The Multifaceted Effects of Flavonoids on Neuroplasticity
Abstract
There has been a significant increase in the incidence of multiple neurodegenerative and terminal diseases in the human population with life expectancy increasing in the current times.
This highlights the urgent need for a more comprehensive understanding of how different aspects of lifestyle, in particular diet, may affect neural functioning and consequently cognitive performance as well as in enhancing overall health. Flavonoids, found in a variety of fruits, vegetables, and derived beverages, provide a new avenue of research that shows a promising influence on different aspects of brain function. However, despite the promising evidence, most bioactive compounds lack strong clinical research efficacy. In the current scoping review, we highlight the effects of Flavonoids on cognition and neural plasticity across vertebrates and invertebrates with special emphasis on the studies conducted in the pond snail, Lymnaea stagnalis, which has emerged to be a functionally dynamic model for studies on learning and memory. In conclusion, we suggest future research directions and discuss the social, cultural, and ethnic dependencies of bioactive compounds that influence how these compounds are used and accepted globally. Bridging the gap between preclinical and clinical studies about the effects of bioactive natural compounds on brain health will surely lead to lifestyle choices such as dietary Flavonoids being used complementarily rather than as replacements to classical drugs bringing about a healthier future.
BACKGROUND
Dependence on bioactive natural compounds for promoting human health
A report in The Lancet states that individuals diagnosed with cognitive disorders are estimated to grow by 115 million by 2050 [1]. As the World’s population ages, age-related impaired executive functions and learning and memory abilities are becoming an enormous public health, social, and economic burden, representing one of the major causes of hospitalization, nursing care, and death worldwide [2–4].
Unfortunately, pharmacological interventions based on synthetic drugs only seem to alleviate symptoms of impaired neuroplasticity [5], without effectively targeting the pathophysiology of cognitive decline. Thus, determining whether and –if so –how human neuroplasticity can be preserved to match extended life expectancy more closely, is both necessary and urgent.
In this complex scenario, growing evidence from translational studies confirmed the potential of dietary bioactive compounds –including polyphenols, terpenoids, polysaccharides, capsaicinoids, carotenoids and tocopherols, triterpenes and phytosterols, alkaloids, saponins, glucosinolates - in preventing and/or improving impaired cognitive functions [6]. Indeed, diet, together with genetic background, aging, hormonal states, comorbidities of chronic disorders, toxin exposures, socioeconomic profiles, and lifestyle behaviours act as a key modulator of neuroplasticity [7, 8].
This Scoping Review is not intended to be an exhaustive review of studies investigating the effects of bioactive compounds on neuroplasticity. Owing to space limitations, we have restricted our discussion to selected bioactive compounds and model organisms. In particular, we focused our attention on Flavonoids (Table 1), as these phytochemical compounds provide a new avenue of research that shows a promising influence on different aspects of brain function [9, 10], including memory, attention, and overall cognitive function [11, 12].
Table 1
Classes of Flavonoids and their Sources
| Class | Flavonoids | Sources |
| Flavanols | Epigallocatechin gallate | Cocoa, grapes, green tea, and red wine |
| Epigallocatechin | Tea, apples, capers, onions, broccoli, strawberries, leeks, and grapefruits | |
| Epicatechin | ||
| Catechin | ||
| Quercetin | ||
| Kaemferol | ||
| Morin | ||
| Galangin | ||
| Flavanones | Eridictyol | Tomatoes, grapefruits, and citrus fruits |
| Hesperetin | ||
| Naringenin | ||
| Naringin | ||
| Flavones | Luteolin | Onions, broccoli, oranges, cabbage, carrot, grapefruit, parsley |
| Wogonin | ||
| Diosmin | ||
| Apigenin | ||
| Isoflavones | Equol | Soy and derivates |
| Daidzein | ||
| Genistein | ||
| Glycerin | ||
| Anthocyanins | Malvidin | Red wine, berry fruits, and beans |
| Hirsutidin | ||
| Pelargonidin | ||
| Cyanidin |
Flavonoids are present in many plants, fruits, vegetables, and leaves [13]. Some examples include compounds found in green tea, such as epicatechin, which have been found to improve attention and cognitive function [13]. Similarly, compounds found in berries, such as anthocyanins, have been found to improve memory and cognitive function [14, 15]. Additionally, compounds like quercetin, have been found to have anti-inflammatory and antioxidant effects, which may also contribute to cognitive enhancement and improve overall immunity and health [16–19].
Thus, in the last decade, an expanding body of research highlights enhanced cognitive performance in various age groups [20–22] after both chronic and acute interventions involving high levels of Flavonoids [23, 24]. In particular, the emerging body of evidence indicates the potential benefits of Flavonoids on attention, working memory [20, 25], and psychomotor processing speed [23, 24]. The data also suggests that the impact of Flavonoids is likely dependent on the dose and flavonoid supplementation could yield cognitive improvements within a short timeframe of 0–6 hours [23, 24]. For example, Devore et al., (2012) investigated the relationship between berry and flavonoid consumption and cognitive decline in≥70 years women [20], by administrating food frequency questionnaires to participants every 4 years from 1980 and in 1995–2001 started measuring their cognitive functions. The study revealed that a higher intake of blueberries and strawberries (i.e., foods rich in Flavonoids [26]) and total Flavonoids was associated with delayed cognitive aging by up to 2.5 years. Thus, this study revealed that a diet rich in Flavonoids, particularly those from berries, might play a role in mitigating cognitive decline in older adults. Similar results have been obtained in the PAQUID (i.e., Personnes Agées Quid) study, which examined 1640 aged 65 or older dementia-free individuals over a 10-year period and, after accounting for age, sex, and education level, demonstrated that higher Flavonoid intake was linked to improved cognitive performances [27]. Finally, in a recent study, Godos et al., (2020) analysed the relationship between dietary flavonoid intake and cognitive health in 808 adults living in southern Italy [28]. By recutting to food frequency questionnaires, estimating polyphenol content using the Phenol-Explorer database (www.phenol-explorer.eu), and assessing the cognitive status using the Short Portable Mental Status Questionnaire [29], the study revealed a significant inverse association between higher dietary intake of total flavonoids and impaired cognitive status. Moreover, specific subclasses of flavonoids, including quercetin, flavan-3-ols, catechins, anthocyanins, and flavonols, were linked to better cognitive health. Thus, the study suggests that greater consumption of flavonoids through diet might be correlated with improved cognitive health in adult individuals residing in the Mediterranean area [28].
Despite the promising results demonstrating the cognitive efficacies of Flavonoids, several other studies show mixed conclusions [25] and there are challenges related to their success in clinical trials [30, 31]. In particular, gaps in scientific validation, knowledge of pharmacokinetics, toxicity, and mechanism of action, are limiting the recommendation of these compounds in clinical studies [32–34]. Moreover, most Flavonoids go through a rapid metabolism, have non-specific targeting, poor solubility, as well as lack brain-blood-barrier permeability [35, 36].
In this complex scenario, translational studies are necessary to predict a direct relationship between Flavonoid intake, enhanced cognitive function, and/or protection against neurodegeneration. This may be extremely useful for both clinical treatment interventions and preventive approaches.
Thus, in the next sections, we present important discoveries on the effects of flavonoid-rich compounds on cognitive functions in different model organisms, highlighting the advantages of invertebrate models in this research field [37]. A special focus will be on the pond snail Lymnaea stagnalis, as –over the last decade –it has become a valuable model organism for studying the memory-enhancing effects of different bioactive compounds [37–39].
Finally, we will provide potential solutions to address research gaps and guide future research. Specifically, we will focus on the social, cultural, and ethnic dependencies on these products, the issues related to potential adverse reactions, and challenges in monitoring safety, as well as their use to complement and not as a substitute to ‘classical drugs’ for cognitive decline and memory loss.
MODEL ORGANISMS FOR PRECLINICAL STUDIES
To promote a better understanding of the multifaceted effects of Flavonoids on brain plasticity, research on multiple model organisms needs to occur. It must always be borne in mind that animal models no matter their origin or complexity can never fully substitute for a human central nervous system. This is especially true when the human nervous system’s functionality is altered by neurodegenerative processes that result in neuropsychiatric disorders. With those caveats in mind model organisms are providing essential information on the mechanisms of action of different bioactive compounds [40–42].
The models most often used are rodent models (i.e., rats and mice) [43] as they offer genetic tools that can be useful to validate the function of specific genes, or their role in more complex functions, including neuroplasticity [44]. In this regard, Singh et al. (2022) recently reviewed the antioxidant and memory-enhancing properties of plant-derived polyphenols such as Flavonoids, phenolic acids, stilbenes, lignans, and non-phenolic compounds like bacoside-A, withaferin-A, ginkgolide-B, withanolide-A, and bilobalide [45].
In that regard, other researchers proposed the use of nano-herb conjugates to improve permeability in the brain to attenuate oxidative stress effectively overcoming the limited ability of many prospective bioactive compounds to cross the blood-brain barrier [46, 47].
Flavonoids, encompassing phytochemical compounds and dietary additions, possess substantial nutritional worth and antioxidant characteristics. These components have been applied to address oxidative stress in therapeutic contexts, aiming to alleviate the negative impacts of this stressor on the aging brain. [38–42]. For example, a recent publication [43] reviewed the therapeutic potential of phytoestrogens rich in Flavonoids, like genistein, daidzein, and resveratrol, in memory restoration in aging and different neurological disorder. Estrogen in females plays a major role in health as estrogen possesses antioxidative, anti-apoptotic, and anti-inflammatory actions [44]. There is growing evidence of the ability of estrogen and its receptors to epigenetically regulate the expressions of genes involved in memory functions [51–53]. Therefore, a reduction in estrogen signalling as occurs in menopause [54, 55] represents a risk for age-related memory decline and neurodegenerative disorders. However, phytoestrogens show neuroprotective, neurogenic, and memory restoration potential in aged estrous female rodents, Alzheimer’s disease models, and human subjects [56]. Previous studies reported that menopause is responsible for multiple metabolic changes such as dyslipidemia and enhanced adiposity, leading to behavioural alterations including cognitive decline [57–60]. Unfortunately, hormone replacement therapy has not been effective, and it sometimes showed detrimental effects on memory functions [61].
In this context, Bahndari et al. (2022) reported that dietary supplementation using stem powder of Tinospora cordifolia (a medicinal plant belonging to the family Menispermaceae rich in Flavonoids, tannins, and steroids [62]) for 12 weeks improved the learning and memory behaviour in high-fat diet-fed acyclic-aged female rats [63]. Molecular analysis of the glial marker GFAP and the microglial protein Iba1 showed a significant decline in the expressions of these proteins, indicating a reduction of neuroinflammation in the hippocampus and the prefrontal cortex of T. cordifolia–supplemented rats, compared with high-fat diet-fed acyclic aged female rats [63]. Furthermore, those authors found a significant increase of the anti-apoptotic proteins AP-1 and Bcl-xL levels and a significant reduction of the pro-apoptotic marker p-BAD in both the hippocampus and prefrontal cortex of these animals, suggesting a pro-cell survival effect of T. cordifolia supplement of high-fat diet-fed acyclic aged female rats [63]. Finally, the T. cordifolia supplement restored the expression of neurotrophic BDNF and Trkβ in the hippocampus and the prefrontal cortex of the high-fat diet-fed acyclic-aged female rats, suggesting T. cordifolia as a potential therapeutic agent to prevent the adverse effects of obesity and obesity-associated brain dysfunctions [64].
Another recent study published by Huang et al., (2021), investigated the neuroprotective effect of the natural flavonoid rhoifolin in rats with streptozotocin-induced Alzheimer’s-like disease [65] and found a significant improvement in memory, cognition, and spatial learning in rhoifolin-treated Alzheimer’s-like disease animals. Moreover, rhoifolin treatment resulted in a significant increase in the hippocampal CA1 pyramidal layer of those animals indicating its neuroprotective properties [65].
The increase in the hippocampal CA1 area further validated the reversal of cognitive dysfunctions caused by the streptozotocin treatment. Furthermore, analysis of oxidative stress markers SOD, CAT, GPX, GRX, and MDA showed a significant improvement in oxidative stress in the hippocampus and frontal cortex.
Thus, this study provided the first evidence of the effect of plant flavonoid, rhoifolin on an Alzheimer -like disease in rat models, representing a promising therapeutic agent for the management of this terrible neurodegenerative disorder [65].
In this complex scenario, because of the complexity of mammalian brains, as well as the multimodal mechanisms of actions of different bioactive compounds, contrasting results are not too surprising [41]. Additionally, the high cost involved in mammalian studies and the increasing difficulties in obtaining ethical approvals for certain types of experimentation may result in researchers considering alternative options [66–68].
In this complex scenario, invertebrates that have a simpler nervous system and also show interesting and important variation across wild populations, represent a more ethical, faster, cheaper but still valid model organisms (with few ethical requirements) to test for the effects of bioactive compounds on brain plasticity and functioning [37].
Being simpler model organisms, the pathways that are affected by such natural compounds can be determined relatively easily [38]. However, as most of these pathways are also evolutionarily preserved and thus would show similarity across taxa, over the last decade, worms flies, bees, snails, and fish have proven to be extremely useful to bridge the gap between preclinical and clinical studies investigating the effects of bioactive compounds on neuroplasticity (Table 2).
Table 2
Some of the most relevant studies on the effects of different bioactive compounds on neuroplasticity in invertebrate model organisms
| Species | Bioactive compound | Effects | Citation |
| Drosophila melanogaster | Adzuki bean | Restoration of the abnormal memory, movement defects, and shortened lifespan in Aβ42-overexpressing flies model of Alzheimer’s disease | [78] |
| Drosophila melanogaster | Citrus sinensis, Citrus maxima, and Citrus paradisi | Improved memory index | [87] |
| Drosophila melanogaster | Ganoderma lucidum, Panax notoginseng Panax ginseng | Improvement of memory deficits induced by an inflammatory status | [88] |
| Drosophila melanogaster | Cyanidin, keracyanin, Kuromanin | Prevention of Aβ-induced neurotoxicity and neurite outgrowth | [89] |
| Drosophila melanogaster | Garcinia binucao | Prevention of alcohol-induced neurotoxic effects on learning, short-term memory, and motor functions | [83] |
| Drosophila melanogaster | Rhodiola rosea | Improved odor-taste reward associative memory | [90] |
| Caenorabditis elegans | Acanthopanax senticosus | Improved the long-term memory of radiation-damaged worms | [91] |
| Caenorabditis elegans | Cranberry extract | Preventive effects through alleviating Aβ toxicity | [92] |
| Lymnaea stagnalis | Quercetin | Enhancement of long-term memory formation, upregulation of the expression levels of CREB1 (a key factor for neuroplasticity), and prevention of the heat-shock-induced upregulation of HSPs | [93–95] |
| Lymnaea stagnalis | Epicatechin | Enhancement of long-term memory formation and reversion of the memory-impairing effects of different stressors | [96–98] |
| Lymnaea stagnalis | Green tea | Enhancement of long-term memory formation and reversion of the memory-impairing effects of different stressors | [98–100] |
| Danio rerio | Quercetin and rutin | Prevention of scopolamine-induced memory impairment | [101] |
| Danio rerio | Silibinin and Naringenin | Prevention of Bisphenol A-induced neurotoxicity | [102] |
Review of some of the literature on the effect of Flavonoids on brain plasticity in different invertebrate species
Over the last three decades, invertebrate models (mainly Molluscs, Arthropods, and Nematodes) have been used as screening tools for drug discovery [40, 69]. Therefore, by combining genetic amenability, low cost, and breeding conditions, these organisms allowed high-throughput screening in a physiological context, representing a needed tool to bridge the gap between traditional in vitro and preclinical animal assays. Thanks to the great advances in comparative genomics, it has been demonstrated that there is a high level of conservation of numerous key physiological pathways across taxa. Thus, while maintaining the simple organization of the invertebrate nervous system [71–73], these organisms not only allowed the characterization of the conserved mechanisms through which the central nervous functions and gets sick but also elucidate the mechanisms of actions of many drugs and compounds [37, 39, 40, 74–76]. Invertebrates have been and still are of fundamental importance in understanding basic neuroscience and in accelerating the pace at which mammalian studies can be translated to humans [40].
Recently, these organisms have been used to detect the mechanisms of action of many dietary bioactive compounds.
As reported in Table 2, most of the studies on the multifaceted effects of bioactive compounds have been performed in the fruit fly Drosophila melanogaster, the worm Caenorhabditis elegans, the pond snail Lymnaea stagnalis, and zebrafish (Danio rerio).
As previously indicated, this section is not intended to be an exhaustive collection of all the studies performed in invertebrate models on all bioactive compounds currently available. That is, for reasons of space, we have selected only recent publications in the most used invertebrate models for biomedical research.
Most of the studies using D. melanogaster and C. elegans as model organisms have been performed on animal models of neurodegenerative diseases and/or aging-related disorders [13, 77–84]. In fact, both these organisms can undergo easy genetic analysis, allowing the discovery of various mutants and the identification of the responsible genes for neurodegenerative diseases.
Therefore, the administration of dietary bioactive compounds and/or food and beverage rich in them in transgenic flies and worms allowed the characterization of the multifaceted effects of bioactive compounds on brain plasticity and functionality. That is, over the last decade, a huge number of bioactive compounds that have been analysed show antioxidant, antiapoptotic, neuroprotective, and anti-inflammatory properties. Moreover, studies involving treatments with these compounds on cognitively impaired animal models showed several beneficial effects in enhancing neuroplasticity and/or extending life span (Table 2).
On the other hand, most of the studies using zebrafish were focused on the effects of various bioactive compounds on neurotoxicity. Danio rerio represents an excellent in vivo model for studying developmental neurotoxicity [85]. Indeed, thanks to their small sizes and abundance of embryos, these organisms are ideal for high-throughput screening in which the compounds tested can simply add in the medium of zebrafish, which will passively diffuse [86].
Importantly, comparative neurogenetic and neuroanatomical analyses reveal high degrees of conservation between the nervous systems of zebrafish and mammals [86]. Therefore, this model organism provides a valid tool in which to investigate the effects of bioactive compounds in preventing and/or modulating neurotoxicity and, on the other hand, to evaluate the potentially toxic effects of bioactive compounds themselves.
Special focus on Lymnaea stagnalis as a model system to understand the effects of natural compounds on learning and memory
Among a wide variety of invertebrate models used in Neuroscience research [37], the freshwater pond snail Lymnaea stagnalis (Linnaeus 1758), has been widely recognized as an ideal model system in which to investigate the action of various bioactive compounds on learning and memory formation [38, 39, 103, 104] (Fig. 1).
Fig. 1
Studies that can be performed using Lymnaea stagnalis as a model organism for Translational Neuroscience research, offering an array of advantages for exploring the conserved mechanisms underlying the effects of bioactive compounds (e.g., Flavonoids), drugs, and environmental stressors on cognitive functions and aging-related processes.
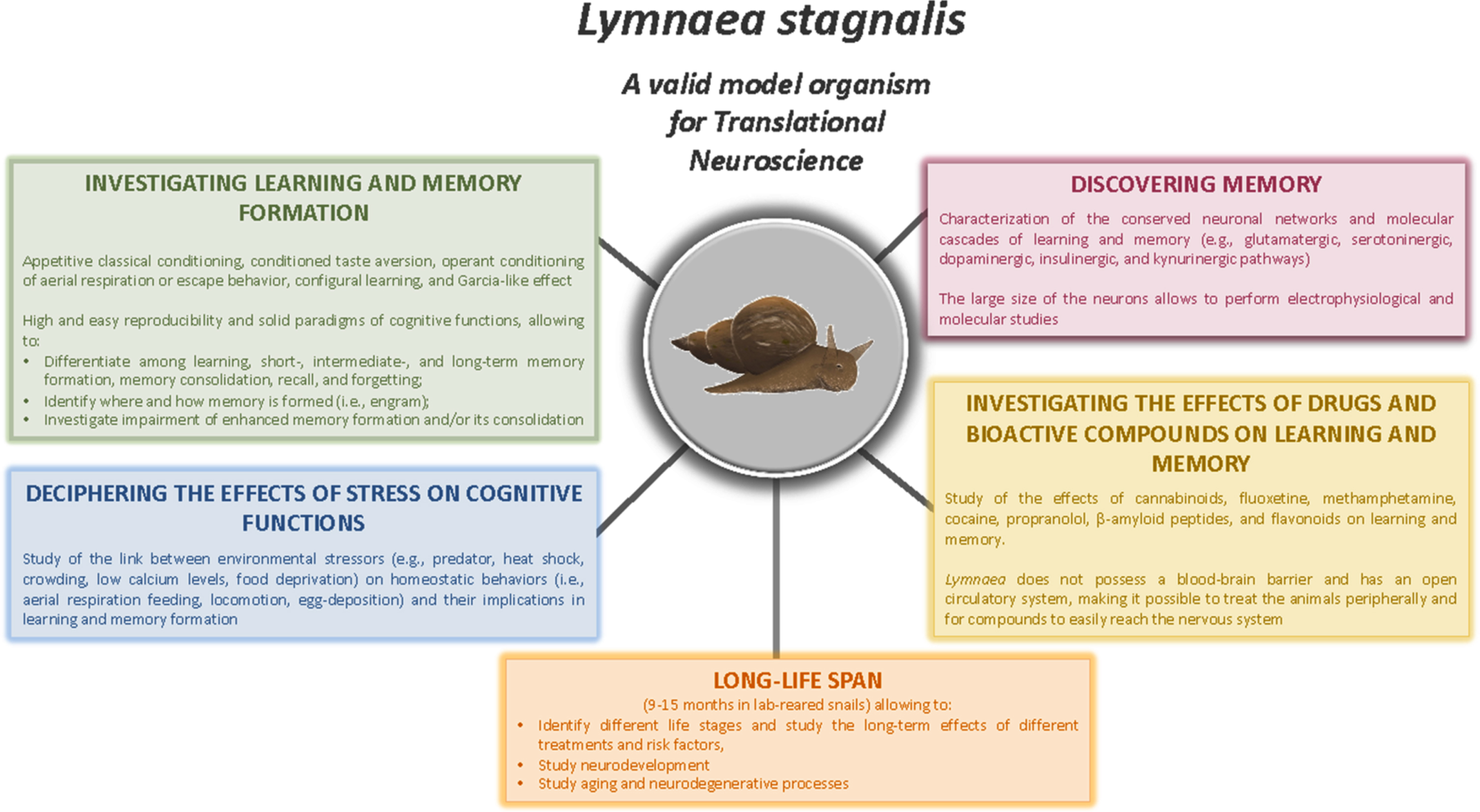
The rich behavioural repertoire that L. stagnalis uses to survive and adapt to its natural environment makes this organism a remarkable model system with which to study not only associative learning and the neuronal and molecular mechanisms of memory formation, but also how different stressors, drugs, and bioactive compounds may modulate (i.e., either enhancing or impairing) learning and memory formation [95, 103, 105–115]. L. stagnalis possesses relatively simple but important homeostatic behaviours whose underlying neuronal circuitry has been well elucidated [116–118]. Moreover, many of these behaviours are tractable and relatively easy to train [119, 120].
At the neuronal level, the nervous system of L. stagnalis consists of about 25000 large (up to 150μm in diameter) neurons, organized in a ring of interconnected ganglia, offering a relatively large amount of biological material that can be analysed molecularly, physiologically, and morphologically [110, 121]. The neurons can be easily removed and placed in culture, where they reform the appropriate synaptic connections [122, 123]. Thus, single neurons can be identified and analysed as part of defined circuits, allowing electrophysiological dissection of the networks involved in relatively simple rhythmic behaviours, such as aerial respiration and feeding [124]. These rhythmic movements are induced by groups of central pattern-generating neurons (CPGs) [125], whose characterization is critical for understanding where and how the nervous system controls these homeostatic behaviours and how the interplay between CPGs and external stimuli participates in the production of adaptive learned behaviours. These CPG circuits can be plastically reconfigured via environmental changes, experiences, and conditioning procedures to optimize the output to meet specific behavioural demands [125].
Importantly, L. stagnalis is an aquatic invertebrate with an open circulatory system, allowing the use of membrane-permeant compounds (including bioactive compounds like Flavonoids) that can be easily absorbed, to unravel the complexity of various signalling pathways and provide new insights into how drugs and molecules can modulate different neuronal functions and behaviours [93–95, 106, 126, 127].
Furthermore, the neuronal plasticity exhibited in the CPG circuits plays an important role in regulating the initiation and temporal output of behavioural rhythms in response to rewarding/aversive stimuli (as occurs in classical conditioning) and action–outcome contingencies (as occurs in operant conditioning) [128, 129]. Therefore, by utilizing both in vitro and semi-intact preparations (which allow monitoring of the behaviour and neural activity simultaneously), the CPGs controlling learning-induced changes and the effects of different compounds (like drugs and bioactive compounds) can be elucidated at the single-cell level in L. stagnalis [123, 130, 131].
Lymnaea stagnalis serves as an excellent system because both quantitative changes in gene expression induced by conditioning and the exposure to bioactive compounds can be studied at the level of single neurons, which may be extremely useful not only for elucidating which molecules participate in the dialogue between the synapse and the nucleus and vice versa during memory and learning but also to elucidate the conserved mechanisms through which Flavonoids and other bioactive compounds exert neuroplastic effects [37, 38]. Importantly, studies such as these cannot easily be performed in most vertebrate preparations because their behaviours are more complex, and the underlying neuronal circuitries are more inaccessible to direct cellular and synaptic analyses [39, 132, 133].
In 2012, Fruson et al. demonstrated that the exposure of Lymnaea to 15 mg l–1 of the flavonoid (–)Epicatechin enhanced long-term memory (LTM) formation for the operant conditioning of aerial respiration, providing the first test of the effect of Flavonoids on invertebrate learning and memory [96].
Indeed, Lymnaea can be operantly conditioned to reduce aerial respiration, the memory of which is altered by environmentally relevant stimuli, so we can reliably assess how different factors alter memory formation [105, 120]. In particular, it has been demonstrated that when snails were operantly conditioned in (–)Epicatechin with a single 0.5 h training session, which typically results in memory lasting ∼3 h, they formed LTM lasting at least 24 h [96]. Additionally, snails exposed to (–)Epicatechin also showed a significant increase in resistance to extinction, consistent with the hypothesis that this flavonoid may induce the formation of a more persistent and stronger LTM. In other words, (–)Epicatechin-enhanced LTM formed faster, persisted longer, and was more resistant to extinction. Thus, this was the first study that paved the way for a new avenue of research using L. stagnalis as a suitable model with which to elucidate behavioural, neuronal, and molecular mechanisms through which bioactive compounds may enhance neuroplasticity.
Additional studies demonstrated that (–)Epicatechin is only able to enhance memory if snails are either trained in (–)Epicatechin-containing pond water or exposed to it immediately after training for the operant conditioning of aerial respiration (i.e., during the consolidation period) [97].
In contrast, pre-treating snails with (–)Epicatechin 1 h before or delaying exposure to (–)Epicatechin 1 h after training did not result in the enhancement of memory formation. Thus, although (–)Epicatechin is a very powerful memory enhancer in Lymnaea as well as in mammals, it must be experienced either during training or immediately after training to effectively enhance memory [134].
As previously reported, learning and subsequent memory formation are influenced by both environmental and lifestyle factors, such as stress and diet [135, 136]. Therefore, while Flavonoids like (–)Epicatechin enhance LTM formation in Lymnaea, by contrast, ecologically relevant stressors, like low-calcium (20 mg l–1) pond water and crowding, suppress LTM formation [137–139].
Thus, in 2014, Knezevic and Lukowiak, demonstrated that exposure to (–)Epicatechin was able to overcome the negative effects of a stressor (i.e., low-calcium [137]) that blocks LTM formation in Lymnaea [140, 141]. Specifically, while snails trained in low-calcium pond water exhibited operant conditioning learning, they did not show LTM, but when epicatechin was added to the low-calcium pond water an LTM enhancement was observed [140]. This was the first evidence in an invertebrate model organism that a naturally occurring bioactive plant compound was able to overcome the suppressive effects of an ecologically relevant stressor on LTM formation. Thus, this study demonstrated that the effects of a memory-impairing stressor can be overcome by diet.
As many foods, like green tea, cocoa powder, and Red Delicious apple peels [142–145] contain substantial amounts of (–)Epicatechin, Swinton et al., (2018) demonstrated that exposure to food products containing (–)Epicatechin in concentrations comparable to human consumption levels (approximately 1 g/day) during training for the operant conditioning of aerial respiration, enhanced LTM formation [127]. In particular, authors demonstrated that food substances containing (–)Epicatechin have a similar ability as the ‘pure’ flavonoid in enhancing memory. As UVB light inactivates (–)Epicatechin [146], following the photo-inactivation of foods containing this flavonoid, their ability to enhance LTM was blocked [127]. Therefore, these data are consistent with the hypothesis that dietary sources of (–)Epicatechin may exert positive benefits on cognitive ability and be able to reverse memory aversive states. L. stagnalis exhibits a higher-order associative learning called configural learning [147, 148]. That is, when snails experience two contrasting stimuli together such as predatory effluent [149] and an appetitive taste (i.e., carrot slurry), they learn and associate risk with food [112]. Thus, following the configural learning training procedure and the establishment of a configural learning LTM, the carrot slurry now elicits a fear state, sometimes referred to as a landscape of fear in the brain, rather than increased feeding [148]. Typically, configural learning memory persists for at least 3 h but not 24 h [150]. However, Batabyal and Lukowiak (2020), showed that green tea exposure (i.e., (–)Epicatechin) following the configural learning training enhances memory persistence if it occurred during the period when memory undergoes the consolidation process [150]. Thus, this study demonstrated for the first time that higher-order associative learning can be enhanced using green tea in an invertebrate taxon.
These promising results obtained by exposing snails to green tea led the researchers to investigate whether Black tea, which is a more popular beverage than green tea and which is derived from the same tea leaves, also enhances LTM formation [99, 151]. Interestingly, Zhang et al., (2018) found that black tea, unlike green tea, depressed homeostatic aerial respiratory behaviour and obstructed LTM formation for the operant conditioning of aerial respiration in L. stagnalis [99]. These differences may be due to the fluoride content in black tea [106, 152]. However, green tea also contains a similar amount of fluoride but it is rich in Flavonoids which are lacking in black tea, and that might lead to the differences observed in terms of cognitive enhancement. Recent studies from this model organism demonstrated the suppressive effects of black tea and fluoride on Lymnaea’s feeding behaviour and cognition [94, 152]. In addition, the exposure of snails to fluoride (1.86 mg/L) for 45-min before, during, or after the configural learning training procedure blocked configural learning memory formation [152]. The above-mentioned effects were long-lasting as one week after a fluoride exposure, snails are still unable to form a configural learning memory. Why these differences? Unlike green tea, black tea leaves go through an oxidation process called “fermentation” and this process substantially reduces (6.16 mg/100 g to 0.49 mg/100 g) the (–)Epicatechin content in black tea [153]. Furthermore, black tea contains more caffeine than green tea, but substantially more flavan-3-ols like thearubigins and theaflavins [153], which –in turn - may alter cognition [153]. These studies suggest that although both green and black teas come from the same plant (Camellia sinensis), the different compositions in bioactive compounds may result in different effects on neuroplasticity.
Along with (–)Epicatechin, another flavonoid widely studied in Lymnaea is quercetin. Quercetin (3,3′,4′,5,7-pentahydroxyfavone) is present in fruits and vegetables, such as apples, berries, onions, asparagus, capers, and red leaf lettuce [154]. Numerous studies have demonstrated quercetin’s antioxidant and neuroprotective properties [155] in aged patients and animal models of neurodegenerative diseases [156]. Thus, studies performed in L. stagnalis may be extremely useful in exploring the effects of these compounds enhancing memory formation and recall. Recently Batabyal et al., (2021) demonstrated that the exposure of snails to quercetin for 1 h, either before or after configural learning enhanced LTM up to 48 h [150]. Interestingly, the enhanced LTM phenotype as a result of quercetin exposure in L. stagnalis was most pronounced when quercetin was experienced during the consolidation phase; or when snails were exposed to it during memory reconsolidation.
Consistent with these behavioural findings it was also shown that the exposure to quercetin for 1 h induced a significant upregulation of the orthologous of the transcription factor CAMP responsive element binding protein 1 (CREB1) in the Lymnaea’s central nervous system. Importantly, in snails as in mammals, CREB1 plays a key role in neuroplasticity [38]. Similarly, Rivi et al., (2021) provided the first support for quercetin-modulated enhancement of cognitive function in an invertebrate model after an operant conditioning procedure [94]. That is when snails were exposed to quercetin for 1 h, 3 h before or after a single 0.5 h training session, which typically results in memory lasting ∼ 3 h, they formed an LTM lasting for at least 24 h [94]. Additionally, the authors assessed the effects of the combined presentation of a single reinforcing stimulus (at 24 h post-training or 24 h before training) and quercetin exposure on both LTM formation and reconsolidation.
These results suggested that, when applied within 3 h of critical periods of memory, quercetin enhances learning acquisition, memory consolidation, memory recall, and memory reconsolidation [94].
Interestingly, when those authors trained a naïve cohort of snails in hypoxic pond water and quercetin to determine whether this exposure resulted in enhanced LTM formation, quite unexpectedly, snails entered a sleep-like quiescent state that persisted for at least 2 h after ending the exposure [157]. The experiments suggest that this state might be a survival mode for these organisms when they cannot induce a physiological stress response of elevated Heat Shock Proteins’ (HSPs) expression under a hypoxic environment.
Indeed, quercetin has proven to be a heat shock protein blocker [95, 108, 109, 158, 159]. In Lymnaea, the heat stress associated with exposure to 30°C pond water for 1 h led to a rapid (within 30 min) upregulation of the mRNA levels of both HSP40 and HSP70, reaching a peak of expression within 2–4 h of exposure [95, 160]. It was further demonstrated that the heat shock stressor-induced enhancement of LTM formation for both operant conditioning of aerial respiration and the Garcia effect (i.e., a ‘special form’ of conditioned taste aversion [95]) occurred as a result of the upregulation of HSPs by the heat shock stressor in snails [159]. However, the enhancing effect of the thermal stimulus on memory was obstructed if quercetin was presented before (but not after) the heat shock [95, 161]. Thus, studies from Lymnaea suggested that the exposure to quercetin and the heat shock results in opposite effects on LTM formation: when quercetin is applied before the heat shock, the upregulation of HSPs is blocked and LTM is not observed, whereas experiencing quercetin alone before or after the operant conditioning of aerial respiration or configural learning training, enhances LTM formation, consolidation, and recall. Thus, all these studies highlight the advantages of using L. stagnalis as a very useful model system in gaining an understanding of how bioactive compounds, such as the Flavonoids quercetin and epicatechin, may improve neuroplasticity in healthy organisms.
BRIDGING THE GAP BETWEEN PRECLINICAL AND CLINICAL STUDIES
Because of the ongoing process of aging experienced by modern society, the increasing prevalence of neurodegenerative diseases is becoming a global public health concern. Unfortunately, to date, there are no effective therapies to slow, stop, or reverse the progression of these diseases [162]. However, many studies have suggested that modification of lifestyle factors, such as the introduction of a balanced diet, can delay or prevent the onset of neurodegenerative diseases and psychiatric disorders. Diet is currently considered to be a crucial factor in controlling health and protecting against oxidative stress and chronic inflammation, and thus against chronic degenerative and psychiatric diseases [163].
In this context, natural bioactive compounds enhancing endogenous neuroplasticity raise hope for such therapies and preventive approaches. The preclinical studies from both mammals and invertebrates summarized in this paper have demonstrated that the neurorestorative actions of bioactive compounds (especially Flavonoids) are associated with both antioxidant and anti-inflammatory properties and also act through the activation of multiple pathways responsible for synaptogenesis and neurogenesis. Although evolutionarily quite distant from humans, invertebrates show molecular and behavioural properties that make them a wonderful model system to study the effects of dietary supplements and bioactive compounds on neuroplasticity paving the way for future studies in humans. The use of invertebrate models will limit as much as possible the use of mammalian models and allow mammals to be involved only for the validation of the results obtained from invertebrates. This will reduce by several orders of magnitude the costs of numerous studies. Thus, invertebrates as model systems provide a rapid and cost-effective experimental tool for elucidating the causal, neuronal, and molecular changes underlying the effects of different bioactive compounds on neuroplasticity. Thus, these organisms may offer a translational approach that may help gain important knowledge and comprehension in the field of Clinical Neuroscience.
FINAL CONSIDERATIONS AND FUTURE PERSPECTIVES
Inter-ethnic differences in the use of bioactive compounds and their metabolism
Historically the production of medicines and pharmacological treatments began with using plant-based natural medicines (herbal medicine) and prior to the 1800 s and the advent of scientific experimentation, herbal remedies were culturally omnipresent throughout the globe [164–166]. This cultural preference for dietary bioactive compounds or alternative medicines stayed prevalent in many parts of the world [167]. In some countries, traditional herbal remedies which have been used for centuries are still deeply ingrained in the culture. In other countries, modern Western medicine is more heavily relied upon. In many Asian countries, for example, traditional Chinese medicine and Indian Ayurvedic medicine are widely used and accepted [168–170]. These traditional systems use natural compounds such as herbs, minerals, and some cases animal products in their natural medicines [171].
In contrast, many Western cultures tend to rely more heavily on pharmaceutical drugs and place less emphasis on alternative therapies although Native Americans have always relied upon natural plant-based medication for treating ailments [166]. However, there is a growing interest and acceptance of alternative medicine globally as many people are now faced with sub-optimal health conditions due to lifestyle choices and are turning to natural compounds and alternative therapies to address their health concerns. The continued use and popularity of dietary supplements in recent years may be due to various factors, including fear of adverse events associated with prescription medications, cost of prescription medications, over-the-counter availability of dietary supplements, and perceptions that dietary supplements are “natural” or “herbal” and are therefore safer to use [172].
Although the use of food supplements and bioactive compounds is increasing worldwide, cultural preferences for natural compounds or the so-called ‘alternative medicines’ vary greatly around the world [173]. Although dietary supplement use is a worldwide growing phenomenon, only a few studies examine why consumers choose to take bioactive compounds [174]. Thus, future studies are necessary to answer questions like: What factors are primary motivators for the initiation of supplement behaviours as well as the decision-making related to short-term or long-term use? How does the use of bioactive compounds vary across cultures? How do motivations differ across different segments of the population? How do social norms influence and increase their use? Answering these questions is important considering that ethical differences reflect differences in drug and bioactive compound metabolism [175]. Therefore, examining ethnic differences in metabolic processes across groups is both urgent and important to define and predict the pharmacokinetics of dietary bioactive compounds and their potential interaction with ‘classical drugs’.
Issues related to potential adverse reactions and challenges in monitoring safety
Although the intake of bioactive compounds has shown promising potential beneficial effects on neuroplasticity [176], many of them remain untested and their use is poorly monitored [177, 178]. Unfortunately, there is still inadequate knowledge of their mode of action, potential adverse reactions, contraindications, and interactions with existing ‘orthodox’ drugs to promote both the safe and rational use of these compounds. Since safety is the major issue with the use of bioactive compounds and dietary supplements, it becomes imperative, that relevant regulatory authorities put in place appropriate measures to protect public health by ensuring that all herbal medicines are safe and of suitable quality [179]. Importantly, as by law, dietary supplements are not intended to diagnose, treat, prevent, or cure any disease, FDA-approved evidence of safety and efficacy is not needed before their appearance on the market. However, if these compounds are used improperly there could be a risk of adverse effects [180, 181]. However, their potential importance needs to be placed in scientific research to understand the nuances of the action of such compounds as most show a multifaceted effect working across different physiological pathways [182, 183]. Moreover, healthcare professionals are often poorly informed on how bioactive compounds may affect (both positively and negatively) the health of their patients and the efficiency and safety of the therapies. Thus, as with other medicines for human use, it has become mandatory that bioactive compounds are covered in every country of the world by a drug regulatory framework to ensure that they conform to the required standards of safety, quality, and efficacy. This is the only way in which the use of bioactive compounds in potential complementary or alternative cognitive therapeutics and preventive approaches will be possible.
Complementary Versus Alternative
The use of bioactive compounds to prevent and/or treat disorders is not typically part of conventional medical care or training when their origins come from outside of usual Western practice. Importantly, when describing these approaches, people often use “alternative” and “complementary” medicine interchangeably. However, the two terms refer to different concepts. If a non-mainstream approach is used together with conventional medicine, it’s considered “complementary”, whereas if a non-mainstream approach is used in place of conventional medicine, it’s considered “alternative” [184]. As most people in Western countries use bioactive compounds together with conventional drugs, the term ‘complementary should be preferred.
To sum up, this Scoping Review emphasizes the significance of different model systems that could act as valid tools for studying the diverse qualities of bioactive compounds like Flavonoids in preventing and/or treating cognitive decline. Nevertheless, there’s still a lack of enough data regarding their best doses, how well the body can absorb them, distinctions between various chemical forms, and potential interactions with other dietary elements and ‘traditional’ drugs.
Although more research in this area is necessary, results from preclinical studies are promising and support the benefits of the intake of food products rich in these substances. Thus, we hope that in the near future, the results from preclinical studies (using both invertebrates and vertebrates) may provide important information on how to combine longer life expectancy with more years free of cognitive impairment.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report
REFERENCES
[1] | Nichols E , Steinmetz JD , Vollset SE , Fukutaki K , Chalek J , Abd-Allah F , et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. The Lancet Public Health. (2022) ;7: :e105–25. https://doi.org/10.1016/S2468-2667(21)00249-8 |
[2] | Tucker-Drob EM . Cognitive Aging and Dementia: A Life Span Perspective. Annu Rev Dev Psychol. (2019) ;1: :177–96. https://doi.org/10.1146/annurev-devpsych-121318-085204 |
[3] | Mild cognitive impairment 2023: Small change, big. . . . Neurology Academy n.d. https://neurologyacademy.org/articles/mild-cognitive-impairment-2023-small-change-big-impact (accessed May 4, 2023). |
[4] | Konar A , Singh P , Thakur MK . Age-associated Cognitive Decline: Insights into Molecular Switches and Recovery Avenues. Aging Dis. (2016) ;7: :121–9. https://doi.org/10.14336/AD.2015.1004 |
[5] | Erkkinen MG , Kim M-O , Geschwind MD . Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb Perspect Biol. (2018) ;10: :a033118. https://doi.org/10.1101/cshperspect.a033118 |
[6] | Mohd Sairazi NS , Sirajudeen KNS . Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid Based Complement Alternat Med. (2020) ;2020: :6565396. https://doi.org/10.1155/2020/6565396 |
[7] | Hughes TF , Ganguli M . Modifiable Midlife Risk Factors for Late-Life Cognitive Impairment and Dementia. Curr Psychiatry Rev. (2009) ;5: :73–92. |
[8] | Guo J , Huang X , Dou L , Yan M , Shen T , Tang W , et al. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Sig Transduct Target Ther. (2022) ;7: :1–40. https://doi.org/10.1038/s41392-022-01251-0 |
[9] | Panche AN , Diwan AD , Chandra SR . Flavonoids: An overview. J Nutr Sci. (2016) ;5: :e47. https://doi.org/10.1017/jns.2016.41 |
[10] | Ullah A , Munir S , Badshah SL , Khan N , Ghani L , Poulson BG , et al. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules. (2020) ;25: :5243. https://doi.org/10.3390/molecules25225243 |
[11] | Arias A , Feijoo G , Moreira MT . Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innovative Food Science & Emerging Technologies. (2022) ;77: :102974. https://doi.org/10.1016/j.ifset.2022.102974 |
[12] | Rana A , Samtiya M , Dhewa T , Mishra V , Aluko RE . Health benefits of polyphenols: A concise review. Journal of Food Biochemistry. (2022) ;46: :e14264. https://doi.org/10.1111/jfbc.14264 |
[13] | Baba Y , Inagaki S , Nakagawa S , Kaneko T , Kobayashi M , Takihara T . Effect of Daily Intake of Green Tea Catechins on Cognitive Function in Middle-Aged and Older Subjects: A Randomized, Placebo-Controlled Study. Molecules. (2020) ;25: :4265. https://doi.org/10.3390/molecules25184265 |
[14] | Tran PHL , Tran TTD . Blueberry Supplementation in Neuronal Health and Protective Technologies for Efficient Delivery of Blueberry Anthocyanins. Biomolecules. (2021) ;11: :102. https://doi.org/10.3390/biom11010102 |
[15] | Ahles S , Joris PJ , Plat J . Effects of Berry Anthocyanins on Cognitive Performance, Vascular Function and Cardiometabolic Risk Markers: A Systematic Review of Randomized Placebo-Controlled Intervention Studies in Humans. Int J Mol Sci. (2021) ;22: :6482. https://doi.org/10.3390/ijms22126482 |
[16] | Li Y , Yao J , Han C , Yang J , Chaudhry MT , Wang S , et al. Quercetin, Inflammation and Immunity. Nutrients. (2016) ;8: :167. https://doi.org/10.3390/nu8030167 |
[17] | Chiang M-C , Tsai T-Y , Wang C-J . The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. International Journal of Molecular Sciences. (2023) ;24: :6328. https://doi.org/10.3390/ijms24076328 |
[18] | Shabir I , Kumar Pandey V , Shams R , Dar AH , Dash KK , Khan SA , et al. Promising bioactive properties of quercetin for potential food applications and health benefits: A review. Front Nutr. (2022) ;9: :999752. https://doi.org/10.3389/fnut.2022.999752 |
[19] | Al-Khayri JM , Sahana GR , Nagella P , Joseph BV , Alessa FM , Al-Mssallem MQ . Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules. (2022) ;27: :2901. https://doi.org/10.3390/molecules27092901 |
[20] | Devore EE , Kang JH , Breteler MMB , Grodstein F . Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. (2012) ;72: :135–43. https://doi.org/10.1002/ana.23594 |
[21] | Hagan KA , Munger KL , Ascherio A , Grodstein F . Epidemiology of Major Neurodegenerative Diseases in Women: Contribution of the Nurses’ Health Study. Am J Public Health. (2016) ;106: :1650–5. https://doi.org/10.2105/AJPH.2016.303324 |
[22] | Jennings A , Steves CJ , Macgregor A , Spector T , Cassidy A . Increased habitual flavonoid intake predicts attenuation of cognitive ageing in twins. BMC Medicine. (2021) ;19: :185. https://doi.org/10.1186/s12916-021-02057-7 |
[23] | Lamport DJ , Dye L , Wightman JD , Lawton CL . The effects of flavonoid and other polyphenol consumption on cognitive performance: A systematic research review of human experimental and epidemiological studies. Nutrition and Aging. (2012) ;1: :5–25. https://doi.org/10.3233/NUA-2012-0002 |
[24] | Bell L , Lamport DJ , Butler LT , Williams CM . A Review of the Cognitive Effects Observed in Humans Following Acute Supplementation with Flavonoids, and Their Associated Mechanisms of Action. Nutrients. (2015) ;7: :10290–306. https://doi.org/10.3390/nu7125538 |
[25] | Gillette-Guyonnet S , Secher M , Vellas B . Nutrition and neurodegeneration: Epidemiological evidence and challenges for future research. Br J Clin Pharmacol. (2013) ;75: :738–55. https://doi.org/10.1111/bcp.12058 |
[26] | Szajdek A , Borowska EJ . Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum Nutr. (2008) ;63: :147–56. https://doi.org/10.1007/s11130-008-0097-5 |
[27] | Letenneur L , Proust-Lima C , Le Gouge A , Dartigues J , Barberger-Gateau P . Flavonoid Intake and Cognitive Decline over a 10-Year Period. American Journal of Epidemiology. (2007) ;165: :1364–71. https://doi.org/10.1093/aje/kwm036 |
[28] | Godos J , Caraci F , Castellano S , Currenti W , Galvano F , Ferri R , et al. Association Between Dietary Flavonoids Intake and Cognitive Function in an Italian Cohort. Biomolecules. (2020) ;10: :1300. https://doi.org/10.3390/biom10091300 |
[29] | Craig CL , Marshall AL , Sjöström M , Bauman AE , Booth ML , Ainsworth BE , et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) ;35: :1381–95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB |
[30] | Paller CJ , Denmeade SR , Carducci MA . Challenges of Conducting Clinical Trials of Natural Products to Combat Cancer. Clin Adv Hematol Oncol. (2016) ;14: :447–55. |
[31] | Atanasov AG , Zotchev SB , Dirsch VM , Supuran CT . Natural products in drug discovery: Advances and opportunities. Nat Rev Drug Discov. (2021) ;20: :200–16. https://doi.org/10.1038/s41573-020-00114-z |
[32] | Dehelean CA , Marcovici I , Soica C , Mioc M , Coricovac D , Iurciuc S , et al. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules. (2021) ;26: :1109. https://doi.org/10.3390/molecules26041109 |
[33] | Seyhan AA . Lost in translation: The valley of death across preclinical and clinical divide - identification of problems and overcoming obstacles. Translational Medicine Communications. (2019) ;4: :18. https://doi.org/10.1186/s41231-019-0050-7 |
[34] | Emmerich CH , Gamboa LM , Hofmann MCJ , Bonin-Andresen M , Arbach O , Schendel P , et al. Improving target assessment in biomedical research: The GOT-IT recommendations. Nat Rev Drug Discov. (2021) ;20: :64–81. https://doi.org/10.1038/s41573-020-0087-3 |
[35] | Martínez-Ballesta Mc , Gil-Izquierdo Á , García-Viguera C , Domínguez-Perles R . Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods. (2018) ;7: :72. https://doi.org/10.3390/foods7050072 |
[36] | Islam F , Khadija JF , Harun-Or-Rashid M , Rahaman MS , Nafady MH , Islam MR , et al. Bioactive Compounds and Their Derivatives: An Insight into Prospective Phytotherapeutic Approach against Alzheimer’s Disease. Oxidative Medicine and Cellular Longevity. (2022) ;2022: :e5100904. https://doi.org/10.1155/2022/5100904 |
[37] | Rivi V , Benatti C , Rigillo G , Blom JMC . Invertebrates as models of learning and memory: Investigating neural and molecular mechanisms. Journal of Experimental Biology. (2023) ;226: :jeb244844. https://doi.org/10.1242/jeb.244844 |
[38] | Rivi V , Benatti C , Colliva C , Radighieri G , Brunello N , Tascedda F , et al. Lymnaea stagnalis as model for translational neuroscience research: From pond to bench. Neuroscience & Biobehavioral Reviews. (2020) ;108: :602–16. https://doi.org/10.1016/j.neubiorev.2019.11.020 |
[39] | Rivi V , Benatti C , Lukowiak K , Colliva C , Alboni S , Tascedda F , et al. What can we teach Lymnaea and what can Lymnaea teach us? Biol Rev Camb Philos Soc. (2021) ;96: :1590–602. https://doi.org/10.1111/brv.12716 |
[40] | Tascedda F , Malagoli D , Accorsi A , Rigillo G , Blom JMC , Ottaviani E . Molluscs as models for translational medicine. Med Sci Monit Basic Res. (2015) ;21: :96–9. https://doi.org/10.12659/MSMBR.894221 |
[41] | Tello JA , Williams HE , Eppler RM , Steinhilb ML , Khanna M . Animal Models of Neurodegenerative Disease: Recent Advances in Fly Highlight Innovative Approaches to Drug Discovery. Front Mol Neurosci. (2022) ;15: :883358. https://doi.org/10.3389/fnmol.2022.883358 |
[42] | Yin P , Li S , Li X-J , Yang W . New pathogenic insights from large animal models of neurodegenerative diseases. Protein Cell. (2022) ;13: :707–20. https://doi.org/10.1007/s13238-022-00912-8 |
[43] | Baker M , Hong S-I , Kang S , Choi D-S . Rodent models for psychiatric disorders: Problems and promises. Laboratory Animal Research. (2020) ;36: :9. https://doi.org/10.1186/s42826-020-00039-z |
[44] | Singh P , Sivanandam TM , Konar A , Thakur MK . Role of nutraceuticals in cognition during aging and related disorders. Neurochem Int. (2021) ;143: :104928. https://doi.org/10.1016/j.neuint.2020.104928 |
[45] | Singh P , Barman B , Thakur MK . Oxidative stress-mediated memory impairment during aging and its therapeutic intervention by natural bioactive compounds. Frontiers in Aging Neuroscience. (2022) ;14: . |
[46] | Ganesan P , Ko H-M , Kim I-S , Choi D-K . Recent trends in the development of nanophytobioactive compounds and delivery systems for their possible role in reducing oxidative stress in Parkinson’s disease models. Int J Nanomedicine. (2015) ;10: :6757–72. https://doi.org/10.2147/IJN.S93918 |
[47] | Upadhyay RK . Drug delivery systems, CNS protection, and the blood brain barrier. Biomed Res Int. (2014) ;2014: :869269. https://doi.org/10.1155/2014/869269 |
[48] | Zeb A . Concept, mechanism, and applications of phenolic antioxidants in foods. J Food Biochem. (2020) ;44: :e13394. https://doi.org/10.1111/jfbc.13394 |
[49] | González-Fuentes J , Selva J , Moya C , Castro-Vázquez L , Lozano MV , Marcos P , et al. Neuroprotective Natural Molecules, From Food to Brain. Frontiers in Neuroscience. (2018) ;12: . |
[50] | Anand S , Bharadvaja N . Potential Benefits of Nutraceuticals for Oxidative Stress Management. Rev Bras Farmacogn. (2022) ;32: :211–20. https://doi.org/10.1007/s43450-022-00246-w |
[51] | Wilson ME , Westberry JM , Prewitt AK . Dynamic Regulation of Estrogen Receptor-Alpha Gene Expression in the Brain: A Role for Promoter Methylation? Front Neuroendocrinol. (2008) ;29: :375–85. https://doi.org/10.1016/j.yfrne.2008.03.002 |
[52] | Bean LA , Ianov L , Foster TC . Estrogen Receptors, the Hippocampus, and Memory. Neuroscientist. (2014) ;20: :534–45. https://doi.org/10.1177/1073858413519865 |
[53] | Fortress AM , Frick KM . Epigenetic regulation of estrogen-dependent memory. Frontiers in Neuroendocrinology. (2014) ;35: :530–49. https://doi.org/10.1016/j.yfrne.2014.05.001 |
[54] | Ledford H . How menopause reshapes the brain. Nature. (2023) ;617: :25–7. https://doi.org/10.1038/d41586-023-01474-3 |
[55] | Graham F . Daily briefing: Menopause is driven by the brain, not just the ovaries. Nature. 2023. https://doi.org/10.1038/d41586-023-01534-8 |
[56] | Singh P , Paramanik V . Neuromodulating roles of estrogen and phytoestrogens in cognitive therapeutics through epigenetic modifications during aging. Frontiers in Aging Neuroscience. (2022) ;14: . |
[57] | Ko S-H , Kim H-S . Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients. (2020) ;12: :202. https://doi.org/10.3390/nu12010202 |
[58] | Ko S-H , Jung Y . Energy Metabolism Changes and Dysregulated Lipid Metabolism in Postmenopausal Women. Nutrients. (2021) ;13: :4556. https://doi.org/10.3390/nu13124556 |
[59] | Hyvärinen M , Juppi H-K , Taskinen S , Karppinen JE , Karvinen S , Tammelin TH , et al. Metabolic health, menopause, and physical activity—a 4-year follow-up study. Int J Obes. (2022) ;46: :544–54. https://doi.org/10.1038/s41366-021-01022-x |
[60] | Conde DM , Verdade RC , Valadares ALR , Mella LFB , Pedro AO , Costa-Paiva L . Menopause and cognitive impairment: A narrative review of current knowledge. World J Psychiatry. (2021) ;11: :412–28. https://doi.org/10.5498/wjp.v11.i8.412 |
[61] | Lethaby A , Hogervorst E , Richards M , Yesufu A , Yaffe K . Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev. (2008) ;2008: :CD003122. https://doi.org/10.1002/14651858.CD003122.pub2 |
[62] | Singh D , Chaudhuri PK . Chemistry and Pharmacology of Tinospora cordifolia. Nat Prod Commun. (2017) ;12: :299–308. |
[63] | Bhandari A , Sunkaria A , Kaur G . Dietary Supplementation With Tinospora cordifolia Improves Anxiety-Type Behavior and Cognitive Impairments in Middle-Aged Acyclic Female Rats. Frontiers in Aging Neuroscience. (2022) ;14: . |
[64] | Mishra R , Manchanda S , Gupta M , Kaur T , Saini V , Sharma A , et al. Tinospora cordifolia ameliorates anxiety-like behavior and improves cognitive functions in acute sleep deprived rats. Sci Rep. (2016) ;6: :25564. https://doi.org/10.1038/srep25564 |
[65] | Huang X , Wu S , Wei Y , Meng S , Jiang R . Effect of the plant flavonoid, rhoifolin, on memory and cognition in a rat model of Alzheimer’s disease. Tropical Journal of Pharmaceutical Research. (1481) ;20: :1481–7. https://doi.org/10.4314/tjpr.v20i7.22 |
[66] | Van Norman GA . Limitations of Animal Studies for Predicting Toxicity in Clinical Trials. JACC Basic Transl Sci. (2019) ;4: :845–54. https://doi.org/10.1016/j.jacbts.2019.10.008 |
[67] | Speakman JR . The physiological costs of reproduction in small mammals. Philos Trans R Soc Lond B Biol Sci. (2008) ;363: :375–98. https://doi.org/10.1098/rstb.2007.2145 |
[68] | Robinson NB , Krieger K , Khan FM , Huffman W , Chang M , Naik A , et al. The current state of animal models in research: A review. International Journal of Surgery. (2019) ;72: :9–13. https://doi.org/10.1016/j.ijsu.2019.10.015 |
[69] | Ségalat L . Invertebrate Animal Models of Diseases as Screening Tools in Drug Discovery. ACS Chem Biol. (2007) ;2: :231–6. https://doi.org/10.1021/cb700009m |
[70] | Wg P , Cl H , Dh G . Evolutionary conservation and divergence of the human brain transcriptome. Genome Biology. (2021) ;22: . https://doi.org/10.1186/s13059-020-02257-z |
[71] | Carew TJ , Walters ET , Kandel ER . Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. (1981) ;1: :1426–37. https://doi.org/10.1523/JNEUROSCI.01-12-01426.1981 |
[72] | Lederhendler II , Alkon DL . Associatively reduced withdrawal from shadows in Hermissenda: A direct behavioral analog of photoreceptor responses to brief light steps. Behavioral and Neural Biology. (1987) ;47: :227–49. https://doi.org/10.1016/S0163-1047(87)90370-0 |
[73] | Strausfeld NJ . Arthropod Brains: Evolution, Functional Elegance, and Historical Significance. Harvard University Press; 2012. https://doi.org/10.2307/j.ctv1dp0v2h |
[74] | Ottaviani E , Franceschi C . The neuroimmunology of stress from invertebrates to man. Prog Neurobiol. (1996) ;48: :421–40. https://doi.org/10.1016/0301-0082(95)00049-6 |
[75] | Giunti S , Andersen N , Rayes D , De Rosa MJ . Drug discovery: Insights from the invertebrate Caenorhabditis elegans. Pharmacol Res Perspect. (2021) ;9: :e00721. https://doi.org/10.1002/prp2.721 |
[76] | Katanaev VL , Di Falco S , Khotimchenko Y . The Anticancer Drug Discovery Potential of Marine Invertebrates from Russian Pacific. Marine Drugs. (2019) ;17: :474. https://doi.org/10.3390/md17080474 |
[77] | Proshkina E , Lashmanova E , Dobrovolskaya E , Zemskaya N , Kudryavtseva A , Shaposhnikov M , et al. Geroprotective and Radioprotective Activity of Quercetin, (-)-Epicatechin, and Ibuprofen in Drosophila melanogaster. Front Pharmacol. (2016) ;7: :505. https://doi.org/10.3389/fphar.2016.00505 |
[78] | Miyazaki H , Okamoto Y , Motoi A , Watanabe T , Katayama S , Kawahara S-I , et al. Adzuki bean (Vigna angularis) extract reduces amyloid-β aggregation and delays cognitive impairment in Drosophila models of Alzheimer’s disease. Nutr Res Pract. (2019) ;13: :64–9. https://doi.org/10.4162/nrp.2019.13.1.64 |
[79] | Siddique YH , Naz F , Jyoti S . Effect of capsaicin on the oxidative stress and dopamine content in the transgenic Drosophila model of Parkinson’s disease. Acta Biol Hung. (2018) ;69: :115–24. https://doi.org/10.1556/018.69.2018.2.1 |
[80] | Siddique YH , Naz F , Jyoti S . Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson’s disease. Biomed Res Int. (2014) ;2014: :606928. https://doi.org/10.1155/2014/606928 |
[81] | Ortega-Arellano HF , Jimenez-Del-Rio M , Velez-Pardo C . Dmp53, basket and drICE gene knockdown and polyphenol gallic acid increase life span and locomotor activity in a Drosophila Parkinson’s disease model. Genet Mol Biol. (2013) ;36: :608–15. https://doi.org/10.1590/S1415-47572013000400020 |
[82] | Abbas S , Wink M . Green Tea Extract Induces the Resistance of Caenorhabditis elegans against Oxidative Stress. Antioxidants (Basel). (2014) ;3: :129–43. https://doi.org/10.3390/antiox3010129 |
[83] | Tantengco OAG , Tan JJE , Tan NRC , Sison MCC , Medina PMB . Garcinia binucao crude ethanolic leaf extract prevents alcohol-induced neurotoxic effects on learning, short-term memory, and motor functions in Drosophila melanogaster. J App Pharm Sci. (2018) ;8,: :106–12. https://doi.org/10.7324/JAPS.2018.81014 |
[84] | Wilson MA , Shukitt-Hale B , Kalt W , Ingram DK , Joseph JA , Wolkow CA . Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. (2006) ;5: :59–68. https://doi.org/10.1111/j.1474-9726.2006.00192.x |
[85] | Zhao Y , Yang Q , Liu D , Liu T , Xing L . Neurotoxicity of nanoparticles: Insight from studies in zebrafish. Ecotoxicology and Environmental Safety. (2022) ;242: :113896. https://doi.org/10.1016/j.ecoenv.2022.113896 |
[86] | Mathias JR , Saxena MT , Mumm JS . Advances in zebrafish chemical screening technologies. Future Med Chem. (2012) ;4: :1811–22. https://doi.org/10.4155/fmc.12.115 |
[87] | [PDF] Effect of citrus peels-supplemented diet on longevity, memory index, redox status, cholinergic and monoaminergic enzymes in Drosophila melanogaster model by Ganiyu Oboh, Damilola M. Olatunde, Ayokunle O. Ademosun, Opeyemi B. Ogunsuyi . 10.1111/jfbc.13616.sOA.mg n.d. |
[88] | Zhao Q , Liu Y , Zhang S , Zhao Y , Wang C , Li K , et al. Studies on the Regulation and Molecular Mechanism of Panax Ginseng Saponins on Senescence and Related Behaviors of Drosophila melanogaster. Front Aging Neurosci. (2022) ;14: :870326. https://doi.org/10.3389/fnagi.2022.870326 |
[89] | Suttisansanee U , Charoenkiatkul S , Jongruaysup B , Tabtimsri S , Siriwan D , Temviriyanukul P . Mulberry Fruit Cultivar ‘Chiang Mai’ Prevents Beta-Amyloid Toxicity in PC12 Neuronal Cells and in a Drosophila Model of Alzheimer’s Disease. Molecules. (2020) ;25: :1837. https://doi.org/10.3390/molecules25081837 |
[90] | Michels B , Zwaka H , Bartels R , Lushchak O , Franke K , Endres T , et al. Memory enhancement by ferulic acid ester across species. Sci Adv. (2018) ;4: :eaat6994. https://doi.org/10.1126/sciadv.aat6994 |
[91] | Liu M , Xiong Y , Shan S , Zhu Y , Zeng D , Shi Y , et al. Eleutheroside E Enhances the Long-Term Memory of Radiation-Damaged C. elegans through G-Protein-Coupled Receptor and Neuropeptide Signaling Pathways. J Nat Prod. (2020) ;83: :3315–23. https://doi.org/10.1021/acs.jnatprod.0c00650 |
[92] | Guo H , Dong Y-Q , Ye B-P . Cranberry extract supplementation exerts preventive effects through alleviating Aβ toxicity in Caenorhabditis elegans model of Alzheimer’s disease. Chin J Nat Med. (2016) ;14: :427–33. https://doi.org/10.1016/S1875-5364(16)30039-5 |
[93] | Batabyal A , Rivi V , Benatti C , Blom JMC , Lukowiak K . Long-term memory of configural learning is enhanced via CREB upregulation by the flavonoid quercetin in Lymnaea stagnalis. Journal of Experimental Biology. (2021) ;224: :jeb242761. https://doi.org/10.1242/jeb.242761 |
[94] | Rivi V , Batabyal A , Benatti C , Blom JM , Tascedda F , Lukowiak K . A flavonoid, quercetin, is capable of enhancing long-term memory formation if encountered at different times in the learning, memory formation, and memory recall continuum. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2021. https://doi.org/10.1007/s00359-021-01522-1 |
[95] | Rivi V , Batabyal A , Juego K , Kakadiya M , Benatti C , Blom JMC , et al. To eat or not to eat: A Garcia effect in pond snails (Lymnaea stagnalis). J Comp Physiol A. (2021) ;207: :479–95. https://doi.org/10.1007/s00359-021-01491-5 |
[96] | Fruson L , Dalesman S , Lukowiak K . A flavonol present in cocoa [(–)epicatechin] enhances snail memory. Journal of Experimental Biology. (2012) ;215: :3566–76. https://doi.org/10.1242/jeb.070300 |
[97] | Fernell M , Swinton C , Lukowiak K . Epicatechin, a component of dark chocolate, enhances memory formation if applied during the memory consolidation period. Commun Integr Biol. (2016) ;9: :e1205772. https://doi.org/10.1080/19420889.2016.1205772 |
[98] | Swinton E , de Freitas E , Swinton C , Shymansky T , Hiles E , Zhang J , et al. Green tea and cocoa enhance cognition in Lymnaea. Communicative & Integrative Biology. (2018) ;11: :e1434390. https://doi.org/10.1080/19420889.2018.1434390 |
[99] | Zhang J , de Freitas E , Lukowiak K . Black tea differs from green tea: It suppresses long-term memory formation in Lymnaea. Communicative & Integrative Biology. (2018) ;11: :1–4. https://doi.org/10.1080/19420889.2018.1491245 |
[100] | Batabyal A , Lukowiak K . Configural learning memory can be transformed from intermediate-term to long-term in pond snail Lymnaea stagnalis. Physiol Behav. (2021) ;239: :113509. https://doi.org/10.1016/j.physbeh.2021.113509 |
[101] | Richetti SK , Blank M , Capiotti KM , Piato AL , Bogo MR , Vianna MR , et al. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav Brain Res. (2011) ;217: :10–5. https://doi.org/10.1016/j.bbr.2010.09.027 |
[102] | Thayumanavan G , Jeyabalan S , Fuloria S , Sekar M , Ravi M , Selvaraj LK , et al. Silibinin and Naringenin against Bisphenol A-Induced Neurotoxicity in Zebrafish Model—Potential Flavonoid Molecules for New Drug Design, Development, and Therapy for Neurological Disorders. Molecules. (2022) ;27: :2572. https://doi.org/10.3390/molecules27082572 |
[103] | Fodor I , Hussein AA , Benjamin PR , Koene JM , Pirger Z . The unlimited potential of the great pond snail, Lymnaea stagnalis. ELife. (2020) ;9: :e56962. https://doi.org/10.7554/eLife.56962 |
[104] | Kuroda R , Abe M . The pond snail Lymnaea stagnalis. EvoDevo. (2020) ;11: :24. https://doi.org/10.1186/s13227-020-00169-4 |
[105] | Lukowiak K , Martens K , Rosenegger D , Browning K , de Caigny P , Orr M . The perception of stress alters adaptive behaviours in Lymnaea stagnalis. Journal of Experimental Biology. (2008) ;211: :1747–56. https://doi.org/10.1242/jeb.014886 |
[106] | Rivi V , Batabyal A , Wiley B , Benatti C , Tascedda F , Blom JMC , et al. Fluoride affects memory by altering the transcriptional activity in the central nervous system of Lymnaea stagnalis. NeuroToxicology. (2022) ;92: :61–6. https://doi.org/10.1016/j.neuro.2022.07.007 |
[107] | Fernell M , Rivi V , Batabyal A , Lukowiak K . The temperature sensitivity of memory formation and persistence is altered by cold acclimation in a pond snail. Journal of Experimental Biology. (2021) ;224: :jeb242513. https://doi.org/10.1242/jeb.242513 |
[108] | Rivi V , Batabyal A , Benatti C , Blom JMC , Lukowiak K . Nature versus nurture in heat stress induced learning between inbred and outbred populations of Lymnaea stagnalis. Journal of Thermal Biology. (2022) ;103: :103170. https://doi.org/10.1016/j.jtherbio.2021.103170 |
[109] | Rivi V , Batabyal A , Benatti C , Tascedda F , Blom JM , Lukowiak K . Too Hot to Eat: Wild and Lab-Bred Lymnaea stagnalis Differ in Feeding Response Following Repeated Heat Exposure. The Biological Bulletin. 2022: :000–000. https://doi.org/10.1086/720948 |
[110] | Cristina B , Veronica R , Silvia A , Andrea G , Sara C , Luca P , et al. Identification and characterization of the kynurenine pathway in the pond snail Lymnaea stagnalis. Sci Rep. (2022) ;12: :15617. https://doi.org/10.1038/s41598-022-19652-0 |
[111] | Benatti C , Rivi V , Colliva C , Radighieri G , Tascedda F , Blom JMC . Redefining operant conditioning of escape behaviour in Lymnaea stagnalis. Invertebrate Survival Journal. 2020: :129–37. https://doi.org/10.25431/1824-307X/isj.v0i0.129-137 |
[112] | Batabyal A , Chau D , Rivi V , Lukowiak K . Risk in one is not risk in all: Snails show differential decision making under high- and low-risk environments. Animal Behaviour. (2022) ;190: :53–60. https://doi.org/10.1016/j.anbehav.2022.05.013 |
[113] | Rivi V , Benatti C , Actis P , Tascedda F , Blom JMC . Behavioral and transcriptional effects of short or prolonged fasting on the memory performances of Lymnaea stagnalis. Neuroendocrinology. 2022. https://doi.org/10.1159/000527489 |
[114] | Rivi V , Batabyal A , Benatti C , Tascedda F , Blom JMC , Lukowiak K . Aspirin reverts lipopolysaccharide-induced learning and memory impairment: First evidence from an invertebrate model system. Naunyn Schmiedebergs Arch Pharmacol. 2022. https://doi.org/10.1007/s00210-022-02286-4 |
[115] | Rivi V , Batabyal A , Benatti C , Blom JM , Tascedda F , Lukowiak K . Novel taste, sickness, and memory: Lipopolysaccharide to induce a Garcia-like effect in inbred and wild strains of Lymnaea stagnalis. Physiology & Behavior. (2023) ;263: :114137. https://doi.org/10.1016/j.physbeh.2023.114137 |
[116] | Lukowiak K , Martens K , Orr M , Parvez K , Rosenegger D , Sangha S . Modulation of aerial respiratory behaviour in a pond snail. Respir Physiol Neurobiol. (2006) ;154: :61–72. https://doi.org/10.1016/j.resp.2006.02.009 |
[117] | Benjamin P , Kemenes G . Lymnaea learning and memory. Scholarpedia. (2010) ;5: :4247. https://doi.org/10.4249/scholarpedia.4247 |
[118] | Benjamin PR , Kemenes G . Invertebrate Models to Study Learning and Memory: Lymnaea. In: Squire LR, editor. Encyclopedia of Neuroscience, Oxford: Academic Press; 2009, p. 197-204. https://doi.org/10.1016/B978-008045046-9.00804-4 |
[119] | Kemenes G , Staras K , Benjamin PR . In Vitro Appetitive Classical Conditioning of the Feeding Response in the Pond Snail Lymnaea stagnalis. Journal of Neurophysiology. (1997) ;78: :2351–62. https://doi.org/10.1152/jn.1997.78.5.2351 |
[120] | Lukowiak null , Ringseis null , Spencer null , Wildering null , Syed null . Operant conditioning of aerial respiratory behaviour in Lymnaea stagnalis. J Exp Biol. (1996) ;199: :683–91. https://doi.org/10.1242/jeb.199.3.683 |
[121] | Fodor I , Hussein AA , Benjamin PR , Koene JM , Pirger Z . The unlimited potential of the great pond snail, Lymnaea stagnalis. ELife. (2020) ;9: :e56962. https://doi.org/10.7554/eLife.56962 |
[122] | Syed NI , Harrison D , Winlow W . Respiratory behavior in the pond snail Lymnaea stagnalis. J Comp Physiol A. (1991) ;169: :541–55. https://doi.org/10.1007/BF00193545 |
[123] | Syed NI , Bulloch AG , Lukowiak K . In Vitro reconstruction of the respiratory central pattern generator of the mollusk Lymnaea. Science. (1990) ;250: :282–5. https://doi.org/10.1126/science.2218532 |
[124] | Feng Z-P , Zhang Z , van Kesteren R , Straub V , van Nierop P , Jin K , et al. Transcriptome analysis of the central nervous system of the mollusc Lymnaea stagnalis. BMC Genomics. (2009) ;10: :451. https://doi.org/10.1186/1471-2164-10-451 |
[125] | Katz PS . Evolution of central pattern generators and rhythmic behaviours. Philosophical Transactions of the Royal Society B: Biological Sciences. (2016) ;371: :20150057. https://doi.org/10.1098/rstb.2015.0057 |
[126] | Benatti C , Colliva C , Blom JMC , Ottaviani E , Tascedda F . Transcriptional effect of serotonin in the ganglia of Lymnaea stagnalis. Invertebrate Survival Journal. (2017) ;14: :251–8. https://doi.org/10.25431/1824-307X/isj.v14i1.251-258 |
[127] | Swinton E , de Freitas E , Swinton C , Shymansky T , Hiles E , Zhang J , et al. Green tea and cocoa enhance cognition in Lymnaea. Commun Integr Biol. (2018) ;11: :e1434390s. https://doi.org/10.1080/19420889.2018.1434390 |
[128] | Ito E , Kobayashi S , Kojima S , Sadamoto H , Hatakeyama D . Associative Learning in the Pond Snail, Lymnaea stagnalis. Jzoo. (1999) ;16: :711–23. https://doi.org/10.2108/zsj.16.711 |
[129] | Nakai J , Totani Y , Kojima S , Sakakibara M , Ito E . Features of behavioral changes underlying conditioned taste aversion in the pond snail Lymnaea stagnalis. Invert Neurosci. (2020) ;20: :8. https://doi.org/10.1007/s10158-020-00241-7 |
[130] | Spencer GE , Syed NI , Lukowiak K . Neural Changes after Operant Conditioning of the Aerial Respiratory Behavior in Lymnaea stagnalis. J Neurosci. (1999) ;19: :1836–43. https://doi.org/10.1523/JNEUROSCI.19-05-01836.1999 |
[131] | McComb C , Meems R , Syed N , Lukowiak K . Electrophysiological Differences in the CPG Aerial Respiratory Behavior Between Juvenile and Adult Lymnaea. Journal of Neurophysiology. (2003) ;90: :983–92. https://doi.org/10.1152/jn.00263.2003 |
[132] | Bond AM , Ming G , Song H . Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell. (2015) ;17: :385–95. https://doi.org/10.1016/j.stem.2015.09.003 |
[133] | Cherubini E , Gustincich S , Robinson H . The mammalian transcriptome and the cellular complexity of the brain. J Physiol. (2006) ;575: :319–20. https://doi.org/10.1113/jphysiol.2006.118364 |
[134] | Letenneur L , Proust-Lima C , Le Gouge A , Dartigues J , Barberger-Gateau P . Flavonoid Intake and Cognitive Decline over a 10-Year Period. American Journal of Epidemiology. (2007) ;165: :1364–71. https://doi.org/10.1093/aje/kwm036 |
[135] | Lukowiak K , Orr M , de Caigny P , Lukowiak KS , Rosenegger D , Han JI , et al. Ecologically relevant stressors modify long-term memory formation in a model system. Behav Brain Res. (2010) ;214: :18–24. https://doi.org/10.1016/j.bbr.2010.05.011 |
[136] | Lukowiak K , Sunada H , Teskey M , Lukowiak K , Dalesman S . Environmentally relevant stressors alter memory formation in the pond snail Lymnaea. J Exp Biol. (2014) ;217: :76–83. https://doi.org/10.1242/jeb.089441 |
[137] | Dalesman S , Lukowiak K . Effect of acute exposure to low environmental calcium on respiration and locomotion in Lymnaea stagnalis (L.). J Exp Biol. (2010) ;213: :1471–6. https://doi.org/10.1242/jeb.040493 |
[138] | Dodd S , Rothwell CM , Lukowiak K . Strain-specific effects of crowding on long-term memory formation in Lymnaea. Comp Biochem Physiol A Mol Integr Physiol. (2018) ;222: :43–51. https://doi.org/10.1016/j.cbpa.2018.04.010 |
[139] | De Caigny P , Lukowiak K . Crowding, an environmental stressor, blocks long-term memory formation in Lymnaea. J Exp Biol. (2008) ;211: :2678–88. https://doi.org/10.1242/jeb.020347 |
[140] | Knezevic B , Lukowiak K . The flavonol epicatechin reverses the suppressive effects of a stressor on long-term memory formation. Journal of Experimental Biology. (2014) ;217: :4004–9. https://doi.org/10.1242/jeb.110726 |
[141] | Knezevic B , Komatsuzaki Y , de Freitas E , Lukowiak K . A flavanoid component of chocolate quickly reverses an imposed memory deficit. Journal of Experimental Biology. (2016) ;219: :816–23. https://doi.org/10.1242/jeb.130765 |
[142] | Sokolov AN , Pavlova MA , Klosterhalfen S , Enck P . Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neuroscience & Biobehavioral Reviews. (2013) ;37: :2445–53. https://doi.org/10.1016/j.neubiorev.2013.06.013 |
[143] | Das D , Nag S , De S , Hazarika AK , Sabhapondit S , Tudu B , et al. Electrochemical Detection of Epicatechin in Green Tea Using Quercetin-Imprinted Polymer Graphite Electrode. IEEE Sensors Journal. (2021) ;21: :26526–33. https://doi.org/10.1109/JSEN.2021.3122145 |
[144] | Gasper A , Hollands W , Casgrain A , Saha S , Teucher B , Dainty JR , et al. Consumption of both low and high (-)-epicatechin apple puree attenuates platelet reactivity and increases plasma concentrations of nitric oxide metabolites: a randomized controlled trial. Arch Biochem Biophys. (2014) ;559: :29–37. https://doi.org/10.1016/j.abb.2014.05.026 |
[145] | Si H , Lai C-Q , Liu D . Dietary Epicatechin, A Novel Anti-aging Bioactive Small Molecule. Curr Med Chem. (2021) ;28: :3–18. https://doi.org/10.2174/0929867327666191230104958 |
[146] | Shi M , Nie Y , Zheng X-Q , Lu J-L , Liang Y-R , Ye J-H . Ultraviolet B (UVB) Photosensitivities of Tea Catechins and the Relevant Chemical Conversions. Molecules. (2016) ;21: :1345. https://doi.org/10.3390/molecules21101345 |
[147] | Swinton C , Swinton E , Shymansky T , Hughes E , Zhang J , Rothwell C , et al. Configural learning: a higher form of learning in Lymnaea. Journal of Experimental Biology. (2019) ;222: :jeb190405s. https://doi.org/10.1242/jeb.190405 |
[148] | Kagan D , Lukowiak K . Configural learning in freshly collected, smart, wild Lymnaea. Journal of Experimental Biology. (2019) ;222: :jeb212886. https://doi.org/10.1242/jeb.212886 |
[149] | Batabyal A , Lukowiak K . Tracking the path of predator recognition in a predator-naive population of the pond snail. Behavioral Ecology. (2023) ;34: :125–35. https://doi.org/10.1093/beheco/arac107 |
[150] | Batabyal A , Lukowiak K . Configural learning memory can be transformed from intermediate-term to long-term in pond snail Lymnaea stagnalis. Physiol Behav. (2021) ;239: :113509. https://doi.org/10.1016/j.physbeh.2021.113509 |
[151] | Khan N , Mukhtar H . Tea and Health: Studies in Humans. Curr Pharm Des. (2013) ;19: :6141–7. |
[152] | Wiley B , Batabyal A , Lukowiak K . Fluoride alters feeding and memory in Lymnaea stagnalis. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. (2022) ;208: :267–77. https://doi.org/10.1007/s00359-021-01528-9 |
[153] | Chow H-HS , Hakim IA . Pharmacokinetic and chemoprevention studies on tea in humans. Pharmacol Res. (2011) ;64: :105–12. https://doi.org/10.1016/j.phrs.2011.05.007 |
[154] | Olthof MR , Hollman PCH , Vree TB , Katan MB . Bioavailabilities of Quercetin-3-Glucoside and Quercetin-4′-Glucoside Do Not Differ in Humans. The Journal of Nutrition. (2000) ;130: :1200–3. https://doi.org/10.1093/jn/130.5.1200 |
[155] | Anand David AV , Arulmoli R , Parasuraman S . Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn Rev. (2016) ;10: :84–9. https://doi.org/10.4103/0973-7847.194044. |
[156] | Amanzadeh E , Esmaeili A , Rahgozar S , Nourbakhshnia M . Application of quercetin in neurological disorders: from nutrition to nanomedicine. Rev Neurosci. (2019) ;30: :555–72. https://doi.org/10.1515/revneuro-2018-0080 |
[157] | Rivi V , Batabyal A , Benatti C , Tascedda F , Blom JMC , Lukowiak K . A Novel Behavioral Display in Lymnaea Induced by Quercetin and Hypoxia. The Biological Bulletin. 2023: :000–000. https://doi.org/10.1086/725689 |
[158] | Teskey ML , Lukowiak KS , Riaz H , Dalesman S , Lukowiak K . What’s hot: the enhancing effects of thermal stress on long-term memory formation in Lymnaea stagnalis. Journal of Experimental Biology. (2012) ;215: :4322–9. https://doi.org/10.1242/jeb.075960 |
[159] | Tan R , Lukowiak K . Combining Factors That Individually Enhance Memory in Lymnaea. Biol Bull. (2018) ;234: :37–44. https://doi.org/10.1086/697197 |
[160] | Foster NL , Lukowiak K , Henry TB . Time-related expression profiles for heat shock protein gene transcripts (HSP40, HSP70) in the central nervous system of Lymnaea stagnalis exposed to thermal stress. Commun Integr Biol. (2015) ;8: :e1040954. https://doi.org/10.1080/19420889.2015.1040954 |
[161] | Sunada H , Riaz H , de Freitas E , Lukowiak K , Swinton C , Swinton E , et al. Heat stress enhances LTM formation in Lymnaea: role of HSPs and DNA methylation. Journal of Experimental Biology. (2016) ;219: :1337–45. https://doi.org/10.1242/jeb.134296 |
[162] | Grodzicki W , Dziendzikowska K . The Role of Selected Bioactive Compounds in the Prevention of Alzheimer’s Disease. Antioxidants (Basel). (2020) ;9: :229. https://doi.org/10.3390/antiox9030229 |
[163] | Sharifi-Rad M , Anil Kumar NV , Zucca P , Varoni EM , Dini L , Panzarini E , et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol. (2020) ;11: :694. https://doi.org/10.3389/fphys.2020.00694 |
[164] | Nathan M . The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Ann Intern Med. (1999) ;130: :459. https://doi.org/10.7326/0003-4819-130-5-199903020-00024 |
[165] | Schulz V , Hänsel R , Tyler VE . Rational Phytotherapy: A Physicians’ Guide to Herbal Medicine. Springer Science & Business Media; 2012. |
[166] | Tyler EBR Virginia M . Handbook of Psychotropic Herbs: A Scientific Analysis of Herbal Remedies for Psychiatric Conditions. New York: Routledge; 2016. https://doi.org/10.4324/9780203047910 |
[167] | Tran N , Pham B , Le L . Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology (Basel). (2020) ;9: :252. https://doi.org/10.3390/biology9090252 |
[168] | Liu W , Yang B , Yang L , Kaur J , Jessop C , Fadhil R , et al. Therapeutic Effects of Ten Commonly Used Chinese Herbs and Their Bioactive Compounds on Cancers. Evid Based Complement Alternat Med. (2019) ;2019: :6057837. https://doi.org/10.1155/2019/6057837 |
[169] | Xu R-S . Some bioactive natural products from chinese medicinal plants. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry, vol. 21, Elsevier; 2000, p. 729-72. https://doi.org/10.1016/S1572-5995(00)80017-4 |
[170] | Khare CP , editor. Indian Herbal Remedies. Berlin, Heidelberg: Springer; 2004. https://doi.org/10.1007/978-3-642-18659-2 |
[171] | Gogtay NJ , Bhatt HA , Dalvi SS , Kshirsagar NA . The use and safety of non-allopathic Indian medicines. Drug Saf. (2002) ;25: :1005–19. https://doi.org/10.2165/00002018-200225140-00003 |
[172] | Astin JA . Why patients use alternative medicine: results of a national study. JAMA. (1998) ;279: :1548–53. https://doi.org/10.1001/jama.279.19.1548 |
[173] | Zörgo S , Purebl G , Zana Á . A qualitative study of culturally embedded factors in complementary and alternative medicine use. BMC Complement Altern Med. (2018) ;18: :25. https://doi.org/10.1186/s12906-018-2093-0 |
[174] | Jasti S , Siega-Riz AM , Bentley ME . Dietary Supplement Use in the Context of Health Disparities: Cultural, Ethnic and Demographic Determinants of Use. The Journal of Nutrition. (2003) ;133: :2010S–2013S. https://doi.org/10.1093/jn/133.6.2010S |
[175] | Zhou HH , Liu ZQ . Ethnic differences in drug metabolism. Clin Chem Lab Med. (2000) ;38: :899–903. https://doi.org/10.1515/CCLM.2000.131 |
[176] | Qiao J , Wang C , Chen Y , Yu S , Liu Y , Yu S , et al. Herbal/Natural Compounds Resist Hallmarks of Brain Aging: From Molecular Mechanisms to Therapeutic Strategies. Antioxidants. (2023) ;12: :920. https://doi.org/10.3390/antiox12040920 |
[177] | Ekor M . The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. (2014) ;4: :177. https://doi.org/10.3389/fphar.2013.00177 |
[178] | Onder G , Liperoti R . Herbal Medications. JAMA. (2016) ;315: :1068. https://doi.org/10.1001/jama.2015.19388 |
[179] | Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases - PMC n.d. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6432691/ (accessed May 4, 2023). |
[180] | Wierzejska RE . Dietary Supplements—For Whom? The Current State of Knowledge about the Health Effects of Selected Supplement Use. Int J Environ Res Public Health. (2021) ;18: :8897. https://doi.org/10.3390/ijerph18178897 |
[181] | Nutrition C for FS and A. Dietary Supplements. FDA. 2023. https://www.fda.gov/food/dietary-supplements (accessed May 4, 2023). |
[182] | Laar A , Amoah Ampah E , Fernandez Y , Senyo Amevinya G , Nortey P , Benyah F , et al. ‘What the herbal medicine can do for me in a week, the orthodox does in a year’: Perceived efficacy of local alternative therapies influences medication adherence in patients with atherosclerotic cardiovascular disease. Health Expectations. (2021) ;24: :444–55. https://doi.org/10.1111/hex.13185 |
[183] | Ameade EPK , Ibrahim M , Ibrahim H-S , Habib RH , Gbedema SY . Concurrent Use of Herbal and Orthodox Medicines among Residents of Tamale, Northern Ghana, Who Patronize Hospitals and Herbal Clinics. Evid Based Complement Alternat Med. (2018) ;2018: :1289125. https://doi.org/10.1155/2018/1289125 |
[184] | Tabish SA . Complementary and Alternative Healthcare: Is it Evidence-based? Int J Health Sci (Qassim). (2008) ;2: :V–IX. |



