Telangiectasias induced by combination tucatinib and ado-trastuzumab emtansine in a patient with metastatic breast cancer
Abstract
BACKGROUND:
Tucatinib is a tyrosine kinase inhibitor currently used in salvage therapy for human epidermal growth factor receptor 2 (HER2)-positive breast and colorectal cancer. The use of tucatinib alone or in combination with ado-trastuzumab emtansine (T-DM1) in the treatment of advanced HER2-positive cancers is rapidly expanding.
OBJECTIVE/METHODS:
We report the case of a 66-year-old female who presented to the dermatology clinic with a one-year history of widespread telangiectasias that began after initiation of combination chemotherapy with tucatinib and T-DM1 for metastatic HER2-positive invasive ductal carcinoma.
RESULTS:
The patient’s lesions regressed upon cessation of combination therapy and reappeared in the setting of tucatinib re-initiation, with gradual improvement over the following four months following electrocautery to the affected regions.
CONCLUSIONS:
We postulate that telangiectasias may be a previously unreported dermatologic side effect of combination treatment with tucatinib and T-DM1. Electrocautery is a safe and effective procedure to reduce the appearance of telangiectasias and improve patient satisfaction during chemotherapy.
1.Background
Tucatinib is a tyrosine kinase inhibitor currently used in salvage therapy for human epidermal growth factor receptor 2 (HER2)-positive breast and colorectal cancer, with a clinical range that is rapidly expanding [1–5]. We report the case of a 66-year-old female who presented to the dermatology clinic with a one-year history of widespread telangiectasias that began after initiation of combination chemotherapy with tucatinib and ado-trastuzumab emtansine (T-DM1) for HER2-positive invasive ductal carcinoma. The lesions regressed upon cessation of combination therapy and reappeared in the setting of tucatinib re-initiation, with gradual improvement over the following four months. As such, we postulate that telangiectasias may be a previously unreported dermatologic side effect of combination treatment with tucatinib and T-DM1.
2.Case presentation
The patient is a 66-year-old female with a history of well-controlled hypertension and a family history of ovarian cancer in a paternal aunt. In 2014, she was diagnosed with a locally advanced, HER2-positive, estrogen and progesterone receptor-negative invasive ductal carcinoma of the right breast (Stage IIIA; pT1c, pN2, M0). Genetic testing was negative for germline mutations. Treatment included 6 cycles of neoadjuvant docetaxel, carboplatin, trastuzumab, and pertuzumab, with an additional 6 months of trastuzumab, which was tolerated well; bilateral mastectomy and axillary node dissection given patient preference; and radiation therapy. In 2017, the patient was diagnosed with metastatic HER2-positive, weakly estrogen receptor-positive, and progesterone receptor-negative disease in the left supraclavicular, mediastinal, axillary, and subpectoral lymph nodes (Stage IV; cT3, cN2, cM1). She was started on docetaxel, trastuzumab, and pertuzumab; after 6 cycles, docetaxel was replaced with letrozole. After seven months of treatment, tumor markers were elevated, and disease progression was noted on PET scan. At this time, fulvestrant was initiated but ultimately discontinued within two months due to intolerable side effects.
In March 2020, the patient began fourth-line treatment of tucatinib in combination with ado-trastuzumab emtansine (T-DM1). For the first year of treatment, she received 300 mg oral tucatinib twice daily and 3.6 mg/kg intravenous T-DM1 every 3 weeks. While she generally tolerated this treatment, she did report neuropathy, arthralgias, and myalgias. One year into treatment, because of progressing neuropathy, the dose of T-DM1 was reduced to 3.0 mg/kg every 3 weeks. Approximately two years into treatment, she presented to the oncology clinic reporting the onset of “red dots” on the body. She was referred to dermatology, where numerous scattered telangiectasias were observed, predominately on the upper chest, neck, and shoulders. The dermatologic examination was otherwise unremarkable, except for mild rosacea on the bilateral cheeks. At this time, the patient was counseled that the rash was likely secondary to her chemotherapy regimen, laser therapy was briefly discussed as a potential future treatment, and she was advised to return for follow-up in 3 months to evaluate whether the lesions had progressed further. During follow-up, the patient reported that the telangiectasis had persisted and begun to spread to her arms as well. She described a negative impact on her emotional well-being due to the rash and stated that she no longer dressed as she desired out of fear of judgment. She was offered laser treatment but ultimately deferred due to cost.
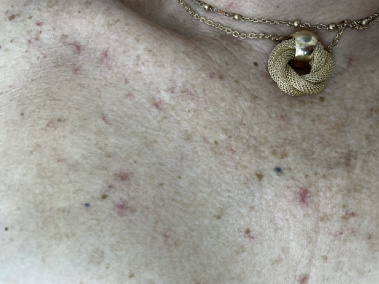
During this time, an elevation in her liver function tests, including bilirubin, aspartate aminotransferase (AST), and alkaline phosphatase was observed with AST increasing from 21 to 61, total bilirubin from 0.3 to 2.1, and alkaline phosphatase from 107 to 136. Of note, her platelet count remained within normal limits. Over the subsequent year, she developed worsening neuropathy, and in January 2023, the decision was made to hold all treatments for three weeks to allow for an improvement in side effects. On the subsequent oncology visit, the decision to discontinue T-DM1 was made. At this time, tucatinib was reinitiated at a reduced dose of 250 mg twice daily. One month later, she presented to the dermatology clinic for a third time due to continued concern over the telangiectasias. She stated that the lesions had begun to disappear in the time that the chemotherapy regimen was discontinued. However, upon reinitiating tucatinib, she noticed that the lesions began to recur, as demonstrated in Figs 1 and 2. She denied melena, mucosal bleeding except for a few episodes of epistaxis, and associated pain, itch, and discomfort at the site of the lesions. She reported that the lesions were still cosmetically bothersome and treatment options were once again discussed. Given the cost associated with laser treatment, the patient elected for treatment with electrocautery. The most prominent telangiectasias were treated with electrocautery on the same day, and the procedure was well tolerated by the patient.
Fig. 1.
Telangiectasias arising during combination chemotherapy.
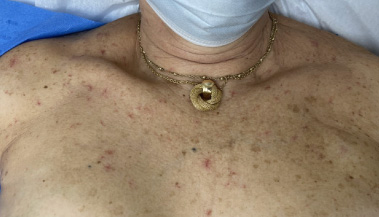
Fig. 2.
Telangiectasias, close-up view.
During a subsequent 3-month dermatology follow-up, the patient reported significant improvement in the regions treated with electrocautery, stating that her emotional well-being had improved significantly following the procedure. On exam, the treated lesions had regressed in size and pigmentation. The patient also described an overall reduction in the number of new telangiectasias and a mild decline in the pigmentation of older, untreated lesions after her dose of tucatinib had been lowered. This patient report was supported by findings on the dermatologic exam. The patient indicated that she was interested in receiving electrocautery treatment again for the remaining lesions.
3.Discussion and conclusions
This patient developed telangiectasias during treatment with T-DM1 and tucatinib. Telangiectasias have not been documented as a side effect of tucatinib; however, T-DM1-associated telangiectasis have been described in several isolated case reports. [6–8] Given that the patient’s lesions appeared during combination T-DM1 and tucatinib treatment, regressed after combination chemotherapy was held, and returned in the context of tucatinib re-initiation with delayed improvement, we believe this may represent a unique and previously undocumented side effect of combination treatment with tucatinib and T-DM1.
Tucatinib is a tyrosine kinase inhibitor currently used in salvage therapy for HER2-positive breast and colorectal cancer, with a clinical range that is rapidly expanding [1–5]. Tucatinib binds to and inhibits the tyrosine kinase domain on HER2 intracellularly, thus suppressing its phosphorylation [9]. Tucatinib is highly selective toward HER2 and has minimal inhibitory effects on the epidermal growth factor receptor (EGFR) [9]. Trastuzumab, a component of T-DM1, is a monoclonal antibody that blocks HER2 homodimerization by binding to the extracellular or juxtamembrane domains of the receptor [9]. Binding to the extracellular domain allows trastuzumab to trigger an immune response against cancer cells that overexpress HER2 via antibody-dependent cellular cytotoxicity, whereas binding to the juxtamembrane domain allows for blockage of HER2-HER3 dimerization regardless of whether a ligand is present [9]. Both drugs therefore inhibit the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) cell survival and proliferation pathways that are implicated in HER2-positive cancers. However, they function through different mechanisms: tucatinib acts intracellularly while trastuzumab works extracellularly. The similar mechanism of action of these drugs provides a biologically plausible explanation for the observed synergy when they are given in combination [9,10].
Presently, telangiectasias have not been documented as a side effect of tucatinib therapy; however, a phase I study of tucatinib reported adverse dermatologic effects including acne, dermatitis acneiform, and skin exfoliation [11]. On the other hand, T-DM1-associated telangiectasis have been documented in the literature [6–8]. The mechanism by which T-DM1 may cause telangiectasias remains to be fully elucidated, but proposed mechanisms include T-DM1-induced transaminitis and hepatic injury, disruption of cytoskeletal microtubules by emtansine, or emtansine-induced vascular dilation [6–8]. Given that transaminitis can occur with tucatinib use as well, and this patient experienced a rise in several markers of hepatic injury and persistent telangiectasias in the context of tucatinib use without T-DM1, we postulate that tucatinib-induced liver injury may a possible mechanism contributing to the development of telangiectasias in this patient. Another possibility is the influence of HER2 and other molecules further downstream in the MAPK and PI3K pathways, as both tucatinib and T-DM1 share HER2 and these pathways in common. Nevertheless, it is most likely that the use of tucatinib in combination with T-DM1 contributed to the initial presentation of telangiectasias, given the reports of T-DM1-associated telangiectasias. Given the expanding use of tucatinib in oncology regimens, future studies may aim to elucidate the adverse side effects that may result from combination chemotherapy with T-DM1 and tucatinib.
The use of tucatinib alone or in combination with T-DM1 in the treatment of advanced HER2-positive breast and colorectal cancers is continuously increasing. As such, it is vital that providers are aware of possible dermatologic side effects, such as telangiectasias, that may be associated with this regimen. Such knowledge allows providers to counsel and educate patients and provide treatment options such as electrocautery or laser therapy to improve patient satisfaction and well-being while receiving life-saving chemotherapy.
Acknowledgements
The authors would like to thank the patient for her willingness to share her experience.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient prior to the writing of this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was not funded.
Author contributions
Conception: ADM, GFR.
Interpretation or analysis of data: ADM, GFR.
Preparation of the manuscript: GFR, AS.
Revision for important intellectual content: ADM.
Supervision: ADM.
References
[1] | Murthy R, Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: A non-randomized, open-label, phase 1b study, Lancet Oncol, 19: : 880–888, (2018) . |
[2] | Murthy R, Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer, N Engl J Med, 382: : 597–609, (2019) . |
[3] | Strickler J, Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): Initial results from the MOUNTAINEER trial, Ann Oncol, 30, : (2019) . |
[4] | Ulrich L, , Okines AFC, Treating advanced unresectable or metastatic HER2-positive breast cancer: A spotlight on tucatinib, Breast Cancer: Targets and Therapy, 13: : 361–381, (2021) . |
[5] | Ahn D, , Walden D, , Bekaii-Saab T, Tucatinib: An investigational novel therapeutic agent for the treatment of HER-2 colorectal cancer, Expert Opin Investig Drugs, 31: : 437–441, (2022) . |
[6] | Sibaud V, Ado-trastuzumab emtansine-associated telangiectasias in metastatic breast cancer: A case series, Breast Cancer Res Treat, 146: : 451–456, (2014) . |
[7] | Gursoy P, , Acar A, , Acikalin T, Ado-trastuzumab emtansine associated spider telangiectasia, J Oncol Pharm Pract, 28: : 986–988, (2022) . |
[8] | Kwon Y, Telangiectasia and pulmonary arterial hypertension following treatment with trastuzumab emtansine: A case report, Chest, 149: : 103–105, (2016) . |
[9] | Kulukian A, Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models, Mol Cancer Ther, 19: : 976–987, (2020) . |
[10] | Sirhan Z, , Thyagarajan A, , Sahu RP, The efficacy of tucatinib-based therapeutic approaches for HER2-positive breast cancer, Mil Med Res, 9: : 39, (2022) . |
[11] | Moulder SL, Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2(+)-advanced solid tumors, with an expansion cohort in HER2(+) metastatic breast cancer (MBC), Clin Cancer Res, 23: : 3529–3536, (2017) . |




