PD-L1 expression and CD8 positive lymphocytes in human neoplasms: A tissue microarray study on 11,838 tumor samples
Abstract
BACKGROUND:
Programmed death ligand 1 (PD-L1) is the target of immune checkpoint inhibitor therapies in a growing number of tumor types, but a unanimous picture on PD-L1 expression across cancer types is lacking.
MATERIALS AND METHODS:
We analyzed immunohistochemical PD-L1 expression in 11,838 samples from 118 human tumor types and its relationship with tumor infiltrating CD8 positive lymphocytes.
RESULTS:
At a cut-off level of 10% positive tumor cells, PD-L1 positivity was seen in 85 of 118 (72%) tumor types, including thymoma (100% positive), Hodgkin’s lymphoma (93%), anaplastic thyroid carcinoma (76%), Kaposi sarcoma (71%), sarcomatoid urothelial carcinoma (71%), and squamous cell carcinoma of the penis (67%), cervix (65%), floor of the mouth (61%), the lung (53%), and pharynx (50%). In immune cells, PD-L1 positivity was detectable in 103 (87%) tumor types, including tumors of haematopoetic and lymphoid tissues (75% to 100%), Warthin tumors of the parotid glands (95%) and Merkel cell carcinoma (82%). PD-L1 positivity in tumor cells was significantly correlated with the number of intratumoral CD8 positive lymphocytes across all tumor types as well as in individual tumor types, including serous carcinoma of the ovary, invasive breast carcinoma of no special type, intestinal gastric adenocarcinoma, and liposarcoma (
CONCLUSIONS:
PD-L1 expression in tumor and inflammatory cells is found in a wide range of human tumor types. Higher rates of tumor infiltrating CD8 positive lymphocytes in PD-L1 positive than in PD-L1 negative cancers suggest that the antitumor immune response may trigger tumoral PD-L1 expression.
1.Introduction
Immune checkpoint inhibitor (CPI) therapies targeting the programmed death 1/programmed death ligand 1 (PD-L1) pathway are increasingly employed in a growing number of tumor types [1]. However, not all patients react favorably to these drugs. PD-L1 immunohistochemistry is often applied to select patients with high likelihood to respond favorably to checkpoint inhibitors but criteria for “PD-L1 positivity” vary between tumor types and sometimes also between drugs. The proportion of PD-L1 positive tumor cells (tumor proportion score, TPS), the percentage of positive immune cells (immune cell score; ICS) or the combination of both (combined positivity score; CPS) are applied at different thresholds to define positive cases [2]. The significant role of PD-L1 for the immune microenvironment of tumors is illustrated by associations between PD-L1 expression in tumor cells and elevated numbers of intratumoral CD8 positive cytotoxic T-lymphocytes which were found in several tumor types [3, 4, 5, 6].
More than 2,800 studies have analyzed cancers of various types for PD-L1 expression by immunohistochemistry. For most tumor types, however, the reported frequencies of PD-L1 positivity vary quite considerably. For example, the reported rate of PD-L1 positivity ranges from 0–92% in prostate cancer [7, 8], 1.7%–75% in breast cancer [9, 10], 5.5–89% in colorectal cancer [11, 12], 22–68% in head & neck squamous cell carcinomas [13, 14], 5.2–65% in stomach cancer [15, 16], 3.9–63% in small cell lung cancer [17, 18], 3.1–82% in liver cell carcinomas [19, 20], 17–72% in malignant mesothelioma [21, 22], 10–92% in malignant melanoma [23, 24], 0–100% in chondrosarcoma [24, 25], 0–100% in liposarcoma [24, 26], and 7–100% in angiosarcoma [19, 27]. Technical factors, staining protocols, antibodies used, definitions of thresholds to determine positivity, as well as a possible selection bias with respect to the analyzed tumors have been proposed as causes for these discrepancies. To better understand the relative importance of PD-L1 expression in different tumor types and its relationship with T-lymphocyte counts, a comprehensive study analyzing large numbers of tumors of different kinds under highly standardized conditions is required.
This study was designed to collect comparable data on the rate of PD-L1 expression in a broad range of different tissues using the same predefined scoring criteria. For this purpose, more than 14,800 tissue samples with preexisting data on intratumoral CD8 positive lymphocytes from 118 different tumor types and subtypes as well as 76 non-neoplastic tissue types were evaluated by immunohistochemistry in a tissue microarray (TMA) format.
2.Materials and methods
2.1Experimental subjects
Tissue Microarrays (TMAs). The normal tissue TMA was composed of 8 samples from 8 different donors for each of 76 different normal tissue types (608 samples on one slide). The cancer TMAs contained a total of 14,897 primary tumors from 118 tumor types and subtypes. The composition of both normal and cancer TMAs is described in detail in the results section. All samples were from the archives of the Institutes of Pathology, University Hospital of Hamburg, Germany, the Institute of Pathology, Clinical Center Osnabrueck, Germany, and Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. The TMA manufacturing process was described earlier in detail [28, 29]. In brief, one tissue spot (diameter: 0.6 mm) was transmitted from a cancer containing donor block in an empty recipient paraffin block. The density of CD8
2.2Immunohistochemistry (IHC)
Freshly cut TMA sections were immunostained on one day and in one experiment. Slides were deparaffinized with xylol, rehydrated through a graded alcohol series and exposed to heat-induced retrieval for 5 minutes in an autoclave at 121
2.3Antibody comparison
To evaluate the impact of antibody selection on PD-L1 immunohistochemistry data, staining properties of MSVA-711R, Cell Signaling Technology E1L3N, Roche SP142, and Roche SP263 were compared in normal tissues with known physiological PD-L1 expression as detailed in Supplementary Fig. S1. Immunohistochemistry protocols and automated staining systems were employed as recommended by the antibody vendors and are listed in Supplementary Table S1. To determine the sensitivity and specificity of each antibody, consensus sets of unequivocally PD-L1 positive and unequivocally PD-L1 negative tissue samples were identified from a tissue microarray with 352 high grade muscle invasive urinary bladder cancers. Consecutive sections were taken from the TMA and stained with the 4 antibodies. For maximal standardization of the PD-L1 status calling, neural network and digital image analysis were used as described in the Supplementary Methods. For MSVA-711R, the consensus set contained 96 cancers that were consistently positive with E1L3N, SP142, and SP263, and 188 cancers that were consistently negative with E1L3N, SP142, and SP263. For E1L3N, the consensus set contained cancers that were consistently positive (
2.4Statistics
Statistical calculations were performed with JMP
3.Results
3.1Technical issue
A total of 11,838 (79.6%) of 14,879 tumor samples were interpretable in the TMA analysis. The remaining 3,059 (20.4%) samples were not analyzable due to the lack of unequivocal tumor cells or loss of the tissue spot during the technical procedures. On the normal tissue TMA, sufficient numbers of samples were always interpretable for each tissue to determine PD-L1 expression.
3.2Antibody comparison
Table 1
Sensitivity and specificity of 4 anti-PD-L1 antibodies. Consensus set: Tumors with unequivocal presence or absence of PD-L1 expression that were used to determine specificity and sensitivity (antibody performance) for each of the indicated anti-PD-L1 antibodies
| Antibody | |||||
| MSVA-711R | E1L3N | SP142 | SP263 | ||
| Consensus set result | PD-L1 positive ( | 96 | 93 | 102 | 98 |
| PD-L1 negative ( | 188 | 199 | 200 | 192 | |
| Antibody performance | True positive ( | 92 | 92 | 92 | 92 |
| True negative ( | 187 | 187 | 187 | 187 | |
| False positive ( | 1 | 12 | 13 | 5 | |
| False negative ( | 4 | 1 | 10 | 6 | |
| Sensitivity | 0.958 | 0.989 | 0.902 | 0.939 | |
| Specificity | 0.995 | 0.940 | 0.935 | 0.974 | |
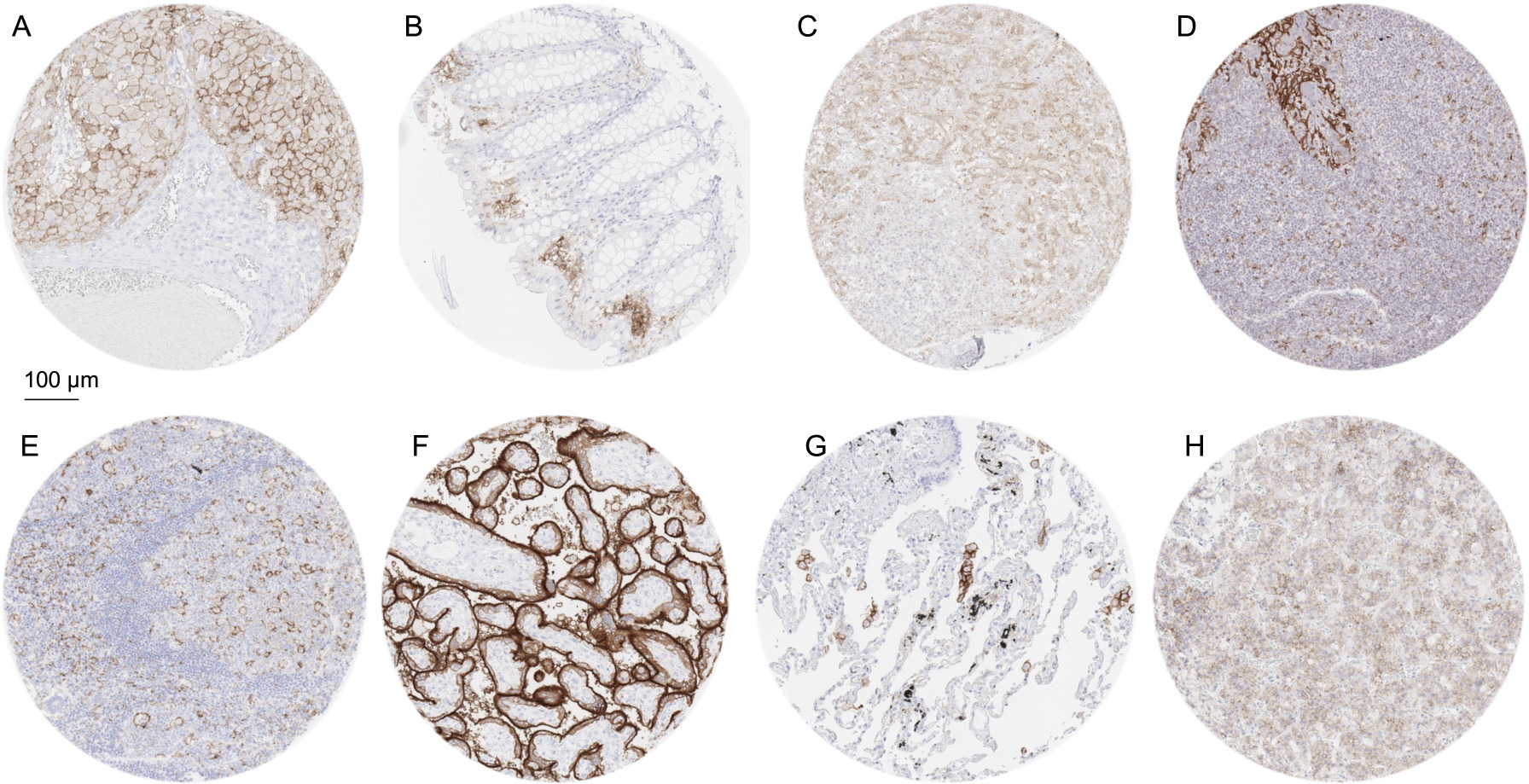
Figure 1.
PD-L1 immunostaining of normal cells using MSVA-711R. The panels show a membranous PD-L1 positivity of Corpus luteum cells in the ovary (A), macrophages in colon epithelium (B), small (littoral) blood vessels in the spleen (C), a fraction of crypt epithelial cells and macrophages of the tonsil (D), dendritic cells and macrophages in a lymph node (E), surface membranes of the syncytiotrophoblast in the placenta (F), alveolar macrophages in the lung (G) and of a fraction of epithelial cells in the adenohypophysis.
Representative images of our comparison of 4 anti-PD-L1 antibodies are shown in Supplementary Fig. S1. All antibodies showed the expected staining in normal tonsil epithelium, placenta, corpus luteum of the ovary, macrophages, and blood vessels. The comparatively low staining intensity observed with SP142 is in line with many earlier reports (reviewed in [34]). The results of the consensus set testing and the calculated sensitivity and specificity of each of the 4 antibodies are shown in Table 1. All antibodies proved to be highly specific and sensitive, with comparable performance.
3.3PD-L1 staining pattern in normal tissue
A moderate to strong membranous PD-L1 immunostaining was found in alveolar macrophages of the lung, macrophages in the endometrium of the pregnant uterus and of the gastrointestinal tract, corpus luteum cells of the ovary, surface cell layers of the syncytiotrophoblast and chorion cells of the placenta, thymic epithelial cells, a fraction of squamous epithelial cells of the tonsil crypts as well as in dendritic cells and macrophages of lymphoid tissues. A weak to moderate PD-L1 staining was also observed in a fraction of epithelial cells of the adenohypophysis and in venous sinuses in the spleen (littoral cells). In addition, weak staining was found in fibrils of the anterior lobe of the pituitary gland. Representative images of PD-L1 positive normal tissues are shown in Fig. 1. PD-L1 staining was absent in epithelial cells of adrenal gland, thyroid gland, parathyroid gland, breast, respiratory epithelium, gastrointestinal tract, esophagus, gallbladder, pancreas, liver, cervix, endometrium, fallopian tube, epididymis, kidney, urinary bladder, prostate, seminal vesicle, testis, skin, as well as in muscle cells, fat, aorta, cerebellum, and the cerebrum.
3.4PD-L1 in neoplastic tissue
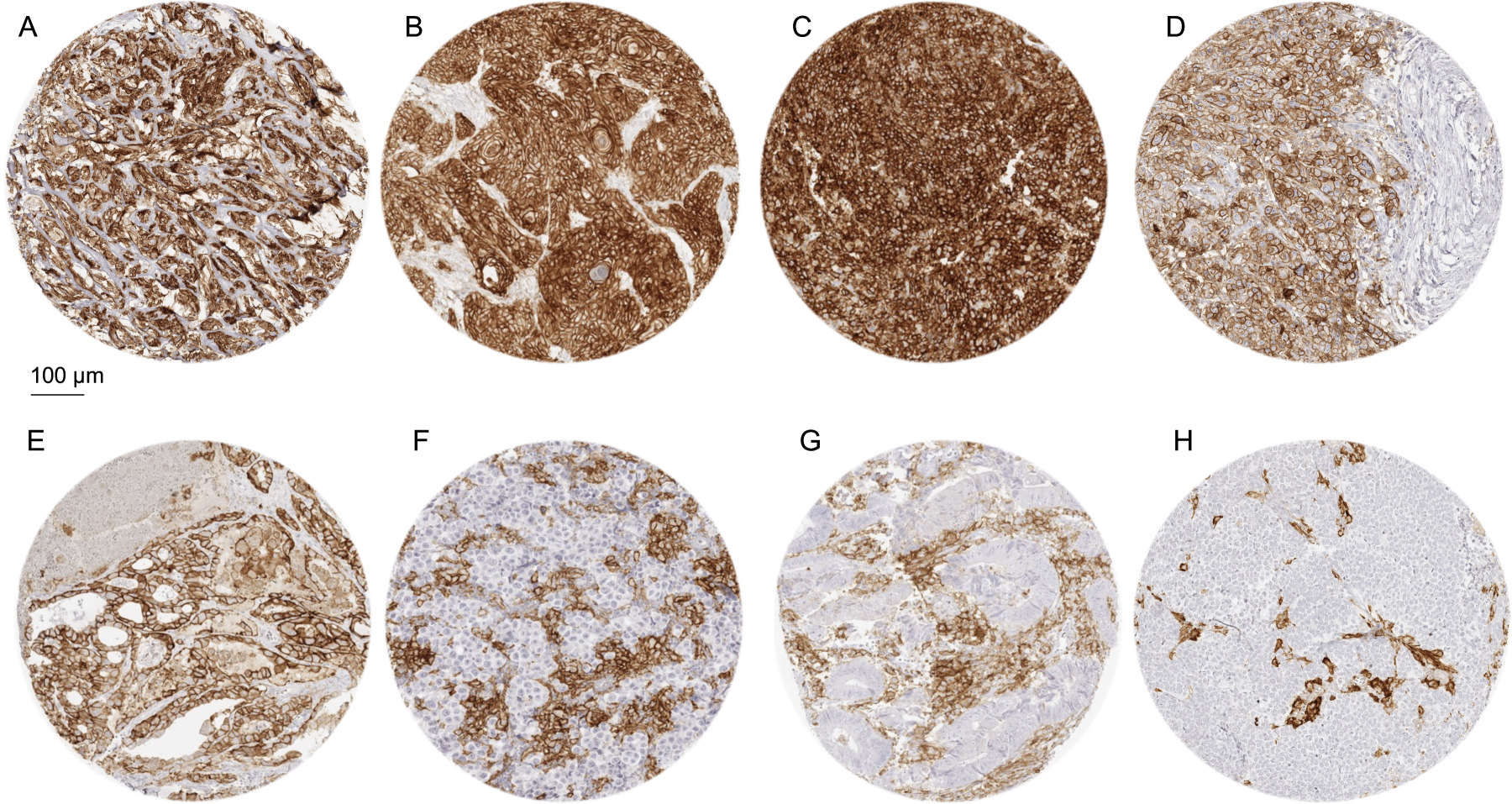
Figure 2.
PD-L1 immunostaining in cancer using MSVA-711R. The panels show a strong, predominantly membranous PD-L1 immunostaining of tumor cells in an epitheloid malignant mesothelioma (A), a muscle-invasive urothelial carcinoma (B), a squamous cell carcinoma of the oral cavity (C), and an anaplastic thyroid cancer (D). A papillary carcinoma of the thyroid shows a membranous staining of both cancer cells (strong intensity) and macrophages (moderate intensity) (E). Cases of seminoma (F), colorectal adenocarcinoma (G), and a Merkel cell carcinoma of the skin (H) do not show tumor cell staining but contain macrophages with intense PD-L1 positivity.
If a cut-off level of
3.5PD-L1 and CD8 expression
Data on intratumoral CD8
Table 2
PD-L1 in human tumor cells and immune cells
| PD-L1 in tumor cells | PD-L1 in immune cells | ||||||
| Tumor entity | On TMA ( | Analyzable ( | Negative (%) | Positive (%) | Negative (%) | Few (%) | Many (%) |
| Tumors of the skin | |||||||
| Pilomatrixoma | 35 | 29 | 69.0 | 31.0 | 75.9 | 6.9 | 17.2 |
| Basal cell carcinoma | 88 | 68 | 95.6 | 4.4 | 67.6 | 8.8 | 23.5 |
| Benign nevus | 29 | 26 | 100.0 | 0.0 | 92.3 | 7.7 | 0.0 |
| Squamous cell carcinoma of the skin | 90 | 83 | 55.4 | 44.6 | 57.8 | 18.1 | 24.1 |
| Malignant melanoma | 46 | 39 | 87.2 | 12.8 | 66.7 | 17.9 | 15.4 |
| Merkel cell carcinoma | 46 | 45 | 97.8 | 2.2 | 17.8 | 28.9 | 53.3 |
| Basal cell adenoma of the salivary gland | 15 | 13 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| Tumors of the lung, pleura and thymus | |||||||
| Adenocarcinoma of the lung | 196 | 99 | 58.6 | 41.4 | 47.5 | 17.2 | 35.4 |
| Squamous cell carcinoma of the lung | 80 | 40 | 47.5 | 52.5 | 65.0 | 12.5 | 22.5 |
| Small cell carcinoma of the lung | 16 | 16 | 93.8 | 6.3 | 31.3 | 25.0 | 43.8 |
| Mesothelioma, epitheloid | 39 | 33 | 87.9 | 12.1 | 75.8 | 9.1 | 15.2 |
| Mesothelioma, other types | 76 | 71 | 64.8 | 35.2 | 85.9 | 5.6 | 8.5 |
| Thymoma | 29 | 25 | 0.0 | 100.0 | 56.0 | 28.0 | 16.0 |
| Tumors of the female genital tract | |||||||
| Squamous cell carcinoma of the vagina | 30 | 29 | 65.5 | 34.5 | 65.5 | 24.1 | 10.3 |
| Squamous cell carcinoma of the vulva | 80 | 77 | 58.4 | 41.6 | 51.9 | 24.7 | 23.4 |
| Squamous cell carcinoma of the cervix | 80 | 76 | 35.5 | 64.5 | 43.4 | 19.7 | 36.8 |
| Endometrioid endometrial carcinoma | 186 | 146 | 94.5 | 5.5 | 77.4 | 9.6 | 13.0 |
| Endometrial serous carcinoma | 32 | 23 | 91.3 | 8.7 | 65.2 | 13.0 | 21.7 |
| Carcinosarcoma of the uterus | 48 | 37 | 97.3 | 2.7 | 78.4 | 5.4 | 16.2 |
| Endometrial carcinoma, high grade, G3 | 13 | 7 | 85.7 | 14.3 | 14.3 | 42.9 | 42.9 |
| Endometrial clear cell carcinoma | 8 | 4 | 100.0 | 0.0 | 50.0 | 25.0 | 25.0 |
| Endometrioid carcinoma of the ovary | 73 | 53 | 84.9 | 15.1 | 73.6 | 18.9 | 7.5 |
| Serous carcinoma of the ovary | 509 | 398 | 84.2 | 15.8 | 58.0 | 16.1 | 25.9 |
| Mucinous carcinoma of the ovary | 70 | 48 | 100.0 | 0.0 | 79.2 | 16.7 | 4.2 |
| Clear cell carcinoma of the ovary | 50 | 40 | 77.5 | 22.5 | 72.5 | 17.5 | 10.0 |
| Carcinosarcoma of the ovary | 47 | 37 | 83.8 | 16.2 | 81.1 | 8.1 | 10.8 |
| Tumors of the breast | |||||||
| Invasive breast carcinoma of no special type | 1345 | 1120 | 94.6 | 5.4 | 79.7 | 7.1 | 13.1 |
| Lobular carcinoma of the breast | 251 | 199 | 99.0 | 1.0 | 91.5 | 6.0 | 2.5 |
| Medullary carcinoma of the breast | 11 | 9 | 66.7 | 33.3 | 0.0 | 0.0 | 100.0 |
| Tubular carcinoma of the breast | 9 | 4 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| Mucinous carcinoma of the breast | 36 | 24 | 100.0 | 0.0 | 87.5 | 12.5 | 0.0 |
| Tumors of the digestive system | |||||||
| Adenomatous polyp, low-grade dysplasia | 50 | 43 | 97.7 | 2.3 | 51.2 | 25.6 | 23.3 |
| Adenomatous polyp, high-grade dysplasia | 50 | 46 | 95.7 | 4.3 | 23.9 | 23.9 | 52.2 |
| Adenocarcinoma of the colon | 1882 | 1408 | 96.2 | 3.8 | 52.0 | 37.4 | 10.6 |
| Gastric adenocarcinoma, diffuse type | 176 | 130 | 97.7 | 2.3 | 90.0 | 6.2 | 3.8 |
| Gastric adenocarcinoma, intestinal type | 174 | 131 | 76.3 | 23.7 | 48.1 | 24.4 | 27.5 |
| Gastric adenocarcinoma, mixed type | 62 | 53 | 84.9 | 15.1 | 66.0 | 17.0 | 17.0 |
| Adenocarcinoma of the esophagus | 83 | 60 | 90.0 | 10.0 | 46.7 | 28.3 | 25.0 |
| Squamous cell carcinoma of the esophagus | 76 | 48 | 54.2 | 45.8 | 33.3 | 31.3 | 35.4 |
| Squamous cell carcinoma of the anal canal | 89 | 84 | 63.1 | 36.9 | 47.6 | 26.2 | 26.2 |
| Cholangiocarcinoma | 113 | 94 | 91.5 | 8.5 | 76.6 | 11.7 | 11.7 |
| Hepatocellular carcinoma | 50 | 48 | 97.9 | 2.1 | 75.0 | 14.6 | 10.4 |
| Ductal adenocarcinoma of the pancreas | 612 | 448 | 89.1 | 10.9 | 79.9 | 16.1 | 4.0 |
| Pancreatic/Ampullary adenocarcinoma | 89 | 61 | 88.5 | 11.5 | 63.9 | 24.6 | 11.5 |
| Acinar cell carcinoma of the pancreas | 16 | 11 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| Gastrointestinal stromal tumor (GIST) | 50 | 45 | 75.6 | 24.4 | 73.3 | 20.0 | 6.7 |
| Tumors of the urinary system | |||||||
| Non-invasive papillary urothelial carcinoma, pTa G2 low grade | 177 | 148 | 99.3 | 0.7 | 87.2 | 6.8 | 6.1 |
| Non-invasive papillary urothelial carcinoma, pTa G2 high grade | 141 | 128 | 99.2 | 0.8 | 89.1 | 4.7 | 6.3 |
| Non-invasive papillary urothelial carcinoma, pTa G3 | 187 | 150 | 93.3 | 6.7 | 64.0 | 17.3 | 18.7 |
| Urothelial carcinoma, pT2-4 G3 | 1206 | 936 | 70.8 | 29.2 | 67.5 | 16.1 | 16.3 |
| Small cell neuroendocrine carcinoma of the bladder | 20 | 20 | 100.0 | 0.0 | 50.0 | 25.0 | 25.0 |
|
Table 2, continued | |||||||
| PD-L1 in tumor cells | PD-L1 in immune cells | ||||||
| Tumor entity | On TMA ( | Analyzable ( | Negative (%) | Positive (%) | Negative (%) | Few (%) | Many (%) |
| Sarcomatoid urothelial carcinoma | 25 | 24 | 29.2 | 70.8 | 91.7 | 4.2 | 4.2 |
| Clear cell renal cell carcinoma | 857 | 665 | 95.0 | 5.0 | 91.4 | 5.6 | 3.0 |
| Papillary renal cell carcinoma | 255 | 199 | 84.4 | 15.6 | 85.4 | 8.0 | 6.5 |
| Clear cell (tubulo) papillary renal cell carcinoma | 21 | 15 | 100.0 | 0.0 | 93.3 | 0.0 | 6.7 |
| Chromophobe renal cell carcinoma | 131 | 100 | 85.0 | 15.0 | 100.0 | 0.0 | 0.0 |
| Oncocytoma | 177 | 141 | 68.8 | 31.2 | 93.6 | 5.0 | 1.4 |
| Tumors of the male genital organs | |||||||
| Adenocarcinoma of the prostate, Gleason 3 | 83 | 70 | 100.0 | 0.0 | 92.9 | 1.4 | 5.7 |
| Adenocarcinoma of the prostate, Gleason 4 | 80 | 67 | 97.0 | 3.0 | 86.6 | 10.4 | 3.0 |
| Adenocarcinoma of the prostate, Gleason 5 | 85 | 68 | 92.6 | 7.4 | 69.1 | 11.8 | 19.1 |
| Adenocarcinoma of the prostate (recurrence) | 258 | 210 | 96.7 | 3.3 | 94.3 | 3.3 | 2.4 |
| Small cell neuroendocrine carcinoma of the prostate | 19 | 17 | 100.0 | 0.0 | 52.9 | 35.3 | 11.8 |
| Seminoma | 621 | 475 | 100.0 | 0.0 | 24.2 | 24.0 | 51.8 |
| Embryonal carcinoma of the testis | 50 | 35 | 100.0 | 0.0 | 14.3 | 22.9 | 62.9 |
| Yolk sac tumor | 50 | 27 | 100.0 | 0.0 | 25.9 | 25.9 | 48.1 |
| Teratoma | 50 | 25 | 100.0 | 0.0 | 96.0 | 4.0 | 0.0 |
| Squamous cell carcinoma of the penis | 80 | 75 | 33.3 | 66.7 | 33.3 | 22.7 | 44.0 |
| Tumors of endocrine organs | |||||||
| Adenoma of the thyroid gland | 113 | 99 | 84.8 | 15.2 | 96.0 | 0.0 | 4.0 |
| Papillary thyroid carcinoma | 391 | 345 | 69.0 | 31.0 | 78.8 | 13.0 | 8.1 |
| Follicular thyroid carcinoma | 154 | 130 | 67.7 | 32.3 | 97.7 | 1.5 | 0.8 |
| Medullary thyroid carcinoma | 111 | 90 | 84.4 | 15.6 | 94.4 | 4.4 | 1.1 |
| Anaplastic thyroid carcinoma | 45 | 38 | 23.7 | 76.3 | 57.9 | 15.8 | 26.3 |
| Adrenal cortical adenoma | 50 | 42 | 100.0 | 0.0 | 95.2 | 4.8 | 0.0 |
| Adrenal cortical carcinoma | 26 | 25 | 92.0 | 8.0 | 96.0 | 4.0 | 0.0 |
| Phaeochromocytoma | 50 | 50 | 68.0 | 32.0 | 82.0 | 14.0 | 4.0 |
| Appendix, neuroendocrine tumor (NET) | 22 | 14 | 92.9 | 7.1 | 92.9 | 0.0 | 7.1 |
| Colorectal, neuroendocrine tumor (NET) | 12 | 12 | 100.0 | 0.0 | 91.7 | 8.3 | 0.0 |
| Ileum, neuroendocrine tumor (NET) | 49 | 47 | 100.0 | 0.0 | 95.7 | 4.3 | 0.0 |
| Lung, neuroendocrine tumor (NET) | 19 | 18 | 94.4 | 5.6 | 100.0 | 0.0 | 0.0 |
| Pancreas, neuroendocrine tumor (NET) | 97 | 49 | 89.8 | 10.2 | 87.8 | 6.1 | 6.1 |
| Colorectal, neuroendocrine carcinoma (NEC) | 12 | 12 | 91.7 | 8.3 | 50.0 | 33.3 | 16.7 |
| Gallbladder, neuroendocrine carcinoma (NEC) | 4 | 4 | 100.0 | 0.0 | 25.0 | 25.0 | 50.0 |
| Pancreas, neuroendocrine carcinoma (NEC) | 14 | 12 | 100.0 | 0.0 | 75.0 | 16.7 | 8.3 |
| Tumors of haemotopoetic and lymphoid tissues | |||||||
| Hodgkin Lymphoma | 103 | 43 | 7.0 | 93.0 | 0.0 | 2.3 | 97.7 |
| Small lymphocytic lymphoma, B-cell type (B-SLL/B-CLL) | 50 | 49 | 100.0 | 0.0 | 2.0 | 55.1 | 42.9 |
| Diffuse large B cell lymphoma (DLBCL) | 114 | 109 | 80.7 | 19.3 | 17.4 | 10.1 | 72.5 |
| Follicular lymphoma | 88 | 86 | 100.0 | 0.0 | 1.2 | 19.8 | 79.1 |
| T-cell Non Hodgkin lymphoma | 24 | 24 | 83.3 | 16.7 | 16.7 | 4.2 | 79.2 |
| Mantle cell lymphoma | 18 | 18 | 100.0 | 0.0 | 5.6 | 16.7 | 77.8 |
| Marginal zone lymphoma | 16 | 16 | 100.0 | 0.0 | 0.0 | 37.5 | 62.5 |
| Diffuse large B-cell lymphoma (DLBCL) in the testis | 16 | 16 | 87.5 | 12.5 | 12.5 | 12.5 | 75.0 |
| Burkitt lymphoma | 5 | 4 | 100.0 | 0.0 | 25.0 | 50.0 | 25.0 |
| Tumors of soft tissue and bone | |||||||
| Tendosynovial giant cell tumor | 45 | 45 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| Granular cell tumor | 53 | 48 | 97.9 | 2.1 | 100.0 | 0.0 | 0.0 |
| Leiomyoma | 50 | 41 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| Leiomyosarcoma | 87 | 76 | 89.5 | 10.5 | 89.5 | 9.2 | 1.3 |
| Liposarcoma | 132 | 105 | 85.7 | 14.3 | 93.3 | 4.8 | 1.9 |
| Malignant peripheral nerve sheath tumor (MPNST) | 13 | 12 | 91.7 | 8.3 | 91.7 | 8.3 | 0.0 |
| Myofibrosarcoma | 26 | 26 | 69.2 | 30.8 | 84.6 | 7.7 | 7.7 |
| Angiosarcoma | 73 | 67 | 65.7 | 34.3 | 70.1 | 17.9 | 11.9 |
| Angiomyolipoma | 91 | 88 | 95.5 | 4.5 | 87.5 | 9.1 | 3.4 |
| Dermatofibrosarcoma protuberans | 21 | 16 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| Ganglioneuroma | 14 | 11 | 81.8 | 18.2 | 100.0 | 0.0 | 0.0 |
| Kaposi sarcoma | 8 | 7 | 28.6 | 71.4 | 71.4 | 0.0 | 28.6 |
| Neurofibroma | 117 | 90 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 |
|
Table 2, continued | |||||||
|---|---|---|---|---|---|---|---|
| PD-L1 in tumor cells | PD-L1 in immune cells | ||||||
| Tumor entity | On TMA ( | Analyzable ( | Negative (%) | Positive (%) | Negative (%) | Few (%) | Many (%) |
| Sarcoma, not otherwise specified (NOS) | 74 | 70 | 62.9 | 37.1 | 98.6 | 1.4 | 0.0 |
| Paraganglioma | 41 | 37 | 94.6 | 5.4 | 83.8 | 8.1 | 8.1 |
| Ewing sarcoma | 23 | 20 | 95.0 | 5.0 | 95.0 | 5.0 | 0.0 |
| Rhabdomyosarcoma | 6 | 6 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| Schwannoma | 121 | 100 | 98.0 | 2.0 | 99.0 | 1.0 | 0.0 |
| Synovial sarcoma | 12 | 11 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| Osteosarcoma | 43 | 32 | 100.0 | 0.0 | 96.9 | 3.1 | 0.0 |
| Chondrosarcoma | 38 | 19 | 68.4 | 31.6 | 100.0 | 0.0 | 0.0 |
Table 3
PD-L1 n human tumor cells and intratumoral CD8 positive (CD8
| PD-L1 IHC in tumor cells |
| CD8 | ||
|---|---|---|---|---|
| All cancers | Negative | 5,016 | 254.2 | |
| Positive | 484 | 612.2 | ||
| Mesothelioma, epitheloid | Negative | 29 | 261.6 | 0.0864 |
| Positive | 4 | 537.9 | ||
| Mesothelioma, other types | Negative | 18 | 231.8 | 0.1312 |
| Positive | 10 | 446.7 | ||
| Endometrioid carcinoma of the ovary | Negative | 28 | 124.0 | 0.9657 |
| Positive | 5 | 117.0 | ||
| Serous carcinoma of the ovary | Negative | 279 | 142.0 | |
| Positive | 43 | 532.1 | ||
| Clear cell carcinoma of the ovary | Negative | 7 | 18.2 | 0.7127 |
| Positive | 2 | 11.9 | ||
| Carcinosarcoma of the ovary | Negative | 20 | 117.0 | 0.5896 |
| Positive | 4 | 54.5 | ||
| Invasive breast carcinoma of no special type | Negative | 997 | 294.6 | |
| Positive | 58 | 699.0 | ||
| Lobular carcinoma of the breast | Negative | 134 | 199.7 | 0.7999 |
| Positive | 1 | 134.0 | ||
| Medullary carcinoma of the breast | Negative | 6 | 1470.0 | 0.1396 |
| Positive | 3 | 2872.1 | ||
| Adenocarcinoma of the colon | Negative | 1229 | 259.3 | |
| Positive | 52 | 692.9 | ||
| Clear cell renal cell carcinoma | Negative | 568 | 435.6 | |
| Positive | 31 | 1164.2 | ||
| Papillary cell renal cell carcinoma | Negative | 127 | 233.9 | 0.2765 |
| Positive | 27 | 337.3 | ||
| Oncocytoma | Negative | 57 | 74.0 | 0.3923 |
| Positive | 31 | 100.2 | ||
| Gastric adenocarcinoma, diffuse type | Negative | 69 | 260.3 | 0.7595 |
| Positive | 2 | 352.8 | ||
| Gastric adenocarcinoma, intestinal type | Negative | 61 | 324.7 | |
| Positive | 15 | 1142.5 | ||
| Gastric adenocarcinoma, mixed type | Negative | 45 | 386.4 | 0.1399 |
| Positive | 8 | 751.9 | ||
| Ductal carcinoma of the pancreas | Negative | 351 | 222.2 | 0.1014 |
| Positive | 42 | 301.3 | ||
| Pancreatic/Ampullary adenocarcinoma | Negative | 34 | 268.8 | 0.1313 |
| Positive | 4 | 654.7 | ||
| Sarcomatoid urothelial carcinoma | Negative | 7 | 229.3 | 0.2457 |
| Positive | 17 | 714.1 | ||
| Granular cell tumor | Negative | 19 | 61.9 | 0.0681 |
| Positive | 1 | 158.0 | ||
| Leiomyosarcoma | Negative | 32 | 82.7 | 0.1047 |
| Positive | 3 | 245.5 |
|
Table 3, continued | ||||
|---|---|---|---|---|
| PD-L1 IHC in tumor cells |
| CD8 | ||
| Liposarcoma | Negative | 56 | 88.3 | |
| Positive | 10 | 1086.6 | ||
| Malignant peripheral nerve sheath tumor (MPNST) | Negative | 11 | 100.9 | 0.0007 |
| Positive | 1 | 1130.0 | ||
| Myofibrosacroma | Negative | 18 | 53.0 | 0.0587 |
| Positive | 8 | 1028.3 | ||
| Angiosarcoma | Negative | 22 | 105.6 | 0.0042 |
| Positive | 17 | 610.5 | ||
| Angiomyolipoma | Negative | 84 | 178.9 | 0.9519 |
| Positive | 4 | 165.9 | ||
| Ganglioneuroma | Negative | 9 | 32.4 | 0.0339 |
| Positive | 2 | 97.2 | ||
| Kaposi sarcoma | Negative | 2 | 304.3 | 0.6885 |
| Positive | 5 | 393.7 | ||
| Sarcoma, not otherwise specified (NOS) | Negative | 44 | 66.8 | 0.0004 |
| Positive | 26 | 677.0 | ||
| Paraganglioma | Negative | 35 | 150.6 | 0.9313 |
| Positive | 2 | 167.7 | ||
| Primitive neuroectodermal tumor (PNET) | Negative | 19 | 65.7 | 0.5439 |
| Positive | 1 | 0.0 | ||
| Schwannoma | Negative | 98 | 81.1 | 0.3795 |
| Positive | 2 | 180.4 | ||
| Chondrosarcoma | Negative | 5 | 481.4 | 0.8664 |
| Positive | 4 | 397.6 | ||
Figure 3.
Ranking order of PD-L1 immunostaining in human tumors. Only staining in tumor cells is shown.
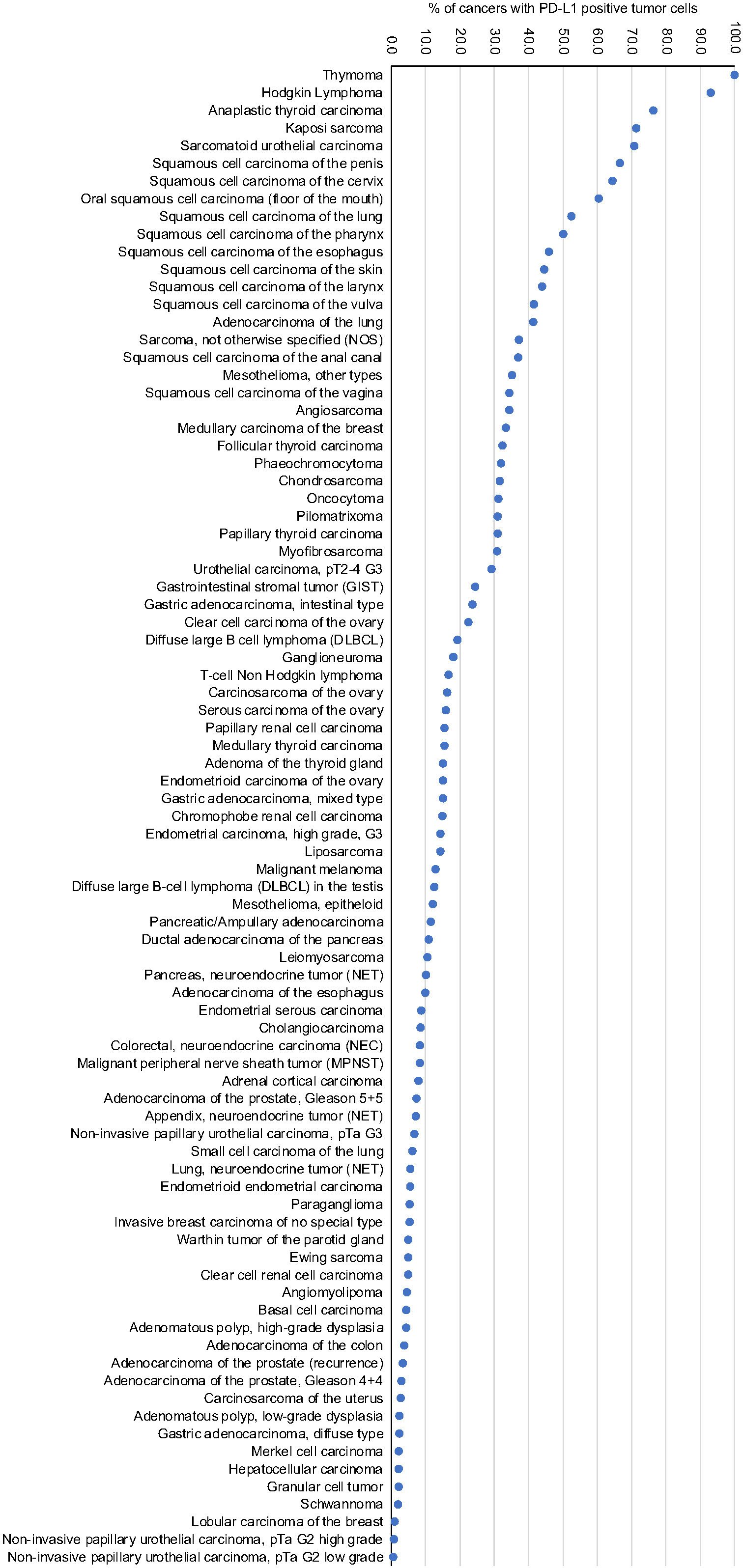
Figure 4.
Graphical comparison of PD-L1 data from this study (
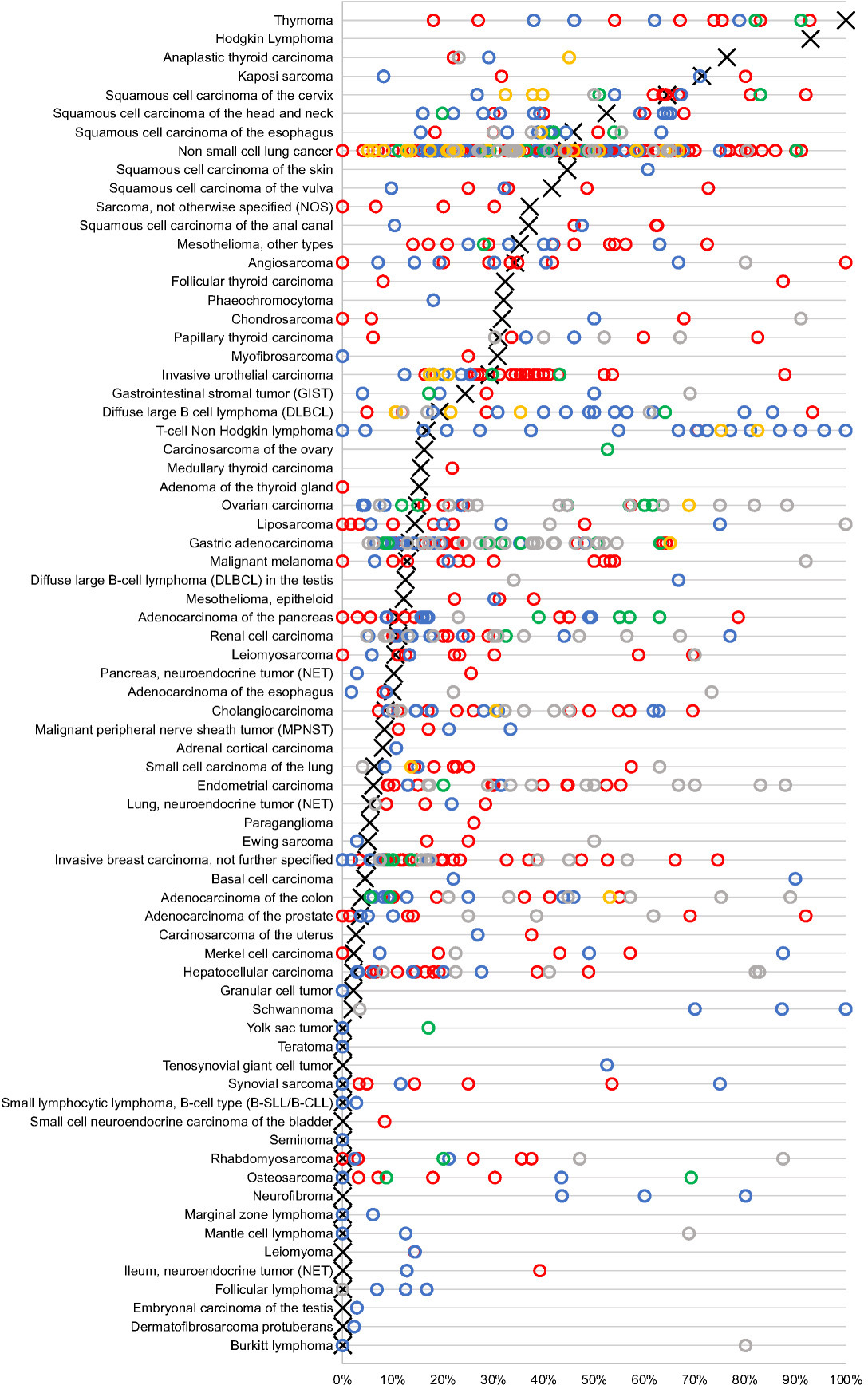
4.Discussion
The analysis of more than 14,000 tumors in a highly standardized way enabled us to define the relative importance of PD-L1 expression across 118 important human tumor entities and to define its relationship with tumor infiltrating CD8 positive lymphocytes. A Medline Search using the terms “PD-L1
Rather underestimated causes for discrepant PD-L1 data include slide ageing and difficulties in the distinction of tumor associated macrophages from tumor cells. Others and we had earlier demonstrated that the immunostaining intensity on stored formalin-fixed tissue sections decreases over time [53, 54] and that a significant reduction of staining may already occur 2 weeks after a tissue section has been taken [55]. This may be a relevant source of discrepant staining results particularly in clinical studies, where sections are often taken long before the analysis is made. In case of PD-L1, where macrophages often express the target protein at high levels, and where low thresholds are used for defining tumor cell positivity, it appears also likely that the quantity of tissue analyzed per patient and difficulties in the distinction of PD-L1 positive macrophages from cancer cells may have contributed to interpretation difficulties. That the analysis of larger tissue fragments more often leads to the perception of PD-L1 positivity than the analysis of small portions is shown by significant differences in data derived from TMA and from large section studies. For example, in 16 studies utilizing cut-off levels of 1% or 5% to define PD-L1 positivity in lung adenocarcinomas with the E1L3N antibody, the average positivity rate was 26% for TMA analyses but 41% for conventional large section staining. While these data might suggest that relevant PD-L1 findings are missed on TMAs, it is also evident that interpretation errors – such as mistaking macrophages for tumor cells – are more likely to occur on large sections [56]. Moreover, TMA studies comparing multiple samples per tumor versus only one sample per tumor have regularly found a significant relationship between the quantity of analyzed tissue and IHC positivity rate [56, 57, 58, 59]. Only recently, it was shown that posttranslational glycolysation of the PD-L1 protein can negatively affect binding of anti-PD-L1 antibodies in formalin fixed tissue samples [60]. Therefore, it has been suggested that tissue samples should be pretreated with deglycolysing reagents to reduce the risk of false-negative PD-L1 IHC findings. In our study, such a systematic change in staining protocol would potentially result in a higher overall number of PD-L1 positive tumors. However, because all tumor types would be equally affected, the relative ranking of PD-L1 positive tumor types would not change.
Groups of cancers that are of special interest based on our data include cancers with very high and very low rate of PD-L1 expression in cancer cells and tumors with a particularly high density of tumor associated PD-L1 inflammatory cells. The group of tumors with highest rates of PD-L1 positivity in tumor cells includes several tumor entities already approved for treatment with CPIs, such as Hodgkin lymphoma, squamous cell carcinomas of the head and neck, urothelial cancers and malignant mesothelioma. If the response to CPIs is indeed driven by tumoral PD-L1 expression in these tumors, cancers with a comparably high PD-L1 expression such as penile carcinoma, squamous cell carcinomas of the esophagus and the anal canal or anaplastic thyroid cancer should also represent premium targets for CPIs. Evidence for clinical responses already exists for anaplastic thyroid cancer [61], squamous cell cancers of the head and neck [62, 63, 64, 65], oral cavity [66], esophagus [67, 68] and skin [69], and a clinical trial is ongoing for squamous cell carcinoma of the cervix [70].
Cancers with a very low rate of tumoral PD-L1 expression for example include prostate cancer, a tumor known for is particularly poor response to CPIs [71] but also cancers such as Merkel cell carcinoma and small cell lung cancer which are both approved for CPI therapy. It is of note, that Merkel cell carcinoma (82.2%) and small cell lung cancer (68.7%) belong to these tumor types with the highest rates of PD-L1 positive immune cells in our analysis. These findings fit well with experimental data highlighting the particularly important role of PD-L1 expressing immune cells. For example, in colon and breast cancer mice models, anti–PD-L1 treatment changed the activity of tumor macrophages from an immune-suppressive to an immune-stimulatory state with an increase in activated CD8 positive cytotoxic T cells [72]. Triple negative breast cancer is the first tumor entity where the indication for CPI atezolizumab solely depends on the presence of intratumoral PD-L1 positive immune cells and is independent of whether tumor cells express PD-L1 [73, 74].
Our data also show that an elevated density of CD8 positive intratumoral lymphocytes in PD-L1 expressing tumors is a general feature occurring across all cancer types. This observation is consistent with various reports describing associations between PD-L1 positivity in tumor cells and high numbers of tumor infiltrating lymphocytes in various individual cancer types [3, 4, 5, 6]. Studies have also demonstrated that PD-L1 positivity is statistically linked to high mutation burden and microsatellite instability [75]. Altogether, these observations are well consistent with a model suggesting that PD-L1 is one of several immune-escape mechanisms that can be activated in highly immunogenic cancer cells in response to “lymphocyte attack”.
In summary, the results of our study provide a ranking order of cancer types according to their PD-L1 expression in tumor and inflammatory cells. A consistently higher rate of tumor infiltrating CD8 positive lymphocytes in PD-L1 positive than in PD-L1 negative cancers corroborates the concept that tumoral PD-L1 expression is driven by a hostile immune environment.
Author contributions
Conception: KM, TK, RS, GS.
Interpretation or analysis of data: MK, EB, MJS, SDR, MK, CHM, NCB, TM, ML, AM, AML, DH, CF, NG, FJ, TSC, SS, EB, SM, AHM.
Preparation of the manuscript: KM, TK, GS.
Revision for important intellectual content: KM, TK, RS, GS, DH.
Supervision: KM, TK, RS, GS.
All authors agree to be accountable for the content of the work.
Supplementary data
The supplementary files are available to download from http://dx.doi.org/10.3233/CBM-220030.
Acknowledgments
We are grateful to Melanie Witt, Laura Behm, Inge Brandt, Maren Eisenberg, and Sünje Seekamp for excellent technical assistance.
Conflict of interest
The PD-L1 antibody clone MSVA-711R was provided by MS Validated Antibodies GmbH (owned by a family member of GS).
References
[1] | H. Hayashi and K. Nakagawa, Combination therapy with PD-1 or PD-L1 inhibitors for cancer, Int J Clin Oncol 25: ((2020) ), 818–830. |
[2] | F. Giunchi, T. Gevaert, M. Scarpelli and M. Fiorentino, Status of Programmed Death Ligand 1 (PD-L1) by Immunohistochemistry and Scoring Algorithms, Curr Drug Targets 21: ((2020) ), 1286–1292. |
[3] | M. Kitsou, G.D. Ayiomamitis and A. Zaravinos, High expression of immune checkpoints is associated with the TIL load, mutation rate and patient survival in colorectal cancer, Int J Oncol 57: ((2020) ), 237–248. |
[4] | T. Yagi, Y. Baba, T. Ishimoto, M. Iwatsuki, Y. Miyamoto, N. Yoshida, M. Watanabe and H. Baba, PD-L1 Expression, Tumor-infiltrating Lymphocytes, and Clinical Outcome in Patients With Surgically Resected Esophageal Cancer, Ann Surg 269: ((2019) ), 471–478. |
[5] | J.R. Webb, K. Milne, D.R. Kroeger and B.H. Nelson, PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer, Gynecol Oncol 141: ((2016) ), 293–302. |
[6] | A. Cimino-Mathews, E. Thompson, J.M. Taube, X. Ye, Y. Lu, A. Meeker, H. Xu, R. Sharma, K. Lecksell, T.C. Cornish, N. Cuka, P. Argani and L.A. Emens, PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas, Hum Pathol 47: ((2016) ), 52–63. |
[7] | C.D. Fankhauser, P.J. Schuffler, S. Gillessen, A. Omlin, N.J. Rupp, J.H. Rueschoff, T. Hermanns, C. Poyet, T. Sulser, H. Moch and P.J. Wild, Comprehensive immunohistochemical analysis of PD-L1 shows scarce expression in castration-resistant prostate cancer, Oncotarget 9: ((2018) ), 10284–10293. |
[8] | N. Ness, S. Andersen, M.R. Khanehkenari, C.V. Nordbakken, A. Valkov, E.E. Paulsen, Y. Nordby, R.M. Bremnes, T. Donnem, L.T. Busund and E. Richardsen, The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort, Oncotarget 8: ((2017) ), 26789–26801. |
[9] | H. Sahin Ozkan, M.U. Ugurlu, P.F. Yumuk and H. Kaya, Prognostic Role of Immune Markers in Triple Negative Breast Carcinoma, Pathol Oncol Res 26: ((2020) ), 2733–2745. |
[10] | H.R. Ali, S.E. Glont, F.M. Blows, E. Provenzano, S.J. Dawson, B. Liu, L. Hiller, J. Dunn, C.J. Poole, S. Bowden, H.M. Earl, P.D. Pharoah and C. Caldas, PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes, Ann Oncol 26: ((2015) ), 1488–93. |
[11] | H.L. Ho, T.Y. Chou, S.H. Yang, J.K. Jiang, W.S. Chen, Y. Chao and H.W. Teng, PD-L1 is a double-edged sword in colorectal cancer: the prognostic value of PD-L1 depends on the cell type expressing PD-L1, J Cancer Res Clin Oncol 145: ((2019) ), 1785–1794. |
[12] | Y. Masugi, R. Nishihara, J. Yang, K. Mima, A. da Silva, Y. Shi, K. Inamura, Y. Cao, M. Song, J.A. Nowak, X. Liao, K. Nosho, A.T. Chan, M. Giannakis, A.J. Bass, F.S. Hodi, G.J. Freeman, S. Rodig, C.S. Fuchs, Z.R. Qian and S. Ogino, Tumour CD274 (PD-L1) expression and T cells in colorectal cancer, Gut 66: ((2017) ), 1463–1473. |
[13] | A.M. Hong, P. Ferguson, T. Dodds, D. Jones, M. Li, J. Yang and R.A. Scolyer, Significant association of PD-L1 expression with human papillomavirus positivity and its prognostic impact in oropharyngeal cancer, Oral Oncol 92: ((2019) ), 33–39. |
[14] | R.F. Koncar, R. Feldman, E.M. Bahassi and N. Hashemi Sadraei, Comparative molecular profiling of HPV-induced squamous cell carcinomas, Cancer Med 6: ((2017) ), 1673–1685. |
[15] | M. Ashizawa, M. Saito, A.K.T. Min, D. Ujiie, K. Saito, T. Sato, T. Kikuchi, H. Okayama, S. Fujita, H. Endo, W. Sakamoto, T. Momma, S. Ohki, A. Goto and K. Kono, Prognostic role of ARID1A negative expression in gastric cancer, Sci Rep 9: ((2019) ), 6769. |
[16] | Y. Geng, H. Wang, C. Lu, Q. Li, B. Xu, J. Jiang and C. Wu, Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance, Int J Clin Oncol 20: ((2015) ), 273–81. |
[17] | E. Gkika, M. Benndorf, B. Oerther, F. Mohammad, S. Beitinger, S. Adebahr, M. Carles, T. Schimek-Jasch, C. Zamboglou, B.C. Frye, F. Bamberg, C.F. Waller, M. Werner, A.L. Grosu, U. Nestle and G. Kayser, Immunohistochemistry and Radiomic Features for Survival Prediction in Small Cell Lung Cancer, Front Oncol 10: ((2020) ), 1161. |
[18] | C. Sun, L. Zhang, W. Zhang, Y. Liu, B. Chen, S. Zhao, W. Li, L. Wang, L. Ye, K. Jia, H. Wang, C. Wu, Y. He and C. Zhou, Expression of PD-1 and PD-L1 on Tumor-Infiltrating Lymphocytes Predicts Prognosis in Patients with Small-Cell Lung Cancer, Onco Targets Ther 13: ((2020) ), 6475–6483. |
[19] | S. Inaguma, Z. Wang, J. Lasota, M. Sarlomo-Rikala, P.A. McCue, H. Ikeda and M. Miettinen, Comprehensive Immunohistochemical Study of Programmed Cell Death Ligand 1 (PD-L1): Analysis in 5536 Cases Revealed Consistent Expression in Trophoblastic Tumors, Am J Surg Pathol 40: ((2016) ), 1133–42. |
[20] | G. Kan and W. Dong, The expression of PD-L1 APE1 and P53 in hepatocellular carcinoma and its relationship to clinical pathology, Eur Rev Med Pharmacol Sci 19: ((2015) ), 3063–71. |
[21] | B.H. Nguyen, R. Montgomery, M. Fadia, J. Wang and S. Ali, PD-L1 expression associated with worse survival outcome in malignant pleural mesothelioma, Asia Pac J Clin Oncol 14: ((2018) ), 69–73. |
[22] | C. Combaz-Lair, F. Galateau-Salle, A. McLeer-Florin, N. Le Stang, L. David-Boudet, M. Duruisseaux, G.R. Ferretti, E. Brambilla, S. Lebecque and S. Lantuejoul, Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas, Hum Pathol 52: ((2016) ), 9–18. |
[23] | S. Kintsler, M.A. Cassataro, M. Drosch, P. Holenya, R. Knuechel and T. Braunschweig, Expression of programmed death ligand (PD-L1) in different tumors. Comparison of several current available antibody clones and antibody profiling, Ann Diagn Pathol 41: ((2019) ), 24–37. |
[24] | Z. Gatalica, C. Snyder, T. Maney, A. Ghazalpour, D.A. Holterman, N. Xiao, P. Overberg, I. Rose, G.D. Basu, S. Vranic, H.T. Lynch, D.D. Von Hoff and O. Hamid, Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type, Cancer Epidemiol Biomarkers Prev 23: ((2014) ), 2965–70. |
[25] | A. Torabi, C.N. Amaya, F.H. Wians Jr., and B.A. Bryan, PD-1 and PD-L1 expression in bone and soft tissue sarcomas, Pathology 49: ((2017) ), 506–513. |
[26] | H.K. Park, M. Kim, M. Sung, S.E. Lee, Y.J. Kim and Y.L. Choi, Status of programmed death-ligand 1 expression in sarcomas, J Transl Med 16: ((2018) ), 303. |
[27] | P.B. Googe, K. Flores, F. Jenkins, B. Merritt, S.J. Moschos and J.E. Grilley-Olson, Immune Checkpoint Markers in Superficial Angiosarcomas: PD-L1, PD-1, CD8, LAG-3, and Tumor-Infiltrating Lymphocytes, Am J Dermatopathol 43: ((2021) ), 556–559. |
[28] | A.M. Dancau, R. Simon, M. Mirlacher and G. Sauter, Tissue Microarrays, Methods Mol Biol 1381: ((2016) ), 53–65. |
[29] | J. Kononen, L. Bubendorf, A. Kallioniemi, M. Barlund, P. Schraml, S. Leighton, J. Torhorst, M.J. Mihatsch, G. Sauter and O.P. Kallioniemi, Tissue microarrays for high-throughput molecular profiling of tumor specimens, Nat Med 4: ((1998) ), 844–7. |
[30] | N.C. Blessin, R. Abu-Hashem, T. Mandelkow, W. Li, R. Simon, C. Hube-Magg, C. Möller-Koop, M. Witt, F. Büscheck, C. Fraune, A.M. Luebke, K. Möller, F. Jacobsen, F. Lutz, M. Lennartz, S. Steurer, G. Sauter, D. Höflmayer, M.C. Tsourlakis, A. Hinsch, E. Burandt, W. Wilczak, S. Minner and T. Clauditz, Prevalance of proliferating CD8+ cells in normal lymphatic tissues, inflammation and cancer, Journal of Translational Medicine (submitted) ((2020) ). |
[31] | JMP®V, SAS Institute Inc, Cary, NC, https://www.jmp.com ((2019) ). |
[32] | R-Core-Team, R: A language and environment for statistical computing, V. R Foundation for Statistical Computing, Austria ed. eds., https://www.R-project.org/ ((2019) ). |
[33] | S. Tippmann, Programming tools: Adventures with R, Nature 517: ((2015) ), 109–10. |
[34] | R. Buttner, J.R. Gosney, B.G. Skov, J. Adam, N. Motoi, K.J. Bloom, M. Dietel, J.W. Longshore, F. Lopez-Rios, F. Penault-Llorca, G. Viale, A.C. Wotherspoon, K.M. Kerr and M.S. Tsao, Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer, J Clin Oncol 35: ((2017) ), 3867–3876. |
[35] | G.J. Hanna, A.J. Kacew, A.R. Tanguturi, H.J. Grote, V. Vergara, B. Brunkhorst, G. Rabinowits, M. Thakuria, N.R. LeBoeuf, C. Ihling, J.A. DeCaprio and J.H. Lorch, Association of Programmed Death 1 Protein Ligand (PD-L1) Expression With Prognosis in Merkel Cell Carcinoma, Front Med (Lausanne) 7: ((2020) ), 198. |
[36] | Y. Toda, K. Kohashi, Y. Yamada, M. Yoshimoto, S. Ishihara, Y. Ito, T. Iwasaki, H. Yamamoto, Y. Matsumoto, Y. Nakashima, M. Mawatari and Y. Oda, PD-L1 and IDO1 expression and tumor-infiltrating lymphocytes in osteosarcoma patients: comparative study of primary and metastatic lesions, J Cancer Res Clin Oncol 146: ((2020) ), 2607–2620. |
[37] | K. Zwaenepoel, J. Jacobs, A. De Meulenaere, K. Silence, E. Smits, V. Siozopoulou, E. Hauben, C. Rolfo, S. Rottey and P. Pauwels, CD70 and PD-L1 in anaplastic thyroid cancer – promising targets for immunotherapy, Histopathology 71: ((2017) ), 357–365. |
[38] | I. Calik, M. Calik, G. Turken, I.H. Ozercan, A.F. Dagli, G. Artas and B. Sarikaya, Intratumoral Cytotoxic T-Lymphocyte Density and PD-L1 Expression Are Prognostic Biomarkers for Patients with Colorectal Cancer, Medicina (Kaunas) 55: ((2019) ). |
[39] | A.C. Eriksen, F.B. Sorensen, J. Lindebjerg, H. Hager, R. dePont Christensen, S. Kjaer-Frifeldt and T.F. Hansen, Programmed Death Ligand-1 expression in stage II colon cancer – experiences from a nationwide populationbased cohort, BMC Cancer 19: ((2019) ), 142. |
[40] | E.D. Thompson, M. Zahurak, A. Murphy, T. Cornish, N. Cuka, E. Abdelfatah, S. Yang, M. Duncan, N. Ahuja, J.M. Taube, R.A. Anders and R.J. Kelly, Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma, Gut 66: ((2017) ), 794–801. |
[41] | R. Saito, H. Abe, A. Kunita, H. Yamashita, Y. Seto and M. Fukayama, Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1(+) immune cells in Epstein-Barr virus-associated gastric cancer: the prognostic implications, Mod Pathol 30: ((2017) ), 427–439. |
[42] | S. Sahin, S. Batur, O. Aydin, T. Ozturk, A. Turna and B. Oz, Programmed Death-Ligand-1 Expression in Non-Small Cell Lung Cancer and Prognosis, Balkan Med J 36: ((2019) ), 184–189. |
[43] | T. Tokito, K. Azuma, A. Kawahara, H. Ishii, K. Yamada, N. Matsuo, T. Kinoshita, N. Mizukami, H. Ono, M. Kage and T. Hoshino, Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy, Eur J Cancer 55: ((2016) ), 7–14. |
[44] | L. Shi, S.J. Zhang, J. Chen, S.X. Lu, X.J. Fan, J.H. Tong, C. Chow, E.K. Tin, S.L. Chan, C.C. Chong, P.B. Lai, K.F. To, N. Wong and A.W. Chan, A comparability study of immunohistochemical assays for PD-L1 expression in hepatocellular carcinoma, Mod Pathol 32: ((2019) ), 1646–1656. |
[45] | D.J. Pinato, F.A. Mauri, P. Spina, O. Cain, A. Siddique, R. Goldin, S. Victor, C. Pizio, A.U. Akarca, R.L. Boldorini, L. Mazzucchelli, J.R.M. Black, S. Shetty, T. Marafioti and R. Sharma, Clinical implications of heterogeneity in PD-L1 immunohistochemical detection in hepatocellular carcinoma: the Blueprint-HCC study, Br J Cancer 120: ((2019) ), 1033–1036. |
[46] | M.J. Ratcliffe, A. Sharpe, A. Midha, C. Barker, M. Scott, P. Scorer, H. Al-Masri, M.C. Rebelatto and J. Walker, Agreement between Programmed Cell Death Ligand-1 Diagnostic Assays across Multiple Protein Expression Cutoffs in Non-Small Cell Lung Cancer, Clin Cancer Res 23: ((2017) ), 3585–3591. |
[47] | E. Torlakovic, H.J. Lim, J. Adam, P. Barnes, G. Bigras, A.W.H. Chan, C.C. Cheung, J.H. Chung, C. Couture, P.O. Fiset, D. Fujimoto, G. Han, F.R. Hirsch, M. Ilie, D. Ionescu, C. Li, E. Munari, K. Okuda, M.J. Ratcliffe, D.L. Rimm, C. Ross, R. Roge, A.H. Scheel, R.A. Soo, P.E. Swanson, M. Tretiakova, K.F. To, G.W. Vainer, H. Wang, Z. Xu, D. Zielinski and M.S. Tsao, “Interchangeability” of PD-L1 immunohistochemistry assays: a meta-analysis of diagnostic accuracy, Mod Pathol 33: ((2020) ), 4–17. |
[48] | Q.H. Zhou, K.W. Li, X. Chen, H.X. He, S.M. Peng, S.R. Peng, Q. Wang, Z.A. Li, Y.R. Tao, W.L. Cai, R.Y. Liu and H. Huang, HHLA2 and PD-L1 co-expression predicts poor prognosis in patients with clear cell renal cell carcinoma, |
[49] | A.M. Valentini, F. Di Pinto, F. Cariola, V. Guerra, G. Giannelli, M.L. Caruso and M. Pirrelli, PD-L1 expression in colorectal cancer defines three subsets of tumor immune microenvironments, Oncotarget 9: ((2018) ), 8584–8596. |
[50] | L.H. Schmidt, A. Kummel, D. Gorlich, M. Mohr, S. Brockling, J.H. Mikesch, I. Grunewald, A. Marra, A.M. Schultheis, E. Wardelmann, C. Muller-Tidow, T. Spieker, C. Schliemann, W.E. Berdel, R. Wiewrodt and W. Hartmann, PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups, PLoS One 10: ((2015) ), e0136023. |
[51] | S. Kim, M.Y. Kim, J. Koh, H. Go, D.S. Lee, Y.K. Jeon and D.H. Chung, Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas, Eur J Cancer 51: ((2015) ), 2698–707. |
[52] | S. Rahn, S. Kruger, R. Mennrich, L. Goebel, D. Wesch, H.H. Oberg, I. Vogel, M. Ebsen, C. Rocken, O. Helm and S. Sebens, POLE Score: a comprehensive profiling of programmed death 1 ligand 1 expression in pancreatic ductal adenocarcinoma, Oncotarget 10: ((2019) ), 1572–1588. |
[53] | M. Mirlacher, M. Kasper, M. Storz, Y. Knecht, U. Durmuller, R. Simon, M.J. Mihatsch and G. Sauter, Influence of slide aging on results of translational research studies using immunohistochemistry, Mod Pathol 17: ((2004) ), 1414–20. |
[54] | U. Manne, R.B. Myers, S. Srivastava and W.E. Grizzle, Re: loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer, J Natl Cancer Inst 89: ((1997) ), 585–6. |
[55] | T.W. Jacobs, J.E. Prioleau, I.E. Stillman and S.J. Schnitt, Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer, J Natl Cancer Inst 88: ((1996) ), 1054–9. |
[56] | J. Torhorst, C. Bucher, J. Kononen, P. Haas, M. Zuber, O.R. Kochli, F. Mross, H. Dieterich, H. Moch, M. Mihatsch, O.P. Kallioniemi and G. Sauter, Tissue microarrays for rapid linking of molecular changes to clinical endpoints, Am J Pathol 159: ((2001) ), 2249–56. |
[57] | M.A. Rubin, R. Dunn, M. Strawderman and K.J. Pienta, Tissue microarray sampling strategy for prostate cancer biomarker analysis, Am J Surg Pathol 26: ((2002) ), 312–9. |
[58] | A. Hoos, M.J. Urist, A. Stojadinovic, S. Mastorides, M.E. Dudas, D.H. Leung, D. Kuo, M.F. Brennan, J.J. Lewis and C. Cordon-Cardo, Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors, Am J Pathol 158: ((2001) ), 1245–51. |
[59] | R.L. Camp, L.A. Charette and D.L. Rimm, Validation of tissue microarray technology in breast carcinoma, Lab Invest 80: ((2000) ), 1943–9. |
[60] | H.H. Lee, Y.N. Wang, W. Xia, C.H. Chen, K.M. Rau, L. Ye, Y. Wei, C.K. Chou, S.C. Wang, M. Yan, C.Y. Tu, T.C. Hsia, S.F. Chiang, K.S.C. Chao, I.I. Wistuba, J.L. Hsu, G.N. Hortobagyi and M.C. Hung, Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy, Cancer Cell 36: ((2019) ), 168–178.e4. |
[61] | R. Kollipara, B. Schneider, M. Radovich, S. Babu and P.J. Kiel, Exceptional Response with Immunotherapy in a Patient with Anaplastic Thyroid Cancer, Oncologist 22: ((2017) ), 1149–1151. |
[62] | W. Abbas, S. Gupta, V. Goel, R.R. Rao, P. Pankaj, D. Tripathi, P.P. Patil and S. Popli, Real-World Experience of Immunotherapy from India in Recurrent Squamous Cell Carcinoma of Head and Neck Cancer, South Asian J Cancer 10: ((2021) ), 72–75. |
[63] | Y. Sato, N. Fukuda, X. Wang, T. Urasaki, A. Ohmoto, K. Nakano, M. Yunokawa, M. Ono, Y. Sato, H. Mitani, J. Tomomatsu and S. Takahashi, Efficacy of Nivolumab for Head and Neck Cancer Patients with Primary Sites and Histological Subtypes Excluded from the CheckMate-141 Trial, Cancer Manag Res 12: ((2020) ), 4161–4168. |
[64] | P. Szturz and J.B. Vermorken, Management of recurrent and metastatic oral cavity cancer: Raising the bar a step higher, Oral Oncol 101: ((2020) ), 104492. |
[65] | R.L. Ferris, G. Blumenschein, Jr., J. Fayette, J. Guigay, A.D. Colevas, L. Licitra, K.J. Harrington, S. Kasper, E.E. Vokes, C. Even, F. Worden, N.F. Saba, L.C.I. Docampo, R. Haddad, T. Rordorf, N. Kiyota, M. Tahara, M. Lynch, V. Jayaprakash, L. Li and M.L. Gillison, Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression, Oral Oncol 81: ((2018) ), 45–51. |
[66] | J.D. Schoenfeld, G.J. Hanna, V.Y. Jo, B. Rawal, Y.H. Chen, P.S. Catalano, A. Lako, Z. Ciantra, J.L. Weirather, S. Criscitiello, A. Luoma, N. Chau, J. Lorch, J.I. Kass, D. Annino, L. Goguen, A. Desai, B. Ross, H.J. Shah, H.A. Jacene, D.N. Margalit, R.B. Tishler, K.W. Wucherpfennig, S.J. Rodig, R. Uppaluri and R.I. Haddad, Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial, JAMA Oncol 6: ((2020) ), 1563–1570. |
[67] | T. Satoh, K. Kato, T. Ura, Y. Hamamoto, T. Kojima, T. Tsushima, S. Hironaka, H. Hara, S. Iwasa, K. Muro, H. Yasui, K. Minashi, K. Yamaguchi, A. Ohtsu, Y. Doki, Y. Matsumura and Y. Kitagawa, Five-year follow-up of nivolumab treatment in Japanese patients with esophageal squamous-cell carcinoma (ATTRACTION-1/ONO-4538-07), Esophagus 18: ((2021) ), 835–843. |
[68] | M. Takahashi, K. Kato, M. Okada, K. Chin, S. Kadowaki, Y. Hamamoto, Y. Doki, Y. Kubota, H. Kawakami, T. Ogata, H. Hara, M. Muto, Y. Nakashima, R. Ishihara, M. Tsuda, S. Motoyama, M. Kodani and Y. Kitagawa, Nivolumab versus chemotherapy in Japanese patients with advanced esophageal squamous cell carcinoma: a subgroup analysis of a multicenter, randomized, open-label, phase 3 trial (ATTRACTION-3), Esophagus 18: ((2021) ), 90–99. |
[69] | A. Wessely, T. Steeb, U. Leiter, C. Garbe, C. Berking and M.V. Heppt, Immune Checkpoint Blockade in Advanced Cutaneous Squamous Cell Carcinoma: What Do We Currently Know in 2020?, |
[70] | J.F. Grau, L. Farinas-Madrid and A. Oaknin, A randomized phase III trial of platinum chemotherapy plus paclitaxel with bevacizumab and atezolizumab versus platinum chemotherapy plus paclitaxel and bevacizumab in metastatic (stage IVB), persistent, or recurrent carcinoma of the cervix: the BEATcc study (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030), Int J Gynecol Cancer 30: ((2020) ), 139–143. |
[71] | M.C. Comiskey, M.C. Dallos and C.G. Drake, Immunotherapy in Prostate Cancer: Teaching an Old Dog New Tricks, Curr Oncol Rep 20: ((2018) ), 75. |
[72] | H. Xiong, S. Mittman, R. Rodriguez, M. Moskalenko, P. Pacheco-Sanchez, Y. Yang, D. Nickles and R. Cubas, Anti-PD-L1 Treatment Results in Functional Remodeling of the Macrophage Compartment, Cancer Res 79: ((2019) ), 1493–1506. |
[73] | R.S. Hoda, E. Brogi, C.H. Dos Anjos, A. Grabenstetter, K. Ventura, S. Patil, P. Selenica, B. Weigelt, J.S. Reis-Filho, T. Traina, M. Robson, L. Norton and H.Y. Wen, Clinical and pathologic features associated with PD-L1 (SP142) expression in stromal tumor-infiltrating immune cells of triple-negative breast carcinoma, Mod Pathol 33: ((2020) ), 2221–2232. |
[74] | P. Schmid, S. Adams, H.S. Rugo, A. Schneeweiss, C.H. Barrios, H. Iwata, V. Dieras, R. Hegg, S.A. Im, G. Shaw Wright, V. Henschel, L. Molinero, S.Y. Chui, R. Funke, A. Husain, E.P. Winer, S. Loi, L.A. Emens and IMpassion130 Trial Investigators, Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer, N Engl J Med 379: ((2018) ), 2108–2121. |
[75] | R.S.P. Huang, J. Haberberger, E. Severson, D.L. Duncan, A. Hemmerich, C. Edgerly, N.L. Ferguson, E. Williams, J. Elvin, J.A. Vergilio, J.K. Killian, D.I. Lin, J. Tse, M. Hiemenz, C. Owens, N. Danziger, P.S. Hegde, J. Venstrom, B. Alexander, J.S. Ross and S.H. Ramkissoon, A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and microsatellite instability in 48,782 cases, Mod Pathol 34: ((2021) ), 252–263. |




