Diagnostic and prognostic utility of TROP-2, SLP-2, and CXCL12 expression in papillary thyroid carcinoma
Abstract
BACKGROUND:
Papillary thyroid carcinoma (PTC) is the most frequent thyroid malignancy. Histopathological examination is widely accepted as the gold standard test for the diagnosis of PTC. However, the histopathological examination sometimes can’t differentiate PTC from other thyroid diseases. Differentiating PTC from other thyroid diseases is essential for a therapeutic approach and prognosis.
OBJECTIVES:
The current study was performed to investigate the utility of TROP-2, SPL-2, and CXCL12 mRNA and protein expression in discriminating PTC from other thyroid diseases that mimic PTC.
METHODS:
The current study was performed on 75 cases of surgically resected thyroid glands. The cases were distributed in two groups: the PTC group and the non-PTC group. The PTC group consisted of 35 cases (25 patients of the classic PTC variant and 10 patients of the PTC follicular variant). The non-PTC group consisted of 40 cases (10 cases were multinodular goiter, 5 cases were Graves’ disease, 5 cases were Hashimoto thyroiditis, 15 patients were follicular adenoma (FA) and 5 cases were follicular carcinoma). TROP-2, SPL-2, and CXCL12 mRNA expression were estimated by qRT-PCR, and protein expression was estimated by immunohistochemistry.
RESULTS:
There were upregulated TROP-2, SPL-2, and CXCL12 mRNA and protein expressions in PTC compared to non-PTC (
CONCLUSION:
mRNA expression of TROP-2, SPL-2, and CXCL12 among PTC cases increased in larger tumor size, tumor stages III and IV, and LN metastasis. Moreover, there was an increase in CXCL-12 gene expression among PTC cases with extra-thyroid extension. Thus, TROP-2, SPL-2, and CXCL12 expressions could be possible diagnostic and prognostic markers in PTC.
Abbreviations
| PTC | Papillary thyroid carcinoma |
| TROP-2 | Trophoblast cell surface antigen-2 |
| Tacstd2 | the tumor associated calcium signal |
| transducer 2 | |
| SLP-2 | Stomatin-like protein-2 |
| CXCL12 | Chemokine (C-X-C motif) ligand 12 |
| ROC | Receiver Operator Curve |
1.Introduction
Papillary thyroid carcinoma (PTC) is the most prevalent primary malignant thyroid tumor. It represents about 80% of thyroid cancer cases worldwide. It has a low aggressive behavior and a favorable outcome [1]. In Egypt, primary thyroid tumors constitute 1.96% of malignant tumors and represent 74.7% of malignant endocrine neoplasms. PTC represents the most prevalent primary malignant thyroid tumor (70.94% of all thyroid malignant tumors) [2].
Histopathological examination is widely accepted as the gold standard test in PTC diagnosis. Most PTC cases can be diagnosed and categorized depending on the histopathologic examination. However, the histopathologic examination sometimes can’t differentiate between PTC and other similar diseases [3].
To remove this discrepancy and reach a final diagnosis, a variety of immunohistochemical and biochemical markers have been investigated. Several studies used immunohistochemical markers to distinguish PTC from other thyroid lesions such as HBME-1, galectin-3, and cytokeratin 19. Some difficulties were associated with those markers including low specificity, variable sensitivity, and background staining. So, we require novel markers with high sensitivity and specificity to improve PTC diagnosis [3].
Trophoblast cell surface antigen-2 (TROP-2) is a transmembrane glycoprotein encoded by the tumor-associated calcium signal transducer 2 (Tacstd2) gene. Originally, it was found in human trophoblast and choriocarcinoma cell lines. It acts as a cell surface receptor where it recognizes certain ligands and increases the intracellular calcium [4]. Also, it is an oncogene that is upregulated in several human cancers with low to no expression in normal tissues [5]. Its upregulation has been correlated with poor prognosis in different cancers [6], as it has pro-proliferative and pro-invasive properties [9, 10]. Moreover, it is an immunotherapeutic target [3]. In thyroid cancer, TROP-2 was significantly elevated [22, 31, 1]. Additionally, TROP-2 immunostaining may differentiate PTC from other thyroid carcinomas or normal follicular tissues [25, 7, 8].
Stomatin-like protein-2 (SLP-2) belongs to the stomatin family. It is identified as a mitochondrial inner membrane protein encoded by the gene STOML2. It plays a role in controlling mitochondrial membrane stability and function [13]. It was strongly associated with many human cancers, such as gallbladder and cervical cancers [11, 12]. Liu et al. [10] found that SLP-2 mRNA and protein expression were overexpressed in PTC.
Chemokine (C-X-C motif) ligand 12 (CXCL12), also named stromal cell-derived factor-1 (SDF-1), belongs to the chemokine family that binds to CXCR4. The biological effect of CXCL12 is attributed to CXCR4-mediated activation of G protein-coupled signaling molecules, including ERK1/2, MAPK, JNK, and AKT [14, 15]. Also, it has a powerful chemotactic ability to lymphocytes. Moreover, it has an important role in angiogenesis, carcinogenesis, and tumor migration [17]. Chung et al. [35] demonstrated that CXCL12 was exclusively upregulated in PTC whatever the histopathological subtype when compared to other thyroid lesions. Also, Zhu et al. [36] found that CXCL12 was exclusively expressed in PTC tissue samples while it was not expressed in benign thyroid lesions and other thyroid malignancies.
Distinguishing PTC from other thyroid lesions is important because it determines the therapeutic intervention and prognosis. However, the pathological findings sometimes can’t differentiate between PTC and other thyroid lesions. Thus, our study aimed to evaluate the utility of TROP-2, SPL-2, and CXCL12 mRNA and protein expression in distinguishing PTC from other thyroid lesions that mimic PTC.
Table 1
Primer sequence of the studied genes
| Forward | Reverse | |
|---|---|---|
| TROP2 | 5’-ACAACGATGGCCTCTACG AC-3’ | 5’-GTCCAGGTCTGAGTGGT TGAA-3’ |
| SLP-2 | 5’-GTGACTCTCGACAATGTAAC-3’ | 5’-TGATCTCATAACGGAGGCAG-3’ |
| CXCL12 | 5’-ATTCTCAACACTCCAAACTGTGC | 5’-ACTTTAGCTTCGGGTCAATGC-3’ |
|
| 5’-CACGAAACTACCTTCAACTCC-3’ | 5’-CATACTCCTGCTTGCTGATC-3’ |
2.Material and methods
This study was conducted on 75 surgically resected thyroid glands after written informed consent from patients attending the Zagazig University Hospitals and Surgical Oncology Department, Ismailia Teaching Oncology Hospital, Egypt during the period between October 2021 and March 2023. The cases were distributed in two groups: the PTC and the non-PTC groups. The PTC group consisted of 35 cases (25 patients of the classic PTC variant and 10 patients of the follicular variant PTC (FVPTC)). The non-PTC group consisted of 40 cases (10 cases were multinodular goiter, 5 patients were Graves’ disease, 5 patients were Hashimoto thyroiditis, 15 patients were follicular adenoma (FA) and 5 cases were follicular carcinoma). Exclusion criteria included patients with indefinite diagnosis or equivocal features. The classification and evaluation of thyroid tumors were performed depending on the 4th edition WHO Classification of Tumors of Endocrine Organs in 2017 [18].
Immediately after surgery, the resected tissues were divided into 2 parts. The first was preserved at
Clinicopathological data including age, sex, tumor size, lymph node involvement, and distant spread were recorded. PTC patients were staged according to the pathological TNM system of the American Joint Committee of Cancer 8th edition 2017. Our study got approval from the institute review board (IRB), (ZU-IRB # 10022/25-10-2022).
2.1RNA extraction, cDNA synthesis, and qRT-PCR
RNA extraction from the homogenates of thyroid tissues was carried out by GENEzolTM (Geneaid). Nanodrop spectrophotometry (ND 1000-NanoDrop®) was used to estimate the quality and the quantity of the extracted RNA. Then, TOPscriptTM cDNA Synthesis Kit (Enzynomics) was used for the synthesis of cDNA. Real-time polymerase chain reaction was carried out using Rotor-Gene Q 2 Plex (Qiagen, Hilden, Germany). The relative gene expressions of TROP-2, SLP-2 and CXCL12 were calculated with the 2-ΔΔct method.
2.2Histopathological examination of thyroid tissues
Thyroid tissues were preserved in formalin. After paraffin block formation, tissue sectioning into 4-micrometer sections was performed. Staining with hematoxylin and eosin was performed [20].
2.3Immunohistochemical staining
The staining was performed on 4-micrometer thickness tissue samples. Absolute ethyl alcohol was used to remove the paraffin-xylene solution. Tissue sections were incubated for an hour with an anti-TROP-2 antibody (Santa Cruz Biotechnology, USA) at 1:40 dilution, an anti-SLP-2 antibody (Proteintech Group, Chicago, IL, USA) at 1:200 dilution, and an anti-CXCL12 antibody (R&D Systems, Minneapolis, MN, USA) at 1:50 dilution. Mayer’s hematoxylin was used as a counterstain.
2.4Assessment of immunohistochemical stains
Regarding TROP-2 expression, the extent of membranous staining was evaluated. Samples with no staining or membranous staining in less than 5% of cells were classified as negative and membranous staining of more than 5% of the cells was categorized as positive. Positive cases were graded as 1
Regarding SLP-2 expression, the extent of cytoplasmic staining was evaluated. Samples with no staining or staining less than 10% of cells were classified as negative and cases with a cytoplasmic expression of more than 10% were categorized as positive [1].
Regarding CXCL12 expression, the extent of cytoplasmic staining was graded from 0 to 3
2.5Statistical analysis
The continuous data were represented as mean
Table 2
Basic characteristics among both studied groups
| Group I (non-PTC) | Group II (PTC) |
| |||
|---|---|---|---|---|---|
| Age | 44.5 | 46.7 | 0.676 | 0.508 | |
| Gender | Male | 10 (25%) | 7 (20%) | 0.266 | 0.605 |
| Female | 30 (75%) | 28 (80%) | |||
| Positive TROP-2 IHC expression | 2 (5%) | 34 (97.1%) | 63.4 | ||
| Positive SLP-2 IHC expression | 3 (7.5%) | 31 (88.6%) | 49.3 | ||
| Positive CXCL-12 IHC expression | 1 (2.5%) | 33 (94.3%) | 63.5 | ||
| TROP-2 gene expression | 1.18 | 4.49 | 77.2 | ||
| SLP-2 gene expression | 1.15 | 3.03 | 46.7 | ||
| CXCL-12 gene expression | 1.09 | 2.52 | 16.9 | ||
*: a significant difference; **: a highly significant difference.
Table 3
Relation between genes expression and basic characteristics of the lesion among studied PTC cases
| Variables | PTC cases | TROP-2 gene expression |
| SLP-2 gene expression |
| CXCL-12 gene expression |
| ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor size | |||||||||||
| | 12 | (34.3%) | 4.28 | 2.81 | 2.07 | ||||||
| | 23 | (65.7%) | 4.59 | 3.15 | 2.76 | ||||||
| TNM stage | |||||||||||
| I–II | 31 | (88.6%) | 4.45 | 0.008* | 2.98 | 0.002* | 2.38 | ||||
| III–IV | 4 | (11.4%) | 4.8 | 3.4 | 3.6 | ||||||
| T stage | |||||||||||
| I–II | 25 | (71.4%) | 4.4 | 2.93 | 2.3 | ||||||
| III–IV | 10 | (28.6%) | 4.75 | 3.3 | 3.16 | ||||||
| LN metastasis | |||||||||||
| Positive | 12 | (34.3%) | 4.69 | 3.25 | 3.03 | ||||||
| Negative | 23 | (65.7%) | 4.38 | 2.92 | 2.26 | ||||||
| Extra-thyroid extension | |||||||||||
| Positive | 2 | (5.7%) | 4.8 | 0.08 | 3.35 | 0.09 | 3.8 | ||||
| Negative | 33 | (94.3%) | 4.47 | 3.01 | 2.44 | ||||||
*: significant difference; **: highly significant difference.
3.Results
3.1Clinicopathological features
There was no significant difference in age between PTC and non-PTC patients (
Regarding PTC cases, twelve patients (34.3%) had tumor sizes less than 2 cm while 23 (65.7%) patients had tumor sizes more than 2 cm. Thirty-one patients (88.6%) were I–II TNM stage while 4 patients (11.4%) were III–IV TNM stage. Twenty-five patients (71.4%) were I–II T stage while 10 patients (28.6%) were III–IV T stage. Twelve patients (34.3%) had lymph node metastasis and two patients (5.7%) had extra-thyroid extension (Table 3).
3.2Histopathological and immunohistochemical results
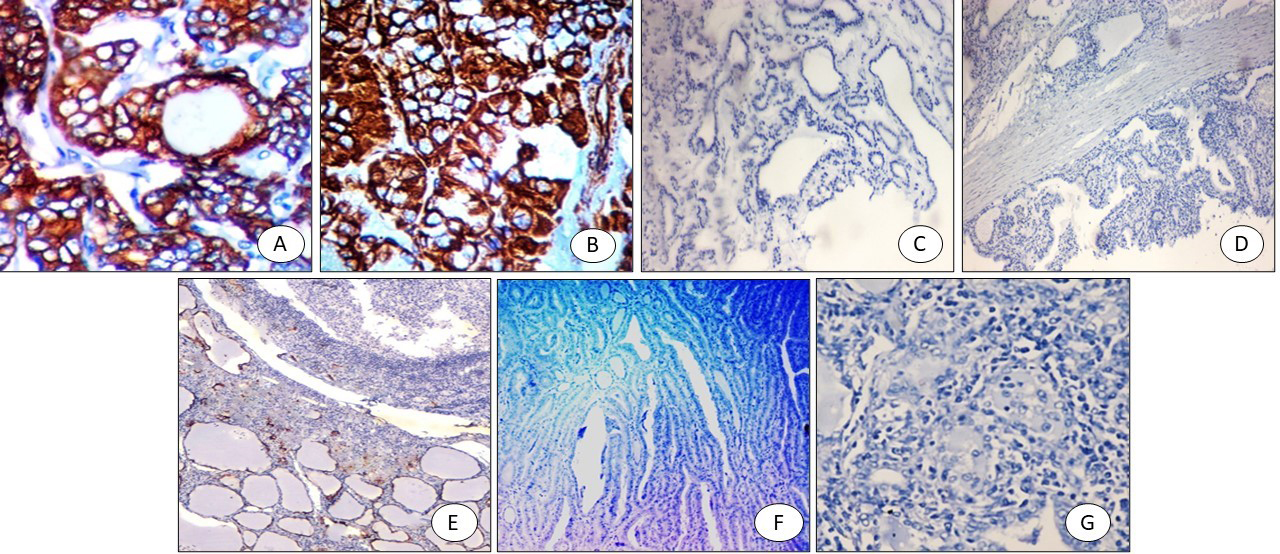
H&E staining showed that PTC sections had papillary projections with nuclear features of PTC (Fig. 1A). FVPTC sections showed capsulated tumors with prominent nuclear features of PTC (Fig. 1B). Colloid goiter sections showed variable-sized follicles with flat lining (Fig. 1C). Graves’ disease showed hyperplastic thyroid follicles with papillary projections (Fig. 1D). Hashimoto thyroiditis sections showed prominent lymphoid follicles with abundant Hurthle cells (Fig. 1E). Follicular adenoma sections showed intact capsules (Fig. 1F). Follicular carcinoma showed thickened capsules with full thickness penetration (Fig. 1G) with prominent atypia and mitosis (Fig. 1H).
Figure 1.
(A) PTC: papillary projections with nuclear features of PTC (X 200), (B) FVPTC: capsulated tumor with prominent nuclear features of PTC (X200), (C) Colloid goiter: variable sized follicles with flat lining (X100), (D) Graves’ disease: hyperplastic thyroid follicles with papillary projections (X200), (E) Hashimoto thyroiditis: prominent lymphoid follicles with abundant hurthle cells (X100), (F) follicular adenoma with intact capsule (X100), (G) Follicular carcinoma; thickened capsule with full thickness penetration (X100), (H) Follicular carcinoma with prominent atypia and mitosis (X200).
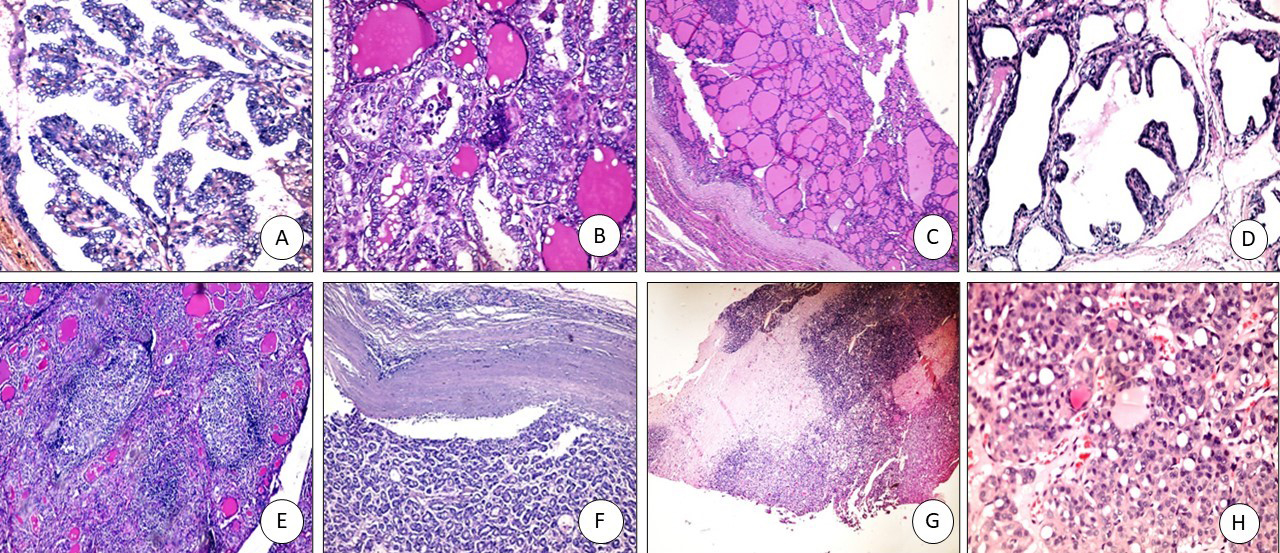
Figure 2.
TROP2 immunostaining: (A) PTC positive membranous staining (x400), (B) FVPTC: positive membranous staining (x200), (C) Colloid goiter: negative (x200), (D) Graves’ disease with papillary hyperplasia-negative (x400), (E) Hashimoto negative (x100), (F) Follicular adenoma negative (x200), (G) Follicular carcinoma negative (x200).
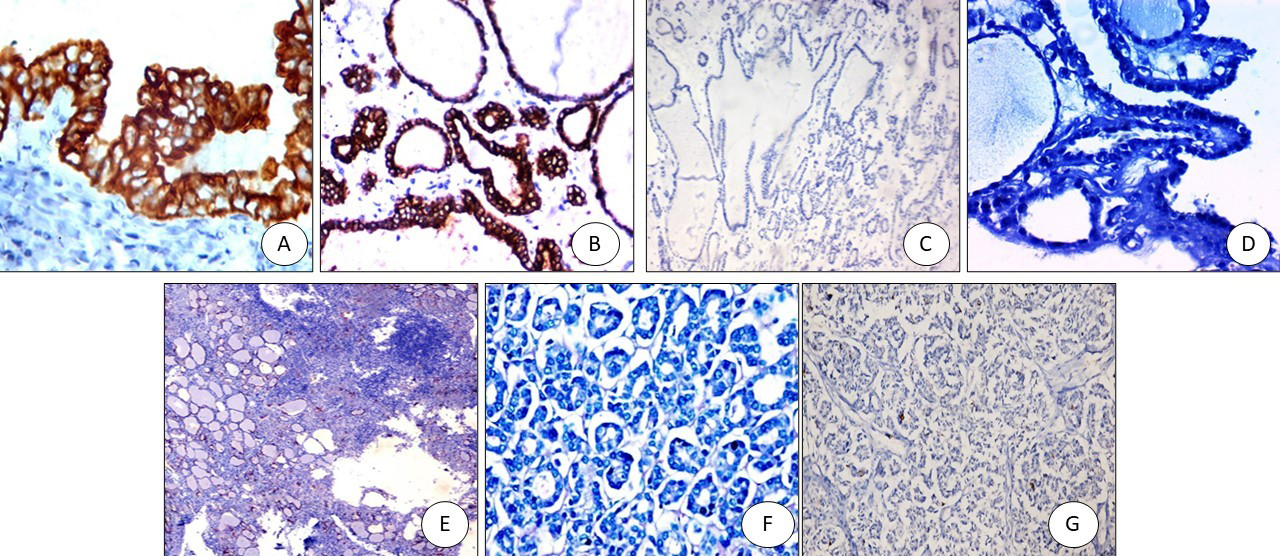
Figure 3.
SLP2 immunostaining: (A) PTC positive cytoplasmic staining (x200), (B) FVPTC: positive cytoplasmic staining (x200), (C) Colloid goiter: negative (x200), (D) Graves’ disease with papillary hyperplasia-negative (x200), (E) Hashimoto negative (x200), (F) Follicular adenoma negative (x200), (G) Follicular carcinoma negative (x200).
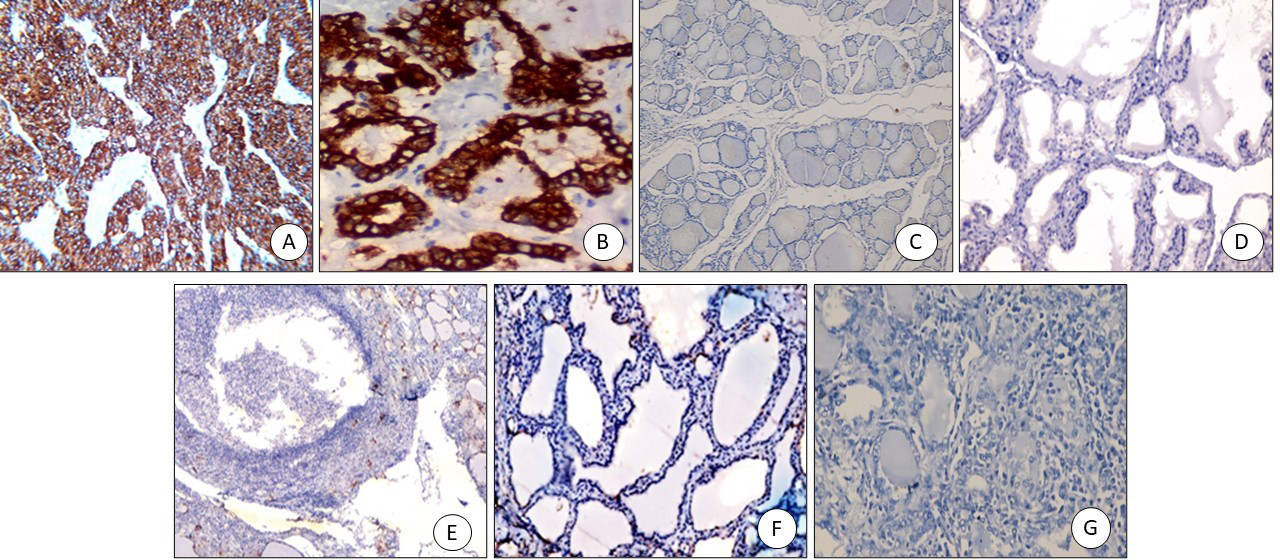
Figure 4.
CXCL2 immunostaining: (A) PTC positive cytoplasmic staining (x400), (B) FVPTC: positive cytoplasmic staining (x200), (C) Colloid goitre: negative (x200), (D) Graves’ disease with papillary hyperplasia-negative (x200), (E) Hashimoto negative (x200), (F) Follicular adenoma negative (x200), (G) Follicular carcinoma negative (x200).
Our results revealed significant positive immunohistochemical expression of (TROP2, SLP2, and CXCL2) in the PTC group compared to the non-PTC group (Figs 2–4 respectively).
3.3Relation between mRNA and protein expression and clinicopathological features in PTC
There was a highly statistically significant elevation in TROP-2, SPL-2, and CXCL12 mRNA and protein expression among the PTC group (
Table 4
Relation between immune-histochemistry results of TROP-2, SLP-2, CXCL-12 and tumor characteristics
| TROP2 |
| SLP-2 |
| CXCL-12 |
| |||||
| Positive | Negative | Positive | Negative | Positive | Negative | |||||
| T size | 11 (91.7%) | 1 (8.3%) | 0.343 | 9 (75%) | 3 (25%) | 0.106 | 12 (100%) | 0 (0.0%) | 0.536 | |
| 23 (100%) | 0 | 22 (95.7%) | 1 (4.3%) | 21 (91.3%) | 2 (8.7%) | |||||
| LN metastasis | Yes ( | 12 (100%) | 0 | 0.665 | 12 (100%) | 0 | 0.275 | 10 (83.3%) | 2 (16.7%) | 0.111 |
| No ( | 22 (95.7%) | 1 (4.3%) | 19 (82.6%) | 4 (17.4%) | 23 (100%) | 0 (0.0%) | ||||
| Distant metastasis | M0 ( | 33 (97.1%) | 1 (2.9%) | 0.971 | 30 (88.2%) | 4 (11.8%) | 0.971 | 32 (94.1%) | 2 (5.9%) | 0.943 |
| M1 ( | 1 (100%) | 0 | 1 (100%) | 0 | 1 (100%) | |||||
| Extra-thyroid extension | Yes ( | 2 (100%) | 0 | 0.943 | 2 (100%) | 0 | 0.793 | 2 (100%) | 0 | 0.889 |
| No ( | 32 (97%) | 1 (3%) | 29 (87.9%) | 4 (12.1%) | 31 (93.9%) | 2 (6.1%) | ||||
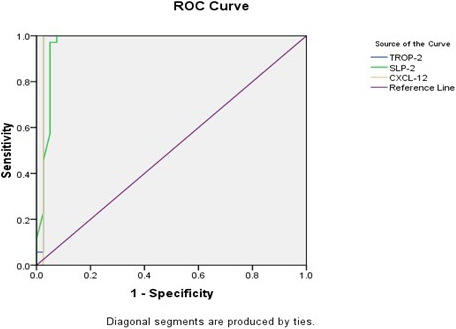
3.4Receiver Operator Curve (ROC) for TROP2, SLP2 and CXCL2
Receiver Operator Curve (ROC) analysis of TROP-2 gene expression detected that the sensitivity was 94.3%, the specificity was 97.5%, the positive predictive value (PPV) was 97.1%, the negative predictive value (NPV) was 95.1%, and the accuracy was 96% at a cut-off
Table 5
Receiver Operator Curve (ROC) curve analysis of studied gene expression as diagnostic predictors of PTC cases
| Variable(s) | AUC | Cut-off | Sensitivity | Specificity | PPV | NPV | Accuracy |
|
|---|---|---|---|---|---|---|---|---|
| TROP-2 gene | 0.976 | 94.3% | 97.5% | 97.1% | 95.1% | 96% | ||
| SLP-2 gene | 0.966 | 97.1% | 92.5% | 91.9% | 97.4% | 94.7% | ||
| CXCL-12 gene | 0.975 | 94.3% | 97.5% | 97.1% | 95.1% | 96% |
Table 6
Function of the proteins encoded by the studied genes
| Gene | Protein | Function | Reference |
|---|---|---|---|
| Tumor associated calcium signal transducer 2 (Tacstd2) | Trophoblast cell surface antigen-2 (TROP-2) | Regulates the level of intracellular calcium regulates epithelial cell migration and proliferation, cell growth, transformation, regeneration, and proliferation. | Lin et al., 2012 |
| Stomatin-like protein 2 (STOML2) | Stomatin-like protein-2 (SLP-2) | Intracellular calcium ion homeostasis, mitochondrion organization, lipid localization, positive regulation of interleukin-2 production, mitochondrial protein processing. | Wang et al., 2014Xiao et al., 2015 |
| C-X-C motif chemokine ligand 12 | Chemokine (C-X-C motif) ligand 12 (CXCL12), stromal cell-derived factor-1 (SDF-1) | Chemoattractant active on T-lymphocytes and monocytes. Activates the CXCR4 to induce a rapid and transient rise in the level of intracellular calcium ions and chemotaxis. During embryonic development, B-cell lymphopoiesis, myelopoiesis in bone marrow and heart ventricular septum formation. | Lu et al., 2009 Roland et al., 2003 Lee et al., 2020 |
Figure 5.
Receiver Operator Curve (ROC) for TROP2, SLP2 and CXCL2.
4.Discussion
Regarding TROP-2 IHC expression, only 5% (2/40) of non-PTC cases showed positive TROP-2 expression. This result was similar to Addati et al. [22] who found that TROP-2 was positive in 6/56 of non-neoplastic samples, and Abdou et al. [3] who found that TROP-2 was positive in 4/21 of non-neoplastic samples. Saffar et al. found that TROP-2 was positive in 5/71 samples of the non-PTC group and all from Hashimoto thyroiditis samples. However, Nesreen et al. [24], Simms et al. [25], and Murtezaoglu et al. [26] did not find any positivity in non-neoplastic samples.
The only benign lesion that showed positive TROP-2 expression was Hashimoto thyroiditis which is attributed to the abundance of Hurthle cells in this lesion. These cells have endogenous biotin activity that may show odd positive staining patterns with several antibodies [27]. In addition, follicular cells in Hashimoto thyroiditis may show PTC-like nuclear changes and may carry the same genetic mutation as classical PTC and may express PTC-associated proteins. This mutation activates the mitogen-activated protein kinases (MAPK) pathway, the same pathway through which TROP-2 conveys its signals. Moreover, chronic lymphocytic thyroiditis has follicular epithelial dysplasia displaying cytological atypia resembling PTC with a similar immunohistochemical profile [28].
In our study, none of the follicular carcinoma samples showed positive TROP-2 expression. This is similar to earlier studies reported by Simms et al. [25], Bychkov et al. [8], Liu et al. [7], and Abdel Raouf et al. [29].
Our study showed positive membranous TROP-2 expression in 97.1% of PTC cases (34 out of 35). These results were consistent with the research conducted by Yang et al. [1], Zargari et al. [21], and Saffer et al. [23]. TROP-2 positivity was 96%, 93%, and 98% in them, respectively. On the other hand, the results were different from the research performed by Abdou et al. [3], Murtezaoglu et al. [26], Bychkov et al. [8], and Lui et al. [7] that reported lower TROP-2 expression in PTC (71.4%, 50%, 81.5%, and 81.6% respectively).
The current study showed that there is a significant difference in TROP-2 positive expression between PTC cases and non-PTC cases. This result is compatible with Abdou et al. [3], Addati et al. [22], Saffer et al. [23], Bychkov et al. [8], and Lui et al. [7] that reported that TROP-2 is a new marker in the diagnosis of PTC.
The diagnostic validity of TROP-2 in the PTC samples showed 94.3% sensitivity and 97.5% specificity, 97.1% PPV, 95.1% NPV and the accuracy was 96%. Similarly, Yang et al. [1] detected that TROP-2 sensitivity in PTC was 96% [1], on the other hand, Bychkov et al. [8] detected that TROP-2 sensitivity in PTC was 75%, but the specificity was 98.4%.
None of the clinicopathological parameters was related to TROP-2 IHC expression. This result was similar to the previous studies performed by Yang et al. [1], Bychkov et al. [8], and Kong et al. [30]. However, TROP-2 positive expression may indicate poor PTC prognosis and be associated with advanced tumor stage as detected by Guan et al. [31].
As regards SLP-2 expression, the current study showed that only 7.5% (3/35) of the non-PTC cases showed positive SLP-2 expression, and 88.6% of PTC cases (31 out of 35) showed positive cytoplasmic expression. This finding was compatible with that found by Yang et al. [1] who found 83.3% of PTC cases showing positive SLP-2 expression. Also, they stated that the mRNA expression and positive SLP-2 staining in PTC were higher than in follicular adenoma with no association with any of the clinicopathological features similar to our findings. They reported that SLP-2 sensitivity was 83.3%, specificity was 79.3%, PPV was 80.6%, NPV was 82.1%, and accuracy was 81.4%.
Liu et al. [7] reported that SLP-2 mRNA and protein expressions were upregulated in PTC cases. 72% (77 out of 107) of PTC cases were positive for SLP2 expression. Similar to our findings, upregulated SLP-2 m RNA was correlated with larger tumor sizes, advanced tumor stages, and lymph node metastasis. They concluded that SLP-2 is a marker for the diagnosis of classic PTC. However, no differences in SLP-2 expression regarding age, gender, or multifocal tumors were detected. SLP-2 expression was strongly correlated with primary tumor size where its expression in T1 cases (tumor
Bartolome et al. [32] reported that 97 of 150 (65%) PTCs were SLP-2 positive. Benign thyroid tissue did not have SLP-2 immunostaining. Follicular carcinomas and anaplastic carcinomas (except in one case) showed no or weak/focal SLP-2 expression. They indicated that SLP-2 was a possible marker for the recognition of high-risk PTC. SLP-2 expression was higher in the classic PTC than in the follicular variant of PTC. Different from our findings, significant positive correlations were detected between increased SLP-2 and lymph node spread, extra-thyroid spread, and higher tumor stages. Similar to our findings, no correlations were detected between SLP-2 expression and other clinicopathological features of the patients (age, gender, tumor size).
Additionally, the expression of SLP-2 was significantly associated with clinicopathological features and the expression of Ki-67, a cell proliferation marker. Moreover, western blot results showed that SLP-2 was upregulated by TGF-
As regards CXCL12 IHC, our study reported that 94.3% (33 out of 35) of PTC cases showed positive CXCL12 expression. This result was compatible with Conley-LaComb et al. [33]. CXCL12 had 90.8% sensitivity, 96.8% specificity, 98.9% PPV, 76.9% NPV, and 92.2% accuracy in tissue specimens of PTC and with Jung et al. [34] that reported 93.6% sensitivity, 88.6% specificity, 91.7% PPV, 91.2% NPV, and 91.5% accuracy and suggested that CXCL12 may be a supplementary diagnostic marker for PTC. Lee et al. [17] showed that CXCL12 immunoreactivity had 98.3% sensitivity, 93.5% specificity, 96.6% PPV, 96.7% NPV, and 96.6% accuracy in TMA specimens. Chung et al. [35] found that CXCL12 expression was exclusively detected in PTC compared to other thyroid diseases. 91.1% of the variant PTCs and 90.6% of the classical type were associated with CXCL12 immunohistochemical expression, irrespective of the histological subtype. On the other side, very few thyroid lesions, excluding PTC, were positive for CXCL12. The Validity of CXCL12 in PTC showed 90.8% sensitivity, 96.8% specificity, 98.9% PPV, 76.9% NPV, and 92.2% accuracy.
In contrast, Zhu et al. [36] found that CXCL12 expression was detected in 75% of PTC cases and 10% of follicular adenoma cases but was not detected in other studied cases.
In our study, TROP-2, SLP-2, and CXCL-12 mRNA expressions were upregulated in PTC. Similarly, Liu et al. [10] were the first to detect SLP-2 overexpression (mRNA and protein levels) in PTC samples. Similar to our findings, SLP-2 upregulation was significantly associated with LN involvement and tumor stage where it was correlated with tumors
Regarding the potential mechanisms of TROP-2 in PTC carcinogenesis, it was found to enhance the invasion of thyroid cancer by inducing MMP2 through ERK and JNK pathways [31]. Also, CXCL12 plays an important role in angiogenesis and carcinogenesis, including tumor migration [17]. Its effect is related to CXCR4-mediated activation of G protein-coupled signaling molecules, including ERK1/2, MAPK, JNK, and AKT [14, 15]. In anaplastic thyroid cancer cells in particular, CXCL12/CXCR4 axis-mediated migration has been reported which is associated with AKT and ERK activation [16]. Zhu et al. [36] added that CXCL12/CXCR4/CXCR7 axis may contribute to thyroid cancer development by regulating cancer cell migration/invasion via AKT and ERK signaling and MMP-2 activation.
SLP-2 is upregulated in mitochondrial stress and interacts with prohibitins and chaperones in the mitochondrial inner membrane. It is required for stress-induced mitochondrial hyperfusion. Its upregulation increases the mitochondrial membrane phospholipid cardiolipin content and binds to cardiolipin to induce mitochondrial biogenesis [13]. Its expression is regulated by transforming growth factor-
5.Conclusion
TROP-2, SPL-2, and CXCL12 expressions could be possible diagnostic and prognostic markers in PTC. mRNA and protein expression of the three markers were increased in PTC cases compared to non-PTC. They were also significantly increased in PTC cases with larger tumor size, tumor stages III and IV, and LN metastasis. Moreover, there was a statistically significant increase in CXCL-12 gene expression among PTC cases with extra-thyroid extension.
Funding
None.
Author contributions
Conception: Amany Selim Attia, Samia Hussein, and Reham Sameh.
Interpretation or analysis of data: Hend Sameh, Amr Khalil, Ahmad Barakat Waley, and Ihab Matar.
Preparation of the manuscript: Amany Selim Attia and Samia Hussein.
Revision for important intellectual content: Reham Sameh and Samia Hussein.
Supervision: Reham Sameh and Samia Hussein.
Acknowledgments
None.
Conflict of interest
None.
References
[1] | X. Yang, Y. Hu, H. Shi, C. Zhang, Z. Wang et al., The diagnostic value of TROP-2, SLP-2 and CD56 expression in papillary thyroid carcinoma, European Archives of Oto-Rhino-Laryngology 275: ((2018) ), 2127–2134. doi: 10.1007/s00405-018-5045-x. |
[2] | N. Mokhtar, A. Salama, O. Badawy, E. Khoshed and G. Mohamed, Cancer Pathology Registry 2000–2011, Cairo [Egypt], Malignant endocrine system tumors, eds.: Cairo Press, (2015) , 182–192. |
[3] | A. Abdou, M. Shabaan, R. Abdallha and N. Nabil, Diagnostic value of TROP-2 and CK19 expression in papillary thyroid carcinoma in both surgical and cytological specimens, Clinical Pathology 12: ((2019) ), 1–16. doi: 10.1177/2632010X19863047. |
[4] | J.C. Lin, Y.Y. Wu, J.Y. Wu, T.C. Lin, C.T. Wu, Y.L. Chang, Y.S. Jou, T.M. Hong and P.C. Yang, TROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinoma, EMBO Mol Med 4: (6) ((2012) ), 472–485. |
[5] | M. Trerotola, P. Cantanelli, E. Guerra, R. Tripaldi, A.L. Aloisi, V. Bonasera, R. Lattanzio, R. de Lange, U.H. Weidle, M. Piantelli et al., Upregulation of Trop-2 quantitatively stimulates human cancer growth, Oncogene 32: (2) ((2013) ), 222–233. |
[6] | T. Liu, Y. Liu, X. Bao, J. Tian, Y. Liu and X. Yang, Overexpression of TROP2 predicts poor prognosis of patients with cervical cancer and promotes the proliferation and invasion of cervical cancer cells by regulating ERK signaling pathway, PLoS One 8: (9) ((2013) ), e75864. |
[7] | H. Liu, J. Shi and F. Lin, The potential diagnostic utility of TROP-2 in thyroid neoplasms, Appl Immunohistochem Mol Morphol 25: (8) ((2017) ), 525–533. |
[8] | A. Bychkov, P. Sampatanukul, S. Shuangshoti and S. Keelawat, TROP-2 immunohistochemistry: A highly accurate method in the differential diagnosis of papillary thyroid carcinoma, Pathology 48: (5) ((2016) ), 425–433. |
[9] | X.D. Wang, Q. Wang, X.L. Chen, J.F. Huang, Y. Yin, P. Da and H. Wu, Trop2 inhibition suppresses the proliferation and invasion of laryngeal carcinoma cells via the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway, Mol Med Rep 12: (1) ((2015) ), 865–870. |
[10] | H. Lin, H. Zhang, J. Wang, M. Lu, F. Zheng, C. Wang, X. Tang, N. Xu, R. Chen, D. Zhang et al., A novel human Fab antibody for Trop2 inhibits breast cancer growth in vitro and in vivo, Int J Cancer 134: (5) ((2014) ), 1239–1249. |
[11] | W.X. Wang, Q.F. Lin, D. Shen, S.P. Liu, W.D. Mao, G. Ma and W.D. Qi, Clinicopathological significance of SLP-2 overexpression in human gallbladder cancer, Tumor Biol 35: (1) ((2014) ), 419–423. |
[12] | B. Xiao, Z. Xie, L. Guo, J. Wu and H. Zhang, Stomatin-like protein 2 expression is associated with clinical survival in patients with cervical cancer, Int J Clin Exp Pathol 8: (2) ((2015) Feb 1), 1804–1809. PMID: 25973071; PMCID: PMC4396211. |
[13] | D.A. Christie, P. Mitsopoulos, J. Blagih et al., Stomatin-like protein 2 deficiency in T cells is associated with altered mitochondrial respiration and defective CD4+ T cell responses, J Immunol 189: ((2012) ), 4349–4360. |
[14] | D.Y. Lu, C.H. Tang, W.L. Yeh, K.L. Wong, C.P. Lin, Y.H. Chen, C.H. Lai, Y.F. Chen, Y.M. Leung and W.M. Fu, SDF-1alpha up-regulates interleukin-6 through CXCR4, PI3K/Akt, ERK, and NF-kappaB-dependent pathway in microglia, Eur J Pharmacol 613: ((2009) ), 146–154. |
[15] | J. Roland, B.J. Murphy, B. Ahr, V. Robert-Hebmann, V. Delauzun, K.E. Nye, C. Devaux and M. Biard-Piechaczyk, Role of the intra-cellular domains of CXCR4 in SDF-1-mediated signaling, Blood 101: ((2003) ), 399–406. |
[16] | V. De Falco, V. Guarino, E. Avilla, M.D. Castellone, P. Salerno, G. Salvatore, P. Faviana, F. Basolo, M. Santoro and R.M. Melillo, Biological role and potential therapeutic targeting of the chemokine receptor CXCR4 in undifferentiated thyroid cancer, Cancer Res 67: ((2007) ), 11821–11829. |
[17] | S.A. Lee, J.H. Choi, S.J. Cho, J.W. Chang and Y.H. Maeng, The clinical usefulness of chemokine C-X-C Motif Ligand 12 as a diagnostic marker for Papillary Thyroid Carcinoma, Indian J Pathol Microbiol 63: ((2020) ), 544–550. |
[18] | K. Kakudo, A. Bychkov, Y. Bai, Y. Li, Z. Liu et al., The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives, Pathol Int 68: ((2018) ), 641–664. |
[19] | H. Sun, Q. Chen, W. Liu, Y. Liu, S. Ruan, C. Zhu, Y. Ruan, S. Ying and P. Lin, TROP2 modulates the progression in papillary thyroid carcinoma, J Cancer 12: (22) ((2021) Sep 27), 6883–6893. doi: 10.7150/jca.62461. PMID: 34659576; PMCID: PMC8518010. |
[20] | H.B. Al-Sabaawy, A.M. Rahawi and S.S. Al-Mahmood, Standard techniques for formalin-fixed paraffin-embedded tissue: A Pathologist’s perspective, Iraqi Journal of Veterinary Sciences 35: ((2021) ), 127–135. |
[21] | N. Zargari and M. Mokhtari, Evaluation of Diagnostic utility of immunohistochemistry markers of TROP-2 and HBME-1 in the diagnosis of thyroid carcinoma, Eur Thyroid J 8: ((2019) ), 1–6. |
[22] | T. Addati, G. Achille, M. Centrone et al., TROP-2 expression in papillary thyroid cancer: A preliminary cyto-histological study, Cytopathology 26: ((2015) ), 303–311. |
[23] | H. Saffar, B. Jahanbin, F. Ameli, F. Farhang, S.M. Tavangar and H. Saffar, Diagnostic value of TROP2 expression in papillary thyroid carcinoma, Appl Immunohistochem Mol Morphol 29: (3) ((2021) Mar 1), 218–222. |
[24] | H.H. Nesreen, S.Z. Manal and A.M. Mohamed, Diagnostic utility of trophoblastic cell surface antigen 2 immunohistochemical expression in papillary thyroid carcinoma, J Pathol Nepal 8: ((2018) ), 1235–1243. |
[25] | A. Simms, R.P. Jacob, C. Cohen and M.T. Siddiqui, TROP-2 expression in papillary thyroid carcinoma: Potential diagnostic utility. Diagn Cytopathol 44: ((2016) ), 26–31. |
[26] | A.R. Murtezaoglu and H. Gucer, Diagnostic value of TROP-2 expression in papillary thyroid carcinoma and comparison with HBME-1, galectin-3 and cytokeratin 19, Pol J Pathol 68: ((2017) ), 1-10.12. |
[27] | G. Bussolati, P. Gugliotta, M. Volante, M. Pace and M.. Papotti, Retrieved endogenous biotin: A novel marker and a potential pitfall in diagnostic immunohistochemistry, Histopathology 31: ((1997) ), 400–407. |
[28] | M.H. Chui, C.A. Cassol, S.L. Asa and O. Mete, Follicular epithelial dysplasia of the thyroid: Morphological and immunohistochemical characterization of a putative preneoplastic lesion to papillary thyroid carcinoma in chronic lymphocytic thyroiditis, Virchows Arch 462: ((2013) ), 557–563. |
[29] | S.M. Abdel Raouf, H. Atia and S.A. Soliman, Diagnostic utility of immunohistochemical markers Trop2 and CD56 in differentiating follicular derived thyroid lesions, Prensa Med Argent 106: ((2020) ), 6. |
[30] | J.S. Kong, H.J. Kim, M.J. Kim, A. Kim, D. Lee, K. Han, S. Park, J.S. Koh and J.K. Myung, The significance of TROP2 expression in predicting BRAF mutations in papillary thyroid carcinoma, J Pathol Transl Med 52: (1) ((2018) ), 14–20. |
[31] | H. Guan, Z. Guo, W. Liang, H. Li, G. Wei, L. Xu, H. Xiao and Y. Li, Trop2 enhances invasion of thyroid cancer by inducing MMP2 through ERK and JNK pathways, BMC Cancer 17: (1) ((2017) Jul 14), 486. |
[32] | A. Bartolome, S. Boskovic, I. Paunovic, V. Bozic and D. Cvejic, Stomatin-like protein 2 overexpression in papillary thyroid carcinoma is significantly associated with high-risk clinicopathological parameters and BRAFV600E mutation, APMIS 124: (4) ((2016) ), 271–277. |
[33] | M.K. Conley-LaComb, L. Semaan, R. Singareddy, Y. Li, E.I. Heath, S. Kim et al., Pharmacological targeting of CXCL12/CXCR4 signaling in prostate cancer bone metastasis, Mol Cancer 15: ((2016) ), 68. |
[34] | Y.Y. Jung, I.A. Park, M.A. Kim, H.S. Min, J.K. Won and H.S. Ryu, Application of chemokine CXC motif ligand 12 as a novel diagnostic marker in preoperative fine-needle aspiration biopsy for papillary thyroid carcinoma, Acta Cytol 57: ((2013) ), 447–454. |
[35] | S.Y. Chung, E.S. Park, S.Y. Park, J.Y. Song and H.S. Ryu, CXC motif ligand 12 as a novel diagnostic marker for papillary thyroid carcinoma, Head Neck 36: ((2014) ), 1005–1012. |
[36] | X. Zhu, Q. Bai, Y. Lu, Y. Lu, L. Zhu, X. Zhou and L. Wu, Expression and function of CXCL12/CXCR4/CXCR7 in thyroid cancer, Int J Oncol 48: ((2016) ), 2321–2329. |
[37] | Z. Liu, Y. Yang, Y. Zhang, X. Ye, L. Wang and G. Xu, Stomatin-like protein 2 is associated with the clinicopathological features of human papillary thyroid cancer and is regulated by TGF-β in thyroid cancer cells, Oncology Reports 31: ((2014) ), 153–160. |
[38] | C. Eloy, J. Santos, J. Cameselle-Teijeiro, P. Soares and M. Sobrinho-Simões, TGF-beta/Smad pathway and BRAF mutation play different roles in circumscribed and infiltrative papillary thyroid carcinoma, Virchows Arch 460: ((2012) ), 587–600. |
[39] | C. Montero-Conde, J.M. Martin-Campos, E. Lerma et al., Molecular profiling related to poor prognosis in thyroid carcinoma. Combining gene expression data and biological information, Oncogene 27: ((2008) ), 1554–1561. |




