Comparison of CINtec PLUS cytology and cobas HPV test for triaging Canadian patients with LSIL cytology referred to colposcopy: A two-year prospective study
Abstract
OBJECTIVES & METHODS:
CINtec PLUS and cobas HPV tests were compared for triaging patients referred to colposcopy with a history of LSIL cytology in a 2-year prospective study. Cervical specimens were tested once at enrollment, and test positivity rates determined. Test performance was ascertained with cervical intraepithelial neoplasia grade 2 or worse (CIN2
RESULTS:
In all ages, (19–76 years,
CONCLUSIONS:
CINtec PLUS or cobas HPV test could serve as a predictor of CIN3
1.Introduction
While some countries have successfully transitioned to human papillomavirus (HPV) primary cervical cancer screening, Papanicolaou cytology remains the mode of primary screening in many jurisdictions, including Canada, for a variety of reasons. In cytology-based cervical cancer screening, a large number of patients are diagnosed as having borderline or low-grade abnormal cytology who are managed at considerable costs, while only a small fraction is at risk. It could be beneficial to consider currently available triage options, especially in managing patients referred to colposcopy with a history of low-grade squamous intraepithelial lesion (LSIL), in routine colposcopy clinical practice.
LSIL accounts for a large proportion of abnormal cytology in routine screening but it regresses in the majority of cases. However, a small fraction has high grade squamous intraepithelial lesions (HSIL) or could be at risk of progression to HSIL and cervical cancer. Due to this risk, those found to have LSIL in routine screening are either referred to colposcopy directly or managed cytologically, with those having persistent abnormalities being referred to colposcopy [1, 2, 3]. In colposcopy clinics, LSIL cases are typically followed with cytology, colposcopy, and biopsy as indicated, for an extended period. With the majority being not at risk, this is excessive and unnecessary for most patients and associated with considerable negative health effects due to distress over prolonged period, increased anxiety at every clinic visit, unnecessary invasive procedures, and overtreatment etc., leading to poorer quality of life [4, 5]. An effective triage of LSIL referral patients can identify those at increased risk who need to remain under care and return those not at immediate risk to routine screening [4, 6], thus eliminating potential negative health effects and reducing systemic costs.
The CINtec PLUS cytology (Roche Diagnostics) has emerged as an effective biomarker-based adjunct test for triaging patients having atypical squamous cells of undetermined significance (ASCUS) or LSIL in cytology screening [5, 7, 8, 9, 10, 11] and those testing positive for high-risk human papillomavirus (hr-HPV) in HPV primary screening [12, 13, 14, 15]. CINtec PLUS is a dual-stain immunocytochemical test which detects p16 and Ki-67 proteins that are over expressed in cervical cells with transforming HPV infection. As the expression of p16 and Ki-67 is mutually exclusive in normal cells, the co-detection of these proteins simultaneously within the same cervical epithelial cell serves as a specific marker of HPV-mediated oncogenic transformation and predictor of cervical cancer risk [5, 7, 8, 9, 10, 16]. CINtec PLUS has been shown to be more sensitive than cytology with equal specificity, and more specific than HPV testing with relatively comparable sensitivity for detecting cervical intraepithelial neoplasia grade 2 or worse (CIN2
The ALTS LSIL study precluded LSIL-HPV triage as 83% of LSIL cases tested hr-HPV positive [24]. However, this study was conducted in patients mostly
We conducted a study to assess positivity rates of CINtec PLUS and cobas HPV tests along with genotype 16/18-specific risk threshold among those referred to colposcopy with a history of LSIL, to identify those at increased risk and thus potentially reduce the proportion requiring further colposcopy clinic visits and follow-up, and prospectively determined clinical efficacy of the two tests to detect CIN2
2.Methods
2.1Ontario cervical cancer screening guidelines
In the province of Ontario, Canada, liquid-based Papanicolaou cytology is being used for primary cervical cancer screening. In this system, if cytology is normal, triennial screening continues. For those with LSIL cytology, either direct referral to colposcopy or repeat cytology at 6-month intervals is recommended; for those having persistent atypical squamous cells of undetermined significance (ASCUS) or worse in repeat cytology, colposcopy is recommended [1]. In colposcopy clinics, all referred patients undergo cytology and colposcopic examination with biopsies of any lesions detected, and further follow-up clinical pathways depend on specific criteria as previously described [16].
2.2Study design and protocol
The study was designed to assess CINtec PLUS cytology and HPV test positivity at baseline (enrollment) to identify the proportion potentially at increased risk, and therefore, requiring continued follow-up in the colposcopy clinic, and conversely, the proportion that could be returned to routine screening, thus improving overall clinical and systemic efficiency. In relation to this, the study was also designed to determine clinical efficacy of CINtec PLUS and HPV tests to detect CIN2
The study was conducted within the Ontario cervical screening guidelines. The study population comprised of patients with a history of LSIL cytology referred to the colposcopy clinic at Juravinski Hospital, Hamilton, Canada. All study patients were attended to per standard of care, with cervical specimens collected for cytology, and colposcopy and biopsies performed per routine clinical practice. Cytology was carried out as part of routine patient care, and CINtec PLUS and cobas HPV tests were performed once at enrollment using the residual cervical specimens for the study purpose. Patients’ baseline data were recorded, and the study cohort remaining under care in the colposcopy clinic was followed up to 2 years to determine disease outcome. Biopsy confirmed CIN2
2.3Ethics
The study was approved by the Hamilton Integrated Research Ethics Board (HiREB) and Newfoundland and Labrador Health Research Ethics Board (HREB). All participants were informed verbally and in writing about the study, use of their residual cervical specimens for CINtec PLUS and HPV testing, and the need to periodically review their medical records during follow-up. Those consenting to participate were enrolled with written informed consent.
2.4Patient enrolment criteria
Patients with a history of LSIL cytology who had not received treatment were eligible. Enrolment criteria included: 1) patients who had LSIL cytology in routine primary screening and who were directly referred to colposcopy, 2) those who were found to have LSIL cytology initially in routine primary screening and who upon repeat cytology found to have persistent ASCUS or LSIL and referred to colposcopy, and 3) those who were diagnosed as having LSIL among patients being followed in the colposcopy clinic. There were no age limits. Pregnant persons and those without a cervix were excluded. Eligible patients were enrolled consecutively from November 2017 through February 2019.
2.5Study specimens
Cervical specimens were collected into ThinPrep PreservCyt
2.6CINtec PLUS cytology
Slides were prepared on a ThinPrep processor (T5000, Hologic Inc) using special ThinPrep slides (Hologic, Inc) and stained using CINtec PLUS test kits within 48 hours and processed on BenchMark ULTRA system (Roche Diagnostics) per manufacturer’s instructions.
The CINtec PLUS slides were initially evaluated independently by one of two experienced cytotechnologists who were trained to read these slides. Smears were determined to be positive if at least one cervical epithelial cell showed both a brownish cytoplasmic immunostaining for p16 and a red nuclear immunostaining for Ki-67 regardless of cellular morphology. If the dual staining was not observed, the smear was considered negative. Smears were deemed unsatisfactory if they did not contain an adequate number of cells (
2.7cobas HPV test
The cobas HPV test was performed on the Roche 4800 automated platform per manufacturer’s instructions. Results were reported as positive for genotypes 16 and/or 18, and/or 12 OHR types, or negative for 14 hr-HPV types, per standard practice.
2.8Cervical biopsy
Biopsies were performed by colposcopists per standard clinical practice. Three sections of each biopsy sample were processed with hematoxylin and eosin (H&E) staining per routine practice. p16 immunostaining (CINtec
2.9Results management
CINtec PLUS and HPV tests were conducted independently. Cytotechnologists and the study pathologists were blinded to test results as well as cytology and biopsy results obtained at baseline. Colposcopy clinicians did not have access to CINtec PLUS or HPV results at the time of initial patient evaluation. Only HPV results were provided subsequently to clinicians to aid in patient management.
2.10Data analysis
Statistical analysis was performed using SPSS for Windows, versions 23 and 27, Excel, Microsoft Office Professional Plus, 2013, MedCalc, 2021, and Social Science Statistics website, 2020 initially at baseline, as previously described [16], and after two-years of prospective follow-up. Qualitative variables were studied through different frequencies. Descriptive statistics were prepared for test results, distribution of cytology grades and HPV genotypes. Study data were analyzed using contingency tables to determine test positivity rates, and the diagnostic indices of CINtec PLUS and HPV testing. Receiver operating characteristic (ROC) analyses were performed for CINtec PLUS and HPV testing in detecting CIN2
Table 1
CINtec PLUS and HPV test results by age groups
| Test | Result | All ages, | |||
|---|---|---|---|---|---|
| CINtec | Positive | 265 (44.3%) | 107 (50.0%) | 158 (41.1%) | |
| PLUS | Negative | 333 (55.7%) | 107 (50.0%) | 226 (58.9%) | compared between women |
| HPV | Positive | 331 (55.4%) | 135 (63.1%) | 196 (51.0%) | |
| Negative | 267 (44.6%) | 79 (36.9%) | 188 (49.0%) | compared between women | |
Figure 1.
Study scheme: patient enrollment, CINtec PLUS and HPV test results and biopsy outcome of clinical assessments at baseline and during follow-up.
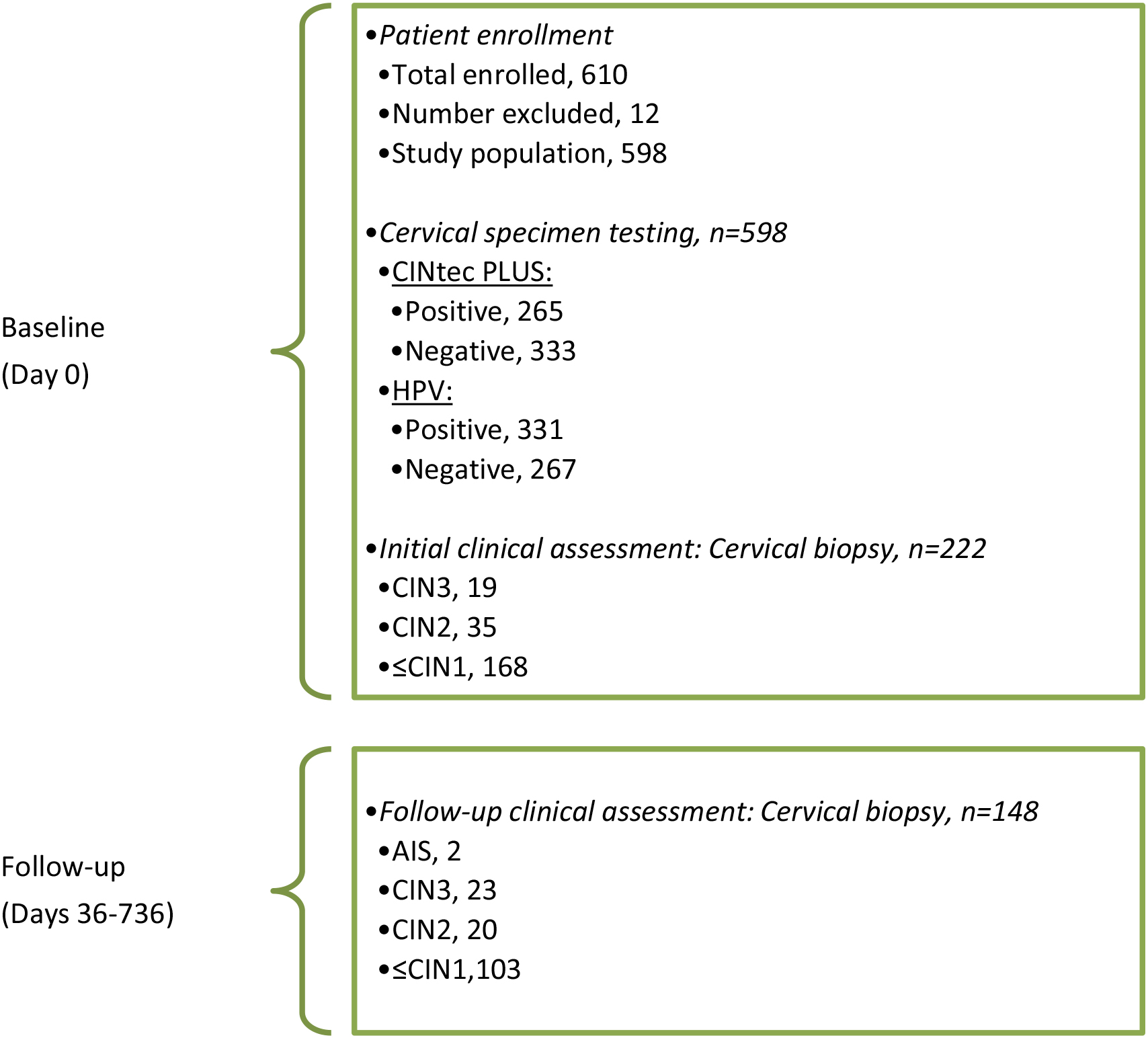
AIS, Adenocarcinoma in situ; CIN3, Cervical intraepithelial neoplasia grade 3; CIN2, CIN grade 2;
3.Results
3.1Study population
A total of 610 patients meeting the study criteria were enrolled in the study. Of these, 12 were excluded due to insufficient or no cervical specimen for CINtec PLUS and/or HPV testing, or invalid CINtec PLUS or HPV test results, leaving 598 patients in the study with evaluable results (Fig. 1). Age ranged from 19 to 76 years (median, 33.0), with 384 (64.2%)
Although the index referral cytology was LSIL in all patients enrolled per study criterion, cytology performed at the time of enrollment showed a heterogeneous cytological grade as expected. The time interval between the index referral LSIL cytology immediately prior to the colposcopy clinic visit and cytology performed in the colposcopy clinic at the time of enrollment ranged from
Of the 598 patients in the study, per standard practice, biopsies were only performed when clinically indicated. As such, there were 222 evaluable cervical biopsy results available at baseline, and among them 54 (24.3%) were diagnosed as CIN2
3.2CINtec PLUS and HPV test results
Table 1 shows CINtec PLUS and cobas HPV results for the total population of 598 for all ages, and those
Figure 2.
Temporal distribution of CIN2
3.3Performance of CINtec PLUS and HPV tests to detect CIN2+ +
Of the 598 patients in all ages, a total of 356 (59.5%) had evaluable biopsy results. Among the 356, as indicated above, biopsy confirmed CIN2
Table 3 illustrates the performance of CINtec PLUS in comparison with HPV testing in detecting 99 CIN2
Table 3 shows the performance of CINtec PLUS compared with HPV testing in detecting 44 CIN3
3.4Performance of CINtec PLUS and HPV tests to detect incident CIN2+
Of the 45 incident CIN2
3.5ROC analysis
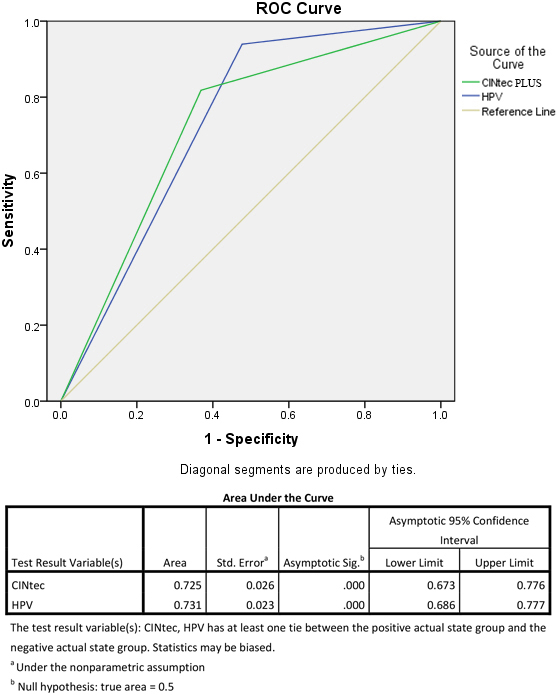
Figure 3 shows the results of ROC analysis comparing the overall performance characteristics of CINtec PLUS with HPV testing in detecting CIN2
3.6Genotype specific risk threshold to detect CIN2+ +
Table 4 shows HPV genotypes 16/18-specific results in comparison with hr-HPV testing to detect CIN2
Table 2
Diagnostic indices of CINtec PLUS and HPV tests for detecting CIN2
| Test | Sensitivity | Specificity | Positive predictive value | Negative predictive value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| % (95% CI) |
|
| % (95% CI) |
|
| % (95% CI) |
|
| % (95% CI) |
| |
| All ages ( | ||||||||||||
| CINtec PLUS | 81/99 | 81.8 (72.8–88.9) | 0.009 | 136/257 | 52.9 (46.6–59.2) | 81/202 | 40.1 (36.3–44.0) | 0.407 | 136/154 | 88.3 (83.0–92.1) | 0.131 | |
| HPV | 93/99 | 93.9 (87.3–97.7) | 94/257 | 36.6 (30.7–42.8) | 93/256 | 36.3 (33.9–38.8) | 94/100 | 94.0 (87.7–97.2) | ||||
| CINtec PLUS | 38/50 | 76.0 (61.8–86.9) | 0.012 | 40/78 | 51.3 (39.7–62.8) | 0.023 | 38/76 | 50.0 (43.2–56.9) | 0.741 | 40/52 | 76.9 (66.1–85.1) | 0.159 |
| HPV | 47/50 | 94.0 (83.5–98.8) | 26/78 | 33.3 (23.1–44.9) | 47/99 | 47.5 (43.2–51.8) | 26/29 | 89.7 (73.5–96.5) | ||||
| CINtec PLUS | 43/49 | 87.8 (75.2–95.4) | 0.294 | 96/179 | 53.6 (46.0–61.1) | 0.003 | 43/126 | 34.1 (30.0–38.5) | 0.384 | 96/102 | 94.1 (88.2–97.2) | 0.063 |
| HPV | 46/49 | 93.9 (83.1–98.7) | 68/179 | 38.0 (30.6–45.5) | 46/157 | 29.3 (26.6–32.2) | 68/71 | 95.8 (88.2–98.6) | ||||
Table 3
Diagnostic indices of CINtec PLUS and HPV tests for detecting CIN3
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| % (95% CI) |
|
| % (95% CI) |
|
| % (95%CI) |
|
| % (95% CI) |
| |
| All ages ( | ||||||||||||
| CINtec PLUS | 41/44 | 93.2 (81.3–98.6) | 1.000 | 151/312 | 48.4 (42.7–54.1) | 41/202 | 20.3 (18.2–22.6) | 0.234 | 151/154 | 98.1 (94.4–99.3) | 0.589 | |
| HPV | 41/44 | 93.2 (81.3–98.6) | 97/312 | 31.1 (26.0–36.6) | 41/256 | 16.0 (14.6–17.5) | 97/100 | 97.0 (91.5–99.0) | ||||
| CINtec PLUS | 22/24 | 91.7 (73.0–99.0) | 0.549 | 50/104 | 48.1 (38.2–58.1) | 0.002 | 22/76 | 29.0 (24.6–33.7) | 0.390 | 50/52 | 96.2 (86.7–99.0) | 0.928 |
| HPV | 23/24 | 95.8 (78.9–99.9) | 28/104 | 26.9 (18.7–36.5) | 23/99 | 23.2 (20.8–25.9) | 28/29 | 96.6 (80.0–99.5) | ||||
| CINtec PLUS | 19/20 | 95.0 (75.1–99.9) | 0.549 | 101/208 | 48.6 (41.6–55.6) | 0.001 | 19/126 | 15.1 (13.1–17.3) | 0.368 | 101/102 | 99.0 (93.7–99.9) | 0.363 |
| HPV | 18/20 | 90.0 (68.3–98.8) | 69/208 | 33.2 (26.8–40.0) | 18/157 | 11.5 (9.8–13.4) | 69/71 | 97.2 (90.1–99.2) | ||||
Table 4
HPV genotypes 16/18-specific testing compared with hr-HPV testing to detect CIN2
| Test | CIN2 | CIN3 | ||||||||||
| Sensitivity | Specificity | Sensitivity | Specificity | |||||||||
|
| % |
|
| % |
|
| % |
|
| % |
| |
| All ages ( | ||||||||||||
| HPV16/18 | 46/99 | 46.5% | 225/257 | 87.5% | 23/44 | 52.3% | 257/312 | 82.4% | ||||
| hr-HPV | 93/99 | 93.9% | 94/257 | 36.6% | 41/44 | 93.2% | 97/312 | 31.1% | ||||
| HPV16/18 | 20/50 | 40.0% | 67/78 | 85.9% | 13/24 | 54.2% | 86/104 | 82.7% | ||||
| hr-HPV | 47/50 | 94.0% | 26/78 | 33.3% | 23/24 | 95.8% | 28/104 | 26.9% | ||||
| HPV16/18 | 26/49 | 53.1% | 158/179 | 88.3% | 10/20 | 50.0% | 0.006 | 171/208 | 82.2% | |||
| hr-HPV | 46/49 | 93.9% | 68/179 | 38.0% | 18/20 | 90.0% | 69/208 | 33.2% | ||||
| Based on 356 patients in all ages with biopsy. CIN2 | ||||||||||||
| neoplasia grade 3 or worse (includes 2 cases of Adenocarcinoma in situ). | ||||||||||||
Figure 3.
ROC curve for CINtec PLUS compared to HPV in detected CIN2
16/18-specific testing was 46.5% sensitive vs 93.9% for hr-HPV testing; for CIN3
4.Discussion
Our study showed an overall CIN2
Our CINtec PLUS positivity rate of 44.3% in LSIL was similar to other studies [11, 19], while the HPV positivity rate of 55.4% was significantly lower than those reported in concurrent LSIL [27, 28] (Table 1). This could be attributed to lesion regression in a large proportion of the LSIL referrals by the time they are seen in the colposcopy clinic, leading to lower HPV prevalence and test positivity rate, thus making HPV testing more effective in this setting as described previously [16]. Based on the above positivity rates, in all ages, CINtec PLUS would reduce the LSIL referral population requiring further investigations and follow-up in a colposcopy clinic by 55.7% (333/598) vs 44.6% (267/598) for HPV testing; these proportions would be higher in those
For CIN2
The use of CINtec PLUS in LSIL triage especially for patients
Although there are differences in the overall sensitivities and specificities of CINtec PLUS and HPV reported in other studies [8, 23, 31, 32] it is important to note that our study showed both tests having an identical high level of sensitivity to detect CIN3
We assessed the application of HPV genotypes 16/18-specific threshold in LSIL triage as these genotypes account for approximately 70% of cervical cancer [35, 36]. Our 16/18 positive proportion of 28% among LSILs is similar to those reported in other studies [23, 32]. If this threshold is used, it would mean reducing the number of LSIL referral cases requiring additional follow-up by more than 2/3rds. While this will greatly improve efficiency, it is significantly less sensitive for detecting both CIN2
In this study, we evaluated the application of CINtec PLUS and HPV tests for triaging LSIL referral patients since their risk stratification and management remain of clinical and programmatic importance in settings where cytology-based cervical screening is used. Overall, triaging LSIL referral populations could help to reduce the number of patients requiring further colposcopy clinic visits and additional investigations, thus decreasing burden on colposcopy clinics and eliminating potential negative health effects, consequently aiding in better patient care and resource management [20]. One aspect that can be difficult to quantify though is the reduction in patients’ anxiety and peace of mind by not having to continue colposcopy clinic visits for extended periods [5]. While not the focus of this work, reductions in number of patients requiring further clinic visits would also lead system efficiency by reducing waitlists and wait times for those who truly need colposcopy. Although it may be difficult to quantify the reduction in systems costs due to regional and programmatic differences [5], there are general system cost efficiencies to be found [5, 27]. Cost-effectiveness modelling with robust economics methodologies will provide important perspectives when assessing patient management strategies as health systems evolve [22].
Our study was carried out in a real-world colposcopy clinic setting without any intervention for study purpose. This shed some light on the lesion regression rate during the interval between referral LSIL cytology and colposcopy, and this has implications in risk stratification and follow-up, and could also impact the outcome of triage tests. Our data on the delay from referral LSIL to colposcopy may be generalizable in Canadian settings, but this may vary in other jurisdictions. One of the limitations of the study was that only a proportion underwent biopsy as clinically indicated and this prevented ascertaining biopsy-based disease outcome in the total study population. Regardless, in addition to 54 CIN2
5.Conclusions
Either CINtec PLUS cytology or the cobas HPV test could serve as a predictor of CIN3
Authors’ contributions
LG: Project administration, investigation, methodology, laboratory technical support, resources, data curation and analysis, writing-original draft and writing-review and editing; SR: Conceptualization, funding acquisition, methodology, supervision, data analysis, writing-review and editing; DJ: Project administration, supervision, data curation, resources, laboratory technical support, and writing-review and editing; RA and MS: Investigation, data analysis, validation, and writing-review and editing; RN: Investigation, data curation, laboratory technical support, and writing-review and editing; AEM: Study co-ordination and technical support; AW: Data base management and data analysis; PW: data analysis, writing-review and editing; DC, LE and GZ: Resources, and writing-review and editing; MC: Resources, methodology, supervision, and writing-review and editing.
All authors reviewed and approved the final version for submission. All authors attest they meet the ICMJE criteria for authorship.
Funding support
This study was supported by a research grant from Roche Diagnostics, Montreal, Canada. Roche Diagnostics also provided BenchMark ULTRA system, CINtec PLUS and cobas HPV test kits for the study, and training in CINtec PLUS testing, reading and interpretation. The study was investigator initiated and conducted independently. Roche Diagnostics offered suggestions with the study design, but did not have any input in the conduct, data analysis, or preparation and reporting of results of this study.
Acknowledgments
We thank Dr. Michele D’Elia, Roche Diagnostics, Montreal, Canada for his commitment, funding support and provision of BenchMark Ultra system and test kits for the study; Carolynn Frain, Roche Diagnostics, Montreal, Canada for CINtec PLUS cytology training. We also thank Mary Jane Carrier, and other colposcopy clinic medical and nursing staff, Juravinski Hospital, Hamilton for assistance with patient enrolment; Thomas Barrett and Kenneth Melvin, Regional Cytology Laboratory, Eastern Health, St. John’s for CINtec PLUS cytology support; Holly Edwards and Tania Smith, Eastern Health, St. John’s for administrative assistance. We acknowledge Dr. Diane Lamoureux, Hôpital Pierre Boucher, Longueuil, Quebec for adjudicating discrepant CINtec PLUS results.
Conflict of interest
Sam Ratnam received research grant from Roche Diagnostics. Max Chernesky received research grant unrelated to this study from Roche Diagnostics. Other members of the study team declare no conflict of interests.
References
[1] | Cancer Care Ontario, Ontario Cervical Screening Guidelines Summary, (2016) . |
[2] | T.C. Wright Jr, I.S. Massad, C.J. Dunton et al., 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests, J Low Genit Tract Dis 11: (4) ((2007) ), 201–222. |
[3] | L.S. Massad, M.H. Einstein, W.K. Huh et al., 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors, Journal of Lower Genital Tract Disease 17: (5 Suppl 1) ((2013) ), S1–S27. |
[4] | J. Cuzick, J.T. Cox, G. Zhang et al., Human papillomavirus testing for triage of women with low-grade squamous intraepithelial lesions, International Journal of Cancer 132: (4) ((2013) ), 959–966. |
[5] | W.A. Tjalma, E. Kim and K. Vandeweyer, The impact on women’s health and the cervical cancer screening budget of primary HPV screening with dual-stain cytology triage in Belgium, Eur J Obstet Gynecol Reprod Biol 212: ((2017) ), 171–181. |
[6] | G. Ronco, J. Cuzick, N. Segnan et al., HPV triage for low grade (L-SIL) cytology is appropriate for women over 35 in mass cervical cancer screening using liquid based cytology, European Journal of Cancer 43: (3) ((2007) ), 476–480. |
[7] | W.A. Tjalma, Diagnostic performance of dual-staining cytology for cervical cancer screening: A systematic literature review, European Journal of Obstetrics, Gynecology, and Reproductive Biology 210: ((2017) ), 275–280. |
[8] | H. Sun, K. Shen and D. Cao, Progress in immunocytochemical staining for cervical cancer screening, Cancer Management and Research 11: ((2019) ), 1817–1827. |
[9] | M. Sun, Y. Shen, M.L. Ren and Y.M. Dong, Meta-analysis on the performance of p16/Ki-67 dual immunostaining in detecting high-grade cervical intraepithelial neoplasm, J Cancer Res Ther 14: (Supplement) ((2018) ), S587–S593. |
[10] | L. Yu, L. Fei, X. Liu, X. Pi, L. Wang and S. Chen, Application of p16/Ki-67 dual-staining cytology in cervical cancers, Journal of Cancer 10: (12) ((2019) ), 2654–2660. |
[11] | C. Bergeron, H. Ikenberg, M. Sideri et al., Prospective evaluation of p16/Ki-67 dual-stained cytology for managing women with abnormal papanicolaou cytology: PALMS study results, Cancer Cytopathology 123: (6) ((2015) ), 373–381. |
[12] | M.A. Clarke, L.C. Cheung, P.E. Castle et al., Five-year risk of cervical precancer following p16-Ki-67 dual-stain triage of HPV-positive women, JAMA Oncology 5: (2) ((2019) ), 181–186. |
[13] | P. Guan, R. Howell-Jones, N. Li et al., Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer, International Journal of Cancer 131: (10) ((2012) ), 2349–2359. |
[14] | T.C. Wright Jr, C.M. Behrens, J. Ranger-Moore et al., Triaging HPV-positive women with k16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial, Gynecologic Oncology 144: (1) ((2017) ), 51–56. |
[15] | N. Wentzensen, M.A. Clarke, R. Bremer et al., Clinical evaluation of human papillomavirus screening with p16/Ki-67 dual stain triage in a large organized cervical cancer screening program, JAMA Internal Medicine 179: (7) ((2019) ), 881–888. |
[16] | S. Ratnam, D. Jang, L. Gilbert et al., CINtec PLUS and cobas HPV testing for triaging Canadian women referred to colposcopy with a history of low-grade squamous intraepithelial lesion: Baseline findings, Papillomavirus Research 10: ((2020) ), 100206. |
[17] | D. Schmidt, C. Bergeron, K.J. Denton and R. Ridder, p16/Ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou, Cancer Cytopathology 119: (3) ((2011) ), 158–166. |
[18] | H. Ikenberg, C. Bergeron, D. Schmidt et al., Screening for cervical cancer precursors with P16/Ki-67 dual-stained cytology: Results of the PALMS study, J Natl Cancer Inst 105: (20) ((2013) ), 1550–1557. |
[19] | C. White, S. Bakhiet, M. Bates et al., Triage of LSIL/ASC-US with p16/Ki-67 dual staining and human papillomavirus testing: A 2-year prospective study, Cytopathology 27: (4) ((2016) ), 269–276. |
[20] | E. Peeters, N. Wentzensen, C. Bergeron and M. Arbyn, Meta-analysis of the accuracy of p16 or p16/Ki-67 immunocytochemistry versus HPV testing for the detection of CIN2+/ CIN3+ in triage of women with minor abnormal cytology, Cancer Cytopathology 127: (3) ((2019) ), 169–180. |
[21] | J.C. Possati-Resende, J.H.T.G. Fregnani, L.M. Kerr, E.C. Mauad, A. Longatto-Filho and C. Scapulatempo-Neto, The accuracy of p16/Ki-67 and HPV test in the detect of CIN2/3 in women diagnosed with ASC-US or LSIL, PLOS ONE 10: (7) ((2015) ), e0134445. |
[22] | N. Wentzensen, M. Schiffman, T. Palmer and M. Arbyn, Triage of HPV positive women in cervical cancer screening, Journal of Clinical Virology 76: (Suppl 1) ((2016) ), S49–S55. |
[23] | M. El-Zein, W. Gotlieb, L. Gilbert et al., Dual staining for p16-Ki-67 to detect high-grade cervical lesions: Results from the screening tirage ascertaining intraepithelial neoplasia by immunostain testing study, Int. J. Cancer 148: (2) ((2021) ), 492–501. |
[24] | ASCUS-LSIL Triage Study (ALTS) Group, Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance, Am J Obstet Gynecol 188: (6) ((2003) ), 1383–1392. |
[25] | W.K. Huh, K.A. Ault, D. Chelmow et al., Use of primary high-risk human papillomavirus test for cervical cancer screening: Interim clinical guidance, Gynecological Oncology 125: (2) ((2015) ), 330–337. |
[26] | D. Solomon, M. Schiffman and R. Tarone, ALTS Study Group, Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: Baseline results from a randomized trial, J Natl Cancer Inst 93: (4) ((2001) ), 293–299. |
[27] | M. Arbyn, P. Martin-Hirsch, F. Buntinx et al., Triage of women with equivocal or low-grade cervical cytology results: A meta-analysis of the HPV positivity rate, J Cell Mol Med 13: (4) ((2009) ), 648–659. |
[28] | M. Arbyn, L. Xu, F. Verdoodt et al., Genotyping for human papillomavirus types 16 and 18 in women with minor cervical lesions, Annals of Internal Medicine 166: (2) ((2017) ), 118–127. |
[29] | H. Slater, FDA expands use of CINtec PLUS cytology test for women who are HPV-Positive, Cancer Network, (2020) . Available at: https://www.cancernetwork.com/view/fda-expands-use-of-cintec-plus-cytology-test-for-women-who-are-hpv-positive, accessed April 04, 2021. |
[30] | K.C.K. Prigenzi, T. Heinke, R.C. Salim and G.R. de Azevedo Focchi, Dual p16 and Ki-67 expression in liquid-based cervical cytological samples compared to pap cytology findings, biopsies, and HPV testing in cervical cancer screening: A diagnostic accuracy study, Acta Cytol 62: (2) ((2018) ), 104–114. |
[31] | Y. Zhu, C. Ren, L. Yang, X. Zhang, L. Liu and Z. Wang, Performance of p16/Ki67 immunostaining, HPV E6/E7 mRNA testing, and HPV DNA assay to detect high-grade cervical dysplasia in women with ASCUS, BMC Cancer 19: (1) ((2019) ), 271. |
[32] | M. McMenamin, M. McKenna and A. McDowell, Clinical utility of CINtec PLUS triage in equivocal cervical cytology and human papillomavirus primary screening, Am J Clin Pathol 150: (6) ((2018) ), 512–521. |
[33] | D. Das, M. Sengupta, K. Basu, M. Tirkey, C. Datta and U. Chatterjee, Role of p16/Ki-67 dual immunostaining in detection of cervical cancer precursors, Journal of Cytology 35: (3) ((2018) ), 153–158. |
[34] | C. Ren, Y. Zhu, L. Yang et al., Prognostic and diagnostic validity of p16/Ki-67, HPV E6/E7 mRNA, and HPV DNA in women with ASCUS: A follow-up study, Virology Journal 16: ((2019) ), 143. |
[35] | N. Munoz, X. Bosch, S. de Sanjose et al., Epidemiologic classification of human papillomavirus types associated with cervical cancer, The New England Journal of Medicine 348: ((2003) ), 518–527. |
[36] | M.J. Khan, P.E. Castle, A.T. Lorincz et al., The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice, Journal of the National Cancer Institute 97: (14) ((2005) ), 1072–1079. |
[37] | J.T. Cox, P.E. Castle, C.M. Behrens et al., Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotypeing for HPV16/18: Results from the ATHENA HPV study, American Journal of Obstetrics & Gynecology 208: (3) ((2013) ), 184.e1–184.e11. |
[38] | M.A. Clarke, L.C. Cheung, P.E. Castle et al., Five-year risk of cervical precancer following p16/Ki-67 dual-stain triage of HPV-positive women, JAMA Oncology 5: (2) ((2019) ), 181–186. |
[39] | M. Sun, Y. Shen, M.L. Ren and Y.M. Dong, Meta-analysis on the performance of p16/Ki-67 dual immunostaining in detecting high-grade cervical intraepithelial neoplasm, Journal of Cancer Research and Therapeutics ((2018) ), S587–93. |
[40] | S. de Sanjose, W.G. Quint, L. Alemany et al., Human papillomavirus genotype attribution in invasive cervical cancer; a retrospective cross-sectional worldwide study, Lancet Oncology 11: (11) ((2010) ), 1048–1056. |
[41] | T.C. Wright, M.H. Stoler, C.M. Behrens, A. Sharma, G. Zhang and T.L. Wright, Primary cervical cancer screening with human papillomavirus: End of study results from the ATHENA study using HPV as the first-line screening test, Gynecologic Oncology 136: (2) ((2015) ), 189–197. |



