Pleiotrophin serum level and metastasis occurrence in breast cancer patients
Abstract
BACKGROUND:
Breast cancer (BC) cases in Makassar, Indonesia, are on the rise, with 2723 cases recorded in 2018. Tumor cells in the blood indicate metastasis, emphasizing the need for early diagnosis and monitoring. Pleiotrophin (PTN) is associated with various human malignancies, and recent studies suggest a correlation between PTN expression and advanced BC stages; therefore, PTN could serve as an independent predictor of metastasis. This study aimed to determine the correlation between serum PTN level, histopathological grading, and metastasis occurrence in BC patients in Makassar, Indonesia.
METHODS:
This study used an observational cross-sectional design. Pleiotrophin serum levels were examined using enzyme-linked immunosorbent assays. This study used a t-test and ROC curve analysis for the statistical tests.
RESULTS:
Of the 64 samples used in this study, metastasis was present in 26 cases and absent in 38 samples. The mean PTN serum levels in metastatic and non-metastatic breast cancer patients were 4.311 and 1.253, respectively. The PTN receiver operating characteristic curve showed an area under the curve of 2.47 ng/dL, which was statistically significant (p < 0.001). A significant relationship was found between PTN level and metastasis (p < 0.001). The correlation coefficient was 0.791, indicating a positive correlation.
CONCLUSION:
This study revealed that the serum PTN level among breast cancer patients had a cut-off value of 2.47 ng/dL. The research established a clear correlation between PTN level and metastasis occurrence in breast cancer patients, indicating a higher likelihood of distant metastasis with elevated PTN concentration.
1.Introduction
Breast cancer (BC) is a critical public health problem worldwide, especially for female patients. In 2020, 2.26 million new cases and 684 996 deaths due to BC were recorded. BC is the most significant cause of cancer deaths worldwide [1] and the most frequent cancer reported in Indonesia, representing 38.6% and 16.6% of new cancer cases in female and male patients, respectively [2]. In Makassar, Indonesia, 2723 BC cases were recorded in 2018, an increase over prior years [3]. Metastasis is often considered the final stage of BC progression and indicates a poor prognosis. Detection of tumor cells in the blood is an early indicator of metastasis, emphasizing the need for early diagnosis and monitoring [4].
Pleiotrophin (PTN) is a potential biomarker of various human malignancies, including BC. However, its correlation with BC in Indonesian patients needs to be clarified. Noninvasive biomarkers, such as those found in biological fluids, including blood, urine, sputum, and serum, are crucial for early-stage BC diagnosis as they can offer insights into disease progression and treatment response. Recent studies suggest a potential correlation between PTN expression and advanced BC stage, indicating that PTN could be an independent predictor of metastasis [5–7]. Given the importance of prognosis biomarkers in BC management, this study aimed to determine the correlation between serum PTN level, histopathological grading, and metastasis occurrence in Makassar BC patients.
2.Methods
This study was a cross-sectional observational study. This research protocol was approved by the the Faculty of Medicine Ethics Committee of the Universitas Hasanuddin–Dr. Wahidin Sudirohusodo Hospital, Makassar, Indonesia. Data was collected from the hospital’s medical records. The research participants were breast cancer patients with invasive mammary carcinomas who met the research criteria based on the order of admission to the hospital (consecutive sampling). The inclusion criteria consisted of female patients with non-metastatic or de novo metastatic breast cancer that was diagnosed histopathologically as an invasive mammary carcinoma. The exclusion criteria included female patients with were recurrent breast cancer, were accompanied by other malignancies, were treated with chemotherapy and radiotherapy, and coexisted with Alzheimer’s disease, ischemic brain injury, trauma, or renal injury.
2.1.Histopathological grading of breast cancer
Histopathological grading is divided into three groups based on an anatomical pathology examination: low, moderate, and high grade, and is determined from the mitotic index, tubular formation, and nuclear pleomorphism (based on the Scarff-Bloom-Richardson modification).
2.2.Breast cancer without metastases
Breast cancers with no clinical symptoms or radiological signs of metastasis after x-ray examination, abdominal ultrasonography, and other examinations according to indications were considered breast cancers without metastases.
2.3.Metastatic breast cancer
In contrast, metastases that were present when breast cancer was first diagnosed (de novo) were considered metastatic breast cancers. Breast cancer patients who presented for the first time with clinical symptoms and radiological signs of metastasis as proven by x-ray examination, abdominal ultrasound, or other indicating examinations were considered metastatic breast cancer patients.
2.4.Pleiotrophin levels
The level of PTN in people with breast cancer was determined using the enzyme-linked immunosorbent assay (ELISA) technique using the blood serum of patients with breast cancer.
2.5.Receiver operating characteristic (ROC) analysis
This study used an ROC analysis to obtain the cut-off value for PTN levels.
2.6.Statistical analysis
The data analysis was conducted using SPSS version 26. The statistical analyses included t-tests and an ROC curve analysis to determine the PTN cut-off value. A result was considered significant if the p-value was <0.05.
3.Results
3.1.Sample characteristics
The 64 samples included in this study consisted of 26 (41%) breast cancer patients with metastasis and 38 (59%) without metastasis. The characteristics of the study samples are listed in Table 1.
Table 1
Study sample characteristics
| Characteristic | Sample (n) | Percentage (%) |
| Age (years) | ||
| ≤50 | 40 | 63.00 |
| >50 | 24 | 37.00 |
| Metastasis status | ||
| Metastasis | 26 | 41.00 |
| No Metastasis | 38 | 59.00 |
| Metastasis location | ||
| Lungs | 13 | 50.00 |
| Bones | 5 | 19.23 |
| Liver | 3 | 11.54 |
| Lungs and Bones | 2 | 7.69 |
| Lungs, Bones, and Brain | 1 | 3.85 |
| Lungs, Bones, and Liver | 1 | 3.85 |
| Lungs and Liver | 1 | 3.85 |
| Histopathology grade | ||
| Low | 15 | 23.40 |
| Moderate | 22 | 34.40 |
| High | 27 | 42.20 |
| Pleiotrophin level | ||
| ≥2.47 (increasing) | 30 | 47.00 |
| <2.47 (low) | 34 | 53.00 |
3.2.Relationship between PTN and breast cancer metastasis
The statistical analysis of the relationship between PTN level and metastasis status using an independent t-test is shown in Table 2.
Table 2
Independent sample T-test of PTN level and breast cancer metastasis status
| Variable | Pleiotrophin level (mean ± SD) | p-value | Mean difference (95% CI) |
| Metastasis | 4.311 (1.553) | <0.001 | 3.058 (2.436–3.680) |
| No metastasis | 1.253 (0.935) |
Table 2 reveals that the average PTN levels in patients with and without metastasis were 4.311 and 1.253, respectively. Therefore, breast cancer patients with metastasis showed higher levels of PTN than those in patients without metastasis.
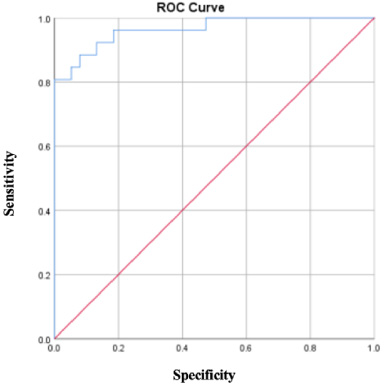
The cut-off PTN level based on ROC analysis was 2.47 ng/dL, whereby increased levels of PTN were represented by values ≥2.47 ng/dL. Figure 1 shows an area under the curve (AUC) value of 2.47 ng/dL, which was statistically significant (p < 0.001); therefore, the PTN cut-off value can be used to predict the presence of distant metastasis.
Fig. 1.
The pleiotrophin ROC curve shows the diagnostic accuracy and determines the optimal cut-off value of 2.47 ng/dL, a sensitivity value of 0.8, and a specificity that moves up to 1.
3.3.The strength of the relationship between pleiotrophin level and metastasis status
Table 3
Spearman correlation test of pleiotrophin level and metastasis status.
| Variable | Metastasis status |
| Pleiotrophin level | r = 0.791 |
| p-value = <0.001 | |
| N = 64 |
According to Table 3, the p-value (2-tailed) was <0.001, which was below the alpha value; therefore, a significant (meaningful) relationship existed between PTN level and metastasis. The correlation coefficient was 0.791, indicating a solid relationship between these two variables. In addition, the correlation coefficient was positive, specifically 0.791, indicating a positive correlation. Therefore, higher PTN levels in breast cancer patients appear to correlate to a greater likelihood of metastasis.
4.Discussion
Pleiotrophin is encoded by the PTN gene, which is approximately 116 kb and is located on chromosome seven in the human genome (7q33 band). PTN is expressed as a 168-residue protein, with the mature 136-residue form typically secreted into the extracellular space through the normal cell secretion pathway. Pleiotrophin is a multifunctional growth factor belonging to a highly conserved family of human genes. It regulates multiple cellular functions, including cell proliferation and angiogenesis in endothelial cells. It is expressed during the development of several solid tumors and functions in tumor cell autocrine stimulation and/or stromal tissue and blood supply recruitment to developing tumors. Numerous reports indicate a positive correlation between PTN and in vivo or in vitro angiogenesis. In vitro studies suggest that PTN controls endothelial cell migration and/or proliferation. The PTN examination in this study revealed values ranging from 0.009 to 7.14 ng/dL. To determine the cut-off value, we conducted an ROC analysis. We obtained a significant AUC of 2.47, indicating that the PTN cut-off value can be used to predict distant metastasis. Therefore, the cut-off value of 2.47 ng/dL was used, whereby PTN levels ≥2.47 ng/dL were considered overexpressed, and levels <2.47 ng/dL were deemed not overexpressed. In this study, 24 subjects (92%) had PTN overexpression with metastasis, while two subjects (8%) showed no overexpression. This aligns with the results of Ma et al., who discovered that an increase in serum PTN level was associated with TNM staging, histopathological grading, and distant metastasis [6].
Metastasis is a complex, sequential process that begins when a tumor reaches a specific size and requires the formation of new blood vessels (angiogenesis) to meet its metabolic and proliferative needs. Cells that spread from the primary tumor through the bloodstream enter the immune system. Once they survive the anti-tumor response, they are transported to the capillary walls of secondary organs. Once they survive the anti-tumor response, they are transported to the capillary walls of secondary organs, where they remain. These cells eventually leave the circulation system through invasion, migrate through the endothelial walls of blood vessels and the extracellular matrix, and enter secondary organs. Micrometastases then form in these new locations, eventually disrupting the physiological state of the patient [8]. Breast cancer metastasis is defined as the spread of the tumor beyond the breast, chest wall, and regional ipsilateral lymph nodes. The most common sites of BC metastases are the bones, lungs, liver, lymph nodes, and brain. Breast cancers with positive hormone receptors tend to metastasize to the bones initially, while those with negative hormone receptors and/or HER-2 positive are more frequently associated with visceral organ metastases [9]. In this study, distant metastases were found in 26 participants (41%), which is consistent with data from the Ministry of Health indicating that most patients in Indonesia seek treatment in the advanced stages. Various factors contribute to the high number of BC patients seeking medical attention, including the prevalence of alternative treatments in society, which often result in the growth and development of cancers, leading to severe cases and possible death for cancer patients. Another factor is the presence of misinformation in society. These myths and misconceptions increase the fear of cancer patients to seek medical treatment as the primary means of addressing their condition. This study’s most common metastasis locations were the lungs in 13 subjects (50 %) and bones in 5 subjects (19.23%). This aligns with existing literature, which states that the bones, lungs, liver, lymph nodes, and brain are the most common sites for BC metastasis [9].
The PTN values of the 64 breast cancer patients ranged from 0.009 to 7.14 ng/dL. This study assessed the relationship between PTN level and metastasis status. This study assessed the relationship between PTN level and metastasis status using the independent t-test. The average PTN level in patients with metastasis was 4.311, higher than the 1.253 found in patients without metastasis. The Spearman correlation test yielded a Sig. (2-tailed) <0.001 was below the alpha value and indicated a significant relationship between PTN levels and metastasis. The correlation coefficient was 0.791, which signified a strong relationship between the two factors. Thus, it can be interpreted that higher PTN levels in breast cancer patients are associated with an increased likelihood of metastasis. Pleiotrophin has been reported to be crucial in regulating several cellular processes. Higher pleiotrophin levels in serum result from the release of the protein from tumor cells, leading to an increase in PTN that parallels the cancer stage. In addition, PTN is considered to have oncogenic potential since its expression increases in breast, prostate, pancreas, stomach, lung, colon, and ovarian cancers. The close association of PTN with metastasis has been reported, with high expression of this protein in aggressive breast cancers. That study concluded that the pharmacologic or genetic suppression of PTN reduced tumor-associated neutrophil accumulation, restored immunity, enhanced T-cell activation, and weakened metastasis [5,10,11].
The limitations of this study included the measurement of variables, particularly the occurrence of distant metastasis, which was performed using chest X-rays, abdominal ultrasounds, and relevant examinations based on complaints. Ideally, the measurements should be conducted using PET scans. In addition, this study utilized a cross-sectional design, which limited the ability to assess whether PTN overexpression in the early stages would lead to subsequent distant metastasis.
5.Conclusion
This study revealed that the serum PTN levels among breast cancer patients had a cut-off value of 2.47 ng/dL. The research established a clear correlation between PTN level and metastasis occurrence in breast cancer patients, indicating a higher likelihood of distant metastasis with elevated PTN concentration. Furthermore, a robust positive correlation was identified, emphasizing a solid association between PTN level and distant metastasis in breast cancer patients. These results provide valuable insights into the potential role of PTN as a biomarker for breast cancer metastasis.
Acknowledgements
The researchers would like to express their sincere gratitude to the Faculty of Medicine at Hasanuddin University in Makassar, Indonesia, for their generous support of this research.
Ethics approval
The study was approved by the Research Ethics Committee of the Faculty of Medicine Universitas Hasanuddin, Makassar, Indonesia, number 172/UN4.6.4.5.31/PP.36/2023. We promised that the participants’ data would be anonymized or maintained private, that the rights or interests of participants would not be invaded, and that informed consent would be obtained from all participants.
Competing interests
No competing interests were reported.
Data availability statement
Data is accessible upon justifiable request.
Funding
Self-funding.
Contributors
MI: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
DJF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Software, Validation, Visualization, Writing – original draft.
SAS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
IJG: Conceptualization, Investigation, Methodology, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Software, Validation, Visualization, Writing – original draft.
NS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
ES: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
CAA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
MF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
References
[1] | Łukasiewicz S, , Czeczelewski M, , Forma A, , Baj J, , Sitarz R, , Stanisławek A, Breast cancer—epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—an updated review, Cancers (Basel) [Internet], 13: (17): 4287, (2021) . Available from: https://www.mdpi.com/2072-6694/13/17/4287. |
[2] | Sung H, , Ferlay J, , Siegel RL, , Laversanne M, , Soerjomataram I, , Jemal A , Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries , CA Cancer J Clin [Internet], 71: (3): 209–249, (2021) . Available from: https://onlinelibrary.wiley.com/doi/10.3322/caac.21660. |
[3] | Prihantono P, , Rusli R, , Christeven R, , Faruk M, Cancer incidence and mortality in a tertiary hospital in indonesia: an 18-year data review, Ethiop J Health Sci [Internet], 33: (3): 515–522, (2023) . Available from: http://www.ncbi.nlm.nih.gov/pubmed/37576162. |
[4] | Lawrence R, , Watters M, , Davies CR, , Pantel K, , Lu Y-J, Circulating tumour cells for early detection of clinically relevant cancer, Nat Rev Clin Oncol [Internet], 20: (7): 487–500, (2023) . Available from: https://www.nature.com/articles/s41571-023-00781-y. |
[5] | Zhou J, , Yang Y, , Zhang Y, , Liu H, , Dou Q, A meta-analysis on the role of pleiotrophin (PTN) as a prognostic factor in cancer, PLoS One, 13: (11): e0207473, (2018) . |
[6] | Ma J, , Kong Y, , Nan H, , Qu S, , Fu X, , Jiang L , Pleiotrophin as a potential biomarker in breast cancer patients, Clin Chim Acta, 466: : 6–12, (2017) . |
[7] | Scully OJ, , Bay B-H, , Yip G, , Yu Y, Breast cancer metastasis, Cancer Genomics Proteomics, 9: (5): 311–320, (2012) . |
[8] | Rakha EA, , Ellis IO, Breast cancer prognostic classification in the molecular era: the role of histological grade, Breast Cancer Res, 12: (207): 260–271, (2010) . |
[9] | Morrow M, , Burstein HJ, , Harris JR, . Malignant tumors of the breast. Cancer of the Breast: Cancer: Principles & Practice of Oncology, . DeVita VT, , Lawrence TS, , Rosenberg SA (eds), 10th ed. Lippincott Williams & Wilkins (LWW); (2016) . |
[10] | Ganguly D, , Schmidt MO, , Coleman M, , Ngo T-VC, , Sorrelle N, , Dominguez ATA , Pleiotrophin drives a prometastatic immune niche in breast cancer, J Exp Med, 220: (5)(2023) . |
[11] | Papadimitriou E, , Pantazaka E, , Castana P, , Tsalios T, , Polyzos A, , Beis D, Pleiotrophin and its receptor protein tyrosine phosphatase beta/zeta regulators of angiogenesis and cancer, Biochim Biophys Acta, 1866: (2): 252–265, (2016) . |




