PD-1 inhibitor combined with Docetaxel exerts synergistic anti-prostate cancer effect in mice by down-regulating the expression of PI3K/AKT/NFKB-P65/PD-L1 signaling pathway
Abstract
BACKGROUND:
Docetaxel is a yew compound antitumor agent with accurate antitumor efficacy, but its application is limited due to the high and serious adverse effects, and finding effective combination therapy options is a viable strategy. Immune checkpoint inhibitors have become hotspots in enhancing anti-tumor immunity by blocking immune checkpoint signaling pathways, but their response rate to monotherapy use is not high and the efficacy is minimal.
OBJECTIVE:
To explore the anti-tumor effects and mechanisms of the combination of PD-1 inhibitors and Docetaxel through in vivo experiments and develop a feasible combination treatment for the therapy of prostate cancer.
METHODS:
Tumor-bearing mice were subcutaneously injected with 0.1 ml RM-1 cells. Treatment were taken when the tumor growed up to 3 mm, after which the tumor and spleen were removed to test the antitumor effect with Flow cytometric (FACS) analysis, Immunohistochemistry, Western Blot.
RESULTS:
In this experiment, we found that PD-1 inhibitors combined with Docetaxel had a synergistic effect on mouse prostate cancer, inhibited the growth of prostate cancer, improved survival and reduced adverse reactions, increased spleen and tumor infiltrative CD4+ and CD8+ T cells, especially in group combination with low-dose Docetaxel, and were related to the PI3K/AKT/NFKB-P65/PD-L1 signaling pathway.
CONCLUSION:
Our study confirms that PD-1 inhibitors in combination with Docetaxel are a viable combination strategy and provide a safe and effective combination option for the clinical treatment of prostate cancer.
1.Introduction
Prostate cancer is one of male malignant tumors, ranking second in male incidence rate and fifth in mortality, which seriously threatens male life and health [1, 2]. Non-surgical treatments for prostate cancer include androgen deprivation therapy (ADT), radiation therapy (RT), ablation therapy, chemotherapy, and emerging immunotherapies [3]. A previous clinical study showed that Docetaxel chemotherapy increased survival and improved pain and quality of life [4]. However, the tissue specificity and side effects of Docetaxel as a first-line chemotherapeutic drug at clinical dose still need to be solved.
Tumor immune escape refers to the phenomenon that tumor cells grow and metastasize by avoiding being recognized and attacked by the immune system through various mechanisms [5]. It is an important pathway for tumor survival and growth. Studies have shown that many cytokines and tumor derived exosomes in the tumor microenvironment can induce the expression of PD-L1 and promote tumor immune escape [6]. PD-1 negatively regulates immune responses, leading to tumor cells being able to evade immune surveillance and being highly resistant to conventional chemotherapy. The application of anti-PD-1/PD-L1 antibodies as checkpoint inhibitors is rapidly becoming a promising treatment for tumors [7]. Blocking the interaction between PD-1 and PD-L1 can restore the immune function of exhausted CD8+T cells [8]. PD-1 has received extensive attention because of its role in inducing T cell immune checkpoint response. Therefore, PD-L1/PD-1-based immunotherapy can help restore the body’s clearance of cancer cells, thereby enhancing the killing effect of Docetaxel on cancer cells. However, some studies have found that Docetaxel treatment can upregulate the expression of NFKB-P65, and then upregulate the level of PD-L1 in cancer cells, affect the immune escape of tumor cells, and play an important role in chemotherapy resistance [9]. Previous studies have confirmed that the PI3K/AKT signaling pathway plays an important role in the development of cancer [10]. The activation of PI3K/AKT/NFKB signaling pathway in prostate cancer is negatively associated with patient biochemical progression and survival, and it is a possible predicted target of cancer recurrence, and is closely related to prognosis [11].
It’s meaningful to explore the mechanism of the combination treatment of prostate cancer in mice. The purpose of this study is to use the PD-1 inhibitor Camrelizumab in combination with Docetaxel, a first-line chemotherapy drug, to observe its effect on experimental treatment of prostate cancer in vivo and impact on immune function, and try to explain this kind of synergetic anti-tumor effcet through PI3K/AKT/NFKB-P65/PD-L1 signaling pathway, as to provide a theoretical and experimental basis for new ideas to clinical treatment of prostate cancer.
2.Materials and methods
2.1Materials
2.1.1Mouse and cell line
Mouse RM-1 prostate cancer cell line was purchased from Shanghai cell bank of Chinese Academy of Sciences; C57BL/6 mice were purchased from Hunan slaker Jingda experimental animal Co. All animals were raised under standard conditions: temperature (23
2.1.2Drugs and reagents
The RMPI1640 culture medium was purchased from Dalian Meilun Biotechnology (catalog number: MA0215-Oct-13G, Changsha, Hunan). The Fetal Bovine Serum was purchesed from WISENT Corporation (catalog number: 086150001, Nanjing, Jiangsu). The Penicillin G potassium salt, Streptomycin sesquisulfate and TRYPSIN were purchased from Solarbio (catalog number: 109D024, 3810-74-0, Beijing, China). The Red Blood Cell Lysis Buffer was purchased from Boster Biological Technology (catalog number: NH4CL2009, Changsha,Hunan). PD-1 inhibitor Carrelizumab for injection was perchased from SUNCADIA BIO PHARMACEUTICALS CO., Ltd (catalog number: 202106070F, Suzhou, Jiangsu). Docetaxel for injection was perchased from Yangzijiang Pharmaceutical Group Co., Ltd (company name: Yangzijiang Pharmaceutical Group Co., Ltd, catalog number: 21070911, Taizhou, Jiangsu).
2.1.3Antibodies
Anti-Mouse CD4 FITC (catalog number: 350042U025) and Anti-Mouse CD8a FITC (catalog number: 351886U025) were purchased from Tonbo BioSciences. PI3 Kinase p85 (19H8) Rabbit mAb(catalog number: 6), AKT(pan)(11E7) Rabbit mAb (catalog number: 3) were purchased from Cell signaling technology. Anti-NF-kB p65 Rb pAb (company name: Wanleibio, catalog number: NO7111273), Anti-P-NF-kB p65 Rb pAb (company name: Wanleibio, catalog number: NO8242169), Anti-BCL2 Rb pAb (company name: Wanleibio, catalog number: N10091556) and Anti-BAX Rb pAb (company name: Wanleibio, catalog number: NO9201637) were purchased from Wanleibio. Anti-PD-L1 Rabbit pAb (company name: Servicebio, catalog number: AC220523073) was purchased from Servicebio. Beta Actin (catalog number: JJ1231) was purchased from Zen Bioscience.
2.2Methods
2.2.1Cell culture
RM-1 cells were placed in RPMI1640 culture medium containing 10% bovine serum (FBS) and double antibodies, cultured in a cell incubator at 37∘C and 5% CO2. When the growth density of RM-1 cells reached about 80%, they were digested and passaged with 0.25% trypsin for subsequent experiments.
2.2.2Construction of mouse prostate cancer model
RM-1 cells cultured to logarithmic phase were collected and resuspended in sterile PBS to prepare 1
2.2.3Experiment grouping and processing
The experimental group included 8 mice per group: Control (PBS), Docetaxel (10 mg/kg), PD-1 inhibitor (200
2.2.4Flow cytometricFACSanalysis
The freshly removed spleen was ground with red blood cell lysate, and a single cell suspension was made by centrifugation, diluted to 1
2.2.5Immunohistochemistry (IHC)
The extracted tumor tissue was embedded by paraffin into tissue wax blocks. After deparaffinzing and rehydrating the paraffin section, Antigen retrieval, Blocking endogenous peroxidase activity. Serum sealing, Primary antibody incubation with PD-L1, CD4 and CD8, Secondary antibody incubatio, DAB chromogenic reaction, Nucleus counterstaining, Dehydration and mounting, we visualize staining of tissue under a microscope, acquisitive and analysis image. The nucleus of hematoxylin stained is blue, and the positive expression of DAB is brownish yellow.All the images were analysed by Image J.
2.2.6Western Blot
The isolated tumor tissue added with cell lysis buffer was homogenated on ice with tissue grinding machine to tissue homogenate with no obvious tissue block and then lysed at
4∘C for 30 min. The well-lysed tissue homogenate was put into a frozen centrifuge and centrifuged
at 4∘C at 12,000 rpm, and the supernatant was taken as the tumor tissue protein. Protein quantification was performed by Bradford method.Mixed the supernatant with 0.01 M PBS, 5
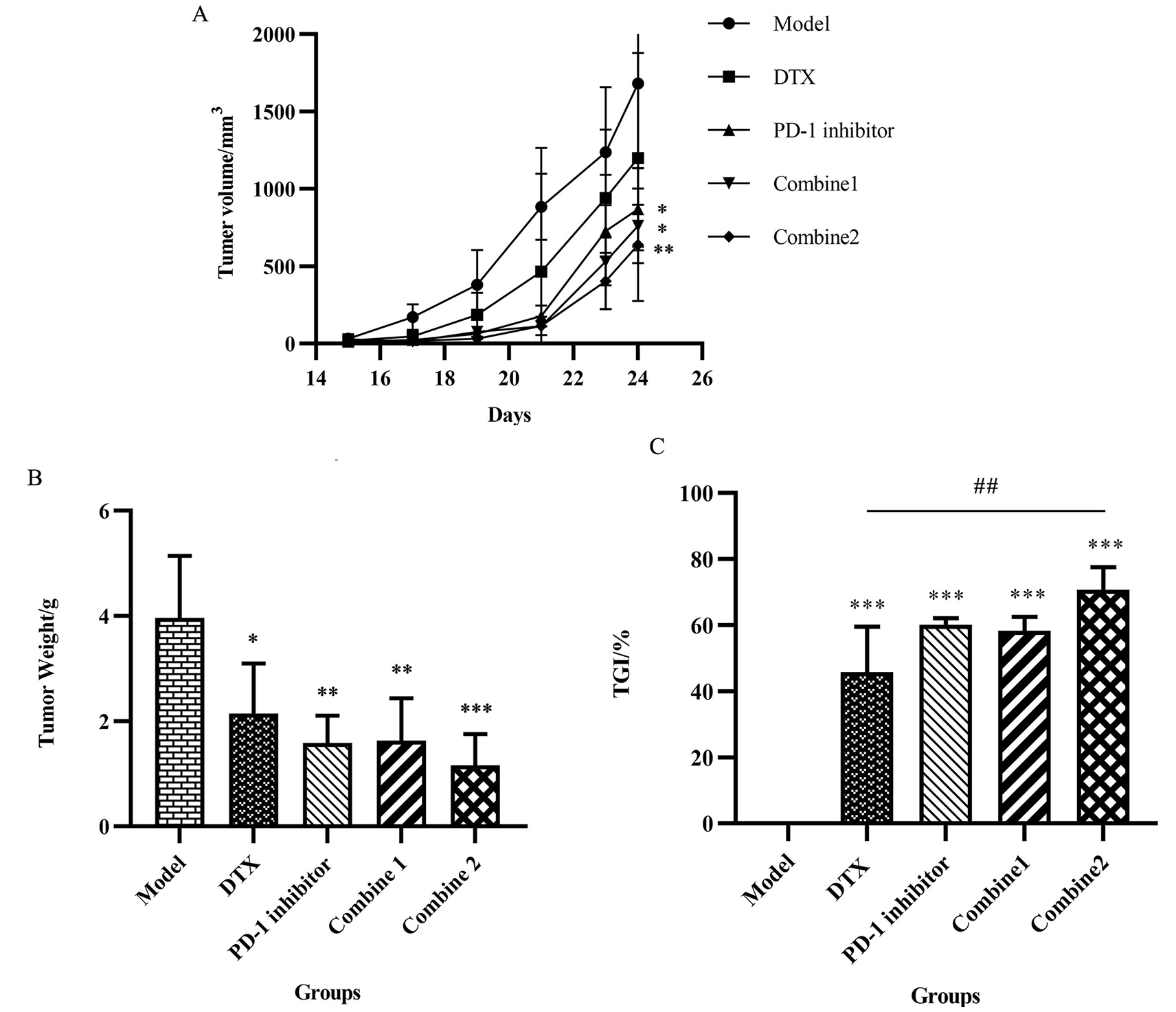
Figure 1.
PD-1 inhibitor combined with Docetaxel inhibited tumor development. (A) After successful tumor implantation, the long and short diameters of tumor were measured daily with vernier calipers. Tumor volume
2.2.7Statistical analysis
Graphpad prism version 8.0.2 software was used for statistical processing. Data were expressed as mean
3.Results
3.1PD-1 inhibitor combined with Docetaxel inhibited tumor development
To evaluate the efficacy of PD-1 inhibitor combined with Docetaxel in the treatment of prostate cancer in mice, we determined the dosing schedule according to the drug label dose conversion and tracked the changes in body weight and tumor volume during the treatment. After three courses of drug treatment, the mice were sacrificed by cutting their necks, and the tumor, spleen, liver and kidney tissue were removed for subsequent test.
We made the mice tumor growth curve in the process of treatment according to the experimental mice tumor volume changes over time to prove the consistent anti-tumor effect.It can be seen that during the treatment, the growth speed of the mice tumor was slowed down after given PD-1 inhibitor and DTX alone, and in the group combine 1 and combine 2 showed a better inhibition result compared to other groups, which meant that the combination of these two drug can obviously play a synergistic anti-tumor-growth effect (Fig. 1A).
At the end of the experiment, we tripped down the tumor and record the weight, calculate the tumor inhibition rate
3.2PD-1 inhibitor combined with Docetaxel improved survival in mice without significant toxicity
We performed survival analysis on the death of the mice, and it was not difficult to see that the untreated control group had a high mortality rate to more than 50% at the end of the experiment. However, drug treatment improved the survival rate of mice, from lower than 40% to about 70% and the most significant prolonged survival time was in the combine 2 group (Fig. 2A).
Figure 2.
PD-1 inhibitor combined with Docetaxel improved survival in mice without significant toxicity. (A) During the treatment process, the death number and date was recorded.According to the data, the survival of mice over the time was presented as a percentage by the survival curve. (B) To test if the treatment dosage affected the mice body weight, we tracted the body weight over the time,and the results were showed with the body weight-time curve. (C) The weight of liver, spleen and kidney were recorded with body weight to figure out if our therapy would induce the tumidness toxicity of the organ. The visceral index was calculated by Liver index
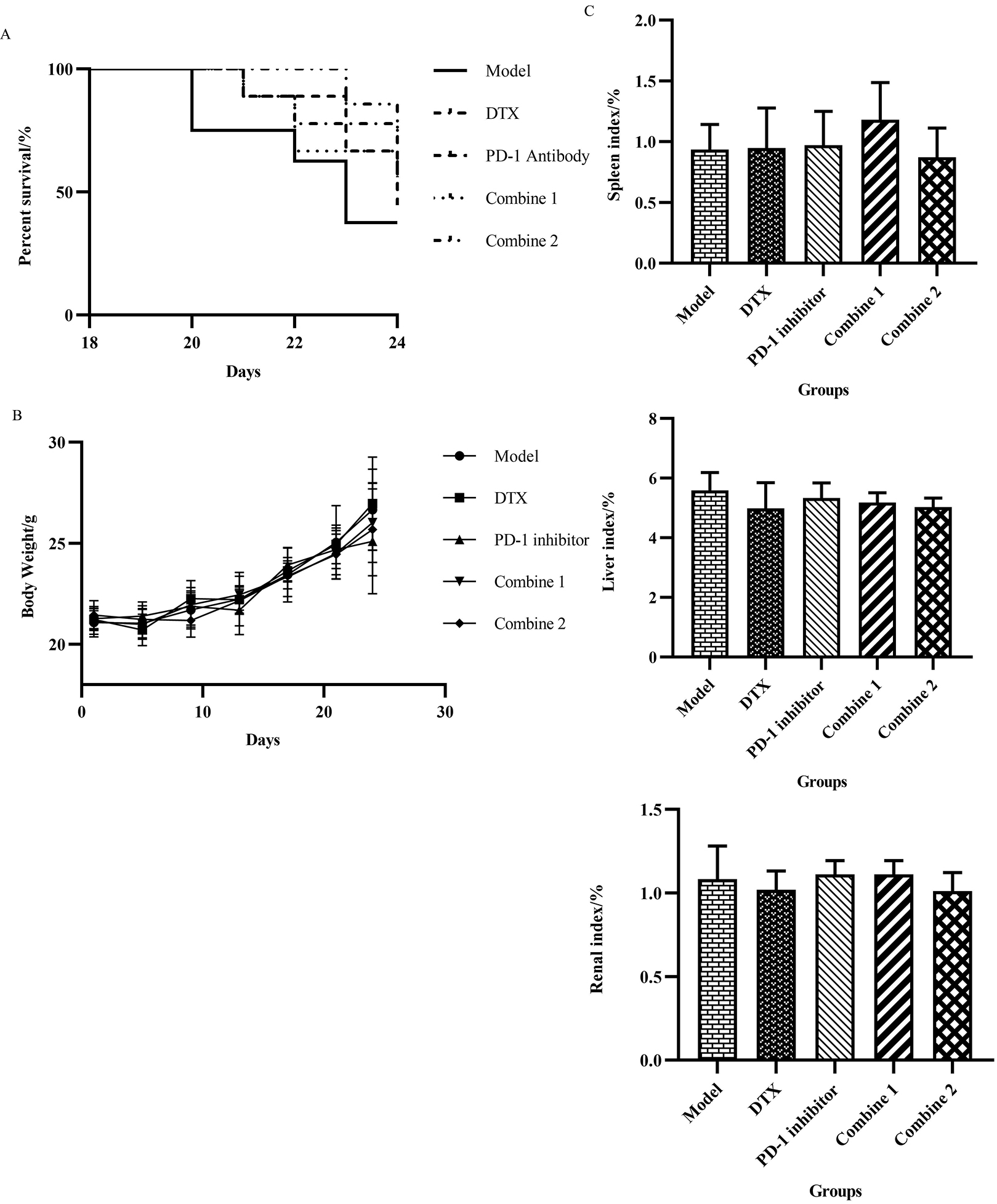
According to our record of the mice body weight throughout the whole treatment, the weight of the mice continued to increase, but with no difference between the groups (Fig. 2B). Then we calculated the visceral index
All the above results indicate that the combination of PD-1 inhibitor and Docetaxel administration regimen is safe and effective, which has little effect on the physiological state of mice, and can effectively inhibit the progression of tumor and improve the survival rate of mice. Especially when the dose of docetaxel is reduced by half, the combination effect can achieve the best.
3.3PD-1 inhibitor combined with Docetaxel enhanced the proportion of CD4+ and CD8+T cells in spleen
It has been shown that Docetaxel group can affect the proliferation of spleen CD4+ and CD8+T cells, and PD-1 inhibitor combined with Docetaxel can enhance the function of immune cells in cancer models, and play a powerful anti-tumor effect on [12]. PD-1 inhibitors inhibit tumor immune escape by blocking PD-L1/PD-1 binding. Among other cancers, PD-1 inhibitors rely on CD8+T cells to exert antitumor effects [13].
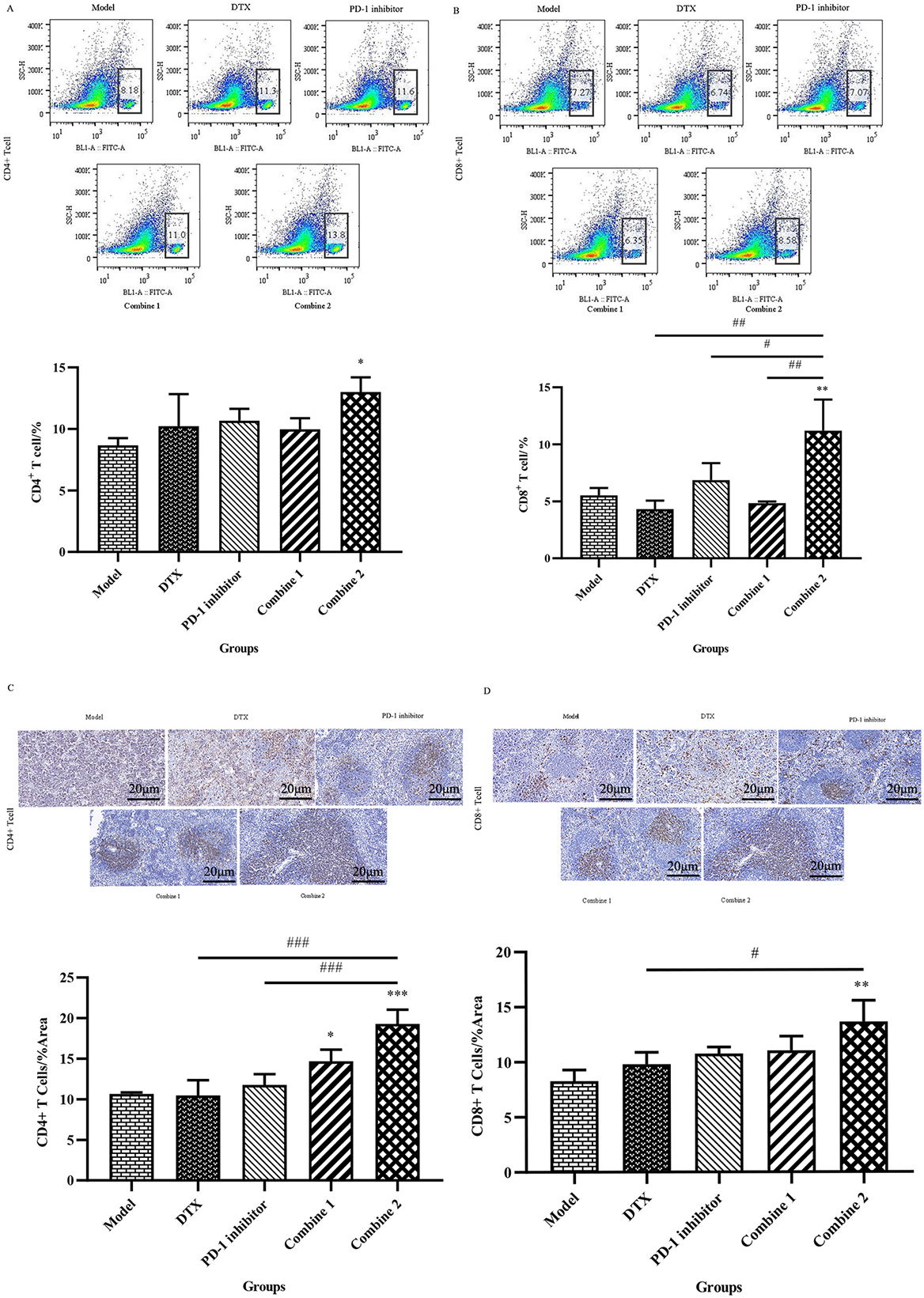
Therefore, to test the effects of CD4+ and CD8+T cells in the spleen of tumor-bearing mice after drug treatment, we demonstrated that PD-1 inhibitor combined with Docetaxel upregulated CD4+ and CD8+T cells by Flow cytometry and Immunohistochemical staining method.In the combine 2 group,there was an obvious increase in spleen CD4+T cells from 8.3% to 14.2%, and the number of spleen CD8+T cells raised from 5.06% to 9.18%,which meant the amount of this two kind T cells were upregulated almost twice time. In another word, PD-1 inhibitor combined with Docetaxel can enhance the immune function by upregualated the proportion of CD4+ and CD8+T cells in spleen (Fig. 3A & B). As we can see from the results, the spleen CD4+ and CD8+T cells in tumor-bearing mice was low due to the development of prostate cancer, which may be a result of disordered of immuesystem in cancer. After given the treatment of PD-1 inhibitor Camrelizumab and chemotherapy Docetaxel, there was a upregulation trend in the spleen CD4+ and CD8+T cells, and that kind of increasing effect was matched to the anti-tumor effect (Fig. 3C & D).
Figure 3.
PD-1 inhibitor combined with Docetaxel enhanced the proportion of CD4+ and CD8+T cells in spleen. (A) Spleens were harvested after mice being sacrificed. The spleen tissue was then grind to a uniform splenocyte suspension with a 40
3.4PD-1 inhibitor combined with Docetaxel decreased tumor PD-L1 expression and increases tumor-infiltrating CD4+ and CD8+T cells
The interaction of PD-L1 and its receptor PD-1 on T cells inactivates the anti-tumor immune response, and PD-L1 expression concerns with poor prognosis [14]. It has been found that Docetaxel upregulates PD-L1 expression and induces drug resistance and immune escape [15]. Thus, PD-1 inhibitor combined with Docetaxel may reverse the PD-L1 overexpression caused by Docetaxel and enhance the antitumor effects by blocking the PD-L1/PD-1 axis. We analyzed the expression of PD-L1 in tumor by immunohistochemistry and found that the Docetaxel monotherapy group upregulated PD-L1 expression in tumor tissue. This kind of results may convey a message to us that high dose of Docetaxel may cause a adverse effect due to its increase in PD-1 inhibition. However, this upregulation was reversed by PD-1 inhibitor combined with Docetaxel and inhence exerted a better anti-tumor effect by blocking PD-L1, especially in the regimen of the combined group 2, this reversal was more obvious, consistent with the results of tumor volume growth changes (Fig. 4A).
Figure 4.
PD-l inhibitor combined with Docetaxel decreased tumor PD-LI expression and increases tumor-infiltrating CD4+ and CD8+T cells. (A) Tumors were harvested after mice being sacrificed and then fixed with 4% paraformaldehyde for 24 hours.The fixed tissues wereembedded in paraffin and cut into 4um thin sections on a paraffin microtomefor immunohistochemical experiments.After dewaxing, antigen repair, blocking endogenous peroxidase, serum sealing and PD-L1 antibody incubaticn, DAB chromogenic reaction, nucleus counterstaining, dehydration and moounting were performed. The prepared sections were examined under the microscope, and images were collected and analyzed by Image J.All the data were presented as means
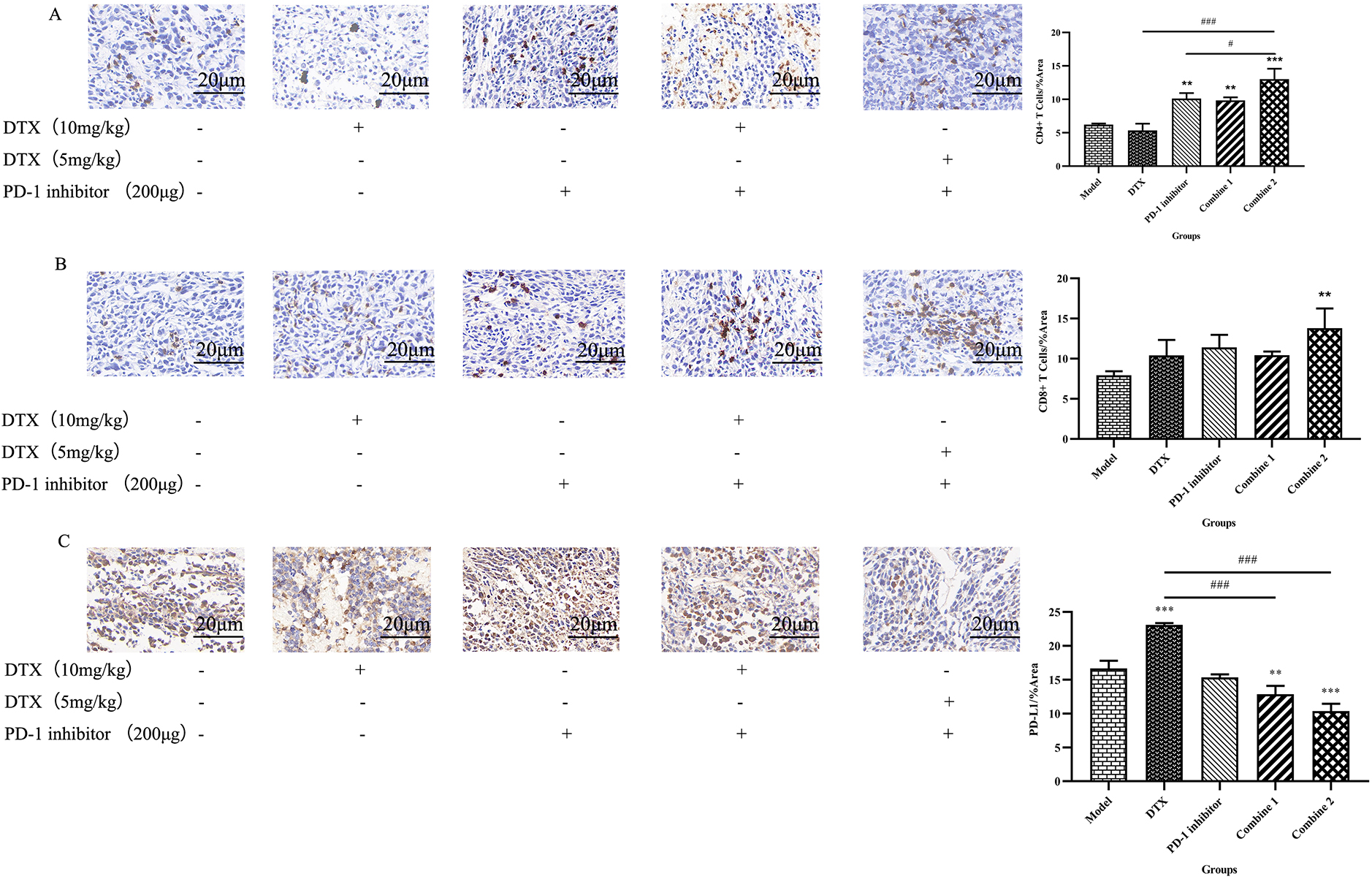
PD-1 plays a crucial role in suppressing immune responses and promoting self-tolerance by regulating T-cell activity, activating antigen-specific T-cell activity, and inhibiting regulatory T-cell apoptosis [16]. Immune checkpoint inhibitors can block PD-1 to restore T cell proliferation and function. It has been shown that Docetaxel combined with immunotherapy can significantly increase CD4+ and CD8+ T cells in tumor and blood, reduce tumor metastasis, and play a better anti-tumor effect [17]. Therefore, in our experiments, invasive CD4+ and CD8+T cells in tumor tissue were detected by Immunohistochemistry (Fig. 4B & C). The pathological test results showed that the immune function in untraeted mice decreased due to the tumor growth, so the survival status of tumor-bearing mice was bad. After different treatment, the tumor-infiltrating CD4+ and CD8+T cells improved at some degree. It’s clearly that compared to monotherapy of Docetaxel and PD-1 inhibitor Camrelizumab used alone, PD-1 inhibitor combined with Docetaxel can further strengthen the recruitment effect of immune cells in tumor tissues, restore the number of CD4+ and CD8+T cells and enhance the mouse immune system.
3.5PD-1 inhibitor combined with Docetaxel exerted synergistic effects by inhibiting the PI3K/AKT/NFKB-P65/PD-L1 signaling pathway
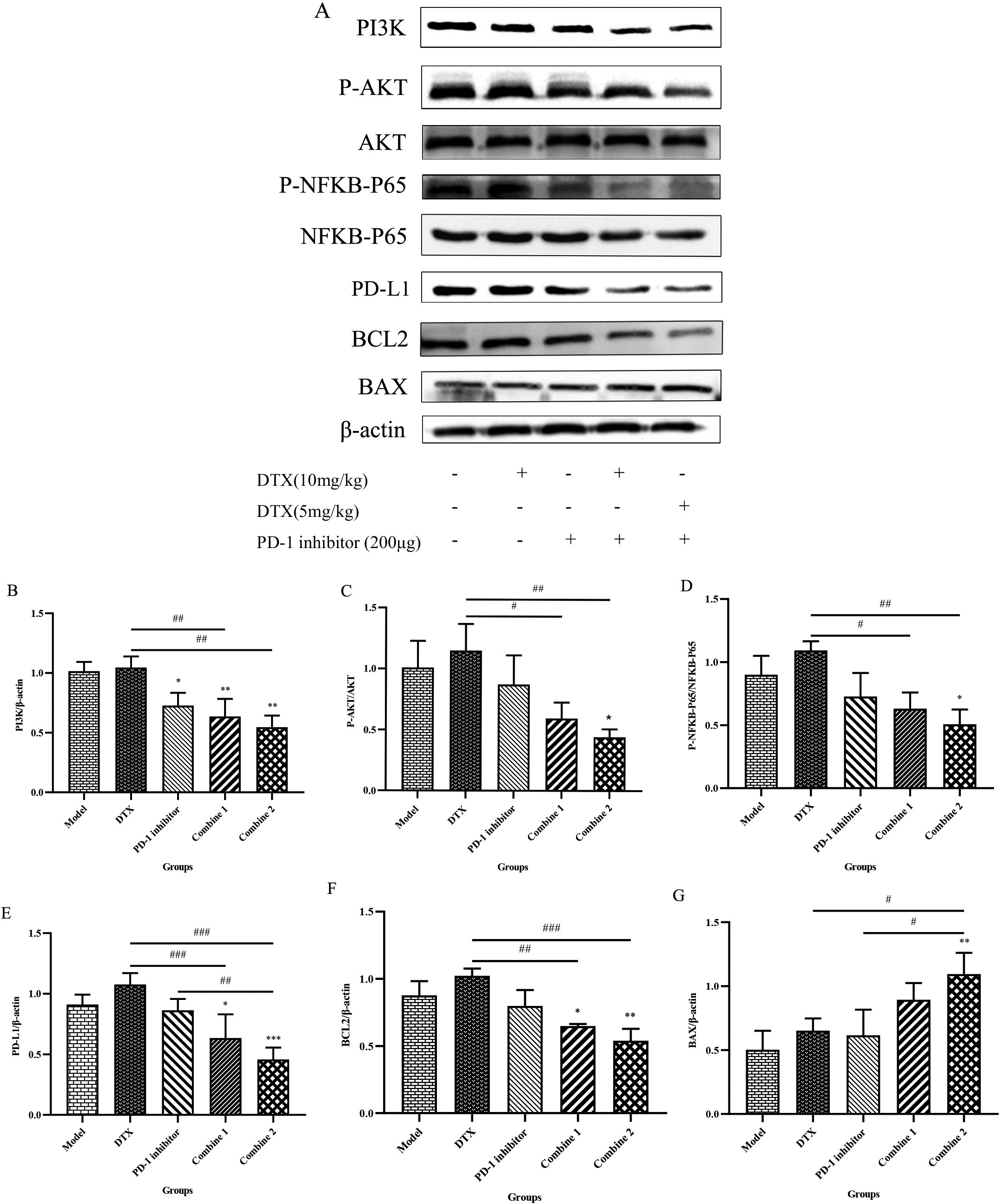
The above experimental data presented in this study have confirmed the good synergistic anticancer effect of PD-1 inhibitor combined with Docetaxel at both the animal and tissue level, and the possible mechanism of the synergistic effect will be explored at the molecular level next. Previous studies have confirmed that the activation of NFKB is associated with the biochemical progression of prostate cancer, and blocking NFKB expression may be a therapeutic target to inhibit prostate cancer proliferation [18, 19]. We examined the PI3K, P-AKT, AKT, P-NFKB-P65, NFKB-P65, PD-L1, BCL2 and BAX expression in the tumors by western blot to see the possible signaling pathway of their synergistic effects in combination treatment (Fig. 5A). The results showed that compared to Model group, Docetaxel alone induced higher PI3K, P-AKT, AKT, P-NFKB-P65, NFKB-P65, PD-L1, BCL2 and decreased BAX expression, which probably caused drug resistance or immune escape while causing anti-tumor effect at the same time, but other groups can progress the apoptosis protein of the tumor and inhibite the classic PI3K/AKT/NFKB-P65 signaling pathway (Fig. 5B & C & D). Besides, in the combine groups, PD-1 inhibitor combined with Docetaxel reversed the results of Docetaxel activeation of PI3K/AKT/NFKB-P65/PD-L1 axis and showed superior inhobitory results, which may explain why it’s workable to exerted synergistic anti-tumor effects, and this kind of reversation was more obvious (Fig. 5E & F & G).
Figure 5.
PD-1 inhibitor combined with Docetaxel exerted synergistic effects by inhibiting the NFKB-P65/PD-L1 signaling pathway. (A) Tumors were harvested after mice being sacrificed. The prepared protein samples were subjected to western blot in 10% separation gel and 5% concentrated gel, incubated overnight with Anti-PI3K, Anti-AKT, Anti-BCL2, Anti-BAX, Anti-NF-kB p65, Anti-P-NF-kB p65 Rabbit pAb and Anti-PD-L1 Rabbit pAb, and prtein bands were visualized with the AlphaImager HP gel imaging system. (B) The gels of PI3K and
4.Discussion
Docetaxel is a taxel compound with broad-spectrum antitumor activity [20]. In two prospective phase III trials, Docetaxel showed obvious survival benefits, and the median survival of patients was prolonged. Docetaxel was approved by FDA as a first-line chemotherapy drug [21, 22]. Some other studies have found in other cancer patients that the combination of immunosuppressants and chemotherapy drugs can effectively prolong the progression free survival rate and the overall survival rate without increasing toxicity [23]. Tuyen et al. [24] found that the combined treatment of chemotherapy drug gemcitabine and PD-1 inhibitor in liver cancer mice was helpful to enhance the immune response and significantly prolong the median survival time. The results of this in-vivo study demonstrated that PD-1 inhibitor combined with Docetaxel is a safe and effective combination therapy for prostate cancer in mice. This regimen can synergistically inhibit the tumor growth rate of tumor-bearing mice, promote the killing effect of mouse immune system on tumor, and significantly prolong the survival time of mice without affecting body weight and visceral poison.
In recent years, immune checkpoint inhibitors (ICIS) targeting the PD-1/PD-L1 axis have become one of the main directions of cancer immunotherapy to reverse immunosuppression and restore the antitumor activity of the immune system [25]. However, at present, the effect of immunotherapy for this cancer is limited, and only 15%
In addition, we also found that in high dose Docetaxel group, it will cause the activation of PI3K/AKT/NFKB-P65 /PD-L1 signaling pathway, perhaps may be associated with the development of drug resistance caused by Docetaxel, but this activation in combination groups is reversed, especially when downregulate Docetaxel for half dose then combined to PD-1 inhibition, the synergistic antitumor effect became more obvious. More and more studies have shown that PD-L1 is upregulated in prostate cancer tissues and is associated with poor prognosis [32, 33]. Previous studies have identified NF-KB as a probal target for prostate cancer therapy because it plays a role in tumorigenesis and therapy-resistant [34]. The activation of the PI3K/AKT signaling pathway binds to downstream effectors to control protein synthesis, transcription, cell survival, apoptosis, proliferation, autophagy, and metabolism, and plays an improtant role in drug resistance mechanisms of prostate cancer [35]. Some studies have confirmed that this signaling pathway is activated after paclitaxel-type chemotherapy treatment to produce drug resistance, triggering the cancer cell survival of the anti-apoptotic pathway [36, 37, 38]. NFKB is a downstream signaling molecule of the AKT signaling pathway, and its activation is PI3K/AKT-dependent [39].Hence it may indicate us that inhibit the activation of NFKB-P65 could be a workable therapy to deal with the drug-resistence caused by chenmotherapy.
Therefore, we established a prostate adenocarcinoma model at the animal level, and through the combination of PD-1 inhibitors to treat prostate cancer, we blocked the interaction between PD-1 and PD-L1, and observed whether the adverse reactions caused by the upregulation of PD-L1 by chemotherapeutic drugs could be reversed. This experiment confirms the synergistic effect of the combination of the two in the treatment of mouse prostate cancer. Then we test the expression of PI3K, AKT, P-NFKB-P65, NFKB-P65, PD-L1 by western blot. As the results showed, although the chemotherapy Docetaxel used alone induce the upregulation of these protein amount, the combine group can reverse the drug-fast by decreasing this signaling pathway. That’s to say this kind of synergistic effect is actualized by inhibiting PI3K/AKT/NFKB-P65/PD-L1 signaling pathway, which may become a key treatment target of the prostate cancer. In addition, we tested the expression of BCL2 and BAX, which are the key factors of the cell apoptosis. BCL2 is a member of anti-apoptotic family, inhibiting apoptosis by blocking pro-apoptotic family members and plays a role in chemoresistance [40]. The BAX gene is the most important apoptotic gene in human body and belongs to the BCL2 gene family. The encoded BAX protein can form heterodimers with BCL2 and produce an inhibitory effect on BCL2. There is study finding that the change expresion of BAX and BCL2 is related to the development of prostate cancer [41]. As the western blot results showed, after combine treatment, the BAX were upregulated and the anti-apoptotic BCL2 were decreased, which meant a great effect on inducing tumor cell death.
This is the first study to explore the anti-tumor effect of PD-1 inhibitor Camrelizumab combined with Docetaxel and the role of PI3K/AKT/NFKB-P65/PD-L1 signaling pathway in the treatment process in mice prostate cancer caused by RM-1 prostate cancer cell. Our experiment revealed that immune-checkpoint inhibitors: PD-1 inhibitor combined with chemotherapy medicine: Docetaxel can work together to strengthen the anti-tumor results, improve the survival percentage of mice and bring no toxicity. This synergistic effect is associated with the recover of exhausted CD4+ and CD8+T cells by downregulating the expression of PI3K/AKT/NFKB-P65/PD-L1 signaling pathway, and promote the apoptosis of tumor cell. We hope our studey could provide experimental data and research basis for the new clinical treatment scheme for male prostate cancer.
Acknowledgments
All funding for the report came from the Natural Science Foundation of Jiangxi Province (Science and Technology Bureau of Yichun City, ¥60,000, NO20202BABL206079). The fundation provided the money, drugs, animals, and some reagents needed for the experiment.This study was approved by the Medical Ethics Committee of Yichun University (NO.2022 (027)).
Author contributions
ZS designed and carried out all the experiment, tested the results, analyzed the data and wrote the manuscript, had full responsibility for all aspects of the work. WB and XY helped the part of animal experiment. WY, DP and CY helped record the data and some tests. XH helped provide drugs. CC and YW helped supervision and final approval of the version to be submitted.The authors declare that they have no competing interests, and all authors should confirm its accuracy.
References
[1] | R.L. Siegel, K.D. Miller, H.E. Fuchs et al., Cancer statistics, CA: A Cancer Journal for Clinicians 72: ((2022) ), 7–33. |
[2] | C. Zijie and L. Qiang, Understanding the Global Cancer Statistics 2018: implications for cancer control. Science China (Life Sciences) 64: (6) ((2021) ), 1017–1020. |
[3] | A.J. Evans, Treatment effects in prostate cancer, Modern Pathology 31: (1) ((2018) ), 110–121. |
[4] | I.F. Tannock, W.R. De, W.R. Berry et al., Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer, New England Journal of Medicine 351: (15) ((2004) ), 1502–1512. |
[5] | X. Jiang, J. Wang, X. Deng et al., Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape, Molecular Cancer 18: (1) ((2019) ). 1–17. |
[6] | F. Xie, M. Xu, J. Lu et al., The role of exosomal PD-L1 in tumor progression and immunotherapy, Molecular Cancer 18: (1) ((2019) ), 1–10. |
[7] | X. Wu, Z. Gu, Y. Chen et al., Application of PD-1 blockade in cancer immunotherapy, Computational and Structural Biotechnology Journal 17: ((2019) ), 661–674. |
[8] | R. Zhao, Y. Song, Y. Wang et al., PD-1/PD-L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway, Cell Proliferation 52: (3) ((2019) ), e12571. |
[9] | Z. Wang, X. Zhang, W. Li et al., ATM/NEMO signaling modulates the expression of PD-L1 following Docetaxel chemotherapy in prostate cancer, Journal for Immunotherapy of Cancer 9: (7) ((2021) ). |
[10] | T. Li and G. Wang, Computer-aided targeting of the PI3K/Akt/mTOR pathway: Toxicity reduction and therapeutic opportunities, International Journal of Molecular Sciences 15: (10) ((2014) ), 18856–18891. |
[11] | N. Torrealba, R. Vera, B. Fraile et al., TGF-β/PI3K/AKT/ mTOR/NF-kB pathway. Clinicopathological features in prostate cancer, The Aging Male ((2019) ). |
[12] | Y. Cao, Q. Wang, Y. Du et al., L-arginine and docetaxel synergistically enhance anti-tumor immunity by modifying the immune status of tumor-bearing mice, International Immunopharmacology 35: ((2016) ), 7–14. |
[13] | C.E. Gao, M. Zhang, Q. Song et al., PD-1 inhibitors dependent CD8+ T cells inhibit mouse colon cancer cell metastasis, OncoTargets and Therapy 12: ((2019) ), 6961. |
[14] | T.K. Choueiri, D.J. Figueroa, A.P. Fay et al., Correlation of PD-L1 Tumor Expression and Treatment Outcomes in Patients with Renal Cell Carcinoma Receiving Sunitinib or Pazopanib: Results from COMPARZ, a Randomized Controlled TrialPD-L1 Correlation with Outcome in RCC Patients in COMPARZ, Clinical Cancer Research 21: (5) ((2015) ), 1071–1077. |
[15] | W. Zhang, J. Xin, J. Lai et al., LncRNA LINC00184 promotes docetaxel resistance and immune escape via miR-105-5p/PD-L1 axis in prostate cancer, Immunobiology 227: (1) ((2022) ), 152163. |
[16] | Y. Han, D. Liu and L. Li, PD-1/PD-L1 pathway: current researches in cancer, American Journal of Cancer Research 10: (3) ((2020) ), 727. |
[17] | H. Maulhardt, A. Marin, H. Hesseltine et al., Submicron particle docetaxel intratumoral injection in combination with anti-mCTLA-4 into 4T1-Luc orthotopic implants reduces primary tumor and metastatic pulmonary lesions, Medical Oncology 38: (9) ((2021) ), 1–13. |
[18] | J.R. Cansino, R. Vera, F.R.D. Bethencourt et al., Prostate specific antigen and NF-kB in prostatic disease: relation with malignancy, Actas Urol Esp 35: ((2011) ), 16–21. |
[19] | M. Zupancic, B. Pospihalj, S. Cerovic et al., Significance of nuclear factor – kappa beta activation on prostate needle biopsy samples in the evaluation of Gleason score 6 prostatic carcinoma indolence, Radiol Oncol 54: ((2020) ), 194–200. |
[20] | L.A. Kraus, S.K. Samuel, S.M. Schmid et al., The mechanism of action of Docetaxel (Taxotere®) in xenograft models is not limited to bcl-2 phosphorylation, Investigational New Drugs 21: (3) ((2003) ), 259–268. |
[21] | D.P. Petrylak, C.M. Tangen, M.H.A. Hussain et al., Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer, New England Journal of Medicine 351: (15) ((2004) ), 1513–1520. |
[22] | I.F. Tannock, W.R. De, W.R. Berry et al., Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer, New England Journal of Medicine 351: (15) ((2004) ), 1502–1512. |
[23] | H. Yu, P. Chen, L. Xia et al., PD-1/PD-L1 inhibitor plus chemotherapy versus bevacizumab plus chemotherapy in first-line treatment for non-squamous non-small-cell lung cancer, Journal for Immunotherapy of Cancer 9: (11) ((2021) ). |
[24] | T.T.B. Ho, A. Nasti, A. Seki et al., Combination of gemcitabine and anti-PD-1 antibody enhances the anticancer effect of M1 macrophages and the Th1 response in a murine model of pancreatic cancer liver metastasis, Journal for Immunotherapy of Cancer 8: (2) ((2020) ). |
[25] | Y. Xu, G. Song, S. Xie et al., The roles of PD-1/PD-L1 in the prognosis and immunotherapy of prostate cancer, Molecular Therapy 29: (6) ((2021) ), 1958–1969. |
[26] | T. Kamada, Y. Togashi, C. Tay et al., PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer, Proceedings of the National Academy of Sciences 116: (20) ((2019) ), 9999–10008. |
[27] | S. Champiat, R. Ferrara, C. Massard et al., Hyperprogressive disease: recognizing a novel pattern to improve patient management, Nature Reviews Clinical Oncology 15: (12) ((2018) ), 748–762. |
[28] | P. Zhang, D.M. Su, M. Liang et al., Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis, Molecular Immunology 45: (5) ((2008) ), 1470–1476. |
[29] | L. Tran, C.T. Allen, R. Xiao et al., Cisplatin Alters Antitumor Immunity and Synergizes with PD-1/PD-L1 Inhibition in Head and Neck Squamous Cell CarcinomaCisplatin, PD-1, and Antitumor Immunity in Head and Neck Cancer, Cancer Immunology Research 5: (12) ((2017) ), 1141–1151. |
[30] | S. Grabosch, M. Bulatovic, F. Zeng et al., Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles, Oncogene 38: (13) ((2019) ), 2380–2393. |
[31] | D.K.L. Van, G. Goel, K. Ramanan et al., 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers, Journal for Immunotherapy of Cancer 4: (1) ((2016) ), 1–8. |
[32] | H. Gevensleben, D. Dietrich, C. Golletz et al., The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate CancerPD-L1 Is Highly Expressed in Primary Prostate Cancer, Clinical Cancer Research 22: (8) ((2016) ), 1969–1977. |
[33] | S. Gan, J. Ye, J. Li et al., LRP11 activates β-catenin to induce PD-L1 expression in prostate cancer, Journal of Drug Targeting 28: (5) ((2020) ), 508–515. |
[34] | D. Verzella, M. Fischietti, D. Capece et al., Targeting the NF-κB pathway in prostate cancer: a promising therapeutic approach? Current Drug Targets 17: (3) ((2016) ), 311–320. |
[35] | T. Pungsrinont, J. Kallenbach and A. Baniahmad, Role of PI3K-AKT-mTOR pathway as a pro-survival signaling and resistance-mediating mechanism to therapy of prostate cancer, International Journal of Molecular Sciences 22: (20) ((2021) ), 11088. |
[36] | D.Z. Qian, B.L.S. Rademacher, J. Pittsenbarger et al., CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel-induced cytotoxicity, The Prostate 70: (4) ((2010) ), 433–442. |
[37] | K.K. Brown, L. Montaser-Kouhsari, A.H. Beck et al., MERIT40 is an Akt substrate that promotes resolution of DNA damage induced by chemotherapy, Cell Reports 11: (9) ((2015) ), 1358–1366. |
[38] | B.R. Davies, H. Greenwood, P. Dudley et al., Preclinical Pharmacology of AZD5363, an Inhibitor of AKT: Pharmacodynamics, Antitumor Activity, and Correlation of Monotherapy Activity with Genetic BackgroundAZD5363, an Oral Inhibitor of AKT, Molecular Cancer Therapeutics 11: (4) ((2012) ), 873–887. |
[39] | V.S. Reddy, R.E. Harskamp, M.W. Van Ginkel et al., Interleukin-18 stimulates fibronectin expression in primary human cardiac fibroblasts via PI3K-Akt-dependent NF-κB activation, Journal of Cellular Physiology 215: (3) ((2008) ), 697–707. |
[40] | S. Maji, S. Panda, S.K. Samal et al., Bcl-2 antiapoptotic family proteins and chemoresistance in cancer, Advances in Cancer Research 137: ((2018) ), 37–75. |
[41] | N. Torrealba, G. Rodriguez-Berriguete, B. Fraile et al., PI3K pathway and Bcl-2 family. Clinicopathological features in prostate cancer, The Aging Male 21: (3) ((2018) ), 211–222. |




