Cathepsin B and Muscular Strength are Independently Associated with Cognitive Control
Abstract
Although muscular strength has been linked to greater cognitive function across different cognitive domains, the mechanism(s) through which this occurs remain(s) poorly understood. Indeed, while an emerging body of literature suggests peripheral myokines released from muscular contractions may play a role in this relationship, additional research is needed to understand this link. Accordingly, this study sought to compare the influences of a particular myokine, Cathepsin B (CTSB), and muscular strength on hippocampal-dependent relational memory and cognitive control in 40 adults (age = 50.0±7.3 yrs). Overnight fasted venous blood draws were taken to assess plasma CTSB and muscular strength was assessed as maximal isokinetic strength testing using a Biodex dynamometer. Cognitive performance was assessed using a Spatial Reconstruction Task to assess relational memory and a modified Flanker task to assess cognitive control. Neuroelectric function for cognitive control was assessed using event-related potentials (ERPs) recorded during the Flanker task. Initial bivariate correlational analyses revealed that neither sex, age, lean body mass, or muscular strength was associated with CTSB. However, CTSB was inversely associated with reaction time and fractional peak latency of the P3 component of the Flanker task. Muscular strength was also inversely associated with reaction time and positively associated with relational memory performance. However, the influence of muscular strength on relational memory did not persist following adjustment for covariates. Greater circulating CTSB was selectively associated with greater cognitive control as well as faster information processing speed. These findings are the first to link circulating CTSB to both cognitive control and neuroelectric function. Future intervention studies are needed to examine the effects of changes in muscular strength, circulating myokines, and different domains of cognitive function.
INTRODUCTION
Regular engagement in physical activity is one of the most efficacious lifestyle approaches for en-hancing aerobic and muscular fitness [1, 2]. Furthermore, an increasing body of research suggests the benefits of physical activity may include greater cognitive function [3, 4]. Thus, it stands to reason that any cognitive benefits may be partially associated with enhanced aerobic and muscular fitness characteristics: physiological and functional outcomes of exercise interventions. Indeed, targeted exercise intervention studies have shown improvements in cognitive tasks designed to assess specific cognitive domains (e.g., cognitive control, memory) [5, 6]. Additionally, cross-sectional studies have found greater levels of aerobic fitness to be associated with enhanced performance during cognitive tasks [7, 8], improved brain structure [9, 10], and improved brain function as shown by neuroelectrical indices [11] for both adults [12] and children [13]. These benefits have been found to extend across different cognitive domains, including cognitive control [14] and hippocampal-dependent memory function [15].
The benefits to hippocampal-dependent memory function and cognitive control may be of particular importance, as both domains are critical for health and daily function. The hippocampus has been suggested to play a disproportionately important role in memory binding and relational memory processing. Furthermore, the hippocampus is able to communicate with other structures including the amygdala as part of the medial temporal lobe to bind converging inputs from various processors and mediate representations of the relationships between elements of scenes or events [16, 17]. This allows integration of the variety of different events that occur in daily life into relational frameworks [18, 19]. Cognitive control can be broadly defined as the ability to pursue goal-directed behavior regardless of more habitual or immediately-compelling behaviors. It is involved in a wide variety of cognitive functions including perception, action, decision making, planning, and problem solving [20]. Cognitive control has been widely accepted to be composed of three core functions: inhibition, working memory, and cognitive flexibility [21]. These functions support higher-order cognitive abilities such as problem solving and planning to ultimately allow individuals to learn, coordinate, and regulate actions [21]. In addition to behavioral performance measures (e.g., accuracy, reaction time), the use of event-related potentials (ERPs) to assess neuroelectrical activity provides insight into the effects of health behaviors, such as routine physical activity, on the neural underpinnings of cognitive control. ERPs are voltage deflections extracted from electroencephalographic (EEG) activity that occur in response to, or in preparation for, a stimulus or action and correspond to specific cognitive, sensory, or motor processes [22]. Specific to this study, the stimulus-locked amplitude and latency of a specific positive-going ERP component, called the P3 or P300, are thought to index the attentional resource allocation and information processing speed during task performance, respectively. Previous work has linked aerobic fitness and chronic exercise to the P3 component [23]. There is a growing support for the association between measures of aerobic fitness characteristics (e.g., VO2 peak) and cognitive health. Comparatively, there is a distinct lack in research examining the link between muscular fitness characteristics (e.g., peak knee extension torque) and cognitive outcomes.
Research suggesting resistance training improves executive [24] and episodic memory function [25] has begun to emerge, with a handful of studies also reporting changes in specific ERP components [26]. Nevertheless, it remains unclear whether muscular strength explains variance in cognitive function and neuroelectric function. This limitation presents challenges in developing effective intervention strategies to optimize cognitive function and maintain cognitive health using targeted exercise strategies. Further, while recent research has identified specific endocrine mediators that may be involved in this process, their links with different domains of cognitive function are unclear. Addressing this limitation is of particular importance, as the cognitive benefits associated with endurance and resistance training may occur through divergent molecular mechanisms [27]. Exercise-sensitive secreted bioactives are referred to as exerkines [28], with exerkines synthesized from skeletal muscle released in response to contraction categorized as myokines [28]. Thus, these myokines provide a potential peripheral pathway. Exercise-induced changes of the protease, cathepsin B (CTSB), is a potential myokine that has been suggested with neuro-cognitive improvements by Moon et al. They observed CTSB to be increased and associated with spatial object recognition, a function of hippocampal-dependent memory, after long-term running [29]. Subsequent studies have examined the association between exercise and CTSB and reported mixed results. Chronic exercise studies have reported no changes in serum CTSB following a six-week high intensity interval training intervention [30], an inverse association between habitual exercise and plasma CTSB [31], higher levels of CTSB in older athletes compared to age-matched sedentary controls [32], and increased CTSB following twelve-weeks of resistance training [33] and twenty-six weeks of moderate-to-vigorous aerobic training [34]. Acute exercise studies have reported more consistent findings with one study reporting a significant, albeit small, increase in CTSB following a single bout of high-intensity interval training [35] and another reporting increased CTSB following an acute bout of exercise for fencers but not swimmer or sedentary individuals [36]. Despite this growing body of literature, it is important to note that Moon et al. and Gaitan et al. are the only investigators who have directly examined the changes in CTSB after an exercise intervention and the association of this change with cognitive function [29, 34]. Unfortunately, there remains a paucity of knowledge regarding the influence of muscle strength, CTSB, and specific domains of cognitive function, and additional research is needed to comprehensively characterize the role of CTSB in cognitive function and brain health. Accordingly, the present work aimed to assess: 1) the association between muscular strength and hippocampal-dependent relational memory, cognitive control, and neuroelectric function; and 2) relationships between circulating CTSB and hippocampal-dependent relational memory, cognitive control, and neuroelectric function. The central hypothesis was that individuals with greater muscular strength [37, 38] and/or elevated CTSB plasma concentrations would exhibit greater cognitive function as evidenced by superior behavioral performance during a relational memory and cognitive control task. The secondary hypothesis was that the benefits of greater muscular strength and circulating CTSB would independently extend to neuroelectric function, as evidenced by greater amplitude and an earlier-shifted latency of the P3 component.
MATERIAL AND METHODS
Study population
Baseline secondary data were collected from 40 healthy middle-aged adults (50.0±7.3 yrs; 21 females) prior to participation in a previous intervention (clinical trial: NCT03029975) [39] to address the study aims. The study and its experimental procedures were approved by the University of Illinois Institutional Review Board and were in accordance with the use of human participants in research as outlined in the Declaration of Helsinki. Participants were eligible for the study if they were 40–64 years of age, were free of chronic cardiovascular or metabolic diseases, had a BMI between 18.5–35 kg·m–2, and did not participate in resistance exercise training over the past year. Exclusion criteria included musculoskeletal conditions or injuries in the previous year, excessive alcohol intake (> 10 drinks·wk–1), abnormal protein intake (< 0.66 or > 1.80 g kg–1·day–1), history of tobacco or marijuana use, reported use of medication or supplements known to affect measured outcomes, exercise or dietary restrictions, or uncontrolled hypertension. Dietary intake was examined through a 3-day diet record using the automated self-administered 24-hr (ASA24) dietary assessment tool (version 2016, National Cancer Institute, Bethesda, MD).
Procedures
Following screening, participants underwent blood pressure and anthropometrics assessment and completed several questionnaires to evaluate eligibility. Participants who met the inclusion criteria were familiarized with the protocol and equipment through an initial strength test session. For body composition assessment (by dual-energy X-ray absorptiometry (DEXA)), fasted blood collection, and muscle strength and performance testing, participants were instructed to abstain from strenuous exercise for 72 hours and fast overnight before visiting. For cognitive assessments, participants were instructed to fast for a minimum of 4 hours.
Body composition
DEXA scans (Hologic QDR 4500A, Bedford, MA) were performed to assess lean body mass (LBM). LBM was quantified by subtracting bone from total lean mass using the APEX (Hologic, Bedford, MA) software. Before initiating the scan, participants were instructed to void their bladder and remove any metal or other effects that may interfere with the analysis.
Blood collection and analysis
Venous blood was collected in EDTA-containing vacutainers and samples were centrifuged; the plasma was then frozen in –80°C for future analysis. Circulating CTSB (Abcam, Cambridge, UK) was analyzed by commercial enzyme-linked immunosorbent assay (ELISA) following manufacturer’s instructions [40].
Muscle strength assessment
Muscle strength was measured after blood collection. Participants were familiarized with the procedure and performed practice sessions prior to assessment of muscle strength as previously described [41]. Briefly, maximal voluntary isometric contraction (MVIC) at 60° and isokinetic peak torque at 60°·sec–1, 120°·sec–1, and 180°·sec–1 was assessed on the dominant leg with a Biodex dynamometer (Biodex System 3, Shirley, NY). Peak torque was calculated by the dynamometer software. [42]. Additionally, handgrip strength of the dominant hand (Sammons Preston Rolyan, Bolingbrook, IL) and lower body 1-RM were assessed on the leg press, and leg extension machine using previously established protocols [43].
Eriksen flanker task
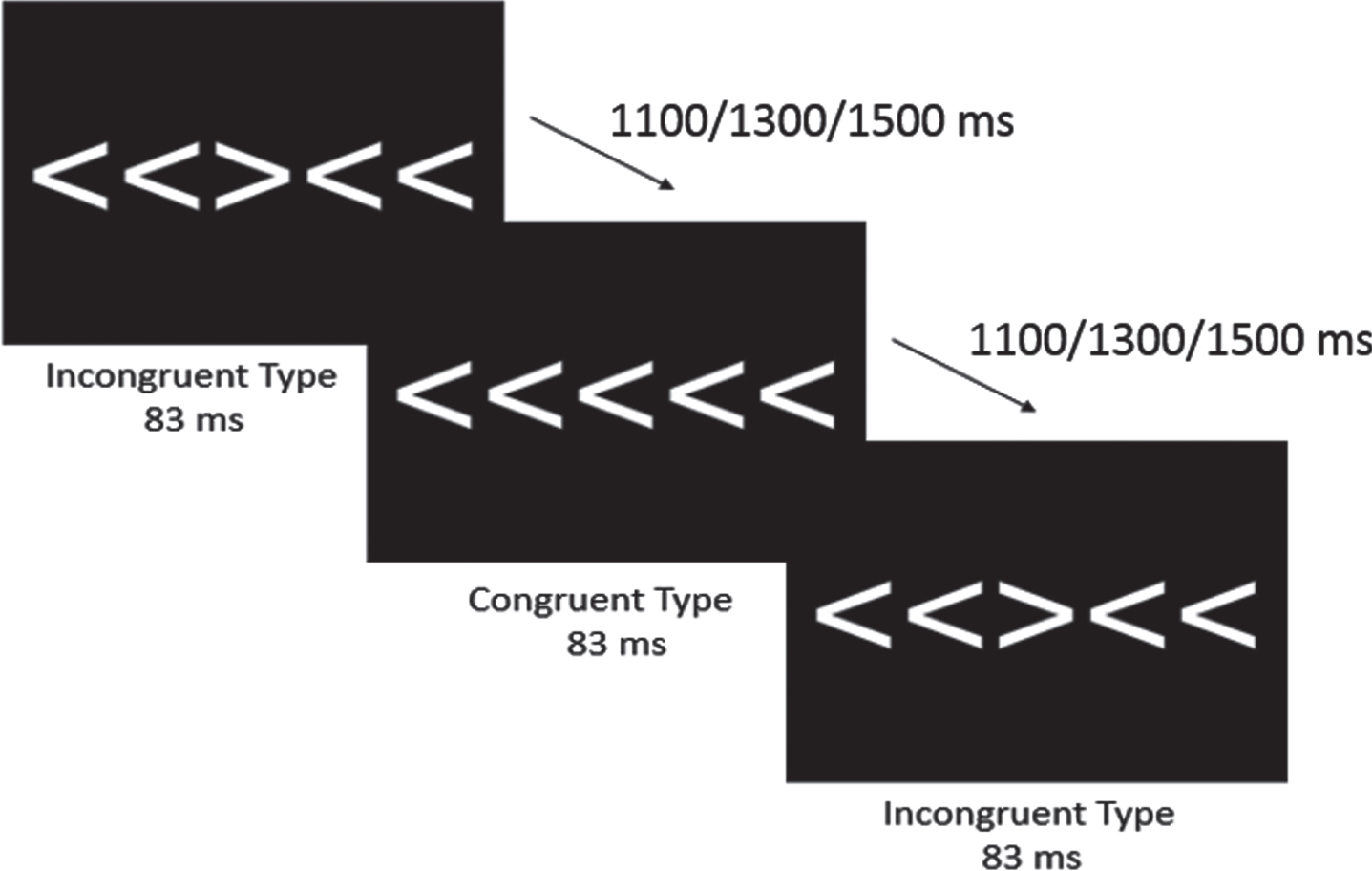
Attentional inhibition was assessed using a modified Eriksen Flanker task [44]. As shown in Fig. 1, participants viewed five 3 cm tall white arrows on a black computer screen directly in front of them and were instructed, using standardized language, to respond on a 4-button response pad (Current Designs, Philadelphia, PA, USA) to the direction of the central arrow that was flanked by two arrows on either side. Specifically, participants pressed the left-most button with their left thumb when the central arrow pointed to the left (e.g., ‘< ’) and pressed the right-most button with their right thumb when the central arrow pointed to the right (e.g., ‘> ’). Upon receiving the instructions, the task began with 40 practice trials followed by two blocks of 100 trials consisting of 50 randomly ordered congruent and incongruent trials. Congruent trials consisted of the flanking arrows facing the same direction as the central arrow (e.g., ‘> > > > > ’), while incongruent trials consisted of the flanking arrows facing the opposite direction of the central arrow (e.g., ‘> > < > > ’). The arrows were presented using the Neuroscan Stim software (Compumedics, Charlotte, NC) for 83 ms with a 1000 ms response window and equiprobable jittered inter-trial intervals of 1100, 1300, and 1500 ms. Behavioral measures of interest included mean accuracy and reaction time (RT) for both congruent and incongruent trials.
Fig. 1
Modified Eriksen Flanker Task. Both congruent and incongruent conditions were presented for 83 ms, with equiprobable jittered inter-trial interval of 1100, 1300, or 1500 ms.
Event-related potentials
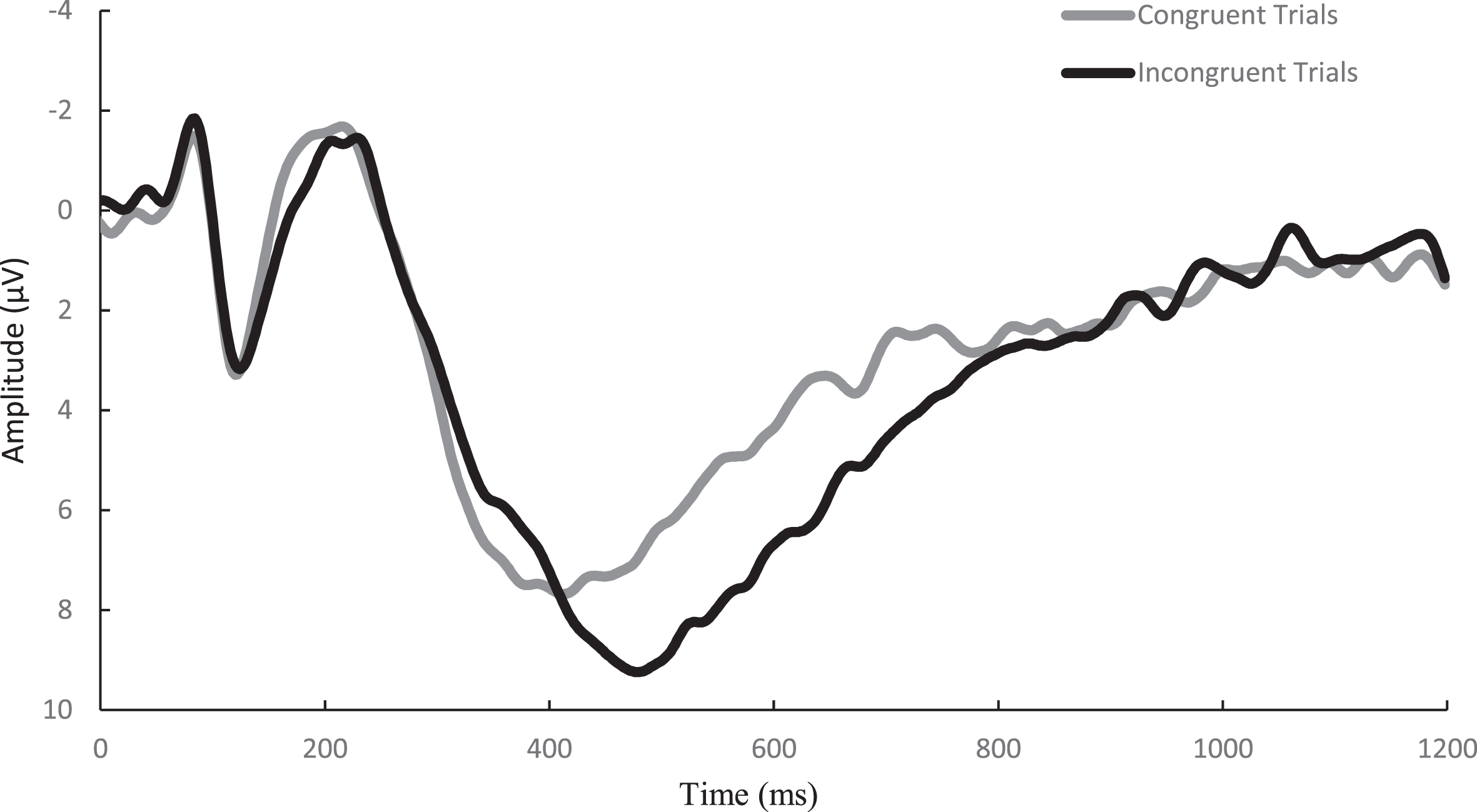
Similar to previously published studies [45], EEG activity was recorded across 64 sensors arranged in the international 10–20 system via a Neuroscan Quik-cap (Compumedics, Charlotte, NC). Electrooculographic (EOG) activity was recorded between a set of four electrodes placed at the outer canthus of each eye and above and below the left orbit in order to account for eye movement and blinks. A midline sensor placed between Cz and CPz served as a reference sensor and AFz served as the ground. Online, using a Neuroscan SynampsRT amplifier (Compumedics, Charlotte, NC), the continuous EEG signal was digitized at a sampling rate of 500 Hz and amplified 500 times to an online low-pass 70-Hz filter with a direct current and a 60-Hz notch filter. Impedance values for all electrodes were maintained ≤10 kohms. Signal processing and analysis were performed in Matlab (Mathworks, Natick, MA, version 2017b) using the EEGLab and ERPlab toolbox plug-ins [46, 47]. Offline, the EEG data were re-referenced to an average between the two mastoids and merged with behavioral data. The data were then submitted to a 0.1-Hz high-pass filter before using an independent components analysis (ICA) to reject eye-blink artifacts [48]. ICA and vertical EOG channel correlations greater than 0.35 were suggested to be eye-blinks and rejected. ICA-corrected data were segmented for each trial beginning –200 ms prior to the onset of the stimulus and continuing to 1200 ms post onset as stimulus-locked epochs. The –200 ms to stimulus onset was used as the baseline correction. Finally, data were filtered using a 30-Hz zero phase shift low-pass filter. Epochs were rejected if a moving window, defined as a 100-ms width and 50-ms step) peak-to-peak amplitude exceeded 100μV. Correct responses from individuals with artifact-free trials of greater than 80% across both the congruent and incongruent conditions were reported. Topographic grand average plots were constructed using a stylized topographic map plugin for EEGLAB/ERPLAB [49]. Based on evidence observed from post-hoc topographic images, a 6-sensor region of interest (ROI) comprised of C1, CZ, C2, CPZ, CP1, and CP2 electrodes was used to assess the P3 ERP component and is presented in Fig. 2. The P3 was defined as the localized respective positive peaks along with the corresponding latencies occurring between the 300–600 ms window post-stimulus onset. To ensure the assessment of the entire component, amplitude was measured as the area under the positively deflecting curve (AUC) between the 200–600 ms window for the P3. Similarly, the onset of each component was assessed through fractional peak latency (FPL), or the latency at which 50% of maximum amplitude was achieved prior to the onset to the maximal amplitude between the 200–600 ms window post-stimulus for the P3. A waveform depicting the congruent and incongruent waveforms at the P3 ROI is presented in Fig. 3.
Fig. 2
Topographical distribution plots of the P3 amplitude for the congruent and incongruent conditions, averaged from 300 to 600 ms post-stimulus. The 6-channel region of interest (C1, CZ, C2, CP1, CPZ, and CP2) outlined in black was used for analyses.
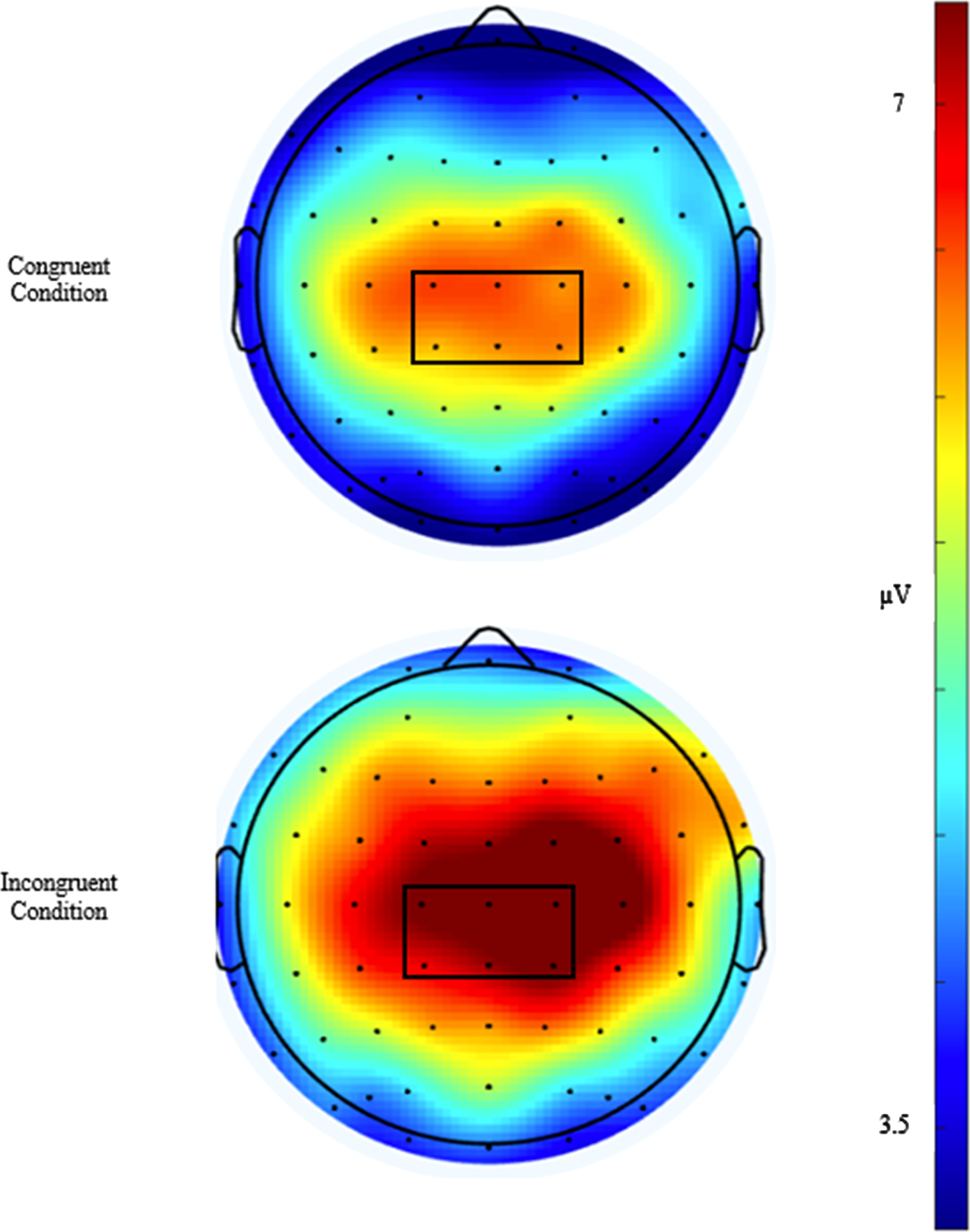
Fig. 3
Grand average waveform of the P3 for the congruent and incongruent trials of the Flanker task over the 6-sensor region of interest.
Spatial reconstruction task
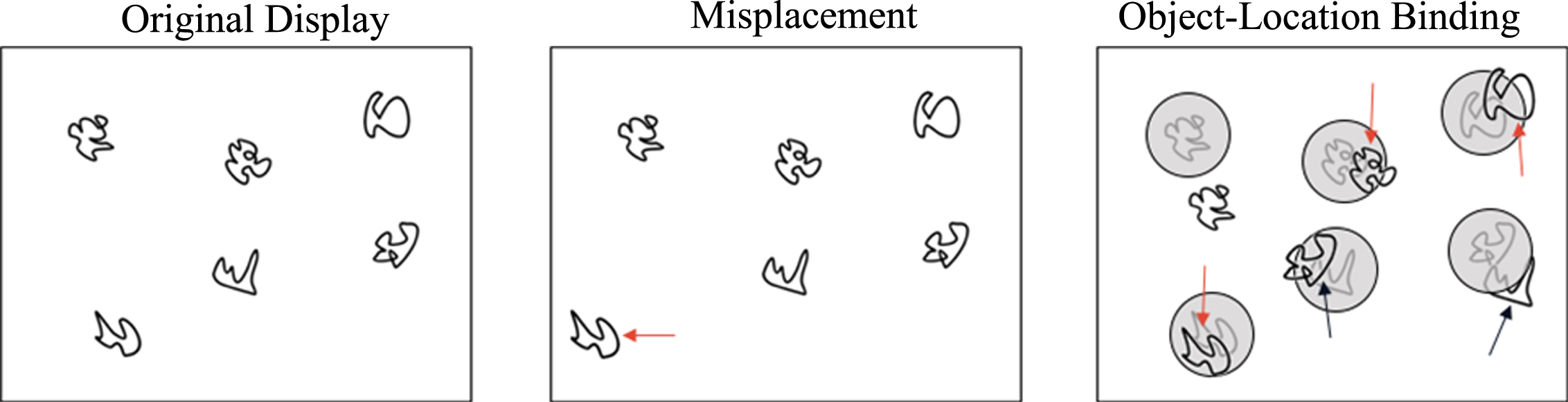
Hippocampal-dependent relational memory ability was assessed through a computerized spatial reconstruction task using Presentation Software (Neurobehavioral Systems, Berkeley, CA, USA). As shown in Fig. 4, participants were instructed to reconstruct an array of 6 abstract figures during the task. Briefly, participants were first introduced to the figures in the center of the screen that would later be used during reconstruction. After 6 s, the figures disappeared and reappeared in a random array on the screen. The identity of the figures was replaced by small squares and the participants were given 12 s to individually select each square to study its location. The squares disappeared and, after a 2 s fixation, the original figures were arranged in a row across the top of the screen. During the test phase, the participants were instructed to reconstruct the randomized array that was previously shown to them. Participants were not given a time limit to reconstruct the array and only moved on to the subsequent trial after they were satisfied with their reconstruction. Each participant completed a total of 20 trials (4 blocks of 5 trials each). Similar to previous publications [50], performance was assessed using two metrics: misplacement and object-location binding. A brief description of these metrics is depicted in Fig. 5. Specifically, misplacement was calculated as the average measure of distance (in pixels) between the objects studied and reconstructed locations, with a higher score indicating poorer performance. Object-location binding was defined as the number of times the participant correctly placed an item within a pre-defined radius around its studied location. Object-binding location was scored between 0-6 for each trial, with a higher score indicated better performance, and performance was averaged across trials.
Fig. 4
Hippocampal-dependent relational memory was assessed using a Spatial Reconstruction Task.
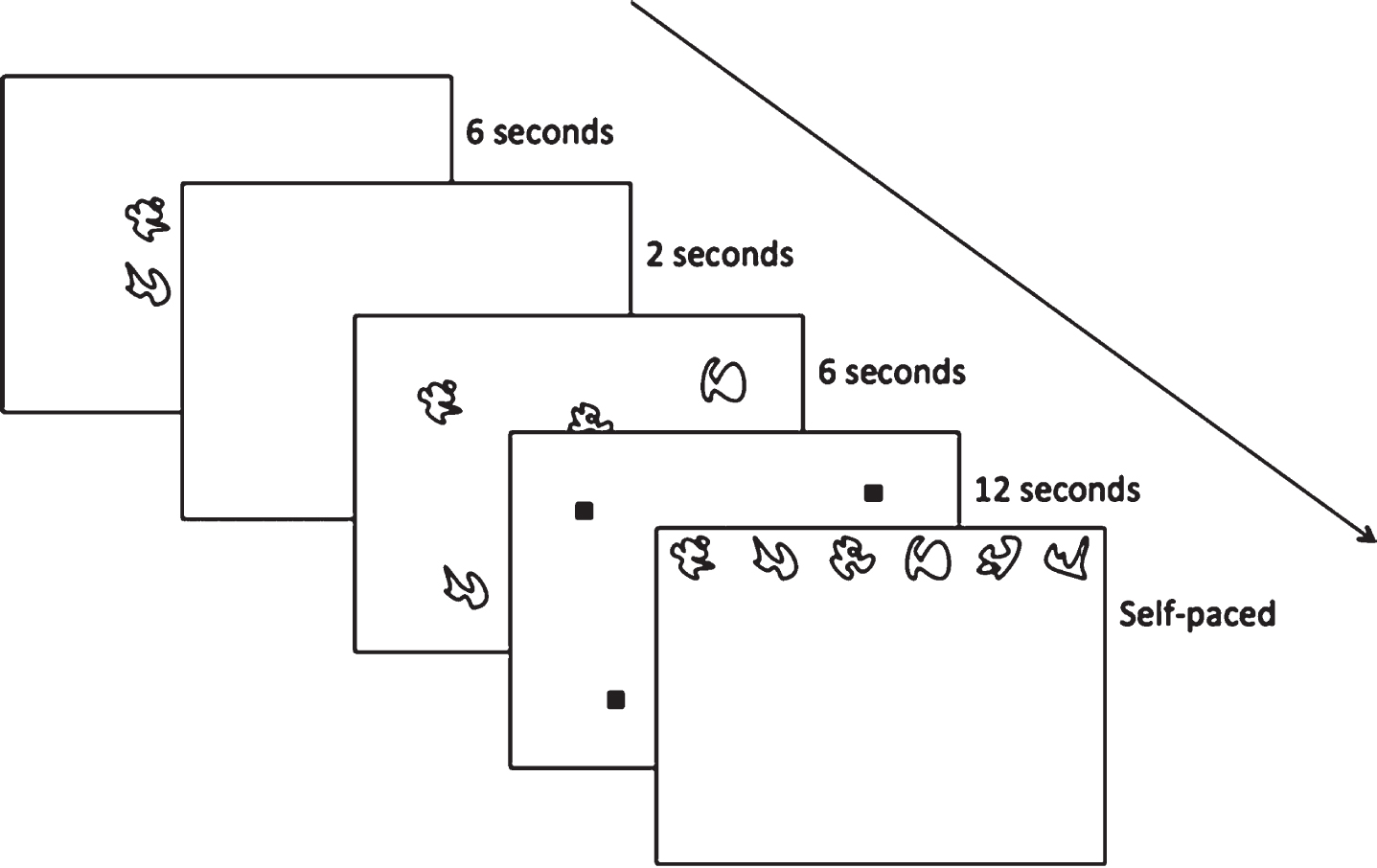
Fig. 5
Misplacement was calculated as the average distance (in pixels) between the original and reconstructed location. Object-binding location was defined as the number of times the participant correctly placed a figure within a pre-defined radius of its original location.
Statistical analyses
Based on a priori power calculation to estimate sample size (alpha = 0.05, beta = 0.80) for linear multiple regression, using an effect size of 0.35 (f2) determined that a total sample size of 32 participants would be sufficient for the primary cognitive outcomes. As the sample included a total of 40 participants, this study was adequately powered. Indeed, post hoc power analyses showed the power of the current sample to exceed 0.90. Isokinetic strength was measured as the aggregate of the respective z-scores of peak torque at each velocity. Normality was assessed using the Shapiro-Wilks test, Q-Q plots, histograms, and boxplot analysis. One outlier (exceeding 3 standard deviations from the mean) was removed from the Flanker behavioral task analysis. CTSB and the fractional peak latency of the congruent P3 were transformed using a negative reciprocal and reciprocal square root, respectively. Preliminary analyses to assess the associations between participant characteristics, CTSB, or muscular strength with behavioral outcomes and neuroelectrical indices were conducted through bivariate correlations. Subsequent analyses utilized a hierarchical linear regression model to assess the contribution of the primary variables of interest (e.g., CTSB, isokinetic strength) on cognitive outcomes while controlling for confounding variables based on bivariate correlations. Thus, for cognitive outcomes found to be significantly associated with either CTSB or isokinetic strength, the regression model is as follows: Step 1 consisted of the confounding variables determined via bivariate correlations, while Step 2 included both CTSB and isokinetic strength to ascertain whether CTSB and muscular strength significantly improved the model and/or were significant predictors following adjustment of covariates. Overall fit of the model was assessed through ANOVA F-statistic, with change in R2 between steps used to determine the change in variation that could be explained by the addition of each predictor variable. The influence of each predictor on behavioral and neuroelectrical outcomes was assessed through standardized β values. This analysis was performed separately for each dependent variable. For all analyses reported, alpha levels of p < 0.05 were specified to indicate statistical significance. Due to the novelty of the outcomes and smaller sample size, alpha levels of 0.05 < p < 0.10 were also specified to indicate trending statistical significance [51]. All analyses were performed using SPSS version 26 (IBM, Somers, NY).
RESULTS
Participant characteristics
Table 1 presents the characteristics of the participants. Analyses of bivariate correlations revealed sex (Male = 1, Female = 2) to be negatively correlated with all strength outcomes (p < 0.001), but positively associated with RT for the incongruent condition (r = 0.35, p = 0.035) and area under the curve for the congruent condition (r = 0.45, p = 0.006). Thus, males had greater strength, slower RT, and a reduced area under the curve during the congruent condition. Age had a significant negative association with congruent accuracy (rho = –0.35, p = 0.036) and a trend-level negative correlation was observed for fractional peak latency of the incongruent condition (r = –0.29, p = 0.091). LBM was positively correlated with all strength measures (p < 0.001) but negatively correlated with area under the curve for both the congruent (r = –0.48, p = 0.003) and incongruent (r = –0.34, p = 0.044) conditions. Furthermore, LBM was negatively correlated with incongruent RT at the trend-level (r = –0.28, p = 0.093). Relative protein intake was associated with incongruent accuracy (r = 0.36, p = 0.030) but no other outcomes including sex, age, or LBM. A summary of the correlation analyses can be found on Table 2.
Table 1
Participant Characteristics
| N = 40 | |
| Sex (F, M) | 21, 19 |
| Age | 50.0±7.3 |
| Lean Body Mass (kg) | 51.4±12.4 |
| Relative Protein Intake (g·kg–1·day–1) | 1.08±0.36 |
| Myokine | |
| CTSB (ng/mL) | 44.7±32.3 |
| Strength | |
| Knee Extension MVIC (N·m) | 179.5±70.8 |
| Leg Press 1-RM (kg) | 108.6±39.9 |
| Leg Extension 1-RM (kg) | 67.5±17.3 |
| Handgrip Strength (kg) | 42.1±13.4 |
| Knee Extension Torque 60°/sec (N·m) | 138.6±46.0 |
| Knee Extension Torque 120°/sec (N·m) | 98.3±40.4 |
| Knee Extension Torque 180°/sec (N·m) | 75.3±35.0 |
| Isokinetic Z-Score Sum | –0.14±2.7 |
Note: Data presented as mean±SD. MVIC = Maximal Voluntary Isometric Contraction.
Table 2
Correlations Between Task Outcomes and Participant Characteristics
| Variable | Sex | Age | LBM | CTSB | MVIC | Leg Press | Leg Extension | Grip Strength | Isokinetic Strength |
| CTSB | –0.01 | –0.06 | –0.08 | ||||||
| MVIC | –0.68* | –0.14 | 0.69* | –0.21 | |||||
| Leg Press | –0.71* | –0.24 | 0.68* | 0.09 | |||||
| Leg Extension | –0.82* | –0.20 | 0.77* | 0.05 | |||||
| Grip Strength | –0.70* | –0.17 | 0.66* | –0.17 | |||||
| Isokinetic Strength | –0.75* | –0.25 | 0.73* | –0.05 | |||||
| Relative Protein Intake | –0.04 | 0.05 | 0.03 | –0.01 | –0.03 | 0.02 | –0.17 | 0.09 | 0.16 |
| Congruent Accuracya | –0.07 | –0.35* | 0.14 | 0.19 | 0.05 | 0.00 | 0.09 | –0.01 | 0.24 |
| Incongruent Accuracy | –0.03 | –0.20 | –0.06 | 0.30† | –0.13 | 0.02 | –0.05 | 0.11 | 0.11 |
| Congruent RT | 0.25 | 0.07 | –0.13 | –0.44* | –0.26 | –0.15 | –0.18 | –0.24 | –0.46* |
| Incongruent RT | 0.35* | 0.15 | –0.28† | –0.39* | –0.42* | –0.20 | –0.27 | –0.37* | –0.55* |
| P3 Congruent AUC | 0.45* | 0.10 | –0.48* | 0.11 | –0.42* | –0.19 | –0.27 | –0.22 | –0.30† |
| P3 Incongruent AUC | 0.24 | 0.00 | –0.34* | 0.08 | –0.27 | –0.13 | –0.10 | –0.09 | –0.05 |
| P3 Congruent FPL | –0.03 | 0.04 | 0.04 | –0.35* | 0.30† | –0.12 | –0.10 | –0.08 | 0.03 |
| P3 Incongruent FPL | –0.08 | –0.29† | 0.14 | –0.39* | 0.27 | 0.12 | 0.05 | 0.16 | –0.01 |
| Misplacement | 0.16 | 0.16 | –0.17 | 0.08 | –0.08 | –0.14 | –0.23 | –0.05 | –0.32† |
| Object Location Binding | –0.24 | –0.12 | 0.27 | –0.04 | 0.18 | 0.20 | 0.31† | 0.11 | 0.34* |
Note: a –Congruent accuracy coefficients are presented as Spearman’s Rho as accuracy scores of the congruent condition exhibited a non-normal distribution following transformations. MVIC = Maximal Voluntary Isometric Contraction; RT = Reaction Time; AUC = Area under curve; FPL = Fractional peak latency; *P < 0.05, †< 0.10.
Cathepsin B (CTSB)
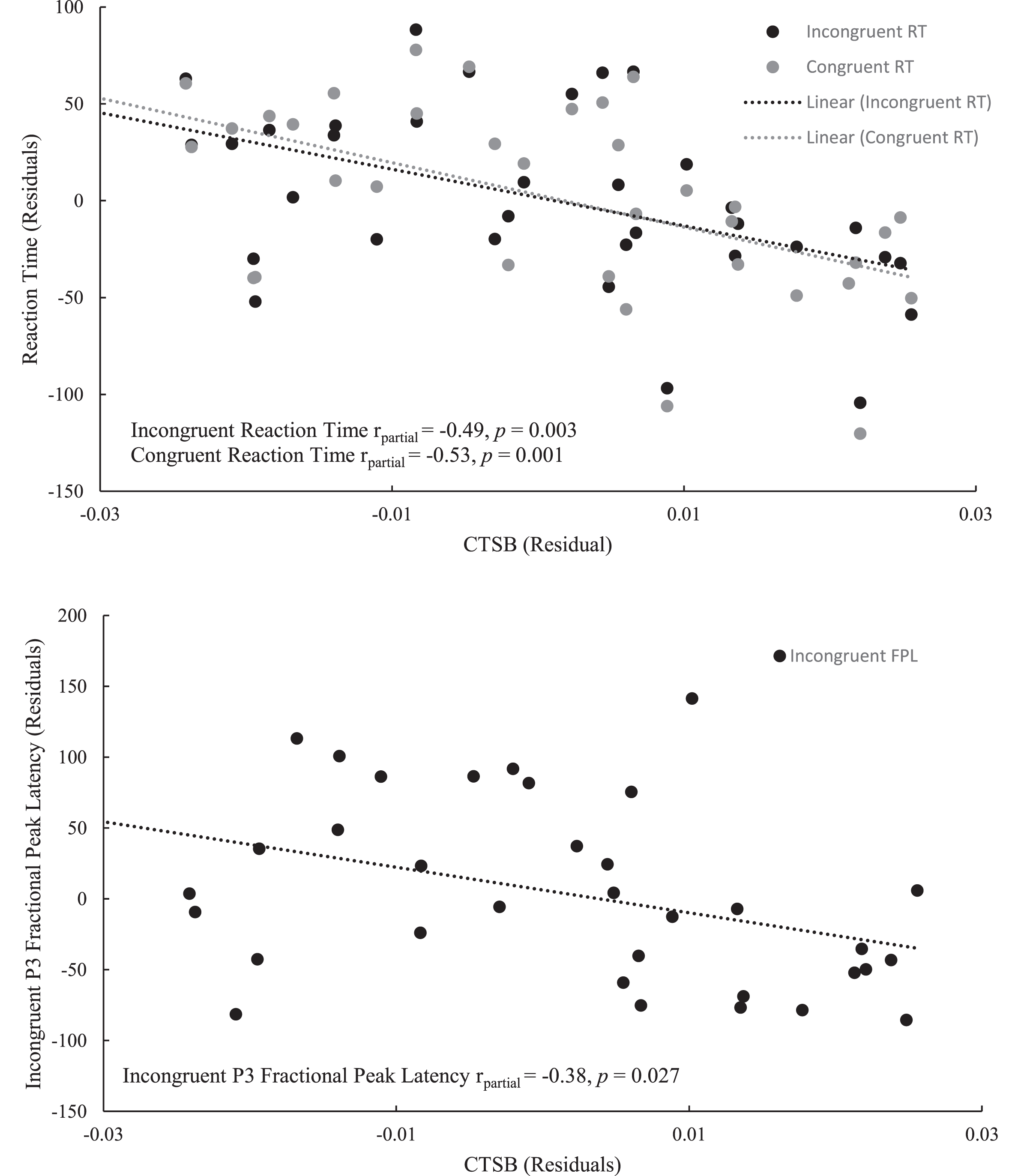
Bivariate correlation analyses revealed an association between CTSB and accuracy for the incongruent condition at the trend-level (r = 0.30, p = 0.083). CTSB was also negatively correlated with both congruent (r = –0.44, p = 0.007) and incongruent (r = –0.39, p = 0.019) RTs, as well as the fractional peak latency of the P3 component, for both congruent (r = –0.35, p = 0.042) and incongruent (r = –0.40, p = 0.019) conditions. Furthermore, CTSB was not found to be associated with either LBM (r = –0.09, p = 0.614), muscular strength (r = –0.05, p = 0.745), or relative protein intake (r = –0.01, p = 0.944). Results of the bivariate correlation analyses are presented in Table 2. Regression analyses revealed that neither sex nor LBM were associated with either congruent or incongruent RT. However, the association between CTSB and faster RT for both the congruent (β= –0.46, p = 0.001) and incongruent (β= –0.42, p = 0.003) trials of the Flanker task were sustained upon adjusting for covariates. Similarly, individuals with higher levels of CTSB had an earlier fractional peak latency, even after adjusting for muscular strength and the covariates of sex, LBM, and age (β= –0.44, p = 0.008). The results of the regression analyses for CTSB are presented in Table 3 with the partial correlation plots shown in Fig. 6.
Table 3
Regression analyses describing the association of CTSB and isokinetic strength with behavioral performance and event related potentials
| β | R2 | Adjusted R2 | Model P | ||
| Flanker Task | Congruent Reaction Time | ||||
| Step 1 | 0.08 | 0.02 | 0.28 | ||
| Sex | 0.42 | ||||
| Lean Body Mass | 0.21 | ||||
| Step 2 | 0.50 | 0.43 | < 0.01 | ||
| Sex | –0.07 | ||||
| Lean Body Mass | 0.34 | ||||
| CTSB | –0.46* | ||||
| Isokinetic Strength | –0.78* | ||||
| Incongruent Reaction Time | |||||
| Step 1 | 0.12 | 0.07 | 0.12 | ||
| Sex | 0.21 | ||||
| Lean Body Mass | 0.97 | ||||
| Step 2 | 0.50 | 0.44 | < 0.01 | ||
| Sex | –0.11 | ||||
| Lean Body Mass | 0.15 | ||||
| CTSB | –0.42* | ||||
| Isokinetic Strength | –0.76* | ||||
| Event Related Potentials | Incongruent FPL | ||||
| Step 1 | 0.09 | 0.01 | 0.38 | ||
| Sex | 0.04 | ||||
| Age | –0.27 | ||||
| Lean Body Mass | 0.14 | ||||
| Step 2 | 0.34 | 0.23 | 0.03 | ||
| Sex | –0.33 | ||||
| Age | –0.41* | ||||
| Lean Body Mass | 0.13 | ||||
| CTSB | –0.44* | ||||
| Isokinetic Strength | –0.48† | ||||
| Spatial Reconstruction Task | Misplacement | ||||
| Step 1 | 0.03 | –0.03 | 0.61 | ||
| Sex | 0.08 | ||||
| Lean Body Mass | –0.10 | ||||
| Step 2 | 0.12 | < 0.01 | |||
| Sex | 0.06 | ||||
| Lean Body Mass | –0.46† | ||||
| CTSB | –0.13 | ||||
| Isokinetic Strength | 0.07 | ||||
| Object-Location Binding | |||||
| Step 1 | 0.08 | 0.02 | 0.28 | ||
| Sex | –0.06 | ||||
| Lean Body Mass | 0.23 | ||||
| Step 2 | 0.12 | 0.01 | 0.40 | ||
| Sex | –0.02 | ||||
| Lean Body Mass | 0.34 | ||||
| CTSB | 0.10 | ||||
| Isokinetic Strength | 0.11 |
Note: *P < 0.05, †< 0.10.
Fig. 6
Partial correlation between CTSB and the Flanker outcomes: reaction time for both congruent and incongruent trials (above), P3 fractional peak latency for the incongruent trials (below).
Muscular strength
Relative protein intake was not associated with any strength measures (p > 0.05). Isokinetic strength was negatively associated with both congruent (r = –0.46, p = 0.005) and incongruent (r = –0.55, p = 0.001) RT. Furthermore, isokinetic strength was negatively correlated at the trend-level with misplacement (r = –0.32, p = 0.061) and positively correlated with object location binding (r = 0.35, p = 0.043). Analyses of neuroelectrical indices revealed isokinetic strength to have a trend-level negative correlation with area under the curve for the congruent trials (r = –0.30, p = 0.081). Regression analyses revealed that the relationship between greater isokinetic strength and faster RT during both the congruent (β= –0.78, p = 0.001) and incongruent (β= –0.79, p = 0.001) conditions of the Flanker task was sustained even after adjusting for CTSB as well as covariates. However, isokinetic strength was not associated with performance during the spatial reconstruction task nor neuroelectric indices upon adjusting for sex and LBM. A summary of the regression analyses for isokinetic strength is presented in Table 3. Other strength measures presented varied associations with cognitive outcomes. MVIC was negatively associated with incongruent RT (r = –0.42, p = 0.010) and area under the curve for the congruent condition (r = –0.42, p = 0.010) but positively associated with fractional peak latency of congruent trials (r = 0.30, p = 0.078) at the trend-level. Further regression analyses found the relationship between MVIC and incongruent RT to be sustained after adjusting for CTSB and covariates (β= –0.56, p = 0.010). However, the association between MVIC and congruent area under the curve was extinguished (β= –0.13, p = 0.599) after adjusting for covariates. Leg extension 1RM had a trend-level association with object binding location (r = 0.31, p = 0.067) but not behavioral or neuroelectrical outcomes of the Flanker task. Similar to MVIC, handgrip strength was negatively associated with incongruent RT (r = –0.37, p = 0.031) but was not associated with neuroelectrical outcomes. However, unlike MVIC, the relationship between handgrip strength and incongruent RT (β= –0.35, p = 0.116) was not sustained after adjusting for CTSB and covariates.
DISCUSSION
The present study aimed to address novel questions regarding the links between muscular strength, circulating CTSB, and cognitive function in healthy adults. CTSB and isokinetic strength were independently associated with faster RTs and information processing speed during an attentional inhibition task. To our knowledge, this is the first study to observe associations between the peripheral CTSB and neuroelectric function during cognitive control performance.
Due to the limited literature related to CTSB, its role in brain health and function remains unclear. While previous studies have demonstrated CTSB to have a role in hippocampal-dependent memory function [29, 31], its role across different cognitive domains has not been examined. Analysis of behavioral data in the current work revealed an inverse relationship between CTSB and RT across both the congruent and incongruent condition of the Flanker task, thereby suggesting that individuals with greater levels of CTSB respond faster. Furthermore, CTSB was also inversely associated with fractional peak latency of the P3 component across both conditions suggesting faster information processing speeds. Thus, this study reports the novel findings that CTSB may play a role in cognitive control by modulating processing speed. While these findings are promising, there remain significant challenges in elucidating the function of CTSB in the context of cognitive function due to the paucity of research. Regardless, these findings contribute to the current research on the association between CTSB and other biomarkers of cognitive health and neurological disorders. CTSB functions as a lysosomal hydrolase and has an important role in processing and degradation of neuronal proteins [52]. Recent research has also suggested CTSB may also indirectly influence neurogenesis [29]. Specifically, Moon et al. reported the addition of recombinant CTSB upregulated gene expression for doublecortin and brain-derived neurotrophic factor (BDNF) [29]. These genes and their associated proteins have previously been shown to support neurogenesis [53, 54], with studies also reporting that BDNF mediates improvements in cognitive control [55]. Therefore, CTSB may influence cognitive control by enhancing BDNF expression. Alternatively, CTSB may provide cognitive benefits in a manner independent of BDNF. Indeed, Alzheimer’s Disease (AD) research has suggested CTSB may provide some level of protection against the onset and progression of AD by degrading amyloid-β (Aβ) peptides [56]. These findings are pertinent to this study, as one of the trademarks of AD is impairment in cognitive control [57]. Further, Gaitan et al. reported increases in plasma levels of CTSB, but not BDNF, to be positively associated with changes in California Verbal Learning Test-II (CVLT) performance following a 26-week aerobic exercise intervention in individuals with AD [34]. Thus, it remains a distinct possibility that CTSB may directly influence cognitive control or through a pathway independent of BDNF. Clearly, more research is needed, as it remains unclear whether the effects of CTSB differ based on neurological status.
Muscular strength was also independently associated with cognitive control. Despite the emerging cognitive benefits of resistance training and the positive association between strength and cognitive control [38], the mechanisms through which the benefits are elicited are poorly understood. Consistent with these findings, isokinetic strength was positively associated with performance during the spatial reconstruction task and negatively associated with RT. Furthermore, both handgrip and isometric strength were associated with faster reaction times during incongruent trials. This indicates stronger individuals respond faster, particularly in conditions that require greater attentional inhibition, thus exhibiting greater cognitive control. Furthermore, upon controlling for possible confounding variables, both isometric and isokinetic strength were inversely associated with reaction time. However, the relationship between isokinetic strength and spatial reconstruction performance was not sustained following adjustment of covariates. Based on cross-sectional evidence, the findings of the present work are consistent with other works that suggest that resistance training may provide disproportionate benefits to cognitive control compared to other cognitive domains [6]. Additional experimental trials testing the effects of different exercise modes (e.g., aerobic and resistance training) on general and specific domains of cognitive function are necessary for confirmation of these potential benefits.
One of the unexpected findings was that there were no significant associations observed between CTSB and hippocampal-dependent memory function. While the positive association between isokine-tic strength and relational memory is consistent with previously reported studies [37, 58], after controlling for sex and LBM, the association was not sustained. Thus, while these results suggest strength may not be associated with memory performance, the strength magnitude of the correlations between sex, lean body mass, and strength challenges this interpretation. As such, it may be premature to draw such a conclusion. Furthermore, other factors in our sample may have altered this relationship, as this was primarily focused on middle-aged adults. Although the age range of this study sample has previously been suggested for early onset decline in muscle mass, strength [59], and cognition [60], this restriction in age range likely lessened the variability in function of this sample. As such, additional studies with larger samples across a wider age range would allow for a more comprehensive investigation on the role of muscular strength and myokines on cognitive function while better controlling for the impact of age. Additionally, CTSB was not associated with relational memory function. This is in contrast to a previously reported aerobic intervention study which found changes in CTSB concentration to be associated with improvements in memory function [29]. This inconsistency may be a result of differences in relational memory assessment. The aforementioned study assessed memory through the Rey-Osterrieth Complex Figure and Modified Complex Figure tasks before and after the intervention, respectively [29]. While both tasks have been validated as a measure of memory function [61], the complexity of the tasks has also been suggested to reflect cognitive control [62].
Several limitations should be considered while interpreting the findings of this work. First, this study does not exclude participants based on obesity. As a growing body of literature has reported the detrimental effects of obesity across different domains of cognitive function [63], the participant’s weight status may have contributed to the outcomes reported in this study. Another important limitation to this study is the cross-sectional nature of this study. Thus, it is not possible to infer causal effects between cognitive control and CTSB or muscular strength. In order to better understand the directionality and mechanisms of this association, preclinical and clinical experimental approaches are necessary. Ideally, studies should be designed to influence the possible mediators of exercise-induced cognitive changes so as to understand whether the benefits are driven by muscular adaptations, circulating myokines, or some other mechanism. For future preclinical research, tissue-specific CTSB knockout studies would clarify the sources of peripheral CTSB and their possible neurotrophic effects. For future clinical research, examining single-nucleotide polymorphisms (SNPs) of CTSB and their effects on allocation, neurotrophic benefits, exercise response, and risk of psychiatric disorders could shed greater light on the mechanism and function of CTSB. In addition, other variables such as IQ [65], education, or socio-economic status [66] have been shown to influence cognitive function across different domains and may have contributed to the findings of this study. However, this risk is somewhat mitigated as relevant variables based on bivariate analysis were adjusted for. Nevertheless, future efforts should account for additional variables that may influence the relationship between muscular strength, myokines, and cognitive function.
Beyond these limitations, differences in methodology may have affected the findings of this study. Previous studies have used different methods of quantifying CTSB, such as utilizing different measurement methods [30, 35] or different manufacturers [33]. Specifically, this study quantified the concentration of circulating CTSB while Nicolini et al. examined total CTSB [30, 35]. Although no study thus far has observed a specific manufacturer to provide more effective or reliable results to quantify CTSB, it is possible that the methodology used in this study could have influenced the reported outcomes.
These limitations and confounding factors not-withstanding, this study is the first to examine the relationship between cognitive control and CTSB in humans and presents multiple strengths including using highly validated and robust assessments of muscular strength and LBM, as well as the introduction of a brain-based measure to assess neuroelectric function. Isokinetic strength is the most reliable method of assessing strength to the extent that it is considered as the gold standard [67] and often used as a reference to compare other muscular strength assessment instruments [68]. Additionally, this study presents multiple different methods of strength assessment, including handgrip strength, which can be readily utilized in both research and clinical settings. Similarly, dual energy X-ray absorptiometry has been considered the reference standard in assessing LBM [69].
Research has repeatedly shown physical fitness (i.e., VO2 peak scores) to have a positive influence on cognitive health and function. However, the role of muscular strength and the endocrine mediators remain unclear. The work presented here provides important evidence demonstrating the possible role of circulating CTSB in supporting response times and neuroelectrical processing speed as reflected both in behavioral outcomes and neuroelectrical indices.
ACKNOWLEDGMENTS
This work was supported by the National Cattlemen’s Beef Association. The sponsor was only involved in financial support of the project, without involvement in design, data collection, and analysis, nor interpretation and dissemination of the report.
FUNDING
Authors have no funding to report.
CONFLICTS OF INTEREST
None of the authors have a conflict of interest to report.
REFERENCES
[1] | Cheng YJ , Macera CA , Addy CL , Sy FS , Wieland D , Blair SN . Effects of physical activity on exercise tests and respiratory function. Br J Sports Med [Internet]. (2003) [cited 2021 Nov 18];37: (6):521–8. Available from: https://bjsm.bmj.com/ |
[2] | Leblanc A , Taylor BA , Thompson PD , Capizzi JA , Clarkson PM , Michael White C , et al. Relationships between physical activity and muscular strength among healthy adults across the lifespan. Springerplus. (2015) ;4: (1):557. |
[3] | Jedrziewski MK , Lee VMY , Trojanowski JQ . Physical activity and cognitive health. Alzheimer’s Dement. (2007) ;3: (2):98–108. |
[4] | Etnier JL , Nowell PM , Landers DM , Sibley BA . A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. (2006) ;52: (1):119–30. |
[5] | Hoffmann CM , Petrov ME , Lee RE . Aerobic physical activity to improve memory and executive function in sedentary adults without cognitive impairment: A systematic review and meta-analysis. Prev Med Reports [Internet]. (2021) ;23: (October 2020):101496. Available from: https://doi.org/10.1016/j.pmedr.2021.101496 |
[6] | Landrigan JF , Bell T , Crowe M , Clay OJ , Mirman D . Lifting cognition: a meta-analysis of effects of resistance exercise on cognition [Internet]. Vol. 84, Psychological Research. Springer; (2020) [cited 2021 May 2]. pp. 1167–83. Available from: https://doi.org/10.1007/s00426-019-01145-x |
[7] | Barnes DE , Yaffe K , Satariano WA , Tager IB . A Longitudinal Study of Cardiorespiratory Fitness and Cognitive Function in Healthy Older Adults. J Am Geriatr Soc [Internet]. (2003) Apr 1 [cited 2021 Mar 21];51: (4):459–767. Available from: https://doi.wiley.com/10.1046/j.1532-5415.2003.51153.x |
[8] | Åberg MAI , Pedersen NL , Toré NEK , Svartengren M , Johnsson T , Cooper-Kuhn CM , et al. Cardiovascular fitness is associated with cognition in young adulthood. Proc Natl Acad Sci U S A [Internet]. 2009 [cited 2020 Apr 27];106: (49):20906–11. Available from: 10.1073pnas.0905307106 |
[9] | Walther K , Birdsill AC , Glisky EL , Ryan L . Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp [Internet]. (2010) Dec 8 [cited 2020 Feb 28];31: (7):1052–64. Available from: https://doi.wiley.com/10.1002/hbm.20916 |
[10] | Weinstein AM , Voss MW , Prakash RS , Chaddock L , Szabo A , White SM , et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. (2012) ;26: (5):811–9. |
[11] | Song T-F , Chi L , Chu C-H , Chen F-T , Zhou C , Chang Y-K . Obesity, Cardiovascular Fitness, and Inhibition Function: An Electrophysiological Study. Front Psychol [Internet]. (2016) Jul 27 [cited 2020 Apr 14];07: (JUL):1124. Available from: https://journal.frontiersin.org/Article/10.3389/fpsyg.2016.01124/abstract |
[12] | Dupuy O , Gauthier CJ , Fraser SA , Desjardins-Crèpeau L , Desjardins M , Mekary S , et al. Higher levels of cardiovascular fitness are associated with better executive function and prefrontal oxygenation in younger and older women. Front Hum Neurosci [Internet]. (2015) Feb 18 [cited 2021 Jun 21];9: (FEB):66. Available from: www.frontiersin.org |
[13] | Donnelly JE , Hillman CH , Castelli D , Etnier JL , Lee S , Tomporowski P , et al. Physical activity, fitness, cognitive function, and academic achievement in children: A systematic review. Vol. 48, Medicine and Science in Sports and Exercise. 2016. pp. 1197-222. |
[14] | Smith PJ , Blumenthal JA , Hoffman BM , Cooper H , Strauman TA , Welsh-Bohmer K , et al. Aerobic Exercise and Neurocognitive Performance: a Meta-Analytic Review of Randomized Controlled Trials. Psychosom Med. (2010) ;72: (3):239–52. |
[15] | Erickson KI , Voss MW , Prakash RS , Basak C , Szabo A , Chaddock L , et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. (2011) ;108: (7):Åberg, Maria A. I., et al. Cardiovascular Fitness. |
[16] | Cohen NJ , Eichenbaum H . Memory, Amnesia, and the Hippocampal System. MIT Press; (1993) . |
[17] | Eichenbaum H , Otto T , Cohen NJ . Does it still make sense to develop a declarative memory theory of hippocampal function? Behav Brain Sci. (1994) ;17: (3):492–3. |
[18] | Cohen NJ , Eichenbaum H . Memory, Amnesia and the Hippocampal System. MIT Press; (1995) . |
[19] | Eichenbaum H . Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. (2004) ;44: (1):109–20. |
[20] | Cohen JD . Cognitive Control: Core Constructs and Current Considerations. In: Egner T, editor. TheWiley handbook of cognitive control. John Wiley & Sons, Ltd; (2017) . pp. 1–28. |
[21] | Diamond A . Executive functions. Annu Rev Psychol [Internet]. (2013) Jan 2 [cited 2021 Mar 21];64: :135–68. Available from: www.annualreviews.org |
[22] | Luck SJ . An Introduction to the Event-Related Potential Technique. 1st ed. Cambridge, MA: Bradford Book; (2005) . |
[23] | Kao SC , Cadenas-Sanchez C , Shigeta TT , Walk AM , Chang YK , Pontifex MB , et al. A systematic review of physical activity and cardiorespiratory fitness on P3b. Psychophysiology. (2020) ;57: (7). |
[24] | Soga K , Masaki H , Gerber M , Ludyga S . Acute and Long-term Effects of Resistance Training on Executive Function. J Cogn Enhanc [Internet]. (2018) Jun 7 [cited 2021 Jun 15];2: (2):200–7. Available from: https://doi.org/10.1007/s41465-018-0079-y |
[25] | Loprinzi PD , Frith E , Edwards MK . Resistance exercise and episodic memory function: a systematic review [Internet]. Vol. 38, Clinical Physiology and Functional Imaging. Blackwell Publishing Ltd; (2018) [cited 2021 Jun 15]. pp. 923–9. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/cpf.12507 |
[26] | Herold F , Törpel A , Schega L , Müller NG . Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements - A systematic review [Internet]. Vol. 16, European Review of Aging and Physical Activity. BioMed Central Ltd.; (2019) [cited 2021 May 10]. pp. 1–33. Available from: https://doi.org/10.1186/s11556-019-0217-2 |
[27] | Cassilhas RC , Lee KS , Fernandes J , Oliveira MGM , Tufik S , Meeusen R , et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. (2012) ;202: :309–17. |
[28] | Severinsen MCK , Pedersen BK . Muscle–Organ Crosstalk: The Emerging Roles of Myokines [Internet]. Vol. 41, Endocrine Reviews. Endocrine Society; (2020) [cited 2021 Jun 15]. pp. 594–609. Available from: https://academic.oup.com/edrv594 |
[29] | Moon HY , Becke A , Berron D , Becker B , Sah N , Benoni G , et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. (2016) ;24: (2):332–40. |
[30] | Nicolini C , Toepp S , Harasym D , Michalski B , Fahnestock M , Gibala MJ , et al. No changes in corticospinal excitability, biochemical markers, and working memory after six weeks of high-intensity interval training in sedentary males. Physiol Rep. (2019) ;7: (11). |
[31] | De la Rosa A , Solana E , Corpas R , Bartrés-Faz D , Pallàs M , Vina J , et al. Long-term exercise training improves memory in middle-aged men and modulates peripheral levels of BDNF and Cathepsin B. Sci Rep. (2019) ;9: (1):1–11. |
[32] | Mucher P , Batmyagmar D , Perkmann T , Repl M , Radakovics A , Ponocny-Seliger E , et al. Basal myokine levels are associated with quality of life and depressed mood in older adults. Psychophysiology. (2021) ;58: (5):1–14. |
[33] | Sung K-Y , Kang S , Park JY , Park KM . Effects of Myokine Factors on Exercise Types in Obese Women. Exerc Sci. (2017) ;26: (4):275–80. |
[34] | Gaitán JM , Moon HY , Stremlau M , Dubal DB , Cook DB , Okonkwo OC , et al. Effects of Aerobic Exercise Training on Systemic Biomarkers and Cognition in Late Middle-Aged Adults at Risk for Alzheimer’s Disease. Front Endocrinol (Lausanne). (2021) ;12: (May):1–18. |
[35] | Nicolini C , Michalski B , Toepp SL , Turco C V , D’Hoine T , Harasym D et al. A Single Bout of High-intensity Interval Exercise Increases Corticospinal Excitability, Brain-derived Neurotrophic Factor, and Uncarboxylated Osteolcalcin in Sedentary, Healthy Males. Neuroscience [Internet]. (2020) ;437: :242–55. Available from: https://doi.org/10.1016/j.neuroscience.2020.03.042 |
[36] | Gokce E , Gunes E , Ari F , Hayme S , Nalcaci E . Comparison of the effects of open- And closed-skill exercise on cognition and peripheral proteins: A cross-sectional study. PLoS One. (2021) ;16: (6 June):1–17. |
[37] | Sui SX , Holloway-Kew KL , Hyde NK , Williams LJ , Leach S , Pasco JA . Muscle strength and gait speed rather than lean mass are better indicators for poor cognitive function in older men. Sci Rep [Internet]. (2020) Dec 1 [cited 2021 Jun 18];10: (1):1–9. Available from: https://doi.org/10.1038/s41598-020-67251-8 |
[38] | Frith E , Loprinzi PD . The Association between Lower Extremity Muscular Strength and Cognitive Function in a National Sample of Older Adults. J Lifestyle Med [Internet]. (2018) Jul 31 [cited 2021 May 16];8: (2):99–104. Available from: /pmc/articles/PMC6239135/. |
[39] | McKenna CF , Salvador AF , Hughes RL , Scaroni SE , Alamilla RA , Askow AT , et al. Higher protein intake during resistance training does not potentiate strength, but modulates gut microbiota, in middle-aged adults: A randomized control trial. Am J Physiol - Endocrinol Metab [Internet]. (2021) [cited 2021 Jul 12];320: (5):E900–13. Available from: https://www.ajpendo.org |
[40] | Thangavelu B , Boutté AM . Single Molecule Assay for Ultrasensitive Detection of Cathepsin B in Human Blood. 2021 [cited 2021 Nov 8]; Available from: https://doi.org/10.1021/acsomega.1c00180 |
[41] | Cramer JT , Jenkins NDM , Mustad VA , Weir JP . Isokinetic Dynamometry in Healthy Versus Sarcopenic and Malnourished Elderly: Beyond Simple Measurements of Muscle Strength. J Appl Gerontol. (2017) ;36: (6):709–32. |
[42] | De Ruiter CJ , Kooistra RD , Paalman MI , De Haan A . Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol [Internet]. (2004) Nov [cited 2021 Jun 30];97: (5):1693–701. Available from: https://www.jap.org1693 |
[43] | Verdijk LB , Van Loon L , Meijer K , Savelberg HHCM . One-repetition maximum strength test represents a valid means to assess leg strength in vivo in humans. J Sports Sci [Internet]. (2009) [cited 2021 Dec 1];27: (1):59–68. https://www.tandfonline.com/doi/abs/10.1080/02640410802428089 |
[44] | Eriksen BA , Eriksen CW . Effects of noise letters upon the identification of a target letter in a nonsearch task. Vol. 16, Perception & Psychophysics. 1974. |
[45] | Edwards CG , Walk AM , Thompson S V , Reeser GE , Dilger RN , Erdman JW et al. Dietary lutein plus zeaxanthin and choline intake is interactively associated with cognitive flexibility in middle-adulthood in adults with overweight and obesity. Nutr Neurosci [Internet]. 2021 [cited 2021 May 3]; Available from: https://www.tandfonline.com/doi/abs/10.1080/1028415X.2020.1866867 |
[46] | Delorme A , Makeig S . EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. (2004) ;134: (1):9–21. |
[47] | Lopez-Calderon J , Luck SJ . ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci [Internet]. (2014) Apr 14 [cited 2021 May 3];8: (1 APR):213. Available from: https://journal.frontiersin.org/article/10.3389/fnhum.2014.00213/abstract |
[48] | Comon P . Independent component analysis, A new concept? Signal Processing [Internet]. (1994) [cited 2021 May 3];;36: (3):287–314.Available from: https://hal.archives-ouvertes.fr/hal-00417283 |
[49] | Pontifex MB . Stylized Topographic Map Plugin for EEGLAB/ERPLAB [Internet]. 2019 [cited 2021 May 17]. Available from: https://education.msu.edu/kin/hbcl/software.html |
[50] | Cannavale CN , Hassevoort KM , Edwards CG , Thompson SV , Burd NA , Holscher HD , et al. Serum lutein is related to relational memory performance. Nutrients. (2019) ;11: (4):1–10. |
[51] | Wasserstein RL , Lazar NA . The ASA’s Statement on p-Values: Context, Process, and Purpose. Am Stat [Internet]. (2016) [cited 2021 Nov 16];70: (2):129–33. Available from: https://www.tandfonline.com/action/journalInformation?journalCode=utas20 |
[52] | Stoka V , Turk V , Turk B . Lysosomal cathepsins and their regulation in aging and neurodegeneration. Vol. 32, Ageing Research Reviews. Elsevier Ireland Ltd; (2016) . pp. 22–37. |
[53] | Couillard-Despres S , Winner B , Schaubeck S , Aigner R , Vroemen M , Weidner N , et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. (2005) ;21: (1):1–14. |
[54] | Liu PZ , Nusslock R . Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. (2018) ;12: (FEB):1–6. |
[55] | Leckie RL , Oberlin LE , Voss MW , Prakash RS , Szabo-Reed A , Chaddock-Heyman L , et al. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci [Internet]. (2014) Dec 11 [cited 2021 Jun 26];8: (DEC):985. Available from: www.frontiersin.org |
[56] | Murphy MP , Levine H . Alzheimer’s disease and the amyloid-β peptide [Internet]. Vol. 19, Journal of Alzheimer’s Disease. IOS Press; (2010) [cited 2021 Jun 26]. pp. 311–23. Available from: /pmc/articles/PMC509/ |
[57] | Swanberg MM , Tractenberg RE , Mohs R , Thal LJ , Cummings JL . Executive dysfunction in Alzheimer disease. Arch Neurol. (2004) ;61: (4):556–60. |
[58] | Perrig-Chiello P , Perrig WJ , Ehrsam I R , Staehelln HB , Krings F . The effects of resistance training on well-being and memory in elderly volunteers [Internet]. Vol. 27, Age and Ageing. 1998 [cited 2020 Apr 15]. Available from: https://academic.oup.com/ageing/article-abstract/27/4/469/32644 |
[59] | Janssen I , Heymsfield SB , Ross R . Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J Am Geriatr Soc [Internet]. (2002) May 1 [cited 2021 Nov 19];50: (5):889–96. Available from: https://onlinelibrary.wiley.com/doi/full/10.1046/j.1532-5415.2002.50216.x |
[60] | Rönnlund M , Nyberg L , Bäckman L , Nilsson LG . Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychol Aging [Internet]. (2005) Mar [cited 2021 Nov 19];20: (1):3–18. Available from: record/2005-02476-001 |
[61] | Casarotti A , Papagno C , Zarino B . Modified Taylor Complex Figure: Normative data from 290 adults. J Neuropsychol. (2014) ;8: (2):186–98. |
[62] | Ogino T , Watanabe K , Nakano K , Kado Y , Morooka T , Takeuchi A , et al. Predicting executive function task scores with the Rey-Osterrieth Complex Figure. Brain Dev [Internet]. (2009) [cited 2021 Nov 21];31: (1):52–7. Available from: www.elsevier.com/locate/braindev |
[63] | Yang Y , Shields GS , Guo C , Liu Y . Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci Biobehav Rev [Internet]. (2018) ;84: (2):225–44. Available from: https://doi.org/10.1016/j.neubiorev.2017.11.020 |
[64] | Hologic. Hologic APEX 3.4/4.0 Data Dictionary. Bedford, MA; (2012) . pp. 1–80. |
[65] | Fritsch T , Mcclendon MJ , Smyth KA , Lerner AJ , Friedland RP , Larsen JD . Cognitive Functioning in Healthy Aging: The Role of Reserve and Lifestyle Factors Early in Life. (2007) [cited 2021 Sep 19];47: (3):307–22. Available from: https://academic.oup.com/gerontologist/article/47/3/307/562695 |
[66] | Cagney KA , Lauderdale DS . Education, Wealth, and Cognitive Function in Later Life. J Gerontol [Internet]. (2002) [cited 2021 Nov 16];57: (2):163–72. Available from: https://academic.oup.com/psychsocgerontology/article/57/2/P163/600165 |
[67] | Stark T , Walker B , Phillips JK , Fejer R , Beck R . Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: A systematic review [Internet]. Vol. 3, PM and R. JohnWiley & Sons, Ltd; (2011) [cited 2021 May 16]. pp. 472–9. Available from: https://onlinelibrary.wiley.com/doi/full/10.1016/j.pmrj.2010.10.025 |
[68] | Steffen K , Nilstad A , Kristianslund EK , Myklebust G , Bahr R , Krosshaug T . Association between lower extremity muscle strength and noncontact ACL injuries. Med Sci Sports Exerc. (2016) ;48: (11):2082–9. |
[69] | Buckinx F , Landi F , Cesari M , Fielding RA , Visser M , Engelke K , et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle [Internet]. (2018) Apr 1 [cited 2021 Sep 19];9: (2):269–78. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jcsm.12268 |



