Early onset of severe lymphopenia during definitive radiotherapy correlates with mean body dose and predicts poor survival in cervical cancer
Abstract
BACKGROUND:
Lymphopenia during definitive radiotherapy (RT) has been shown to reduce survival in patients with cervical cancer. However, there are few studies on the significance of onset time of lymphopenia during RT in patients with cervical cancer.
OBJECTIVE:
This study aimed to exam the prognostic significance of early onset of severe lymphopenia (EOSL) during definitive RT in patients with cervical cancer.
METHODS:
Newly diagnosed cervical cancer patients treated with definitive RT from January 2015 to December 2019 were eligible for this retrospective study. EOSL was defined as first onset of grade 3–4 lymphopenia
RESULTS:
A total of 104 patients were included and 59.6% had EOSL. MBD (
CONCLUSIONS:
EOSL during definitive RT correlates with MBD and predicts poor survival in patients with cervical cancer.
1.Introduction
Cervical cancer is one of the most common malignant tumors in women [1]. Definitive concurrent chemoradiotherapy (CCRT) is the preferred treatment for patients with localized cervical cancer who are not amenable to surgery [2]. However, there is still room for improvement in the prognosis of cervical cancer with a reported 5-year disease-free survival of less than 70% [3]. It is important to study risk factors for survival to guide personized treatment.
Immune system plays an important role in the development of malignant tumors [4] and is also essential for tumor response and eradication [5, 6]. Lymphocytes have been recognized as major actors in the fight against tumor progression. Lymphopenia is a common side effect of anti-cancer treatment and has been reported to be an independent prognostic factor for poor survival in several cancer types [7]. The incidence rate of grade 3–4 lymphopenia during CCRT was as high as 89% in cervical cancer [8]. Previous studies showed that pre-, during-, and post-treatment lymphopenia may be associated with decreased survival in patients with locally advanced cervical cancer [8, 9, 10].
The onset time of lymphopenia during radiotherapy (RT) was clinically relevant in patients with lung cancer [11]: the median overall survival (OS) and progression-free survival (PFS) for patients with low absolute lymphocyte count (ALC) (
2.Methods
2.1Patient selection
This study conforms with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by institutional ethics committee of the University of Hong Kong-Shenzhen Hospital (NO: [2019]049). Individual consent from each patient for this retrospective analysis was waived. Newly diagnosed cervical cancer patients treated with definitive RT from January 2015 to December 2019 were identified from patient database of the department (
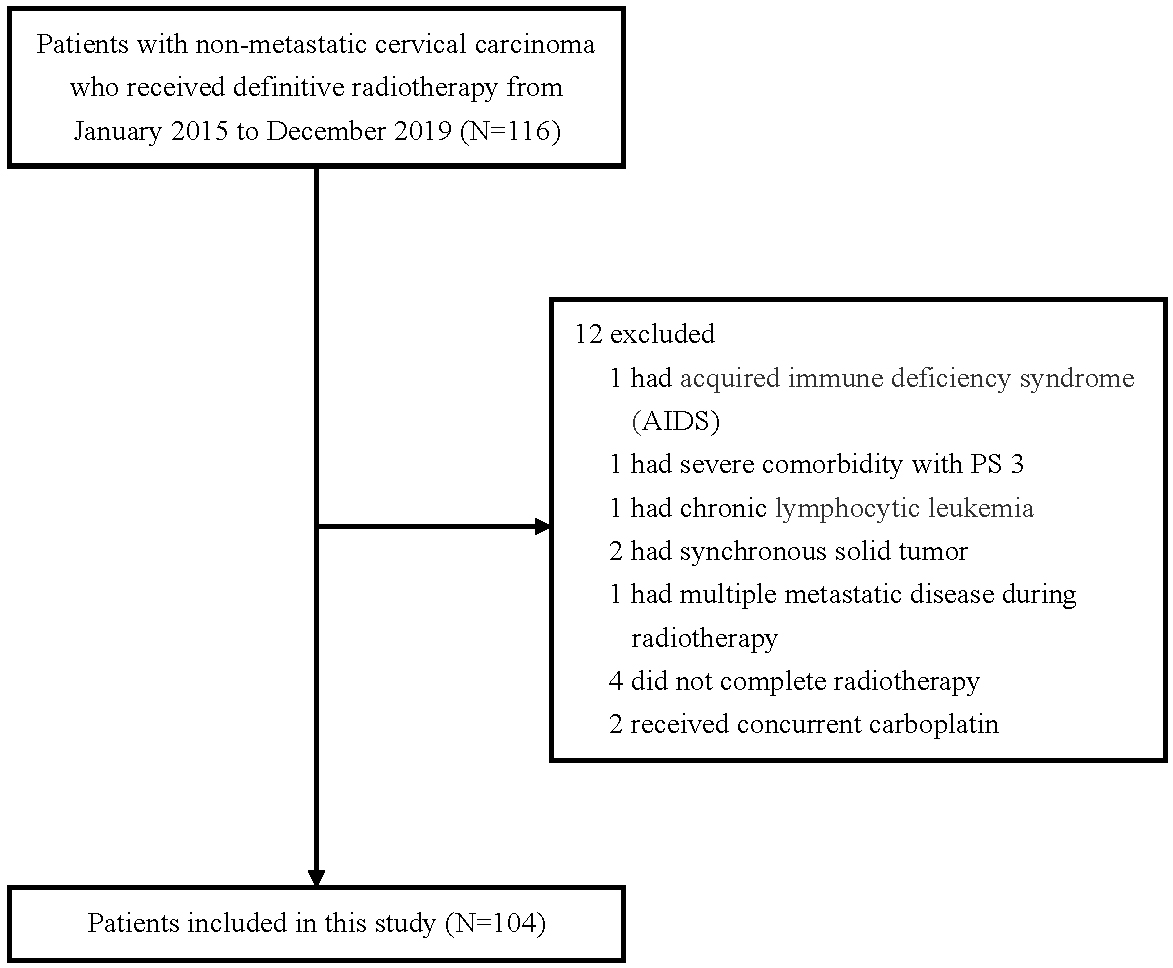
Figure 1.
Selection process of study population.
2.2Investigations, treatment and follow up
According to the practice guideline of the department, all patients had gynecological physical examination by experienced gynecologists, contrast computed tomography (CT) of thorax, abdomen and pelvis or positron emission tomography (PET)-CT, and contrast magnetic resonance imaging (MRI) of pelvis (if no contraindication of MRI) for baseline staging. Cystoscopy or sigmoidoscopy were indicated only if bladder or rectum invasion was suspected. Patients also had serial CBC, liver and renal function tests before and weekly during RT. Patients received EBRT followed by brachytherapy with or without concurrent cisplatin or IC. Two types of EBRT techniques were used namely RapidArc or three-dimensional conformal radiotherapy (3D-CRT). GTV (Gross Tumor Volume)-T: the primary tumor from hyper intense signal of T2-weighted MRI images. GTV-N: locoregional pathologically enlarged lymph nodes. CTV (Clinical Target Volume)-T included cervix, parametrium bilaterally, uterus and part of vagina. CTV-N: GTV-N
Brachytherapy was guided by CT or MRI and was started 4–5 weeks after the start of EBRT,
Concurrent cisplatin was applied at 40 mg/m
After completion of RT, patients were followed up every 3 to 4 months for the first 2 years, every 4 to 6 months for third to fifth year and annually thereafter. Surveillance evaluations included physical examination, tumor marker (optional), imaging studies (optional) such as pelvic MRI, CT chest and abdomen or PET-CT, and biopsy if clinically indicated on follow up.
2.3Data collection
Variables of interest in this study included patient demographics, clinicopathologic findings, and treatment related data. All these data were collected from hospital’s electronic medical records. Common Terminology Criteria for Adverse Events (CTCAE) v4.03 was used to grade the myelosuppression. Grade 3 lymphopenia was defined as ALC
2.4Statistical analysis
Data was analyzed using SPSS version 25.0 (SPSS, Inc., Chicago, IL, USA) statistical software and R version 3.6.1(R Core Team, Vienna, Austria). Demographics, clinicopathologic findings, and treatment related characteristics were described as median (interquartile range [IQR]) values of continuous variables and percentages for categorical variables. Means between groups were tested for normal distribution with Shapiro-Wilk tests followed by either a student’s
3.Results
3.1Patient characteristics
A total of 104 women were included in this study. The median age was 52.5 years. The FIGO (2018) stage distribution was 3 (2.9%), 31 (29.8%), 66 (63.5%), and 4 (3.8%) for stage I, II, III and IVA, respectively. One hundred and one (97.1%) had squamous cell carcinoma. Seventeen (16.3%) patients received IC before RT. Fifteen (14.4%) patients had RT alone. The demographics, clinicopathologic findings, and treatment characteristics of the patients were listed in Table 1.
Table 1
Patient characteristics and clinical outcomes
| Features | No. (%) or Median (IQR) |
|---|---|
| Age | 52.5 (46–63) |
| | 72 (69.2) |
| | 32 (30.8) |
| ECOG | |
| 0–1 | 90 (86.5) |
| 2 | 14 (13.5) |
| FIGO stage (2018) | |
| I | 3 (2.9) |
| II | 31 (29.8) |
| III | 66 (63.5) |
| IVA | 4 (3.8) |
| Pathology | |
| Squamous cell carcinoma | 101 (97.1) |
| Adenocarcinoma | 2 (1.9) |
| Adenosquamous cell carcinoma | 1 (1.0) |
| Body mass index | 23.1 (20.1–24.9) |
| Induction chemotherapy | |
| Yes | 17 (16.3) |
| No | 87 (83.7) |
| Concurrent cisplatin | |
| Yes | 89 (85.6) |
| No | 15 (14.4) |
| Concurrent cisplatin cycles | 5 (3.75–6) |
| EBRT technique | |
| 3D-CRT | 27 (26.0) |
| RapidArc | 77 (74.0) |
| Mean body dose | 12.38 (10.52–14.33) |
| Pre-RT WBC (cells | 6.77 (5.45–8.12) |
| Pre-RT ANC (cells | 4.41 (3.36–5.57) |
| Pre-RT ALC (cells | 1.74 (1.32–2.05) |
| Pre-RT PLT (cells | 268 (217.5–324.3) |
| Pre-RT AMC (cells | 0.34 (0.26–0.43) |
| Pre-RT HGB (g/L) | 119 (103–131) |
| During-RT ALC nadir (cells | 0.22 (0.17–0.29) |
| Grade of lymphopenia during RT | |
| 1 | 0 (0.0) |
| 2 | 5 (4.8) |
| 3 | 64 (61.5) |
| 4 | 35 (33.7) |
| Early onset of severe lymphopenia | |
| Yes | 62 (59.6) |
| No | 42 (40.4) |
| Post-RT local residual or recurrent disease | |
| Yes | 12 (11.5) |
| No | 92 (88.5) |
| Post-RT regional lymph nodes metastases | |
| Yes | 5 (4.8) |
| No | 99 (95.2) |
| Post-RT distant metastases | |
| Yes | 15 (14.4) |
| No | 89 (85.6) |
| Disease progression | |
| Yes | 26 (25) |
| No | 78 (75) |
| Death | |
| Yes | 22 (21.2) |
| No | 82 (78.8) |
IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; FIGO, Federation of Gynecology and Obstetrics; EBRT, external beam radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy; WBC, white blood cell; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; PLT, platelet; AMC, absolute monocyte count; HGB, hemoglobin; RT, radiotherapy.
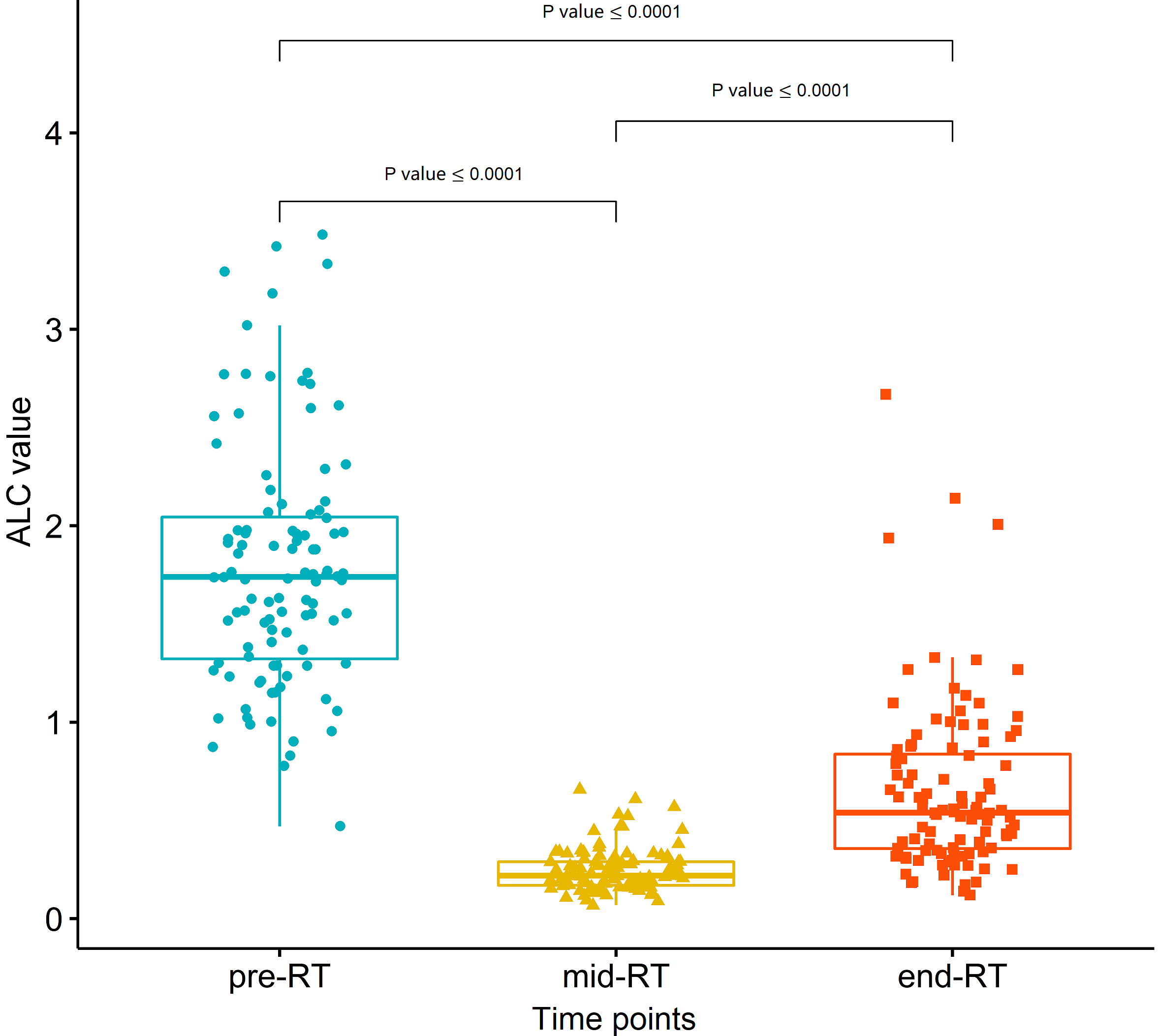
Figure 2.
The change and comparison of absolute lymphocyte count (ALC) at different time points (pre- RT, mid-RT, and end-RT). The values (median, interquartile range) of pre-RT ALC, mid-RT ALC nadir, and end-RT ALC were 1.74 (1.32–2.05)
3.2Pattern and characteristics of lymphopenia and potential contributing factors of early onset of severe lymphopenia during RT
ALC of all patients significantly declined during RT and generally recovered to some extent at the end of RT (Wilcoxon test, all
Figure 3.
Distribution of the time of first onset of severe lymphopenia (FOSL) from the start of radiotherapy (RT) (median 20 days, interquartile range 14–27 days). The number (percentage) of patients with FOSL at the 1
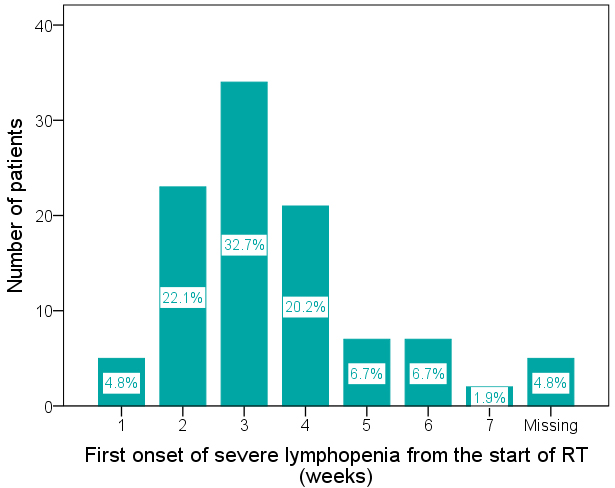
Figure 4.
Forest plot of univariate and multivariate logistic regression model for early onset of severe lymphopenia (EOSL). (ECOG, Eastern Cooperative Oncology Group; FIGO, Federation of Gynecology and Obstetrics; EBRT, external beam radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy; RT, radiotherapy; WBC, white blood cell; HGB, hemoglobin; PLT, platelet; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count; OR, odds ratio; CI, confidence interval.)
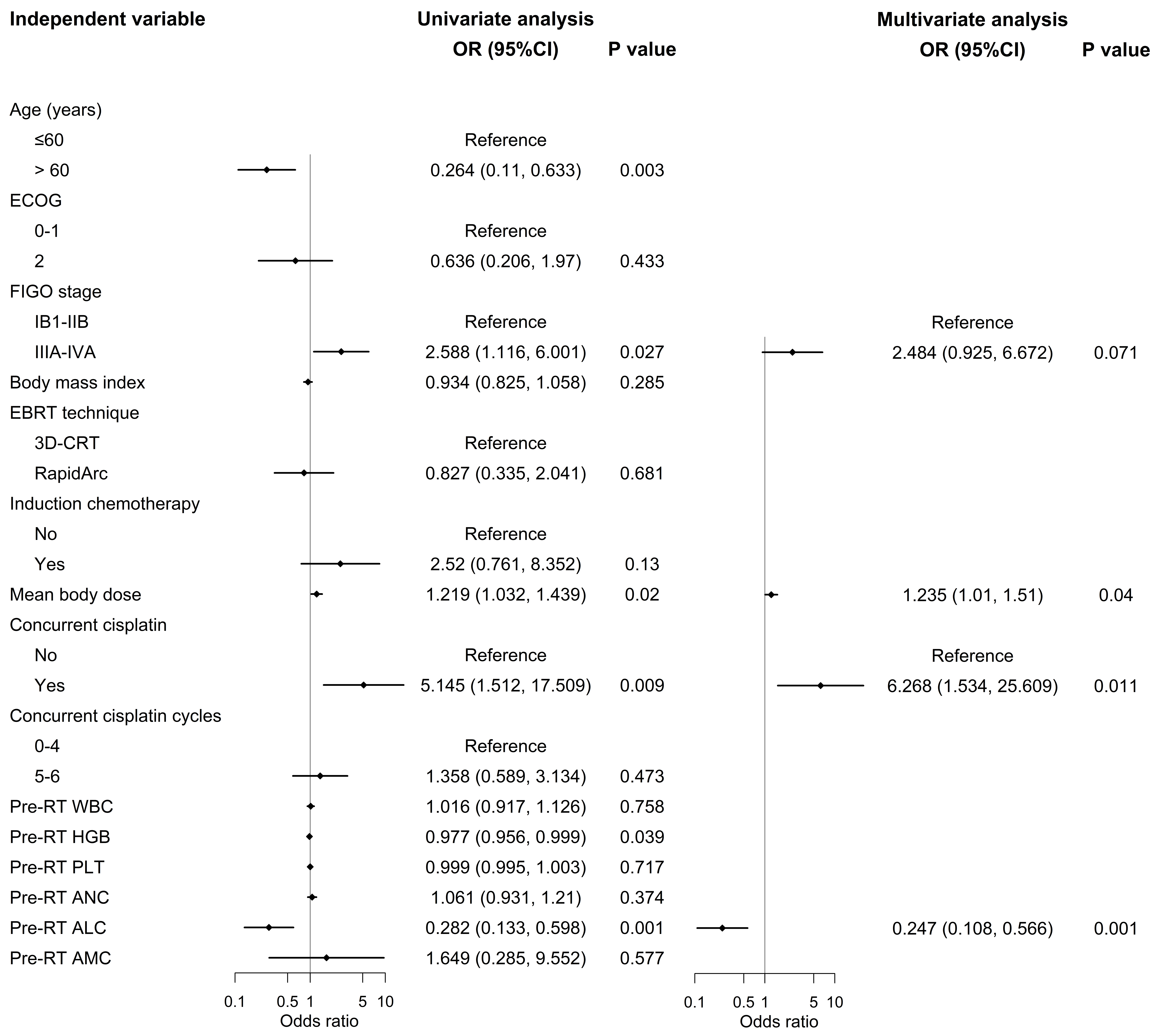
On univariate logistic regression analysis, higher MBD (
3.3Correlation between mean body dose (MBD) and risk of early onset of severe lymphopenia (EOSL)
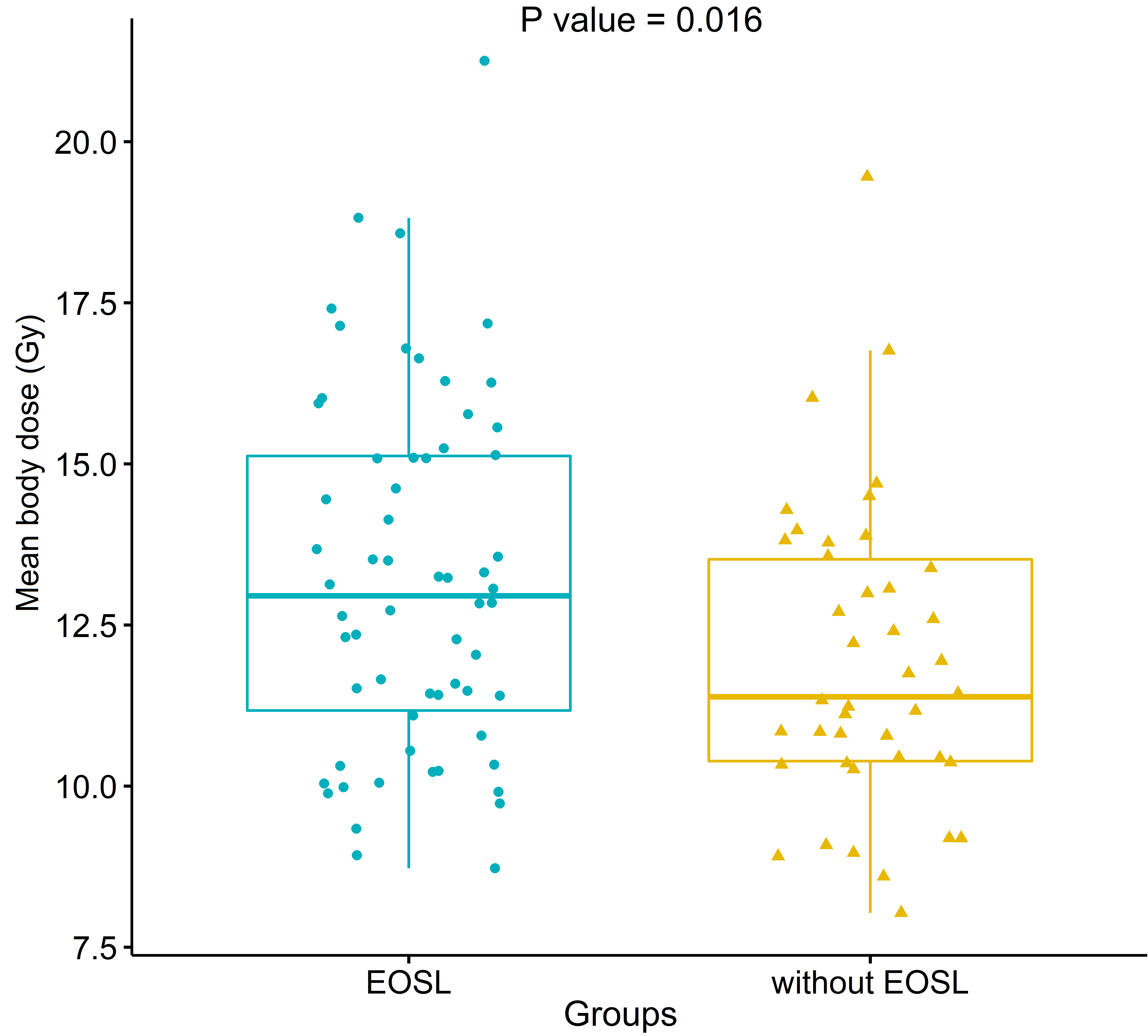
Patients with EOSL had higher MBD (mean
Figure 5.
The comparison of mean body dose (MBD) between patients with or without early onset of severe lymphopenia (EOSL). Patients with EOSL had higher MBD (mean
3.4Early onset of severe lymphopenia and survival
With a median follow-up of 26.2 (IQR 16.2–39.0) months, the estimated 2-year OS and PFS for all patients were 82.0% and 76.4%, respectively. Patients with EOSL had decreased OS (2-yr 75.1% vs. 91.1%, HR [95%CI]
Figure 6.
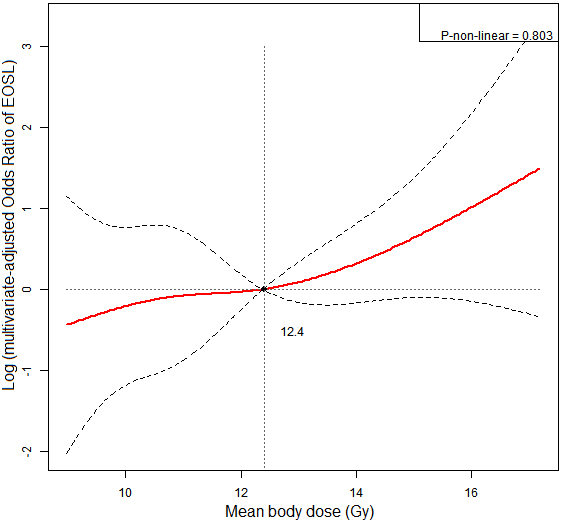
Restricted cubic spline (RCS) model to illustrate dose-response relationship between mean body dose (MBD) and risk of early onset of severe lymphopenia (EOSL). The dashed lines represented the 95% confidence intervals. MBD was linearly correlated with risk of EOSL.
Under univariate analysis, EOSL (
Table 2
Factors associated with the overall survival for all 104 patients
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) |
| HR (95%CI) |
| |
| Age ( | 0.338 (0.099–1.147) | 0.082 | NI | |
| ECOG (2/0–1) | 2.024 (0.679–6.040) | 0.206 | NI | |
| FIGO stage (IIIA-IVA/ IB1-IIB) | 1.945 (0.654–5.786) | 0.232 | NI | |
| EBRT technique (Rapidarc/3D-CRT) | 1.080 (0.421–2.768) | 0.873 | NI | |
| Concurrent cisplatin (Yes/ No) | 1.106 (0.317–3.852) | 0.875 | NI | |
| Concurrent cisplatin cycles (5-6/0-4) | 0.416 (0.174–0.995) | 0.049 | 0.245(0.088–0.679) | 0.007 |
| Induction chemotherapy (Yes/ No) | 1.665 (0.480–5.771) | 0.421 | NI | |
| Pre-RT WBC (Per 10 | 1.072 (0.979–1.174) | 0.135 | NI | |
| Pre-RT ANC (Per 10 | 1.095 (1.001–1.198) | 0.047 | 1.115 (1.020–1.218) | 0.016 |
| Pre-RT ALC (Per 10 | 0.430 (0.184–1.001) | 0.050 | NI | |
| Pre-RT HGB (Per g/L) | 0.965 (0.943–0.988) | 0.004 | 0.964(0.938–0.991) | 0.010 |
| Pre-RT PLT (Per 10 | 1.002 (0.998–1.005) | 0.316 | NI | |
| Pre-RT AMC (Per 10 | 0.821 (0.048–14.145) | 0.892 | ||
| Mean body dose (Per Gy) | 1.259 (1.094–1.449) | 0.001 | 1.254 (1.065–1.477) | 0.007 |
| EOSL (Yes/ No) | 3.351 (1.124–9.991) | 0.030 | 3.845 (1.196-12.360) | 0.024 |
ECOG, Eastern Cooperative Oncology Group; FIGO, Federation of Gynecology and Obstetrics; EBRT, external beam radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy; RT, radiotherapy; WBC, white blood cell; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; HGB, hemoglobin; PLT, platelet; AMC, absolute monocyte count; EOSL, early onset of severe lymphopenia; HR, hazard ratio; CI, confidence interval; NI, not included.
Figure 7.
Kaplan-Meier curves of overall survival (OS) and progression-free survival (PFS). A. OS curves of patients with or without EOSL during radiotherapy (RT) (2-yr 75.1% vs. 91.1%,
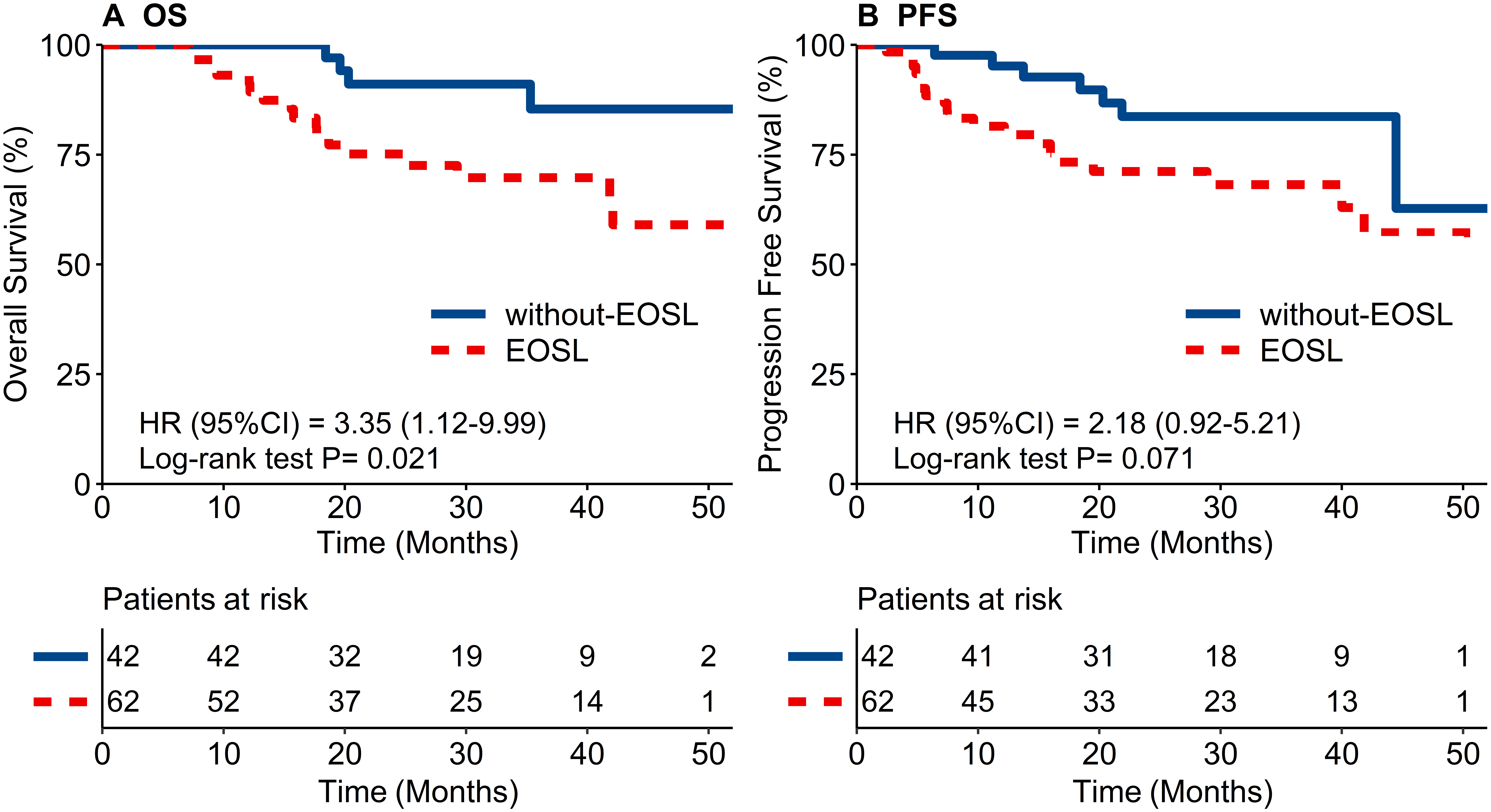
Figure 8.
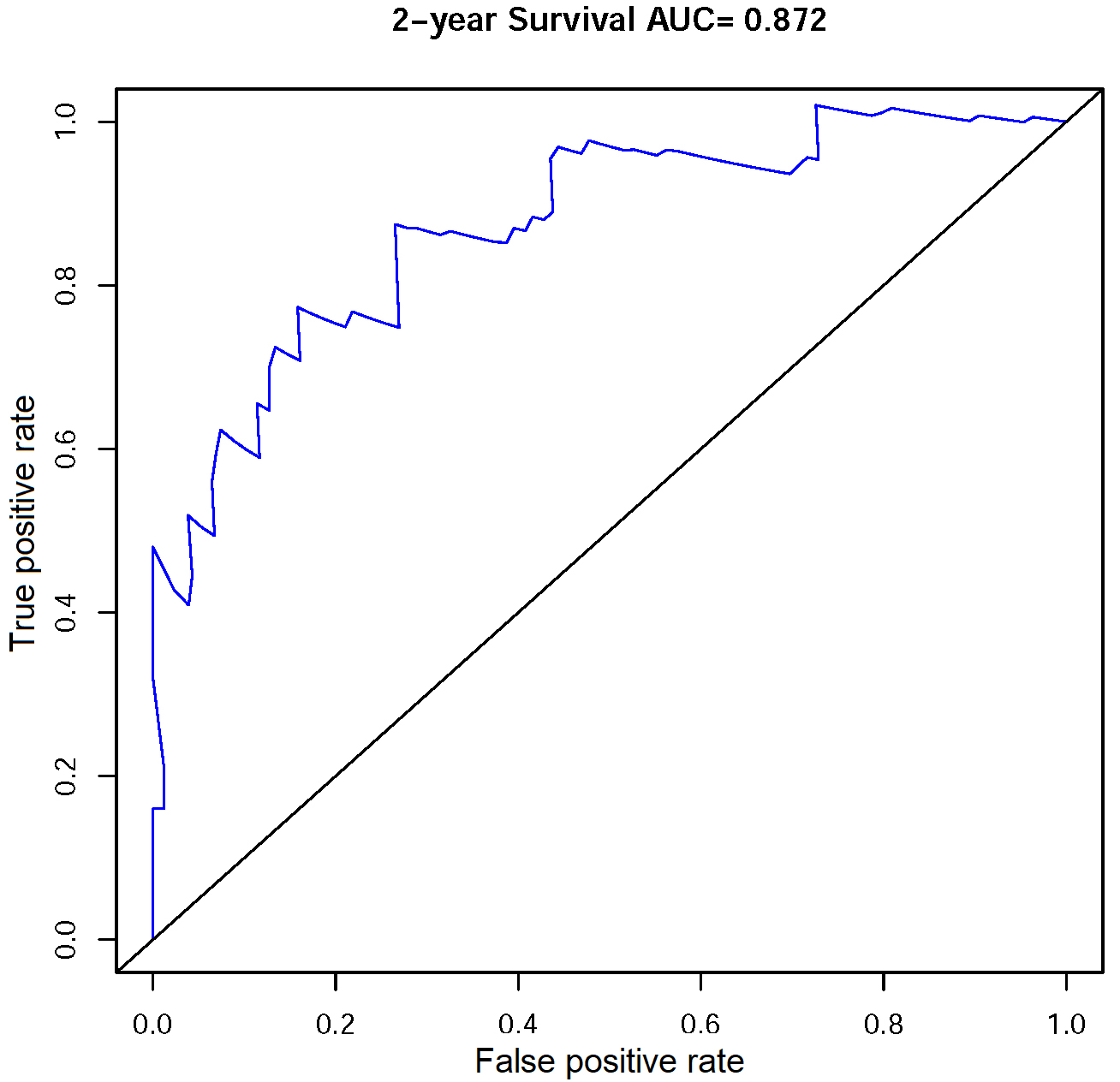
Receiver operator characteristic (ROC) curve of the multivariate COX model for OS prediction.
4.Discussion
Previous studies showed that lymphopenia during RT was common and correlated with survival in patients with cervical cancer [8, 9, 12]. The current study confirmed that the incidence rate of severe lymphopenia was as high as 95.2% in cervical cancer patients treated with definitive (chemo)RT, which was much higher than those observed in other solid tumors [7], such as brain tumors (20–40%), head and neck cancers (48–71%), and thoracic malignancies (40–50%). Therefore investigating lymphopenia and seeking ways to minimize the risk and severity of lymphopenia in cervical cancer are clinically important topics.
In this study the authors demonstrated that the median time of FOSL was 20 days (about 3 weeks) from the start of RT. Patients with EOSL had decreased OS (2-yr 75.1% vs. 91.1%,
Lymphocytes are extremely sensitive to radiation, LD
Although we successfully demonstrated significant association between MBD and risk of EOSL, higher MBD was independently associated with poor OS (HR 1.254, 95%CI 1.065–1.477,
The role of chemotherapy on lymphopenia has not been fully investigated. In a study in patients treated with IC for advanced ovarian cancer, ALC increased significantly post-IC compared with the pre-IC values (
Neutrophils are important components of immune system as well. Contrary to lymphocytes, neutrophils were found to be negatively correlated with survival for patients with cancers. Wisdom et al. showed that neutrophils promoted tumor resistance to RT [23]. High ANC during CCRT was associated with poor local control and survival in cervical cancer patients [23]. In NSCLC, high baseline ANC was a significant survival covariate (HR 1.07, 95% CI 1.02–1.11,
This study had some limitations. First, the retrospective nature of the study created discordance on the timing of blood test, confounding the interpretation of the results. Second, lymphocyte subtypes had different roles in the treatment outcomes of RT [18, 19]. However we did not test lymphocyte subtypes and did not collect patients’ blood for future research routinely in clinical practice, so there was lack of data on the lymphocyte subtype changes and their impact on the treatment outcome in our patients. Third, we did not exam the relationship of dose to immune organ at risk (OAR) and EOSL, which might have important role in radiation-induced lymphopenia. Finally, the absence of a validation cohort was also a limitation of our study. However, we have demonstrated some interesting results with a linear association between MBD and risk of EOSL and the prognostic effect of EOSL on survival.
In conclusion, our study demonstrated that EOSL during definitive RT correlated with MBD and predicted poor survival in patients with cervical cancer. There are several questions remain to be answered, such as is radiation related lymphopenia truly a driver of inferior survival? Efforts to differentiate this from other contributing factors are required. Furthermore, additional research to explore treatment approaches that preserve or restore ALC during definitive RT will be important to improve the treatment outcome of cervical cancer patients with the guidance of these results.
Author contributions:
Li Yang: Primary data collection, data analysis, manu-script writing and study design and manuscript approval.
Zhiyuan Xu: Corresponding Author, data collection and double check, data analysis, result interpretation, study design and manuscript approval.
Lingyu Ma: Data double check, data analysis, result interpretation, and manuscript approval.
Qin Liu and Amy TY Chang: Manuscript edit and manuscript approval.
Qian Wang, Jiandong Zha, Jinliang Zhang, Xiaoqin Jiang, Jingjing Zhang: data collection and patient follow-up and manuscript approval.
Feng-Ming (Spring) Kong: Study design, data analysis, result interpretation, manuscript edits and approval.
Linlang Guo: Corresponding Author, study design oversight, data quality control, results check and manuscript approval.
Funding
This project was supported by the grants from Health Commission of Guangdong Province, China (Grant No.: B2020100); HKUSZH Seed Funding, High Level-Hospital Program, Health Commission of Guangdong Province, China (Grant No.: HKUSZH201902031); Shenzhen Key Medical Discipline Construction Fund (Grant No.: SZXK014); and Shenzhen Science and Technology Program (Grant NO.: KQTD201804111 85028798).
Acknowledgments
The authors acknowledge Dr. Weiwei Chen for useful discussion.
Conflict of interest
No.
References
[1] | H. Sung, J. Ferlay, R.L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 countries, CA Cancer J Clin 71: ((2021) ), 209–249. |
[2] | W.J. Koh, N.R. Abu-Rustum, S. Bean, K. Bradley, S.M. Campos, K.R. Cho, H.S. Chon, C. Chu, R. Clark, D. Cohn, M.A. Crispens, S. Damast, O. Dorigo, P.J. Eifel, C.M. Fisher, P. Frederick, D.K. Gaffney, E. Han, W.K. Huh, J.R. Lurain, A. Mariani, D. Mutch, C. Nagel, L. Nekhlyudov, A.N. Fader, S.W. Remmenga, R.K. Reynolds, T. Tillmanns, S. Ueda, E. Wyse, C.M. Yashar, N.R. McMillian and J.L. Scavone, Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology, J Natl Compr Canc Netw 17: ((2019) ), 64–84. |
[3] | M.N. Brodeur, R. Dejean, M.C. Beauchemin, V. Samouëlian, B. Cormier, O.M. Bacha, T. Warkus and M. Barkati, Oncologic outcomes in the era of modern radiation therapy using FIGO 2018 staging system for cervical cancer, Gynecol Oncol 162: ((2021) ), 277–283. |
[4] | D. Mittal, M.M. Gubin, R.D. Schreiber and M.J. Smyth, New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape, Curr Opin Immunol 27: ((2014) ), 16–25. |
[5] | M.H. Melis, K.L. Simpson, S.J. Dovedi, A. Welman, M. MacFarlane, C. Dive, J. Honeychurch and T.M. Illidge, Sustained tumour eradication after induced caspase-3 activation and synchronous tumour apoptosis requires an intact host immune response, Cell Death Differ 20: ((2013) ), 765–773. |
[6] | A. Ko, A. Kanehisa, I. Martins, L. Senovilla, C. Chargari, D. Dugue, G. Mariño, O. Kepp, M. Michaud, J.L. Perfettini, G. Kroemer and E. Deutsch, Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling, Cell Death Differ 21: ((2014) ), 92–99. |
[7] | B.P. Venkatesulu, S. Mallick, S.H. Lin and S. Krishnan, A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors, Crit Rev Oncol Hematol 123: ((2018) ), 42–51. |
[8] | O. Cho, M. Chun, S.J. Chang, Y.T. Oh and O.K. Noh, Prognostic value of severe lymphopenia during pelvic concurrent chemoradiotherapy in cervical cancer, Anticancer Res 36: ((2016) ), 3541–3547. |
[9] | E.S. Wu, T. Oduyebo, L.P. Cobb, D. Cholakian, X. Kong, A.N. Fader, K.L. Levinson, E.J. Tanner, 3rd, R.L. Stone, A. Piotrowski, S. Grossman and K.L. Roche, Lymphopenia and its association with survival in patients with locally advanced cervical cancer, Gynecol Oncol 140: ((2016) ), 76–82. |
[10] | X. Han, Q. Yang, J. Zhang and J. Cao, Correlation between changes in the number of peripheral blood lymphocytes and survival rate in patients with cervical cancer after radio-chemotherapy, Cancer Radiother 25: ((2021) ), 72–76. |
[11] | J.H. Joo, S.Y. Song, J. Park, E.K. Choi, S.Y. Jeong and W. Choi, Lymphocyte depletion by radiation therapy alone is associated with poor survival in non-small cell lung cancer, International Journal of Radiation Oncology Biology Physics 96: ((2016) ), E478. |
[12] | L. Yang, Z.Y. Xu, A.T. Chang, Q. Wang, X. Chen, L. Shen, S.K. Hui, K.W. Lee, W. Chan, Y. Zhou, F. Chen, J. Zha, J.Y. Jin and F.M. Kong, A potential survival impact of blood immune cells in patients with cervical carcinoma treated with concurrent chemoradiotherapy, International Journal of Radiation Oncology Biology Physics 105: ((2019) ), E343. |
[13] | N. Nakamura, Y. Kusunoki and M. Akiyama, Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay, Radiat Res 123: ((1990) ), 224–227. |
[14] | N. Joseph, A. McWilliam, J. Kennedy, K. Haslett, J. Mahil, A. Gavarraju, H. Mistry, M. Van Herk, C. Faivre-Finn and A. Choudhury, Post-treatment lymphocytopaenia, integral body dose and overall survival in lung cancer patients treated with radical radiotherapy, Radiother Oncol 135: ((2019) ), 115–119. |
[15] | T. Saito, R. Toya, T. Matsuyama, A. Semba and N. Oya, Dosimetric predictors of treatment-related lymphopenia induced by palliative radiotherapy: predictive ability of dose-volume parameters based on body surface contour, Radiol Oncol 51: ((2017) ), 228–234. |
[16] | R. Davuluri, W. Jiang, P. Fang, C. Xu, R. Komaki, D.R. Gomez, J. Welsh, J.D. Cox, C.H. Crane, C.C. Hsu and S.H. Lin, Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy, Int J Radiat Oncol Biol Phys 99: ((2017) ), 128–135. |
[17] | G.P. Swanson, S.G. Jhavar and K. Hammonds, The effect of pelvic radiation alone on lymphocyte subgroups, Clin Transl Radiat Oncol 23: ((2020) ), 100–102. |
[18] | E.S. Jordanova, A. Gorter, O. Ayachi, F. Prins, L.G. Durrant, G.G. Kenter, S.H. van der Burg and G.J. Fleuren, Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res 14: ((2008) ), 2028–2035. |
[19] | M. Ozsahin, N.E. Crompton, S. Gourgou, A. Kramar, L. Li, Y. Shi, W.J. Sozzi, A. Zouhair, R.O. Mirimanoff and D. Azria, CD4 and CD8 T-lymphocyte apoptosis can predict radiation-induced late toxicity: a prospective study in 399 patients, Clin Cancer Res 11: ((2005) ), 7426–7433. |
[20] | Y. Yoshino, A. Taguchi, M. Takao, T. Kashiyama, A. Furusawa, M. Uno, S. Okada, N. Kino and T. Yasugi, Lymphopenia after induction chemotherapy correlates with incomplete surgical resection in patients with advanced ovarian cancer, Int J Clin Oncol 24: ((2019) ), 428–436. |
[21] | J.L. Campian, X. Ye, M. Brock and S.A. Grossman, Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer, Cancer Invest 31: ((2013) ), 183-188. |
[22] | M. Di Gioacchino, L. Di Giampaolo, N. Verna, M. Reale, M.B. Di Sciascio, A.R. Volpe, M. Carmignani, J. Ponti, R. Paganelli, E. Sabbioni and P. Boscolo, In vitro effects of platinum compounds on lymphocyte proliferation and cytokine release, Ann Clin Lab Sci 34: ((2004) ), 195–202. |
[23] | A.J. Wisdom, C.S. Hong, A.J. Lin, Y. Xiang, D.E. Cooper, J. Zhang, E.S. Xu, H.C. Kuo, Y.M. Mowery, D.J. Carpenter, K.T. Kadakia, J.E. Himes, L. Luo, Y. Ma, N. Williams, D.M. Cardona, M. Haldar, Y. Diao, S. Markovina, J.K. Schwarz and D.G. Kirsch, Neutrophils promote tumor resistance to radiation therapy, Proc Natl Acad Sci U S A 116: ((2019) ), 18584–18589. |
[24] | A. Salem, H. Mistry, A. Backen, C. Hodgson, P. Koh, E. Dean, L. Priest, K. Haslett, I. Trigonis, A. Jackson, M.C. Asselin, C. Dive, A. Renehan, C. Faivre-Finn and F. Blackhall, Cell death, inflammation, tumor burden, and proliferation blood biomarkers predict lung cancer radiotherapy response and correlate with tumor volume and proliferation imaging, Clin Lung Cancer 19: ((2018) ), 239–248e7. |




