Hypermethylation of the BRCA2 gene promoter and its co-hypermethylation with the BRCA1 gene promoter in patients with breast cancer
Abstract
BACKGROUND:
The BRCA2 gene is an important tumour suppressor in breast cancer, and alterations in BRCA2 may lead to cancer progression. The aim of the study was to investigate the association of hypermethylation of the BRCA2 gene promoter and its co-hypermethylation with the BRCA1 gene promoter with the development and course of breast cancer in women.
METHODS:
This study included 74 women with breast cancer (tumour tissue samples and peripheral blood) and 62 women without oncological pathology (peripheral blood) – control group.
RESULTS:
Hypermethylation of the BRCA2 gene was significantly more frequently detected in the tumour tissue of women with breast cancer compared to their peripheral blood and peripheral blood of control subjects (p= 0.0006 and p= 0.00001, respectively). Hypermethylation of BRCA2 was more frequently detected in patients with breast cancer over the age of 50 and in patients with higher Ki67 expression levels (p= 0.045 and p= 0.045, respectively). There was a high frequency of unmethylated BRCA1 and BRCA2 gene combination in women of the control group compared to women with breast cancer, both in blood samples and tumour tissue samples (p= 0.014 and p= 0.00001, respectively).
CONCLUSION:
Our study confirms the hypothesis that BRCA2 hypermethylation plays an important role in the pathogenesis of breast cancer and the importance of assessing its co-hypermethylation with BRCA1 in predicting the course of the disease.
1.Introduction
Breast cancer is an important medical and social problem. Breast cancer in women reduces life expectancy and worsens its quality, starting from the working age. It is known that Central Asian and Eastern European countries have higher mortality rates from breast and cervical cancer and later diagnosis compared to countries in other parts of the WHO European Region [1]. In particular, in Ukraine, according to the National Cancer Registry of Ukraine, breast cancer is the leading cause of morbidity and death from malignant tumours among women [2]. Thus, in 2021, 14036 new cases of this disease were registered in women and 4732 deaths (the number of cases does not include the data from the Donetsk and Luhansk regions, the Autonomous Republic of Crimea and the city of Sevastopol). Only 31.9% of cases were detected during preventive check-ups and 47.3% of newly diagnosed cases had stage II disease. At the same time, the incidence of breast cancer among women in Ukraine has been steadily increasing every year over the past decade.
There are population differences in the frequencies of pathogenic variants of the BRCA1 and BRCA2 genes and epigenetic events, which may be associated with different incidence rates and features of breast cancer [3, 4, 5]. Ecology, climate, residence and lifestyle may be other factors that may also have population differences [4, 6]. In addition, it is worth remembering that even hereditary cancer can be polygenic in nature, with varying degrees of contribution from modifier genes [7].
The BRCA2 gene is an important tumour suppressor in breast cancer, and alterations in BRCA2 may lead to cancer progression. However, the BRCA2 gene is rarely mutated, and it is suspected that the loss of function is mediated mainly by its epigenetic regulation [8]. Since current treatment strategies aim to identify pathogenic variants in both genes, it is essential to study the combination of different “functionality” of these genes in patients with breast cancer.
The aim of the study was to investigate the association of hypermethylation of the BRCA2 gene promoter and its co-hypermethylation with the BRCA1 gene promoter with the development and course of breast cancer in women.
2.Materials and methods
2.1Study population
The study involved 74 patients with newly diagnosed breast cancer who were treated at the Department of Oncology of the Bogomolets National Medical University at the Kyiv City Clinical Oncology Centre. Patients underwent a standard clinical, laboratory, instrumental and molecular pathological examination, as well as epigenetic testing for hypermethylation of the BRCA2 gene promoter region in tumour tissue samples and peripheral blood. The study was based on the case-control principle. 62 women in the control group, who were examined for pathology of the female reproductive system, did not have malignant breast tumours. Their peripheral blood was used as a biological material for the study. Women in both groups were asked about their family history and cancer heredity.
The study was approved by the Commission on Bioethical Expertise and Research Ethics of Bogomolets National Medical University (0120U100871). Informed consent was obtained from each participant included in this study.
2.2Sample collection
The materials used to determine the hypermethylation of the BRCA2 gene promoter region were peripheral blood from women in the control group and paired samples of tumour tissue and peripheral blood from patients with breast cancer. All tumour tissue samples obtained during resection/biopsy and peripheral blood samples from the main and control groups were collected and stored using “DNA/RNA Shield” preservative (Zymo Research Irvine, CA, USA).
2.3DNA extraction
Genomic DNA was extracted using “Quick-DNA™ Miniprep Plus Kit” (Zymo Research Irvine, CA, USA). The extracted DNA was stored at -18∘C.
2.4Methylation-specific polymerase (MSP) chain reaction
Sodium bisulfite conversion of genomic DNA was carried out using the “EZ DNA Methylation-Gold Kit” (Zymo Research Irvine, CA, USA) following the manufacturer’s instructions. The modified DNA was then used as a matrix. The PCR reaction was performed using “ZymoTaq PreMix kit” (Zymo Research, US Irvine, CA, USA) and specific primer pairs (Metabion, Bayern, Germany). The methylated primers were as follows: GACGGTTGGGATGTTTGATAAGG and reverse: AATCTATCCCCTCACGCTTCTCC. The un-methylated primers were as follows, forward: AGGG TGGTTTGGGATTTTTAAGG, and, reverse: TCACAC TTCTCCCAACAACAACC [9].
Figure 1.
Electrophoregram of methylated (M) and unmethylated (U) DNA amplification products in the promoter region of the BRCA2 gene. L – 50-bp DNA ladder; K+ – methylated control fragment; K- – unmethylated control fragment; Samples 1, 4, 5 – UU; Samples 2, 3, 6 – MU.
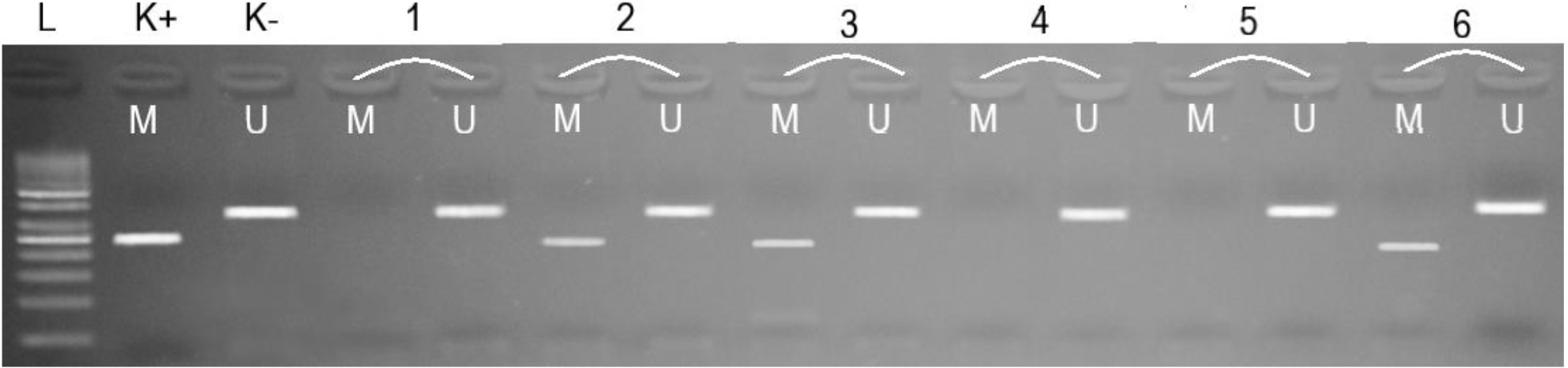
The reaction products were separated by agarose gel electrophoresis and analysed according to the presence or absence of amplification of fragments of methylated DNA (M) – 250 bp and unmethylated DNA (U) – 337 bp (Fig. 1) [10]. The “Human Methylated & Unmethylated DNA Set” (Zymo Research Irvine, CA, USA) was used as methylated and unmethylated controls.
2.5Analysis of the status of combined hypermethylation (comethylation) of the promoter regions of the BRCA1 and BRCA2 genes
In order to analyse the effect of the presence or absence of cometylation of the BRCA1 and BRCA2 gene promoter regions, we used the available data on hypermethylation of the BRCA1 gene promoter region, which were previously published for the same study groups [5].
2.6Statistical analysis
Data were analysed using SPSS v.26 software (SPSS Inc, Chicago, IL, USA). Differences in the distribution of categorical variables between the studied groups and subgroups were assessed using χ2 (or χ2 with Yates correction) and odds ratio (OR) with a 95% confidence interval. Clinical and pathological characteristics of the women with BC were tested for normal distribution using the Kolmogorov-Smirnov test. Then, the probability of differences in the quantitative results was determined using ANOVA or the Kruskal-Wallis test followed by post-hoc analysis with the Bonferroni correction. P values less than 0.05 were considered statistically significant.
3.Results
Table 1
Frequency of hypermethylation of the BRCA2 gene promoter region in the study groups
| Groups of investigations | Hypermethylation of promoter region of BRCA2 gene | |
|---|---|---|
| MU | UU | |
| Control, blood samples, (n= 62) | 3 (4.8%) | 59 (95.2%) |
| BC, blood samples, (n= 74) | 12 (16.2%) | 62 (83.8%) |
| BC, tumour samples, (n= 74) | 31 (41.9%) | 43 (58.1%) |
M – methylated; U – unmethylated.
In paired biological samples from patients with breast cancer (tumour tissue and peripheral blood), the frequency of detection of hypermethylation of the BRCA2 promoter region was higher than in peripheral blood samples from women in the control group (Table 1).
Hypermethylation of the promoter region of the BRCA2 gene in both patients of the main study group and women of the control group was detected in the heterozygous state. The frequency of BRCA2 hypermethylation determined in tumour tissue samples of breast cancer patients was significantly higher compared to the control group (χ2= 22.77, p= 0.00001, OR = 14.18 (4.07–49.41)). Although the frequency of BRCA2 gene hypermethylation in the blood of patients with breast cancer was higher compared to the frequency in women in the control group, these differences were not significant χ2= 3.37, p= 0.07, OR = 3.81 (1.02–14.17)). When comparing the frequencies of BRCA2 hypermethylation in paired samples of breast cancer patients, a higher frequency of hypermethylation was found in tumour tissue samples (χ2= 11.83, p= 0.0006, OR = 3.72 (1.72–8.06)).
Table 2
Clinical and pathological characteristics of patients with breast cancer depending on the status of hypermethylation of the BRCA2 gene promoter region
| Clinical and pathological | BC, blood samples (n= 74) | BC, tumour samples (n=74) | ||
|---|---|---|---|---|
| characteristics | MU | UU | MU | UU |
| Number of samples | 12 | 62 | 31 | 43 |
| Age, years | 56.9 ± 12.8 | 51.7 ± 14.3 | 55.0 ± 14.0 | 50.7 ± 14.1 |
| p value | 0.15 | 0.11 | ||
| Age groups | ||||
| Up to 50 years | 3 (25.0%) | 36 (58.1%) | 10 (32.3%) | 24 (55.8%) |
| After 50 years | 9 (75.0%) | 26 (41.9%) | 21 (67.7%) | 19 (44.2%) |
| p value | 0.07 | 0.045 | ||
| Diagnosis | ||||
| Bilateral | 0 (0.0%) | 4 (6.5%) | 2 (6.5%) | 2 (4.7%) |
| Dex | 7 (58.3%) | 23 (37.1%) | 14 (45.2%) | 16 (37.2%) |
| Sin | 5 (41.7%) | 35 (56.5%) | 15 (48.4%) | 25 (58.1%) |
| p value | 0.32 | 0.70 | ||
| Stage | ||||
| I + II | 8 (66.7%) | 47 (75.8%) | 21 (67.7%) | 34 (79.1%) |
| III + IV | 4 (33.3%) | 15 (24.2%) | 10 (32.3%) | 9 (20.9%) |
| p value | 0.51 | 0.27 | ||
| Histological type | ||||
| Ductal | 10 (83.3%) | 52 (83.9%) | 27 (87.1%) | 35 (81.4%) |
| Others | 2 (16.7%) | 10 (16.1%) | 4 (12.9%) | 8 (18.6%) |
| p value | 0.96 | 0.51 | ||
| Estrogen receptor | ||||
| Positive | 9 (75.0%) | 48 (77.4%) | 21 (67.7%) | 36 (83.7%) |
| Negative | 3 (25.0%) | 14 (22.6%) | 10 (32.3%) | 7 (16.3%) |
| p value | 0.86 | 0.11 | ||
| Progesterone receptor | ||||
| Positive | 6 (50.0%) | 46 (74.2%) | 18 (58.1%) | 34 (79.1%) |
| Negative | 6 (50.0%) | 16 (25.8%) | 13 (41.9%) | 9 (20.9%) |
| p value | 0.09 | 0.051 | ||
| HER2/neu | ||||
| Positive | 1 (8.3%) | 10 (16.1%) | 5 (16.1%) | 6 (14.0%) |
| Negative | 11 (91.7%) | 52 (83.9%) | 26 (83.9%) | 37 (86.0%) |
| p value | 0.49 | 0.80 | ||
| Ki67, % | 31.4 ± 11.2 | 30.7 ± 15.2 | 33.7 ± 13.0 | 28.9 ± 15.2 |
| p value | 0.76 | 0.18 | ||
| Ki67 groups | ||||
| Low (0%–15%) | 1 (8.3%) | 10 (19.2%) | 1 (3.8%) | 10 (26.3%) |
| Intermediate (16%–29%) | 7 (58.3%) | 19 (36.5%) | 13 (50.0%) | 13 (34.2%) |
| High (⩾ 30%) | 4 (33.3%) | 23 (44.2%) | 12 (46.2%) | 15 (39.5%) |
| | 0.35 | 0.045 | ||
| Molecular subtype | ||||
| TNBC | 3 (25.0%) | 12 (19.4%) | 9 (29.0%) | 6 (14.0%) |
| Luminal A | 6 (50.0%) | 25 (40.35) | 11 (35.5%) | 20 (46.5%) |
| Luminal B | 3 (25.0%) | 23 (37.1%) | 10 (32.3%) | 16 (37.2%) |
| HER2-positive | 0 (0.0%) | 2 (3.2%) | 1 (3.2%) | 1 (2.3%) |
| | 0.76 | 0.44 | ||
| Family history | ||||
| Positive | 5 (41.7%) | 31 (50.0%) | 15 (48.4%) | 21 (48.8%) |
| Negative | 7 (58.3%) | 31 (50.0%) | 16 (51.6%) | 22 (51.2%) |
| | 0.60 | 0.97 | ||
M – methylated; U – unmethylated.
When analysing the associations of BRCA2 promoter hypermethylation with the clinical and pathological characteristics of breast cancer patients, no correlations were found between the status of BRCA2 hypermethylation in blood samples and clinical and pathological characteristics (Table 2).
Instead, an association of BRCA2 hypermethylation status in tumour tissue samples from breast cancer patients and such clinicopathological characteristics as age and Ki67 proliferation index was found. Thus, it was determined that BRCA2 hypermethylation was more frequently detected (Table 2) in patients with breast cancer over 50 years of age
Table 3
Frequency of cometylation of the promoter regions of BRCA1 and BRCA2 genes in the study groups
| Combined hypermethylation | Control, blood samples ( | BC, blood samples ( | BC, tumour samples ( |
|---|---|---|---|
| BRCA1 UU | 57 (91.9%) | 55 (74.3%) | 31 (41.9%) |
| BRCA1 MU | 2 (3.2%) | 7 (9.5%) | 12 (16.2%) |
| BRCA1 UU | 3 (4.8%) | 10 (13.5%) | 17 (23.0%) |
| BRCA1 MU | 0 (0.0%) | 2 (2.7%) | 14 (18.9%) |
M – methylated; U – unmethylated.
Table 4
Relationship between BRCA1 and BRCA2 gene methylation and clinical and pathological characteristics of breast cancer patients
| Clinical and pathological | BC (blood) | BC (tumour) | ||
|---|---|---|---|---|
| characteristics |
BRCA1 UU |
BRCA1 UU |
BRCA1 UU |
BRCA1 UU |
| Age groups | ||||
| Up to 50 years | 26 (47.3%) | 2 (20.0%) | 18 (58.1%) | 4 (23.5%) |
| After 50 years | 29 (52.7%) | 8 (80.0%) | 13 (41.9%) | 13 (76.5%) |
| | 0.20 | 0.046 | ||
| Ki67 groups | ||||
| Low (0%–15%) | 10 (21.7%) | 1 (10.0%) | 9 (34,6%) | 0 (0%) |
| Intermediate (16%–29%) | 10 (21.7%) | 5 (50.0%) | 6 (23.1%) | 5 (35.7%) |
| High ( | 26 (56.5%) | 4 (40.0%) | 11 (42.3%) | 9 (64.3%) |
| | 0.33 | 0.035 | ||
| Progesterone receptor | ||||
| Negative | 12 (21.8%) | 6 (60.0%) | 6 (19.4%) | 7 (41.2%) |
| Positive | 43 (78.2%) | 4 (40.0%) | 25 (80.6%) | 10 (58.8%) |
| | 0.0359 | 0.18 | ||
M – methylated; U – unmethylated.
The next stage of our study was to analyse the prevalence of combined hypermethylation of BRCA1 and BRCA2 genes in the study groups (Table 3).
There was a high frequency of unmethylated BRCA1 and BRCA2 gene promoters combination in women of the control group compared to women with breast cancer, both in blood samples and tumour tissue samples (
For patients with breast cancer, we analysed differences in clinicopathological characteristics depending on the status of BRCA1 and BRCA2 gene methylation. The analysis was performed for all characteristics listed in Table 2. The significant differences identified in this analysis are shown in Table 4.
First of all, we noticed how the previously identified significant differences had changed for age and Ki67 proliferation index (Table 4). Thus, in women over 50 years of age, the frequency of unmethylated BRCA1 (BRCA1 UU) and hypermethylated BRCA2 (BRCA2 MU) in tumour tissue samples was significantly increased (76.5%) compared to patients with a combination of unmethylated statuses of the BRCA1 and BRCA2 gene promoter regions (41.9%) –
4.Discussion
Table 5
Frequency of methylation of the BRCA2 gene promoter in other study groups
| No | Country | Method | Type of material | Case | Control | Study | |||
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | M | U | M | U | ||||
| 1 | France | QAMA | Peripheral blood ( | Peripheral blood ( | 17% | 83% | 16% | 84% | [12] |
| 2 | Ukraine | MSP | Tumor tissue ( | – | 50% | 50% | – | – | [10] |
| 3 | Brazil | MSP | Tumor tissue ( | – | 44% | 56% | [13] | ||
| 4 | Israel | QAMA | Peripheral blood ( | Peripheral blood ( | 0% | 100% | 0% | 100% | [14] |
| 5 | Nigeria | MSP | Peripheral blood ( | – | 57% | 43% | – | – | [15] |
| 6 | Tunis | MSP | Tumor tissue ( | Non-tumor tissue ( | 69%/5% | 31%/95% | 0% | 100% | [16] |
| 7 | Korea | MS-MLPA | Tumor tissue ( | – | 2%/0% | 98%/100% | – | – | [17] |
| 8 | Turkey | MS-MLPA | Tumor tissue ( | – | 0%/0% | 100%/100% | – | – | [18] |
MS-MLPA – methylation-specific multiplex ligation-dependent probe amplification; MSP – methylation-specific PCR; QAMA – quantitative analysis of methylated alleles; M – methylated; U – unmethylated.
Hypermethylation of the BRCA2 promoter leads to low mRNA expression and reduced synthesis of the corresponding protein [11]. The frequency of BRCA2 promoter hypermethylation in breast malignancies has been reported by different research groups to be 0%–69% (Table 5). Here, we report that the frequency of BRCA2 promoter hypermethylation is 41.9% in tumour tissue samples and 16.2% in peripheral blood samples from breast cancer patients from Ukraine. Given the frequency of detection of BRCA2 promoter hypermethylation in the peripheral blood of patients with hereditary breast cancer syndrome and ovarian cancer, where it was 0% [19], BRCA2 promoter hypermethylation cannot be associated with hereditary breast cancer. This was confirmed in our study, where no differences were found in the distribution of gene promoter hypermethylation depending on the family history and heredity. Thus, the frequencies we obtained are comparable to those reported in the current literature. It should be noted that such an analysis in peripheral blood samples of breast cancer patients is less effective than in tumour tissue samples.
We have shown that the frequency of BRCA2 hypermethylation is higher in a subgroup of women with breast cancer over 50 years of age. Our results are indirectly confirmed by the work of Bosviel et al., who also noted a higher level of BRCA2 methylation in elderly patients [12]. In Ukraine, the peak incidence of breast cancer and related mortality is rapidly increasing in women over 50 years of age [2]. That is why the study of BRCA2 promoter hypermethylation, in particular for Ukrainian patients with breast cancer, can potentially be recommended for women in this age subgroup and may be the basis for personalised targeted treatment.
Our study included breast cancer patients with different stages of newly diagnosed disease – from stage I to stage IV. However, no association was found between BRCA2 promoter hypermethylation and the stage at which breast cancer was diagnosed in women. In contrast to our results, Vos et al in their study showed that hypermethylation of the BRCA2 promoter is more common in tumours with a high degree of malignancy [20]. However, when evaluating the association of co-hypermethylation of the studied genes with other clinicopathological characteristics, we found that in patients with BRCA2 promoter hypermethylation or a combination of unmethylated BRCA1 status and BRCA2 promoter hypermethylation, the level of Ki67 expression was higher. It is known that this prognostic biomarker in breast cancer has proven clinical reliability – its high levels are associated with a poor prognosis for survival and an increased risk of recurrence [21, 22].
Another interesting result of this study is that breast cancer patients with a combination of the unmethylated status of the BRCA1 and BRCA2 gene promoter regions were significantly more likely to have positive progesterone receptors. It should be noted that high expression of progesterone receptors, according to some research groups, is more common in tumours with a better prognosis and is associated with better survival [23, 24]. On the other hand, we did not find such a relationship with progesterone receptor expression when analysing the hypermethylation of BRCA1 and BRCA2 gene promoters separately in this study [5]. This may be due to the small sample size. It should also be noted that we did not stratify breast cancer patients according to the strength of receptor expression and receptor types.
When analysing the relationship between family history of cancer in first- and second-order relatives and BRCA2 gene hypermethylation status, we did not find any significant differences. Although there are studies that indicate the transmission of epimutations, in particular, hypermethylation of the BRCA1 promoter, from mother to daughter [25]. And based on this, we hypothesised that in the group of breast cancer patients with a family history, the frequency of BRCA2 promoter hypermethylation would be higher. However, taking into account our previous work, we did not find a link between hypermethylation of the BRCA1 and BRCA2 gene promoters and either hereditary or sporadic breast cancer [5].
The analysis of BRCA1 and BRCA2 promoter regions methylation indicates a certain interaction between them, which has a synergistic effect on the better prognosis in the absence of promoter methylation. Therefore, we assume that further studies of the contribution of hypermethylation of tumour suppressor gene promoters to the development and course of breast cancer, prevention and selection of personalised therapy should be conducted for both BRCA1 and BRCA2 genes, and take into account their interaction. In particular, the demethylating effect of such natural compounds as curcumin, genistein, catechin and quercetin has already been proven [26, 27, 28, 29]. The possibility of their use for preventive and therapeutic purposes (as concomitant therapy) in breast cancer patients is being actively studied. In addition, hypermethylation of the BRCA2 promoter, by analogy with hypermethylation of the BRCA1 promoter, can be a potential marker for predicting chemosensitivity in patients with breast cancer, especially to drugs such as cyclophosphamide, methotrexate, fluorouracil and platinum drugs [30, 31]. Another group of drugs is highly effective targeted drugs – PARP inhibitors. Despite their impressive clinical efficacy, resistance to PARP inhibitors remains a serious problem. The mechanisms of resistance and biological markers that will improve targeting for this type of therapy in patients with breast cancer are being actively studied. Hypermethylation of the BRCA1 and BRCA2 promoters is one of the most promising factors in this direction [32].
5.Conclusions
Hypermethylation of the BRCA2 gene promoter region was significantly more frequently detected in the tumour tissue of women with breast cancer compared to their peripheral blood and peripheral blood of control subjects, thus it is a biological marker of breast cancer risk in women and an early diagnostic marker in case of suspected disease. Hypermethylation of BRCA2 was more frequently detected in patients with breast cancer over the age of 50 and in patients with higher Ki67 expression levels. An association was found between the absence of hypermethylation of the BRCA1 and BRCA2 gene promoter regions and a prognostically better course of the disease, as they also had significantly higher progesterone receptor expression. Our study confirms the hypothesis that BRCA2 hypermethylation plays an important role in the pathogenesis of breast cancer and the importance of assessing its co-hypermethylation with BRCA1 in predicting the course of the disease. Determination of this epigenetic alteration of BRCA2 can be used as a prognostic and predictive marker in breast cancer, as well as for the search/selection of personalised therapy, but further multicentre studies are needed.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Commission on Bioethical Expertise and Research Ethics of Bogomolets National Medical University (approval no. 0120U100871). The written informed consent was obtained from each participant included in this study.
Author contributions
Conception: Zoia Rossokha, Olga Lobanova.
Interpretation or analysis of data: Olga Lobanova, Viktoriia Vershyhora, Nataliia Medvedieva, Olha Dubitska.
Preparation of the manuscript: Liliia Fishchuk, Zoia Rossokha.
Revision for important intellectual content: Valeriy Cheshuk, Roman Vereshchako.
Supervision: Natalia Gorovenko.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
Not applicable.
Conflict of interest
The Authors declare that there is no conflict of interest.
References
[1] | A. Znaor, A. Ryzhov, M.L. Losada, A. Carvalho, V. Smelov, A. Barchuk, M. Valkov, E. Ten, D. Andreasyan, S. Zhizhilashvili, Z. Dushimova, L.D. Zhuikova, A. Egorova, A. Yaumenenka, S. Djanklich, O. Tril and F. Bray, Breast and cervical cancer screening practices in nine countries of Eastern Europe and Central Asia: a population-based survey, J Cancer Policy 38: ((2023) ), 100436. doi: 10.1016/j.jcpo.2023.100436. |
[2] | National Cancer Registry of Ukraine [Internet]. www.ncru.inf.ua. [cited 2023 Sep 20]. Available from: http://www.ncru.inf.ua/. |
[3] | A. Samusieva, S. Serga, S. Klymenko, L. Rybchenko, B. Klimuk, L. Zakhartseva, N. Gorovenko, O. Lobanova, Z. Rossokha, L. Fishchuk, N. Levkovich, N. Medvedieva, O. Popova, V. Cheshuk, M. Inomistova, N. Khranovska, O. Skachkova, Y. Michailovich, O. Ponomarova and I. Kozeretska, Contribution of BRCA1 5382insC mutation to triplene-gative and luminal types of breast cancer in Ukraine, Breast Cancer Res Treat 195: (3) ((2022) ), 453–459. doi: 10.1007/s10549-022-06692-3. |
[4] | S.M. Wang, A global perspective on the ethnic-specific BRCA variation and its implication in clinical application, J Natl Cancer Cent 3: (1) ((2023) ), 14–20. doi: 10.1016/j.jncc.2022.12.001. |
[5] | O. Lobanova, N. Medvedieva, L. Fishchuk, O. Dubitska, V. Cheshuk, R. Vereshchako, L. Zakhartseva, Z. Rossokha and N. Gorovenko, Methylation of promoter region of BRCA1 gene versus pathogenic variants of gene: risk factor or clinical marker of breast cancer, Breast Cancer Res Treat 196: (3) ((2022) ), 505–515. doi: 10.1007/s10549-022-06774-2. |
[6] | A.M. Coletta, S.K. Peterson, L.A. Gatus, K.J. Krause, S.M. Schembre, S.C. Gilchrist, B. Arun, Y.N. You, M.A. Rodriguez-Bigas, L.L. Strong, K.H. Lu and K. Basen-Engquist, Diet, weight management, physical activity and ovarian and breast cancer risk in women with BRCA1/2 pathogenic germline gene variants: systematic review, Hered Cancer Clin Pract 18: ((2020) ), 5. doi: 10.1186/s13053-020-0137-1. |
[7] | O.V. Paliychuk, L.Z. Polishchuk, Z.I. Rossokha and V.F. Chekhun, Molecular-genetic models for prognosis of development of tumors of reproductive system in women with family history of cancer, Exp Oncol 40: (1) ((2018) ), 59–67. |
[8] | H. Pradjatmo, Methylation status and expression of BRCA2 in epithelial ovarian cancers in Indonesia, Asian Pac J Cancer Prev 16: (18) ((2015) ), 8599–8604. doi: 10.7314/apjcp.2015.16.18.8599. |
[9] | J.L. Hilton, J.P. Geisler, J.A. Rathe, M.A. Hattermann-Zogg, B. DeYoung and R.E. Buller, Inactivation of BRCA1 and BRCA2 in ovarian cancer, J Natl Cancer Inst 94: (18) ((2002) ), 1396–1406. doi: 10.1093/jnci/94.18.1396. |
[10] | O.E. Lobanova, Z.I. Rossokha, N.L. Medvedieva, V.E. Cheshuk, R.I. Vereshchako, V.O. Vershyhora, L.Y. Fishchuk, L.M. Zakhartseva and N.G. Gorovenko, Prevalence of BRCA1 and BRCA2 genes promoter hypermethylation in breast cancer tissue, Exp Oncol 43: (1) ((2021) ), 56–60. doi: 10.32471/exp-oncology.2312-8852.vol-43-no-1.15703. |
[11] | M.N. Lee, R.C. Tseng, H.S. Hsu, J.Y. Chen, C. Tzao, W.L. Ho and Y.C. Wang, Epigenetic inactivation of the chromosomal stability control genes BRCA1, BRCA2, and XRCC5 in non-small cell lung cancer, Clin Cancer Res 13: (3) ((2007) ), 832–838. doi: 10.1158/1078-0432.CCR-05-2694. |
[12] | R. Bosviel, J. Durif, J. Guo, M. Mebrek, F. Kwiatkowski, Y.J. Bignon and D.J. Bernard-Gallon, BRCA2 promoter hypermethylation in sporadic breast cancer, OMICS 16: (12) ((2012) ), 707–710. doi: 10.1089/omi.2012.0060. |
[13] | E.A. Ramalho, J.L. Silva-Filho, M.F. Cartaxo, C.B. Cavalcanti, M.J. Rêgo, M.B. Oliveira and E.I. Beltrão, Assessment of changes in the BRCA2 and P53 genes in breast invasive ductal carcinoma in northeast Brazil, Biol Res 47: (1) ((2014) ), 3. doi: 10.1186/0717-6287-47-3. |
[14] | T. Kontorovich, Y. Cohen, U. Nir and E. Friedman, Promoter methylation patterns of ATM, ATR, BRCA1, BRCA2 and p53 as putative cancer risk modifiers in Jewish BRCA1/BRCA2 mutation carriers, Breast Cancer Res Treat 116: (1) ((2009) ), 195–200. doi: 10.1007/s10549-008-0121-3. |
[15] | T. Samuel, B. James, A. Adara-Ali, Y. Fayoda and M. Habeeb, Promoter methylation signatures of BRCA2 and TP53 genes in the serum of some breast cancer patients attending radiotherapy clinic in Lagos, Nigeria, Endocr Abstr 38: ((2015) ), 174. doi: 10.1530/endoabs.38.p174. |
[16] | R. Ben Gacem, M. Hachana, S. Ziadi, K. Amara, F. Ksia, M. Mokni and M. Trimeche, Contribution of epigenetic alteration of BRCA1 and BRCA2 genes in breast carcinomas in Tunisian patients, Cancer Epidemiol 36: (2) ((2012) ), 190–197. doi: 10.1016/j.canep.2011.09.001. |
[17] | E.J. Jung, I.S. Kim, E.Y. Lee, J.E. Kang, S.M. Lee, D.C. Kim, J.Y. Kim and S.T. Park, Comparison of methylation profiling in cancerous and their corresponding normal tissues from korean patients with breast cancer, Ann Lab Med 33: (6) ((2013) ), 431–440. doi: 10.3343/alm.2013.33.6.431. |
[18] | N. Buyru, J. Altinisik, F. Ozdemir, S. Demokan and N. Dalay, Methylation profiles in breast cancer, Cancer Invest 27: (3) ((2009) ), 307–132. doi: 10.1080/07357900802350814. |
[19] | M. Rodríguez-Balada, B. Roig, M. Melé, M. Salvat, L. Martorell, J. Borràs and J. Gumà, Germline promoter hypermethylation in BRCA1 and BRCA2 genes is not present in hereditary breast cancer patients, Clin Transl Oncol 20: (9) ((2018) ), 1226–1231. doi: 10.1007/s12094-018-1837-0. |
[20] | S. Vos, C.B. Moelans and P.J. van Van Diest, BRCA promoter methylation in sporadic versus BRCA germline mutation-related breast cancers, Breast Cancer Res 19: (1) ((2017) ), 64. doi: 10.1186/s13058-017-0856-z. |
[21] | T.O. Nielsen, S.C.Y. Leung, D.L. Rimm, A. Dodson, B. Acs, S. Badve, C. Denkert, M.J. Ellis, S. Fineberg, M. Flowers, H.H. Kreipe, A.V. Laenkholm, H. Pan, F.M. Penault-Llorca, M.Y. Polley, R. Salgado, I.E. Smith, T. Sugie, J.M.S. Bartlett, L.M. McShane, M. Dowsett and D.F. Hayes, Assessment of Ki67 in breast cancer: Updated recommendations from the international Ki67 in breast cancer working group, J Natl Cancer Inst 113: (7) ((2021) ), 808–819. doi: 10.1093/jnci/djaa201. |
[22] | P. Cabrera-Galeana, W. Muñoz-Montaño, F. Lara-Medina, A. Alvarado-Miranda, V. Pérez-Sánchez, C. Villarreal-Garza, R.M. Quintero, F. Porras-Reyes, E. Bargallo-Rocha, I. Del Carmen, A. Mohar and O. Arrieta, Ki67 changes identify worse outcomes in residual breast cancer tumors after neoadjuvant chemotherapy, Oncologist 23: (6) ((2018) ), 670–678. doi: 10.1634/theoncologist.2017-0396. |
[23] | C.A. Purdie, P. Quinlan, L.B. Jordan, A. Ashfield, S. Ogston, J.A. Dewar and A.M. Thompson, Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study, Br J Cancer 110: (3) ((2014) ), 565–572. doi: 10.1038/bjc.2013.756. |
[24] | Z. Li, H. Wei, S. Li, P. Wu and X. Mao, The role of progesterone receptors in breast cancer, Drug Des Devel Ther 16: ((2022) ), 305–314. doi: 10.2147/DDDT.S336643. |
[25] | N. Al-Moghrabi, M. Al-Showimi, N. Al-Yousef, B. Al-Shahrani, B. Karakas, L. Alghofaili, H. Almubarak, S. Madkhali and H. Al Humaidan, Methylation of BRCA1 and MGMT genes in white blood cells are transmitted from mothers to daughters, Clin Epigenetics 10: (1) ((2018) ), 99. doi: 10.1186/s13148-018-0529-5. |
[26] | R. Tong, X. Wu, Y. Liu, Y. Liu, J. Zhou, X. Jiang, L. Zhang, X. He and L. Ma, Curcumin-Induced DNA demethylation in human gastric cancer cells is mediated by the DNA-Damage response pathway, Oxid Med Cell Longev 2020: ((2020) ), 2543504. doi: 10.1155/2020/2543504. |
[27] | Q. Xie, Q. Bai, L.Y. Zou, Q.Y. Zhang, Y. Zhou, H. Chang, L. Yi, J.D. Zhu and M.T. Mi, Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells, Genes Chromosomes Cancer 53: (5) ((2014) ), 422–431. doi: 10.1002/gcc.22154. |
[28] | L.P. Xiang, A. Wang, J.H. Ye, X.Q. Zheng, C.A. Polito, J.L. Lu, Q.S. Li and Y.R. Liang, Suppressive effects of tea catechins on breast cancer, Nutrients 8: (8) ((2016) ), 458. doi: 10.3390/nu8080458. |
[29] | P. Selvakumar, A. Badgeley, P. Murphy, H. Anwar, U. Sharma, K. Lawrence and A. Lakshmikuttyamma, Flavonoids and other polyphenols act as epigenetic modifiers in breast cancer, Nutrients 12: (3) ((2020) ), 761. doi: 10.3390/nu12030761. |
[30] | O.A. Stefansson, H. Hilmarsdottir, K. Olafsdottir, L. Tryggvadottir, A. Sverrisdottir, O.T. Johannsson, J.G. Jonasson, J.E. Eyfjord and S. Sigurdsson, BRCA1 promoter methylation status in 1031 primary breast cancers predicts favorable outcomes following chemotherapy, JNCI Cancer Spectr 4: (2) ((2019) ), pkz100. doi: 10.1093/jncics/pkz100. |
[31] | F. Menghi, K. Banda, P. Kumar, R. Straub, L. Dobrolecki, I.V. Rodriguez, S.E. Yost, H. Chandok, M.R. Radke, G. Somlo, Y. Yuan, M.T. Lewis, E.M. Swisher and E.T. Liu, Genomic and epigenomic BRCA alterations predict adaptive resistance and response to platinum-based therapy in patients with triple-negative breast and ovarian carcinomas, Sci Transl Med 14: (652) ((2022) ), eabn1926. doi: 10.1126/scitranslmed.abn1926. |
[32] | R.E. Miller, A. Leary, C.L. Scott, V. Serra, C.J. Lord, D. Bowtell, D.K. Chang, D.W. Garsed, J. Jonkers, J.A. Ledermann, S. Nik-Zainal, I. Ray-Coquard, S.P. Shah, X. Matias-Guiu, E.M. Swisher and L.R. Yates, ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer, Ann Oncol 31: (12) ((2020) ), 1606–1622. doi: 10.1016/j.annonc.2020.08.2102. |



