Primary breast sarcomas: A 13 case-series study treated in university hospital in central Tunisia over a 25-year period
Abstract
AIM:
To retrospectively study the therapeutic modalities of primary breast sarcomas in view of the data of a local Tunisian experience.
METHODS:
It is a monocentric, descriptive, retrospective study including 13 cases of primary breast sarcoma treated over a period of 25 years (1995–2020) in the oncological radiotherapy department of a university hospital in Sousse, Tunisia.
RESULTS:
In our study, 13 cases of non-metastatic breast sarcomas that has been identified, divided into ten cases of phyllodes sarcomas and three cases of non-phyllodes sarcomas.
Surgically, all our patients had a mastectomy. Among them, seven underwent a lymph node procedure: five underwent axillary lymph node dissection, and two others had primary axillary lymph node biopsy. For the adjuvant treatment, all the patients included in our study received radiotherapy and seven received chemotherapy. Local recurrence occurred on the operative scar in one patient after completion of radiation therapy. Metastatic relapse was described in five patients. The time to onset of metastases varied between two months and five years. Nevertheless, a complete remission was noted in 6 patients with a follow-up varying from four years to 20 years. Two patients were lost to follow-up.
CONCLUSION:
Breast sarcomas remain a very rare entity of aggressive tumors.
The therapeutic approach is poorly codified. For this reason, the therapeutic decision should always be discussed in a multidisciplinary assessment.
1.Introduction
Primary breast sarcomas (PBS) are part of the nosological group of non-epithelial tumors of the breast. They form an extremely rare and heterogeneous group of malignant tumors that share a common origin in embryonic mesenchyme. They account for less than 1% of all breast malignancies, and less than 5% of all soft tissue sarcomas [1]. PBS can be classified into two groups: grade 3 malignant phyllodes tumors (PT) or phyllodes sarcomas (PS), which are the most frequent and specific tumors of the breast, and other non-phyllodes sarcomas (NPS), which are stromal tumors of ubiquitous distribution but of which the mammary location remains infrequent [2].
The diagnosis of PBS must be confirmed by anatomopathological examination [3]. From a therapeutic standpoint, surgical resection with healthy margins is the cornerstone of treatment for all PBS [4]. There is no standard of care regarding adjuvant treatment.
The rarity of this pathology and the restricted sample sizes in literature pose major challenges in terms of diagnosis, treatment and research. Current recommendations are derived from small retrospective case reviews and extrapolated from data on soft tissue sarcomas [5].
The aim of our work was to identify the therapeutic features of PBS in a series of cases treated at a university hospital in central Tunisia.
2.Patients and methods
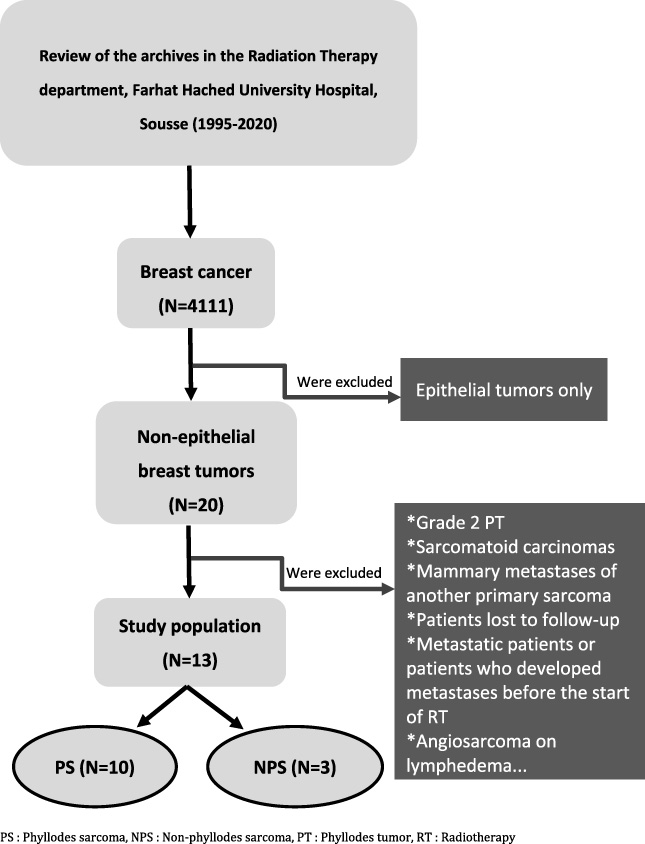
We conducted a retrospective, descriptive, monocentric study of patients with PBS treated in the oncological radiotherapy (RT) department of the university hospital Farhat Hached of Sousse over a 25-year period, from January 1995 to December 2020. We identified 20 cases of PBS. Only 13 cases met the inclusion criteria and were included in our study.
We included all non-metastatic PBS whose diagnosis was confirmed by anatomopathological examination and whose management was carried out in our department.
The following were excluded from our study: malignant tumors of exclusive epithelial origin, borderline PT, sarcomatoid carcinomas, breast metastases of another primary sarcoma, patients with a valid indication for RT but who were lost to follow-up, patients who were metastatic at diagnosis or who had developed metastases before the start of adjuvant RT, angiosarcoma on lymphedema, also known as Stewart-Treves syndrome.
Data collection was based on the clinical medical records and RT data sheets of the patients included in the study, consulted in the oncology RT department in Sousse. We collected patient data, disease data, therapeutic data (surgery, RT and chemotherapy (CT)), anatomopathological data, post-therapeutic surveillance data and patient outcomes.
We described the sociodemographic, clinical and therapeutic characteristics of our patients through the observations collected. We therefore did not use statistical tests on our series. Our results were a description of our case series. Anonymity and confidentiality were ensured from data entry to dissemination of results.
Information was entered using Microsoft Excel 2019®. Zotero was used to compile the list of references.
The bibliographic search was carried out using the Science Direct, PubMed and Google Scholar search engines, using the following keywords: sarcoma, breast, surgery, RT, CT.
3.Results
Between 1995 and 2020, we recorded 13 cases of PBS among 4111 malignant breast tumors treated at our RT department. The incidence of PBS was 0.3%. The 13 cases were divided into three cases of NPS and ten PS. Figure 1 illustrates the patient selection technique. The median age of our patients was 41.4 years, with extremes ranging from 21 to 55 years. Ten patients were nulligravida and eight were premenopausal. Four patients had a family history of cancer.
Fig. 1.
Patient selection chart, Farhat Hached University Hospital, Sousse, 1995–2020.
Seven patients had a history of benign breast pathology. One patient had a history of fibrocystic dystrophy, and the other six had a history of adenofibromas and grade 2 PT.
In our patient cohort, inflammatory skin signs were identified in four cases. These manifestations included skin findings characterized by tension, warmth, neovascularization, and pus discharge. Additionally, a renital mass exhibited an orange peel appearance. Another noteworthy observation was the presence of bloody nipple discharge accompanied by the surrounding skin displaying an orange peel-like texture.
The median tumor size was 10.25 cm, with extremes ranging from 2.5 to 20 cm. Involvement of the left breast was predominant: as many as 11 cases.
Surgically, all our patients underwent mastectomy. Among our study, seven patients, constituting half of the cohort, underwent axillary lymph node dissection and/or axillary lymph node biopsy. In five cases, lymph node dissection was carried out immediately during the surgical procedure. For the remaining two individuals, a primary axillary lymph node biopsy was performed. Notably, one of these biopsies was subsequently completed with dissection due to a positive biopsy result. For adjuvant treatment, all patients included in our study underwent RT, seven of whom underwent CT.
RT was delivered at a dose of 50 Gy in 25 fractions (2 Gy/fraction) or at a dose of 52.2 Gy in 29 fractions (1.8 Gy/fraction), with an additional dose in 4 patients only. The target volume was essentially represented by the chest wall in all patients except two, who had, in addition to parietal RT, locoregional lymph node RT to the internal mammary chain and supra-clavicular recesses in both, and axillary RT in one.
One patient developed local recurrence (LR) on the surgical scar after the end of irradiation. Lung metastases were described in five patients, two of whom had associated brain metastases. The time to onset of metastases ranged from two months to five years. Six patients achieved complete remission (CR), with follow-up ranging from four to 20 years. Two patients were lost to follow-up. For the sake of clarity, we prefer to report all the cases studied in two summary tables:
Table 1 summarizes the clinical and histological characteristics of our patients. Table 2 describes the therapeutic management and evolutionary characteristics of each case.
Table 1
Clinical and histological characteristics of 13 primary breast sarcomas collected, Sousse-Tunisia, 1995–2020
| No | Age (years old) | Tumor location | Skin involvement | Tumor size (cm) | Lymph nodes involvement | Histology |
| 1 | 31 | Left breast | Ø | 20 | Ø | Malignant phyllodes tumour |
| Upper quadrant union | ||||||
| 2 | 39 | Left breast | Tense, warm with neovessels and pus discharge | 13 | 2 homolateral mobile axillary adenopathies | Malignant phyllodes tumour |
| 3 | 41 | Left breast | Ø | 12 | Ø | Malignant phyllodes tumour |
| 4 | 45 | Left breast | Inflammatory signs | 11 | Left axillary satellite node | Malignant phyllodes tumour with rhabdomyoblastic differentiation |
| Lower quadrant | ||||||
| 5 | 52 | Left breast | Ø | 19 | Mass in left axillary fossa > 10 cm | Malignant phyllodes tumour |
| 6 | 55 | Right breast | Ø | 18 | Ø | Malignant phyllodes tumour |
| 7 | 47 | Left breast | Renital mass with orange peel appearance | 12 | Ø | Osteosarcoma on grade 3 phyllodes tumour, with verification through IHC |
| Supra-internal quadrant | ||||||
| 8 | 44 | Left breast | Ø | 4 | Ø | High-grade angiosarcoma |
| External quadrant union | ||||||
| 9 | 49 | Left breast | Ø | 3 | Ø | High-grade leiomyosarcoma |
| Supra-internal quadrant | ||||||
| 10 | 55 | Left breast | Ø | 2.5 | Ø | Fibrosarcoma on grade 3 phyllodes tumour, with verification through IHC |
| External quadrant union | ||||||
| 11 | 32 | Left breast | Bloody nipple discharge + surrounding orange peel skin | 12 | Ø | Fibrosarcoma on grade 3 phyllodes tumour, with verification through IHC |
| Super-external quadrant | ||||||
| 12 | 26 | Left breast | Ø | 7 | Ø | Undifferentiated Pleomorphic Sarcoma |
| Super-external quadrant | ||||||
| 13 | 21 | Right breast | Ø | 7 | Ø | Malignant phyllodes tumour |
| Lower-externel quadrant |
[i] IHC: immunohistochemistry.
Table 2
Therapeutic management and evolution of 13 observations of primary breast sarcoma, Sousse-Tunisia, 1995–2020
| Observation | Surgery | Radiotherapy | Chemotherapy | Evolution |
| 1 | Left mastectomy + lymph node dissection | Left parietal RT at a dose of 50 Gy in CF + 14 Gy supplement on the scar | 4 courses Doxorubicin-Ifosfamide | Lost from sight at the end of RT |
| 3 N−/3 N | ||||
| (negative surgical margins) | ||||
| 2 | Left mastectomy | Left parietal RT at a dose of 52.2 Gy (1,8 Gy/fraction + 18 Gy supplement on the scar | 2 courses Adriamycin | Complete remission for 13 years |
| (Positive posterior resection margin) | ||||
| 3 | Left mastectomy (negative surgical margins) then excision was performed for 3 recurrences, with permeation nodules in the scar area | Left parietal RT at a dose of 50 Gy in CF | Ø | Complete remission for 20 years |
| 4 | Left mastectomy + biopsy of a left axillary lymph node + additional lymph node dissection | Initially refused | 6 courses Adriamycin-Holoxan (parietal recurrence with anterior mediastinal invasion) | 2 years later: Pulmonary and cerebral relapse, patient died |
| Left locoregional RT at a dose of 50 Gy in CF | ||||
| (3 N+/17 N) | (after good evolution under CT) | |||
| 5 | Tumor exision followed by Mastectomy + immediate reconstruction with lymph node dissection | Left locoregional RT including the axillary area at a dose of 50 Gy + 24 Gy supplement on the scar in CF | 3 courses ifosfamide-Holoxan | Suspicious parietal nodule at mastectomy scar of 1 cm at end of RT |
| Surgical revision: enlarged mastectomy | Died 6 months later | |||
| 6 | Right mastectomy + lymph node dissection | Right parietal RT at a dose of 52.2 Gy | Indicated but not done | Pulmonary, intramedullary and cerebral metastases 20 months after end of RT |
| 18 N−/18 N | ||||
| (negative surgical margins) | (1,8 Gy/fraction) | Patient died | ||
| 7 | Left mastectomy | Left parietal RT at a dose of 50 Gy in CF + 24 Gy supplement on recurrent nodule | 3 courses Cyclophosphamide-Epirubicin | Died of lung metastases 2 months after RT was completed |
| (negative surgical margins) | ||||
| 8 | Left mastectomy + lymph node dissection | Left parietal RT at a dose of 50 Gy in CF | Ø | Complete remission for 25 years |
| 8 N−/8 N | ||||
| 9 | Mastectomy | Left parietal RT at a dose of 52.2 Gy | 6 courses Adriamycin monotherapy | Metastatic pulmonary nodule 1 year after metastasectomy |
| (1,8 Gy/fraction) | Complete remission for 7 years | |||
| 10 | lumpectomy completed by simple mastectomy (uncertain surgical limits) | Right parietal RT at a dose of 50 Gy + 14 Gy supplement on the scar in CF | Ø | Complete remission for 5 years, then death from pulmonary metastasis |
| 11 | Mastectomy + lymph node dissection | 50 Gy RT of the left chest wall and internal mammary chain (2 Gy/fraction) | Ø | 6 years of follow-up without recurrence, followed by loss of follow-up |
| 9 N−/9 N | ||||
| (negative surgical margins) | ||||
| 12 | Left mastectomy + left axillary lymph node biopsy | Left parietal RT at a dose of 52.2 Gy | 4 courses Doxorubicine + Isofosfamide | In complete remission for 7 years |
| 1 N−/1 N | (1.8 Gy/fraction) | |||
| 13 | Mastectomy (positive superficial and deep surgical tumor borders) + extended re-excision | Right parietal RT at a dose of 50 Gy in CF | Ø | In complete remission for 4 years |
[i] RT: Radiotherapy, CF: Conventional fractionation, CT: Chemotherapy.
4.Discussion
PBS are extremely rare. The frequency found in our series is close to published figures. Most of PBS arise in young women with an average age of 45 (35–55 years) [6]. Menopausal status has not been well specified in the literature. According to the studies, parity does not influence the onset of the disease [7].
Patients usually present with a rapidly enlarging unilateral breast mass, associated with mastodynia in 20% of cases [3]. In the literature, the median tumor size reported is 9 cm, with extremes ranging from 1 to 40 cm [8]. The presence of palpable adenopathy (ADP) is exceptional. The surrounding skin is usually respected, but skin changes such as ulceration or necrosis may be observed [6]. Involvement of the right breast is more frequent in studies [9], while in others it is more frequent on the left [10].
Diagnosis must be confirmed by anatomopathological examination, often using immunohistochemistry [3,11].
NPS originate from the mesenchymal tissue of the mammary gland, while PS are known as bi-tissue tumors as they combine a benign epithelial component with stromal components [6]. To this date, the question of therapeutic management is still conflicting. Given the comparable disease-free survival (DFS) and overall survival (OS) rates, the management of PS and NPS should be similar [12].
Treatment options include surgery with optimal excision (margin over 1 cm), which is the only potentially curative treatment. Adjuvant treatment with RT and CT remains debatable due to its controversial efficacy.
4.1.Surgery
Surgical treatment remained the upfront standard of care for all patients. Whereas, there is debate about the optimal surgical procedure: wide local excision or mastectomy [13].
Mastectomy has long been considered the reference treatment for PBS [4,7,14,15]. The radical surgical approach was inspired by the study by Berg et al. published in 1962, which found a higher local recurrence (LR) rate with lumpectomy [16].
To date, no double-blind, multicenter studies have been carried out on this subject. Most case reports and current studies describe the treatment of these tumors exclusively by wide local excision.
In fact, both lumpectomy and total mastectomy are feasible if tumor size is less than 5 cm and complete resection with margins over 1 cm can be achieved [1,17].
In our series, all patients underwent mastectomy, either immediately or secondarily after the final histological result of a lumpectomy specimen.
This may be explained by the large tumor size in the majority of cases, which precludes wide resection within the recommended margins. However, negative excision margins are often not achieved, especially as positive margins after conservative surgery represent an independent factor in LR [18–21].
As for the impact of the type of surgery on OS, this remains a controversial subject. In fact, no difference was found between mastectomy and lumpectomy with negative margins in their study of NPS in the literature in relation to five-year survival (relapse-free and specific) [1,22]. Interestingly, other studies have demonstrated better survival results with lumpectomy than with mastectomy [13]. This may be explained by the use of mastectomy when the tumor is intrinsically associated with a poor prognosis, i.e. large size and high tumor grade.
PBS are known for their hematogenous tropism. Thus, lymph node invasion is exceptional, even in cases of clinical suspicion [23]. Because of the low risk of lymph node involvement and the consequent morbidity of axillary surgery, studies do not recommend axillary lymph node dissection, regardless of the type of surgery [1]. According to some authors, lymph node biopsy may be justified if there is any doubt about axillary involvement [17]. In our series, lymph node biopsy was prompted by the presence of a suspicious-looking axillary lymph node on ultrasound mammography.
4.2.Radiotherapy
The post-operative management is still unclear. RT of PBS is widely suggested in the literature and its indications have increased considerably in recent years [15,24].
It is the second therapeutic weapon after surgery.
Several studies have demonstrated that RT has a clear benefit in terms of local control (LC) after complete surgery [17]. For PS, RT was the only independent prognostic factor for LC [15]. Similarly, a meta-analysis and review of the literature showed that the rate of LR after RT was lower than that without RT [25]. Other more recent studies have confirmed the same findings [26].
Adjuvant RT did not improve recurrence-free survival after mastectomy in a more recent study [27].
Several studies recommend the use of adjuvant RT regardless of the type of surgery for NPS. In fact, LC is achieved when wide excision is combined with adjuvant RT, especially if the tumor is large (>5 cm) or the surgical margins are positive [17]. Then, adjuvant RT is recommended after partial mastectomy.
For PS, the 5-year LC for the lumpectomy alone, lumpectomy with adjuvant RT, mastectomy alone and mastectomy with postoperative RT groups was 80.7; 93.3; 92.4; and 100% respectively [21].
In the Boutrus et al. series, although the authors did not demonstrate the contribution of RT after mastectomy as already mentioned, they opted for adjuvant RT after lumpectomy. Indeed, the 5-year LC was 100% with RT versus 34.3% without RT [27]. RT was independently associated with a reduced risk of local failure [23,27].
Finally, the first and only prospective non-randomized trial of adjuvant RT available to date demonstrated a LC rate of 100% after 56 months of median follow-up. However, this study was limited to patients who had undergone breast-conserving surgery with negative surgical margins of malignant PT [24].
Most studies did not support the contribution of RT to OS, given the small numbers involved [15,18,27]. These results could be explained by the indication of adjuvant RT for patients with unfavorable tumor characteristics from the outset.
In other series, adjuvant RT has been determined as a significant factor influencing recurrence-free survival and OS in NPS [1].
A significant survival benefit of adjuvant RT in tumors larger than 5 cm compared to surgery alone has been demonstrated in the SEER database [13].
The recommended tumoricidal dose is 50 Gy in 25 fractions, at a rate of 2 Gy per fraction, on the chest wall after mastectomy, and a dose of 50 Gy in 25 fractions on the whole of the remaining breast, with a further 10 Gy in 5 fractions on the tumor bed after lumpectomy. An escalated dose of more than 60 Gy is recommended if the surgical limits are invaded. Lymph nodes are not included in the radiation fields [1,7,27].
In our series, the tumoricidal dose delivered to the chest wall ranged from 50 to 52.2 Gy. An additional dose of 14 to 24 Gy was delivered in the event of scar recurrence, or in the event of tumoral or doubtful resection limits, to reach a total dose of 64 to 74 Gy.
The widely accepted indications for RT are: tumor size over 5 cm, high histological grade of the tumor, clear surgical margins of less than 1 cm, especially if surgical revision is not possible, and partial mastectomy. Nevertheless, RT should be discussed in the case of large, low-grade sarcomas, as well as for tumors of 2 to 5 cm with an aggressive component and a high histoprognostic grade [1,6,7,14,15,17]. In our series, RT was indicated when tumor size exceeded 5 cm in the majority of cases, or when tumor grade was high.
4.3.Chemotherapy
The role of adjuvant CT in PBS is not well codified, but encouraging results using CT for soft-tissue sarcomas suggest that consideration be given for their use in cases of PBS [6]. Doxorubicin and/or Ifosfamide are the most widely used agents in the treatment of PBS [28]. A meta-analysis has highlighted the contribution of adjuvant CT with Anthracyclines and Ifosfamide, and has shown that adjuvant CT provides a benefit in terms of Relapse-Free Survival [29].
A few retrospective studies have confirmed the contribution of adjuvant CT to OS. For NPS, CT was associated with prolonged DFS [30]. Patients with PS with heterologous malignant elements were considered to have a poor prognosis and were therefore treated with adjuvant CT. They had a longer recurrence-free survival [31]. Indeed, the presence of heterologous sarcomatous elements in the malignant stroma of PT could predict a high risk of recurrence and distant metastases, justifying the use of CT in this case.
However, recent studies have shown that adjuvant CT does not affect survival in patients with breast sarcoma [23].
In our study, adjuvant CT was indicated after radical surgery in half the cases, including 3 cases of PS and 4 cases of NPS, in view of the high tumor size, high grade, presence of ADP and invaded margins.
That said, we cannot conclude that systemic CT is a standard for PBS, but it should be discussed if factors for metastatic relapse are present, in particular large tumor size, stromal proliferation, compromised surgical margins and locoregional invasion [7,17].
PBS have a high recurrence rate and a poor prognosis, with a course that is generally unpredictable. In fact, the dissemination of cancer cells is generally by local invasion or hematogenous spread. As a result, the evolution is marked by local recurrence, especially in cases of incomplete resection, but also by the occurrence of distant metastases. The rate of distant metastases varies considerably from 20% to 40%. Metastases are generally localized to the lungs, but bony, epidural, supra-clavicular, cerebral and pelvic localizations have also been described. Nevertheless, the median survival time is estimated at 108 months, and the 5-year survival rate is variable, ranging from 14% to 90% [13].
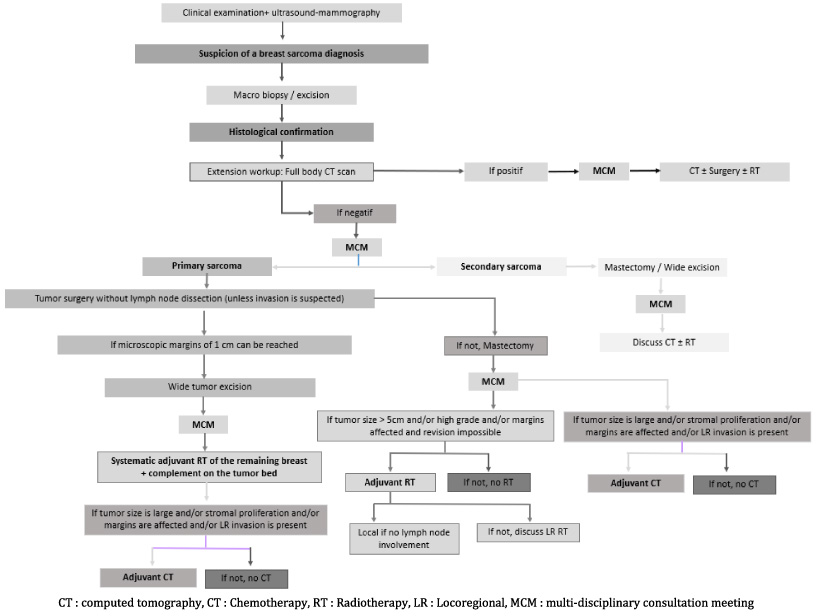
At the end of our work, we proposed a decision algorithm for the therapeutic management of PBS (Fig. 2).
Fig. 2.
Decision matrix for therapeutic management in primary breast sarcoma.
5.Conclusion
The limited prevalence of PBS makes large-scale prospective trials illusory. Therefore, it is important to report small case series and local experiences to help the clinician manage these uncommon cancers.
Early histopathological diagnosis and clinical staging of PBS is essential, not only to improve the overall prognosis of the disease, but also to improve patients’ quality of life after treatment.
While surgery with clear margins remains the mainstay of curative treatment, there is no established consensus on the place of postoperative RT. Nevertheless, it should be considered to improve LC, particularly in patients with high tumor size or grade, surgical excision with margins less than 1 cm and who have had conservative surgery.
There are even fewer data available on the value of adjuvant CT, and no clear benefit can be demonstrated. This is why these rare tumors should be accurately diagnosed and effectively treated in time, and the therapeutic decision must always be discussed in a multidisciplinary assessment.
References
[1] | McGowan TS, , Cummings BJ, , O’Sullivan B, , Catton CN, , Miller N, , Panzarella T, An analysis of 78 breast sarcoma patients without distant metastases at presentation, Int J Radiat Oncol *Biology*Physics, 46: (2): 383–390, (2000) . |
[2] | Lim SZ, , Selvarajan S, , Thike AA, , Nasir NDBMd, , Tan BKT, , Ong KW , Breast sarcomas and malignant phyllodes tumours: comparison of clinicopathological features, treatment strategies, prognostic factors and outcomes, Breast Cancer Res Treat, 159: (2): 229–244, (2016) . |
[3] | Sang NV, , Duc NM, , My TTT, , Ly TT, , Bang LV, , Thong PM, A rare case report of breast sarcoma, Radiol Case Rep, 16: (5): 1047–1050, (2021) . |
[4] | Toesca A, , Spitaleri G, , De Pas T, , Botteri E, , Gentilini O, , Bottiglieri L , Sarcoma of the breast: Outcome and reconstructive options, Clinical Breast Cancer, 12: (6): 438–444, (2012) . |
[5] | Gronchi A, , Miah AB, , Dei Tos AP, , Abecassis N, , Bajpai J, , Bauer S , Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up, Ann Oncol, 32: (11): 1348–1365, (2021) . |
[6] | Grenier J, , Delbaldo C, , Zelek L, , Piedbois P, Tumeurs phyllodes et sarcomes du sein: mise au point, Bulletin du Cancer, 97: (10): 1197–1207, (2010) . |
[7] | Bousquet G, , Confavreux C, , Magné N, , de Lara CT, , Poortmans P, , Senkus E , Outcome and prognostic factors in breast sarcoma: A multicenter study from the rare cancer network, Radiother Oncol, 85: (3): 355–361, (2007) . |
[8] | Kumar S, , Sharma J, , Ralli M, , Singh G, , Sen R, Primary stromal sarcoma of breast: A rare entity. 5, 2016. |
[9] | Gabriele R, , Borghese M, , Corigliano N, , Barbaro M, , Conte M, Phyllodes tumor of the breast. Personal contribution of 21 cases, G Chir, 21: (11–12): 453–456, (2000) . |
[10] | Norris HJ, , Taylor HB, Sarcomas and related mesenchymal tumors of the breast, Cancer, 22: (1): 22–28, (1968) . |
[11] | Li N, , Cusidó MT, , Navarro B, , Tresserra F, , Baulies S, , Ara C , Breast sarcoma. A case report and review of literature, Int J Surg Case Rep, 24: : 203–205, (2016) . |
[12] | Wang F, , Jia Y, , Tong Z, Comparison of the clinical and prognostic features of primary breast sarcomas and malignant phyllodes tumor, Jpn J Clin Oncol, 45: (2): 146–152, (2015) . |
[13] | Yin M, , Mackley HB, , Drabick JJ, , Harvey HA, Primary female breast sarcoma: clinicopathological features, treatment and prognosis, Sci Rep, 6: (1): 31497, (2016) . |
[14] | El Amine Elhadj O, , Nasri M, , Thabet S, , Ben Hassouna J, , Goucha A, , Rahal K , Sarcomes mammaires primitifs : à propos de 30 cas traités à l’institut Salah-Azaiez de Tunis, Cancer/Radiothérapie, 21: (1): 45–50, (2017) . |
[15] | Belkacémi Y, , Bousquet G, , Marsiglia H, , Ray-Coquard I, , Magné N, , Malard Y , Phyllodes tumor of the breast, Int J Radiat Oncol *Biology*Physics, 70: (2): 492–500, (2008) . |
[16] | Berg JW, , Decrosse JJ, , Fracchia AA, , Farrow J, Stromal sarcomas of the breast. A unified approach to connective tissue sarcomas other than cystosarcoma phyllodes, Cancer, 15: (2): 418–424, (1962) . |
[17] | Zelek L, , Llombart-Cussac A, , Terrier P, , Pivot X, , Guinebretiere JM, , Le Pechoux C , Prognostic factors in primary breast sarcomas: A series of patients with long-term follow-up, JCO, 21: (13): 2583–2588, (2003) . |
[18] | Oladeru OT, , Yang DD, , Ma SJ, , Miccio JA, , Orio PF, , Warren LE, Patterns of care and predictors of adjuvant radiation therapy in phyllodes tumor of the breast, Breast J, 26: (7): 1352–1357, (2020) . |
[19] | Choi N, , Kim K, , Shin KH, , Kim Y, , Moon HG, , Park W , The characteristics of local recurrence after breast-conserving surgery alone for malignant and borderline phyllodes tumors of the breast (KROG 16-08), Clinical Breast Cancer, 19: (5): 345–353.e2, (2019) . |
[20] | Pezner RD, , Schultheiss TE, , Paz IB, Malignant phyllodes tumor of the breast: local control rates with surgery alone, Int J Radiat Oncol.Biology.Physics, 71: (3): 710–713, (2008) . |
[21] | Choi N, , Kim K, , Shin KH, , Kim Y, , Moon HG, , Park W , Malignant and borderline phyllodes tumors of the breast: a multicenter study of 362 patients (KROG 16-08), Breast Cancer Res Treat, 171: (2): 335–344, (2018) . |
[22] | Macdonald OK, , Lee CM, , Tward JD, , Chappel CD, , Gaffney Dk, Malignant phyllodes tumor of the female breast: Association of primary therapy with cause-specific survival from the surveillance, epidemiology, and end results (SEER) program, Cancer, 107: (9): 2127–2133, (2006) . |
[23] | Bogach J, , Shakeel S, , Wright FC, , Hong NJL, Phyllodes tumors: a scoping review of the literature, Ann Surg Oncol, 29: (1): 446–459, (2022) . |
[24] | Barth RJ, , Wells WA, , Mitchell SE, , Cole BF, A prospective, multi-institutional study of adjuvant radiotherapy after resection of malignant phyllodes tumors, Ann Surg Oncol, 16: (8): 2288–2294, (2009) . |
[25] | Chao X, , Chen K, , Zeng J, , Bi Z, , Guo M, , Chen Y , Adjuvant radiotherapy and chemotherapy for patients with breast phyllodes tumors: A systematic review and meta-analysis, BMC Cancer, 19: (1): 372, (2019) . |
[26] | Yom CK, . Malignant phyllodes of breast. Translational Research in Breast Cancer [Internet], . Noh DY, , Han W, , Toi M (eds), (Singapore), Springer; 601–612. (2021) [cité 10 mars 2022]. |
[27] | Boutrus RR, , Khair S, , Abdelazim Y, , Nasr S, , Ibraheem MH, , Farahat A , Phyllodes tumors of the breast: Adjuvant radiation therapy revisited, The Breast, 58: : 1–5, (2021) . |
[28] | Le Cesne A, , Antoine E, , Spielmann M, , Le Chevalier T, , Brain E, , Toussaint C , High-dose ifosfamide: Circumvention of resistance to standard-dose ifosfamide in advanced soft tissue sarcomas, JCO, 13: (7): 1600–1608, (1995) . |
[29] | Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: eta-analysis of individual data, The Lancet, 350(9092): 1647–1654, 1997. |
[30] | Gutman H, , Pollock RE, , Ross MI, , Benjamin RS, , Johnston DA, , Janjan NA , Sarcoma of the breast: Implications for extent of therapy. The M. D. Anderson experience, Surgery, 116: (3): 505–509, (1994) . |
[31] | Li Y, , Song Y, , Lang R, , Shi L, , Gao S, , Liu H , Retrospective study of malignant phyllodes tumors of the breast: Younger age, prior fibroadenoma surgery, malignant heterologous elements and surgical margins may predict recurrence, The Breast, 57: : 62–70, (2021) . |




