Proceedings from the Albert Charitable Trust Inaugural Workshop on ‘Understanding the Acute Effects of Exercise on the Brain’
Abstract
An inaugural workshop supported by “The Leo and Anne Albert Charitable Trust,” was held October 4–7, 2019 in Scottsdale, Arizona, to focus on the effects of exercise on the brain and to discuss how physical activity may prevent or delay the onset of aging-related neurodegenerative conditions. The Scientific Program Committee (led by Dr. Jeff Burns) assembled translational, clinical, and basic scientists who research various aspects of the effects of exercise on the body and brain, with the overall goal of gaining a better understanding as to how to delay or prevent neurodegenerative diseases. In particular, research topics included the links between cardiorespiratory fitness, the cerebrovasculature, energy metabolism, peripheral organs, and cognitive function, which are all highly relevant to understanding the effects of acute and chronic exercise on the brain. The Albert Trust workshop participants addressed these and related topics, as well as how other lifestyle interventions, such as diet, affect age-related cognitive decline associated with Alzheimer’s and other neurodegenerative diseases. This report provides a synopsis of the presentations and discussions by the participants, and a delineation of the next steps towards advancing our understanding of the effects of exercise on the aging brain.
INTRODUCTION
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease. With the increase in human lifespan [1] the incidence of AD will likely continue to rise in the coming decades. Risk for the disease is increased in individuals with a family member affected by AD or who have apolipoprotein e4 genotype (APOE4) alleles [2], as well as by physical inactivity and associated conditions, such as arterial hypertension and hypercholestremia [3]. The accumulation of amyloid plaques and neurofibrillary tangles in the hippocampus and cortex result in a progressive loss of brain function accompanied by physical and cognitive decline [4]. To date there are no effective treatment options for AD patients, and pharmaceutical trials have generally resulted in failure [5]. Indeed, the recently approved monoclonal anti-amyloid antibody aducanumab has unclear therapeutic efficacy [6]. Therefore, at present lifestyle interventions hold the most promise for delaying or preventing the onset of dementia [7]. In particular, aerobic physical activity, such as walking, running, swimming or cycling, that improves cardiorespiratory endurance is a low-cost and low-risk intervention [8]. In rodents, running enhances adult hippocampal neurogenesis and neurotrophin levels, and memory function [9, 10]. In animal models of AD, exercise improves pathophysiology and cognitive function [11–13]. In humans, longitudinal epidemiological studies suggest that aerobic exercise benefits memory function during normal aging [14], and reduces the incidence of AD [15, 16]. Moreover, randomized controlled trials indicate that aerobic exercise training in older adults can increase brain volume, blood flow, memory, and executive function [17–19], which may delay or prevent AD onset [7, 20, 21].
Observational studies indicate moderate to high intensity aerobic activity of at least 150 minutes per week may be needed for cognitive benefits in middle-aged and older adults [22, 23]. To better understand the outcomes and mechanisms underlying effects of exercise on human brain function, objective measures of physical activity capacity such as peak oxygen consumption (VO2peak) and cardiorespiratory fitness (CRF) are very important [24]. Research has shown that increased CRF is associated with a larger hippocampal volume and better cognitive function scores in normal aging adults [25], improved functional connectivity in the default mode network [26], and is linked to a reduction in brain atrophy in patients with AD [27]. Similarly, randomized controlled trials suggest that higher intensity exercise, which results in increased CRF, is the optimal approach to improvement and maintenance of memory function in older adults [28–30]. In late-middle-aged adults with increased risk for AD due to family history and APOE4 status, six months of aerobic exercise training increased CRF in correlation with enhanced executive function [31]. Indeed, there is increasing evidence that CRF is an important indicator of brain health both during normal aging and in AD patients [32]. In addition, randomized controlled trial data suggest aerobic exercise may influence brain structure and function in older adults both with and without AD. In early AD, exercise-related increases in VO2peak are associated with improved memory performance and reduced hippocampal atrophy over 6 months [33]. Jeff Burns and colleagues showed that in older adults without cognitive impairment, a dose-response relationship likely exists between aerobic exercise and cognition, beginning with low doses (75 mins/week) and with increased benefits at higher doses (150 and 225 mins/week) in those who adhered to the exercise protocol [29]. Maximizing CRF may be an important therapeutic target for achieving cognitive benefits [34].
In a study of the benefits of acute exercise in chronic stroke survivors (>6 months post-stroke), Swathi Gujral and colleagues showed that an acute bout of exercise in chonic stroke survivors and age-matched controls had broad-based cognitive benefits (i.e., processing speed, memory, global cognition), regardless of intensity (low or moderate) or modality (aerobic or stretching). However, only aerobic exercise resulted in attentional and executive functioning benefits specifically in chronic stroke survivors but not in age-matched controls.
The link between exercise, CRF, cognition and dementia was discussed in this Workshop by Jeff Burns, Swathi Gujral, Eric Vidoni and Jill Morris.
CEREBROVASCULATURE
The mechanisms underlying the association between CRF and brain health remain under investigation. In a healthy brain, cerebral blood flow is augmented in response to a vasodilatory stimulus. Exercise modulates the cerebrovasculature, which may allow for better cerebral perfusion, delivery of oxygen, nutrients, neurotrophins, and other factors that may promote brain function. In animal studies, acute bouts of running increase cerebral blood flow (CBF) in several brain regions, including the hippocampus [35], and chronic voluntary wheel running enhances angiogenesis, neurogenesis and spatial memory function [36]. On the other hand, in mouse models of AD, cerebral hypoperfusion and inflammation are associated with memory impairment [37]. Similarly, cerebrovascular reactivity to CO2 is impaired in patients with mild cognitive impairment [38]. Reductions in global CBF and cerebrovascular reactivity may lower blood flow to critical areas of the brain and augment neurological disease burden [39]. In a collaborative study resulting from the Workshop, Jill Barnes, Sandra Billinger and Patrice Brassard combined data sets to show that middle cerebral artery blood velocity (MCAv) declines across the lifespan with older women showing the most rapid decline after the 6th decade of life [40]. Therefore, interventions are necessary to maintain CBF and cerebrovascular reactivity, and to potentially reduce or delay the onset of cognitive decline or Alzheimer’s disease. In humans, exercise may counteract the age-related decline in CBF [41] and cerebrovascular reactivity [42, 43] findings supported by a collaborative paper that resulted from the Workshop [44]. In addition, gadolinium contrast imaging in humans revealed hippocampal perfusion changes after long-term exercise in young [45] and older adults [46]. However, questions remain with regard to the exercise modality, intensity, duration of the intervention as well as the types of measurements used to assess CBF and cerebrovascular reactivity. In the Workshop various aspects of exercise effects on the cerebral vasculature was discussed by Jill Barnes, Paul Fadel, Jill Morris and Shawn Whitehead.
Cross-sectional studies examining the influence of habitual exercise on cerebrovascular function have reported conflicting results [43, 47–49]. The majority of studies assessing habitual aerobic exercise effects on cerebrovascular function have been performed in older adults. Furthermore, the underlying mechanisms by which exercise may impact the cerebral circulation remains unclear. To address these gaps in the literature, the Barnes lab designed a study to examine cerebrovascular function, determined as cerebrovascular reactivity to CO2, in healthy young adults who were either aerobically trained, resistance trained, or untrained. This is the first study to date, to evaluate cerebrovascular reactivity in resistance trained individuals and to directly compare across different modes of exercise. Contrary to the hypothesis, there was no difference in cerebrovascular reactivity to CO2 between untrained, aerobic trained, or resistance trained healthy young adults. The results suggest that exercise mode does not influence cerebrovascular reactivity in healthy young adults, but may influence resting cerebral hemodynamics in this population [50].
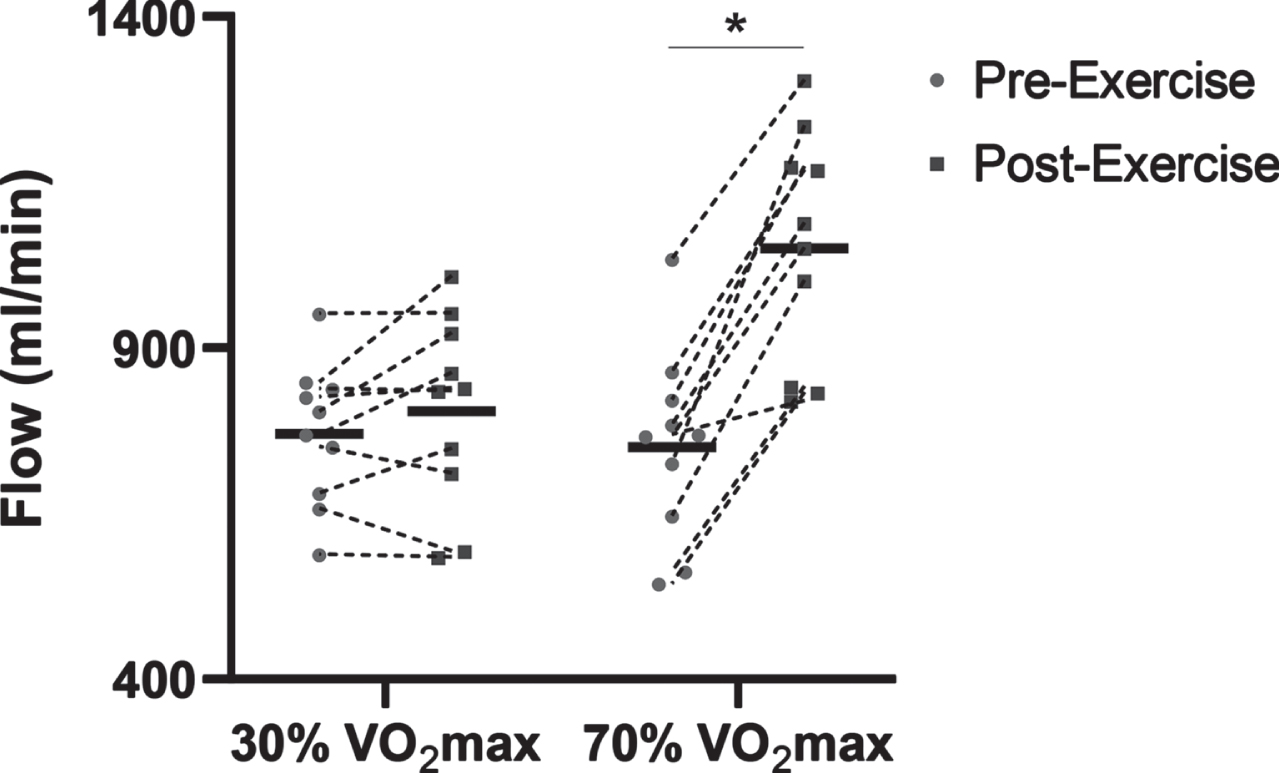
Although there were no differences in cerebrovascular reactivity between healthy adults with different modes of exercise, acute exercise is known to cause shifts in vascular function, and this may be dependent on exercise intensity. Specifically, the function of the peripheral blood vessels is acutely improved after a single bout of moderate intensity aerobic exercise in young adults, and it was hypothesized that the cerebral circulation would respond in a similar manner. Previous studies have shown that CBF during exercise is highest at moderate intensities, but is lower at near-maximal exercise [51]. Yet, it is still unclear what exercise “dose” is necessary to improve CBF and cerebrovascular reactivity after exercise. Therefore, a study to assess cerebral and peripheral vascular function after an acute bout of aerobic exercise at low (30% VO2max) and high (70% VO2max) intensity was conducted in young healthy adults. Data from the Barnes lab show that carotid artery blood flow increased immediately after high intensity exercise, whereas there was no change in carotid artery blood flow after low intensity exercise. This study will help inform future exercise protocols to improve cerebrovascular health in young adults, and then extend this protocol to middle-aged and older adults. This information will help determine the aerobic exercise dose that may be needed to improve cerebrovascular function, promote brain health, and reduce the risk of cognitive decline (Fig. 1).
Fig. 1
Preliminary data showing common carotid artery flow differences before (Pre-Exercise) and immediately after (Post-Exercise) 30 minutes of treadmill-based exercise in n = 10 young adults (4M:6F). The common carotid artery blood flow was significantly increased Post-Exercise with the 70% VO2max treadmill exercise. * P < 0.05 vs. Pre-Exercise.
In related work to understand the cerebrovascular dynamic response during an acute bout of exercise, Sandra Billinger, David C. Poole and colleagues developed a novel method for measuring cerebrovascular response from rest to moderate-intensity exercise in the middle cerebral artery (MCA) [52–54]. The resolution of the MCAv kinetic response has provided unique information for age- and sex-differences with a blunted response in older adults when compared to their younger counterparts (Fig. 2). For those with chronic conditions, the MCAv kinetic response may provide valuable insight into cerebrovascular function. For instance, stroke in the MCA negatively affects the MCAv kinetic response and those with stroke differ compared to age-and sex-matched sedentary individuals. Future work is needed to understand which exercise parameters (frequency, intensity, time and type) possess the greatest influence on cerebrovascular health. The downstream consequences, specifically in the capillary bed, of these slowed kinetics and reduced blood flow amplitude in response to increased metabolic demands have not been resolved, as described below.
Fig. 2
Typical middle cerebral artery velocity (MCAv) at rest and response following the onset of moderate intensity exercise (dashed vertical line, time 0). Solid circles are from a representative young healthy subject, Subject 2. Hollow circles are from an older healthy subject (Subject 20). Hollow squares are from stroke patient (Subject 201) using the ipsilateral MCA. By comparison, note very slow mean response time (MRT) and low amplitude of response in the older subject and absence of any response in the stroke patient [52].
![Typical middle cerebral artery velocity (MCAv) at rest and response following the onset of moderate intensity exercise (dashed vertical line, time 0). Solid circles are from a representative young healthy subject, Subject 2. Hollow circles are from an older healthy subject (Subject 20). Hollow squares are from stroke patient (Subject 201) using the ipsilateral MCA. By comparison, note very slow mean response time (MRT) and low amplitude of response in the older subject and absence of any response in the stroke patient [52].](https://content.iospress.com:443/media/bpl/2022/8-2/bpl-8-2-bpl220146/bpl-8-bpl220146-g002.jpg)
Effective blood-tissue oxygen delivery depends not only on bulk arterial blood flow but crucially on the capillary distribution of that flow. David C. Poole and colleagues have developed a contemporary model of capillary function that replaces extant Kroghian-based models, which more accurately describes oxygen transport especially in muscle and brain [55–58], (Fig. 3). Disease processes that impair red blood cell (RBC) distribution within the capillary bed, such as homogenization of flow, longitudinal recruitment of capillary surface area, capillary hematocrit and glycocalyx function, may restrict blood-tissue oxygen transport as much as a substantial reduction in bulk blood flow. However, present diagnostic approaches that permit clinicians to visualize large arteries and measure blood perfusion regionally cannot assess capillary distribution of that flow. Thus, accurate capillary hemodynamic models are desperately needed to provide insights into the impact of ageing, vascular risk factor and diseases such as Alzheimer’s disease (AD) on capillary function and oxygen transport (Fig. 4) over and above their impact on bulk blood supply [59, 60]. That large vessel alterations are absent in AD has resulted in the prevailing view that vascular impediments are not involved in its etiology: This despite the common risk factors for AD as for stroke and cardiovascular disease. Counter to this notion, capillary hemodynamic derrangements, that impair brain oxygen transport, were found in AD patients [61, 62]. These observations highlight a clear and pressing urgency for application of realistic capillary functional models (Fig. 3), in the steady-state and across metabolic transients, to the brain in health and disease. Key questions that such models will usefully address are listed in Table 1. Development of better visualization techniques and models, likely combined with machine learning, to better understand the role of microvascular dysfunction and oxygenation deficits in AD and other diseases is crucial for this undertaking.
Fig. 3
Contemporary model of brain capillary function developed in skeletal muscle [54] that demonstrates key features of the capillary bed crucial for blood-tissue O2 (and other substrates) exchange. It is anticipated that further development of these more accurate capillary function models will provide invaluable insights into dysfunction in diseases such as Alzheimer’s and dementia.
![Contemporary model of brain capillary function developed in skeletal muscle [54] that demonstrates key features of the capillary bed crucial for blood-tissue O2 (and other substrates) exchange. It is anticipated that further development of these more accurate capillary function models will provide invaluable insights into dysfunction in diseases such as Alzheimer’s and dementia.](https://content.iospress.com:443/media/bpl/2022/8-2/bpl-8-2-bpl220146/bpl-8-bpl220146-g003.jpg)
Fig. 4
Geometry of oxygen (O2) diffusion from red blood cell (RBC) in brain capillary to neurons, astrocytes and pericytes. Effective O2 supply is dependent upon adequate capillary RBC flux and its distribution as well as capillary hematocrit (generally lower than systemic hematocrit) as it determines capillary O2 diffusing capacity [144].
![Geometry of oxygen (O2) diffusion from red blood cell (RBC) in brain capillary to neurons, astrocytes and pericytes. Effective O2 supply is dependent upon adequate capillary RBC flux and its distribution as well as capillary hematocrit (generally lower than systemic hematocrit) as it determines capillary O2 diffusing capacity [144].](https://content.iospress.com:443/media/bpl/2022/8-2/bpl-8-2-bpl220146/bpl-8-bpl220146-g004.jpg)
Table 1
Brain Microcirculation: Pressing questions
| How does brain capillary RBC flux and distribution change from rest-↑metabolic rates in health and disease? |
| Is the necessary homogenization of capillary hematocrit across units impacted in disease? |
| How does this affect blood-tissue O2/substrate delivery? |
| What is the distribution of PO2 s across the capillary, interstitium and intracellular space at rest and during ↑ metabolic rates in health and disease? |
| What might be the role of pericytes in re-distributing RBC distribution? |
| How can regular exercise improve brain capillary function to improve O2 delivery? |
| What is the best exercise format –resistance, endurance and what frequency, duration and intensity? |
Key questions pertaining to brain microcirculation that the model of capillary function shown in Figure 3 could address.
In the Dementia Risk and Dynamic Response to Exercise clinical trial Eric Vidoni and colleagues are characterizing the acute exercise response in cerebral perfusion, and circulating neurotrophic factors in older adults with and without APOE4, the strongest genetic predictor for sporadic, late onset AD. Older adults will undergo single bouts of a moderate intensity exercise intervention. Pre- and post-exercise CBF will be examined using arterial spin labeling, as well as blood-based neurotrophic factors. Indeed, it is very important to accurately measure circulating factors that are responsive to acute exercise. Some of the most dynamic biomarkers, such as neurotrophins, are markedly affected by draw timings and post blood draw processing [63]. Moreover, Jill Morris and colleagues have shown that medication use can markedly affect mitochondrial bioenergetic outcomes, which may add another dimension to trial design in cognitively impaired individuals [64]. It is expected that APOE4 carriers will have poor CBF regulation and will demonstrate blunted neurotrophic response to exercise. Accurately understanding the acute effects of aerobic exercise on cognitive function and brain health is especially important for those at a higher genetic risk of Alzheimer’s disease (Fig. 5).
Fig. 5
Example of study flow for assessing markers of the acute effects of exercise using both MRI and blood-based biomarkers by Eric Vidoni and colleagues NCT04009629.
Exercise modality may also have effects on human cerebrovascular function [65]. In particular, Patrice Brassard addressed the growing interest in high-intensity interval training (HIIT) because of its efficacy in improving cardiovascular and metabolic functions, both in healthy individuals and in people with chronic disease. However, there are important knowledge gaps regarding the impact of acute and chronic HIIT on cerebrovascular function. For example, high-intensity exercise is usually associated with rapid elevations in arterial blood pressure, which could be directly transmitted to the brain without efficient neuroprotective mechanisms. Unless countered by the neuroprotective influences of sympathetic activation or cerebral autoregulation, these acute and repeated surges in blood pressure could potentially increase the risk of hyperperfusion injury predisposing to stroke or blood–brain barrier breakthrough (reviewed in [65, 66]). Recent research shows that the acute cerebral artery blood velocity response to a high-intensity exercise bout is biphasic in young fit individuals: a rapid elevation in cerebral artery blood velocity relative to baseline is followed by a return toward baseline values at the completion of exercise [67, 68]. Both of these studies also reported large elevations in cerebral artery blood velocity during the recovery period following high-intensity exercise. These results suggest that cerebral blood vessels of young fit individuals are challenged during and after a bout of high-intensity exercise. Further research is needed to evaluate how the cerebral vessels respond to rapid surges in blood pressure during and following high-intensity exercise in patients with cardiovascular and cerebrovascular diseases. Knowledge gaps also exist regarding the chronic effects of HIIT on the cerebrovascular function as recently highlighted in a systematic review [69]. Studies reported either no training effect (aerobic exercise training of varied nature and intensity including high-intensity exercise) on cerebral autoregulation in healthy older sedentary participants [70], or a subtle reduction in dynamic cerebral autoregulation following 6 weeks of HIIT to exhaustion in young endurance-trained men [71]. Further research showed an acute increase in MCAv during HIIT, in particular in women, which will further understanding the long-term implications of HIIT on cerebrovascular function in health and disease [72].
Another important consideration in studying cerebral vasculature is race. American Non-Hispanic Black (BL) individuals have a greater prevalence of cerebrovascular and neurocognitive conditions including cognitive dysfunction, and AD, relative to other racial groups. Although it is known that long-term exercise is important for cognitive function and overall brain health, the impact of race remains incompletely understood. This is important because the increased blood flow response leads to elevations in shear stress, a primary stimulus to improve vascular health. Recently, Paul Fadel and colleagues studied forearm blood flow responses to acute bouts of moderate and high-intensity rhythmic handgrip exercise and found that these were lower in young, healthy BL compared to White (WH) men, indicating a reduced peripheral vascular responsiveness [73]. Additional research found that BL men have an overall blunted ability to rapidly vasodilate in the peripheral circulation at the onset of exercise compared with young WH men [74]. Whether these reduced hyperemic responses to exercise are also present in BL women remains unclear. Heightened peripheral vasoconstriction and reduced vasodilation under resting conditions, highlight peripheral vascular differences in young BL compared to WH individuals. In studies performed mainly in WH individuals, cerebral blood flow increases during acute bouts of aerobic exercise. Whether this occurs in BL individuals is unknown. The impact of race on cerebral hemodynamics in response to acute exercise remains to be studied.
There is emerging evidence for a relationship between AD and cerebrovascular disease. Research presented by Shawn Whitehead proposes that white matter inflammation plays a role in linking vascular risk factors and cognitive impairment. Changes to brain volume and white matter integrity can be measured by magnetic resonance imaging (MRI) but may only be visible upon irreversible damage to neurons and axons. Through the application of positron emission tomography (PET) and radio labelled ligands targeting specific proteins, it may be possible to quantify physiological events, such as inflammation and synaptic degeneration, prior to manifestation of the aforementioned damage. Changes to the white matter, in particular chronic activation of microglia, in aging and following cerebrovascular stress, stroke and prodromal AD are related to cognitive impairment. Using a combination of behavioral testing, focused on executive dysfunction, live animal imaging with PET/MRI and brain histopathology, it is suggested that the vascular contribution to chronically activated microglia within the white matter may be prevented or ameliorated by exercise.
SLEEP
Diffusion weighted imaging has been used by J. Carson Smith and colleagues to show acute exercise induces microstructural plasticity within the hippocampus [75]. Hippocampal diffusion was elevated following an acute bout of exercise compared to seated rest; an effect not observed in a whole-brain average or in other control regions. Additional work showed that large-scale brain networks interact with acute exercise related effects on executive function and mood. The default mode network (DMN), where beta-amyloid aggregates in Alzheimer’s disease, the executive control network (ECN), which promotes inhibitory control and dual-task capabilities, and the salience network (SN, also called the cingulo-opercular network), which helps direct attention, is sensitive to emotion, and shows hyperactivation in mood disorders, all interact to support goal directed behavior. Using functional MRI, Smith and colleagues showed that aging-related changes in sleep may benefit from acute exercise through its impact on brain networks that regulate mood states [76]. Thirty minutes of moderate-intensity aerobic exercise increased positive affect and also reduced functional connectivity between the cingulo-opercular network and the hippocampus. Both of these effects after exercise were greater among older adults who showed greater sleep disturbance, measured by wrist actigraphy. Moreover, the exercise-related decrease in functional connectivity was found to mediate the correlation between greater sleep disturbance and exercise-related improvement in positive mood. Another study found that older adults with a longer total sleep time showed greater improvements in executive function after acute exercise, and this effect was mediated by greater volume of the caudate nucleus [77]. The effect of acute exercise on improvement in executive function has also been shown to be related to greater activation of the ECN during the performance of incongruent conditions on the Flanker task [78]. In summary, acute exercise seems to produce complex interactive effects among large-scale brain networks that regulate both mood and cognitive function in older adults, with greater exercise-related effects on mood in poor sleepers, and greater exercise-related effects on executive function in better sleepers.
ENERGY METABOLISM
Supply of energy in the form of ATP, produced from glucose by oxidative phosphorylation in mitochondria, and by aerobic glycolysis in the cytoplasm is needed for brain function. With aging there are deficits in brain energy metabolism. In particular, mitochondria are critically important for cell energy metabolism, calcium homeostasis, cell genesis, cell death and synaptic plasticity [79]. In the brains of AD patients, mitochondria have structural and functional deficits [80] and mitochondrial dysfunction may further AD pathology [81]. AD, and metabolic conditions such as diabetes, are associated with an upregulation in inflammatory markers leading to deficient microglial and astrocytic activation [82] which may affect mitochondrial function [83]. Aerobic exercise upregulates markers of mitochondrial biogenesis in aged mice [84] and reduces markers of inflammation [9, 85]. How different cell types, e.g. hippocampal progenitor cells [86], neurons, astrocytes, are affected by the effects of exercise on mitochondrial biogenesis remain to be defined and are being studied using novel genomic and proteomic approaches [87]. Russell Swerdlow, Jacob Haus and Benjamin Miller discussed aspects of these topics during the Workshop.
Proteostatic quality control mechanisms fail with age, resulting in the accumulation of damaged and dysfunctional proteins, including mitochondrial proteins [88, 89]. Protein breakdown and synthesis (collectively referred to as protein turnover) is the primary mechanism to mitigate accumulation of damaged proteins. Protein turnover is one of the most energetically costly cellular processes [90]. In turn maintaining efficient energy production relies on the maintenance of mitochondrial proteostasis. Therefore, targeting mitochondrial proteostasis and preserving cellular energetics may have significant benefits for tissue function. Aerobic exercise improves cognitive function in older individuals and those who exercise have reduced risk for developing neurodegeneration [91]. The protective benefits of exercise on cognition are well-established, but the underlying mechanisms are relatively unknown. In peripheral tissues, aerobic exercise stimulates mitochondrial turnover [92], increases antioxidant capacity and reduces accumulation of reactive oxygen species damage [93]. The limited research on the topic suggests these adaptations occur in the brain as well [94]. Benjamin Miller and colleagues are currently studying the impact of exercise on mitochondrial protein turnover in the brain. Key methodological developments include the ability to measure synthesis rates of individual proteins using stable isotopes and proteomic approaches, and cell-specific (e.g. neuron versus astrocyte) measurements of turnover [87].
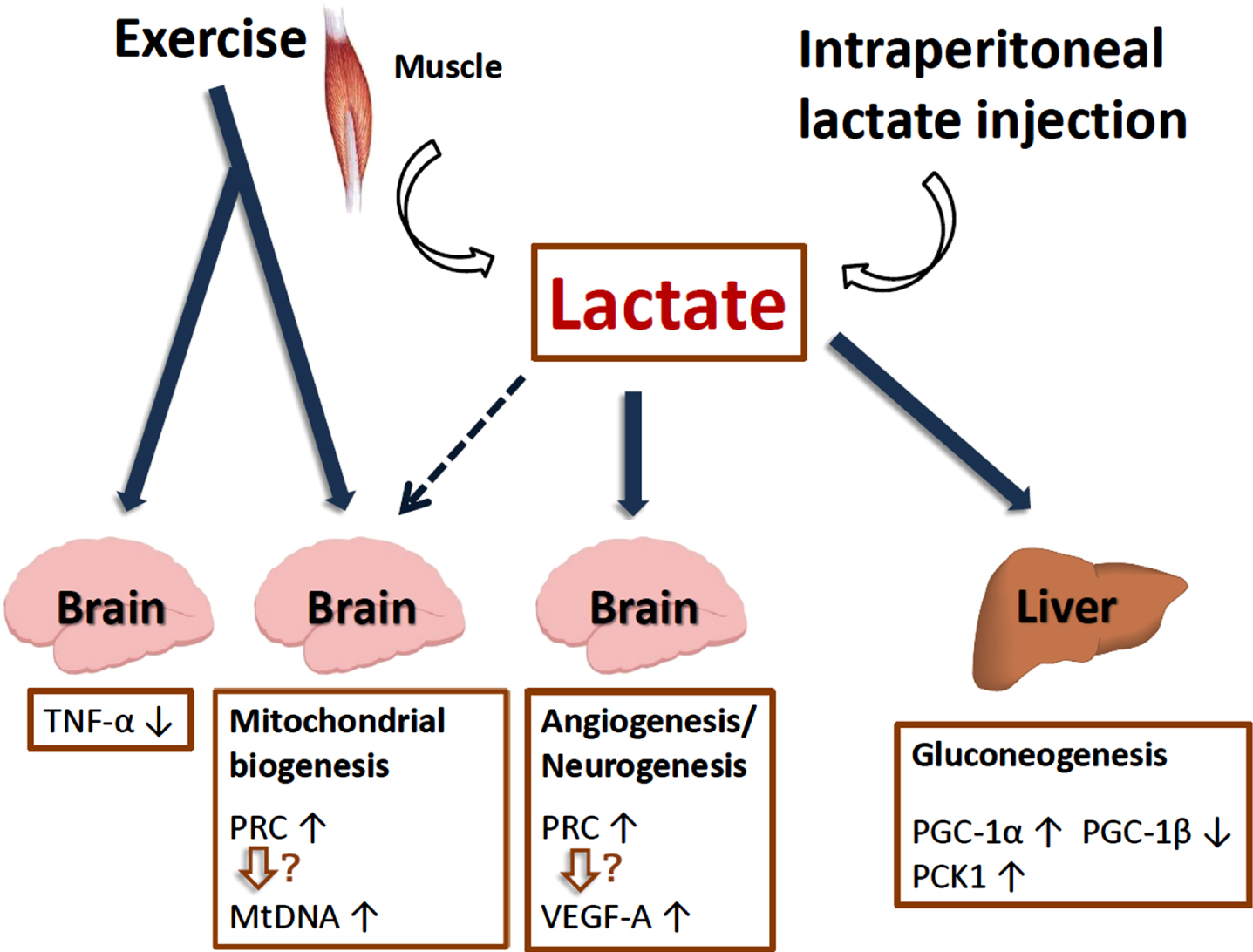
Lactate is a myometabolite produced via exercise that could potentially mediate molecular changes in non-muscle tissues, including mitochondrial biogenesis and turnover in the brain. Lactate can signal through the hydroxycarboxylic receptor (HCAR1), and is also provided to neurons by myelin and myelin-forming cells (oligodendrocytes) by coupling monocarboxylate transporters (MCT) 1 and 2 to neuronal processes [95, 96]. In research by Russell Swerdlow and colleagues, C57BL/6 mice underwent seven weeks of treadmill exercise sessions at intensities intended to exceed the lactate threshold. In liver, mRNA levels of gluconeogenesis-promoting genes increased. Peroxisome-proliferator-activated receptor-γ coactivator-1α (PGC-1α) expression increased, PGC-1β expression decreased, and overall gene expression changes favored respiratory chain down-regulation. In brain, PGC-1α and PGC-1β were unchanged but PGC-1 related co-activator (PRC) expression and mtDNA copy number increased. Brain TNF-α expression fell, and vascular endothelial growth factor (VEGF)-A expression rose. In adjunct experiments, exogenously administered lactate was found to reproduce some but not all observed liver and brain changes. Thus, lactate, an exercise byproduct, could mediate some of exercise’s extra-muscular effects (including the brain), and lactate itself can act as a partial exercise mimetic [84, 97]. Indeed, these findings are supported by studies showing that lactate plays a role in adult neurogenesis, hippocampal BDNF and VEGF-A expression, and learning and memory [98–100] (Fig. 6).
Fig. 6
Lactate plays a role in adult neurogenesis, hippocampal BDNF expression, mitochondrial biogenesis and reduction of inflammation.
Cognitive dysfunction is an emerging chronic complication of diabetes mellitus type 2, which was discussed by Jacob Haus and colleagues. The long-term risk of dementia, and progression of mild cognitive impairment (MCI) to dementia, increases in patients with diabetes. Even with prediabetes, there is an increased risk of dementia which is not related to the future development of diabetes. While the mechanisms for these trends are unclear, diabetes-related cognitive decline has been linked to the formation of advanced glycated end products (AGEs) and their receptors (RAGE). AGEs are a heterogenous class of chemical protein adducts that form spontaneously between amino acids and reactive carbonyl groups, such as sugar aldehydes, that disrupts protein charge and function. Circulating AGEs and other damage associated molecular pattern (DAMP) molecules (i.e. amyloid beta, HMGB1, S100 s) bind to RAGE which induces chronic inflammatory signaling. AGE formation and RAGE expression both increase under conditions of hyperglycemia and oxidative stress. Conversely, a number of inflammatory transmembrane cell surface proteins, such as RAGE, also exist as soluble forms (i.e. soluble RAGE, sRAGE) which suggests that their cleavage and release from the cell surface may be a regulatory mechanism. Individuals with metabolic derangement (i.e insulin resistance, elevated BMI, or increased fat mass) concurrent with cognitive impairment, present with aberrant sRAGE profiles [101]. These findings are consistent with prior reports demonstrating decreased plasma sRAGE in individuals with cognitive impairment, obesity, insulin resistance and type 2 diabetes [102–104] and further supports the protective role of sRAGE in inflammatory diseases. In addition, both acute and chronic exercise appears to be effective in attenuating AGE burden and reducing RAGE expression in metabolic tissues of patients with type 2 diabetes and prediabetes [105–107]. RAGE/sRAGE is a potential therapeutic target for the treatment of diabetes-associated cognitive decline and modulation of neuronal plasticity with exercise.
PERIPHERAL ORGAN-BRAIN CROSSTALK
There is increasing evidence that peripheral organs such as muscle, liver and the gut microbiome play a role in the effects of exercise on the brain. Skeletal muscle is of particular interest, it makes up more than 45% of total body weight, and is important for general health and wellness because of its primary role in movement, as well as in the control of systemic functions such as glucose homeostasis, thermoregulation and fat metabolism. Muscle adapts to a variety of signals to modify its size and function throughout life; however, the adaptability of muscle to increased loading is diminished with age. With aging and neurodegenerative conditions there is a loss of muscle mass and motorneuron function [108] as a result of deleterious changes to multiple components of the neuromuscular system. The degree to which loss of function and mass is triggered by motoneurons or muscle is unclear and is under investigation in the laboratory of Sue Bodine. In particular, the extent to which exercise can modify these changes during normal aging and in neurodegenerative conditions remain to be determined [108]. This is also important given that studies have shown that muscle weakness (sarcopenia) often coincides with cognitive decline [109]. Interestingly, recent research by Marcas Bamman and colleagues pertaining to Parkinson’s Disease, the most common motor neurodegenerative disease, shows that HIIT improves muscle, neuromuscular function and motor function as well as indices of brain neural activity, including increased substantia nigra and frontal activity as measured by fMRI, cognition, and emotional well-being [110]. These findings indicate that exercise is a powerful form of regenerative medicine [111]. However, it should be considered that exercise modality, strength or endurance, can produce distinct behavioral and neural plasticity phenotypes accompanied by differential gene expression in peripheral organs and the brain. Another consideration is the motivation to exercise. Frank Booth and colleagues have shown that signaling pathways within the rat nucleus accumbens may mediate voluntary wheel running motivation [112].
The benefits of exercise may be communicated to the brain via factors secreted from peripheral organs, comprehensively referred to as exerkines [113]. These include myokines [114, 115] such as VEGF [116] irisin [117–119] and Cathepsin B [30, 120–122] as well as the myometabolite lactate [98]. In addition, hepatokines (insulin-like growth factor ([123], clusterin [124], glycerophosphoryl diester phosphodiesterase-like protein [125]), adipokines [126], and blood platelets [127] derived from runners have been shown to play a role in neural plasticity or cognition [128–130]. Conversely, an unhealthy, high-fat and sugar diet can result in metabolic disturbances in the gut microbiome that impair learning and memory [131]. The human gut microbiome consists of billions of gut microbes that can communicate with the central nervous system [132, 133]. Changes and reduction in the diversity of gut microbiome composition have been observed in AD patients, and have been suggested to play a role in inflammatory processes associated with the disease [134–137]. Exercise training in humans increases the microbiome diversity [138], and contributes to delaying the progression of AD in a mouse model [139]. Another form of inter-organ crosstalk is being studied by Paige Geiger and colleagues who hypothesize that tissue crosstalk via transfer of extracellular vesicles (EV; exosomes and microvesicles) could underlie the benefits of exercise in AD patients. Recent studies of EVs have implicated these bilayer-phospholipid enclosed vesicles as key regulators of cell behavior and physiology, including nerve regeneration, synaptic function and behavior. Importantly, rodent studies have demonstrated that intracerebrally administered EVs can scavenge amyloid-β-peptide (Aβ) and these EVs can be deposited in microglia for degradation. Thus, interventions that enhance EV generation, secretion, or Aβ-scavenging could benefit AD patients. Recent findings suggest aerobic exercise improves EV clearance of Aβ in isolated neuronal cells, increases EV number in human serum, and that EVs carrying enhanced amounts of heat shock proteins (HSPs) may clear Aβ through a novel, microglial-independent pathway [129]. This research suggests a novel mechanism of ‘exercise-enriched’ EVs to prevent or delay the development of AD by mitigating protein aggregation, inflammation and cognitive decline (Fig. 7).
Fig. 7
Exercise can increase HSP content in EVs that travel to the brain and impact protein aggregation. HSP: heat shock proteins; EV: Extracellular Vesicles, Aβ: β-Amyloid.

Research pertaining to interactions between peripheral tissues and the brain was discussed by Marcas Bamman, Sue Bodine, Frank Booth, Paige Geiger, Henriette van Praag.
DIET AND BRAIN FUNCTION
The hypothalamus is considered as the main locus of the control of feeding behavior [140]. Neurons expressing the neuropeptide agouti-related peptide (AgRP) in the arcuate nucleus of the hypothalamus are essential for sensing and responding to metabolic signals; increased activity of these neurons is associated with increased food seeking and hunger. AgRP neurons also play a critical role in “top-down” regulation of insulin sensitivity and energy expenditure, thus the appropriate coupling of neuronal activity to metabolic need is likely critical for the maintenance of healthy body weight and metabolic function. Kristen O’Connell and colleagues have demonstrated that consumption of a‘Western’ diet is associated with hyperexcitability and leptin resistance in AgRP neurons, resulting in persistent activation and decoupling of neuronal activity with metabolic state, contributing to obesity and associated comorbidities. Successful therapeutics for improving metabolic health and body weight will likely restore the coupling between AgRP neuronal excitability and metabolic state. Common weight control interventions (low-fat diet, intermittent fasting, exercise) may play a role in restoring AgRP neuronal excitability. Importantly, composition of the diet plays a more important role than calorie intake therein [141].
The detrimental effects of a high-fat diet go beyond disruption of hypothalamic physiology. In addition to promoting obesity and associated comorbidities, excessive consumption of saturated fatty acids and refined sugars – hallmark dietary components of a Western diet – are associated with cognitive impairments in both humans and in experimental animal models [142]. Recent work by Scott Kanoski and colleagues has identified the juvenile and adolescent periods of development as particularly vulnerable stages for learning and memory impairments associated with Western diet consumption in rats. More specifically, free access to a Western diet during these early life developmental periods yields long-lasting deficits in learning and memory processes that rely on the integrity of the hippocampus. These effects appear to be based, in part, on dietary-induced changes in the microbiome. These memory deficits and microbiome changes persist well into adulthood despite a dietary intervention of switching the animals to a healthy control diet for an extended period of time [131]. Whether exercise can reverse or attenuate the gut dysbiosis and long-lasting cognitive impairments associated with early life Western diet consumption is under investigation.
Many signals that communicate nutrient status to the brain originate in the gastrointestinal (GI) tract. Nutrient entry into the GI tract initiates a myriad of physiological responses including secretion of several GI peptides that have paracrine, endocrine, and neuroendocrine action, and that function to aid in the processing and systemic assimilation of nutrients. The small intestine is richly innervated by the autonomic nervous system (ANS), but also contains an enteric nervous system (ENS) and many of the GI peptides secreted in response to a meal either have receptors expressed on local innervation or within the central nervous system (CNS). This has contributed to the idea that a “gut-brain axis” is integral to regulating feeding and body mass. Interestingly, Darleen Sandoval and colleagues have shown that many of the GI peptides that are secreted in response to meal, are also increased with stress. Glucagon like peptide-1, (GLP-1), predominantly secreted from specialized endocrine cells within the distal gut, is increased in response to various types of stress, including exercise [143]. Mice devoid of GLP-1 have impaired exercise capacity suggesting an important physiological role for GLP-1 in exercise performance. However, the exact function of GLP-1 during stress is unclear. Recent work has focused on understanding the source and function of GLP-1 and GLP-1 receptor signaling that increases with stress.
After food intake, amylin (a fibrillogenic protein), is co-secreted with insulin from pancreatic beta cells. Tameka Clemons and colleagues will study the impact of vigorous physical exertion and extended periods of time without food on uncoupling proteins and amylin interaction. Amylin has been shown to function in metabolism through its influence on glucose homeostasis, which includes sending satiety signals to the brain, inhibiting glucagon secretion and delaying gastric emptying. Amylin has also been proposed to have an impact on beta-amyloid. Alzheimer’s disease brains have been shown to have amyloid plaque formation and decreased expression of certain isoforms of uncoupling proteins (UCP). UCP are mitochondrial transporter proteins that create proton leaks across the inner mitochondrial membrane resulting in the dissipation of energy in the form of heat. UCP2 is expressed in pancreatic beta cells, which suggests an additional metabolic role for uncoupling proteins.
The role of diet was highlighted by Kristen O’Connell, Scott Kanoski, Darleen Sandoval and Tameka Clemons.
DISCUSSION AND NEXT STEPS
The Albert Trust Workshop provided new knowledge and discussion on the effects of exercise on cognitive function in the young and elderly. Some points of further discussion and collaboration were raised that included, but were not limited to the following items:
1. Optimizing exercise duration, type, therapeutic window (personalized medicine)
For instance, what is the best exercise modality, aerobic or aerobic plus resistance training? Does the acute response to exercise predict the chronic response to exercise? How do age, sex, trained vs. untrained, or other covariates influence exercise outcomes? Can we perform dose-response trials? Are there non-responder biologicalcharacteristics?
2. What are good proxy measures for effects of exercise on the brain?
What are the readouts from blood, muscle, imaging CSF, brain tissue? Are there outcome anchors that can be standardized, e.g. Dual-energy X-ray absorptiometry (DXA), VO2max, strength test, blood or muscle biomarkers? PD is a good example with Unified Parkinson’s Disease Rating Scale (UPDRS) which is an anchor.
3. Larger scale trials / pragmatic trials that are needed because they allow for more inclusion of subjects and therefore lessen the impact of interindividual variability (and for intervention to rise above the noise)
4. Exercise has multipotent systemic effects that provide feedback to the brain – animal and human studies need to understand the inter-organ interactions mediated by exosomes and exerkines.
5. How do you propose an exercise trial? How do you get reviewers of NIH grants to appreciate the value of the trial?
6. Animal exercise studies: which transgenic mouse models for AD and PD are optimal? Both mice and rats can serve as exercise models. Can we create better models?
7. Team science: collaborations are needed so we can pool data, share resources, samples and models to keep moving the field forward.
ACKNOWLEDGMENTS
The Leo and Anne Albert Charitable Trust fully supported the workshop that led to this publication. Grateful acknowledgment is made to Gene M. Pranzo, Trustee, Jeffrey Burns, scientific program chair and Susan Brogan, meeting planner, for their efforts in advancing awareness of cognitive dysfunction research.
FUNDING
The work was supported by P30AG072973, P30AG035982 (S. Billinger); by NIH R01DK109948, Sarns Family Gift to the University of Michigan School of Kinesiology (J.M. Haus); by NIH R01DK121995, NIH R01DK107282, and by an American Diabetes Association grant (1–19-IBS-252) (D.A. Sandoval), and in part, by NIH grants HL-50306, HL-137156, HL-108328, AG 078060 and AG 19228, the Johnson Cancer Research Center (A 21-0645) and a K-State College of Veterinary Medicine Sustained Momentum for Investigators with Laboratories Established (S.M.I.L.E.) Award (D.C. Poole); by the Ministère de l’Éducation, du Loisir et du Sport du Québec and the Fundation of the Institut Universitaire de Cardiologie et Pneumologie de Québec (P. Brassard).
CONFLICTS OF INTEREST
None to declare.
REFERENCES
[1] | Kontis V , et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet (2017) ;389: (10076):1323–35. |
[2] | Okonkwo OC , et al. Family history of Alzheimer disease predicts hippocampal atrophy in healthy middle-aged adults. Neurology (2012) ;78: (22):1769–76. |
[3] | Norton S , et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol (2014) ;13: (8):788–94. |
[4] | Spires-Jones T.L. , Hyman B.T. , The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron (2014) ;82: (4):756–71. |
[5] | Honig LS , et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N Engl J Med (2018) ;378: (4):321–30. |
[6] | Vaz M , et al. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin Interv Aging (2022) ;17: :797–810. |
[7] | Duzel E , van Praag H , Sendtner M Can physical exercise in old age improve memory and hippocampal function? Brain (2016) ;139: (Pt 3):662–73. |
[8] | Voss MW , et al Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci (2013) ;17: (10):525–44. |
[9] | Cotman CW , Berchtold NC , Christie LA Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci (2007) ;30: (9):464–72. |
[10] | van Praag H , Neurogenesis and exercise: past and future directions Neuromolecular Med (2008) ;10: (2):128–40. |
[11] | Adlard PA , et al Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci (2005) ;25: (17):4217–21. |
[12] | Wu CW , et al Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol (2008) ;105: (5):1585–94. |
[13] | Zhang X , et al. Treadmill Exercise Decreases Abeta Deposition and Counteracts Cognitive Decline in APP/PS1 Mice, Possibly via Hippocampal Microglia Modifications. Front Aging Neurosci (2019) ;11: , 78. |
[14] | Pa J , et al. Effects of Sex, APOE4, and Lifestyle Activities on Cognitive Reserve in Older Adults. Neurology (2022) ;99: (8):e789–e798. |
[15] | Buchman AS , et al Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology (2012) ;78: (17):1323–9. |
[16] | Tan ZS , et al. Physical Activity, Brain Volume, and Dementia Risk: The Framingham Study. J Gerontol A Biol Sci Med Sci (2017) ;72: (6):789–95. |
[17] | Erickson KI , et al Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A (2011) ;108: (7):3017–22. |
[18] | Baker LD , et al Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol (2010) ;67: (1):71–9. |
[19] | Kaufman CS , et al Aerobic exercise improves hippocampal blood flowfor hypertensive Apolipoprotein E4 carriers. J Cereb Blood Flow Metab (2021) ;41: (8):2026–37. |
[20] | Basso JC , Suzuki WA The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast (2017) ;2: (2):127–52. |
[21] | Vivar C , Potter MC , van Praag H All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci (2013) ;15: :189–210. |
[22] | Hayes SM , Forman DE , Verfaellie M Cardiorespiratory Fitness Is Associated With Cognitive Performance in Older But Not Younger Adults. J Gerontol B Psychol Sci Soc Sci (2016) ;71: (3):474–82. |
[23] | Pentikainen H , et al Cardiorespiratory Fitness and Cognition: Longitudinal Associations in the FINGER Study. J Alzheimers Dis (2019) ;68: (3):961–8. |
[24] | Basso JC , et al Examining the Effect of Increased Aerobic Exercise in Moderately Fit Adults on Psychological State and Cognitive Function. Front Hum Neurosci (2022) ;16: :833149. |
[25] | Erickson KI , et al Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus (2009) ;19: (10):1030–9. |
[26] | Voss MW , et al Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia (2010) ;48: (5):1394–406. |
[27] | Burns JM , et al Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology (2008) ;71: (3):210–6. |
[28] | Kovacevic A , et al The effects of aerobic exercise intensity on memory in older adults. Appl Physiol Nutr Metab (2020) ;45: (6):591–600. |
[29] | Vidoni ED , et al Dose-Response of Aerobic Exercise on Cognition: A Community-Based, Pilot Randomized Controlled Trial. PLoS One (2015) ;10: (7):e0131647. |
[30] | Gaitan JM , et al Effects of Aerobic Exercise Training on Systemic Biomarkers and Cognition in Late Middle-Aged Adults at Risk for Alzheimer’s Disease. Front Endocrinol (Lausanne) (2021) ;12: , 660181. |
[31] | Gaitan JM , et al Brain Glucose Metabolism, Cognition, and Cardiorespiratory Fitness Following Exercise Training in Adults at Risk for Alzheimer’s Disease. Brain Plast (2019) ;5: (1):83–95. |
[32] | Sobol NA , et al Change in Fitness and the Relation to Change in Cognition and Neuropsychiatric Symptoms After Aerobic Exercise in Patients with Mild Alzheimer’s Disease. J Alzheimers Dis (2018) ;65: (1):137–45. |
[33] | Morris JK , et al Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS One (2017) ;12: (2):e0170547. |
[34] | Szabo-Reed A , et al COMbined Exercise Trial (COMET) to improve cognition in older adults: Rationale and methods. Contemp Clin Trials (2022) ;118: , 106805. |
[35] | Nishijima T , Torres-Aleman I , Soya H Exercise and cerebrovascular plasticity. Prog Brain Res (2016) ;225: :243–68. |
[36] | van Praag H , et al Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci (2005) ;25: (38):8680–5. |
[37] | Cruz Hernandez JC , et al Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci (2019) ;22: (3):413–20. |
[38] | Tomoto T , et al Cerebral Vasomotor Reactivity in Amnestic Mild Cognitive Impairment. J Alzheimers Dis (2020) ;77: (1):191–202. |
[39] | Mattsson N , et al Association of brain amyloid-beta with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain (2014) ;137: (Pt 5):1550–61. |
[40] | Alwatban MR , et al Effects of age and sex on middle cerebral artery blood velocity and flow pulsatility index across the adult lifespan. J Appl Physiol (2021) ;130: (6):1675–83. |
[41] | Ogoh S , Ainslie PN Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol (2009) ;107: (5):1370–80. |
[42] | Miller KB , et al Cerebrovascular Reactivity and Central Arterial Stiffness in Habitually Exercising Healthy Adults. Front Physiol (2018) ;9: :1096. |
[43] | Barnes JN , et al Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J Appl Physiol (2013) ;114: (10):1383–7. |
[44] | Zeller NP , et al Sex-specific effects of cardiorespiratory fitness on age-related differences in cerebral hemodynamics. J Appl Physiol (2022) ;132: (5):1310–7. |
[45] | Pereira AC , et al An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A (2007) ;104: (13):5638–43. |
[46] | Maass A , et al Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry (2015) ;20: (5):585–93. |
[47] | Bailey DM , et al Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke (2013) ;44: (11):3235–8. |
[48] | Braz ID , et al Impact of aerobic fitness on cerebral blood flow and cerebral vascular responsiveness to CO2 in young and older men. Scand J Med Sci Sports (2017) ;27: (6):634–42. |
[49] | Zhu YS , et al Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and Masters athletes. J Cereb Blood Flow Metab (2013) ;33: (8):1190–6. |
[50] | Corkery AT , et al Influence of habitual aerobic and resistance exercise on cerebrovascular reactivity in healthy young adults. J Appl Physiol (2021) ;130: (6):1928–35. |
[51] | Smith KJ , Ainslie PN Regulation of cerebral blood flow and metabolism during exercise. Exp Physiol (2017) ;102: (11):1356–71. |
[52] | Billinger SA , et al Dynamics of middle cerebral artery blood flow velocity during moderate-intensity exercise. J Appl Physiol (2017) ;122: (5):1125–33. |
[53] | Ward JL , et al Effect of healthy aging and sex on middle cerebral artery blood velocity dynamics during moderate-intensity exercise. Am J Physiol Heart Circ Physiol (2018) ;315: (3):H492–H501. |
[54] | Witte E , et al Exercise intensity and middle cerebral artery dynamics in humans. Respir Physiol Neurobiol (2019) ;262: :32–9. |
[55] | Poole DC , et al. August Krogh’s theory of muscle microvascular control and oxygen delivery: a paradigm shift based on new data. J Physiol (2020) ;598: (20):4473–507. |
[56] | Poole DC , et al Exercise limitations in heart failure with reduced and preserved ejection fraction. J Appl Physiol (2018) ;124: (1):208–24. |
[57] | Angleys H , Ostergaard L Krogh’s capillary recruitment hypothesis, 100 years on: Is the opening of previously closed capillaries necessary to ensure muscle oxygenation during exercise? Am J Physiol Heart Circ Physiol (2020) ;318: (2):H425–H447. |
[58] | Poole DC , et al August Krogh: Muscle capillary function and oxygen delivery. Comp Biochem Physiol A Mol Integr Physiol (2021) ;253: :110852. |
[59] | Ostergaard L , et al Capillary transit time heterogeneity and flow-metabolism coupling after traumatic brain injury. J Cereb Blood Flow Metab (2014) ;34: (10):1585–98. |
[60] | Ostergaard L , et al Capillary dysfunction: its detection and causative role in dementias and stroke. Curr Neurol Neurosci Rep (2015) ;15: (6):37. |
[61] | Eskildsen SF , et al Increased cortical capillary transit time heterogeneity in Alzheimer’s disease: a DSC-MRI perfusion study. Neurobiol Aging (2017) ;50: :107–118. |
[62] | Nielsen RB , et al Capillary dysfunction is associated with symptom severity and neurodegeneration in Alzheimer’s disease. Alzheimers Dement (2017) ;13: (10):1143–53. |
[63] | White D , et al. A methodology for an acute exercise clinical trial called dementia risk and dynamic response to exercise. Sci Rep (2021) ;11: (1):12776. |
[64] | Morris JK , et al Collective effects of age, sex, genotype, and cognitive status on fitness outcomes. Alzheimers Dement (Amst) (2020) ;12: (1):e12058. |
[65] | Lucas SJ , et al High-intensity interval exercise and cerebrovascular health: curiosity, cause, and consequence. J Cereb Blood Flow Metab (2015) ;35: (6):902–11. |
[66] | Calverley TA , et al HIITing the brain with exercise: mechanisms, consequences and practical recommendations. J Physiol (2020) ;598: (13):2513–30. |
[67] | Curtelin D , et al Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J Cereb Blood Flow Metab (2018) ;38: (1):136–50. |
[68] | Labrecque L , et al Comparable blood velocity changes in middle and posterior cerebral arteries during and following acute high-intensity exercise in young fit women. Physiol Ree (2020) ;8: (9):14430. |
[69] | Whitaker AA , et al Effects of high intensity interval exercise on cerebrovascular function: A systematic review. PLoS One (2020) ;15: (10):e0241248. |
[70] | Lewis N , et al Cerebrovascular function in patients with chronic obstructive pulmonary disease: the impact of exercise training. Am J Physiol Heart Circ Physiol (2019) ;316: (2):H380–H391. |
[71] | Drapeau A , et al Six weeks of high-intensity interval training to exhaustion attenuates dynamic cerebral autoregulation without influencing resting cerebral blood velocity in young fit men. Physiol Rep (2019) ;7: (15):e14185. |
[72] | Whitaker AA , et al Cerebrovascular response to an acute bout of low-volume high-intensity interval exercise and recovery in young healthy adults. J Appl Physiol (2022) ;132: (1):236–46. |
[73] | Barbosa TC , et al Attenuated forearm vascular conductance responses to rhythmic handgrip in young African-American compared with Caucasian-American men. Am J Physiol Heart Circ Physiol (2018) ;315: (5):H1316–H1321. |
[74] | Kaur J , et al Attenuated Rapid-Onset Vasodilation to Forearm Muscle Contraction in Black Men. Med Sci Sports Exerc (2021) ;53: (3):590–6. |
[75] | Callow DD , et al Microstructural Plasticity in the Hippocampus of Healthy Older Adults after Acute Exercise. Med Sci Sports Exerc (2021) ;53: (9):1928–36. |
[76] | Alfini AJ , et al Impact of exercise on older adults’ mood is moderated by sleep and mediated by altered brain connectivity. Soc Cogn Affect Neurosci (2020) ;15: (11):1238–51. |
[77] | Won J , et al Caudate Volume Mediates the Interaction between Total Sleep Time and Executive Function after Acute Exercise in Healthy Older Adults. Brain Plast (2019) ;5: (1):69–82. |
[78] | Won J , et al Brain activation during executive control after acute exercise in older adults. Int J Psychophysiol (2019) ;146: , 240–8. |
[79] | Beckervordersandforth R , Mitochondrial Metabolism-Mediated Regulation of Adult Neurogenesis. Brain Plast (2017) ;3: (1):73–87. |
[80] | Swerdlow RH , Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J Alzheimers Dis (2018) ;62: (3):1403–16. |
[81] | Chakravorty A , Jetto CT , Manjithaya R Dysfunctional Mitochondria and Mitophagy as Drivers of Alzheimer’s Disease Pathogenesis. Front Aging Neurosci (2019) ;11: , 311. |
[82] | Heppner FL , Ransohoff RM , Becher B Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci (2015) ;16: (6):358–72. |
[83] | van Horssen J , van Schaik P , Witte M Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci Lett (2019) ;710: , 132931. |
[84] | E L , Burns JM , Swerdlow RH Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol Aging (2014) ;35: (11):2574–83. |
[85] | Wahl D , Cavalier AN , LaRocca TJ Novel Strategies for Healthy Brain Aging. Exerc Sport Sci Rev (2021) ;49: (2):115–25. |
[86] | Steib K , et al Mitochondria modify exercise-induced development of stem cell-derived neurons in the adult brain. J Neurosci (2014) ;34: (19):6624–33. |
[87] | Chucair-Elliott AJ , et al Inducible cell-specific mouse models for paired epigenetic and transcriptomic studies of microglia and astroglia. Commun Biol (2020) ;3: (1):693. |
[88] | Balch WE , et al Adapting proteostasis for disease intervention. Science (2008) ;319: (5865):916–9. |
[89] | Jayaraj GG , Hipp MS , Hartl FU Functional Modules of the Proteostasis Network. Cold Spring Harb Perspect Biol (2020) ;12: (1). |
[90] | Rolfe DF , Brown GC Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev (1997) ;77: (3):731–58. |
[91] | Voss MW , et al Exercise and Hippocampal Memory Systems. Trends Cogn Sci (2019) ;23: (4):318–33. |
[92] | Scalzo RL , et al Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J (2014) ;28: (6):2705–14. |
[93] | Powers SK , Nelson WB , Hudson MB Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med (2011) ;51: (5):942–50. |
[94] | Steiner JL , et al Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol (2011) ;111: (4):1066–71. |
[95] | Bergersen LH , Lactate transport and signaling in the brain:potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab (2015) ;35: (2):176–85. |
[96] | Zalc B The acquisition of myelin: An evolutionary perspective. Brain Res. (2016) ;1641: (Pt A):4–10. |
[97] | E L , et al Lactate administration reproduces specific brain and liver exercise-related changes. J Neurochem (2013) ;127: (1):91–100. |
[98] | El Hayek L , et al Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J Neurosci (2019) ;39: (13):2369–82. |
[99] | Hashimoto T , et al Effect of Exercise on Brain Health: The Potential Role of Lactate as a Myokine. Metabolites (2021) ;11: (12). |
[100] | Morland C , et al Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun (2017) ;8: , 15557. |
[101] | Fuller KNZ , et al Metabolic Derangements Contribute to Reduced sRAGE Isoforms in Subjects with Alzheimer’s Disease. Mediators Inflamm (2018) ;2061376. |
[102] | Fuller KNZ , et al A single high-fat meal alters human soluble RAGE profiles and PBMC RAGE expression with no effect of prior aerobic exercise. Physiol Rep (2018) ;6: (14):e13811. |
[103] | Miranda ER , et al Divergent Changes in Plasma AGEs and sRAGE Isoforms Following an Overnight Fast in T1DM. Nutrients (2019) ;11: (2). |
[104] | Miranda ER , et al Endogenous secretory RAGE increases with improvements in body composition and is associated with markers of adipocyte health. Nutr Metab Cardiovasc Dis (2018) ;28: (11):1155–65. |
[105] | Mahmoud AM , et al Aerobic Exercise Reduces Pro-inflammatory Cytokines in Skeletal Muscle via Downregulation of RAGE in Obese Insulin Resistant Adults. Diabetes (2015) ;64: , A200–A200. |
[106] | Mahmoud AM , et al RAGE Expression in Human Skeletal Muscle Is Normalized Following Aerobic Exercise Training. Diabetes (2013) ;62: , A14–A14. |
[107] | Haus JM , et al Acute Aerobic Exercise Increases Plasma Soluble Receptor of Advanced Glycation Endproducts (sRAGE) in Obese Insulin Resistant Adults. Diabetes (2015) ;64: , A205–A205. |
[108] | Miller BF , et al Muscle-specific changes in protein synthesis with aging and reloading after disuse atrophy. J Cachexia Sarcopenia Muscle (2019) ;10: (6):1195–209. |
[109] | Brinkley TE , et al Workshop on Synergies Between Alzheimer’s Research and Clinical Gerontology and Geriatrics: Current Status and Future Directions. J Gerontol A Biol Sci Med Sci (2018) ;73: (9):1229–37. |
[110] | Lavin KM , et al Rehabilitative Impact of Exercise Training on Human Skeletal Muscle Transcriptional Programs in Parkinson’s Disease. Front Physiol (2020) ;11: , 653. |
[111] | Sanford JA , et al Molecular Transducers of Physical Activity Consortium (MoTrPAC): Mapping the Dynamic Responses to Exercise. Cell (2020) ;181: (7):1464–74. |
[112] | Ruegsegger GN , et al Loss of Cdk5 function in the nucleus accumbens decreases wheel running and may mediate age-related declines in voluntary physical activity. J Physiol (2017) ;595: (1):363–84. |
[113] | Chow LS , et al Exerkines in health, resilience and disease. Nat Rev Endocrinol (2022) ;18: (5):273–89. |
[114] | Pedersen BK , Febbraio MA Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev (2008) ;88: (4):1379–406. |
[115] | F., R.M.a.D., Muscle-to-brain signaling via myokines and myometabolites. Brain Plasticity. 2022:1-21. |
[116] | Fabel K , et al VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci (2003) ;18: (10):2803–12. |
[117] | Wrann CD , et al Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab (2013) ;18: (5):649–59. |
[118] | Lourenco MV , et al Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med (2019) ;25: (1):165–75. |
[119] | Islam MR , et al Exercise hormone irisin is a critical regulator ofcognitive function. Nat Metab (2021) ;3: (8):1058–70. |
[120] | Moon HY , et al Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab (2016) ;24: (2):332–40. |
[121] | Mazo CEM , Edwin R , Shadiow J , Vesia M , Haus JM High Intensity AcuteAerobic Exercise Elicits Alterations in Circulating and SkeletalMuscle Tissue Expression of Neuroprotective Exerkines. BrainPlasticity (2022) ;8: , 5–18. |
[122] | Kim J , McKenna , Colleen F , Salvador AF , Scaroni SE , Askow AT , Cerna J , Cannavale CN , Paluska SA , De L , Michael P , Steven J , Burd NA , Khan NA Cathepsin B and Muscular Strength are Independently Associated with Cognitive Control. Brain Plasticity (2022) ;8: (1):19–33. |
[123] | Trejo JL , Carro E , Torres-Aleman I Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci (2001) ;21: (5):1628–34. |
[124] | De Miguel Z , et al Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature (2021) ;600: (7889):494–9. |
[125] | Horowitz AM , et al Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science (2020) ;369: (6500):167–73. |
[126] | Yau SY , et al Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci U S A (2014) ;111: (44):15810–5. |
[127] | Leiter O , et al Exercise-Induced Activated Platelets Increase Adult Hippocampal Precursor Proliferation and Promote Neuronal Differentiation. Stem Cell Reports (2019) ;12: (4):667–79. |
[128] | Townsend LK , MacPherson REK , Wright DC New Horizon: Exercise and a Focus on Tissue-Brain Crosstalk. J Clin Endocrinol Metab (2021) ;106: (8):2147–63. |
[129] | Von Schulze AT , et al Heat therapy: possible benefits for cognitive function and the aging brain. J Appl Physiol (2020) ;129: (6):1468–76. |
[130] | Formolo DA , Cheng T , Yu J , Kranz GS , Yau SY Central Adiponectin Signaling –A Metabolic Regulator in Support of Brain Plasticity. Brain Plasticity (2022) ;8: , 79–96. |
[131] | Noble EE , et al Gut microbial taxa elevated by dietary sugar disrupt memory function. Transl Psychiatry (2021) ;11: (1):194. |
[132] | Martin CR , et al The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol (2018) ;6: (2):133–48. |
[133] | Lledo PM , Combining Sensory Experiences with Internal Milieu in the Brain. Brain Plasticity. 2022;8. |
[134] | Zhuang ZQ , et al Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J Alzheimers Dis (2018) ;63: (4):1337–46. |
[135] | Vogt NM , et al Gut microbiome alterations in Alzheimer’s disease. Sci Rep (2017) ;7: (1):13537. |
[136] | Cattaneo A , et al Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging (2017) ;49: , 60–8. |
[137] | Connell E , et al Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol Neurodegener (2022) ;17: (1):43. |
[138] | Monda V , et al Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid Med Cell Longev (2017) ;2017: :3831972. |
[139] | Abraham D , et al Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp Gerontol (2019) ;115: :122–31. |
[140] | Alcantara IC , et al Acts of appetite: neural circuits governing theappetitive, consummatory, and terminating phases of feeding. NatMetab (2022) ;4: (7):836–47. |
[141] | Wei W , et al Diet composition, not calorie intake, rapidly altersintrinsic excitability of hypothalamic AgRP/NPY neurons in mice. SciRep (2015) ;5: :16810. |
[142] | Parent MB , et al Memory and eating: A bidirectional relationship implicated in obesity. Neurosci Biobehav Rev (2022) ;132: :110–29. |
[143] | Davis EM , Sandoval DA Glucagon-Like Peptide- Actions and Influence on Pancreatic Hormone Function. Compr Physiol (2020) ;10: (2):577–95. |
[144] | Roca J , et al Effects of training on muscle O2 transport at VO2max. J Appl Physiol (1992) ;73: (3):1067–76. |



