Can serum ferritin serve as a biomarker for the prognosis of gynecological malignant tumors? A retrospective cohort study
Abstract
PURPOSE:
It is widely accepted that there is a strong relationship between iron levels and cancer. This study aimed to investigate the relationship between serum ferritin levels and the severity and prognosis of gynecological malignant tumors.
METHODS:
This retrospective study included patients with gynecological malignant tumors at Sir Run Run Shaw Hospital in the Department of Obstetrics and Gynecology from January 2013 to June 2019. Patients were grouped according to their serum ferritin level: low (
RESULTS:
The 402 total patients were divided into a low (
CONCLUSION:
Although this study did not find serum ferritin to be a significant independent prognosis indicator in gynecological malignant tumors, this study did identify that gynecological malignant tumor patients with high serum ferritin levels have significantly less survival time than patients with low or normal serum ferritin levels.
1.Introduction
Gynecological cancer is one of the main causes of cancer-related mortality in females worldwide [1, 2, 3, 4, 5]. According to the global incidence statistics on new cancer cases in women for the year 2020, there were 314,000 cases of ovarian cancer, 604,000 cases of cervical cancer, and 417,000 cases of endometrial cancer. These malignancies represented 3.4%, 6.5%, and 4.5% of the total female cancer cases, respectively. Correspondingly, the number of deaths attributed to these cancers was 207,000 for ovarian cancer, 342,000 for cervical cancer, and 97,000 for endometrial cancer. These mortality figures accounted for 4.7%, 7.7%, and 2.2% of the overall female cancer-related deaths, respectively [6]. With the rate of gynecological cancer increasing every year [4, 5], there is an urgent need for new biomarkers for the screening, therapy selection, or improving the diagnostic, prognostic performance for gynecological cancer.
The high mortality rate of gynecological cancer can be attributed to two factors [7, 8, 9, 10, 11, 12, 13]: vague and non-specific symptoms, and current biomarkers have low sensitivity and specificity. Both of these factors lead to patients being diagnosed in advanced metastatic stages when the prognosis is poor [7, 10, 11, 12]. Ovarian tumors especially are difficult to diagnose due to these non-specific symptoms and are at most times diagnosed when the tumor mass has reached large proportions, when complications occur, or when disseminated into neighboring organs [7]. Gynecological malignancies, particularly ovarian cancer, exhibit a pronounced mortality burden attributed to challenges associated with early detection [14]. Cancer antigen 125 (CA125) is the most widely used blood biomarker for the progression and monitoring of cancer in general, but it cannot be used to screen ovarian cancer because of too high false-positive rates due to its low specificity and sensitivity. CA125 is also expressed in patients with benign gynecological disorders and epithelial cells; thus, CA125’s low specificity and sensitivity for the early-stage disease are extremely limited [7]. Due to these two factors, it is critical to identify a marker that can be used to improve the current biomarkers’ diagnostic and prognostic values.
Ferritin is a cytosolic protein used for iron storage and is involved in proliferation, angiogenesis, immunosuppression, and carcinogenesis. Serum ferritin is commonly elevated in many cancers, and its increased levels are associated with poor prognosis in cancer patients. Studies have suggested that ferritin might be used as a diagnostic and prognostic biomarker for various malignancies. Studies have also shown that ferritin can discriminate cancer patients, even during early-stage disease, and therefore might be a valuable biomarker and diagnostic marker [13, 15, 16, 17]. With this in mind, this study aimed to investigate the relationship between serum ferritin levels and the severity and prognosis of patients with gynecological malignant tumors.
Hence, the prognostic value of serum ferritin expression was evaluated against other well-known prognostic factors in cancer [18, 19, 20, 21, 22, 23], including CA125, albumin (ALB), C-reactive protein (CRP), carcinoembryonic antigen (CEA), hemoglobin (Hb), white blood cell (WBC), alpha-fetoprotein (AFP), cancer antigen 153 (CA153), and alanine aminotransferase (ALT). ALB is a common indicator of nutritional status, while CRP is a marker that correlates with inflammation [18]. Studies have shown that CEA has the highest sensitivity among other common diagnostic markers in cancer [20]. AFP was one of the first protein tumor markers discovered and is not only used for screening and diagnosis of cancers but also as means to drive therapeutic choice and to monitor treatment [21]. CA153 is a glycoprotein commonly upregulated in epithelial carcinomas, including breast cancer and ovarian cancer [23]. It is an independent predictor of cancer recurrence, a strong prognostic indicator for patients at advanced-stage breast cancer, and the best biomarker in diagnosing pleural effusions in lung cancer [22, 23].
2.Methods
2.1Study design and participants
This retrospective, observational, single-center cohort study included patients with gynecological malignant tumors at Sir Run Run Shaw Hospital (Hangzhou, Zhejiang, China) in the Department of Obstetrics and Gynecology from January 2013 to June 2019. Gynecological cancer patients (endometrial, cervical, or ovarian) who were diagnosed by obstetricians and gynecologists according to international guidelines and in relatively good physical condition with an expected survival time of more than one year were included. Patients already treated with radiotherapy and chemotherapy, that had undergone surgery, with acute or were critically ill (including infection or organ failure), with no available serological data, or with pathological results that suggested a primary tumor that was a non-gynecological malignancy were excluded from the study.
The data included in this study contains patients with gynecological malignant tumors and their age, cancer type, menopause status, BMI, FIGO stage (FIGO 2009) [24], lymphatic metastasis, tumor size, and other baseline characteristics.
This study was approved by the Ethics Committee of Sir Run Run Shaw Hospital (approval No. 2022-610-01). Informed consent was waived by the ethics committee.
2.2Biochemistry
Peripheral blood samples were collected prior to any anti-cancer treatment with the following considered to be the normal range: ALB, 40–55 g/L; Hb, 115–150 g/L; mean corpuscular volume (MCV), 82–100 fL; WBC, 3.5–9.5
2.3Follow-up and outcomes
Follow-up was conducted in the outpatient clinic or by telephone and was ended in June 2019. The primary outcome of this study was death due to gynecological cancer. Overall survival (OS) was defined as the time from the first visit to the date of all-cause death or the last follow-up. When the patient was lost to follow-up, the patient was censored. The survival time of censored patients was defined by the period from the first visit to the last day of follow-up or to the date on which survival was investigated. The exact date of death was recorded from a relative or by a medical report.
Table 1
Clinical characteristics of patients with gynecological malignant tumor and serum ferritin expression
| Characteristic | Serum ferritin* |
| |||
|---|---|---|---|---|---|
| Low ( | Normal ( | High ( | |||
| Age | 73.432 | ||||
| | 37 | 150 | 85 | ||
| | 0 | 32 | 98 | ||
| Cancer type | 24.429 | ||||
| Endometrial | 14 | 54 | 58 | ||
| Cervical | 17 | 71 | 38 | ||
| Ovarian | 6 | 57 | 87 | ||
| Menopause | 120.122 | ||||
| No | 35 | 94 | 21 | ||
| Yes | 2 | 88 | 162 | ||
| BMI (kg/m2) | 5.401 | 0.249 | |||
| | 2 | 6 | 13 | ||
| 18.5–24 | 23 | 96 | 86 | ||
| | 12 | 80 | 84 | ||
| ALB (g/L) | 9.824 | 0.007 | |||
| | 8 | 40 | 66 | ||
| | 29 | 142 | 117 | ||
| WBC ( | 2.889 | 0.577 | |||
| | 1 | 4 | 3 | ||
| 3.5–9.5 | 34 | 172 | 167 | ||
| | 2 | 6 | 13 | ||
| Hb (g/L) | 33.851 | ||||
| | 22 | 28 | 45 | ||
| 115–150 | 15 | 153 | 136 | ||
| | 0 | 1 | 2 | ||
| MCV (fL) | 68.84 | ||||
| | 20 | 19 | 10 | ||
| | 17 | 163 | 173 | ||
| CRP (mg/L) | 32.625 | ||||
| | 33 | 150 | 108 | ||
| | 4 | 30 | 75 | ||
| ALT (U/L) | 0.757 | 0.685 | |||
| | 36 | 171 | 174 | ||
| | 1 | 11 | 9 | ||
| CA125 (U/mL) | 18.623 | ||||
| | 27 | 122 | 86 | ||
| | 10 | 60 | 97 | ||
| CEA (ng/mL) | 1.273 | 0.529 | |||
| | 35 | 164 | 162 | ||
| | 2 | 18 | 21 | ||
| AFP (ng/mL) | 2.803 | 0.246 | |||
| | 36 | 177 | 182 | ||
| | 1 | 5 | 1 | ||
| CA153 (U/mL) | 25.987 | ||||
| | 33 | 156 | 121 | ||
| | 3 | 23 | 60 | ||
| FIGO stage | 49.694 | ||||
| I | 33 | 115 | 75 | ||
| II | 2 | 29 | 25 | ||
| III | 1 | 29 | 25 | ||
| IV | 1 | 9 | 37 | ||
| Lymphatic metastasis | 14.838 | 0.001 | |||
| No | 32 | 141 | 114 | ||
| Yes | 5 | 41 | 69 | ||
| Ascites | 21.628 | ||||
| No | 35 | 155 | 125 | ||
| Yes | 2 | 27 | 58 | ||
| Tumor size (cm)** | 3.223 | 0.2 | |||
| | 25 | 103 | 95 | ||
| | 12 | 79 | 88 | ||
2.4Statistical analysis
The chi-square test was used to compare the correlation between groups of classified data. Spearman rank correlation test was performed to assess the relationship between serum ferritin and other markers. Kaplan-Meier curves and the log-rank test were used to compare the expression level of serum ferritin and the prognosis of gynecological cancer patients. Right censoring was used in survival analysis if the survival time was incomplete. Univariable and multivariable Cox regression models were performed to evaluate the correlation between serum ferritin levels, clinical parameters, pathological parameters, biochemical parameters, and prognosis in patients with gynecological malignant tumors. Multivariate proportional hazards regression was performed with statistically significant factors from the univariate analysis (p-values less than 0.05) and controlling confounding factors, such as age and menopause. Hazard ratios were also calculated.
3.Results
3.1Participant enrollment
A total of 402 patients with gynecological malignant tumors were treated at our hospital during the study and included 126 patients with endometrial cancer, 126 patients with cervical cancer, and 150 patients with ovarian cancer. By the end of follow-up in June 2019, 29 patients died of gynecological malignant tumors, and 351 patients survived, 22 patients were lost to follow-up.
3.2Participant characteristics
The patients were grouped according to their serum ferritin level: low (
3.3Serum ferritin expression and its association with other clinical factors
We examined the subset of gynecological malignant tumor patients with different serum ferritin expressions (Table 1). All three low, normal, and high ferritin groups had more patients that expressed low cancer biomarkers (CEA, AFP, and CA153), high ALB levels, low serum levels (CRP and ALT), positive for ascites, and positive for lymphatic metastasis versus patients that did not. Differences existed within the groups. We identified the high ferritin level group had predominately more elderly patients, more ovarian cancer patients, and more patients that were premenopausal. We identified the low ferritin level group had no elderly patients (
3.4Correlations between serum ferritin and other factors
According to correlation analysis, ALB (
Table 2
Correlations between serum ferritin and other factors
| Characteristic | Correlation coefficients | |
|---|---|---|
| ALB | 0.004 | |
| WBC | 0.166 | 0.010 |
| Hb | 0.052 | 0.296 |
| MCV | 0.191 | |
| CRP | 0.439 | |
| ALT | 0.089 | 0.074 |
| CA125 | 0.253 | |
| CEA | 0.062 | 0.213 |
| AFP | 0.078 | 0.120 |
| CA153 | 0.281 |
ALB, albumin; WBC, white blood cell; Hb, hemoglobin; MCV, mean corpuscular volume; CRP, C-reactive protein; ALT, alanine aminotransferase; CA125, cancer antigen 125; CEA, carcinoembryonic antigen; AFP, alpha fetoprotein; CA153, cancer antigen 153.
3.5Survival analysis of expression levels of serum ferritin in gynecological cancer patients
By the end of the follow-up in June 2019, 29 patients had died, 351 patients survived, and 22 patients were censored. The Kaplan-Meier survival curves revealed that of the three groups analyzed, the high ferritin level group (
Figure 1.
High serum ferritin expression level in gynecological malignancies correlates with poor overall survival. The Kaplan-Meier survival curve for 402 patients revealed that the high ferritin level group (
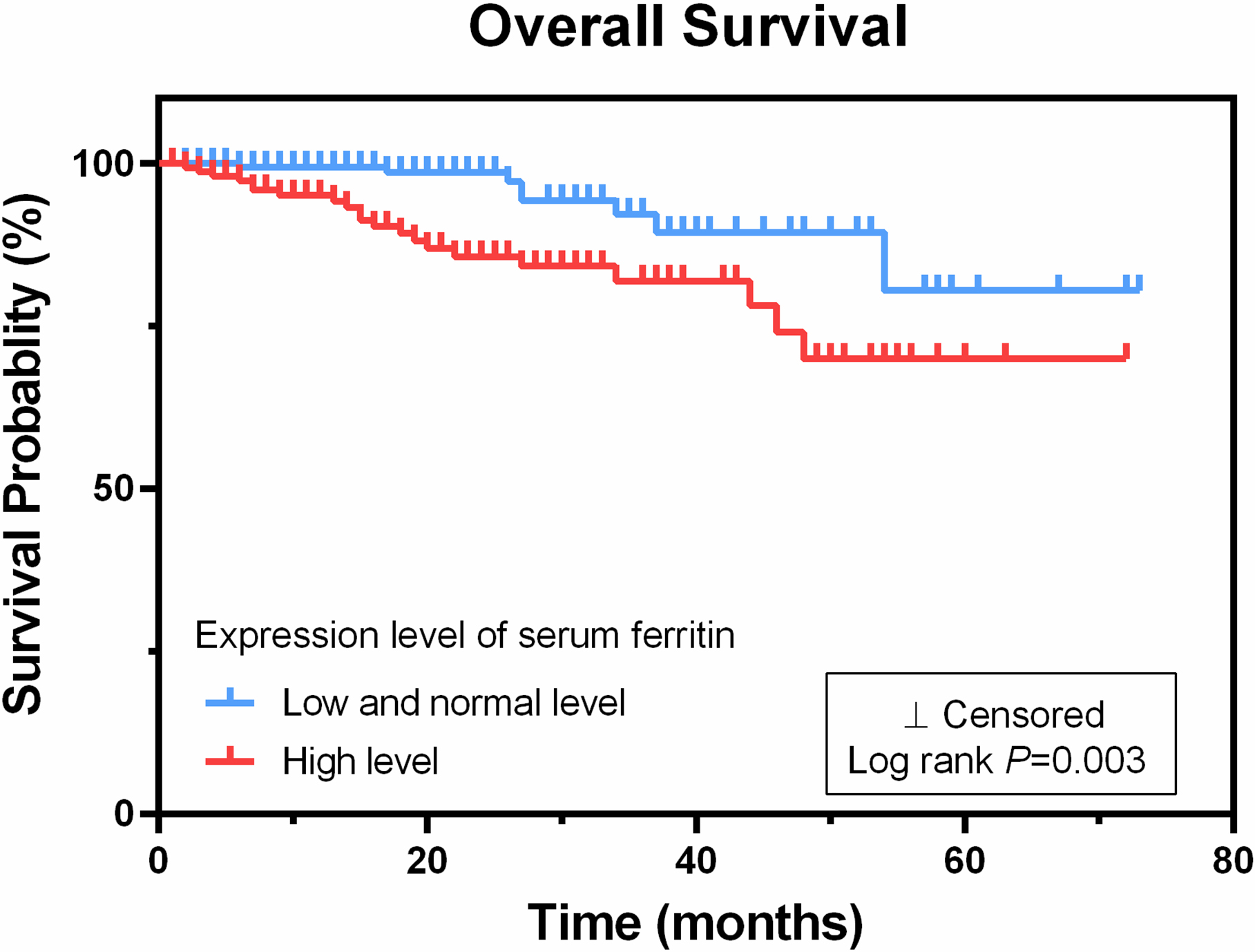
Table 3
Univariable and multivariable analysis in patients with gynecological malignant tumor for OS
| Clinicopathologic factors | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age | 3.964 | 1.885–8.336 | 2.847 | 1.108–7.32 | ||
| Menopause | 4.362 | 1.518–12.536 | 2.729 | 0.737–10.109 | 0.133 | |
| BMI index | 0.563 | 0.445–1.555 | 0.563 | |||
| Albumin | 0.113 | 0.048–0.267 | 0.824 | 0.291–2.33 | 0.715 | |
| WBC | 1.528 | 0.411–5.681 | 0.527 | |||
| Hb | 0.331 | 0.159–0.687 | 0.648 | 0.278–1.51 | 0.315 | |
| MCV | 1.701 | 0.470–6.156 | 0.418 | |||
| CRP | 11.918 | 4.842–29.334 | 7.644 | 2.215–26.374 | 0.001 | |
| ALT | 1.166 | 0.157–8.654 | 0.881 | |||
| CA125 | 9.055 | 3.144–26.075 | 5.445 | 1.365–21.727 | ||
| CEA | 3.917 | 1.780–8.620 | 0.001 | 4.492 | 1.759–11.471 | |
| AFP | 2.814 | 0.655–12.086 | 0.164 | |||
| CA153 | 4.601 | 2.212–9.572 | 1.564 | 0.637–3.84 | 0.329 | |
| Ferritin | 2.72 | 1.324–5.590 | 0.792 | 0.351–1.787 | 0.574 | |
| Lymphatic metastasis | 4.795 | 2.261–10.166 | 1.022 | 0.359–2.909 | 0.968 | |
| Ascites | 6.915 | 3.192–14.979 | 1.048 | 0.359–3.056 | 0.932 | |
| Tumor size | 2.201 | 1.016–4.766 | 0.395 | 0.154–1.012 | 0.053 | |
| FIGO stage | 2.989 | 2.058–4.342 | 1.235 | 0.301–5.074 | 0.77 | |
OS: overall survival; HR, hazard ratio; CI: confidence interval; BMI, body mass index; ALB, albumin; WBC, white blood cell; Hb, hemoglobin; MCV, mean corpuscular volume; CRP, C-reactive protein; ALT, alanine aminotransferase; CA125, cancer antigen 125; CEA, carcinoembryonic antigen; AFP, alpha fetoprotein; CA153, cancer antigen 153; FIGO, Inter-national Federation of Gynecology and Obstetrics.
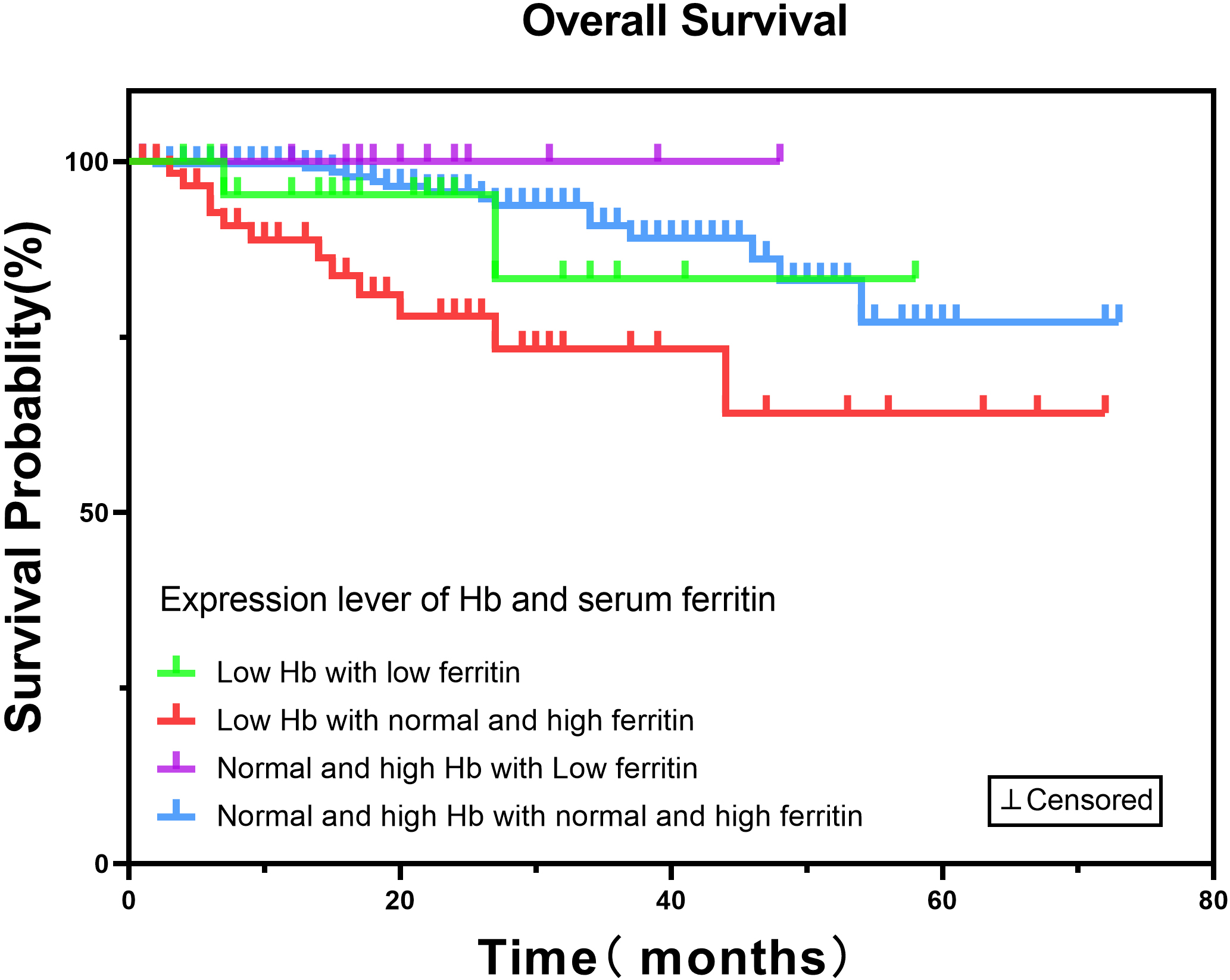
Figure 2.
Low hemoglobin (Hb) and Low serum ferritin expression level in gynecological malignancies has no impact on overall survival. The Kaplan-Meier survival curve for 402 patients revealed that the low Hb (
3.6Association of clinical factors and mortality
Univariable Cox regression analysis identified patients with a high expression level of ferritin (
3.7Independent prognosis indicators in gynecological malignancies
Multivariable Cox analyses after adjusting for age, menopause and other factors revealed four significant parameters as independent prognosis indicators in gynecological malignant tumors: age (HR
Figure 3.
A forest plot of significant independent prognosis indicators in gynecological malignancies. Multivariable Cox analyses revealed four significant parameters as independent prognosis indicators in gynecological malignant tumors: age (hazard ratio [HR]
![A forest plot of significant independent prognosis indicators in gynecological malignancies. Multivariable Cox analyses revealed four significant parameters as independent prognosis indicators in gynecological malignant tumors: age (hazard ratio [HR] = 2.847, 95% CI: 1.108–7.32, P< 0.05), C-reactive protein (CRP) (HR = 7.644, 95% CI: 2.215–26.374, P= 0.001), cancer antigen 125 (CA125) (HR = 5.445, 95% CI: 1.365–21.727, P< 0.05), and carcinoembryonic antigen (CEA) (HR = 4.492, 95% CI: 1.759–11.471, P< 0.05).](https://content.iospress.com:443/media/cbm/2024/39-2/cbm-39-2-cbm230040/cbm-39-cbm230040-g003.jpg)
4.Discussion
This study aimed to investigate the relationship between serum ferritin levels and the severity and prognosis of patients with gynecological malignant tumors. We found that the high ferritin level group had more elderly, menopausal, ovarian cancer patients, high CA125 levels, and more patients in FIGO stage IV, especially patients exhibiting high ferritin levels demonstrated a significantly poorer prognosis compared to their counterparts with lower or normal ferritin levels.
The relationship between ferritin levels and cancer may be due to the relationship between ferritin and oxidative stress [25, 26, 27, 28]. Yildirim et al. [19] found serum ferritin was significantly elevated in lymphatic metastatic lung cancer patients, which is similar to our study’s findings. Furthermore, Lee et al. [18] concluded patients with recurrent or refractory metastatic colorectal cancer had a high expression level of ferritin. Scutiero et al. [29] showed that iron accumulation leads to oxidative stress, causing DNA hypermethylation and histone modifications. These changes due to oxidative stress contribute to the malignant transformation process of cells.
In this study, correlation analyses were performed between serum ferritin level and other factors. WBC, MCV, CRP, CA125, and CA153 were significantly positively correlated with serum ferritin level, whereas ALB was negatively correlated. Based on these results, ferritin was closely related to nutrition, inflammation and tumor markers. This finding implies that the increased serum ferritin was significantly associated with poor survival quality. It was also suggested that ferritin can assist in the diagnosis of gynecological malignant tumors.
It is worth noting that there was no significant correlation between hemoglobin and ferritin in this study, which is consistent with the results of another study [18]. This may be related to the increase of ferritin as an acute phase protein in infection, malignant tumor or chronic inflammation [30]. In addition, when the loss of iron exceeds the absorption or the absorption is lower than the demand, the initial iron storage will be exhausted, resulting in the reduction of ferritin level, and hemoglobin is still normal at this time, which is called non-anaemic iron deficiency (NAID). However, when ferritin decreases further, the hemoglobin concentration will eventually fall below the lower limit of the normal range [31]. And in later Kaplan-Meier survival analyses of hemoglobin and serum ferritin expression revealed that anemia combined with low ferritin had no significant correlation with survival results. It may be related to previous findings that patients with low ferritin were relatively younger, had lower FIGO grade tumors, had fewer ovarian cancers, and thus recoverd more quickly.
The Kaplan-Meier survival analyses of serum erritin expression revealed that the high ferritin level group had significantly less survival time than normal and low ferritin level groups. It indicates that serum ferritin could predict a poor prognosis for patients with gynecological malignant tumors. The univariable Cox regression analysis identified patients with a high level of ferritin (
Though our study did not show significant data for ferritin as an independent prognosis indicator, other studies have. Orlandi et al. [32] showed serum concentrations of hepcidin and ferritin were satisfactory predictors of malignant breast cancer; High ferritin levels were associated with poor prognosis in breast cancer, lung cancer, pancreatic cancer, multiple myeloma, hepatocellular carcinoma and colorectal cancer patients [33].
Our study showed that there is a significant relationship between ferritin levels and gynecological cancer. Still, other studies indicate that there is a relationship between ferritin and anti-cancer efficacy. Ihlow et al. [34] showed that ferritin levels are related to the response to the course and duration of chemotherapy. Shi et al. [35] ferritin levels were related to platinum-based chemotherapy efficacy in patients with lung cancer. Despite an initial response to ovarian cancer therapy, most patients develop chemoresistance and ultimately terminal disease. Basuli et al. [36] reported that this drug resistance might be due to alterations in iron metabolism and retention in excess iron that contributes to tumor growth, thereby recommending a new strategy for the use of iron chelators with anticancer therapy. These studies suggest a significant relationship between ferritin and cancer therapy and may also suggest a need for iron therapeutic management in combination with anti-cancer therapy. It could drastically improve morbidity and mortality of gynecological cancer in the future.
This study has several limitations. The included patients were from one hospital only, resulting in a small sample size and limiting the statistical power or generalizability of the results. It may be particularly true since the population was homogeneous regarding ethnicity and the limited geographical area. We did not include the expression of other proteins related to iron metabolism that may be relevant to the study, such as transferrin or iron regulatory proteins. Finally, this was a retrospective study; therefore, the results should be considered preliminary. Though there were strengths to our study, including studying a combination of different gynecological cancers, a prospective, multicenter study with a larger study population is needed.
5.Conclusion
In this study focusing on gynecological malignancies, we identified a notable prevalence of elevated ferritin levels among elderly patients with menopausal gynecologic tumors. Specifically, individuals within the high-ferritin in the gynecologic tumor population faced an increased risk of mortality. These findings have substantial implications for prognostic assessment and therapeutic approaches in elderly patients with menopausal gynecologic tumors, particularly those afflicted with ovarian cancer, as ferritin levels hold potential as a valuable prognostic indicator. Moreover, patients exhibiting high ferritin levels demonstrated a significantly poorer prognosis compared to their counterparts with lower or normal ferritin levels. However, it is imperative to acknowledge that this study’s scope remains limited to data obtained from a single center, necessitating further validation through extensive, prospective, and multi-center clinical trials.
Ethics approval
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by Taizhou Hospital of ZheJiang Province. This article is a retrospective study. Therefore the Institutional waived the requirement to obtain distinct written informed consent from the patients.
Consent for publication
Not applicable
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was supported by Zhejiang Provincial Medical and Healthy Science and Technology Projects (WKJ-ZJ-2125) and Taizhou Science and Technology Plan Project (23ywb52).
Authors’ contributions
Conception: WZ.
Interpretation or analysis of data: WZ and QC.
Preparation of the manuscript: WZ and QC.
Revision for important intellectual content: YC, JT, RT and MW.
Supervision: JY and YP.
Acknowledgments
None.
References
[1] | P. Gaona-Luviano, L. Medina-Gaona and K. Magaña-Pérez, Epidemiology of ovarian cancer, Chinese Clinical Oncology 9: ((2020) ), 47. |
[2] | C. Chibwesha and J. Stringer, Cervical Cancer as a Global Concern: Contributions of the Dual Epidemics of HPV and HIV, JAMA 322: ((2019) ), 1558–1560. |
[3] | Y. Fan, M. Wang, Y. Mu, S. Mo, A. Zheng and J. Li, Ovarian metastasis in women with cervical carcinoma in stages IA to IIB: A systematic review and meta-analysis, Medicine 99: ((2020) ), e21146. |
[4] | F. Bray, J. Ferlay, I. Soerjomataram, R. Siegel, L. Torre and A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA: A Cancer Journal for Clinicians 68: ((2018) ), 394–424. |
[5] | J. Ferlay, H. Shin, F. Bray, D. Forman, C. Mathers and D. Parkin, Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008, International Journal of Cancer 127: ((2010) ), 2893–2917. |
[6] | H. Sung, J. Ferlay, R. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA: A Cancer Journal for Clinicians 71: ((2021) ), 209–249. |
[7] | J. Zhao, N. Guo, L. Zhang and L. Wang, Serum CA125 in combination with ferritin improves diagnostic accuracy for epithelial ovarian cancer, British Journal of Biomedical Science 75: ((2018) ), 66–70. |
[8] | A. Dijmrescu, V. Gheorman, M. Manolea, S. Vrabie, M. Săndulescu, C. Siloşi, I. Siloşi, M. Radu, M. Popescu-Drigă, M. Novac, V. Pădureanu, A. Istrate-Ofiţeru and L. Boldeanu, Serological and immunohistochemical biomarkers for discrimination between benign and malignant ovarian tumors, Romanian Journal of Morphology and Embryology = Revue Roumaine de Morphologie et Embryologie 60: ((2019) ), 1163–1174. |
[9] | L. Brunette, P. Mhawech-Fauceglia, L. Ji, J. Skeate, H. Brand, K. Lawrenson, S. Walia, M. Chiriva-Internati, S. Groshen, L. Roman, W. Kast and D. Da Silva, Validity and prognostic significance of sperm protein 17 as a tumor biomarker for epithelial ovarian cancer: a retrospective study, BMC Cancer 18: ((2018) ), 970. |
[10] | B. Reid, J. Permuth and T. Sellers, Epidemiology of ovarian cancer: a review, Cancer Biology & Medicine 14: ((2017) ), 9–32. |
[11] | K. Matsuo, H. Machida, R. Mandelbaum, B. Grubbs, L. Roman, A. Sood and D. Gershenson, Mucinous borderline ovarian tumor versus invasive well-differentiated mucinous ovarian cancer: Difference in characteristics and outcomes, Gynecologic Oncology 153: ((2019) ), 230–237. |
[12] | C. Chen, T. Markossian, A. Silva and Y. Tarasenko, Epithelial ovarian cancer mortality among Hispanic women: Sub-ethnic disparities and survival trend across time: An analysis of SEER 1992–2013, Cancer Epidemiology 52: ((2018) ), 134–141. |
[13] | A. Moreira, G. Mesquita and M. Gomes, Ferritin: An Inflammatory Player Keeping Iron at the Core of Pathogen-Host Interactions, Microorganisms 8: ((2020) ). |
[14] | H. Jeong, M. Kwon and Y. Shin, Overexpression of Cancer-Associated Genes via Epigenetic Derepression Mechanisms in Gynecologic Cancer, Frontiers in Oncology 4: ((2014) ), 12. |
[15] | Y. Chi, J. Remsik, V. Kiseliovas, C. Derderian, U. Sener, M. Alghader, F. Saadeh, K. Nikishina, T. Bale, C. Iacobuzio-Donahue, T. Thomas, D. Pe’er, L. Mazutis and A. Boire, Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis, Science (New York, N.Y.) 369: ((2020) ), 276–282. |
[16] | Y. Okazaki, S. Chew, H. Nagai, Y. Yamashita, H. Ohara, L. Jiang, S. Akatsuka, T. Takahashi and S. Toyokuni, Overexpression of miR-199/214 is a distinctive feature of iron-induced and asbestos-induced sarcomatoid mesothelioma in rats, Cancer Science 111: ((2020) ), 2016–2027. |
[17] | G. Camiolo, A. Barbato, C. Giallongo, N. Vicario, A. Romano, N. Parrinello, R. Parenti, J. Sandoval, D. García-Moreno, G. Lazzarino, R. Avola, G. Palumbo, V. Mulero, G. Li Volti, D. Tibullo and F. Di Raimondo, Iron regulates myeloma cell/macrophage interaction and drives resistance to bortezomib, Redox Biology 36: ((2020) ), 101611. |
[18] | S. Lee, A. Song and W. Eo, Serum Ferritin as a Prognostic Biomarker for Survival in Relapsed or Refractory Metastatic Colorectal Cancer, Journal of Cancer 7: ((2016) ), 957–964. |
[19] | A. Yildirim, M. Meral, H. Kaynar, H. Polat and E. Ucar, Relationship between serum levels of some acute-phase proteins and stage of disease and performance status in patients with lung cancer, Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 13: ((2007) ), CR195–200. |
[20] | Y. Gao, J. Wang, Y. Zhou, S. Sheng, S. Qian and X. Huo, Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer, Scientific Reports 8: ((2018) ), 2732. |
[21] | C. Sauzay, A. Petit, A. Bourgeois, J. Barbare, B. Chauffert, A. Galmiche and A. Houessinon, Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma, Clinica Chimica Acta; International Journal of Clinical Chemistry 463: ((2016) ), 39–44. |
[22] | X. Wang, Y. Wu, M. Wang and Y. Wang, CEA, AFP, CA125, CA153 and CA199 in malignant pleural effusions predict the cause, Asian Pacific Journal of Cancer Prevention: APJCP 15: ((2014) ), 363–368. |
[23] | S. Zhao, Y. Mei, Y. Wang, J. Zhu, G. Zheng and R. Ma, Levels of CEA, CA153, CA199, CA724 and AFP in nipple discharge of breast cancer patients, International Journal of Clinical and EXPERIMENTAL MEDicine 8: ((2015) ), 20837–20844. |
[24] | S. Pecorelli, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium, International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics 105: ((2009) ), 103–104. |
[25] | X. Fang, Z. Cai, H. Wang, D. Han, Q. Cheng, P. Zhang, F. Gao, Y. Yu, Z. Song, Q. Wu, P. An, S. Huang, J. Pan, H. Chen, J. Chen, A. Linkermann, J. Min and F. Wang, Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis, Circulation Research 127: ((2020) ), 486–501. |
[26] | M. Wood, V. Gadd, L. Powell, G. Ramm and A. Clouston, Ductular reaction in hereditary hemochromatosis: the link between hepatocyte senescence and fibrosis progression, Hepatology (Baltimore, Md.) 59: ((2014) ), 848–857. |
[27] | A. Picca, R. Mankowski, G. Kamenov, S. Anton, T. Manini, T. Buford, S. Saini, R. Calvani, F. Landi, R. Bernabei, E. Marzetti and C. Leeuwenburgh, Advanced Age Is Associated with Iron Dyshomeostasis and Mitochondrial DNA Damage in Human Skeletal Muscle, Cells 8: ((2019) ). |
[28] | S. Bertoli, E. Paubelle, E. Bérard, E. Saland, X. Thomas, S. Tavitian, M. Larcher, F. Vergez, E. Delabesse, A. Sarry, F. Huguet, C. Larrue, C. Bosc, T. Farge, J. Sarry, M. Michallet and C. Récher, Ferritin heavy/light chain (FTH1/FTL) expression, serum ferritin levels, and their functional as well as prognostic roles in acute myeloid leukemia, European Journal of Haematology 102: ((2019) ), 131–142. |
[29] | G. Scutiero, P. Iannone, G. Bernardi, G. Bonaccorsi, S. Spadaro, C. Volta, P. Greco and L. Nappi, Oxidative Stress and Endometriosis: A Systematic Review of the Literature, Oxidative Medicine and Cellular Longevity 2017: ((2017) ), 7265238. |
[30] | S. Bouri and J. Martin, Investigation of iron deficiency anaemia, Clinical medicine (London, England) 18: ((2018) ), 242–244. |
[31] | G. Clénin, The treatment of iron deficiency without anaemia (in otherwise healthy persons), Swiss Medical Weekly 147: ((2017) ), w14434. |
[32] | R. Orlandi, M. De Bortoli, C. Ciniselli, E. Vaghi, D. Caccia, V. Garrisi, S. Pizzamiglio, S. Veneroni, C. Bonini, R. Agresti, M. Daidone, D. Morelli, C. Camaschella, P. Verderio and I. Bongarzone, Hepcidin and ferritin blood level as noninvasive tools for predicting breast cancer, Annals of Oncology: Official Journal of the European Society for Medical Oncology 25: ((2014) ), 352–357. |
[33] | H. Tingting, S. Di, C. Xiaoping, W. Xiaohong and H. Dong, High preoperative serum ferritin predicted poor prognosis in non-metastatic colorectal cancer, Saudi Medical Journal 38: ((2017) ), 268–275. |
[34] | J. Ihlow, S. Gross, A. Sick, T. Schneider, A. Flörcken, T. Burmeister, S. Türkmen, R. Arnold, B. Dörken and J. Westermann, AML: high serum ferritin at initial diagnosis has a negative impact on long-term survival, Leukemia & Lymphoma 60: ((2019) ), 69–77. |
[35] | H. Shi, X. Li, J. Jiang, W. Zhao, M. Ji and C. Wu, Serum ferritin is elevated in advanced non-small cell lung cancer patients and is associated with efficacy of platinum-based chemotherapy, Journal of Cancer Research and Therapeutics 10: ((2014) ), 681–685. |
[36] | D. Basuli, L. Tesfay, Z. Deng, B. Paul, Y. Yamamoto, G. Ning, W. Xian, F. McKeon, M. Lynch, C. Crum, P. Hegde, M. Brewer, X. Wang, L. Miller, N. Dyment, F. Torti and S. Torti, Iron addiction: a novel therapeutic target in ovarian cancer, Oncogene 36: ((2017) ), 4089–4099. |




