GPX4 inhibits apoptosis of thyroid cancer cells through regulating the FKBP8/Bcl-2 axis
Abstract
GPX4 has attracted much attention as a key molecule of cell ferroptosis, but its role in cell apoptosis is rarely reported, and its role in apoptosis of thyroid cancer (TC) cell has not been reported. The analysis of TCGA database showed that both GPX4 and FKBP8 were highly expressed in TC tumor tissues; The expression of GPX4 and FKBP8 were positively correlated. The immunohistochemical analysis further confirmed that GPX4 and FKBP8 were highly expressed in TC tumor tissues. In addition, the high expression of GPX4 and FKBP8 were both significantly correlated with the poor prognosis of TC. Silencing GPX4 significantly inhibited the proliferation, induced apoptosis of TC cells, and reduced tumor growth in mice. The co-immunoprecipitation assay revealed a physical interaction between GPX4 and FKBP8 observed in the TC cells. Knockdown of FKBP8 significantly inhibited the proliferation and induced apoptosis of TC cells. Rescue experiments suggested that knockdown of FKBP8 could reverse the strengthens of cell proliferation and apoptosis and the higher expression of FKBP8 and Bcl-2 caused by overexpression of GPX4. Our results suggest that the GPX4/FKBP8/Bcl-2 axis promotes TC development by inhibiting TC cell apoptosis, which provides potential molecular targets for TC therapeutic strategies.
1.Introduction
Thyroid cancer (TC) is the most common malignant tumor of the endocrine system. The incidence rate for thyroid cancer from 2008 to 2012 was 7.56/100000, and the mortality rate was 0.52/100000 in China [1]. The incidence and death of thyroid cancer ranked 7th and 22nd in cancer incidence and death, respectively. There are 2 types of differentiated thyroid cancer (DTC) such as Papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), caused by follicular cells while medullary thyroid cancer (MTC) originates from parafollicular C cells. [2]. The overall prognosis of MTC is worse than DTC [3]. Anaplastic thyroid cancer (ATC), produced in thyroid follicular cells, is one of the most aggressive solid tumors in human beings [4]. The median overall survival of patients with ATC is about 6 months. The diagnostic rate of TC has increased significantly due to the utilization of serum thyroid stimulating hormone measurement, ultrasound and fine needle aspiration in recent years [5]. At present, the treatment for TC includes surgery, 131I treatment, and targeted therapy, which results in a 5-year overall survival rate of 96.1% for TC patients surviving one year after initial treatment [5]. However, approximately 30% of DTC patients have disease recurrences during several decades [6]. Research on factors affecting TC development may help to provide a better strategy for TC treatment.
Glutathione peroxidase 4 (GPX4), as a selenoprotein, contains a rare selenocysteine at its active site [7]. It catalyzes the reduction of lipid peroxides by consuming reduced glutathione, and functions as an essential molecule of the anti-ferroptosis defense system [8]. GPX4 was shown to suppress apoptosis in some malignancies, such as glioma and breast cancer [9, 10]. The earlier reports of Zhao et al. mentioned that the down-regulation of GPX4 could induce apoptosis of glioma cells [9]. Ding et al. reported that DMOCPTL, a derivative of natural product parthenolide, could induce ferroptosis in triple-negative breast cancer (TNBC) cells through ubiquitination of GPX4; GPX4 could also inhibit apoptosis of breast cancer cells by inhibiting EGR1 expression [10]. However, the role of GPX4 in TC development remains unclear.
FKBP8, also known as FKBP38, belongs to the family of the FK506-binding proteins (FKBPs) [11, 12]. Studies have shown that FKBP8 localized not only in the inner membrane of endoplasmic reticulum and mitochondria, but also in the Golgi complex and plasma membrane [13]. FKBP8, which consists of a N-terminal Glu-rich region, a calmodulin (CaM) binding site, three TPR motifs, a PPIase domain, and a membrane anchor site, which can be activated by CaM [14]. FKBP8 contains a transmembrane domain near the carboxyl terminal, which is mainly located in the mitochondria. Immunoprecipitation analysis showed that FKBP8 interacted with antiapoptotic factors Bcl-2 and Bcl-XL, and colocalized with these proteins in the mitochondria [15]. Overexpression of FKBP8 inhibited apoptosis of HeLa cells [15]. This result shows that FKBP8 has an anti-apoptotic activity and can be used as a target for cancer treatment. Shimamoto et al. reported that knockdown of FKBP8 expression could significantly reduce the Bcl-2 protein expression [16]. However, molecular mechanisms of FKBP8 in TC development remains elusive.
In this study, we examined the expression of GPX4 and FKBP8 in patients with TC and their correlation with clinicopathological features by immunohistochemistry (IHC). We demonstrated that inhibition of GPX4 expression suppressed growth of TC cells in vitro and in vivo. The GPX4/FKBP8/Bcl-2 axis played an important role in the protection of TC cells against apoptosis. Our data indicates that GPX4 and FKBP8 may serve as potential biomarkers for TC patients, and putative targets for therapeutic interventions in TC.
2.Materials and methods
2.1Clinical samples
The formalin-fixed paraffin-embedded (FFPE) tissue samples and the corresponding clinicopathological data, including gender, age, histological subtypes, TNM stage, were obtained from 20 patients with benign thyroid nodules (BTN) and 159 TC patients who underwent thyroidectomy between February 2008 and February 2021 at the First Affiliated Hospital of Nanchang University. Archival slides of TC patients were evaluated by at least two pathologists. TNM staging was defined according to the 8th edition of the American Joint Committee on Cancer staging system. This study was approved by the Medical Research Ethics Committee of the First Affiliated Hospital of Nanchang University ((2022)CDYFYYLK(06-016)) as per the Declaration of Helsinki.
2.2Cell culture
The 8305C cell, an ATC cell, was purchased from Procell Life Science & Technology Co., Ltd. in September 2021, and was authenticated using STR method. The BCPAP cell, a PTC cell, was obtained from Fuheng Biology in January 2022, and was authenticated using STR method. The 8305C cell and the BCPAP cell were authenticated again using STR method in April and May 2023, respectively. The 8305C cell was cultured in a MEM medium (Procell, China) containing 10% fetal bovine serum (FBS, Gibco, USA) whereas the BCPAP cell was cultured in a DMEM medium (Solarbio, China) containing 10% FBS (Gibco, USA). TC cells were stained using the trypan blue, and the number of viable cells was counted with a hemocytometer.
2.3Western blot analysis
Cells were lysed using Cell lysis buffer for Western and IP (Beyotime, China) supplemented with 1 mM PMSF. Proteins in cell lysates were first separated by SDS-polyacrylamide gel electrophoresis (PAGE); then the gel was transferred to the polyvinylidene fluoride (PVDF) membrane; subsequently, the membrane was incubated with the primary antibody. After the membrane was incubated with the HRP-conjugated secondary antibody, the signals were visualized using the BeyoECL Star kit (Beyotime, China). Antibodies used in the western blot analysis were as follows: anti-GPX4 (#A11243, 1:1000), anti-FKBP8 (#A8701, 1:1000), and anti-Bcl-2 (#19693, 1:1000) were purchased from Abclonal (China); anti-GAPDH (#60004-1-lg, 1:2000), HRP-conjugated Goat Anti-Mouse IgG(H+L) (#SA00001-1, 1:2000), and HRP-conjugated Goat Anti-Rabbit IgG(H+L) (#SA00001-2, 1:2000) were obtained from Proteintech (China).
2.4Quantitave real-time PCR
Total RNA was extracted using TRizol Reagent (Beyotime, China) according to the manufacturer’s instructions. cDNA was synthesized using Evo M-MLV RT kit with gDNA clean for qPCR (Accurate Biology, China). Quantitative real-time PCR was performed using SYBR Green Premix Pro Taq HS qPCR kit (Accurate Biology, China). The sequences of primers for real-time PCR were supplied in Table S1.
2.5Cell viability assay
Cell viability was measured using Cell Counting Kit-8 (CCK-8, Abmole, USA). Briefly, 6
2.6EdU assay
TC cells were plated onto 96-well plates at a density of 6
2.7Apoptosis assay
TC cells were seeded onto a 6-well plate. The next day, approximately 50% confluent cells were transfected with siRNAs or plasmids for 48 hours. Then, flow-cytometry-based apoptosis assay was perform using TransDetect® Annexin V-FITC/PI Cell Apoptosis Detection Kit (Transgen, China). Cells were collected and incubated with 5
2.8RNA interference and gene transfection
The siRNAs targeted for GPX4 or FKBP8 were obtained from Ribobio and transfected using riboFECT CP Transfection Kit (Ribobio, China) according to the manufacturer’s instructions. pCMV6-GPX4 (#RC208065) was obtained from OriGene Technologies (USA) and transfected with Lipofectamine™3000 (Invitrogen, USA) following the manufacturer’s instructions. The Sequence of the human GPX4 shRNA (CCGGGTGGATGAAGATCCAACCCAACTCGAGT TGGGTTGGATCTTCATCCAC) was obtained from Sigma (USA).
2.9Immunohistochemistry (IHC)
FFPE specimens were used for IHC analysis following the criteria: the staining index equals the staining intensity (scores: negative
Figure 1.
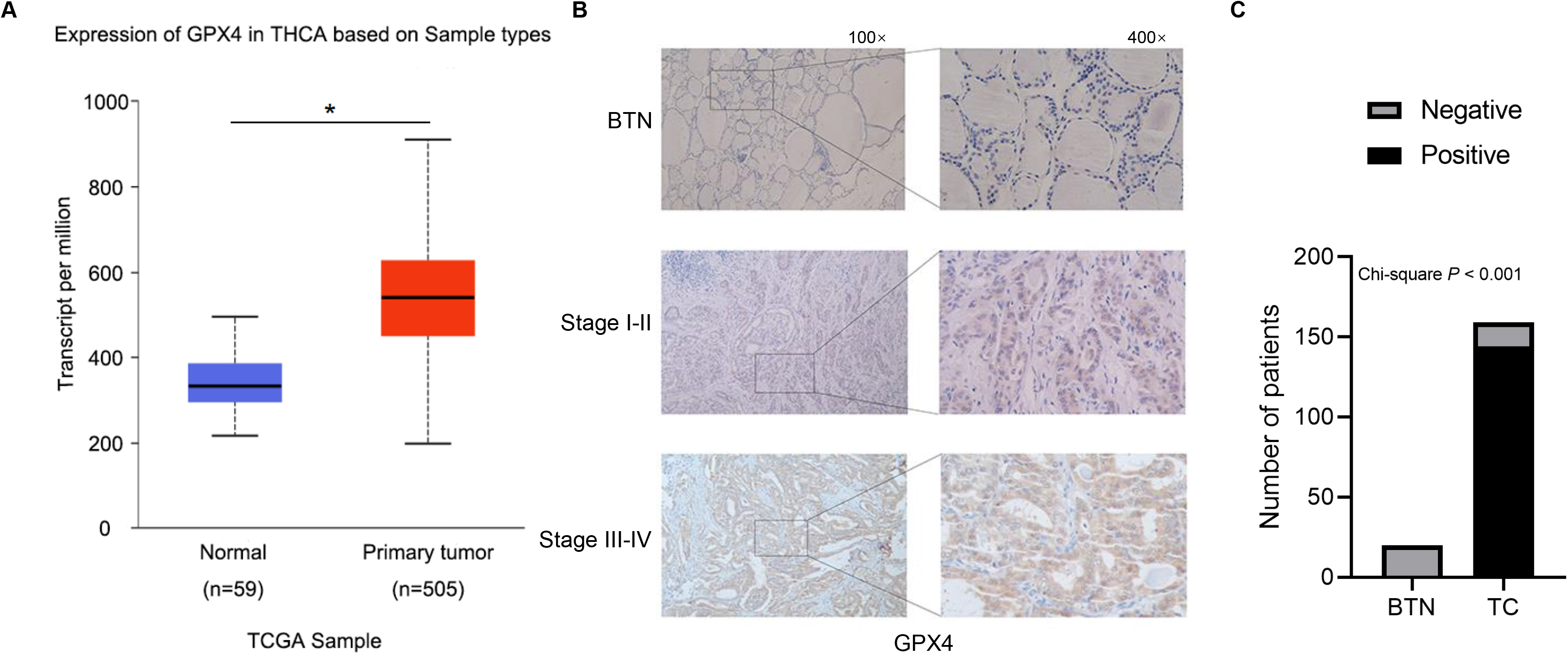
GPX4 expression was upregulated in patients with TC. (A) The expression of GPX4 was compared between TC tumor tissues and normal tissues. Data are from TCGA database. (B) The IHC staining of GPX4 in FFPE slides of BTN patients and TC patients at different clinical stage. The representative images of IHC were shown. (C) The number of GPX4-positive slides in IHC staining was evaluated in patients with benign thyroid nodules (BTN) and thyroid cancer (TC) using the Chi-square analysis. TCGA, The Cancer Genome Atlas; THCA, thyroid cancer; IHC, immunohistochemistry.
2.10Co-immunoprecipitation
Protein extracts were prepared using Cell lysis Buffer for Western and IP (Beyotime, China). Normal IgG (#B900620) from Proteintech, or primary antibodies, including anti-GPX4 (#ab16739) purchased from Abcam (USA), anti-FKBP8 (#sc-166607) obtained from Santa Cruz Biotechnology (USA), which were derived from the mouse, were added to the protein extracts and incubated at 4∘C overnight. Then, 10
2.11Mouse xenograft
All animal experiments were approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Nanchang University (CDYFY-IACUC-202209QR020). Male BALB/c nude mice (4–6 weeks old) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China) and fed a standard animal diet. The 8305C cells with control shRNA or GPX4 shRNA were suspended in PBS and mixed with Matrigel at a 1:1 ratio. The mice were inoculated subcutaneously with cells in the region near the left axilla. Tumors were measured every 3 days, and the tumor volume was calculated using the formula (1/2
Figure 2.
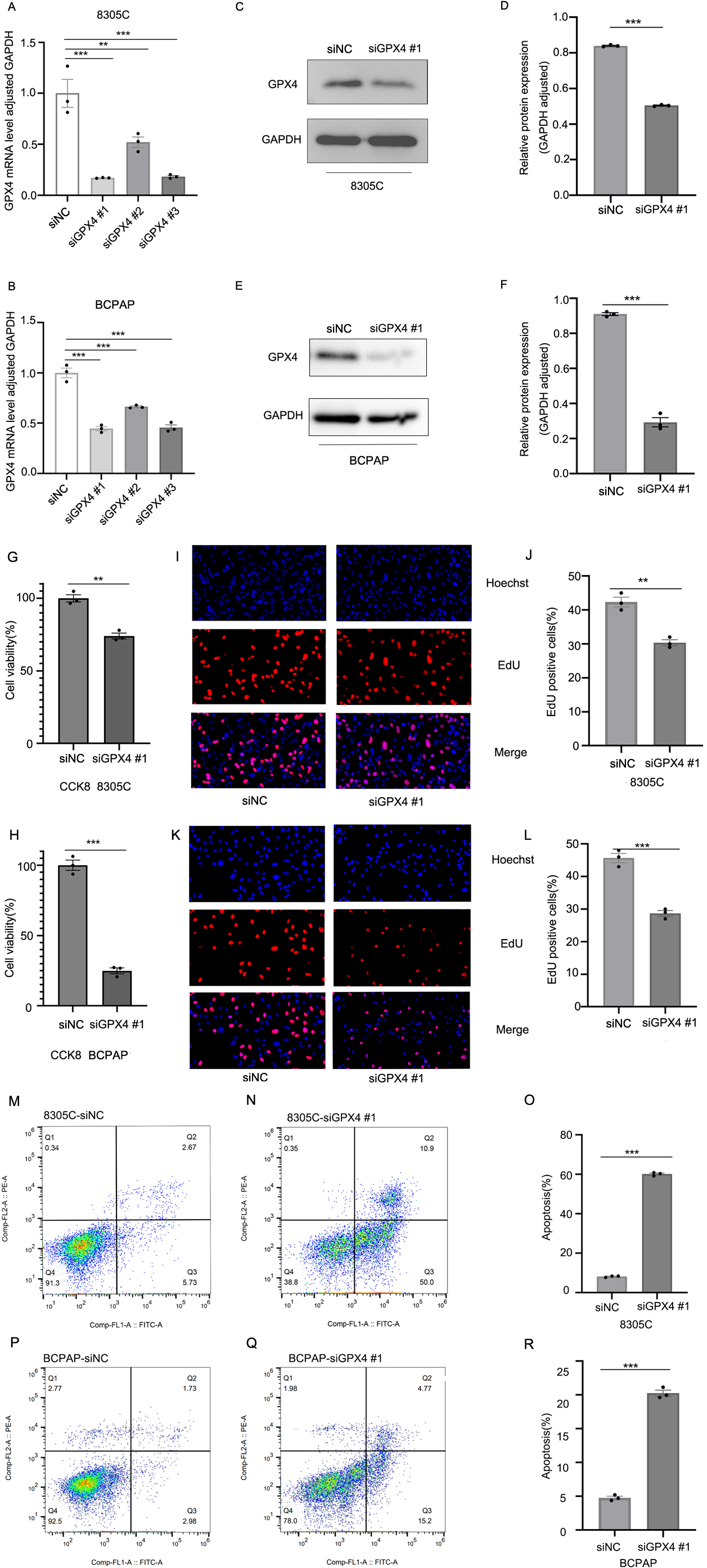
Knockdown of GPX4 expression suppresses the cell proliferation and induces apoptosis in TC cells. The expression of GPX4 mRNA and protein was detected in both 8305C and BCPAP cells transfected with GPX4 siRNAs (siGPX4) or negative control siRNA (siNC) using RT-qPCR (A, B) and western blot analysis (C-F), respectively. Data are represented as mean
2.12Statistical analysis
The statistical analysis was conducted using GraphPad Prism (Version 8.0, GraphPad Software). Statistical significance was defined as a two-sided
Figure 3.
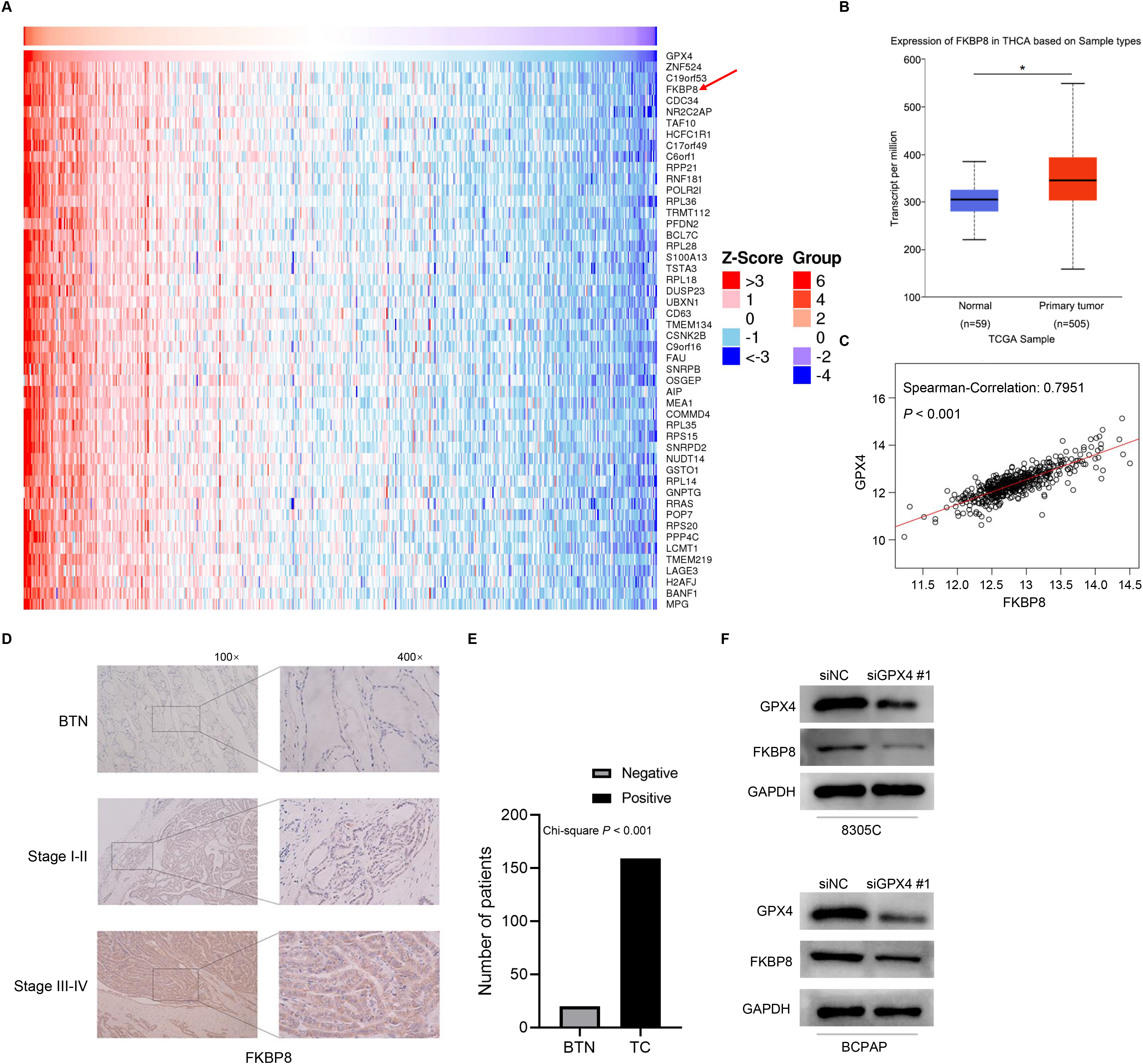
Knockdown of GPX4 expression downregulates the expression of FKBP8. (A) The top 50 genes whose mRNA expression were positively related to the expression of GPX4 mRNA in the TCGA-THCA cohort. The Spearman’ correlation analysis was used to evaluate the association between the expression of GPX4 and that of other genes. (B) The expression of FKBP8 mRNA was compared between TC tumor tissues and normal tissues. Data are from TCGA database. (C) The association between the expression of GPX4 mRNA and the expression of FKBP8 mRNA in the TCGA-THCA cohort was assessed using the Spearman’ correlation analysis. (D) The IHC staining of FKBP8 in FFPE slides of BTN patients and TC patients at different clinical stage. The representative images of IHC were shown. (E) The number of FKBP8-positive slides in IHC staining was evaluated using the Chi-square analysis. (F) Western blot analysis of GPX4 and FKBP8 expression in 8305C and BCPAP cells transfected with siGPX4 or siNC. The representative images of the western blot analysis were shown. TCGA-THCA, The Cancer Genome Atlas-Thyroid Cancer.
3.Results
3.1The high expression of GPX4 in TC patients
The Cancer Genome Atlas (TCGA, https://cancergen ome.nih.gov/) database was used to analyze GPX4 expression in patients with TC. We found that GPX4 mRNA was significantly upregulated in TC tumor tissues compared with normal tissues (Fig. 1A). Next, GPX4 expression was further verified by collecting FFPE slides of 159 TC patients and 20 BTN patients for IHC analysis. GPX4 expression was significantly increased in TC patients compared with BTN patients (Fig. 1B, C). A stepwise increase in GPX4 expression was observed with clinical stage (Fig. 1B, Table S2). The higher expression level of GPX4 was also significantly associated with older age, lymph node metastasis (Table S2). In addition, GPX4 expression was prone to be elevated in ATC and PTC compared with MTC and FTC.
3.2Suppression of GPX4 inhibited the cell proliferation in TC cells
To further investigate the functional role of GPX4 in TC, we tried to knock down the expression of GPX4 in 8305C and BCPAP cells using small interfering RNAs (siRNAs). The quantitative real-time PCR analysis showed that siGPX4 #1 was the most effective siRNA that could knock down GPX4 mRNA expression (Fig. 2A, B). The western blot analysis showed that siGPX4 #1 also effectively suppressed GPX4 protein expression (Fig. 2C–F). CCK-8 and EdU assay were used to determine the effect of suppressing GPX4 on the cell proliferation. Suppression of GPX4 in 8305C and BCPAP cells significantly inhibited the cell proliferation compared with cells with negative control siRNA (siNC) (Fig. 2G–L). To explore the molecular mechanism by which suppression of GPX4 inhibited the cell proliferation, we tested whether apoptosis was induced. Flow cytometry analysis was used to examine the effect of suppressing GPX4 expression on apoptosis of TC cells. The apoptotic cell populations were significantly increased in 8305C or BCPAP cells with siGPX4 compared with those with siNC (Fig. 2M–R).
3.3The high expression of FKBP8 in TC patients
To screen the downstream gene regulated by GPX4 in TC cells, we performed an analysis of correlation between the expression of GPX4 and that of other genes using the Linked Omics database and the RNA-Seq data of TC patients in TCGA database. The expression of FKBP8, a gene located mainly in mitochondria and possessing the anti-apoptotic property, was found to be significantly associated with that of GPX4 (Fig. 3A, C, R
3.4Depletion of FKBP8 expression induces apoptosis of TC cells
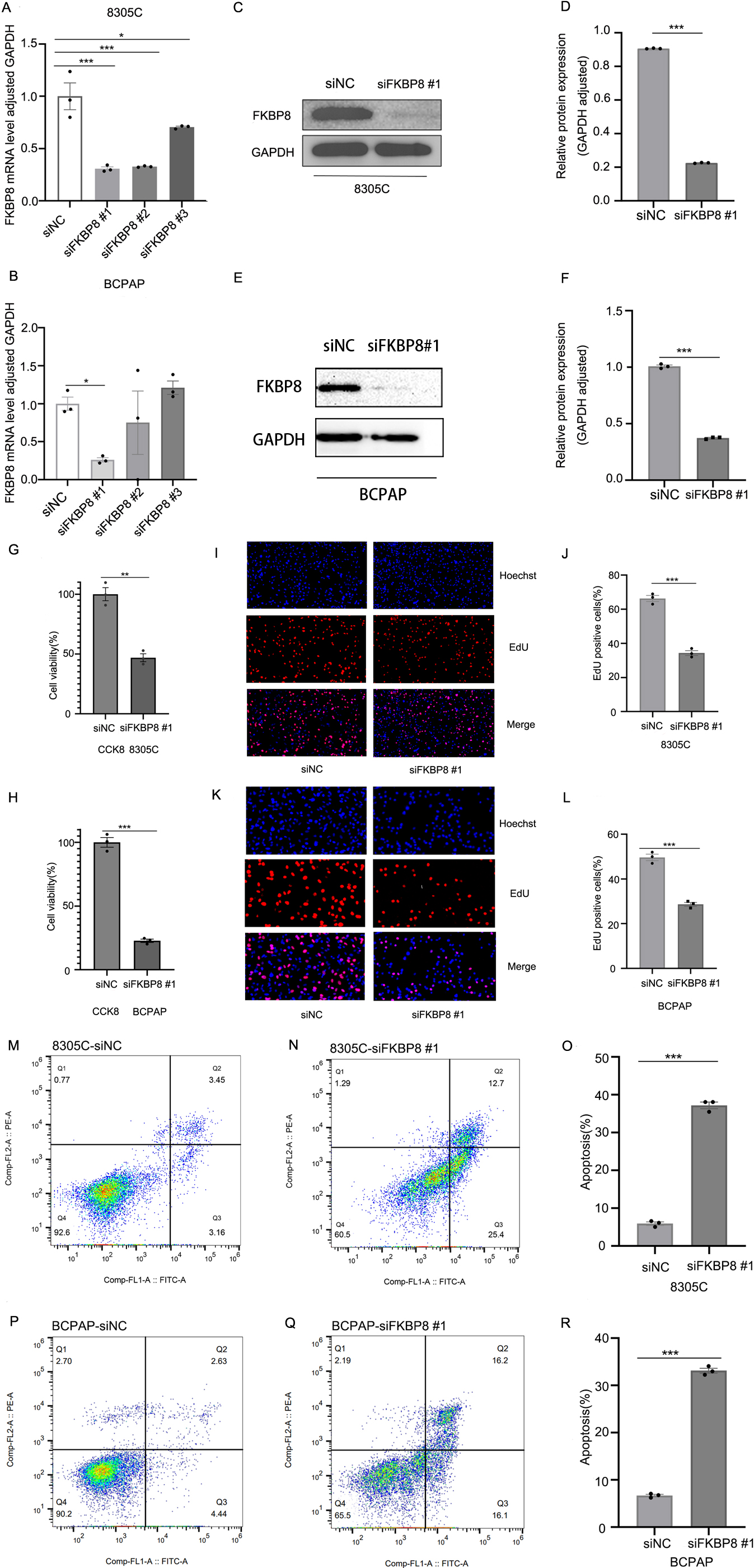
To examine the function of FKBP8 in TC, we tried to knock down the expression of FKBP8 in 8305C and BCPAP cells using siRNAs. The quantitative real-time PCR analysis and the western blot analysis both showed that siFKBP8 #1 was the most effective siRNA that could knock down FKBP8 expression (Fig. 4A–F). CCK-8 and EdU assays suggested that suppression of FKBP8 in TC cells significantly inhibited the cell proliferation compared with those with siNC (Fig. 4G–L). Flow cytometry analysis showed that apoptotic cell populations were significantly higher in TC cells with siFKBP8 than those with siNC (Fig. 4M–R). These results demonstrate that knockdown of FKBP8 expression induces apoptosis of TC cells.
Figure 4.
Knockdown of FKBP8 expression suppresses the cell proliferation and induces apoptosis in TC cells. The expression of FKBP8 mRNA and protein was detected in both 8305C and BCPAP cells transfected with FKBP8 siRNAs (siFKBP8) or negative control siRNA (siNC) using RT-qPCR (A, B) and western blot analysis (C-F), respectively. Data are represented as mean
3.5GPX4 inhibits apoptosis of TC cells through activating the FKBP8/Bcl-2 axis
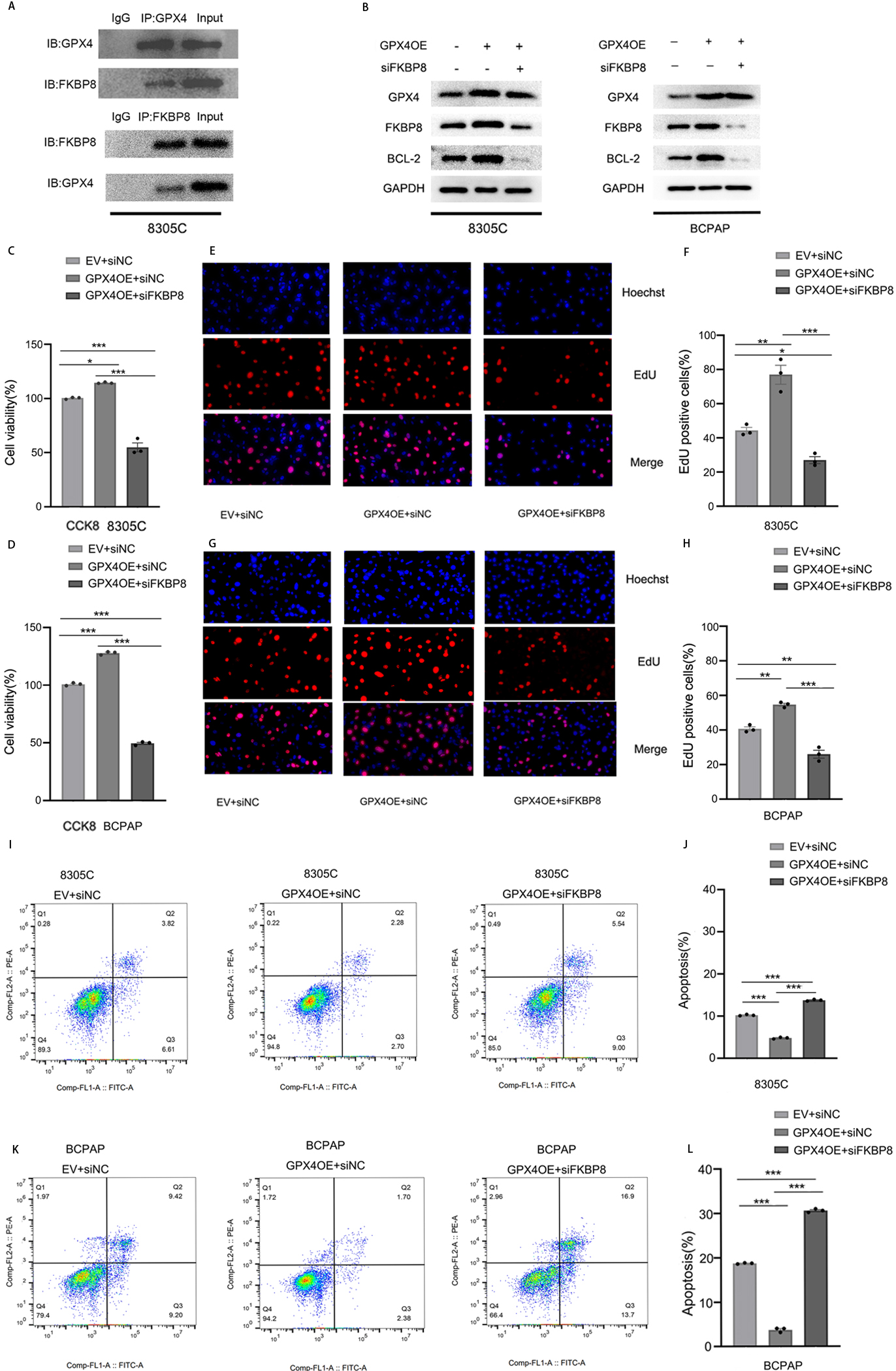
To investigate whether GPX4 directly binds to FKBP8 to regulate FKBP8 gene expression, we performed co-immunoprecipitation assay in 8305C cells. As shown in Fig. 5A, a physical interaction between GPX4 and FKBP8 was observed in the cells. It is reported that knocking down FKBP8 expression decreases the expression of Bcl-2 [16]. We hypothesized that GPX4 might inhibit the apoptosis of TC cells through regulating the FKBP8/Bcl-2 axis. Indeed, overexpression of GPX4 elevated the expression of FKBP8 and Bcl-2 while knockdown of FKBP8 abolished the upregulation of Bcl-2 mediated by overexpressing GPX4 in 8305C and BCPAP cells (Fig. 5B). CCK-8 and EdU assays showed that knockdown of FKBP8 abolished the cell proliferation promoted by overexpression of GPX4 (Fig. 5C–H). Flow cytometry results revealed that knocking down FKBP8 expression augmented cell apoptosis suppressed by increase of GPX4 expression (Fig. 5I–L). These results clearly demonstrate that overexpression of GPX4 inhibits apoptosis of TC cells through activating the FKBP8/Bcl-2 axis.
Figure 5.
GPX4 inhibits apoptosis of TC cells through activating the FKBP8/Bcl-2 axis. (A) Cell lysates of 8305C cells were subjected to immunoprecipitation and immunoblotting with respective antibodies. The representative images of the immunoblotting results were shown. (B) Western blot analysis of GPX4, FKBP8, and Bcl-2 expression in GPX4-overexpressing 8305C and BCPAP cells transfected with siFKBP8 or siNC. The representative images of the western blot analysis were shown. The cell proliferation was determined in GPX4-overexpressing 8305C and BCPAP cells transfected with siFKBP8 or siNC using CCK-8 (C, D) or EdU (E-H) assays. (I-L) The flow-cytometric analysis of apoptosis was performed in GPX4-overexpressing 8305C and BCPAP cells transfected with siFKBP8 or siNC. Data are represented as mean
3.6GPX4 inhibits tumor growth in vivo
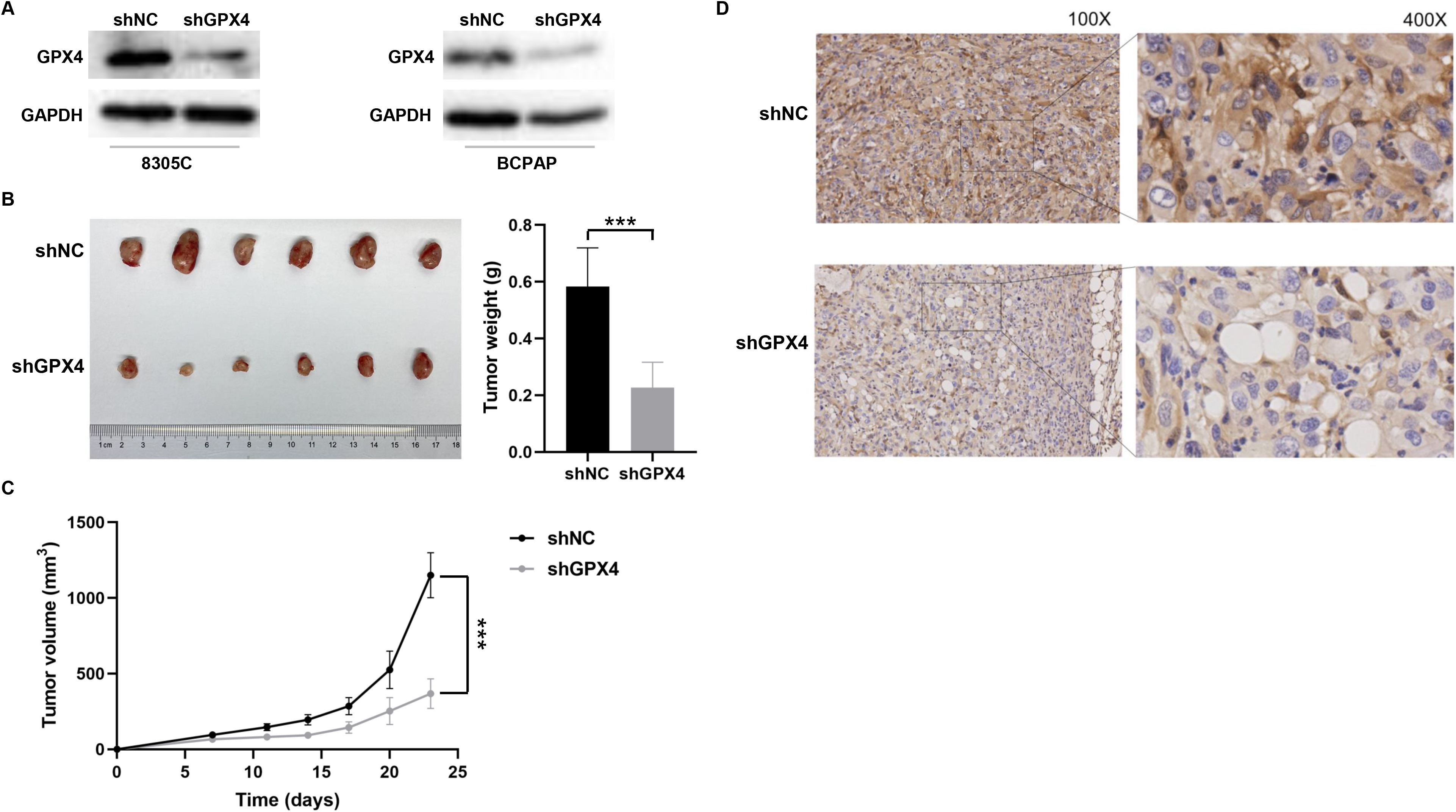
The 8305C cells were transfected with GPX4 shRNA lentivirus (shGPX4) to stably knock down GPX4 expression (Fig. 6A). Approximately 5
Figure 6.
GPX4 promotes tumor growth in vivo. (A) The expression of GPX4 protein was detected in both 8305C and BCPAP cells transfected with GPX4 shRNA lentivirus (shGPX4) or negative control shRNA (shNC) using the western blot analysis. The representative images of the western blot analysis were shown. The 8305C cells transfected with shGPX4 or shNC were subcutaneously injected into BALB/c nude mice (six mice per group).The weight (B) and volume (C) of xenograft tumors were compared between the two groups. (D) The IHC staining of GPX4 in xenograft tumors from the BALB/c nude mice. The representative images of IHC were shown. IHC, immunohistochemistry.
4.Discussion
GPX4 expression is up-regulated in several kinds of tumors. Wei et al. reported that the expression of GPX4 was significantly higher in tumor tissues of endometrial cancer than that in normal endometrial tissues [17]. GPX4 expression was up-regulated in tumor tissues of glioma compared with normal brain tissues; higher GPX4 expression was associated with worser overall survival [9]. Our results showed that higher GPX4 expression was related with several poorer prognostic factors of TC, including older age, lymph node metastasis, and advanced clinical stage. Ding et al. reported that GPX4 induced apoptosis of TNBC cells through up-regulation of EGR1 [10]. Knockdown of GPX4 expression inhibited the proliferation and induced apoptosis in endometrial cancer cells [17]. We demonstrate that GPX4 promotes TC development through inhibition of apoptosis.
FKBP8 plays an important role in mitophagy [18] and apoptosis. An in vivo study with a FKBP8 mouse mutant found that the loss of FKBP8 resulted in increased apoptosis, demonstrating that FKBP8 blocks apoptosis [19]. FKBP8 suppresses apoptosis by regulating localization and stability of Bcl-2 [15]. Cytoplasmic KDM1A could demethylate FKBP8 to increase the stability of Bcl-2 in cells of hepatocellular carcinoma [20]. In this study, FKBP8 expression was higher in TC tumor tissues than that in BTN tissues, and higher expression of FKBP8 was significantly associated with older age, lymph node metastasis, and advanced clinical stage. Depletion of FKBP8 induced TC cell apoptosis and suppressed TC cell proliferation. These results suggest that FKBP8 may promote TC cell growth through suppression of apoptosis.
Bcl-2, an anti-apoptotic protein, participates in the intrinsic pathway of apoptosis [21]. Extracellular vesicles derived from Oesophageal squamous cell carcinoma contained high levels of P4HB, which induced apoptosis via activating the PHGDH/Bcl-2/caspase-3 pathway [22]. Zhou et al. reported that knockdown of SPTBN2 downregulated expression of Bcl-2 to induce apoptosis of TC cells [23]. In this study, overexpression of GPX4 increased the levels of FKBP8 and Bcl-2. However, depletion of FKBP8 abolished the increase of Bcl-2 expression mediated by overexpression of GPX4. Depletion of FKBP8 expression augmented cell apoptosis suppressed by overexpression of GPX4. These results suggest that FKBP8-mediated elevation of Bcl-2 expression is essential for inhibition of apoptosis in TC cells, and GPX4 regulates the cell proliferation and inhibits apoptosis through regulating the FKBP8/Bcl-2 axis in TC cells. Venetoclax (ABT-199), the Bcl-2 selective inhibitor, has been approved for the treatment of acute myeloid leukemia and chronic lymphocytic leukemia [24, 25]. Thus, it is promising to develop drugs targeting GPX4, FKBP8, or Bcl-2, which participates in the suppression of apoptosis, for TC treatments.
Chen et al. reported that knockdown of GPX4 suppressed the cell proliferation and induced ferroptosis in TC cells [26]. To our knowledge, our study is the first to uncover the essential roles of GPX4 and FKBP8 in TC cell apoptosis. We demonstrated that the expression of GPX4 and FKBP8 were both significantly higher in TC tumor tissues than in BTN tissues, and higher levels of GPX4 and FKBP8 were both significantly associated with older age, advanced clinical stage and lymph node metastasis. Inhibition of GPX4 expression suppressed the cell proliferation and induced apoptosis in TC cells. FKBP8 was the target of GPX4. Depletion of FKBP8 abolished upregulation of Bcl-2 mediated by overexpression of GPX4 and augmented the apoptosis suppressed by elevation of GPX4 expression. Thus, we demonstrated the essential role of GPX4 in promoting the cell proliferation and inhibiting apoptosis in TC cells through activating the FKBP8/Bcl-2 axis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was approved by the Medical Research Ethics Committee of the First Affiliated Hospital of Nanchang University ((2022)CDYFYYLK(06-016)) as per the Declaration of Helsinki.
Author contributions
Conception: TD, JY, JJ, YS, SY, CP, XM, and YX.
Interpretation or analysis of data: TD, JY, YY, JJ, YS, SY, CP, and YX.
Preparation of the manuscript: DD, WZ, and PL.
Revision for important intellectual content: DD.
Supervision: DD.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82160527), Science and Technology Department of Jiangxi Province (No. 20203BBGL73201), Jiangxi Provincial Natural Science Foundation (No. 20224ACB216014), Central Funds Guiding the Local Science and Technology Development (20221ZDG020066), Health Commission of Jiangxi Province (No. 20203229, 202130202), The First Affiliated Hospital of Nanchang University (No. YFYPY202002), and the 448 Double Thousand Talents Plan of Jiangxi (No. 449 jxsq2023101088).
Supplementary data
The supplementary files are available to download from http://dx.doi.org/10.3233/CBM-230220.
Acknowledgments
The authors thank the clinical staff who supported this study.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
[1] | L. Du, R. Li, M. Ge, Y. Wang, H. Li, W. Chen and J. He, Incidence and mortality of thyroid cancer in China, 2008–2012, Chin J Cancer Res 31: ((2019) ), 144–151. |
[2] | S. Agarwal, A. Bychkov and C.K. Jung, Emerging Biomarkers in Thyroid Practice and Research, Cancers 14: ((2021) ). |
[3] | S. Gogna, M. Goldberg, D. Samson, M. Gachabayov, D.M. Felsenreich, A. Azim and X.D.E. Dong, Medullary Thyroid Cancer in Patients Older than 45-Epidemiologic Trends and Predictors of Survival, Cancers 12: ((2020) ). |
[4] | E. Molinaro, C. Romei, A. Biagini, E. Sabini, L. Agate, S. Mazzeo, G. Materazzi, S. Sellari-Franceschini, A. Ribechini, L. Torregrossa, F. Basolo, P. Vitti and R. Elisei, Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies, Nat Rev Endocrinol 13: ((2017) ), 644–660. |
[5] | R. Wong, S.G. Farrell and M. Grossmann, Thyroid nodules: diagnosis and management, Med J Aust 209: ((2018) ), 92–98. |
[6] | R.M. Tuttle, D.W. Ball, D. Byrd, R.A. Dilawari, G.M. Doherty, Q.Y. Duh, H. Ehya, W.B. Farrar, R.I. Haddad, F. Kandeel, R.T. Kloos, P. Kopp, D.M. Lamonica, T.R. Loree, W.M. Lydiatt, J.C. McCaffrey, J.A. Olson, Jr., L. Parks, J.A. Ridge, J.P. Shah, S.I. Sherman, C. Sturgeon, S.G. Waguespack, T.N. Wang, L.J. Wirth and National Comprehensive Cancer Network, Thyroid carcinoma, J Natl Compr Canc Netw 8: ((2010) ), 1228–1274. |
[7] | Y. Zhang, R.V. Swanda, L. Nie, X. Liu, C. Wang, H. Lee, G. Lei, C. Mao, P. Koppula, W. Cheng, J. Zhang, Z. Xiao, L. Zhuang, B. Fang, J. Chen, S.B. Qian and B. Gan, mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation, Nat Commun 12: ((2021) ), 1589. |
[8] | H. Lee, F. Zandkarimi, Y. Zhang, J.K. Meena, J. Kim, L. Zhuang, S. Tyagi, L. Ma, T.F. Westbrook, G.R. Steinberg, D. Nakada, B.R. Stockwell and B. Gan, Energy-stress-mediated AMPK activation inhibits ferroptosis, Nat Cell Biol 22: ((2020) ), 225–234. |
[9] | H. Zhao, B. Ji, J. Chen, Q. Huang and X. Lu, Gpx 4 is involved in the proliferation, migration and apoptosis of glioma cells, Pathol Res Pract 213: ((2017) ), 626–633. |
[10] | Y. Ding, X. Chen, C. Liu, W. Ge, Q. Wang, X. Hao, M. Wang, Y. Chen and Q. Zhang, Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells, J Hematol Oncol 14: ((2021) ), 19. |
[11] | Y.K. Banasavadi-Siddegowda, J. Mai, Y. Fan, S. Bhattacharya, D.R. Giovannucci, E.R. Sanchez, G. Fischer and X. Wang, FKBP38 peptidylprolyl isomerase promotes the folding of cystic fibrosis transmembrane conductance regulator in the endoplasmic reticulum, J Biol Chem 286: ((2011) ), 43071–43080. |
[12] | D.M. Hutt, D.M. Roth, M.A. Chalfant, R.T. Youker, J. Matteson, J.L. Brodsky and W.E. Balch, FK506 binding protein 8 peptidylprolyl isomerase activity manages a late stage of cystic fibrosis transmembrane conductance regulator (CFTR) folding and stability, J Biol Chem 287: ((2012) ), 21914–21925. |
[13] | Y.J. Peng, Y.C. Lee, S.J. Fu, Y.C. Chien, Y.F. Liao, T.Y. Chen, C.J. Jeng and C.Y. Tang, FKBP8 Enhances Protein Stability of the CLC-1 Chloride Channel at the Plasma Membrane, Int J Mol Sci 19: ((2018) ). |
[14] | F. Edlich and C. Lücke, From cell death to viral replication: the diverse functions of the membrane-associated FKBP38, Curr Opin Pharmacol 11: ((2011) ), 348–353. |
[15] | M. Shirane and K.I. Nakayama, Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis, Nat Cell Biol 5: ((2003) ), 28–37. |
[16] | S. Shimamoto, M. Tsuchiya, F. Yamaguchi, Y. Kubota, H. Tokumitsu and R. Kobayashi, Ca2+/S100 proteins inhibit the interaction of FKBP38 with Bcl-2 and Hsp90, Biochem J 458: ((2014) ), 141–152. |
[17] | S. Wei, Z. Yu, R. Shi, L. An, Q. Zhang, Q. Zhang, T. Zhang, J. Zhang and H. Wang, GPX4 suppresses ferroptosis to promote malignant progression of endometrial carcinoma via transcriptional activation by ELK1, BMC Cancer 22: ((2022) ), 881. |
[18] | S.M. Yoo, S.I. Yamashita, H. Kim, D. Na, H. Lee, S.J. Kim, D.H. Cho, T. Kanki and Y.K. Jung, FKBP8 LIRL-dependent mitochondrial fragmentation facilitates mitophagy under stress conditions, FASEB J 34: ((2020) ), 2944–2957. |
[19] | R.L. Wong, B.J. Wlodarczyk, K.S. Min, M.L. Scott, S. Kartiko, W. Yu, M.Y. Merriweather, P. Vogel, B.P. Zambrowicz and R.H. Finnell, Mouse Fkbp8 activity is required to inhibit cell death and establish dorso-ventral patterning in the posterior neural tube, Hum Mol Genet 17: ((2008) ), 587–601. |
[20] | S. Lv, X. Zhao, E. Zhang, Y. Yan, X. Ma, N. Li, Q. Zou, L. Sun and T. Song, Lysine demethylase KDM1A promotes cell growth via FKBP8-BCL2 axis in hepatocellular carcinoma, J Biol Chem 298: (2022) 102374. |
[21] | B. Zhang, Y. Fan, P. Cao and K. Tan, Multifaceted roles of HSF1 in cell death: A state-of-the-art review, Biochim Biophys Acta Rev Cancer 1876: ((2021) ), 188591. |
[22] | X. Gao, Y. Wang, F. Lu, X. Chen, D. Yang, Y. Cao, W. Zhang, J. Chen, L. Zheng, G. Wang, M. Fu, L. Ma, Y. Song and Q. Zhan, Extracellular vesicles derived from oesophageal cancer containing P4HB promote muscle wasting via regulating PHGDH/Bcl-2/caspase-3 pathway, J Extracell Vesicles 10: ((2021) ), e12060. |
[23] | X. Zhou, L. Lin, Y. Qi, M. Xu, Q. Xu, Y. Wang and J. Qu, SPTBN2 Promotes the Progression of Thyroid Cancer by Accelerating G1/S Transition and Inhibiting Apoptosis, Dis Markers 2022: ((2022) ), 2562595. |
[24] | S.T. Diepstraten, M.A. Anderson, P.E. Czabotar, G. Lessene, A. Strasser and G.L. Kelly, The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs, Nat Rev Cancer 22: ((2022) ), 45–64. |
[25] | Y.J. Thus, E. Eldering, A.P. Kater and M. Spaargaren, Tipping the balance: toward rational combination therapies to overcome venetoclax resistance in mantle cell lymphoma Leukemia 36: ((2022) ), 2165–2176. |
[26] | H. Chen, F. Peng, J. Xu, G. Wang, Y. Zhao, Increased expression of GPX4 promotes the tumorigenesis of thyroid cancer by inhibiting ferroptosis and predicts poor clinical outcomes Aging (Albany NY) 15: ((2023) ), 230–245. |




