Preoperative albumin-alkaline phosphatase ratio affects the prognosis of patients undergoing hepatocellular carcinoma surgery
Abstract
BACKGROUND:
The correlation between the preoperative albuminalkaline phosphatase ratio (AAPR) and the prognosis of hepatocellular carcinoma (HCC) patients after radical resection is still not comprehensive.
OBJECTIVE:
This study aims to observe the correlation between preoperative AAPR and the prognosis of HCC patients after radical resection.
METHODS:
We constructed a retrospective cohort study and included 656 HCC patients who underwent radical resection. The patients were grouped after determining an optimum AAPR cut-off value. We used the Cox proportional regression model to assess the correlation between preoperative AAPR and the prognosis of HCC patients after radical resection.
RESULTS:
The optimal cut-off value of AAPR for assessing the prognosis of HCC patients after radical resection was 0.52 which was acquired by using X-tile software. Kaplan-Meier analysis curves showed that a low AAPR (
CONCLUSIONS:
The preoperative AAPR level was related to the prognosis of HCC patients after radical resection and can be used as a routine preoperative test, which is important for early detection of high-risk patients and taking personalized adjuvant treatment.
1.Introduction
Primary liver cancer (PLC) is a relatively common malignancy worldwide. It is also a major contributor to cancer deaths. Approximately 75 to 85% of PLC cases are hepatocellular carcinoma (HCC) [1]. There are various treatments for HCC, among which surgical resection of the tumor and liver transplantation have the best therapeutic effects [2]. However, due to a shortage of liver donors, the popularization and application of liver transplantation are limited [3]. Therefore, patients with HCC are most likely to benefit from surgical resection. The technical expertise involved in performing surgical resection of HCC has greatly improved over the years as medical technologies have developed and progressed. Even so, an unacceptably large proportion of HCC patients who undergo surgical resection have the possibility of recurrence and metastasis, and this is detrimental to their prognosis. Therefore, it would be advantageous to explore a simple and useful postoperative prognosis evaluation index for patients, which will potentially help clinicians identify those at high risk early and implement appropriate interventions.
Over the past few years, with the exception of alpha-fetoprotein (AFP) levels, studies have continued to show that the ratios of a number of combined indexes were related to the prognosis of HCC patients that after surgical resection. These included neutrophil-lymphocyte ratio (NLR), albumin-glutamyl transferase ratio (AGR) and albumin-bilirubin (ALBI) [4, 5, 6]. The prognosis of HCC is determined by several factors including liver function. Albumin (ALB) as well as alkaline phosphatase (ALP) are important indexes for the assessment of liver function and they can reflect the status of this organ. ALB is produced by the liver and is closely related to inflammation [7]. It can also inhibit the proliferation of HCC by changing the phosphorylation of Rb protein [8]. As a substance related to both the liver and bones, elevated levels of ALP are known to be closely related to patients’ survival rates for HCC, and often indicate a poor prognosis [9, 10]. Chan and his team first proposed that the AAPR may have prognostic significance in HCC [11], and this index has gradually proven to reflect upon the prognosis of patients with HCC. According to previous studies, there is an association between the preoperative AAPR and the prognosis of HCC patients after liver transplantation as well as in hepatitis B virus (HBV)-related HCC patients after hepatectomy [12, 13]. This index also has a strong correlation with the clinical prognosis among patients with early-diagnosed breast cancer, IB-IIA cervical cancer and those with pancreatic ductal adenocarcinoma that cannot undergo resection [14, 15, 16]. At present, the relationship between preoperative AAPR and the prognosis of HCC patients after radical resection is not comprehensive. We undertook this study primarily to determining the prognostic significance of preoperative AAPR among HCC patients that after radical resection.
2.Materials and methods
2.1Research population
We used the active health management platform to construct a retrospective cohort study. (Registration site: http://www.chictr.org.cn/index.aspx; registration number: ChiCTR2200062446). Briefly, the active health management platform used an advanced medical data management system to manage the patients, and connected and indexed all the diagnosis and treatment records held at the hospital. It contains outpatient, inpatient and physical examination data, covering the diagnosis and treatment regimens, test reports, examination reports, electronic medical records and other medical data of the outpatients, inpatients and those undergoing physical examinations. All the medical information assessed was obtained from the electronic database, including demographic characteristics, medical diagnostic codes, surgical codes, drug prescriptions and death-related data. The information regarded a patient was automatically integrated into this platform whenever someone attended the clinic.
Figure 1.
A flow chart of inclusion and exclusion criteria of the patients in this study. (HCC: hepatocellular carcinoma; AAPR: albuminalkaline phosphatase ratio).
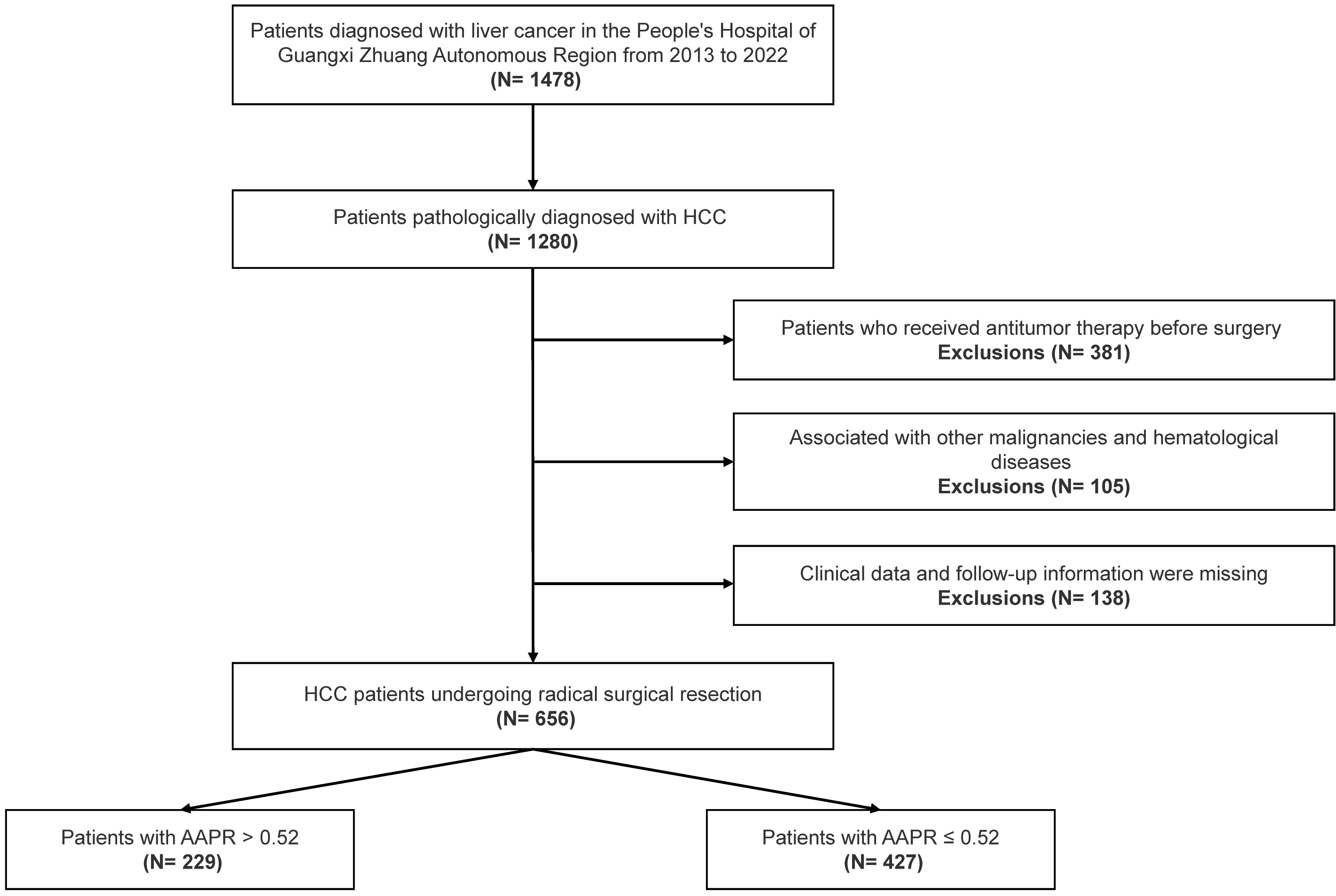
We reviewed inpatients diagnosed with HCC at the Guangxi Academy of Medical Sciences and the People’s Hospital of Guangxi Zhuang Autonomous Region from May 2013 to March 2022. The criteria for inclusion were: (1) HCC was diagnosed for the first time by pathology (2) the patient had undergone radical hepatectomy (negative margin) (3) comprehensive clinico-pathological and follow-up information were available for the patients. The criteria for exclusion were: (1) patients who had received antitumor therapy such as interventional, targeted and immune therapy or they had undergone liver transplantation, before surgery (2) patients with other malignant tumors and hematological diseases (3) those with missing either clinical baseline data or follow-up information. After screening, 656 HCC patients took part in this present study (Fig. 1). The study was approved by the Ethics Committee of Guangxi Academy of Medical Sciences and Guangxi Zhuang Autonomous Region People’s Hospital, and it met the guidelines set by the Helsinki Declaration. As the data was anonymized, individual informed consent was not needed for this study.
2.2Data acquisition and patients’ follow-up
The hospital big data retrieval platform was used to obtain the clinical data for the study subjects, including patients’ general personal data, past medical histories, preoperative blood test results, imaging data and postoperative pathological data. The tumor characteristics included diameter, number, portal vein invasion, microvascular invasion, tumor differentiation grade and recurrence or metastasis after tumor resection, which could all be obtained from the imaging and pathological data. The hospital’s health management cloud platform was used for all the patients with postoperative routine follow-up at 1, 6, 12, 18 24, 30 and 36 months follow-up points within 3 years of discharge. For patients who have been discharged from hospital for more than 3 years, follow-up visits were carried out at least once a year. For some patients who were not able to return regularly at the appointed times, they were followed-up by telephone. The overall survival (OS) was calculated as the interval from the date of surgery until death or the last time the patient was followed up, and the recurrence-free survival (RFS) was calculated as the time interval between the date of surgery and recurrence.
Figure 2.
The cut-off value of AAPR obtained by using the X-tile software was 0.52 where it was shown to have the strongest prognostic ability (A–C). (AAPR: albuminalkaline phosphatase ratio).
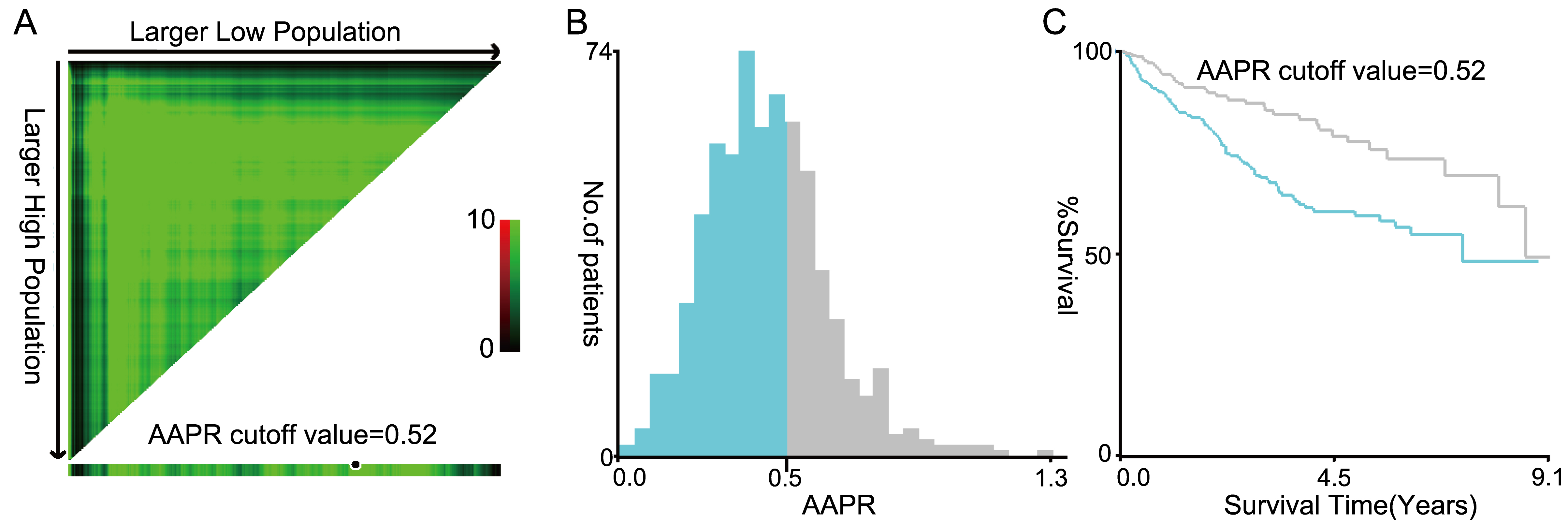
Table 1
Association of AAPR with patients’ clinical data
| Variables | Type | Total ( | AAPR | AAPR | |
|---|---|---|---|---|---|
| Values are expressed as medians (Q1–Q3) or number (%) | |||||
| Age (year) | 53.0 (44.0–62.0) | 54.0 (46.5–63.0) | 50.0 (41.0–61.0) | 0.001 | |
| Gender | 0.981 | ||||
| Female | 120 (18.3%) | 78 (18.3%) | 42 (18.3%) | ||
| Male | 536 (81.7%) | 349 (81.7%) | 187 (81.7%) | ||
| BCLC | 0.007 | ||||
| 0-A | 293 (44.7%) | 170 (39.8%) | 123 (53.7%) | ||
| B | 31 (4.7%) | 23 (5.4%) | 8 (3.5%) | ||
| C | 323 (49.2%) | 227 (53.2%) | 96 (41.9%) | ||
| D | 9 (1.4%) | 7 (1.6%) | 2 (0.9%) | ||
| Child-Pugh | |||||
| A | 599 (91.3%) | 376 (88.1%) | 223 (97.4%) | ||
| B | 57 (8.7%) | 51 (11.9%) | 6 (2.6%) | ||
| Tumor diameter (cm) | 5.0 (3.0–7.7) | 5.5 (3.2–8.6) | 4.2 (2.7–6.0) | ||
| Tumor number | 0.001 | ||||
| 1 | 555 (84.6%) | 347 (81.3%) | 208 (90.8%) | ||
| Multiple | 101 (15.4%) | 80 (18.7%) | 21 (9.2%) | ||
| Differentiation | 0.358 | ||||
| Low | 57 (8.7%) | 42 (9.8%) | 15 (6.6%) | ||
| Middle | 533 (81.2%) | 342 (80.1%) | 191 (83.4%) | ||
| High | 66 (10.1%) | 43 (10.1%) | 23 (10.0%) | ||
| PVTT | 0.001 | ||||
| No | 583 (88.9%) | 367 (85.9%) | 216 (94.3%) | ||
| Yes | 73 (11.1%) | 60 (14.1%) | 13 (5.7%) | ||
| MVI | |||||
| No | 383 (58.4%) | 227 (53.2%) | 156 (68.1%) | ||
| Yes | 273 (41.6%) | 200 (46.8%) | 73 (31.9%) | ||
| Paracancerous tissue infiltration | 0.07 | ||||
| No | 579 (88.3%) | 384 (89.9%) | 195 (85.2%) | ||
| Yes | 77 (11.7%) | 43 (10.1%) | 34 (14.8%) | ||
| Invasion of liver capsule | 0.641 | ||||
| No | 503 (76.7%) | 325 (76.1%) | 178 (77.7%) | ||
| Yes | 153 (23.3%) | 102 (23.9%) | 51 (22.3%) | ||
| Cirrhosis | 0.001 | ||||
| No | 407 (62.0%) | 246 (57.6%) | 161 (70.3%) | ||
| Yes | 249 (38.0%) | 181 (42.4%) | 68 (29.7%) | ||
| HBV-DNA | 0.029 | ||||
| No | 404 (61.6%) | 250 (58.5%) | 154 (67.2%) | ||
| Yes | 252 (38.4%) | 177 (41.5%) | 75 (32.8%) | ||
| AFP (ng/dL) | 80.8 (7.7–800.0) | 101.3 (11.7–800.0) | 65.6 (5.2–800.0) | 0.022 | |
| ALT (IU/L) | 33.0 (23.0–51.0) | 35.0 (24.0–56.5) | 29.0 (21.0–45.0) | ||
| AST (IU/L) | 38.0 (28.0–57.0) | 43.7 (32.0–63.5) | 30.0 (24.0–42.0) | ||
| TBIL ( | 13.5 (10.2–18.3) | 13.4 (10.1–18.9) | 13.6 (10.4–17.2) | 0.596 | |
| ALP (U/L) | 84.0 (68.8–110.0) | 99.0 (84.5–126.0) | 65.0 (55.6–71.0) | ||
| ALB (g/L) | 38.2 (35.1–40.9) | 37.0 (34.0–40.0) | 39.9 (37.6–42.6) | ||
| GGT (IU/L) | 58.1 (35.0–112.2) | 76.0 (46.5–141.0) | 39.0 (26.0–58.0) | ||
| HGB (g/L) | 135.0 (123.0–148.0) | 135.0 (121.5–149.0) | 137.0 (125.0–147.0) | 0.106 | |
| PT (s) | 13.8 (13.2–14.4) | 13.8 (13.2–14.5) | 13.8 (13.2–14.3) | 0.449 | |
AAPR: albumin-alkaline phosphatase ratio; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TBIL: total bilirubin; ALP: alkaline phosphatase; ALB: albumin; GGT: gamma glutamyl transpeptidase; HGB: hemoglobin; PT: prothrombin time; HBV: hepatitis B virus; AFP: alpha-fetoprotein; PVTT: portal vein tumor thrombus; MVI: microvascular infiltration; BCLC: Barcelona Clinic Liver Cancer.
Table 2
Univariate and multivariate analysis of the factors associated with OS
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age (year) | 0.98 (0.97, 1.00) | 0.021 | 0.98 (0.97–1.00) | 0.033 |
| Gender | ||||
| Female | 1 | |||
| Male | 1.66 (1.02, 2.68) | 0.041 | 1.66 (1.02–2.71) | 0.043 |
| ALT (IU/L) | 1.00 (1.00, 1.00) | 0.704 | ||
| AST (IU/L) | 1.00 (1.00, 1.00) | 0.957 | ||
| TBIL ( | 1.00 (0.99, 1.01) | 0.935 | ||
| PT (s) | 1.06 (0.91, 1.23) | 0.461 | ||
| HBV-DNA | ||||
| No | 1 | |||
| Yes | 1.19 (0.85, 1.65) | 0.305 | 0.89 (0.63–1.26) | 0.512 |
| Tumor diameter (cm) | 1.13 (1.09, 1.18) | 1.09 (1.04–1.14) | ||
| Tumor number | ||||
| 1 | 1 | |||
| Multiple | 1.62 (1.05, 2.51) | 0.03 | 1.62 (1.04–2.54) | 0.033 |
| Differentiation | ||||
| Low | 1 | |||
| Middle | 0.86 (0.51, 1.45) | 0.572 | ||
| High | 0.48 (0.22, 1.05) | 0.068 | ||
| PVTT | ||||
| No | 1 | |||
| Yes | 2.39 (1.52, 3.75) | 1.12 (0.66–1.89) | 0.677 | |
| MVI | ||||
| No | 1 | |||
| Yes | 2.62 (1.88, 3.66) | 1.98 (1.38–2.84) | ||
| Paracancerous tissue infiltration | ||||
| No | 1 | |||
| Yes | 0.92 (0.57, 1.48) | 0.731 | ||
| Invasion of liver capsule | ||||
| No | 1 | |||
| Yes | 1.34 (0.93, 1.92) | 0.119 | ||
| Cirrhosis | ||||
| No | 1 | |||
| Yes | 0.93 (0.65, 1.34) | 0.705 | 0.90 (0.62–1.32) | 0.601 |
| Child-Pugh | ||||
| A | 1 | |||
| B | 1.78 (1.02, 3.11) | 0.043 | 1.15 (0.64–2.05) | 0.639 |
| BCLC | ||||
| 0-A | 1 | |||
| B | 1.09 (0.44, 2.70) | 0.856 | ||
| C | 1.43 (1.02, 2.03) | 0.041 | ||
| D | 0.82 (0.11, 5.90) | 0.841 | ||
| AAPR group | ||||
| | 1 | 1 | ||
| | 0.55 (0.38, 0.80) | 0.002 | 0.66 (0.45–0.97) | 0.036 |
OS: overall survival; HR: Hazard ratio; AAPR: albumin-alkaline phosphatase ratio; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TBIL: total bilirubin; HGB: hemoglobin; PT: prothrombin time; HBV: hepatitis B virus; PVTT: portal vein tumor thrombus; MVI: microvascular infiltration; BCLC: Barcelona Clinic Liver Cancer.
2.3Statistical analysis
Table 3
Univariate and multivariate analysis of the factors associated with RFS
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age (year) | 0.98 (0.98, 0.99) | 0.002 | 0.99 (0.98–1.00) | 0.011 |
| Gender | ||||
| Female | 1 | |||
| Male | 1.25 (0.91, 1.70) | 0.169 | 1.18 (0.86–1.62) | 0.307 |
| ALT (IU/L) | 1.00 (1.00, 1.00) | 0.986 | ||
| AST (IU/L) | 1.00 (1.00, 1.00) | 0.514 | ||
| TBIL( | 1.00 (0.98, 1.01) | 0.358 | ||
| PT (s) | 1.05 (0.95, 1.17) | 0.322 | ||
| HBV-DNA | ||||
| No | 1 | |||
| Yes | 1.47 (1.16, 1.85) | 0.001 | 1.21 (0.95–1.54) | 0.118 |
| Tumor diameter (cm) | 1.12 (1.08, 1.15) | 1.07 (1.04–1.11) | ||
| Tumor number | ||||
| 1 | 1 | |||
| Multiple | 1.62 (1.20, 2.19) | 0.002 | 1.55 (1.14–2.11) | 0.005 |
| Differentiation | ||||
| Low | 1 | |||
| Middle | 0.71 (0.49, 1.03) | 0.068 | ||
| High | 0.51 (0.31, 0.87) | 0.012 | ||
| PVTT | ||||
| No | 1 | |||
| Yes | 2.15 (1.53, 3.02) | 1.07 (0.73–1.59) | 0.722 | |
| MVI | ||||
| No | 1 | |||
| Yes | 2.22 (1.76, 2.81) | 1.80 (1.40–2.31) | ||
| Paracancerous tissue infiltration | ||||
| No | 1 | |||
| Yes | 1.06 (0.75, 1.48) | 0.753 | ||
| Invasion of liver capsule | ||||
| No | 1 | |||
| Yes | 1.57 (1.22, 2.03) | |||
| Cirrhosis | ||||
| No | 1 | |||
| Yes | 1.04 (0.82, 1.33) | 0.73 | 0.94 (0.73–1.21) | 0.629 |
| Child-Pugh | ||||
| A | 1 | |||
| B | 1.65 (1.12, 2.43) | 0.011 | 1.09 (0.72–1.63) | 0.687 |
| BCLC | ||||
| 0–A | 1 | |||
| B | 1.84 (1.12, 3.03) | 0.015 | ||
| C | 1.13 (0.88, 1.44) | 0.345 | ||
| D | 0.60 (0.15, 2.43) | 0.473 | ||
| AAPR group | ||||
| | 1 | |||
| | 0.58 (0.45, 0.75) | 0.70 (0.53–0.92) | 0.011 | |
RFS: recurrence-free survival; HR: Hazard ratio; AAPR: albumin-alkaline phosphatase ratio; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TBIL: total bilirubin; HGB: hemoglobin; PT: prothrombin time; HBV: hepatitis B virus; PVTT: portal vein tumor thrombus; MVI: microvascular infiltration; BCLC: Barcelona Clinic Liver Cancer.
We used the X-tile software [17] (https://x-tile.software.informer.com/) to access the optimal cut-off value of AAPR, and used this cut-off value to divide the patients into two groups. Numbers and percentages were used to express the categorical variables. All the continuous variables were presented as medians and interquartile ranges. For baseline data between AAPR
Table 4
Subgroup analysis of prognostic factors
| Subgroup | OS | RFS | ||
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age (year) | ||||
| | 0.63 (0.41, 0.95) | 0.026 | 0.65 (0.49, 0.87) | 0.004 |
| | 0.28 (0.11, 0.71) | 0.008 | 0.37 (0.21, 0.65) | |
| Gender | ||||
| Female | 0.41 (0.14, 1.23) | 0.112 | 0.99 (0.55, 1.81) | 0.983 |
| Male | 0.57 (0.39, 0.85) | 0.005 | 0.52 (0.39, 0.69) | |
| HBV-DNA | ||||
| No | 0.43 (0.26, 0.71) | 0.52 (0.37, 0.73) | ||
| Yes | 0.81 (0.46, 1.42) | 0.466 | 0.74 (0.50, 1.10) | 0.133 |
| Tumor diameter (cm) | ||||
| | 0.65 (0.37, 1.14) | 0.135 | 0.76 (0.53, 1.09) | 0.131 |
| | 0.59 (0.35, 0.99) | 0.044 | 0.53 (0.36, 0.78) | 0.001 |
| Tumor number | ||||
| 1 | 0.56 (0.38, 0.83) | 0.004 | 0.61 (0.47, 0.81) | |
| Multiple | 0.62 (0.23, 1.69) | 0.352 | 0.51 (0.25, 1.05) | 0.066 |
| PVTT | ||||
| No | 0.57 (0.39, 0.85) | 0.006 | 0.61 (0.47, 0.80) | |
| Yes | 0.58 (0.17, 1.97) | 0.384 | 0.59 (0.25, 1.43) | 0.243 |
| MVI | ||||
| No | 0.55 (0.32, 0.94) | 0.027 | 0.63 (0.44, 0.89) | 0.01 |
| Yes | 0.66 (0.40, 1.12) | 0.121 | 0.63 (0.43, 0.93) | 0.019 |
| Cirrhosis | ||||
| No | 0.51 (0.33, 0.78) | 0.002 | 0.55 (0.40, 0.75) | |
| Yes | 0.68 (0.33, 1.38) | 0.28 | 0.67 (0.42, 1.07) | 0.096 |
| Child-Pugh | ||||
| A | 0.58 (0.40, 0.84) | 0.004 | 0.56 (0.43, 0.73) | |
| B | 0.50 (0.07, 3.88) | 0.508 | 3.04 (1.14, 8.15) | 0.027 |
The AAPR
3.Results
3.1Correlation of AAPR with clinical data
X-tile software analysis showed that the optimal AAPR cut-off value for evaluating OS and RFS was 0.52 (Figs 2A–C). On the basis of this cut-off value, the population we studied could be divided into two groups with high (
Table 1 shows the general personal data and clinicopathological characteristics of the participants in each group. In comparison with the high AAPR group, the patients in the low AAPR group were older (
3.2Prognostic factors related to OS and RFS
The impact of AAPR and other clinicopathological features on the prognosis of patients after surgery was investigated using COX regression analysis. Univariate analysis showed significant associations between preoperative AAPR, age, gender, tumor diameter, tumor number, PVTT, MVI, Child-Pugh grade and BCLC stage with OS (
With respect to RFS, univariate analysis showed that preoperative AAPR, age, HBV-DNA, tumor diameter, tumor number, tumor differentiation grade, PVTT, MVI, hepatic capsule invasion and Child-Pugh grade and BCLC stage were significantly related to RFS (
3.3Survival analysis
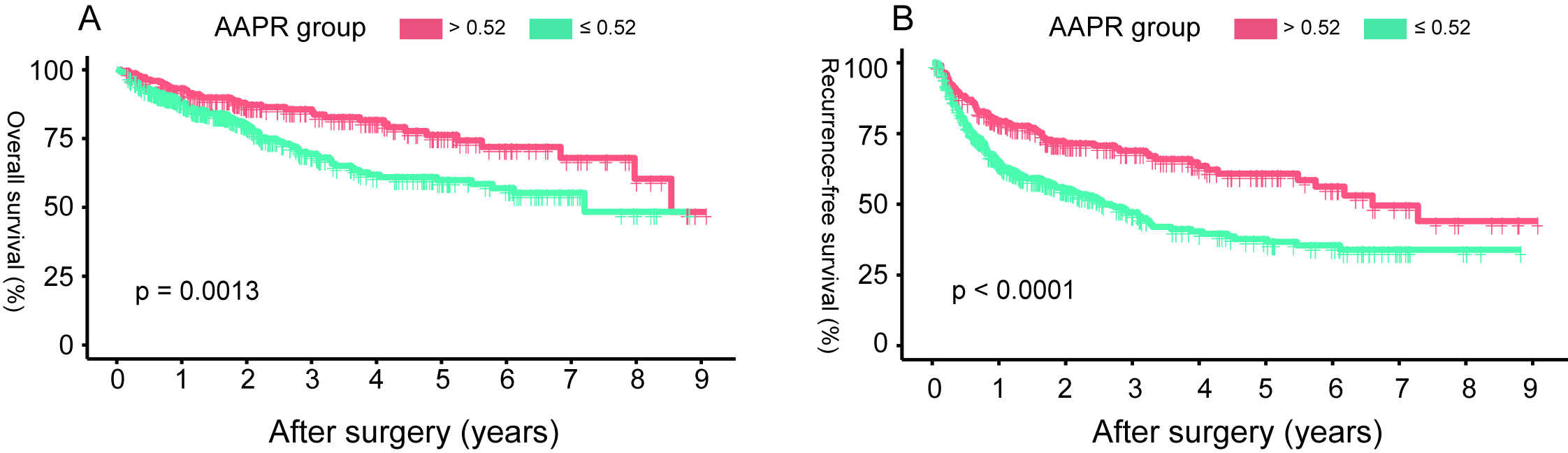
The 1st, 3rd and 5th year OS rates of the high AAPR (
3.4Subgroup analysis
In this study, the preoperative AAPR was negatively correlated with the probability of death and recurrence of HCC patients after radical resection. In order to further confirm the prognostic significance of preoperative AAPR, we conducted subgroup analysis on age, gender, HBV-DNA, tumor diameter, tumor number, PVTT, MVI, cirrhosis and the Child-Pugh grade (Table 4). Based on AAPR
4.Discussion
At present, HCC represents a difficult and challenging malignant tumor to treat. This study was undertaken to further confirm whether preoperative AAPR can be a strong prognostic factor for HCC patients that after radical resection, so as to promote its use in clinical practice. Through our observations of the association between the preoperative AAPR and other clinicopathological features on the prognosis of HCC patients after radical resection, it was found that this ratio exhibited significant independent prognostic value. There was a significant association between a preoperative AAPR
Figure 3.
The overall survival (A) and recurrence-free survival (B) based on the AAPR cut-off value of 0.52 in HCC patients undergoing radical resection. (HCC: hepatocellular carcinoma; AAPR: albuminalkaline phosphatase ratio).
Liver function is one of the important test indexes for HCC patients, and ALB and ALP are the related test indexes of this organ. ALB is abundant in plasma [18]. Its main physiological effects are to maintain colloid osmotic pressure within the body and transport various important substances. ALB also has been shown to have an inhibitory effect on proliferation of liver cancer cells [8]. In addition, inflammation and antioxidant processes are regulated by ALB in different organs of the body, and its plasma level has become a favorable factor for cancer patients [7]. ALB can be used in the treatment of many diseases in the clinic, and it is also one of the important markers of cancer [19]. Its level is related to the prognosis of patients with malignant tumors of the intrahepatic bile duct [20], breasts [21], intestinal tract [22] and esophagus [23]. In addition, some studies have combined the levels of ALB with either bilirubin [24] or fibrinogen [25] and these have become important prognostic factors for HCC patients. ALP is a metalloenzyme, that is ubiquitous in various biological species in nature and it helps a variety of important biological processes to take place. Altered levels of ALP is responsible for many serious diseases [26]. Multiple studies have shown that there is a significant association between ALP levels and poor OS and RFS in HCC patients [9]. In addition, ALP is also related to the prognosis of patients with malignant tumors of the esophagus [27], rectum [28] and pancreas [29]. Therefore, it appears that the levels of ALB and ALP can reflect opposite effects in the occurrence and progression of tumors. Previously related studies showed decreased AAPR were found to correlate with shorter OS in cancer patients, suggesting poor clinical outcomes [30]. In addition to the various cancers previously mentioned, a low AAPR was considered to be an independent risk factor for OS as well as progression-free survival in metastatic nasopharyngeal carcinoma [31]. The AAPR is also related to poor OS and cancer-specific survival in uppertract urothelial carcinoma patients as well as those with non-metastatic renal cell carcinoma who have undergone surgical treatment [32, 33]. This has also been demonstrated in lung malignancies [34, 35]. Related studies pointed out that the c-index and X2-value of AAPR, which can be derived from the circulating levels of albumin and alkaline phosphatase, had the highest prognostic evaluation ability among many liver biochemical indexes [11]. We combined these two closely related liver function indexes and assessed the prognosis of HCC patients after radical resection by employing correlation analysis.
In this retrospective cohort study, we used X-tile software to obtain the optimum cut-off value of 0.52 for the AAPR in order to divide all patients into high (
Our study has the following advantages: Firstly, our study had a long follow-up period, a large sample size, and more convincing conclusions. Secondly the cut-off value in this study were obtained using the X-tile software, a very novel method for optimising the selection of cut-off value. Thirdly, we ensured the consistency of baseline data as much as possible by excluding patients who received antitumor therapy other than surgery, and this reduced the interference caused by other treatments. Finally, a subgroup analysis further confirmed the prognostic effect of the AAPR. However, some limitations existed in this study. Firstly, although our study strictly defined the enrolled patients according to the criteria set out, there was an unavoidable selection bias. Secondly, the data were only obtained from the database of a single medical institution and the study was only conducted on patients who had received surgical treatment. However, there was a considerable number of HCC patients in our Chinese population and they were already in the middle and late stages when diagnosed, these patients would have missed the opportunity for surgery [40], therefore, more similar studies on advanced HCC patients with other treatment modalities (such as hepatic arterial infusion chemotherapy, targeted therapy and immunotherapy) are needed. Thirdly, the AAPR cutoff value needs to be validated in future prospective studies. Fourthly, at present, the molecular regulatory mechanism of the AAPR and poor prognosis is not clear, so this needs to be further explored by establishing a pathological specimen tissue bank. This may also be partly resolved by experimental work on animal models. Finally, all our patients were from China, and most of the etiologies were related to HBV infection. Therefore, this study’s findings may lack some scientific guidance for patients with HCC caused primarily by alcoholic liver disease. Similar studies also need to be performed in other countries and populations around the world.
5.Conclusions
In summary, based on our results, the preoperative AAPR was significantly correlated with the prognosis of OS and RFS among HCC patients that after radical resection. Preoperative AAPR can be routinely tested in the clinic and it can be considered as a biomarker for the clinical management of HCC patients undergoing radical resection. It is helpful for clinicians to detect high-risk patients early, thereby strengthening patient monitoring and intervention. However, the evaluation ability of AAPR needs to be verified by future multicenter studies that utilizes larger cohorts of patients.
Funding
This work was supported by the Major Project of Science and Technology of Guangxi Zhuang Autonomous Region (grant number: Guike-AA22096018), the Specific Research Project of Guangxi for Research Bases and Talents (grant number: Guike-AD21220042) and the National Natural Science Foundation of China (grant/award number: 82260558).
Authors contributions
Conception: Wei Huang, Suosu Wei.
Interpretation or analysis of data: Suosu Wei, Yi Tang.
Preparation of the manuscript: Wei Huang, Hongjun Liu, Junzhang Huang.
Revision for important intellectual content: Xiaofeng Dong, Yi Tang.
Supervision: Yuntian Tang, Jianrong Yang.
Acknowledgments
The authors would like to thank Dr Dev Sooranna of Imperial College London for his language editing of the manuscript.
References
[1] | Y. Miao, Y. Chen and D. Mi, Role of gasdermin family proteins in the occurrence and progression of hepatocellular carcinoma, Heliyon 8: ((2022) ), e11035. |
[2] | L. European Association For The Study Of The, R. European Organisation For and C. Treatment Of, EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma, J Hepatol 56: ((2012) ), 908–43. |
[3] | C.L. Chen, C.S. Kabiling and A.M. Concejero, Why does living donor liver transplantation flourish in Asia? Nat Rev Gastroenterol Hepatol 10: ((2013) ), 746–51. |
[4] | D. Galun, A. Bogdanovic, J. Djokic Kovac, P. Bulajic, Z. Loncar and M. Zuvela, Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative-intent surgery for hepatocellular carcinoma: experience from a developing country, Cancer Manag Res 10: ((2018) ), 977–988. |
[5] | J. Shen, L. Tang, X. Zhang, W. Peng, T. Wen, C. Li, J. Yang and G. Liu, A Novel Index in Hepatocellular Carcinoma Patients After Curative Hepatectomy: Albumin to Gamma-Glutamyltransferase Ratio (AGR), Front Oncol 9: ((2019) ), 817. |
[6] | Y.Y. Wang, J.H. Zhong, Z.Y. Su, J.F. Huang, S.D. Lu, B.D. Xiang, L. Ma, L.N. Qi, B.N. Ou and L.Q. Li, Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma, Br J Surg 103: ((2016) ), 725–734. |
[7] | V. Arroyo, R. Garcia-Martinez and X. Salvatella, Human serum albumin, systemic inflammation, and cirrhosis, J Hepatol 61: ((2014) ), 396–407. |
[8] | S. Nojiri and T. Joh, Albumin suppresses human hepatocellular carcinoma proliferation and the cell cycle, Int J Mol Sci 15: ((2014) ), 5163–74. |
[9] | P. Sun, S. Chen and Y. Li, The association between pretreatment serum alkaline phosphatase and prognosis in hepatocellular carcinoma: A meta-analysis, Medicine (Baltimore) 99: ((2020) ), e19438. |
[10] | M.C. Yu, K.M. Chan, C.F. Lee, Y.S. Lee, F.Z. Eldeen, H.S. Chou, W.C. Lee and M.F. Chen, Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg 15: ((2011) ), 1440–9. |
[11] | A.W. Chan, S.L. Chan, F.K. Mo, G.L. Wong, V.W. Wong, Y.S. Cheung, H.L. Chan, W. Yeo, P.B. Lai and K.F. To, Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma, Dis Markers 2015: ((2015) ), 564057. |
[12] | H. Li, L. Wang, L. Chen, H. Zhao, J. Cai, J. Yao, J. Zheng, Y. Yang and G. Wang, Prognostic Value of Albumin-to-Alkaline Phosphatase Ratio in Hepatocellular Carcinoma Patients Treated with Liver Transplantation, J Cancer 11: ((2020) ), 2171–2180. |
[13] | Q. Li, Z. Lyu, L. Wang, F. Li, Z. Yang and W. Ren, Albumin-to-Alkaline Phosphatase Ratio Associates with Good Prognosis of Hepatitis B Virus-Positive HCC Patients, Onco Targets Ther 13: ((2020) ), 2377–2384. |
[14] | Z.Q. Long, X. Hua, W.W. Zhang, S.W. Lv, J.P. Deng, L. Guo, Z.Y. He and H.X. Lin, Prognostic impact of the pretreatment albumin to alkaline phosphatase ratio for nonmetastatic breast cancer patients, Cancer Manag Res 11: ((2019) ), 4809–4814. |
[15] | C. Zhang, Y. Li, R. Ji, W. Zhang, C. Zhang, Y. Dan, H. Qian and A. He, The Prognostic Significance Of Pretreatment Albumin/alkaline Phosphatase Ratio In Patients With Stage IB-IIA Cervical Cancer, Onco Targets Ther 12: ((2019) ), 9559–9568. |
[16] | K. Zhang, S. Dong, Y.H. Jing, H.F. Gao, L.Y. Chen, Y.Q. Hua, H. Chen and Z. Chen, Albumin-to-alkaline phosphatase ratio serves as a prognostic indicator in unresectable pancreatic ductal adenocarcinoma: a propensity score matching analysis, BMC Cancer 20: ((2020) ), 541. |
[17] | R.L. Camp, M. Dolled-Filhart and D.L. Rimm, X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization, Clin Cancer Res 10: ((2004) ), 7252–9. |
[18] | C.M. Mendez, C.J. McClain and L.S. Marsano, Albumin therapy in clinical practice, Nutr Clin Pract 20: ((2005) ), 314–20. |
[19] | G. Fanali, A. di Masi, V. Trezza, M. Marino, M. Fasano and P. Ascenzi, Human serum albumin: from bench to bedside, Mol Aspects Med 33: ((2012) ), 209–90. |
[20] | J. Shen, T. Wen, C. Li, L. Yan, B. Li and J. Yang, The Prognostic Prediction Role of Preoperative Serum Albumin Level in Patients with Intahepatic Cholangiocarcinoma Following Hepatectomy, Dig Dis 36: ((2018) ), 306–313. |
[21] | C.G. Lis, J.F. Grutsch, P.G. Vashi and C.A. Lammersfeld, Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr 27: ((2003) ), 10–5. |
[22] | B. Nazha, Hypoalbuminemia in colorectal cancer prognosis: Nutritional marker or inflammatory surrogate? World Journal of Gastrointestinal Surgery 7: ((2015) ). |
[23] | Y. Jiang, D. Xu, H. Song, B. Qiu, D. Tian, Z. Li, Y. Ji and J. Wang, Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis, BMJ Open 11: ((2021) ), e048324. |
[24] | D. Feng, M. Wang, J. Hu, S. Li, S. Zhao, H. Li and L. Liu, Prognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma and other liver diseases, Ann Transl Med 8: ((2020) ), 553. |
[25] | Q. Xu, Y. Yan, S. Gu, K. Mao, J. Zhang, P. Huang, Z. Zhou, Z. Chen, S. Zheng, J. Liang, Z. Lin, J. Wang, J. Yan and Z. Xiao, A Novel Inflammation-Based Prognostic Score: The Fibrinogen/Albumin Ratio Predicts Prognoses of Patients after Curative Resection for Hepatocellular Carcinoma, J Immunol Res 2018: ((2018) ), 4925498. |
[26] | D.M. Zaher, M.I. El-Gamal, H.A. Omar, S.N. Aljareh, S.A. Al-Shamma, A.J. Ali, S. Zaib and J. Iqbal, Recent advances with alkaline phosphatase isoenzymes and their inhibitors, Arch Pharm (Weinheim) 353: ((2020) ), e2000011. |
[27] | X.L. Wei, D.S. Zhang, M.M. He, Y. Jin, D.S. Wang, Y.X. Zhou, L. Bai, Z.Z. Li, H.Y. Luo, F.H. Wang and R.H. Xu, The predictive value of alkaline phosphatase and lactate dehydrogenase for overall survival in patients with esophageal squamous cell carcinoma, Tumour Biol 37: ((2016) ), 1879–87. |
[28] | H.Y. Hung, J.S. Chen, Y. Chien, R. Tang, P.S. Hsieh, S. Wen, Y.T. You, J.F. You and J.M. Chiang, Preoperative alkaline phosphatase elevation was associated with poor survival in colorectal cancer patients, Int J Colorectal Dis 32: ((2017) ), 1775–1778. |
[29] | Y. Xiao, J. Lu, W. Chang, Y. Chen, X. Li, D. Li, C. Xu and H. Yang, Dynamic serum alkaline phosphatase is an indicator of overall survival in pancreatic cancer, BMC Cancer 19: ((2019) ), 785. |
[30] | L. An, W.T. Yin and D.W. Sun, Albumin-to-alkaline phosphatase ratio as a promising indicator of prognosis in human cancers: is it possible? BMC Cancer 21: ((2021) ), 247. |
[31] | M. Nie, P. Sun, C. Chen, X. Bi, Y. Wang, H. Yang, P. Liu, Z. Li, Y. Xia and W. Jiang, Albumin-to-Alkaline Phosphatase Ratio: A Novel Prognostic Index of Overall Survival in Cisplatin-based Chemotherapy-treated Patients with Metastatic Nasopharyngeal Carcinoma, J Cancer 8: ((2017) ), 809–815. |
[32] | P. Tan, N. Xie, J. Ai, H. Xu, H. Xu, L. Liu, L. Yang and Q. Wei, The prognostic significance of Albumin-to-Alkaline Phosphatase Ratio in upper tract urothelial carcinoma, Sci Rep 8: ((2018) ), 12311. |
[33] | A. Xia, Y. Chen, J. Chen, Y. Pan, L. Bao and X. Gao, Prognostic value of the albumin-to-alkaline phosphatase ratio on urologic outcomes in patients with non-metastatic renal cell carcinoma following curative nephrectomy, J Cancer 10: ((2019) ), 5494–5503. |
[34] | L. Zhang, H. Zhang, D. Yue, W. Wei, Y. Chen, X. Zhao, J. Zhu, B. Zhang, Z. Zhang and C. Wang, The prognostic value of the preoperative albumin to alkaline phosphatase ratio in patients with non-small cell lung cancer after surgery, Thorac Cancer 10: ((2019) ), 1581–1589. |
[35] | S. Zhou, H. Wang, W. Jiang, Q. Yu and A. Zeng, Prognostic Value of Pretreatment Albumin-to-Alkaline Phosphatase Ratio in Extensive-Disease Small-Cell Lung Cancer: A Retrospective Cohort Study, Cancer Manag Res 12: ((2020) ), 2015–2024. |
[36] | D.J. Erstad and K.K. Tanabe, Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma, Ann Surg Oncol 26: ((2019) ), 1474–1493. |
[37] | F. Luo, M. Li, J. Ding and S. Zheng, The Progress in the Treatment of Hepatocellular Carcinoma With Portal Vein Tumor Thrombus, Front Oncol 11: ((2021) ), 635731. |
[38] | Z.H. Chen, X.P. Zhang, X.R. Cai, S.D. Xie, M.M. Liu, J.X. Lin, X.K. Ma, J. Chen, Q. Lin, M. Dong, X.Y. Wu, J.Y. Wen and R.H. Xu, The Predictive Value of Albumin-to-Alkaline Phosphatase Ratio for Overall Survival of Hepatocellular Carcinoma Patients Treated with Trans-Catheter Arterial Chemoembolization Therapy, J Cancer 9: ((2018) ), 3467–3478. |
[39] | F. Zhang, S.X. Lu, K.S. Hu, Y.H. Gan, Y. Chen, N.L. Ge, B.W. Yang, L. Zhang, R.X. Chen, Z.G. Ren and X. Yin, Albumin-to-alkaline phosphatase ratio as a predictor of tumor recurrence and prognosis in patients with early-stage hepatocellular carcinoma undergoing radiofrequency ablation as initial therapy, Int J Hyperthermia 38: ((2021) ), 1–10. |
[40] | H. Zhou and T. Song, Conversion therapy and maintenance therapy for primary hepatocellular carcinoma, Biosci Trends 15: ((2021) ), 155–160. |




