KIF18A promotes cervical squamous cell carcinoma progression by activating the PI3K/AKT pathway through upregulation of CENPE
Abstract
BACKGROUND:
Cervical cancer is a prevalent malignancy that significantly contributes to morbidity and mortality rates among women in developing nations. Although the association of KIF18A with various cancers has been established, its role in cervical squamous cell carcinoma (CESC) remains elusive.
METHODS:
The KIF18A impact on the progression of CESC and its underlying mechanism were investigated through comprehensive bioinformatics analysis utilizing publicly available datasets. The levels of KIF18A and CENPE were assessed in clinical CESC samples through western blotting and qRT-PCR. To discover the role and molecular pathways of KIF18A in CESC, a combination of experimental approaches, including wound-healing, flow cytometry, CCK-8, and Transwell assay, were employed.
RESULTS:
Our results demonstrate a significant KIF18A expression upregulation in CESC tissues in contrast to healthy tissues. In vitro, KIF18A upregulation was found to enhance cell growth, migration, and invasion and activate the PI3K/AKT signaling pathway while concurrently suppressing apoptosis. Conversely, downregulating KIF18A exhibited contrasting effects. Mechanistically, we observed a positive significant connection between KIF18A and CENPE in CESC cells.
CONCLUSION:
KIF18A promotes tumor growth in CESC by modulating the PI3K/AKT signaling pathway through regulation of CENPE, making it a potential biomarker for diagnosis and prognosis as well as a therapeutic target.
1.Introduction
Cervical cancer is a malignancy tumor that affects the reproductive system of the female and is a major health concern for women worldwide, ranking second only to breast cancer in its effect on women’s health. Despite extensive efforts to promote vaccination and implement cervical cancer screening programs, the global incidence of new cases reached a staggering 604,127 in 2020, leading to an alarming number of over 341,831 fatalities [1]. Even though individuals diagnosed with cervical malignancy in early stage generally have a favorable prognosis, the potential for an adverse outcome escalates in cases of recurrence or metastasis. Cisplatin-based chemotherapy is a commonly recommended treatment for advanced cervical cancer, yet its efficacy remains suboptimal, with a 5-year overall survival rate of only 68% for patients [2, 3]. Cervical squamous cell carcinoma (CESC) constitutes the majority of cervical malignancy cases, with approximately 70%–80% being squamous type and around 20%–25% being adenocarcinomas [4, 5]. Therefore, it remains imperative to investigate the pathway underlying CESC development, detect appropriate markers, and explore novel targets for therapeutic intervention.
KIF18A, a kinesin superfamily member, functions as a molecular motor powered by ATP hydrolysis to generate force and travel through microtubules [6]. Recently, there has been a rising emphasis in the field of molecular oncology research on investigating the expression and significance of KIF18A in tumors [7]. It is worth mentioning that recent investigations have exhibited a strong connection between KIF18A and the development, progression, invasion, and metastasis of various malignant tumors [8, 9]. Additionally, targeting KIF18A has been recognized as a potential therapeutic approach, and its significance as a prognostic indicator for different kinds of cancer like hepatocellular carcinoma, prostate cancer, and lung adenocarcinoma has been well-documented [9, 10, 11]. The KIF18A gene knockdown suppresses the growth, migration, and invasion of malignancy cells of the esophagus while enhancing cellular sensitivity to radiotherapy [12]. The expression of KIF18A in CESC has not yet been investigated for its correlation and clinicopathological significance, highlighting the necessity for further investigation.
Consequently, the goal of this investigation was to discover the connection between KIF18A and the medical features and prognosis of CESC while also elucidating the underlying molecular mechanisms.
2.Materials and methods
2.1Bioinformatic analysis
We acquired gene expression data and health information from the Cancer Genome Atlas (TCGA) dataset, available at https://portal.gdc.cancer.gov. The information was extracted in level 3 HTSeq fragments per kilobase per million (FPKM) format. The mRNA expression profiles datasets, GSE63514 [13] and GSE9750 [14], were acquired from the database of GEO (http://www.ncbi.nlm.nih.gov/geo) for subsequent analysis. We employed the
Table 1
Clinicopathological characteristics of 38 patients with CESC and their associations with KIF18A expression
| Characteristics | Cases ( | KIF18A expression | ||
|---|---|---|---|---|
| Low ( | High ( | |||
| Age | 0.744 | |||
| | 17 | 6 | 11 | |
| | 21 | 9 | 12 | |
| Tumor size | 0.728 | |||
| | 26 | 11 | 15 | |
| | 12 | 4 | 8 | |
| T stage | 0.021* | |||
| T1–2 | 20 | 11 | 9 | |
| T3–4 | 18 | 4 | 14 | |
| N stage | 0.003* | |||
| N0 | 21 | 13 | 8 | |
| N1-2 | 17 | 2 | 15 | |
| FIGO stage | 0.016* | |||
| I | 23 | 13 | 10 | |
| II | 15 | 2 | 13 | |
*P< 0.05 Considered statiscally significant.
2.2Clinical tissues
We obtained samples of tumors tha embedded in paraffin from 38 patients with CESC and treated at Weifang People’s Hospital between 2022 and 2023. We obtained recently collected healthy tissues from eight individuals with CESC undergoing radical resection at Weifang People’s Hospital. All specimens of the tissue were subjected to verification by two expert pathologists. The Ethics Committee of Weifang People’s Hospital provided the authorization for the investigation. The clinicopathological characteristics of the 38 cases diagnosed with CESC were meticulously documented and recorded in Table 1.
2.3Cell lines, cell culture, transfection
The cell lines of HcerEpic and three human CESC (C-33A, CaSki, and SiHa) were obtained from the American Type Culture Collection (ATCC). The cells were grown in DMEM (Gibco, USA) with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS, Gibco, USA) combination. The incubation was conducted in an incubator with a fixed temperature of 37∘C and under a 5% CO2 environment. The KIF18A expression level in cervical cancer cells was assessed using western blot and qRT-PCR. SiHa cells exhibiting high endogenous KIF18A expression were chosen for further investigation. The targeting sequences for KIF18A (5’-GGAGTGATGTATCTAACAATG-3’) and CENPE (5’-GAAGAAATGGAATTGAAATTA-3’) were incorporated into lentiviral vectors, respectively. The lentiviruses for KIF18A overexpression and its negative control were generated by GeneChem (Shanghai, China). The validation of gene suppression or overexpression was verified by employing qRT-PCR and western blot.
2.4Western blotting
Tissue specimens and cells were utilized for obtaining total protein employing a customized RIPA buffer solution from Kangwei, China. The proteins have been extracted by 12.5% SDS-PAGE from Epizyme in China and then transmitted to PVDF membranes from Millipore in America with a diameter of the pore of 0.45 mM. Following blocking with 5% skimmed milk powder for sixty minutes, the sample was rinsed three times utilizing TBST. The membranes subjected to incubation at 4∘C overnight with primary antibodies against KIF18A (1:2000, ab72417), CENPE (1:1000, ab133583), p-PI3K (1:500, ab182651), PI3K (1:1000, ab302958), p-AKT (1:1000, ab38449), AKT (1:1000, ab8933), and GADPH (1:1000, ab8245) obtained from Abcam (Shanghai, China). Subsequently, the membranes were subjected to incubation for 2 h with an antibody conjugated to horseradish peroxidase (HRP) at ambient temperature. The detection of target proteins was achieved using an enhanced ECL kit (Bio-rad, USA).
2.5RNA extraction and qRT-PCR
The TRIzol reagent (Invitrogen, USA) was employed to obtain total RNA from cells and tissues following the directions of the manufacturer. Next, analysis was performed utilizing reverse transcription-PCR and the SYBR Green II kit (Takara, Japan). The relative target gene expression was standardized with respect to the GAPDH expression. The sequences of primer are as follows: KIF18A (forward) 5’-CTAACCGGGCAAAGGACATTA-3’ and (reverse) 5’-ATCTCTGCCTTCTGCTCATTAC-3’; CENPE (forward) 5’-CACCTCATCCAGTTCGCTATT-3’ and (reverse) 5’-CACTCTCTGCTCCTGCATTT-3’; GADPH (forward) 5’-CACCCACTCCTCCACCTTTG-3’ and (reverse) 5’-CCACCACCCTGTTGCTGTAG-3’.
2.6Cell proliferation assay
Cells were first placed in 96-well plates at a density of 1
2.7Cell apoptosis assay
Flow cytometry was utilized to perform the assessment of cell apoptosis according to established protocols. Cells underwent culturing at a density of 1
2.8Scratch wound assay
Cells underwent seeding into 6-well e-plates and overnight culturing. The following day, a 200
2.9Transwell assay
The invasion assay was conducted employing Matri-gel-coated chambers in 24-well Transwell plates with a pore size of 8
2.10Coimmunoprecipitation (Co-IP) assay
The exogenous Co-IP assay was performed by co-transfecting Flag-KIF18A and HA-CENPE into HEK-293T cells. The cells were lysed using an IP buffer containing 20 mM Tris-HCl, 1 mM EDTA, 150 mM NaCl, and 1% NP-40. Additionally, a 1% phosphatase inhibitor cocktail was included in the buffer solution. The lysis process was conducted at 4∘C for a duration of thirty minutes. Following a centrifugation step at 12,000g and 4∘C for ten minutes, the supernatant was collected. Subsequently, it was incubated overnight at 4∘C with magnetic beads (20
2.11Statistical analysis
SPSS 25.0 and GraphPad Prism 8.0.1 were employed to conduct analysis for all experimental data. All assays were performed three times, and the outcomes were represented as the mean
3.Results
Figure 1.
The KIF18A expression is significantly upregulated in cervical cancer. (A) Venn diagram illustrating the overlapping DEGs obtained from TCGA-CESC, GSE63514, and GSE9750 datasets. (B) The expression of KIF18A was analyzed across multiple cancer types using data obtained from the TCGA. (C–E) Volcano plots were employed to identify DEGs in the TCGA-CESC, GSE63514, and GSE9750 datasets, respectively. (F–H) The KIF18A expression level exhibited a significant rise in TCGA-CESC, GSE63514, and GSE9750 datasets. (I, J) KIF18A expression levels were assessed and quantified in CESC samples and their corresponding normal tissues using Western blot analysis and qRT-PCR. * p < 0.05; **p < 0.01; ***p < 0.001.
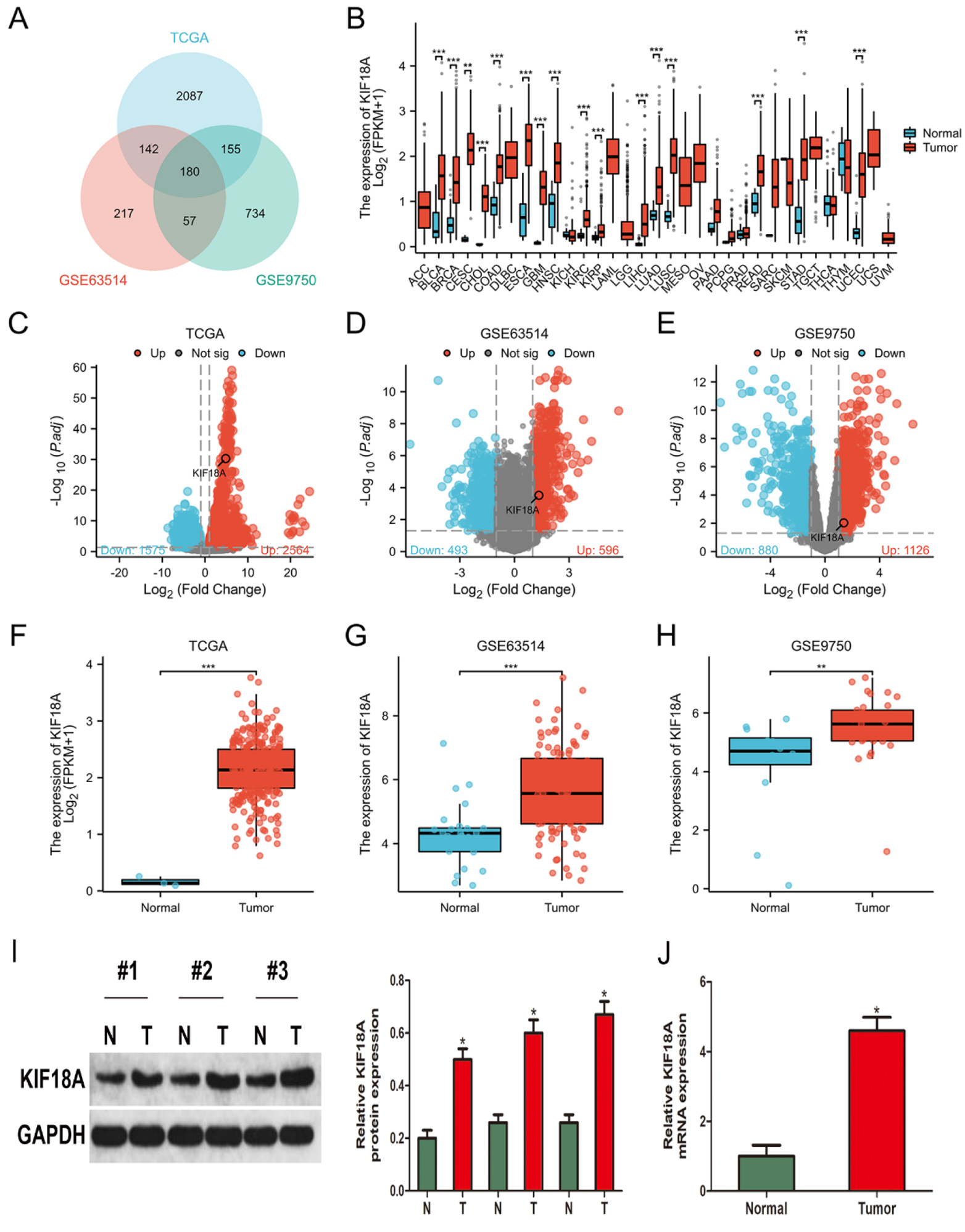
3.1KIF18A expression is significantly upregulated in cervical cancer
To discover the underlying molecular pathways of cervical cancer, we conducted an analysis of gene expression profiles obtained from three different datasets: TCGA-CESC, GSE63514, and GSE9750. By conducting a threshold of adjusted
Figure 2.
KIF18A promotes cell growth and suppresses apoptosis in CESC cells. (A, B) Western blot and qRT-PCR were performed to analyze the KIF18A expression in CESC cell lines and the human cervical epithelial cell line HCerEpiC cells. (C, D) The verification of KIF18A overexpression and knockdown effects was performed using Western blot analysis and qRT-PCR. (E) The CCK-8 assay is utilized to evaluate the growth levels of the SiHa cell line. (F) The flow cytometry technique was utilized for the analysis of apoptosis in SiHa cells. * p < 0.05.
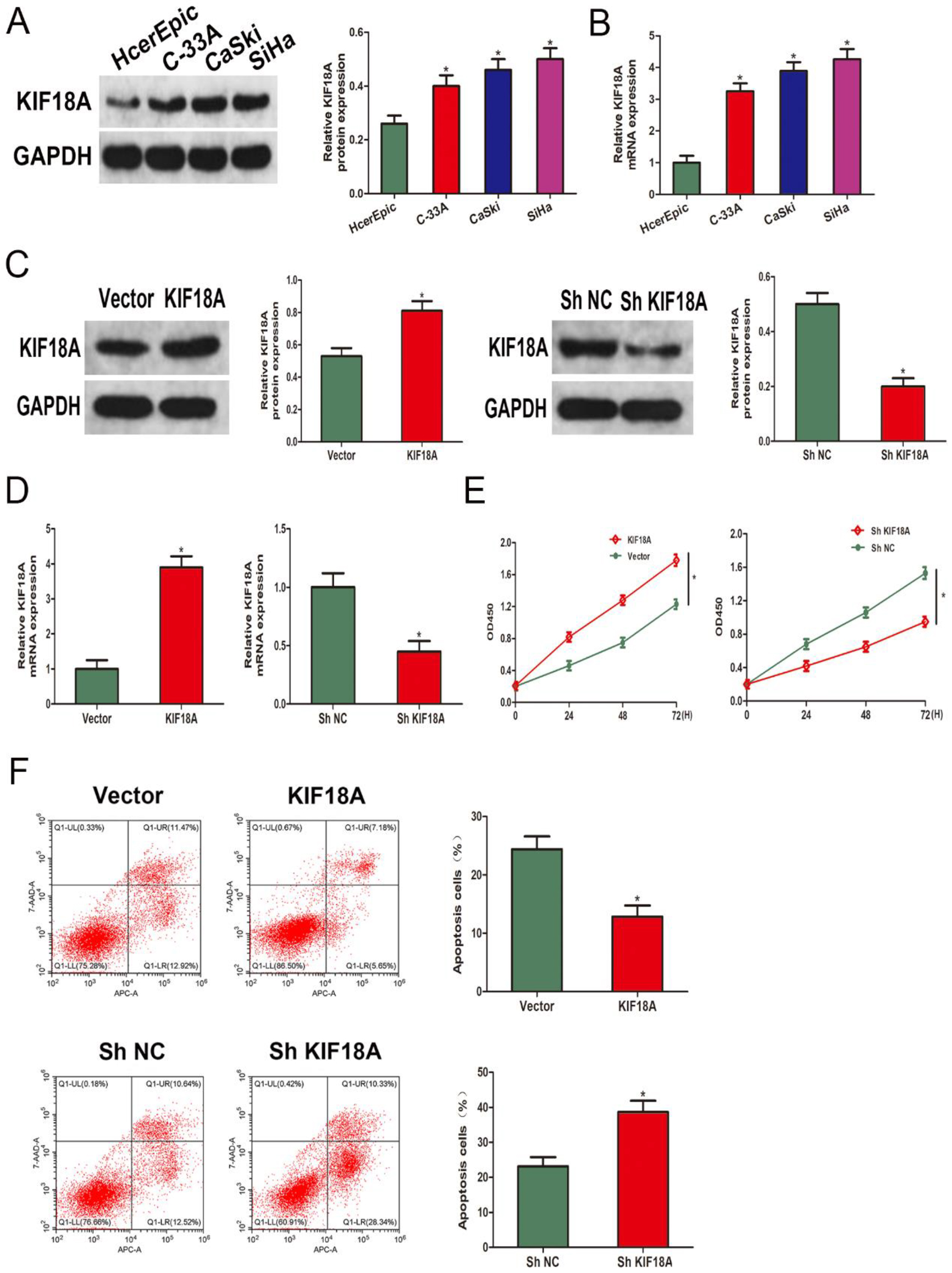
3.2KIF18A promotes cell growth and suppresses apoptosis in CESC cells
The KIF18A mRNA and protein expression were subsequently investigated in CESC cell lines and normal cervical epithelial cells. Significantly elevated KIF18A mRNA and protein levels were observed in CESC cells in comparison with normal cervical epithelial cells (Fig. 2A, B). To further investigate the KIF18A impact, the SiHa cell line was transfected with KIF18A overexpressing lentivirus and shKIF18A lentivirus. The levels of protein and mRNA expression were consequently evaluated to validate the overexpression and knockdown (Fig. 2C, D). The KIF18A impact on the growth of SiHa cells was further investigated using CCK-8 assays. The KIF18A group exhibited significantly enhanced cell viability, whereas a noticeable decrease in cell viability was observed in the ShKIF18A group (Fig. 2E). The upregulation of KIF18A expression was observed to significantly mitigate the occurrence of apoptotic cells in SiHa cells, and this impact could be reversed by downregulating the expression of KIF18A (Fig. 2F).
Figure 3.
KIF18A enhances the CESC cells’ migration and invasion capabilities. (A, B) Evaluation of the migratory potential of SiHa cells using wound-healing assays. (C, D) The invasive capacity of SiHa cells was assessed utilizing Transwell invasion assays. *p < 0.05.
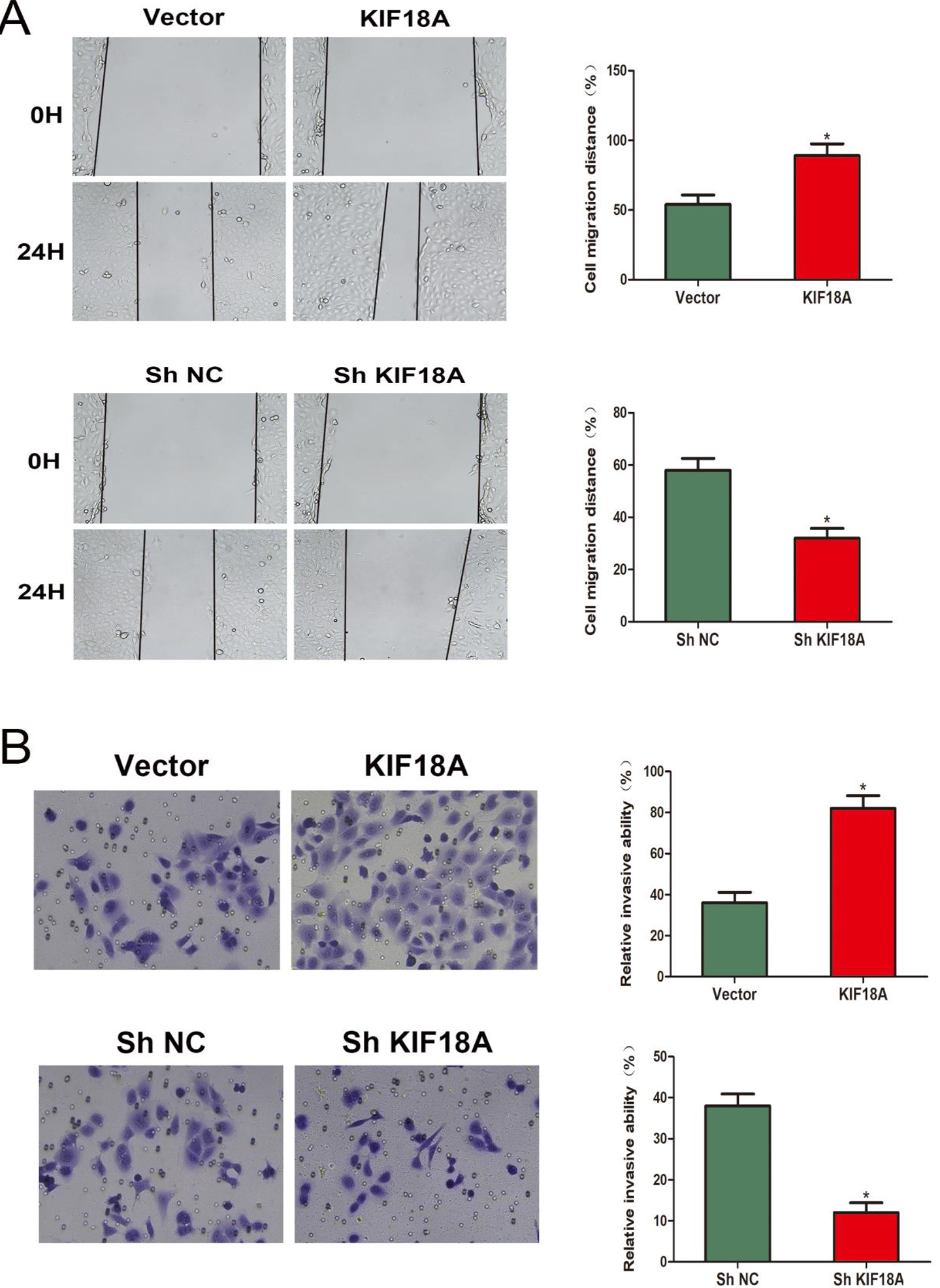
3.3KIF18A promotes the capability of migration and invasion of CESC cells
Consequently, we evaluate the KIF18A impact on CESC cells’ capability of migration and invasion. The migratory capacity of SiHa cells in the wound healing experiment was significantly increased by the overexpression of KIF18A, while the knockdown of KIF18A resulted in a notable reduction in SiHa cell migration (Fig. 3A). The Transwell assay exhibited that the KIF18A upregulation significantly augmented the invasive potential of SiHa cells, while inhibition of KIF18A led to a remarkable reduction in cell invasion (Fig. 3B).
Figure 4.
Detection of genes related to KIF18A and conducting functional enrichment analysis using TCGA database. (A, B) Volcano plot and heatmap analysis were employed to identify KIF18A-associated genes in the TCGA-CESC dataset. (C, D) Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analyses were conducted on genes positively associated with KIF18A in CESC. (E, F) KEGG and GO analyses were conducted on genes that exhibited a negative association with KIF18A in CESC.
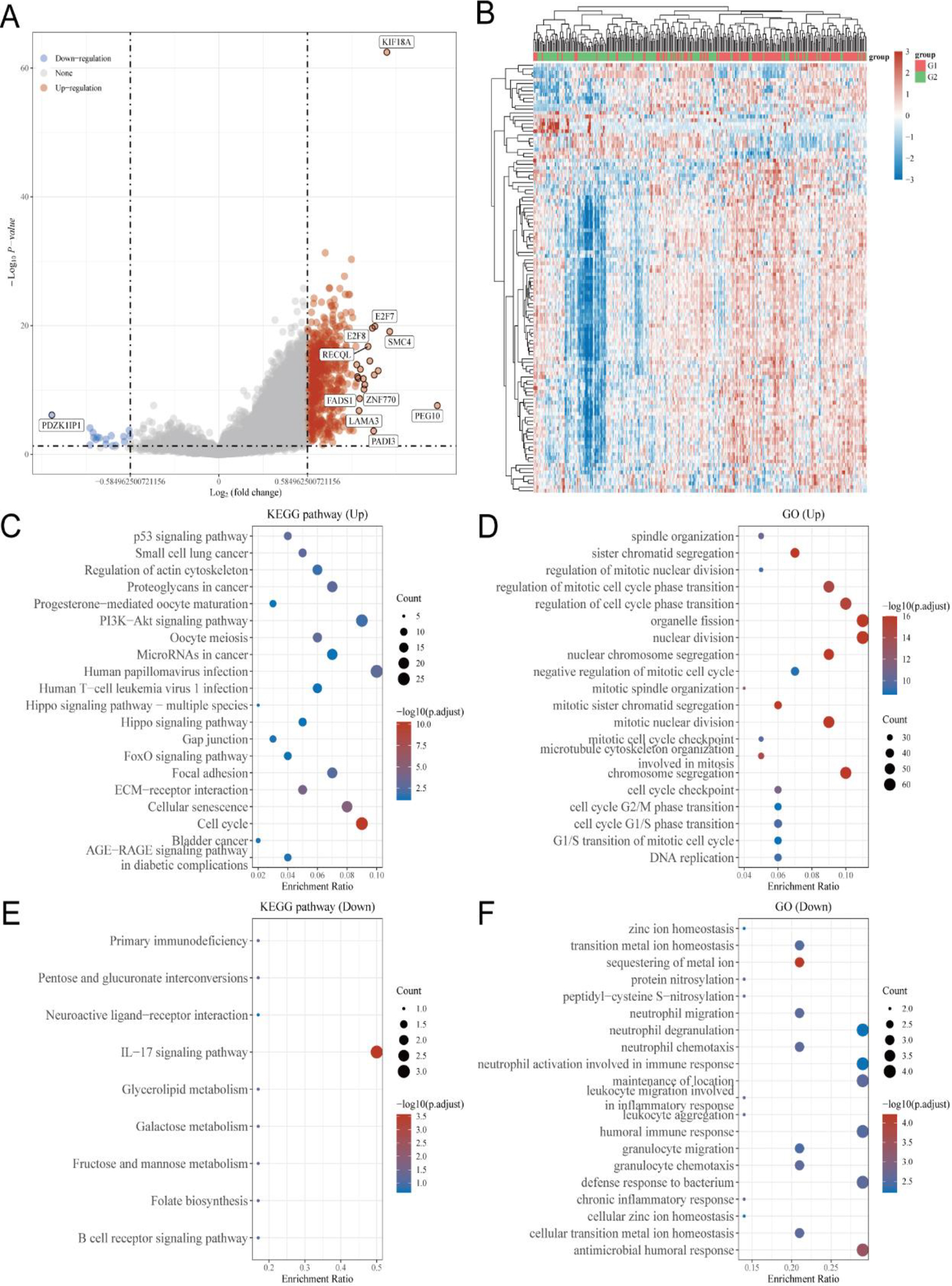
3.4Identification of KIF18A-related genes and conducting functional enrichment analysis using the TCGA database
The TCGA-CESC dataset was utilized to identify 618 up-regulated and 17 down-regulated genes that exhibited a strong association with KIF18A based on a screening criterion of
Figure 5.
The function of KIF18A in CESC cells is mediated through PI3K/AKT pathway modulation. (A) The GSEA analysis revealed significant disparities in the enrichment of the PI3K/AKT pathway between samples showing elevated and reduced expression levels of KIF18A. (B, C) Western Blotting assay revealed the expression of p-PI3K, PI3K, p-AKT, and AKT in SiHa cells. *p < 0.05.
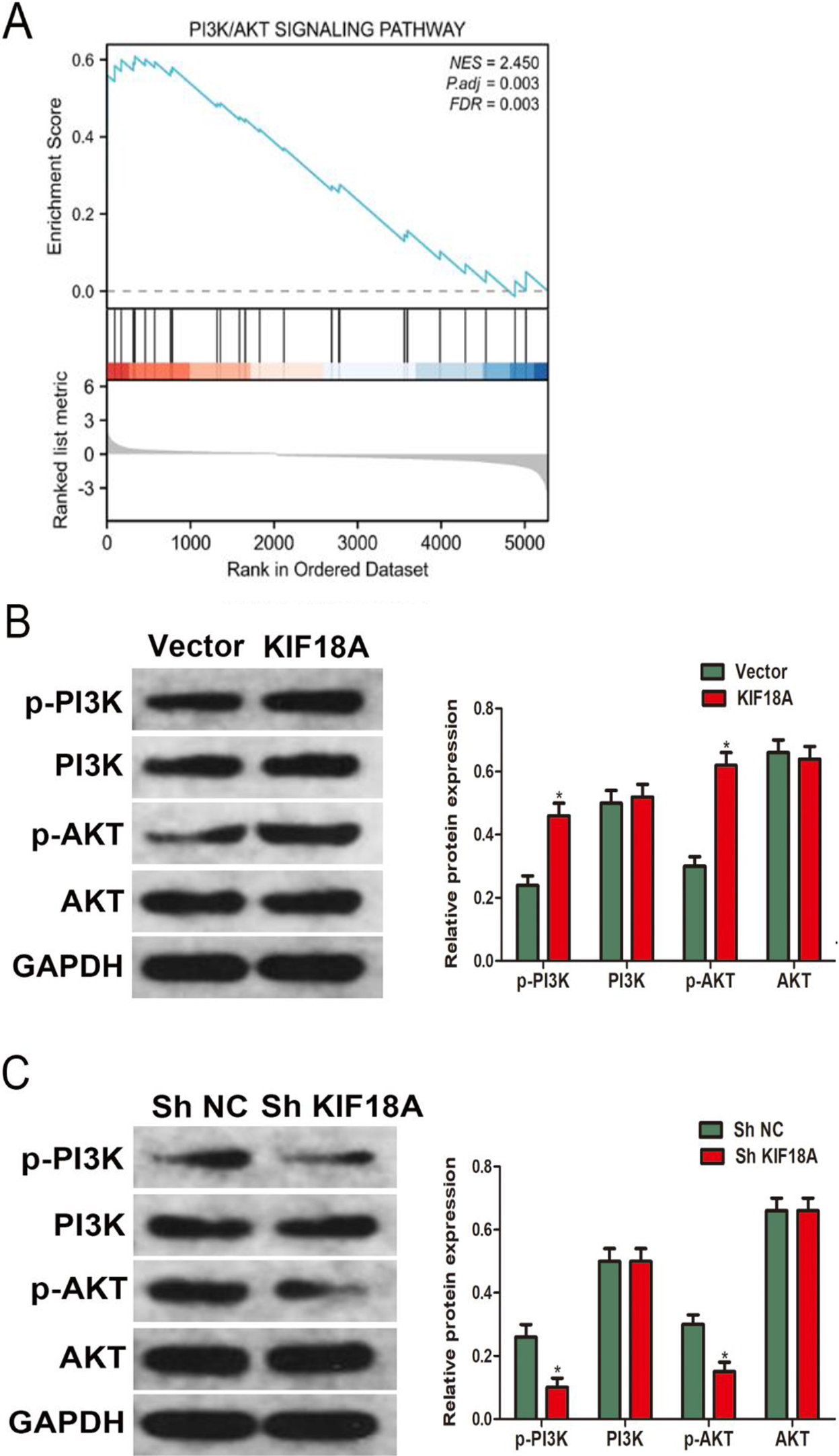
3.5The function of KIF18A in CESC cells is mediated through the PI3K/AKT pathway modulation
A further discovery from gene set enrichment analysis (GSEA) unveiled a significant PI3K/AKT pathway enrichment in cervical cancer samples exhibiting high expression levels of KIF18A (Fig. 5A). According to the results obtained from KEGG and GSEA analyses, we performed western blot analysis in SiHa cells to explore the KIF18A impact on the PI3K/AKT signaling pathway. The western blot analysis findings provided compelling evidence that the upregulation of KIF18A caused a significant rise in p-PI3K and p-AKT levels while exerting no discernible impact on the total levels of PI3K and AKT expression (Fig. 5B). Furthermore, the downregulation of KIF18A resulted in a reduction in the expression levels of p-PI3K and p-AKT (Fig. 5C).
Figure 6.
The expression of CENPE in CESC cells is regulated by KIF18A. (A) The heatmap demonstrates the top 20 genes displaying a strong positive correlation with KIF18A in TCGA-CESC. (B) The expression of CENPE exhibited a significantly positive correlation with KIF18A expression (correlation_spearman
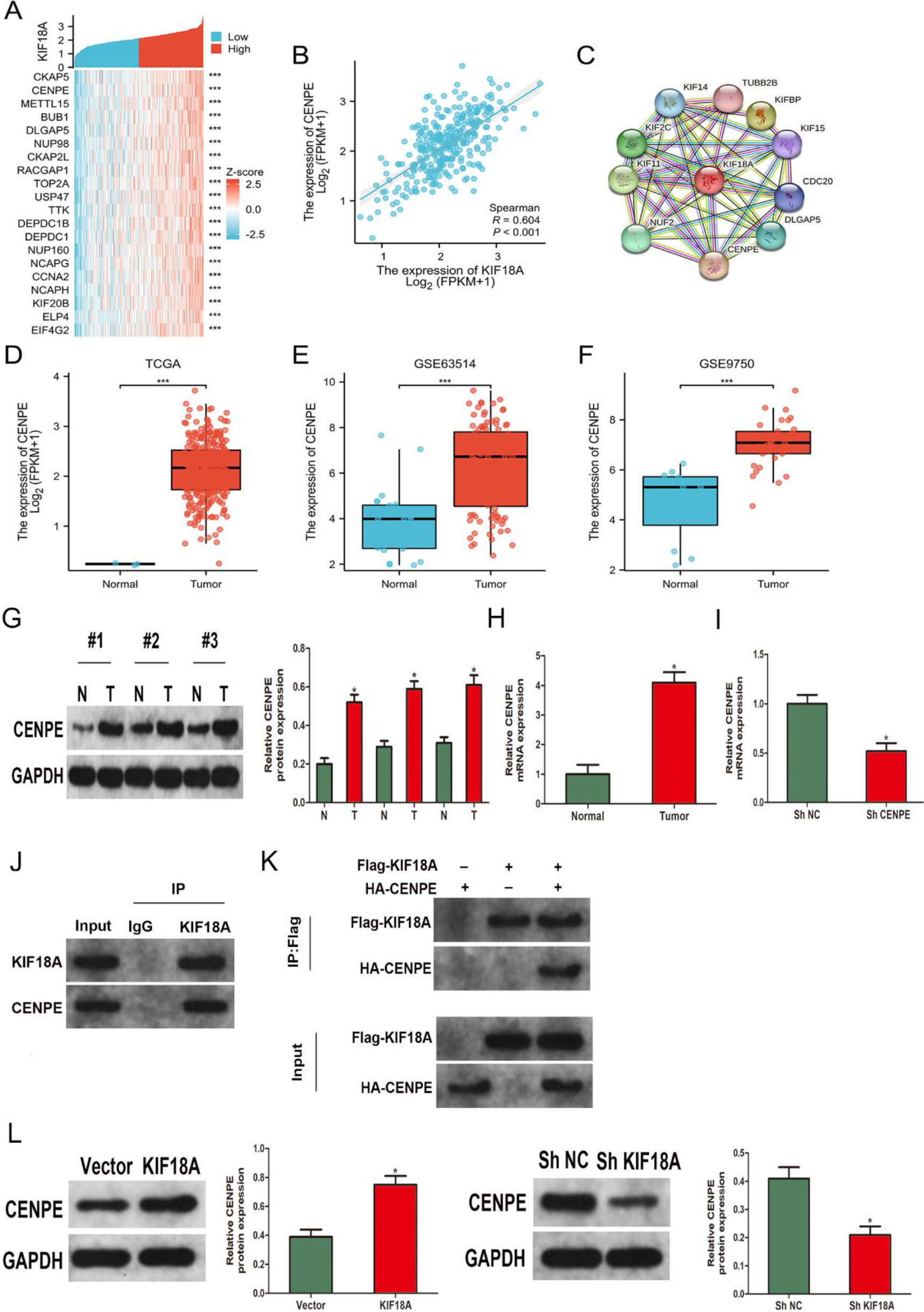
3.6The expression of CENPE in CESC cells is regulated by KIF18A
The TCGA database was utilized to identify co-expressed genes associated with KIF18A in CESC, aiming to obtain a comprehensive understanding of its molecular pathway in CESC. The creation of a heatmap was employed to visually represent the top 20 genes that exhibit a high correlation with KIF18A (Fig. 6A). Our outcomes exhibited a significant positive connection between KIF18A and CENPE expression levels in CESC (Fig. 6B). Additionally, the database of STRING was utilized to generate a protein-protein interaction (PPI) network for KIF18A, unveiling a potential interaction between KIF18A and CENPE (Fig. 6C). The CENPE expression levels were significantly upregulated in cervical malignancy tissues in contrast to healthy tissues in TCGA, GSE63514, and GSE9750 datasets (Fig. 6D–F). The protein expression of KIF18A in tumor tissues and corresponding normal tissues from 3 pairs of CESC patients was evaluated through western blot analysis (Fig. 6G). Moreover, we performed RNA extraction from 38 CESC tissues and 9 normal tissues, along with 9 paired CESC tissues and their corresponding normal tissues, followed by mRNA expression analysis using qRT-PCR (Fig. 6H, Fig. S2). The results revealed elevated levels of KIF18A protein and mRNA expression in CESC tissues compared to normal tissues. The lentiviral infection was utilized to induce stable CENPE knockdown in SiHa cells, thereby confirming the effectiveness of knockdown through qRT-PCR analysis (Fig. 6I). As a consequence, we proceeded to confirm the direct interaction between KIF18A and CENPE. The interaction was validated through endogenous Co-IP assays conducted in SiHa cells (Fig. 6J) as well as exogenous Co-IP assays performed in HEK293T cells (Fig. 6K). Furthermore, Western blot analysis indicated that upregulation of KIF18A expression in SiHa cells led to a significant rise in CENPE levels. Conversely, KIF18A expression downregulation resulted in a notable decrease in CENPE expression (Fig. 6L).
Figure 7.
KIF18A promotes invasion, growth, and activates the PI3K/AKT pathway through its interaction with CENPE in CESC cells. (A) The protein expression levels of p-PI3K, PI3K, p-AKT, and AKT in SiHa cells were assessed by Western blot analysis following the overexpression of KIF18A and/or knockdown of CENPE. (B) After transfection, the invasive possible of SiHa cells was evaluated using the Transwell assay. (C) The proliferation of SiHa cells following transfection was evaluated using the CCK-8 assay. * p < 0.05 vs. the Vector
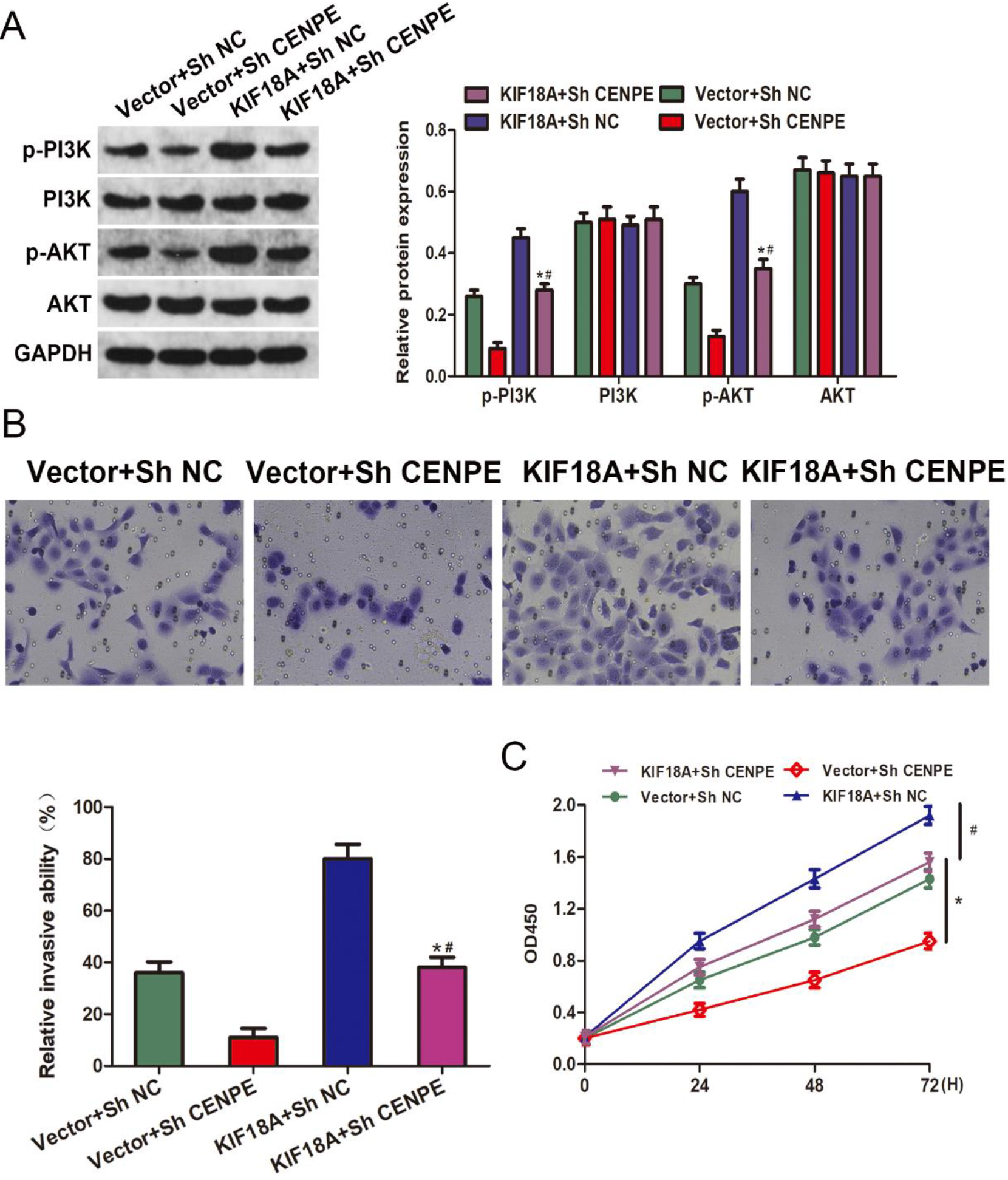
3.7KIF18A promotes invasion and proliferation and activates the PI3K/AKT pathway through its interaction with CENPE in CESC cells
The KIF18A-CENPE axis was further investigated in our study to explore its impact on CESC. The Western blot analysis indicated that the CENPE downregulation effectively attenuated the upregulation of p-PI3K and p-AKT levels induced by KIF18A overexpression (Fig. 7A). Reversal of the enhanced cell invasion resulting from KIF18A overexpression was observed upon silencing of CENPE, as evidenced by the Transwell assay (Fig. 7B). The CCK-8 results demonstrated that the enhanced cellular proliferation induced by KIF18A overexpression was effectively counteracted upon silencing of CENPE (Fig. 7C).
4.Discussion
Cervical cancer is the fourth most common reason for deaths linked to cancer in women globally, presenting a substantial risk to their health and life [15]. In 2017, owing to its vast population, China represented 11.9% of worldwide cervical cancer mortality and 12.3% of life years adjusted for disability linked to cervical cancer [1]. The histopathology findings categorize cervical cancer into subtypes, including CESC, and less common kinds, like neuroendocrine carcinoma, adenosquamous carcinoma, and smooth muscle sarcoma. Squamous cell carcinoma of the cervix is the most common subtype, representing around 80% of cases [16]. Despite the implementation of HPV vaccination and cervical cancer examination in numerous nations, there has been a persistent upward trend observed in both the incidence and mortality rates associated with this disease [17, 18]. Therefore, it is imperative to promptly address the urgent clinical issues of identifying novel biomarkers in CESC and discovering effective target molecules for early detection.
As a kinesin superfamily member, KIF18A serves as a microtubule depolymerase that depends on ATP and binds to microtubules, ensuring efficient intracellular transport and mitotic activities [19]. Given the pivotal role of KIF18A in maintaining chromosomal stability and facilitating cellular division, it is highly plausible that targeting KIF18A at a molecular level could have significant implications for addressing aberrant cell proliferation and cancer development [7]. Additionally, a growing body of evidence has demonstrated significant expression levels of KIF18A in various human malignant tumors, underscoring its pivotal role in tumor initiation, progression, and prognosis across multiple cancer kinds comprising breast cancer, clear cell renal carcinoma, carcinoma of liver cells, and colorectal cancer [11, 20, 21]. Liu et al. [22] demonstrated that KIF18A functions as a PTEN mediator and facilitates the PI3K/AKT signaling pathway activation, thereby promoting colorectal cancer progression. In this study, the analysis of CESC using TCGA, GSE63514, and GSE9750 data exhibited a significant upregulation in the KIF18A expression, which was further validated through examination of clinical specimens. KIF18A expression levels were significantly upregulated in subjects diagnosed with CESC. In vitro, upregulation of KIF18A was observed to enhance cell growth, promote migration and invasion, and suppress apoptosis. Conversely, downregulating the levels of KIF18A had opposite effects.
The PI3K/AKT pathway modulates several mechanisms of the cell, comprising cell growth, proliferation, metabolism, motility, survival, and apoptosis [23, 24]. The PI3K/AKT signaling pathway is often subject to modifications in malignancy, rendering targeted suppression of AKT activation a potentially efficacious strategy for cancer treatment [25]. The present study employed KEGG and GSEA analyses, revealing a significant connection between the upregulated expression of KIF18A and the PI3K/AKT signaling pathway stimulation. Thus, we conducted additional analyses on the protein expression patterns linked to the PI3K/AKT signaling pathway. The overexpression of KIF18A resulted in an upregulation of phosphorylation levels in both PI3K and AKT, while the suppression of KIF18A caused PI3K/AKT signaling pathway inhibition. Hence, our outcomes substantiate the involvement of KIF18A in augmenting the PI3K/AKT signaling pathway, thereby facilitating the acquisition of a malignant phenotype in CESC cells.
The Centrosome-associated protein E (CENPE) is a plus end-directed kinetochore motor protein that exhibits G2 phase-specific accumulation and has a pivotal function in the mitosis mechanism [26]. A comprehensive analysis across multiple cancer types reveals a significant connection between CENPE expression and tumorigenesis. Enhanced CENPE expression is connected with an unfavorable prognosis in colorectal, non-small cell lung, and breast cancers [27, 28, 29]. Furthermore, CENPE plays a crucial role in the malignant growth and migration of pulmonary adenocarcinoma as well as malignancy of the ovaries [30]. To elucidate the underlying molecular mechanisms driving CESC progression mediated by KIF18A, we have identified a potential interaction between KIF18A and CENPE. Furthermore, in vitro investigations demonstrate a positive association between the KIF18A and CENPE expression levels in CESC. Furthermore, the inhibition of CENPE led to the reversal of KIF18A-induced augmentation in cell proliferation, invasion, and phosphorylation levels of PI3K and AKT. Collectively, the findings suggest that KIF18A-mediated regulation of CENPE promotes PI3K/AKT pathway activation in CESC.
5.Conclusion
The findings of this investigation indicate that KIF18A enhances the growth, motility, and infiltration of CESC cells while suppressing apoptosis by modulating the PI3K/AKT signaling pathway through CENPE regulation. Our investigation has revealed a novel finding indicating the involvement of KIF18A in promoting tumor growth in CESC. This significant discovery highlights the potential utility of KIF18A as an invaluable biomarker for both diagnosing and prognosticating CESC while also emphasizing its potential as a treatment target.
Ethics approval and consent to participate
This research was approved by Institutional Review Board of Weifang People’s Hospital (Approval No. KYLL20240223-1) Prior written consent was well informed and signed by all participants.
Availability of data and material
The data used and analysed during the current study are available from the corresponding author on reasonable request.
Author contribution
Conception: Fengyi Sun, Tiantian Zhao.
Interpretation or analysis of data: Fengyi Sun.
Preparation of the manuscript: Fengyi Sun.
Revision for important intellectual content: Tiantian Zhao.
Supervision: Tiantian Zhao.
Competing interests
All authors have no conflicts of interest or financial ties to disclose.
Supplementary data
The supplementary files are available to download from http://dx.doi.org/10.3233/CBM-240074.
References
[1] | M. Guo, J. Xu and J. Du, Trends in cervical cancer mortality in China from 1989 to 2018: An age-period-cohort study and Joinpoint analysis, BMC Public Health 21: (1) ((2021) ), 1329. |
[2] | S.C.S. da Costa, R.C. Bonadio and F.C.G. Gabrielli et al., Neoadjuvant Chemotherapy With Cisplatin and Gemcitabine Followed by Chemoradiation Versus Chemoradiation for Locally Advanced Cervical Cancer: A Randomized Phase II Trial, J Clin Oncol 37: (33) ((2019) ), 3124–31. |
[3] | M. Kagabu, T. Nagasawa, D. Fukagawa et al., Immunotherapy for Uterine Cervical Cancer, Healthcare (Basel) 7: (3) ((2019) ). |
[4] | A. Castanon, R. Landy and P.D. Sasieni, Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int J Cancer 139: (5) ((2016) ), 1040–5. |
[5] | A.F. Rositch, K. Levinson, G. Suneja et al., Epidemiology of Cervical Adenocarcinoma and Squamous Cell Carcinoma Among Women Living With Human Immunodeficiency Virus Compared With the General Population in the United States, Clin Infect Dis 74: (5) ((2022) ), 814–20. |
[6] | N. Hirokawa and Y. Tanaka, Kinesin superfamily proteins (KIFs): Various functions and their relevance for important phenomena in life and diseases, Exp Cell Res 334: (1) ((2015) ), 16–25. |
[7] | B.-Y. Tao, Y.-Y. Liu and H.-Y. Liu et al., Prognostic Biomarker KIF18A and Its Correlations With Immune Infiltrates and Mitosis in Glioma. Front Genet 13: ((2022) ), 852049. |
[8] | L.H. Alfarsi, R. Elansari, M.S. Toss et al., Kinesin family member-18A (KIF18A) is a predictive biomarker of poor benefit from endocrine therapy in early ER+ breast cancer, Breast Cancer Res Treat 173: (1) ((2019) ). |
[9] | W. Luo, M. Liao, Y. Liao et al., The role of kinesin KIF18A in the invasion and metastasis of hepatocellular carcinoma, World J Surg Oncol 16: (1) ((2018) ), 36. |
[10] | H. Zhang, T. Shen, Z. Zhang, Y. Li and Z. Pan, Expression of KIF18A Is Associated with Increased Tumor Stage and Cell Proliferation in Prostate Cancer, Med Sci Monit 25: ((2019) ), 6418–28. |
[11] | Y. Zhong, L. Jiang, H. Lin et al., Overexpression of KIF18A promotes cell proliferation, inhibits apoptosis, and independently predicts unfavorable prognosis in lung adenocarcinoma, IUBMB Life 71: (7) ((2019) ), 942–55. |
[12] | L.-X. Qian, X. Cao, M.-Y. Du et al., KIF18A knockdown reduces proliferation, migration, invasion and enhances radiosensitivity of esophageal cancer, Biochem Biophys Res Commun 557: ((2021) ), 192–8. |
[13] | J.A. den Boon, D. Pyeon, S.S. Wang et al., Molecular transi-tions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling, Proc Natl Acad Sci U S A 112: (25) ((2015) ), E3255–E64. |
[14] | L. Scotto, G. Narayan, S.V. Nandula et al., Identification of copy number gain and overexpressed genes on chromosome arm 20q by an integrative genomic approach in cervical cancer: potential role in progression, Genes Chromosomes Cancer 47: (9) ((2008) ), 755–65. |
[15] | F. Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre and J. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J Clin 68: (6) ((2018) ), 394–424. |
[16] | Q. Jia, J. Song, T. Xu, et al., ZIC5 promotes aggressiveness and cancer stemness in cervical squamous cell carcinoma, Pathol Res Pract 241: ((2023) ), 154268. |
[17] | G. Giannone, A.R. Giuliano, M. Bandini et al., HPV vaccination and HPV-related malignancies: impact, strategies and optimizations toward global immunization coverage, Cancer Treat Rev 111: ((2022) ), 102467. |
[18] | A.R. Kreimer, T. Cernuschi, H. Rees, J.M.L. Brotherton, C. Porras and J. Schiller, Public health opportunities resulting from sufficient HPV vaccine supply and a single-dose vaccination schedule, J Natl Cancer Inst 115: (3) ((2023) ), 246–9. |
[19] | C.E. Walczak, S. Gayek and R. Ohi, Microtubule-depoly-merizing kinesins, Annu Rev Cell Dev Biol 29: ((2013) ), 417–41. |
[20] | E. Marra, F. Palombo, G. Ciliberto and L. Aurisicchio, Kinesin spindle protein SiRNA slows tumor progression, J Cell Physiol 228: (1) ((2013) ), 58–64. |
[21] | U. Eichenlaub-Ritter, Microtubule dynamics and tumor invasion involving MCAK, Cell Cycle 14: (21) ((2015) ), 3353. |
[22] | Y. Liu, M. Sun, B. Zhang and W. Zhao, KIF18A improves migration and invasion of colorectal cancer (CRC) cells through inhibiting PTEN signaling, Aging (Albany NY) 15: (17) ((2023) ), 9182–92. |
[23] | C.-Y. Lee, M.-C. Hsin, P.-N. Chen et al., Arctiin Inhibits Cervical Cancer Cell Migration and Invasion through Suppression of S100A4 Expression via PI3K/Akt Pathway, Pharmaceutics 14: (2) ((2022) ). |
[24] | K. Jiang, L.-Z. Xu, J.-Z. Ning and F. Cheng, FAP promotes clear cell renal cell carcinoma progression via activating the PI3K/AKT/mTOR signaling pathway, Cancer Cell Int 23: (1) (2023): 217. |
[25] | M. Osaki, M. Oshimura and H. Ito, PI3K-Akt pathway: its functions and alterations in human cancer, Apoptosis 9: (6) ((2004) ), 667–76. |
[26] | L. Shan, M. Zhao, Y. Lu et al., CENPE promotes lung adenocarcinoma proliferation and is directly regulated by FOXM1, Int J Oncol 55: (1) ((2019) ), 257–66. |
[27] | L. Fang, Q. Liu, H. Cui, Y. Zheng and C. Wu, Bioinformatics Analysis Highlight Differentially Expressed CCNB1 and PLK1 Genes as Potential Anti-Breast Cancer Drug Targets and Prognostic Markers, Genes (Basel) 13: (4) ((2022) ). |
[28] | X. Hao and T. Qu, Expression of CENPE and its Prognostic Role in Non-small Cell Lung Cancer, Open Med (Wars) 14: ((2019) ), 497–502. |
[29] | Z. Zhang and X. Zhang, Identification of m6A-Related Biomarkers Associated with Prognosis of Colorectal Cancer, Med Sci Monit 27: ((2021) ), e932370. |
[30] | H. Fang, Y. Zhang, C. Lin et al., Primary microcephaly gene CENPE is a novel biomarker and potential therapeutic target for non-WNT/non-SHH medulloblastoma, Front Immunol 14: (2023): 1227143. |




