Tumor-infiltrating lymphocytes are associated with β-catenin overexpression in breast cancer
Abstract
BACKGROUND:
Inhibition of lymphocytes infiltration and activity may impair antitumor immune response and limit treatment responsiveness. Wnt/
OBJECTIVE:
We aimed to investigate whether intratumoral Wnt/
METHODS:
The distribution of stromal TILs, CD8
RESULTS:
Both stromal infiltrated TILs and
CONCLUSIONS:
For the first time, we demonstrated that rather than excluding lymphocytes infiltration as reported in mela-noma, high levels of TILs were associated with
1.Introduction
Despite the improvement of its overall 5-year survival rate in the past 3 decades, breast cancer is still the most prevalent cancer among females worldwide [1]. It is also the leading cause of cancer death among women in less developed regions and the second cause of cancer death in more developed regions [2], posing great threat to women’s health. The interaction between tumor cells and immune system is critical for the development and progression of cancer [3]. Although breast cancer (BC) is not traditionally considered as immunogenic, an increasing number of retrospective and prospective studies have shown that high level of lymphocytes infiltration in BC is associated with improved outcome of patients, particularly in triple negative and HER2-enriched early breast cancer [4, 5, 6]. Moreover, the presence of TILs could favorably influence treatment responsiveness to neoadjuvant chemotherapy [7]. An association between higher levels of TILs and increased trastuzumab benefit in HER2
Wnt pathway plays a key role in the development and function of hematopoietic system [9, 10, 11]. In peripheral immunity, canonical Wnt/
High
In this study, we seek to further explore the clinicalpathological factors that affect T lymphocytes infiltration in breast cancer and the correlation between components of tumor inflammatory infiltrates and Wnt/
2.Materials and methods
2.1Patients’ specimen and clinicopathological data
Female patients with first diagnosed, primary operable breast carcinoma, without clinical distant metastasis, no history of receiving neoadjuvant chemotherapy or preoperative radiation therapy were considered for inclusion in this study. A total number of 96 paraffin-embedded tumor samples were obtained from primary breast cancer patients. Ninety-six tumor samples were reported as infiltrating ductal or lobular breast carcinoma. Median age of the patients is 52 years old (range 28–77). Detailed clinicopathological data of the tumor samples were retrieved from the routine reports of Department of Pathology, the Second Affiliated Hospital of Xi’an Jiaotong University. Immunohistochemical analyses were performed by qualified pathologists, and the cutoffs for ER, PR were 1% [33]. The criteria used to determine the HER2 status was in accordance with the 2013 ASCO/CAP guideline [34]. In samples showing IHC 2
2.2Histopathologic analysis of TILs
Full-face breast cancer slides (5
2.3Immunohistochemical staining
The expression of
2.4Immunohistochemistry evaluation
Figure 1.
Representative images of
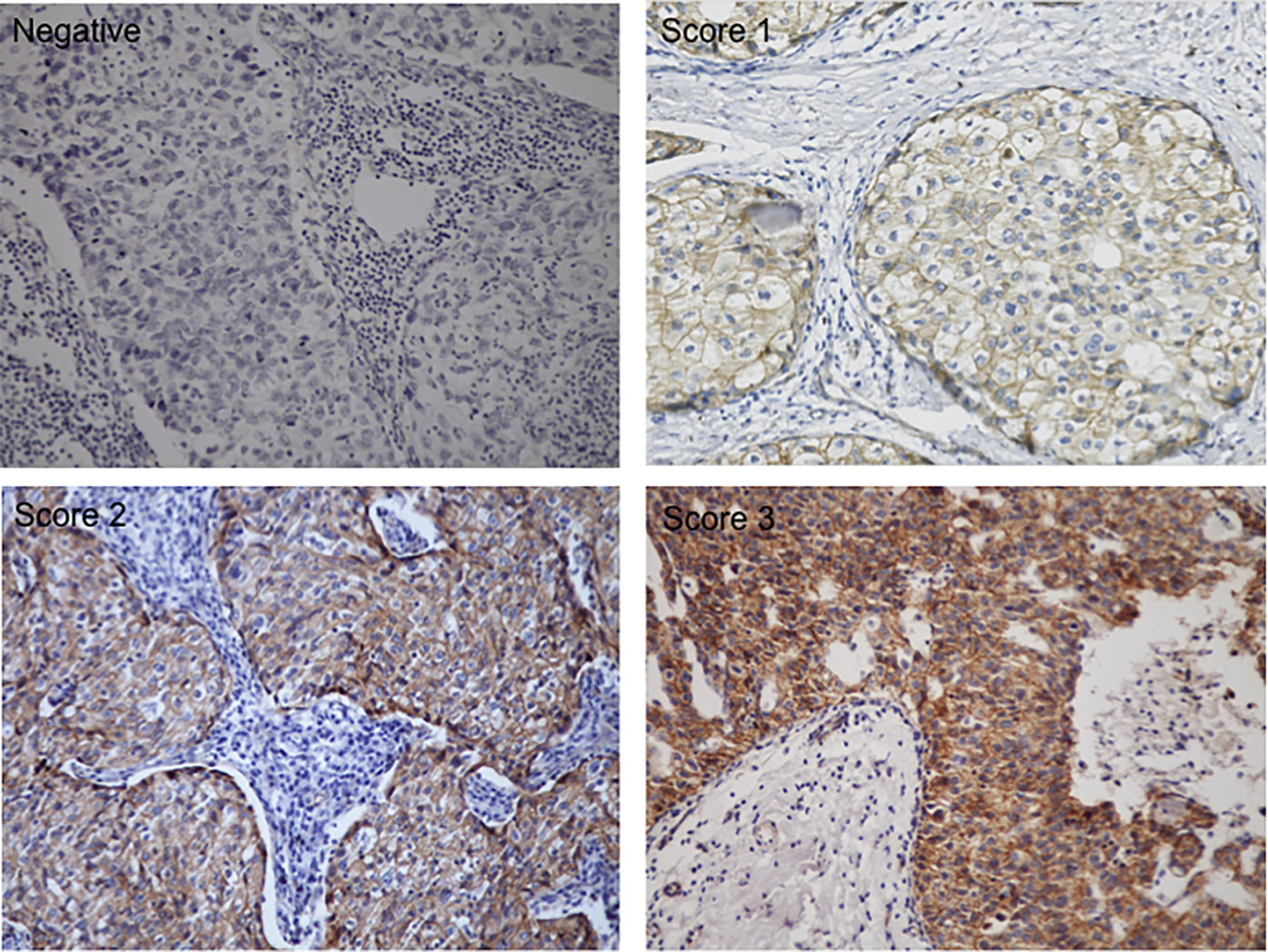
All tumors slides were assessed for both intensity (score 1 to 3, Fig. 1), and the proportion of cells staining positive in the nucleus and/or cytoplasm. Score of 0 if
Figure 2.
Representative immunohistochemistry staining of stromal CD8
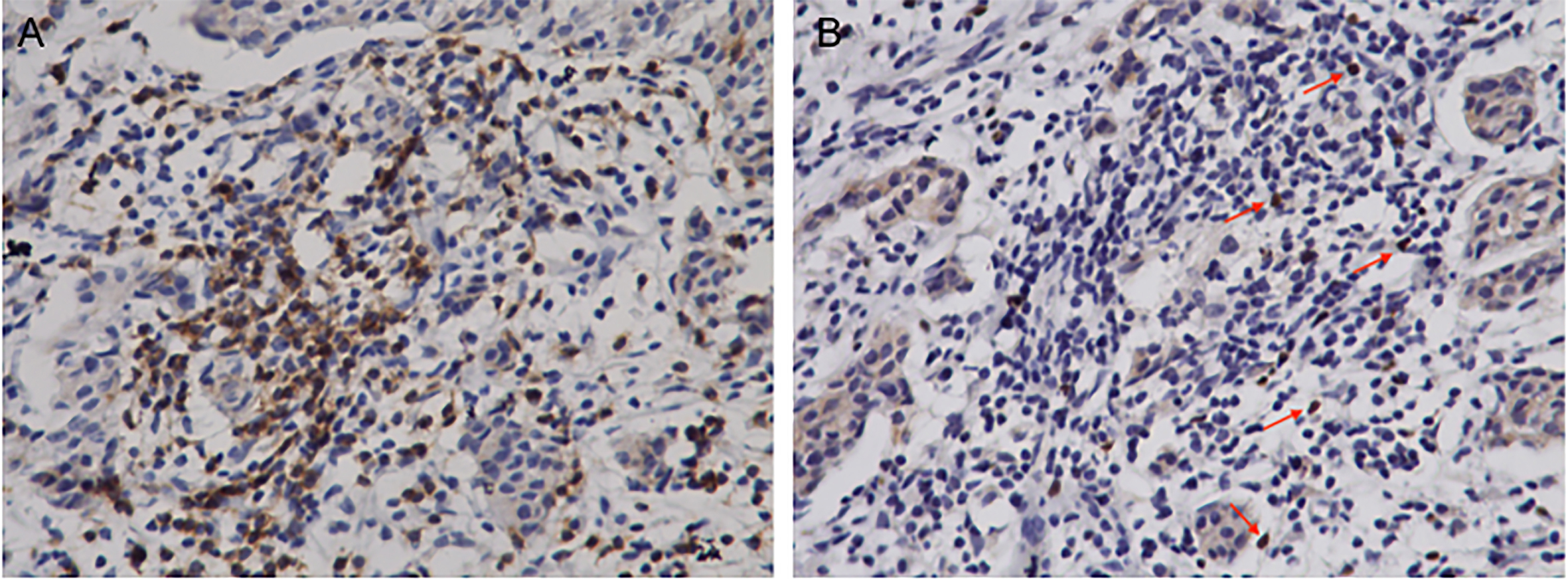
CD8
2.5Statistical analyses
Associations between TILs, CD8
3.Results
3.1Lymphocytes infiltration in breast cancer correlates with tumor grade and ER/PR status
Table 1
Correlation analyses between the presence of stromal TILs and clinicopathologic parameters in 96 primary breast cancer patients
| Number of patients (%) | TILs | ||||
|---|---|---|---|---|---|
| Low ( | intermediate (10–40%) | High ( | p | ||
| Age (years) | |||||
| | 44 (45.8) | 24 (54.5) | 17 (38.6) | 3 (6.8) | 0.773 |
| | 52 (54.2) | 24 (46.2) | 24 (46.2) | 4 (7.6) | |
| Tumor size (mm) | |||||
| | 35 (36.5) | 16 (45.7) | 18 (51.4) | 1 (2.9) | 0.372 |
| 21–50 | 56 (58.3) | 30 (53.6) | 21 (37.5) | 5 (8.9) | |
| | 5 (5.20) | 2 (40.0) | 2 (40.0) | 1 (20.0) | |
| Grade | |||||
| II | 40 (41.7) | 24 (60.0) | 12 (30.0) | 4 (10.0) | 0.020 |
| II | 32 (33.3) | 9 (28.1) | 16 (50.0) | 7 (21.9) | |
| III | 24 (25.0) | 6 (25.0) | 11 (45.8) | 7 (29.2) | |
| Nodal status | |||||
| Negative | 44 (45.8) | 23 (52.3) | 17 (38.6) | 4 (9.1) | 0.736 |
| Positive | 52 (54.2) | 25 (48.1) | 24 (46.2) | 3 (5.7) | |
| HER2 | |||||
| Negative | 56 (58.3) | 32 (57.1) | 20 (35.7) | 4 (7.2) | 0.216 |
| Positive | 40 (41.7) | 16 (40.0) | 21 (52.5) | 3 (7.5) | |
| ER | |||||
| Negative | 28 (29.2) | 7 (25.0) | 16 (57.1) | 5 (17.9) | 0.001 |
| Positive | 68 (70.8) | 41 (60.3) | 25 (36.8) | 2 (2.9) | |
| PR | |||||
| Negative | 42 (43.7) | 15 (35.7) | 22 (52.4) | 5 (11.9) | 0.029 |
| Positive | 54 (56.3) | 33 (61.1) | 19 (35.2) | 2 (3.7) | |
| Stage | |||||
| I | 12 (12.5) | 5 (41.7) | 7 (58.3) | 0 | 0.346 |
| II | 63 (65.6) | 30 (47.6) | 26 (41.3) | 7 (11.1) | |
| III | 21 (21.9) | 13 (61.9) | 8 (38.1) | 0 | |
The clinicopathological information of 96 breast cancer patients recruited in this study is summarized in Table 1. Fifty-two patients (54.2%) presented with clinically detectable axillary lymph node metastasis at diagnosis. Fifty-six of patients (58.3%) had moderately (grade II) or poorly differentiated (grade III) tumors, and 84 patients (87.5%) presented with stage II or III tumors. According to the criteria previously described [35], we divided the distribution of stromal TILs into three levels based on the percentage of TILs that occupied the stromal areas. Increased lymphocytes infiltration in tumor stroma was significantly associated with higher histological grade (
3.2Both stromal lymphocytes infiltration and β
Breast cancer has been classified into four molecular subtypes: luminal types A, luminal types B, HER2-enriched and triple-negative (TNBC) as previously described [38]. According to the immunohistochemical report, tumor specimens included in this study consist of 41 (42.7%) luminal type A, 20 (20.8%) luminal type B, 19 (19.8%) HER2-enriched subtype and 16 (16.7%) triple-negative breast cancer. As shown in Table 2, stromal TILs significantly differed between subtypes of breast cancer (
Table 2
Comparison of stromal lymphocyte infiltration among molecular subtypes of breast cancer
| Subtype | Number of patients (%) | TILs | p | ||
|---|---|---|---|---|---|
| Low ( | Intermediate (10–40%) | High ( | |||
| Luminal A | 41 (42.7) | 27 (66.0) | 13 (31.9) | 1 (2.1) | 0.004 |
| Luminal B | 20 (20.8) | 9 (47.6) | 10 (47.6) | 1 (4.8) | |
| HER2 | 19 (19.8) | 5 (26.3) | 12 (63.2) | 2 (10.5) | |
| TNBC | 16 (16.7) | 4 (22.2) | 7 (44.5) | 5 (33.3) | |
It has been reported that Wnt/
Table 3
Comparison of
| Subtype | Number of patients (%) | Nuclear | p | Cytoplasm | p | ||
|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||||
| Luminal A | 41 (42.7) | 18 (43.9) | 23 (56.1) | 0.051 | 18 (43.9) | 23 (56.1) | 0.000 |
| Luminal B | 20 (20.8) | 10 (50.0) | 10 (50.0) | 9 (45.0) | 11 (55.0) | ||
| HER2 | 19 (19.8) | 8 (42.1) | 11 (57.9) | 5 (26.3) | 14 (73.7) | ||
| TNBC | 16 (16.7) | 5 (31.3) | 11 (68.7) | 3 (18.8) | 13 (81.2) | ||
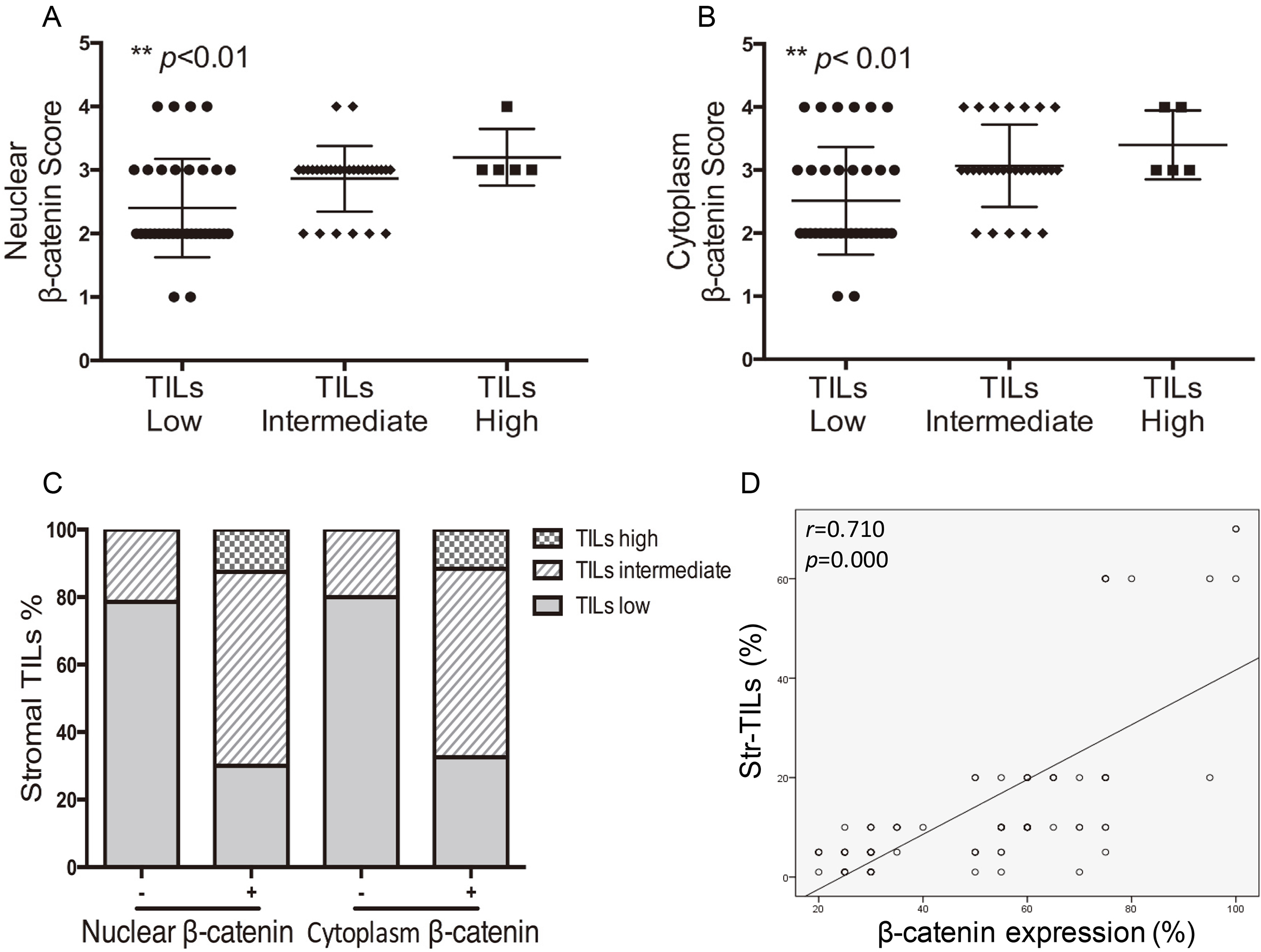
Figure 3.
Correlation between TILs and
3.3High levels of TILs are associated with β
Table 4
Correlation analyses between presence of stromal TILs and
| Nuclear | p | Cytoplasm | p | |||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |||
| TILs Low ( | 33 (68.7) | 15 (31.3) | 0.000 | 28 (58.3) | 20 (41.7) | 0.000 |
| TILs Intermediate (10–40%) | 9 (22.0) | 32 (78.0) | 7 (17.1) | 34 (82.9) | ||
| TILs High ( | 0 | 7 (100) | 0 | 7 (100) | ||
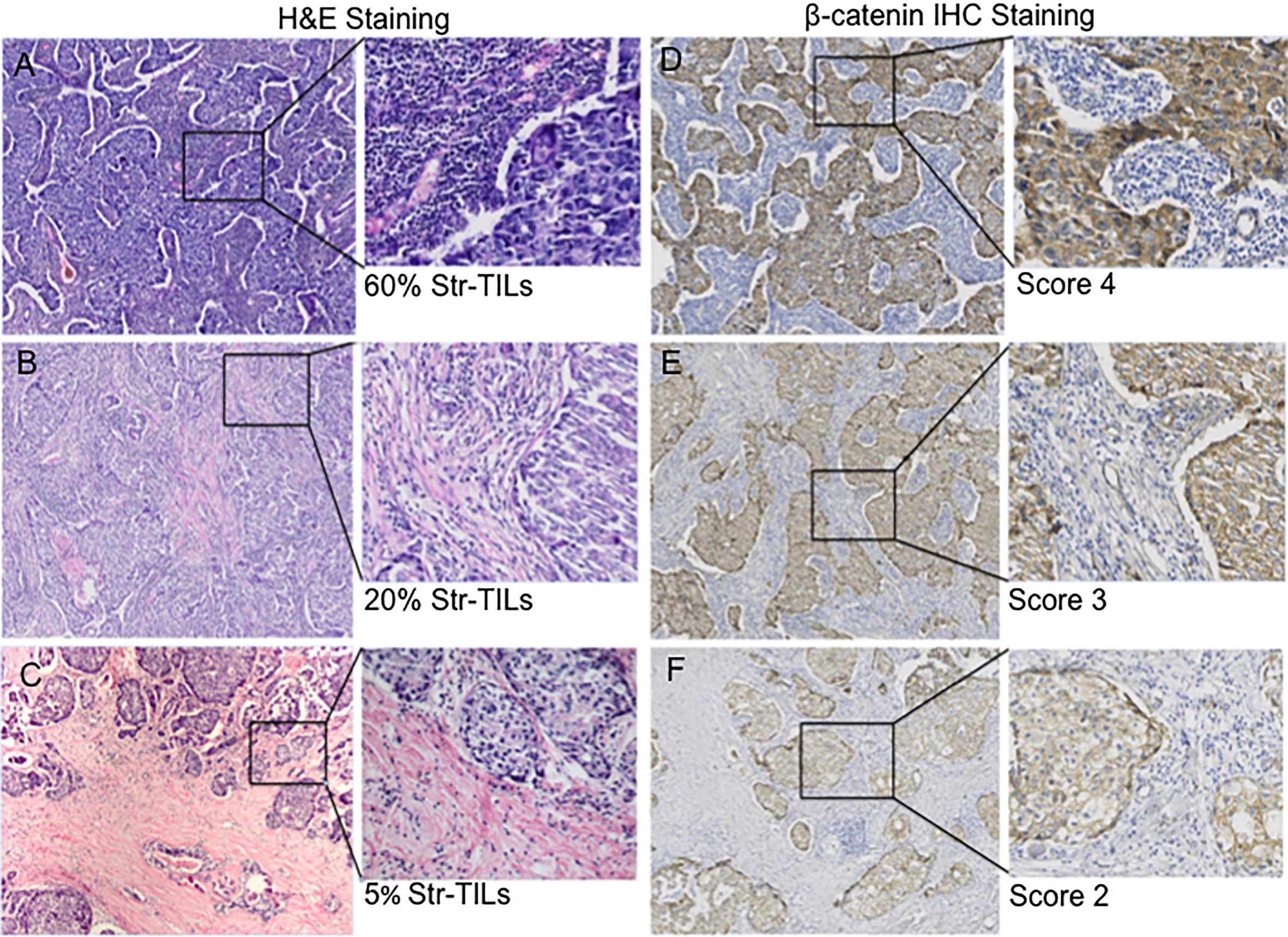
Figure 4.
Correlation of stromal TILs with
In our study, consecutive sections were used for immunohistochemical detection of TILs distribution and
Figure 5.
Association of TIL subsets infiltration with
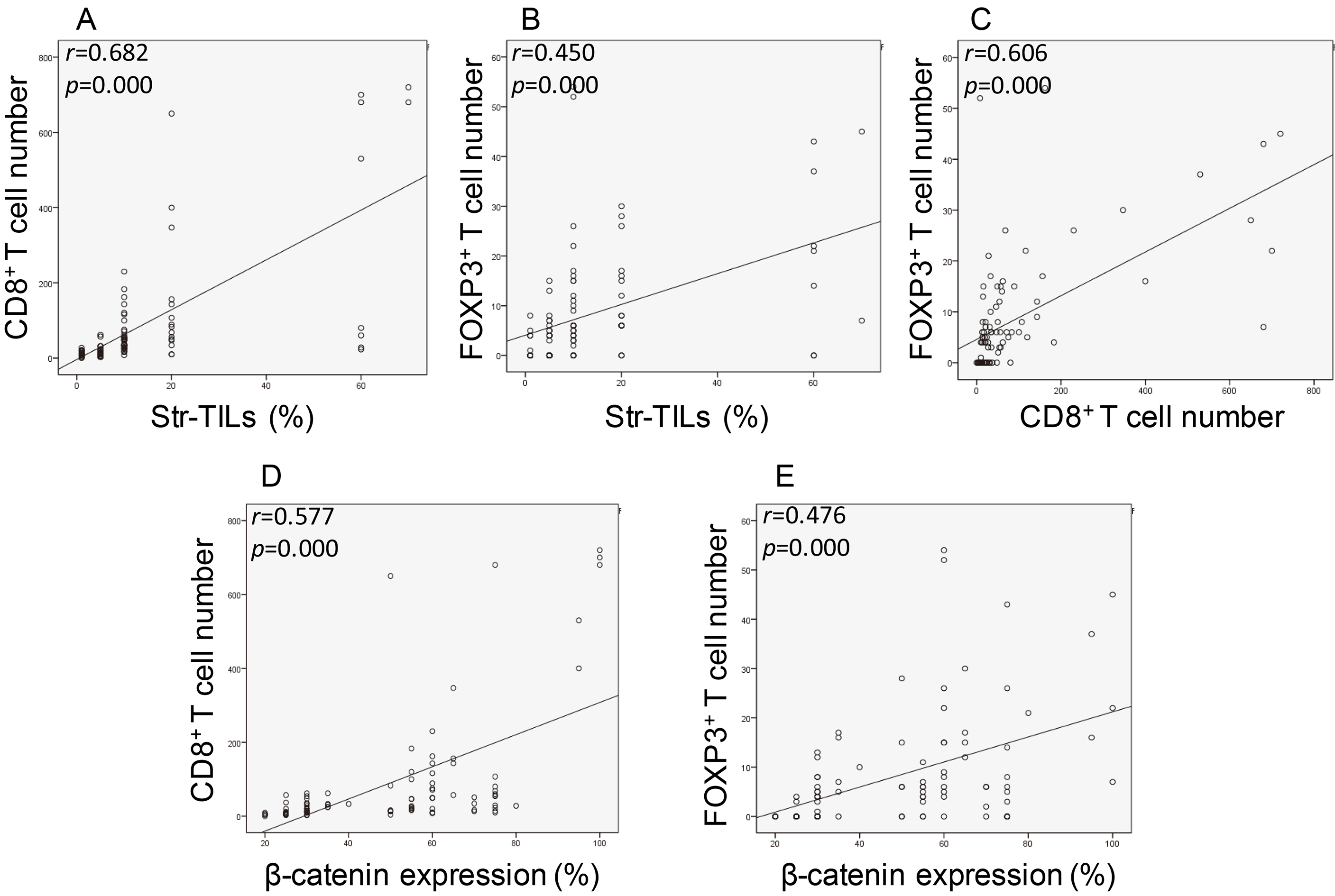
3.4Association of CD8+ + β
We further assessed the association of two TIL subsets, CD8
4.Discussion
TILs that interact with other immune infiltrates in the tumor microenvironment are mainly responsible for intratumoral immune responses. Detection of TILs by immunohistochemical techniques may be an indicator of immune responses to cancer development. In addition, presence of TILs shows predictive value for patients’ responses to neoadjuvant chemotherapy and can potentially help oncologists to identify the subgroup of patients that will receive greater benefit from the therapy [40, 41]. Rather than showing clinical significance among overall breast cancer population, the prognostic or predictive values of TILs seems to be restricted for certain molecular subtypes of breast cancer, mainly TNBC and HER2-enriched subtypes [42, 43]. Our clinicopathological data revealed significant correlations between ER/PR status and stromal TILs. ER/PR negative BC i.e. TNBC or HER2-positive BC tends to have higher level of lymphocytes infiltration, while in luminal type A and B, the percentage is much lower. These results are partly consistent with previous data form other studies [5, 8] showing that the clinical value of TILs should be emphasized in hormone receptor negative BC, i.e. TNBC and HER2
Wnt/
By IHC staining of
Different subgroups of the immune complex within tumor site may exert diverse functions. For example, DCs, Th1 CD4
To sum up, our results demonstrated that lymphocytes infiltration in tumor stroma varies with hormone receptor status, tumor grade and different molecular subtypes of breast cancer. Both stromal lymphocytes infiltration and
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Project No.81274136).
References
[1] | K.D. Miller, R.L. Siegel, C.C. Lin et al., Cancer treament and survivorship statistics, CA Cancer J Clin 66: (4) ((2016) ), 271–289. |
[2] | J. Ferlay, I. Soerjomataram, R. Dikshit et al., Cancer incidence and mortality worldwide: sources, methods, major patterns in GLOBOCAN 2012, Int J Cancer 136: (5) ((2015) ), E359–E386. |
[3] | R.D. Schreiber, L.J. Old and M.J. Smyth, Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion, Science 331: ((2011) ), 1565–1570. |
[4] | L. Carbognin, S. Pilotto, R. Nortilli et al., Predictive and Prognostic Role of Tumor-Infiltrating Lymphocytes for Early Breast Cancer According to Disease Subtypes: Sensitivity Analysis of Randomized Trials in Adjuvant and Neoadjuvant Setting, Oncologist 21: (3) ((2016) ), 283–291. |
[5] | M.V. Dieci, M.C. Mathieu, V. Guarneri et al., Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials, Ann Oncol 26: (8) ((2015) ), 1698–1704. |
[6] | S. Adams, R.J. Gray and S. Demaria et al., Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199, J Clin Oncol 32: (27) ((2014) ), 2959–2966. |
[7] | C. Denkert, S. Loibl, A. Noske et al., Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer, J Clin Oncol 28: (1) ((2010) ), 105–113. |
[8] | S. Loi, S. Michiels, R. Salgado et al., Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial, Ann Oncol 25: (8) ((2014) ), 1544–1550. |
[9] | D.J. Van Den Berg, A.K. Sharma, E. Bruno et al., Role of members of the Wnt gene family in human hematopoiesis, Blood 92: (9) ((1998) ), 3189–3202. |
[10] | F.J. Staal, J. Meeldijk, P. Moerer et al., Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription, Eur J Immunol 31: ((2001) ), 285–293. |
[11] | F.J. Staal, T.C. Luis and M.N. Tiemessen, WNT signalling in the immune system: WNT is spreading its wings, Nature Reviews Immunology 8: ((2008) ), 581–593. |
[12] | L. Gattinoni, Y. Ji and N.P. Restifo, Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy, Clin Cancer Res 16: (19) ((2010) ), 4695–4701. |
[13] | G. Driessens, Y. Zheng, F. Locke et al., β-Catenin Inhibits T Cell Activation by Selective Interference with Linker for Activation of T Cells–Phospholipase C-γ1 Phosphorylation, J Immunol 186: (2) ((2011) ), 784–790. |
[14] | S. Muralidharan, P.J. Hanley, E. Liu et al., Activation of Wnt signaling arrests effector differentiation in human peripheral and cord blood-derived T lymphocytes, J Immunol 187: (10) ((2011) ), 5221–5232. |
[15] | C. Oderup, M. LaJevic and E.C. Butcher, Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance, J Immunol 190: (12) ((2013) ), 6126–6134. |
[16] | T. Reya and H. Clevers, Wnt signalling in stem cells and cancer, Nature 434: (7035) ((2005) ), 843–850. |
[17] | T. Yaguchi, Y. Goto and K. Kido et al., Immune Suppression and Resistance Mediated by Constitutive Activation of Wnt/β-Catenin Signaling in Human Melanoma Cells, J Immunol 189: (5) ((2012) ), 2110–2117. |
[18] | H. Harlin, Y. Meng, A.C. Peterson et al., Chemokine expression in melanoma metastases associated with CD81 T-cell recruitment, Cancer Res 69: (7) ((2009) ), 3077–3085. |
[19] | R.R. Ji, S.D. Chasalow, L. Wang et al., An immune-active tumor microenvironment favors clinical response to ipilimumab, Cancer Immunol Immunother 61: (7) ((2012) ), 1019–1031. |
[20] | O. Hamid and R.D. Carvajal, Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy, Expert Opin Biol Ther 13: (6) ((2013) ), 847–861. |
[21] | S. Spranger, R. Bao and T.F. Gajewski, Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity, Nature 523: (7559) ((2015) ), 231–235. |
[22] | H.E. Fleming, V. Janzen, C. Lo Celso et al., Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo, Cell Stem Cell 2: ((2008) ), 274–283. |
[23] | A.W. Duncan, F.M. Rattis, L.N. DiMascio et al., Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance, Nat Immunol 6: (3) ((2005) ), 314–322. |
[24] | M.A. Forget, Y. Huon, A. Reuben et al., Stimulation of Wnt/β-catenin pathway in human CD8+ T lymphocytes from blood and lung tumors leads to a shared young/memory phenotype, PLoS One 7: (7) ((2012) ), e41074. |
[25] | S.Y. Lin, W. Xia, J.C. Wang et al., Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression, Proc Natl Acad Sci USA 97: (8) ((2000) ), 4262–4266. |
[26] | A.I. Khramtsov, G.F. Khramtsova and M. Tretiakova et al., Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome, Am J Pathol 176: (6) ((2010) ), 2911–2920. |
[27] | X. Ma, W. Yan, Z. Dai et al., Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway, Drug Des Devel Ther 10: ((2016) ), 1419–1441. |
[28] | S.M. Mahmoud, E.C. Paish, D.G. Powe et al., Tumor-Infiltrating CD8+ Lymphocytes Predict Clinical Outcome in Breast Cancer, J Clin Oncol 29: (15) ((2011) ), 1949–1955. |
[29] | F. Liu, R. Lang, J. Zhao et al., CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes, Breast Cancer Res Treat 130: ((2011) ), 645–655. BMC Cancer 15: ((2015) ), 727. |
[30] | S. Liu, J. Lachapelle, S. Leung et al., CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer, Breast Cancer Research 14: ((2002) ), R48. |
[31] | G.J. Bates, S.B. Fox, C. Han et al., Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse, J Clin Oncol 24: ((2006) ), 5373–5380. |
[32] | D. Jiang, Z. Gao, Z. Cai et al., ClinicopathologicalandprognosticsignificanceofFOXP3, BMC Cancer 15: ((2015) ), 727. |
[33] | M.E. Hammond, D.F. Hayes, A.C. Wolff et al., American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer, J Clin Oncol 28: (16) ((2010) ), 2784–2795. |
[34] | A.C. Wolff, M.E. Hammond, D.G. Hicks et al., Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update, J Clin Oncol 31: (31) ((2013) ), 3997–4013. |
[35] | R. Salgado, C. Denkert and S. Demaria et al., The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014, Annals of Oncology 26: ((2014) ), 259–2571. |
[36] | E. Lóez-Knowles, S.J. Zardawi, C.M. McNeil et al., Cytoplasmic Localization of β-Catenin is a Marker of Poor Outcome in Breast Cancer Patients, Cancer Epidemiol Biomarkers Prev 19: (1) ((2010) ), 301–309. |
[37] | L. Demir, S. Yigit, H. Ellidokuz et al., Predictive and prognostic factors in locally advanced breast cancer: effect of intratumoral FOXP3+ Tregs, Clin Exp Metastasis 30: ((2013) ), 1047–1062. |
[38] | O. Yersal and S. Barutca, Biological subtypes of breast cancer: Prognostic and therapeutic implications, World J Clin Oncol 5: (3) ((2014) ), 412–424. |
[39] | N. Dey, B.G. Barwick, C.S. Moreno et al., Wnt signaling in triple negative breast cancer is associated with metastasis, BMC Cancer 13: ((2013) ), 537. |
[40] | C. Denkert, S. Loibl, A. Noske et al., Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer, J Clin Oncol 28: (1) ((2010) ), 105–113. |
[41] | H. Hornychova, B. Melichar, M. Tomsova et al., Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma, Cancer Invest 26: (10) ((2008) ), 1024–1031. |
[42] | Y. Mao, Q. Qu, X. Chen et al., The Prognostic Value of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis, PLoS One 11: (4) ((2016) ), e0152500. |
[43] | L. Carbognin, S. Pilotto, R. Nortilli et al., Predictive and Prognostic Role of Tumor-Infiltrating Lymphocytes for Early Breast Cancer According to Disease Subtypes: Sensitivity Analysis of Randomized Trials in Adjuvant and Neoadjuvant Setting, Oncologist 21: (3) ((2016) ), 283–291. |
[44] | R. Fodde and T. Brabletz, Wnt/beta-catenin signaling in cancer stemness and malignant behavior, Curr Opin Cell Biol 19: (2) ((2007) ), 150–158. |
[45] | M. Krausova and V. Korinek, Wnt signaling in adult intestinal stem cells and cancer, Cell Signal 26: (3) ((2014) ), 570–579. |
[46] | Z. Wang, H. Zhang, J. Hou et al., Clinical implications of β-catenin protein expression in breast cancer, Int J Clin Exp Pathol 8: (11) ((2015) ), 14989. |
[47] | N. Mukherjee, N. Bhattacharya, N. Alam et al., Subtype-specific alterations of the Wnt signaling pathway in breast cancer: clinical and prognostic significance, Cancer Sci 103: (2) ((2012) ), 210–220. |
[48] | F.J. Staal and H.C. Clevers, WNT signalling and hematopoiesis: a WNT-WNT situation, Nat Rev Immunol 5: (1) ((2005) ), 21–30. |
[49] | L. Gattinoni, X.S. Zhong, D.C. Palmer et al., Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells, Nat Med 15: ((2009) ), 808–813. |
[50] | D.M. Zhao, S. Yu and X. Zhou et al., Constitutive activation of Wnt signaling favors generation of memory CD8 T cells, J Immunol 184: ((2010) ), 1191–1199. |
[51] | D. Notani, K.P. Gottimukkala, R.S. Jayani et al., Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner, PLoS Biol 8: ((2010) ), e1000296. |
[52] | X. Liang, C. Fu, W. Cui et al., β-catenin mediates tumor-induced immunosuppression by inhibiting cross-priming of CD8+ T cells, J Leukoc Biol 95: (1) ((2014) ), 179–190. |
[53] | W.H. Fridman, J. Galon and F. Pagès et al., Prognostic and predictive impact of intra- and peritumoral immune infiltrates, Cancer Res 71: (17) ((2011) ), 5601–5605. |
[54] | J. Galon, H.K. Angell, D. Bedognetti et al., The Continuum of Cancer Immunosurveillance: Prognostic, Predictive, and Mechanistic Signature, Immunity 39: (1) ((2013) ), 11–26. |
[55] | N.R. West, S.E. Kost, S.D. Martin et al., Tumour-infiltrating FOXP3+ lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer, Br J Cancer 108: (1) ((2013) ), 155–162. |
[56] | E.M. Shevach, Mechanisms of FOXP3+ T regulatory cell-mediated suppression, Immunity 30: (5) ((2009) ), 636–645. |
[57] | A. Prat, J.S. Parker and O. Karginova et al., Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer, Breast Cancer Res 12: (5) ((2010) ), R68. |




