Circ_0049271 targets the miR-1197/PTRF axis to attenuate the malignancy of osteosarcoma
Abstract
BACKGROUND:
Circular RNAs (circRNAs) perform key regulatory functions in osteosarcoma (OS) tumorigenesis. In this study, we aimed to explore the detailed action mechanisms of circ_0049271 in OS progression.
METHODS:
Cell colony formation, cell counting kit-8, and transwell assays were performed to assess the proliferation and invasion of OS cells. Quantitative reverse transcription-polymerase chain reaction and western blotting were used to determine the expression levels of polymerase 1 and transcript release factor (PTRF), microRNA (miR)-1197, and circ_0049271 in OS cells. Furthermore, RNA immunoprecipitation and dual luciferase assays were conducted to explore the targeted relationships among PTRF, miR-1197, and circ_0049271. Finally, a tumor formation assay was conducted to determine the effects of circ_0049271 on in vivo tumor growth in mice.
RESULTS:
High expression levels of miR-1197 and low levels of circ_0049271 and PTRF were observed in OS cells. circ _0049271 targeted miR-1197 to mediate PTRF expression. Moreover, the proliferation and invasion of OS cells were repressed by circ_0049271 or PTRF overexpression and increased by miR-1197 upregulation. Enforced circ_0049271 also impeded tumor growth in vivo. Upregulation of miR-1197 reversed the antitumor effects of circ_0049271 on OS progression in vitro; however, PTRF overexpression attenuated the cancer-promoting effects of miR-1197 on OS in vitro.
CONCLUSIONS:
Our findings revealed that circ_0049271 targeted the miR-1197/PTRF axis to attenuate the malignancy of OS, suggesting a potential target for its clinical treatment.
1.Introduction
Osteosarcoma (OS) is a highly destructive bone malignancy [1]. The focal zone of OS is in the metaphysis of long bones, where active bone repair and growth occur [2]. Surgical amputation is a key treatment method for OS; however, it causes disability and leads to recurrence [3, 4]. Although significant progress in chemotherapy and radiotherapy has improved the five-year survival rate to approximately 70%, adverse side effects may be observed in patients with OS [5, 6, 7]. Therefore, understanding the pathogenesis of OS is necessary to develop robust strategies for its treatment.
Circular RNAs (circRNAs) are non-coding RNAs (ncRNAs) with a closed covalent loop structure [8, 9]. Lacking 5’-caps and 3’-tails, circRNAs exhibit exonuclease resistance, making them more stable than linear RNAs [10]. The regulatory functions of circRNAs in various cancer types, including OS, have attracted attention [11, 12]. For example, Yang et al. reported that patients with OS with distant metastases and large tumors exhibit high circ_001422 expression [13]. Jiang et al. reported low levels of circ_0000658 in OS cells and tissues [14]. Guan et al. reported that circ_0008259 was underexpressed in OS and that its overexpression distinctly represses the metastasis and proliferation of OS cells [15]. circ_0049271 is downregulated in non-small cell lung cancer (NSCLC), exhibiting diagnostic value in distinguishing patients with NSCLC from healthy subjects [16]. Diminished circ_0049271 expression is closely correlated to the poor prognosis of patients with lung adenocarcinoma [17, 18]. He et al. analyzed the differentially expressed circRNAs in OS and reported poor circ_0049271 expression, suggesting its role as a tumor-suppressive circRNA in OS progression [19]. However, the specific functions and regulatory mechanisms of circ_0049271 in OS tumorigenesis remain unclear.
MicroRNAs (miRNAs) are highly conserved ncRNAs that modulate post-transcriptional functions by binding to the 3'-untranslated region of their target mRNAs [20, 21]. Recently, many studies have demonstrated the vital functions of miRNAs in the progression of OS. For example, miR-21 is expressed at low levels in OS and is an effective biomarker for the onset of OS tumorigenesis [22]. Low miR-505 levels have been reported in OS serum and tissues [23]. miR-505 upregulation inhibits the proliferation and induces the apoptosis of OS cells [23]. miRNAs, such as miR-421 [24], miR-487a [25], and miR-628-5p [26], are overexpressed in OS and considered as oncogenes for OS progression. Moreover, regulatory mechanisms of various circRNA–miRNA networks, such as circ_0046264–miR-940 [27], circ_0000527–miR-646 [28], and circ_0001721–miR-372-3p [29], have been widely investigated in the occurrence and progression of OS. miR-1197 interacts with circRNAs in several human cancers, including circ_0075542 in prostate cancer (PC) [30], circ_0004018 in hepatocellular carcinoma (HCC) [31], and circ_0000429 in NSCLC [32]. However, whether circ_0049271 modulates the role of miR-1197 in OS progression remains unclear.
In this study, we investigated the biological functions of circ_0049271 in OS and explored its regulatory mechanisms via the miR-1197/polymerase 1 and transcript release factor (PTRF) axis. Our results highlight a promising new therapeutic target for OS treatment.
2.Methods
2.1Sources of reagents, cells, and animals
hFOB 1.19 osteoblasts and human-derived OS cell lines (HOS and Saos-2) were purchased from Procell Life Science & Technology Co., Ltd. (Wuhan, China). Another human SW1353-derived OS cell line was provided by the BeNa Culture Collection (Beijing, China). Lipofectamine 3000, fetal bovine serum (FBS), McCoy’s 5A medium, Dulbecco’s Modified Eagle’s Medium (DMEM), and L-15 medium were purchased from Procell Life Science & Technology Co., Ltd. Cytoplasmic and nuclear RNA purification kits were acquired from Norgen BioTek (Thorold, Canada). RNase R was purchased from Geneseed Biotech (Guangzhou, China). An RNA extraction Kit (Cwbio, Beijing, China), an Evo m-mlv reverse transcription reagent, and a SYBR green Kit (Accyrate Biology, Changsha, China) were used for quantitative reverse transcription-polymerase chain reaction (qRT-PCR). The Magna RIP RNA-Binding Protein Immunoprecipitation Kit was purchased from Millipore (Billerica, MA, USA). Cell counting kit-8 (CCK-8), crystal violet, and Matrigel were obtained from Solarbio (Beijing, China). Primary antibodies against PTRF (Cat# ab76919) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Cat# ab8245) and horseradish peroxidase (HRP)-conjugated secondary antibodies (Cat# ab6759) for western blotting were purchased from Abcam (Cambridge, UK). Genomeditech (Shanghai, China) synthesized the pcDNA3.1-circ-circ_0049271 overexpression vector (OE-circ), pcDNA3.1-PTRF, pcDNA3.1 empty vector (OE-NC), miR-1197 mimic (mimic), and mimic-NC. Tumor xenograft models were established using four-week-old BALB/c nude mice (20–26 g) obtained from Charles River Laboratories (Sulzfeld, Germany).
Table 1
Real-time PCR primer list
| Gene | Sequences | |
|---|---|---|
| circ_0049271 | Forward | 5’-AACTTCGCTGAGCAGATTGG-3' |
| Reverse | 5’-GCATGGGGTTCCAGAAG ATA-3' | |
| miR-1197 | Forward | 5'-TCCTGGTATTTGAAGATGCGGT-3’ |
| Reverse | 5'-AGTAGACCATGTGTCCTACGTC-3' | |
| SPOCK1 | Reverse | 5’-AAAGCACAAGGCAGAAAGGA-3' |
| Reverse | 5'-GGGTCAAGCAGGAGGTCATA-3' | |
| PTRF | Forward | 5'-GAGCTGTTAGGACCCGATGG-3’ |
| Reverse | 5'-CCTTCCCATTCCACTCGGAC-3' | |
| TIMP3 | Reverse | 5’-AGCGCAAGGGGCTGAACTATCG-3' |
| Forward | 5'-CGGGTAGCCGAAATTGGAGAGC-3' | |
| U6 | Forward | 5'-CTCGCTTCGGCAGCACA-3' |
| Reverse | 5'-AACGCTTCACGAATTTGCGT-3' | |
| GAPDH | Forward | 5'-AGAAAAACCTGCCAAATATGATGAC-3’ |
| Reverse | 5'-TGGGTGTCGCTGTTGAAGTC-3' | |
2.2Cell culture
HOS and hFOB 1.19 cells were cultivated in DMEM containing 1% penicillin/streptomycin and 10% FBS. Saos-2 cells were cultured in McCoy’s 5A medium supplemented with 1% penicillin/streptomycin and 15% FBS. SW1353 cells were cultured in L-15 medium supplemented with 1% penicillin/streptomycin and 10% FBS. The cultures were all maintained in an incubator at 37∘C with 5% CO2. The cells were cultured until the logarithmic growth phase and then collected for subsequent experiments.
2.3Cell transfection
Lipofectamine 3000 was used to transfect 2
2.4Measurement of cell proliferation
Next, the viabilities of SW1353 and HOS cells were assessed via the CCK-8 assay. Briefly, 1
Cell numbers were estimated using a cell colony experiment. HOS and SW1353 cells in a 6-well plate (3
2.5Transwell invasion assay
SW1353 and HOS cells (2
2.6Western blotting assay
Total protein was extracted from 2
2.7qRT-PCR
Total RNAs from OS samples and cell lines (1
2.8Subcellular location analysis
The cytoplasmic and nuclear RNAs of SW1353 and HOS cells (1
2.9RNase R treatment
RNAs extracted from the SW1353 and HOS cells (1
2.10RNA immunoprecipitation (RIP) assay
HOS and SW1353 cells (1
2.11Bioinformatics analysis
Two online databases, starBase (https://rnasysu.com/ encori/) and CircInteractome (https://circinteractome. nia.nih.gov/index.html), were used to predict the miRNA binding to circ_0049271. Another database, TargetScan (https://www.targetscan.org/vert_71/), was used to predict the target genes of miR-1197. In addition, an mRNA microarray (GSE12865) from Gene Expression Omnibus was used to confirm the downregulated genes in OS samples with adj.
2.12Dual-luciferase reporter assay
To create mutant (MUT) and wild-type (WT) circ_0049271/PTRF, we inserted miR-1197-binding sites on circ_0049271/PTRF sequences into pGL3 vectors. Recombinant vectors and mimic/mimic-NC were introduced into HOS and SW1353 cells (
2.13Murine xenograft model
OE-circ or OE-NC lentiviral vector was introduced into SW1353 cells (1
2.14OS sample collection
Nineteen individuals diagnosed with OS by two pathologists using computed tomography and a surgical biopsy were enrolled in this study. Tumors and matched normal tissues (
2.15Statistical analyses
Data are represented as the mean
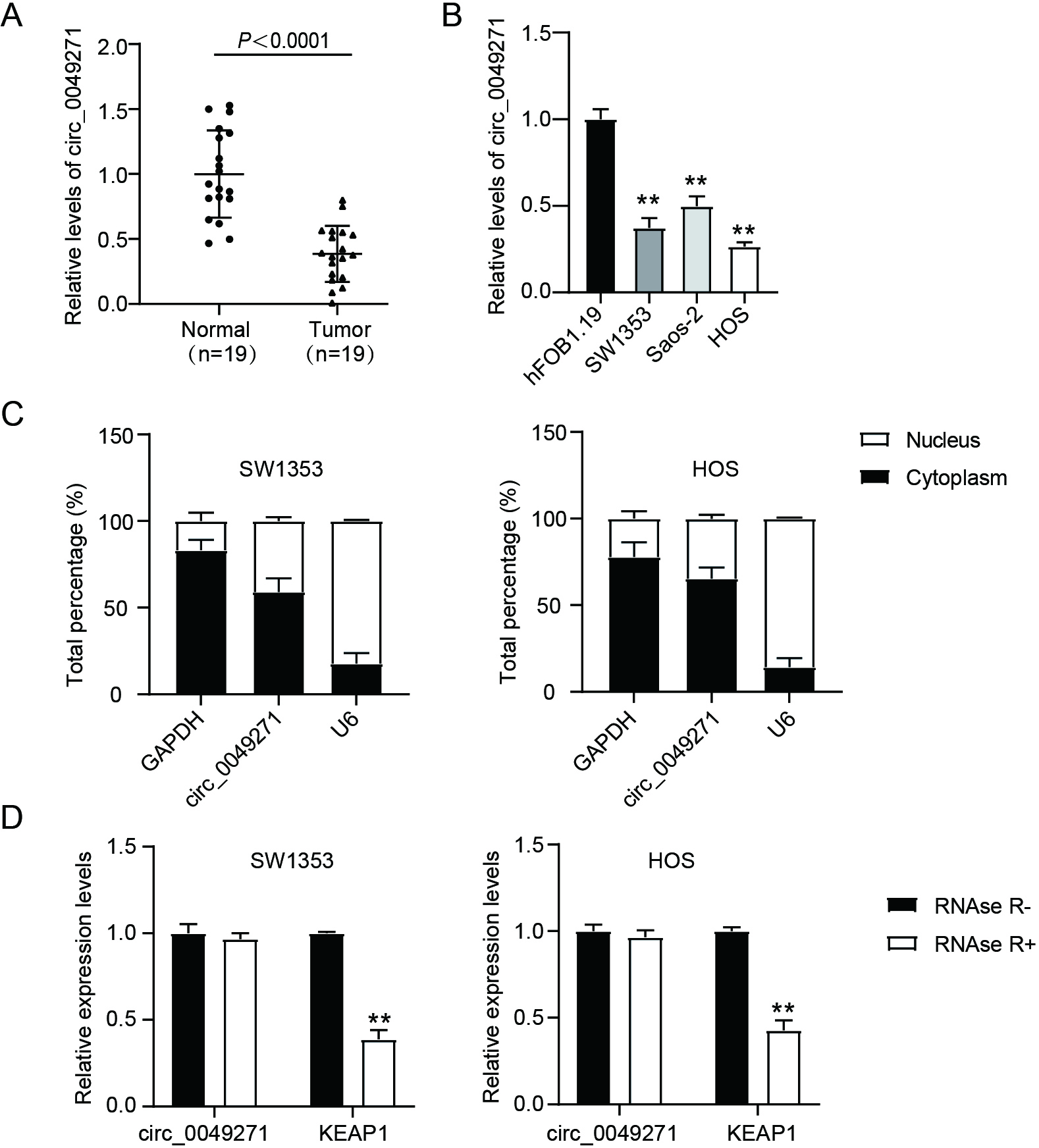
Figure 1.
Circ_0049271 is under-expressed in OS. (A) Circ_0049271 levels in OS normal and tumoral tissues were estimated through qRT-PCR.
3.Results
3.1Circ_0049271 is underexpressed in OS
Here, circ_0049271 expression levels were considerably lower in tumor tissues than in the corresponding normal tissues (Fig. 1A). Moreover, circ_0049271 was underexpressed in SW1353, HOS, and Saos-2 OS cells relative to its expression in hFOB1.19 cells (Fig. 1B). Therefore, SW1353 and HOS cells were selected for subsequent experiments. A subcellular localization assay revealed that circ_0049271 was mainly localized in the cytoplasm (Fig. 1C). To corroborate its circular features, circ_0049271 and its linear transcript, KEAP1, were treated with RNase R. As shown in Fig. 1D, unlike KEAP1, circ_0049271 exhibited resistance to RNase R digestion. These findings suggest that circ_0049271 is underexpressed and functions as a competing endogenous RNA (ceRNA) in OS.
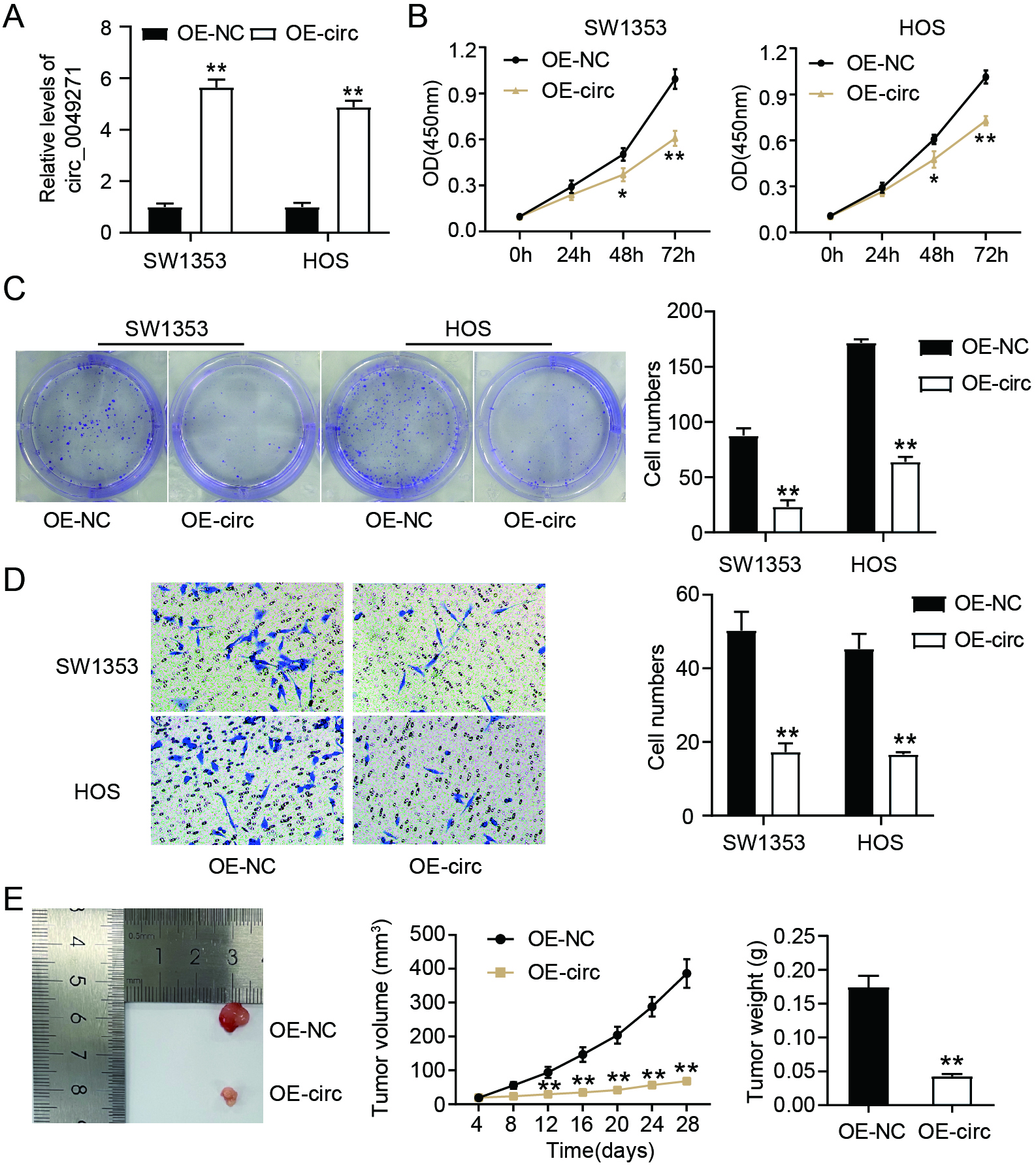
Figure 2.
Enforced circ_0049271 suppresses the invasion and proliferation of OS cells in vitro as well as the growth of tumors in vivo. (A) Circ_0049271 levels in HOS and SW1353, following their OE-circ/NC transfection, were estimated through quantitative RT-PCR. ∗∗P < 0.01 vs. OE-NC. The viability (B) and colony numbers (C) of SW1353 and HOS cells harboring either OE-circ/NC were assessed through the CCK-8 and colony formation experiments. ∗P < 0.05, ∗∗P < 0.01 vs. OE-NC. (D) The invasive capacity of SW1353 and HOS as assessed in the transwell experiment. ∗∗P < 0.01 vs OE-NC. (E) Representative image, volume, and weight of xenograft tumors after injecting the mice with SW1353 cells stably expressing OE-circ/NC. ∗∗P < 0.01 vs. OE-NC.
3.2Enforced circ_0049271 suppresses the invasion and proliferation of OS cells in vitro and tumor growth in vivo
To verify the effects of circ_0049271 on OS cells, it was overexpressed by transfecting SW1353 and HOS cells with OE-circ (Fig. 2A). As shown in Fig. 2B, the viability of OS cells decreased markedly after circ_0049271 overexpression. Moreover, circ_0049271 overexpression reduced the number of OS cell colonies (Fig. 2C) and suppressed the invasion of SW1353 and HOS cells (Fig. 2D). In addition, analysis of the influence of circ_0049271 on solid tumor growth revealed that the injection of stably overexpressing circ_0049271 drastically diminished the tumor volume and weight in SW1353 cells (Fig. 2E). Therefore, circ_0049271 represses OS tumorigenesis in vivo and in vitro.
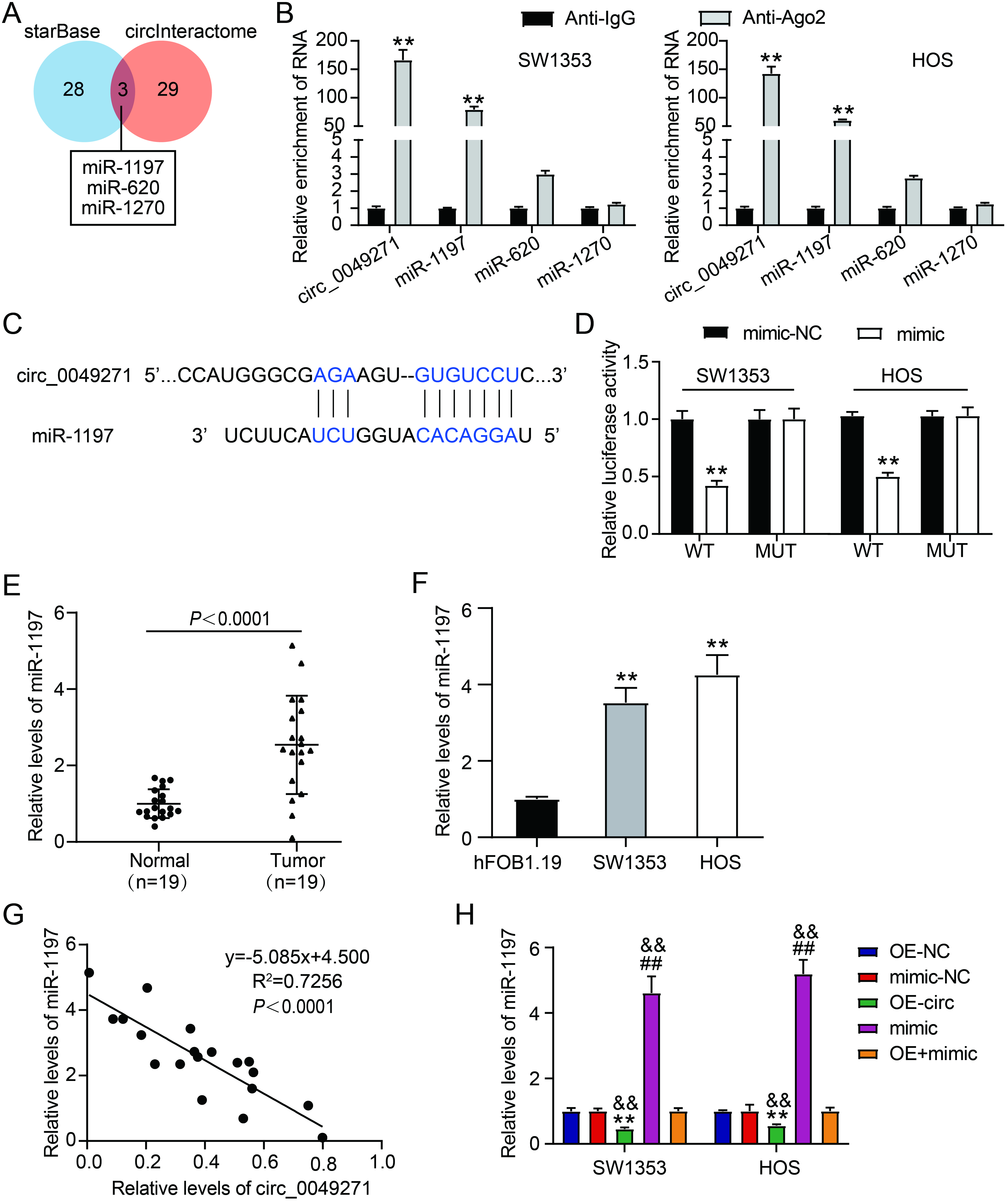
Figure 3.
Circ_0049271 binds to miR-1197. (A) starBase and circInteractome predicted the miRNAs binding to circ_0049271. (B) RIP experiment was accomplished to verify the interaction of miR-1197/miR-620/miR-1270 with circ_0049271. ∗∗P < 0.01 vs Anti-IgG. (C) The binding sites between miR-1197 and circ_0049271. (D) The luciferase activities in HOS and SW1353 transfected with a combination of a pGL3-circ_0049271 WT/MUT and either a miR-1197 mimic/NC were gauged through the dual luciferase experiment. ∗∗P < 0.01 vs. circ_0049271 WT
3.3Circ_0049271 binds to miR-1197
To confirm miRNA binding to circ_0049271, starBase and circInteractome databases were used to predict the binding of three miRNAs (miR-1197, miR-620, and miR-1270) to circ_0049271 (Fig. 3A). RIP assays of SW1353 and HOS cells revealed that, compared to miR-620 and miR-1270, circ_0049271 and miR-1197 were principally enriched by Ago2 antibodies (Fig. 3B). As shown in Fig. 3C, the circInteractome revealed binding sites between circ_0049271 and miR-1197. Moreover, the luciferase reporter assay indicated no obvious changes in the luciferase activity of the circ_0049271 MUT plus mimic and circ_0049271 MUT plus mimic-NC groups. Luciferase activity in the circ_0049271 WT plus mimic group was significantly lower than that in the circ_0049271 WT plus mimic-NC group (Fig. 3D). Relative to the respective NCs, elevated levels of miR-119 were detected in OS tissues (Fig. 3E) and cell lines (Fig. 3F). Circ_0049271 expression exhibited an inverse linear correlation with miR-1197 expression in OS tissues (Fig. 3G). Mimic/mimic-NC was introduced into SW1353 and HOS cells to verify this regulatory relationship. As illustrated in Fig. 3H, miR-1197 expression levels increased after mimic transfection and decreased after OE-circ transfection. Furthermore, transfection with a mimic partially attenuated the repressive effect of OE-circ on miR-1197 expression. Therefore, circ_0049271 targets miR-1197, and these two exhibit a negative regulatory relationship with each other in OS.
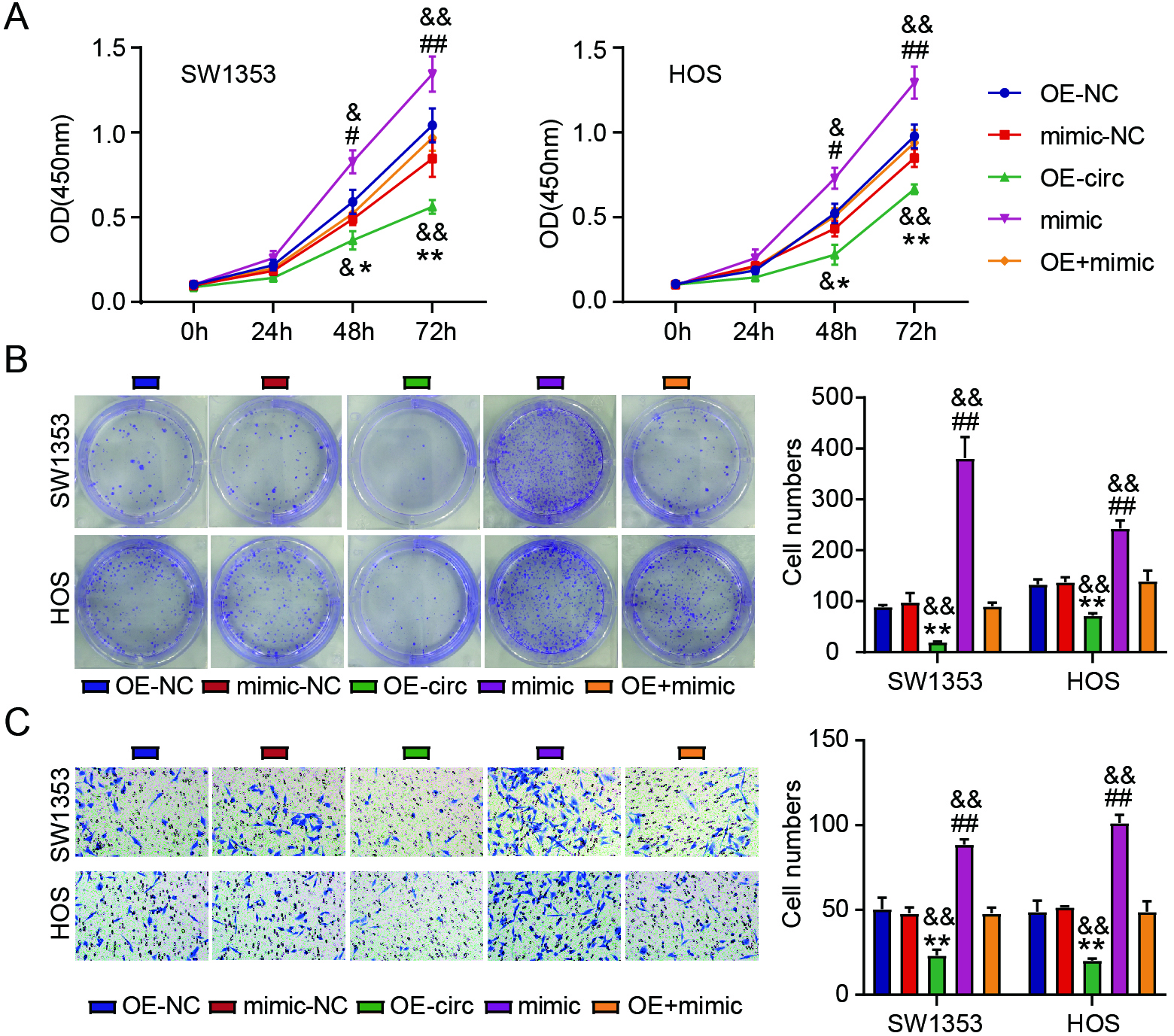
Figure 4.
Circ_0049271 overexpression prohibits the invasion and proliferation of cells through miR-1197 modulation in OS. The viability (A) and colony numbers (B) of SW1353 and HOS cells transfected with OE-circ, OE-NC, mimic, mimic-NC, or OE-circ
3.4Circ_0049271 overexpression abates the proliferation and invasion of OS cells via miR-1197 modulation
Rescue experiments investigated the interaction between circ_0049271 and miR-1197 in SW1353 and HOS cells. Overexpression of miR-1197 distinctly enhanced the viability (Fig. 4A), number (Fig. 4B), and invasion (Fig. 4C) of SW1353 and HOS cells. Impaired proliferation and invasion of OS cells caused by enforced circ_0049271 were considerably reversed by miR-1197 upregulation (Fig. 4A–C). These findings imply that circ_0049271 acts as a ceRNA of miR-1197 in OS progression.
Figure 5.
MiR-1197 targets PTRF. (A) Three genes were overlapped from TargetScan and GSE12865. (B) The levels of SPOCK1, PTRF, and TIMP3 in OS normal and tumoral tissues were estimated via quantitative RT-PCR.
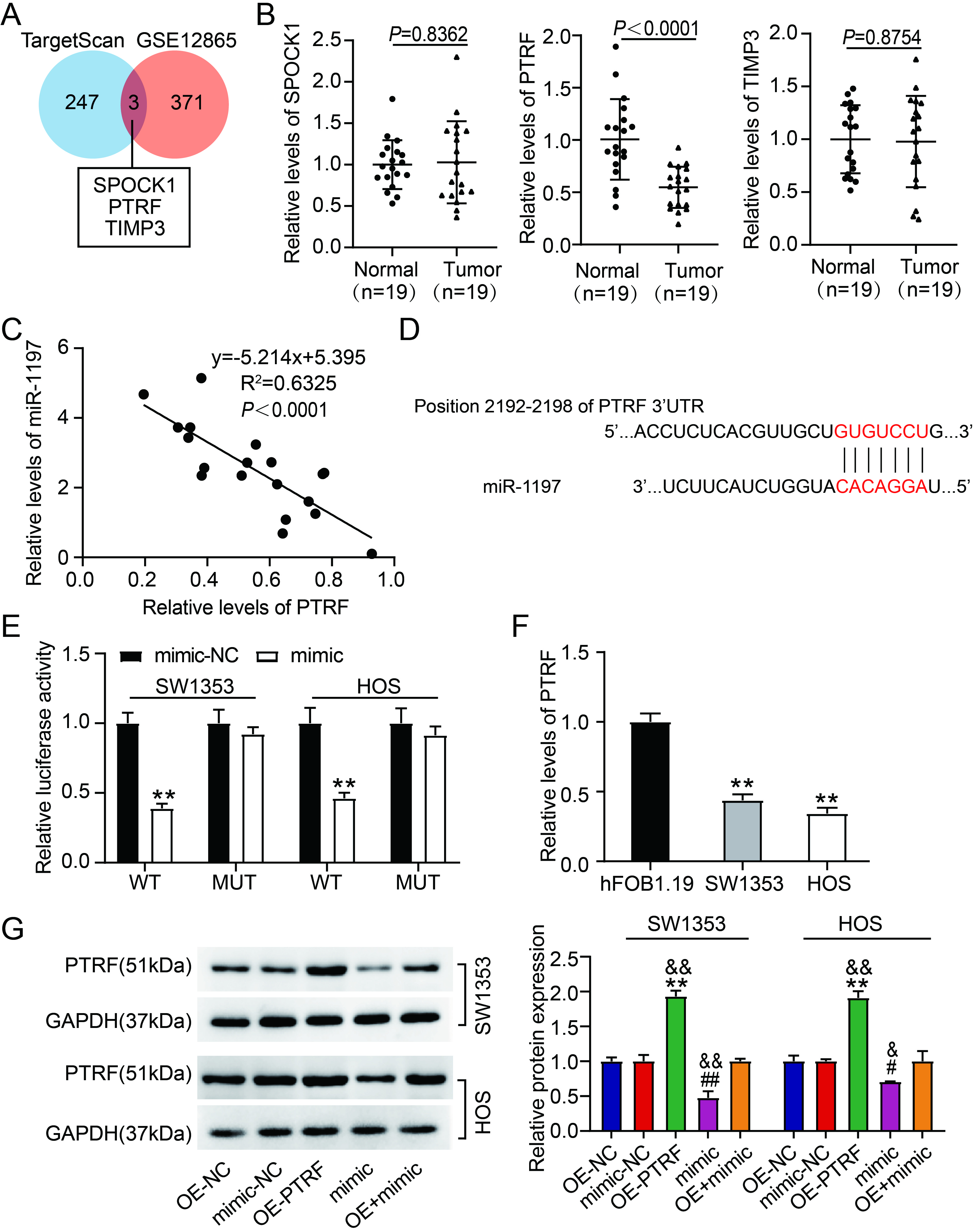
3.5PTRF is a target of miR-1197
TargetScan was used to identify the target genes of miR-1197. An mRNA microarray (GSE12865) was used to identify the downregulated genes in OS samples. Using Venny 2.1.0, three genes (SPOCK1, PTRF, and tissue inhibitor of metalloproteinase 3 [TIMP3]) overlapped between the TargetScan and GSE12865 datasets (Fig. 5A). qRT-PCR revealed that only PTRF expression was reduced and inversely correlated with miR-1197 expression in OS tissues (Fig. 5B–C). Therefore, PTRF was selected for further investigation. Figure 5D shows the TargetScan-predicted binding site of miR-1197 on PTRF. Luciferase reporter assays revealed that combining PTRF WT and mimic markedly diminished the luciferase activity in SW1353 and HOS cells. However, luciferase activity was barely affected by the combination of PTRF MUT and mimic or mimic-NC (Fig. 5E). Reduced PTRF expression was detected in OS cell lines (Fig. 5F). Western blotting revealed that OE-PTRF transfection markedly elevated PTRF protein levels. In contrast, mimic transfection reduced PTRF production in SW1353 and HOS cells (Fig. 5G). Moreover, decreased PTRF protein levels via mimic transfection were reversed by transfecting OE-PTRF into SW1353 and HOS cells (Fig. 5G). These data suggest that miR-1197 targets PTRF and inversely modulates its expression.
Figure 6.
MiR-1197/PTRF axis is associated with the abilities of OS cells to invade and proliferate. The viability (A) and colony numbers (B) of SW1353 and HOS cells carrying OE-PTRF, OE-NC, mimic, mimic-NC, or OE-PTRF
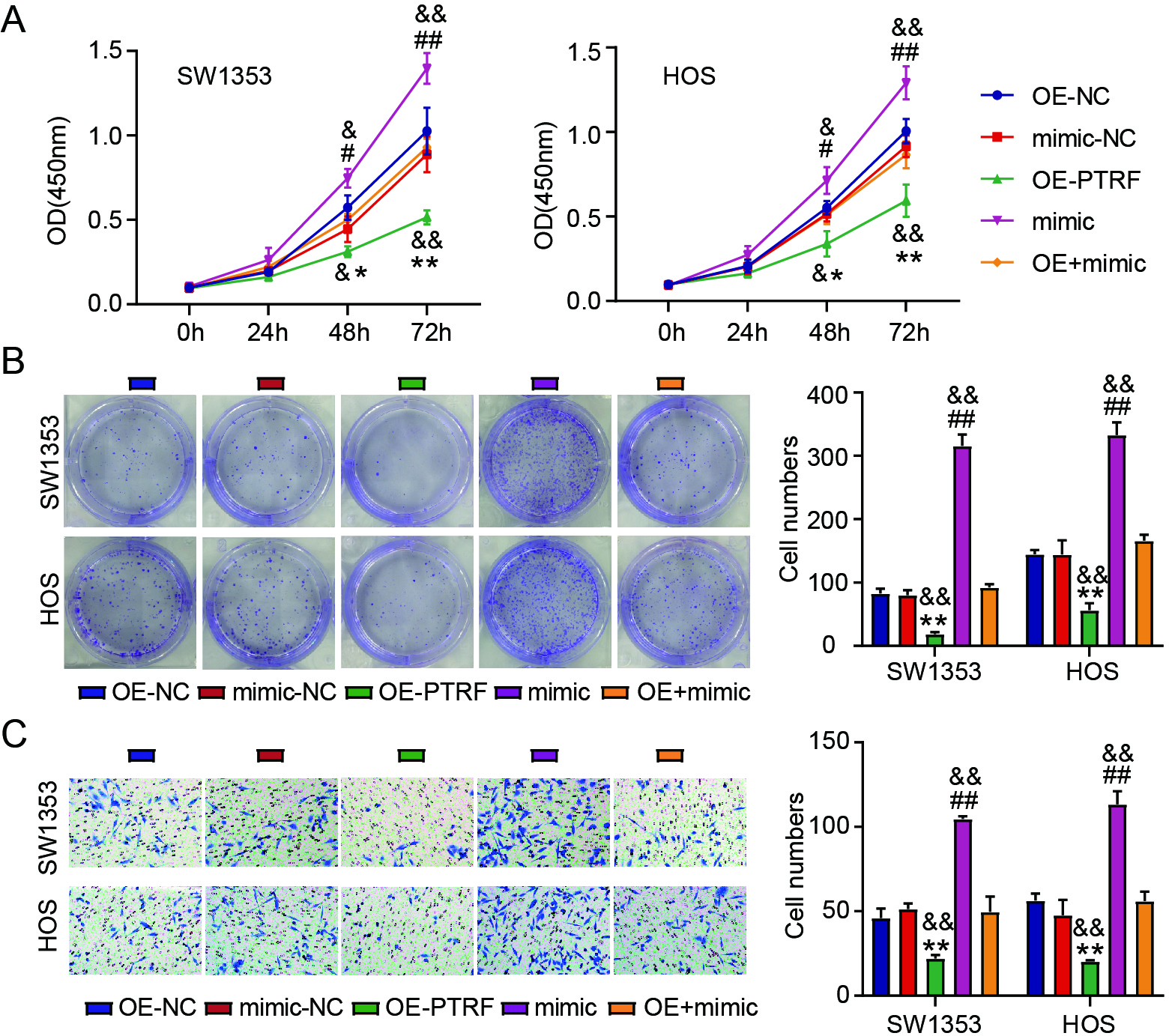
3.6miR-1197/PTRF axis is associated with the proliferation and invasion of OS cells
Next, the interaction between miR-1197 and PTRF during OS progression was verified in vitro. High levels of PTRF decreased the viability (Fig. 6A) and colony number (Fig. 6B) of OS cells. Moreover, PTRF overexpression inhibited OS cell invasion (Fig. 6C). PTRF overexpression further attenuated the promotive effect of miR-1197 upregulation on the proliferation and invasion of OS cells (Fig. 6A–C). Our data suggest that miR-1197 interacts with PTRF during OS progression.
4.Discussion
OS is a malignant bone tumor that originates from bone mesenchymal stem cell lesions [37, 38]. Identification of effective approaches to prevent metastasis and excessive growth is important for the clinical treatment of OS. Our data revealed that circ_0049271 expression was reduced in OS and that its overexpression attenuated OS tumorigenesis. In addition, circ_0049271 prevented OS progression by interacting with the miR-1197/PTRF axis.
Various circRNAs, such as circ_0000190 and circ_0008259 [15, 39], are aberrantly expressed in OS. They are downregulated in OS tissues and are regarded as inhibitors of OS progression [15, 39]. In contrast, circ_0010220 and circ_0032462 are overexpressed and act as oncogenes during OS tumorigenesis [40, 41]. The data in this study indicate that circ_0049271 is underexpressed in OS cells and tissues. Our results lend credence to an earlier study by He et al., wherein they performed bioinformatics analyses to detect low circ_0049271 expression in OS [19]. In our study, we pointed out that overexpression of circ_0049271 exerted repressive effects on cell invasion and proliferation. Moreover, we discovered that the upregulation of circ_0049271 hindered tumor growth in a mouse model. These results suggest that circ_0049271 may function as an anticancer circRNA in OS by suppressing cell invasion and growth.
Many studies have shown that miR-1197 has various regulatory functions in human cancers. For example, miR-1197 overexpression, which plays an important role in the tumorigenicity of the two cancers, has been observed in PC and HCC [30, 31]. However, in the tumorigenesis of NSCLC, low expression levels of miR-1197 have been detected during NSCLC tumorigenesis [32, 42]. In this study, we observed elevated miR-1197 levels in OS cells and tissues. This suggests that miR-1197 may be a risk factor for expediting the carcinogenicity of OS cells. CircRNA–miRNA crosstalk has crucial regulatory effects on the onset and development of OS [27, 28, 29]. miR-1197 has been confirmed to be modulated by circ_0075542 [30], circ_0004018 [31], and circ_0000429 [32] to affect the progression of various cancers except for OS. Therefore, we speculated that miR-1197 may be correlated with circ_0049271 in OS progression. CircInteractome revealed a circ_0049271–miR-1197 binding site, implying that miR-1197 is a downstream target of circ_0049271. To a certain extent, this result substantiates our hypothesis. Further rescue experiments revealed that overexpression of miR-1197 reversed the inhibitory effects of circ_0049271 upregulation on the invasive and proliferative potential of OS cells to a great extent. This finding supports the aforementioned hypothesis. Hence, we suggest that circ_0049271–miR-1197 crosstalk attenuates the malignancy of OS.
PTRF, named cavin-1, partakes in cellular processes by acting as a membrane sensor [43]. However, the specific role of PTRF in cancer progression remains unclear. Multiple studies suggest that PTRF serves as a tumor inhibitor, as its underexpression is accompanied by the development of breast [44] and lung [45] cancers. In contrast, recent studies have reported its cancer-promoting roles in glioma and pancreatic cancer [46, 47]. This study detected poor PTRF expression in OS cells and tissues. A recent study by Huertas-Martínez et al. revealed poor expression of PTRF in Ewing sarcoma, a type of primary bone tumor mainly occurring in adolescents [48]. These findings suggest that PTRF is a tumor-suppressive gene in OS. Additionally, recent investigations have shown that the circRNA–miRNA–mRNA network may be an efficacious treatment option for patients with OS [19, 49]. Based on the data obtained from our study, PTRF was hypothesized to be modulated by the circ_0049271–miR-1197 axis, which influences OS progression. In this study, PTRF was verified as the target gene of miR-1197, suggesting its interaction with miR-1197 to mediate OS tumorigenicity. As shown in Fig. 6, overexpression of PTRF drastically attenuated the proliferation and invasion of cells, which were promoted by miR-1197 upregulation. These data indicate that circ_0049271 impedes the malignancy of OS by repressing cell invasion and proliferation via the miR-1197/PTRF axis.
This study has certain limitations. PTRF is a conserved cytoplasmic protein that exerts its functions via exosome secretion [50, 51]. In OS progression, whether PTRF directly interacts with the circ_0049271/miR-1197 axis or indirectly via exosome secretion warrants further investigation. Moreover, the impact of the circ_0049271/miR-1197/PTRF regulatory axis on tumor growth in vivo needs to be explored further in future studies.
5.Conclusion
To the best of our knowledge, this study is the first to demonstrate that circ_0049271 attenuates the malignancy of OS via the miR-1197/PTRF axis. Our findings suggest that circ_0049271 acts as a tumor-suppressive circRNA in OS progression. Moreover, our results reveal a novel regulatory network underlying the pathogenesis of OS and suggest a potential target for its treatment.
Funding
This work was supported by Wuhan Medical Research Project under the project name of “Experimental study of nano-hydroxyapatite/
Ethics approval
This research received approval from the ethics committee of Fifth hospital in Wuhan (Wuhan, China). The handling of the clinical tissue specimens complied with the Declaration of Helsinki’s ethical precepts. An informed consent form was completed by each patient.
The ethical use of animals has been granted by the Ethics Committee of Fifth hospital in Wuhan (Wuhan, China). Animal experiments were executed in strict accordance with the ARRIVE guidelines.
Consent to participate
All patients submitted an informed consent form.
Consent for publication
The participants provided their consent for the publication of this study.
Availability of data and material
This article includes all of the data that were gathered or examined during this investigation.
Author contributions
Conception: YXW and FX.
Interpretation or analysis of data: HZ and YXW.
Preparation of the manuscript: YXW and FX.
Revision for important intellectual content: YXW, FX and HZ.
Supervision: all authors.
This manuscript has been reviewed and approved by all authors.
Acknowledgments
None.
Conflict of interest
The authors have declared that there were no conflicts of interest.
References
[1] | M.S. Isakoff, S.S. Bielack, P. Meltzer and R. Gorlick, Osteosarcoma: Current Treatment and a Collaborative Pathway to Success, J Clin Oncol 27: (33) ((2015) ), 3029–35. |
[2] | G. Ottaviani and N. Jaffe, The epidemiology of osteosarcoma, Cancer Treat Res, (152) ((2009) ), 3–13. |
[3] | J.L. Ferguson and S.P. Turner, Bone Cancer: Diagnosis and Treatment Principles, Am Fam Physician 4: (98) ((2018) ), 205–13. |
[4] | S.C. Kaste, C.B. Pratt, A.M. Cain, D.J. Jones-Wallace and B.N. Rao, Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features, Cancer 8: (86) ((1999) ), 1602–8. |
[5] | E. Simpson and H.L. Brown, Understanding osteosarcomas, Jaapa 8: (31) ((2018) ), 15–9. |
[6] | C. Zhou, W. Tan, H. Lv, F. Gao and J. Sun, Hypoxia-inducible microRNA-488 regulates apoptosis by targeting Bim in osteosarcoma, Cell Oncol (Dordr) 5: (39) ((2016) ), 463–71. |
[7] | G.A. Marulanda, E.R. Henderson, D.A. Johnson, G.D. Letson and D. Cheong, Orthopedic surgery options for the treatment of primary osteosarcoma, Cancer Control 1: (15) ((2008) ), 13–20. |
[8] | G. Lu, J. Zhang, X. Liu, W. Liu, G. Cao, C. Lv, et al. Regulatory network of two circRNAs and an miRNA with their targeted genes under astilbin treatment in pulmonary fibrosis, J Cell Mol Med 10: (23) ((2019) ), 6720–9. |
[9] | H. Bai, K. Lei, F. Huang, Z. Jiang and X. Zhou, Exo-circRNAs: a new paradigm for anticancer therapy, Mol Cancer 1: (18) ((2019) ), 56. |
[10] | J. Li, J. Yang, P. Zhou, Y. Le, C. Zhou, S. Wang, et al. Circular RNAs in cancer: novel insights into origins, properties, functions and implications, Am J Cancer Res 2: (5) ((2015) ), 472–80. |
[11] | Z. Li, X. Li, D. Xu, X. Chen, S. Li, L. Zhang et al., An update on the roles of circular RNAs in osteosarcoma, Cell Prolif 1: (54) ((2021) ), e12936. |
[12] | Y. Xi, M. Fowdur, Y. Liu, H. Wu, M. He and J. Zhao, Differential expression and bioinformatics analysis of circRNA in osteosarcoma, Biosci Rep 5: (39) ((2019) ). |
[13] | B. Yang, L. Li, G. Tong, Z. Zeng, J. Tan, Z. Su, et al., Circular RNA circ_001422 promotes the progression and metastasis of osteosarcoma via the miR-195-5p/FGF2/PI3K/Akt axis, J Exp Clin Cancer Res 1: (40) ((2021) ), 235. |
[14] | X. Jiang and D. Chen, Circular RNA hsa_circ_0000658 inhibits osteosarcoma cell proliferation and migration via the miR-1227/IRF2 axis, J Cell Mol Med 1: (25) ((2021) ), 510–20. |
[15] | K. Guan, S. Liu, K. Duan, X. Zhang, H. Liu, B. Xu, et al., Hsa_circ_0008259 modulates miR-21-5p and PDCD4 expression to restrain osteosarcoma progression, Aging (Albany NY) 23: (13) ((2021) ), 25484–95. |
[16] | L. Li, D. Sun, X. Li, B. Yang and W. Zhang, Identification of Key circRNAs in Non-Small Cell Lung Cancer, Am J Med Sci 1: (361) ((2021) ), 98–105. |
[17] | L. Gao and L. Zhang, Construction and comprehensive analysis of a ceRNA network to reveal potential prognostic biomarkers for lung adenocarcinoma, BMC Cancer 1: (21) ((2021) ), 849. |
[18] | Y. Liu, X. Wang, L. Bi, H. Huo, S. Yan, Y. Cui, et al., Identification of Differentially Expressed Circular RNAs as miRNA Sponges in Lung Adenocarcinoma, J Oncol, (10) ((2021) ), 5193913. |
[19] | Y. He, H. Zhou, W. Wang, H. Xu and H. Cheng, Construction of a circRNA-miRNA-mRNA Regulatory Network Reveals Potential Mechanism and Treatment Options for Osteosarcoma, Front Genet, (12) ((2021) ), 632359. |
[20] | A.C. Panda, Circular RNAs Act as miRNA Sponges, Advances in Experimental Medicine and Biology, (1087) ((2018) ), 67–79. |
[21] | E. Anastasiadou, L.S. Jacob and F.J. Slack, Non-coding RNA networks in cancer, Nat Rev Cancer 1: (18) ((2018) ), 5–18. |
[22] | S. Wang, F. Ma, Y. Feng, T. Liu and S. He, Role of exosomal miR21 in the tumor microenvironment and osteosarcoma tumorigenesis and progression (Review), Int J Oncol 5: (56) ((2020) ), 1055–63. |
[23] | G. Li, F. Liu, J. Miao and Y. Hu, miR-505 inhibits proliferation of osteosarcoma via HMGB1, FEBS Open Bio 7: (10) ((2020) ), 1251–60. |
[24] | Z. Ren, M. He, T. Shen, K. Wang, Q. Meng, X. Chen et al., MiR-421 promotes the development of osteosarcoma by regulating MCPIP1 expression, Cancer Biol Ther 3: (21) ((2020) ), 231–40. |
[25] | Z. Gu, S. Wu, G. Xu, W. Wu, B. Mao and S. Zhao, miR-487a performs oncogenic functions in osteosarcoma by targeting BTG2 mRNA, Acta Biochim Biophys Sin (Shanghai) 6: (52) ((2020) ), 631–7. |
[26] | J. Wang, Z. Li, X. Wang, Y. Ding and N. Li, The tumor suppressive effect of long non-coding RNA FRMD6-AS2 in uteri corpus endometrial carcinoma, Life Sciences, (243) ((2020) ), 117254. |
[27] | R. Du, B. Fu, G. Sun, B. Ma, M. Deng, X. Zhu et al., Circular RNA circ_0046264 Suppresses Osteosarcoma Progression via microRNA-940/Secreted Frizzled Related Protein 1 Axis, Tohoku J Exp Med 3: (254) ((2021) ), 189–97. |
[28] | X. Wu, L. Yan, Y. Liu and L. Shang, Circ_0000527 promotes osteosarcoma cell progression through modulating miR-646/ARL2 axis, Aging (Albany NY) 4: (13) ((2021) ), 6091–102. |
[29] | Y. Gao, H. Ma, Y. Gao, K. Tao, L. Fu, R. Ren et al., CircRNA Circ_0001721 Promotes the Progression of Osteosarcoma Through miR-372-3p/MAPK7 Axis, Cancer Manag Res, (12) ((2020) ), 8287–302. |
[30] | Y. Han, X. Wen, X. Li, D. Chen, L. Peng, B. Lai et al., Circular RNA hsa_circ_0075542 acts as a sponge for microRNA-1197 to suppress malignant characteristics and promote apoptosis in prostate cancer cells, Bioengineered 1: (12) ((2021) ), 5620–31. |
[31] | H. Wang, Q. Zhang, W. Cui, W. Li and J. Zhang, Circ_0004018 suppresses cell proliferation and migration in hepatocellular carcinoma via miR-1197/PTEN/PI3K/AKT signaling pathway, Cell Cycle 20: (20) ((2021) ), 2125–36. |
[32] | J.Y. Wang, F. Zhang, L. Hong and S.J. Wei, CircRNA_0000429 Regulates Development of NSCLC by Acting as a Sponge of miR-1197 to Control MADD, Cancer Manag Res, (13) ((2021) ), 861–70. |
[33] | Y. Li, R. Shi, G. Zhu, C. Chen, H. Huang, M. Gao et al., Construction of a circular RNA-microRNA-messenger RNA regulatory network of hsa_circ_0043256 in lung cancer by integrated analysis, Thoracic Cancer 1: (13) ((2022) ), 61–75. |
[34] | B. Zhang, W. Guo, C. Sun, H.Q. Duan, B.B. Yu, K. Mu et al., Dysregulated MiR-3150a-3p Promotes Lumbar Intervertebral Disc Degeneration by Targeting Aggrecan, Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, And Pharmacology 6: (45) ((2018) ), 2506–15. |
[35] | V.L. Veenstra, H. Damhofer, C. Waasdorp, A. Steins, H.M. Kocher, J.P. Medema et al., Stromal SPOCK1 supports invasive pancreatic cancer growth, Molecular Oncology 8: (11) ((2017) ), 1050–64. |
[36] | Y. Gao, T. Zou, W. Liang, Z. Zhang and M. Qie, Long non-coding RNA HAND2-AS1 delays cervical cancer progression via its regulation on the microRNA-21-5p/TIMP3/VEGFA axis, Cancer Gene Therapy 6: (28) ((2021) ), 619–33. |
[37] | J. Ritter and S.S. Bielack, Osteosarcoma. Ann Oncol. (21 Suppl 7) ((2010) ), vii320–5. |
[38] | C.A. Arndt, P.S. Rose, A.L. Folpe and N.N. Laack, Common musculoskeletal tumors of childhood and adolescence, Mayo Clin Proc 5: (87) ((2012) ), 475–87. |
[39] | S. Li, Y. Pei, W. Wang, F. Liu, K. Zheng and X. Zhang, Extracellular nanovesicles-transmitted circular RNA has_circ_ 0000190 suppresses osteosarcoma progression, J Cell Mol Med 3: (24) ((2020) ), 2202–14. |
[40] | Z. Lu, C. Wang, X. Lv and W. Dai, Hsa_circ_0010220 regulates miR-198/Syntaxin 6 axis to promote osteosarcoma progression, J Bone Oncol, (28) ((2021) ), 100360. |
[41] | R. Gu, X. Li, X. Yan, Z. Feng and A. Hu, Circular RNA circ_0032462 Enhances Osteosarcoma Cell Progression by Promoting KIF3B Expression, Technol Cancer Res Treat, (19) ((2020) ), 1533033820943217. |
[42] | B. Sun, J. Hua, H. Cui, H. Liu, K. Zhang and H. Zhou, MicroRNA-1197 downregulation inhibits proliferation and migration in human non-small cell lung cancer cells by upregulating HOXC11, Biomed Pharmacother, (117) ((2019) ), 109041. |
[43] | R.G. Parton and M.A. del Pozo, Caveolae as plasma membrane sensors, protectors and organizers, Nat Rev Mol Cell Biol 2: (14) ((2013) ), 98–112. |
[44] | L. Bai, X. Deng, Q. Li, M. Wang, W. An, A. Deli et al., Down-regulation of the cavin family proteins in breast cancer, J Cell Biochem 1: (113) ((2012) ), 322–8. |
[45] | J. Peng, H.Z. Liu, J. Zhong, Z.F. Deng, C.R. Tie, Q. Rao et al., MicroRNA187 is an independent prognostic factor in lung cancer and promotes lung cancer cell invasion via targeting of PTRF, Oncol Rep 5: (36) ((2016) ), 2609–18. |
[46] | K. Yi, Q. Zhan, Q. Wang, Y. Tan, C. Fang, Y. Wang et al., PTRF/cavin-1 remodels phospholipid metabolism to promote tumor proliferation and suppress immune responses in glioblastoma by stabilizing cPLA2, Neuro Oncol 3: (23) ((2021) ), 387–99. |
[47] | L. Liu, H.X. Xu, W.Q. Wang, C.T. Wu, T. Chen, Y. Qin et al., Cavin-1 is essential for the tumor-promoting effect of caveolin-1 and enhances its prognostic potency in pancreatic cancer, Oncogene 21: (33) ((2014) ), 2728–36. |
[48] | J. Huertas-Martínez, F. Court, S. Rello-Varona, D. Herrero-Martín, O. Almacellas-Rabaiget, M. Sáinz-Jaspeado et al., DNA methylation profiling identifies PTRF/Cavin-1 as a novel tumor suppressor in Ewing sarcoma when co-expressed with caveolin-1, Cancer Lett, (386) ((2017) ), 196–207. |
[49] | Y. Qiu, C. Pu, Y. Li and B. Qi, Construction of a circRNA-miRNA-mRNA network based on competitive endogenous RNA reveals the function of circRNAs in osteosarcoma, Cancer Cell Int, (20) ((2020) ), 48. |
[50] | P. Jansa, S.W. Mason, U. Hoffmann-Rohrer and I. Grummt, Cloning and functional characterization of PTRF, a novel protein which induces dissociation of paused ternary transcription complexes, Embo J 10: (17) ((1998) ), 2855–64. |
[51] | M.M. Hill, M. Bastiani, R. Luetterforst, M. Kirkham, A. Kirkham, S.J. Nixon, et al., PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function, Cell 1: (132) ((2008) ), 113–24. |




