogical (hands-on training). How BMS developed the UPL materials and assessed their effectiveness is explained in the next section.
4.Building the UPL with human-centered service design
From the outset, BMS used human-centered service design to build the UPL. As with any human-centered design project, this meant that understanding and designing for people’s unmet needs was of paramount importance. However, since the UPL project was grounded in a service paradigm, the lens was wider. BMS considered how BMS and its patients fit into the broader healthcare ecosystem and how they participate in interactions in which complex healthcare information is communicated.
Tactically, BMS used three specific service design methods to build the Universal Patient Language: systems thinking; co-creation; and prototyping.
4.1.Systems thinking
Patients do not encounter BMS’s communications in a vacuum. Doctors, nurses, and other allied health professionals all have important roles to play, alongside patients, caregivers, and other stakeholders, such as clinical trial coordinators and health insurers. BMS sought contributions from all of these perspectives. During the five years BMS developed and grew the UPL, it engaged with 161 healthcare providers (physicians, nurses, pharmacists), 233 patients, 81 caregivers, and 79 other stakeholders representing everyone from social workers and pharmacists to advocacy groups and insurance companies.
After exposure to these diverse perspectives, BMS began to perceive patient experience and communication more multidimensionally. For instance, regarding drug safety information, different information is more or less relevant depending on where patients are in their health journey. If a patient is working with their healthcare team to decide on a treatment option, the risks of side effects may be more salient. Yet, if a patient is already taking the medication, he or she might be more aware of foods or other medicines that are contraindicated. Thinking about drug communication as part of a system with many players enables BMS to include features in its design that otherwise might not have come to mind.
Finally, BMS also wanted to seek other external perspectives and have them inform its work. Specifically, BMS wanted to leverage best practices and established expertise in communicating about complex medical topics. For this, BMS turned to experts in biomedical communications and medical illustration.
The Association of Medical Illustrators (AMI) was founded in 1945; its mission is to “further the use of visual media to advance life sciences, medicine, and healthcare through a worldwide network of specialized interdisciplinary professionals” [10]. BMS welcomed multiple AMI members to UPL co-design sessions so that they could inform its work with their expertise in areas like risk communication, data visualization, and use of plain language. In addition, all of BMS’s UPL communications were created by individuals with graduate degrees in biomedical communications—trained in both medical science and visual design. Their skill and expertise, combined with the perspectives of patients, caregivers, doctors, nurses, and other stakeholders, were integral to UPL’s development.
4.2.Co-creation
The entire UPL was co-created in 29 hands-on design sessions with the stakeholders outlined above. In recent years, ‘co-creation’ and ‘co-design’ have become buzzwords, meaning different things to diverse people. BMS’s approach was different from a focus group (where most of the time is spent talking) or market testing (where participants are asked to provide opinions on a handful of completed options). Instead, BMS’s co-creation sessions typically lasted about eight hours over two days. With the support of design facilitators, participants collaborated on curated, hands-on activities to build prototypes of new communications that addressed priorities.
Crucially, a variety of BMS participants also played an active role in building these prototypes. Thus, the sessions were not an exercise in producing a platonic ideal from the patients’ perspective. Rather, co-creation was a pragmatic, iterative process to find the sweet spot where the prototypes were achieving BMS’s business objectives (including legal and regulatory compliance) while also addressing the unmet needs of a variety of stakeholders: patients, caregivers, physicians, and extended healthcare teams.
4.3.Prototyping
Traditionally, prototyping is associated with large-scale industrial design. For example, an auto manufacturer or aerospace company builds a series of new car or aircraft prototypes prior to mass production. While it might seem strange to think of prototyping within the context of patient communications, BMS suggests the same principles apply.
Especially in the pharmaceutical industry, where there is significant administrative overhead with every publicly released communication, prototyping enables BMS to explore many more ideas and see which ones will resonate with patients. In fact, during the course of its UPL work, BMS produced hundreds of prototypes, ranging from large-scale ones built in co-creation with markers, glue, and scissors to more refined prototypes that were validated with patients in one-on-one feedback sessions. The process of soliciting feedback on prototypes for further iteration provided BMS with thousands of opportunities to understand patient perspectives and incorporate them into finished materials.
BMS’s prototyping mindset extended beyond the creation of individual patient materials to how BMS created and improved the UPL. Hypothetically, a straightforward way to craft the UPL would be initially to invest significant time to build the principles, tools, and training, and then to use these resources to create BMS’s patient communications. However, BMS took a prototyping approach and flipped the order of operations upside down.
BMS spent about a month co-creating an initial prototype of the UPL principles with a diverse set of subject matter experts both inside and outside BMS. With those principles in hand, BMS immediately turned its attention to individual patient communications, beginning with drug safety information. Day-to-day, BMS’s focus was on a specific patient communication with a specific purpose. Then, periodically, BMS would step back and generalize its findings, creating new UPL tools and refining existing ones based on what worked in practice. This explains why BMS refers to the UPL as a living prototype—always ready, never finished. Each time BMS builds a new patient communication, it learns something new that can be used to further refine the UPL’s resources. Hence, the UPL’s resources become a distillation of the lessons learned from co-design with patients, caregivers, doctors, nurses, and other stakeholders.
To put this another way, BMS’s confidence in the UPL and its constituent resources is driven by the quantity and quality of interactions BMS has experienced with people representing many diverse perspectives in healthcare. It also is grounded in real communications pieces that both leverage and inform the UPL.
5.Applying the UPL to patient communications
Overall, BMS has undertaken more than 25 UPL projects, resulting in dozens of new patient-facing materials, ranging from brochures to websites to call center scripts. BMS has applied the UPL to a broad array of topics, including clinical trials, drug safety information, how a drug works, health insurance, and broad disease-state education that is not tied to any one product.
UPL’s first application was to redesign how BMS communicates drug safety information. Before and during treatment, patients need to understand the treatment’s risks, benefits, and drug interactions, as well as other important information on how to take the medicine. Traditionally, drug safety information is developed within a fixed, text-only template working within specific FDA guidance. The goal was to redesign drug safety information to make it more inviting for patients to read and potentially easier to understand. Drug safety information was particularly attractive to address and envisage because, once approved, it is reused in a variety of contexts.
Looking at the traditional drug safety information compared to the UPL version, the contrast is striking (Fig. 2).
Fig. 2.
Traditional drug safety information vs UPL drug safety information.

The UPL design in Fig. 2 reflects dozens of design decisions that align to the UPL principles. The example suggests:
To communicate visually, BMS incorporated icons to help patients navigate their way around the document and included an image of the actual pill because that is something patients told BMS in co-creation they wanted to see.
To demonstrate empathy for patients and caregivers, each of the subheadings was re-phrased as a question. BMS learned in co-creation that these were questions patients wanted to ask about their medicine, either before or during treatment.
To format materials for understanding, BMS changed the layout of the document, using tables to make it easier for patients to find the information most relevant to them.
To share qualified, quantified data, BMS added percentages and natural frequencies showing how often side effects were observed during clinical trials.
BMS evaluated the UPL drug safety information for one specific product, conducting two small-scale studies with patients. First, BMS conducted qualitative interviews with 10 patients who were drug candidates but did not necessarily have any experience with the medicine. From these interviews, BMS learned patients appreciated seeing the information clearly broken down and found the use of color and bolding very helpful.
BMS also conducted a survey with 33 patients, who were asked to evaluate statements on a 5-point Likert scale (1 = strongly disagree; 5 = strongly agree). Seventeen respondents were shown the traditional format, and 16 were shown the new UPL format for drug safety information. On average, respondents found that the UPL version made it easier to identify the most relevant information (3.29 vs. 4.13); more effectively communicated the importance of following the instructions (3.29 vs. 4.13); and made the respondents more interested in learning about the drug (3.18 vs. 4.06) (see Table1).
Table 1
Survey results of 33 participants shown traditional format vs new UPL format
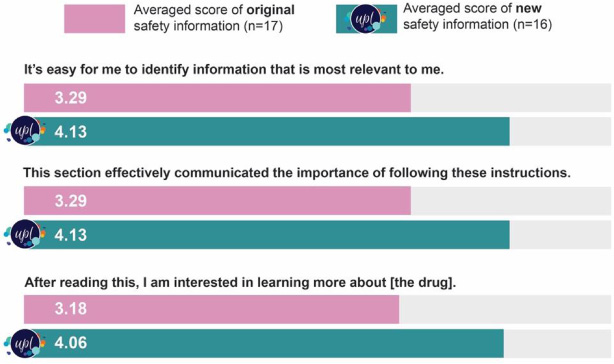 |
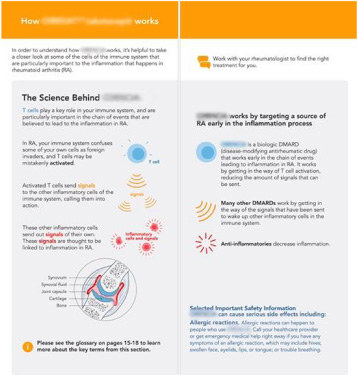
Fig. 3.
UPL redesign of how a drug works.
Another application of the UPL was to reconsider the promotional materials that explained how one particular drug works. BMS’s traditional explanation for how the drug works was quite complicated. It used words patients found difficult to understand and did not resonate with patient knowledge or experience. The UPL version was a complete redesign, following UPL principles and leveraging the UPL toolset (Fig. 3):
To use plain language, BMS greatly simplified the story, removing references to many elements within the immune system’s cascade, such as ‘macrophage,’ ‘cytokines,’ and ‘antigen-presenting cells.’ In co-creation, patients told BMS those terms were so foreign that they diminished their interest in the topic. BMS worked with the patients to craft a new story that focused on the actions of T cells.
To communicate visually, BMS used a visual analogy of a cell phone or wi-fi signal to represent the complicated idea of immune cascades. This visual analogy was actually created by the patients in co-creation, because they felt it helped communicate the key idea of parts of the immune system signaling other parts.
To enable patient learning, BMS provided a labelled illustration of a joint to visually identify clinical jargon and demonstrate the immune response to inflamed joints.
To further understand the impact of the UPL version that explains how the drug works, BMS conducted video interviews with 13 rheumatoid arthritis patients (who had experience with the specific drug). BMS showed all participants the traditional and UPL explanations (alternating the order in which it was shown to counter a first-seen bias). Eleven of the 13 interviewed patients (∼85%) found the UPL version was more useful and generated additional confidence to engage with healthcare providers about the management of their treatment. Twelve of 13 patients (∼92%) said the UPL version was easier to understand than the prior version. Although the evaluation was limited by a small sample size (and the fact the participants already were familiar with the specific drug), the participants clearly favored the UPL-generated version.
From a business perspective, the UPL’s benefits also were evident. After updating the brand’s website to include a variety of UPL materials (not just the explanation of how the drug works), the number of site visitors who took a follow-on action on the site (such as clicking a link) jumped from 9% to 38%. Internal teams also report that UPL materials are more in demand than the previous materials.
6.Conclusion
As the healthcare landscape becomes more patient-centric, the U.S. pharmaceutical industry is devoting more effort to enhance patient communications and a patient’s overall experience as a health consumer. Health literacy is becoming increasingly relevant for the pharmaceutical industry as these trends continue.
For BMS, the Universal Patient Language (UPL) is one important way to incorporate health literacy into patient-facing communications. The UPL is a set of resources—comprising principles, tools, and stewardship—that help communicate complex topics to patients. BMS built the UPL using human-centered service design, collaborating repeatedly with patients, caregivers, physicians, nurses, allied health care providers, and experts in biomedical communication and medical illustration. BMS has created dozens of UPL patient communications internally.
BMS’s small-scale evaluation studies suggest patients respond well to UPL-generated materials and find them more inviting. Although some UPL materials for clinical trials have been used in translation around the world, most UPL work has occurred in the U.S. The adoption of the UPL for different countries, cultures, healthcare systems, and languages provides diverse opportunities for future exploration and implementation.
As BMS developed the UPL, it began to appreciate that many of the UPL’s elements are not specific to BMS or the pharmaceutical industry. While some aspects are proprietary, BMS publicly released a significant portion of the UPL, which is available at www.UPL.org. BMS believes the UPL materials on the website can be useful to anyone who works in health literacy or who creates patient communications.
BMS recommends the UPL’s use by health literacy practitioners and hopes it contributes to enhanced health communications from healthcare organizations to patients, caregivers, consumers, and health professionals.
References
[1] | J. Donohue, A history of drug advertising: the evolving roles of consumers and consumer protection, Milbank Q 84: (4) ((2006) ), 659–699. doi:10.1111/j.1468-0009.2006.00464.x. |
[2] | https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm069984.pdf. Retrieved June 1, 2019. |
[3] | S. Barello, G. Graffigna and E. Vegni, Patient engagement as an emerging challenge for healthcare services: mapping the literature, Nurs Res Pract ((2012) ). doi:10.1155/2012/905934. |
[4] | M. Wenzel, N. Henne and Y. Zöllner, Beyond the pill: The move towards value-added services in the pharmaceutical industry, J. Med. Mark 14: (2–3) ((2014) ), 91–98. doi:10.1177/1745790414556564. |
[5] | https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm069984.pdf. Retrieved June 1, 2019. |
[6] | https://health.gov/communication/literacy/issuebrief. Retrieved June 1, 2019. |
[7] | https://health.gov/communication/literacy/quickguide/factsbasic.htm. Retrieved June 1, 2019. |
[8] | https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/LawsActsandRules/ucm084159.htm. Retrieved June 1, 2019. “On January 24, 2006, the U.S. Food and Drug Administration (FDA) issued final regulations governing the content and format of prescribing information (PI) for human drug and biological products. The rule is commonly referred to as the ‘Physician Labeling Rule’ (PLR) because it addresses prescription drug labeling that is used by prescribers and other health care providers…. The goal of the PLR content and format requirements ... is to enhance the safe and effective use of prescription drug products by providing health care providers with clear and concise PI that is easier to access, read, and use. The PLR format also makes PI more accessible for use with electronic prescribing tools and other electronic information resources.”. |
[9] | https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm069984.pdf. Retrieved June 1, 2019. |
[10] | https://www.ami.org/about-ami. Retrieved June 1, 2019. |



