Changes in Hippocampal Androgen Receptor Density and Behavior in Sprague-Dawley Male Rats Exposed to a Low-Pressure Blast Wave
Abstract
Objective:
The purpose of this study was to examine the effect of exposure of a low-intensity blast wave on androgen receptor (AR) density in the hippocampus and the potential influence on behavioral and cognitive responses.
Methods:
Sprague-Dawley rats were randomly assigned to either a blast exposed group (n = 27) or an unexposed (control) group (n = 10). Animals were treated identically, except that rats within the control group were not exposed to any of the characteristics of the blast wave. Behavior measures were conducted on day seven post-exposure. The rats were initially assessed in the elevated plus maze followed by the acoustic startle response paradigm. Spatial memory performance using the Morris water-maze test was assessed at 8-days post-exposure, for seven consecutive days. Following all behavioral tests AR immunofluorescence staining was performed in different hippocampal subregions.
Results:
A significant elevation in anxiety index (p < 0.001) and impaired learning (p < 0.015) and spatial memory (p < 0.0015) were noted in exposed rats. In addition, a significant attenuation of the AR was noted in the CA1 (p = 0.006) and dentate gyrus (p = 0.031) subregions of the hippocampus in blast exposed animals. Correlational analyses revealed significant associations between AR and both anxiety index (r = –.36, p = 0.031) and memory (r = –0.38, p = 0.019).
Conclusions:
The results of this study demonstrate that exposure to a low-pressure blast wave resulted in a decrease in AR density, which was associated with significant behavioral and cognitive changes.
INTRODUCTION
Intense military training has been reported to cause significant reductions in resting total testosterone concentrations [1, 2]. The intense nature of military training is also associated with reductions in body mass, lean body mass and power [1, 2]. During military deployments the physiological, environmental and psychological demands have been reported to cause a myriad of issues that increase the occurrence of both physical and psychological injury [3]. Exposure to both low- and high-pressure blast waves during deployments can result in brain injuries ranging from mild to severe. Although high pressure blast waves will often result in severe brain injury, exposure to a low-pressure blast wave can also exert significant force on brain tissue without actual penetration and may cause changes in neural function. These injuries are considered to be on the mild end of the traumatic brain injury spectrum (mTBI) but are the most common form (∼75%) of TBI in soldiers [4, 5]. More than 300,000 service members who have returned from deployment are believed to meet the diagnostic criteria for mTBI [4]. Damage from these less intense forms of blast-exposure is associated with a number of functional deficits including memory problems, difficulty concentrating, impaired decision making, depression, apathy and anxiety [6, 7]. Many of these cognitive deficits are thought to be a result of a depression in neurotrophins such as Brain-derived neurotropic factor (BDNF) which are associated with neural plasticity and connectivity processes [8].
A number of investigations have suggested that testosterone may provide a protective effect of neurotrophins. Testosterone is thought to exert neurotrophic effects via its aromatization to estradiol and/or by transformation into 5a-dihydrotestosterone [9]. In addition, precursors of testosterone both dehydroepiandrosterone (DHEA) and its sulfate ester DHEA sulfate (DHEAS) have been reported to promote neuroprotection [10]. In a recent study of active duty military personnel undergoing 12 days of survival training Taylor and colleagues [11] indicated that changes in salivary nerve growth factor appeared to be mediated by changes in testosterone. Interestingly, Fenchel and colleagues [12] reported that a decrease in androgen receptor density was associated with an extreme behavioral response (posttraumatic stress disorder (PTSD)-phenotype) to a predator scent stress (PSS) in rats. The PSS is an animal model related to the pathophysiology associated with PTSD, at least in some individuals [13]. Androgens have also been shown to have positive effects on mood and anxiety [14, 15]. Testosterone has been shown to modulate multiple neurotransmitter systems [16], and androgen administration directly into the hippocampus has been demonstrated to relieve anxiety [17]. In addition, androgens are also involved in the modulation of learning and memory and have positive effects on cognitive performance [15, 18, 19]. Therefore, considering that the pathogenesis of PTSD and mTBI are thought to have common biomolecular and morphological pathways [20], it is likely that changes in androgen and estrogen receptors may also influence behavioral responses associated with mTBI. Thus, the primary purpose of this study was to examine the effect of exposure of a low-intensity blast wave on androgen receptor (AR) density in the hippocampus and the potential influence on behavioral and cognitive responses.
MATERIALS AND METHODS
All procedures were carried out under strict compliance with ethical principles and guidelines of the NIH Guide for the Care and Use of Laboratory Animals. All treatment and testing procedures were approved by the Animal Care Committee of Ben-Gurion University of the Negev, Israel (IL-83-12-2012).
Animals
A total of 37 (4-month-old) male Sprague–Dawley rats (Envigo RMS, Israel) weighing 200–250 gm were used in this study. The animals were housed four per cage in a vivarium with stable temperature and 12 h light/dark cycle (lights off: 08 : 00 p.m.) with unlimited access to food and water.
Experimental design
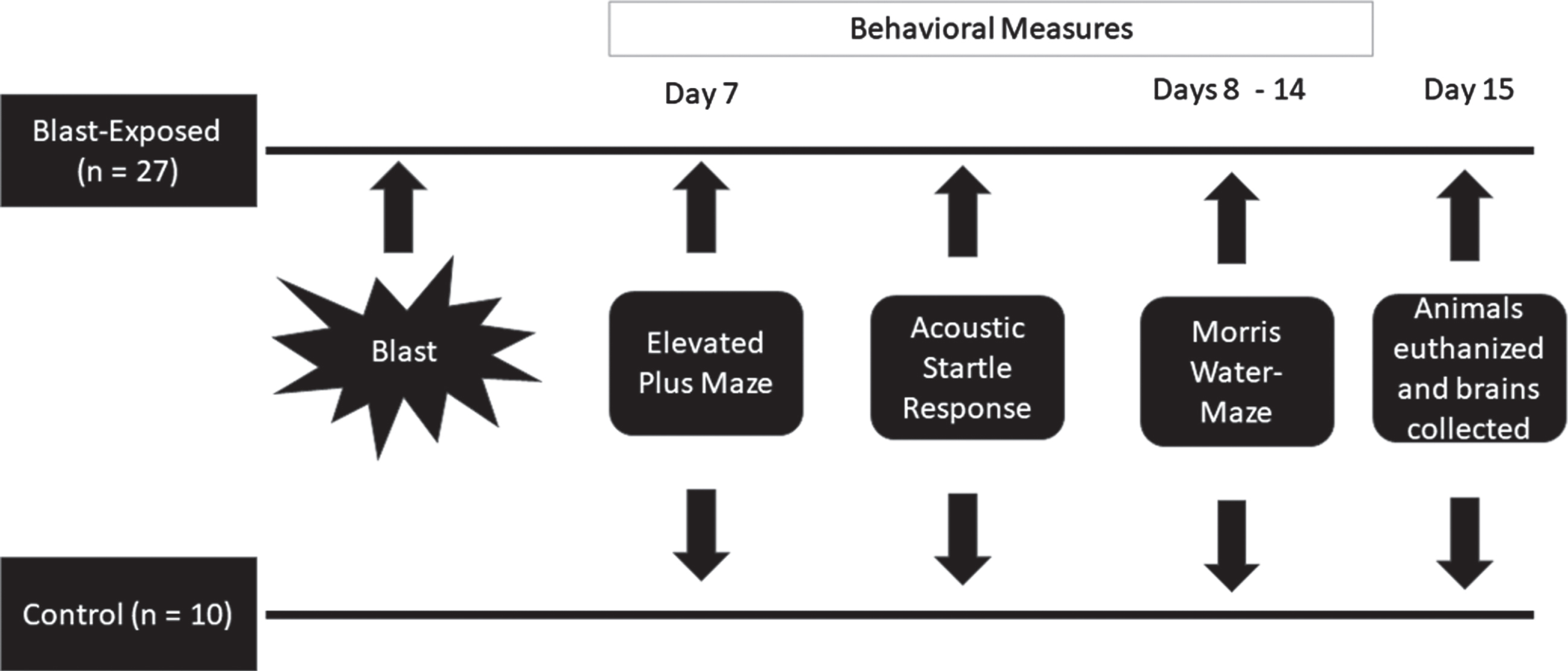
Rats were randomly assigned into one of two groups, either a blast exposed group (n = 27) or an unexposed (control) group (n = 10). Animals in both groups were treated identically, except that the rats within the control group were not exposed to any of the characteristics of the blast wave. Behavior measures were conducted on day seven. The rats were initially assessed in the elevated plus maze followed, 1 hour later, by the acoustic startle response paradigm. Spatial memory performance using the Morris water-maze test was assessed at 8 days post-exposure, for seven consecutive days. Twenty-four hours after the behavioral tests (15 days post-exposure) animals were sacrificed, and their brains were collected for immunofluorescence staining in different hippocampal subregions. Figure 1 provides a description of the experimental design.
Fig. 1
Experimental Design.
Blast wave exposure
The experimental tool used an exploding wire technique to generate small-scale cylindrical and spherical blast waves, as previously described [21–23]. Briefly, each rat was restrained in a custom flexible harness located on a platform, which was placed 265 mm from the wire. Movement was restricted to 3–5 cm during the blast exposure. Pressure values were recorded using a Kistler 211B3 piezoelectric pressure transducer mounted on a perpendicular wall. Rats were subjected to a single blast wave, without any body shielding, resulting in a full body exposure to the blast wave. Following the blast, rats were returned to their home cage.
Blast wave details
A 0.4 mm diameter 70 mm length copper wire and a charging voltage of 4.2 kV were used to generate the blast wave. The discharge current was about 500 kA (estimated). The short pulse at t = 0 was associated with the electro-magnetic pulse generated by the capacitor discharge. Previous research has reported that this low-pressure blast wave results in a mean peak over-pressure of 95 kPa (13.77 psi) (rise time of 0.01 ms) that is sustained for a duration of 0.19 ms and leads to a peak impulse of 10.8×10–3 kPa·s–1 [22]. The over-pressure wave is followed with a negative pressure wave that is sustained for more than 0.66 ms with a peak negative pressure of –40 kPa (–5.8 psi). This blast protocol is reported to result in a sound pressure level of 193 dB and a light intensity of approximately 5 Mlux [22], which is similar to that experienced during exposure to a M84 stun grenade at a distance of 1.5 m (3.1 Mlux). The peak overpressure deviations between the different experimental trials was between 1–3%. Following the blast rats were returned to their home cage. Exposure to this experimental blast wave has been validated to elicit distinct behavioral and morphological responses modelling mTBI-like, PTSD-like and comorbid mTBI-PTSD-like behaviors [22].
Neurological severity score (NSS)
To determine that the blast wave did not result in vast neurological deficits, we employed the NSS. The NSS was performed 1 hour following the initial blast wave exposure. NSS assesses somatomotor and somatosensory function by evaluating the animals’ activities in motor, sensory, reflexes, beam walking, and beam balancing tasks [24]. Specifically, the following were assessed: ability to exit from a circle (3-point scale), gait on a wide surface (3-point scale), gait on a narrow surface (4-point scale), effort to remain on a narrow surface (2-point scale), reflexes (5-point scale), seeking behavior (2-point scale), beam walking (3-point scale), and beam balance (3- point scale). An observer, who was blind to the different treatment groups, tested the animals.
Behavioral assessments
All behavioral tests were performed in a closed, quiet, light-controlled room in the Faculty of Medicine, Anxiety and Stress Research Unit, Ben-Gurion University between 10 : 00–16 : 00 hr. All results were recorded and analyzed using an EthoVision automated tracking system (Noldus Information Technology, The Netherlands).
Elevated plus-maze (EPM)
The maze is a plus-shaped platform with two opposing open and two opposing closed arms (surrounded by 41 cm high opaque walls on three sides) [26]. Rats were placed on the central platform facing an open arm and allowed to explore the maze for 5 min. Each session was videotaped and subsequently scored by an independent observer. Arm entry was defined as entering an arm with all four paws. Behaviors assessed were: time spent (duration) in open and closed arms and on the central platform; number of open and closed arm entries; and total exploration (entries into all arms). Total exploration was calculated as the number of entries into any arm of the maze to distinguish between impaired exploratory behavior, exploration limited to closed arms (avoidance) and free exploration. “Anxiety Index”, an index that integrates the EPM behavioral measures, was calculated as follows:
Anxiety Index values range from 0–1 where an increase in the index expresses increased anxiety-like behavior.
Acoustic startle response
Startle response was measured using two ventilated startle chambers (SR-LAB system, San Diego Instruments, San Diego, CA). The SR-LAB calibration unit was used routinely to ensure consistent stabilimeter sensitivity between test chambers and over time. Each Plexiglas cylinder rested on a platform inside a sound-proofed, ventilated chamber. Movement inside the tube was detected by a piezoelectric accelerometer below the frame. Sound levels within each test chamber were measured routinely using a sound level meter to ensure consistent presentation. Each test session started with a 5 min acclimatization period to background white noise of 68 dB, followed by 30 acoustic startle trial stimuli in six blocks (110 dB white noise of 40 ms duration with 30 or 45 sec inter-trial interval). Behavioral assessment consisted of mean startle amplitude (averaged over all 30 trials) and percent of startle habituation to repeated presentation of the acoustic pulse. Percent habituation defined as the percent change between the response to the first block of sound stimuli and the last block of sound stimuli was calculated as follows:
Morris water maze (MWM)
Spatial learning and memory were assessed by performance in a hippocampal-dependent visuospatial learning task in the MWM according to a test modified from the procedure of Morris [25]. Animals were trained in a pool 1.8 m in diameter and 0.6 m high, filled half-way with water at 24°±1°C. A 10 cm square transparent platform was hidden in a constant position in the pool submerged 1 cm below water level. Within the testing room only distal visual-spatial cues were available to the rats for location of the submerged platform. Rats were given four trials per day, to find the hidden platform over four consecutive days (acquisition phase). The escape latency, (i.e., the time required by the rat to find and climb onto the platform), was recorded for up to 120 s. Each rat was allowed to remain on the platform for 30 s and was then removed to its home cage. If the rat did not find the platform within 120 s, it was manually placed on the platform and returned to its home cage after 30 s. To assess reference memory at the end of learning, a probe or extinction trial was given. Twenty-four hours after the last acquisition day, the island was removed, and the search strategy of the rat was monitored to evaluate whether it used spatial memory to search for the island in the quadrant where it had previously been located. On days 6−7 the platform was placed at the opposite end of the pool, and the rat was retrained in four daily sessions (reversal phase).
Tissue preparation
All animals were euthanized 24 hours after the last behavioral assessment. Animals were deeply anesthetized via an intraperitoneal injection of a ketamine and xylazine mixture (70 mg kg–1, 6 mg kg–1, respectively) and perfused transcardially with cold 0.9% physiological saline followed by 4% paraformaldehyde (Sigma-Aldrich) in 0.1 M phosphate buffer (pH 7.4). Brains were quickly removed, postfixed in the same fixative for 12 h at 4°C, and were cryoprotected overnight in 30% sucrose in 0.1 M phosphate buffer at 4°C. Brains were frozen on dry ice and stored at –80°C. Serial coronal sections (10μm) at the level of dorsal hippocampus were collected for each animal, using a cryostat (Leica CM 1850) and mounted on coated slides.
Immunofluorescence
Sliced sections of brain tissue were air dried and washed three times in phosphate buffer saline (PBS) containing Tween 20 (PBS/T) (Sigma-Adrich, Israel). Sections were then incubated for 60 min in a blocking solution (normal goat serum in PBS) and then kept overnight at 4°C with the primary antibodies against AR (rabbit polyclonal anti-AR receptor antibody, Cat Number: ab74272, Lot Number: GR3179947-6, (1 : 250), Abcam plc, UK). After three washes in PBS/T, sections were incubated for 2 h in DyLight-488 labeled goat-anti-rabbit IgG (1 : 500; KPL, MD, USA) in PBS containing 2% normal goat or horse serum. The sections were then washed and mounted with mounting medium (Vectastain; Vector Laboratories, CA, USA). Brain sections of the two experimental groups were processed at the same time and under identical conditions to ensure reliable comparisons and to maintain stringency in tissue preparation and staining conditions. Control staining was performed in the absence of the primary antibodies. Additionally, fluorescent labels were swapped to test cross-reactivity, and sections were incubated without primary antibodies to assess non-specific binding of the secondary antibodies.
Quantification
Each brain region was defined under the microscope according to cytoarchitectural landmarks [27]. The regions of interest were outlined and computer-aided estimation was used to calculate the number of AR-ir cells in the pyramidal layer of CA1 and CA3, and in the granular layer of DG. Measurements occurred in a 30,000 mm2 for CA1 and CA3 areas and also 4800μm2 in DG area and were digitized using microscopic images (Leica microscope DM4500B; Leica Microsystems, Wetzlar, Germany) and a DFC340FX digital imaging camera (Leica Microsystems). Measurements were taken in each sub-region from both brain hemispheres. The number of positive cells expressing AR-ir, was determined with the Leica LAS software (version 3.8). Sections from the brains of different groups of rats were processed at the same time and under identical conditions to ensure reliable comparisons and to maintain stringency in tissue preparation and staining conditions. Seven representative sections of the hippocampus were chosen (between Bregma – 2.30 and Bregma – 3.60) from each animal, from each group. Sections from the brains of different groups of rats were processed at the same time and under identical conditions to ensure reliable comparisons and to maintain stringency in tissue preparation and staining conditions. Control staining was performed in the absence of the primary antibodies. Additionally, secondary fluorescent labels were swapped to test for cross-reactivity, and sections were incubated without primary antibodies to test for non-specific binding of the secondary antibodies. The sections were analyzed by two observers blinded to the treatment protocol.
Statistical analyses
In consideration of the multiple responses of each rat during the various phases of the MWM, a linear mixed model analysis of repeated measurement was used to examine spatial learning and memory. An unpaired student’s t test was to compare differences in the remaining behavioral and molecular results. Pearson’s product-moment correlation was used to determine selected bivariate correlations. All statistical analyses were analyzed using SPSS v26 software (SPSS Inc., Chicago, IL), and an alpha level of p < 0.05 was used to determine statistical significance. All data are reported as means±SEM. Using the methods of Cohen [28] for determining sample size and power, and results from previously published data [22], it was determined that 10 animals per group would provide for a statistical power of 80% with an α level of 0.05 (two-tailed).
RESULTS
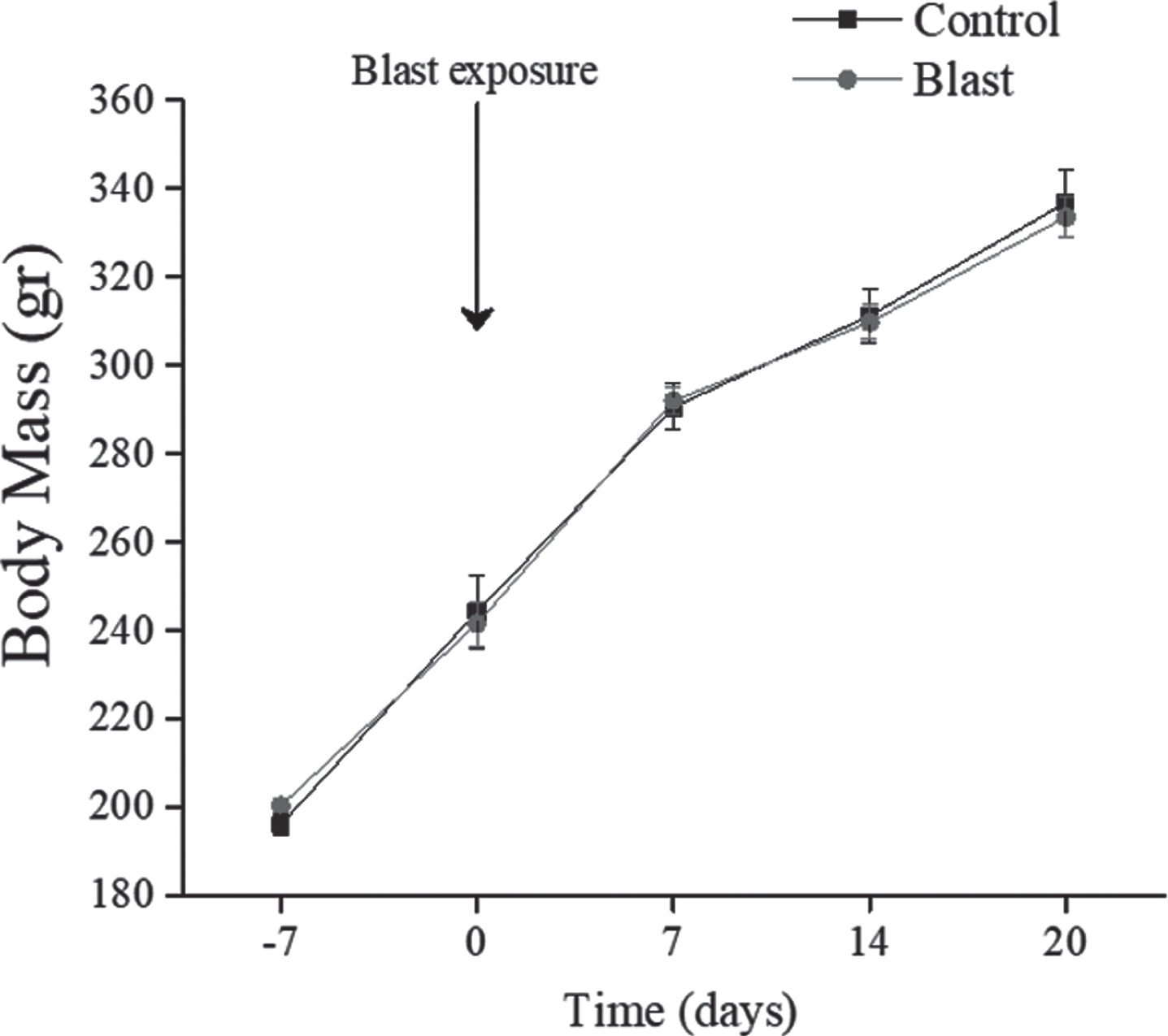
No significant differences in body weight (see Fig. 2) were found between all groups and weight gain over the 20 days post-blast exposure was comparable in all groups.
Fig. 2
Body Mass Changes. All data are reported as means±SEM.
No significant differences were observed between control and exposed animals in reflex responses, motor coordination, motor strength, or sensory function. All animals were graded as zero, suggesting that differences in behavioral and cognitive tasks were not related to abnormal motor function required of the animals to complete the behavioral tasks.
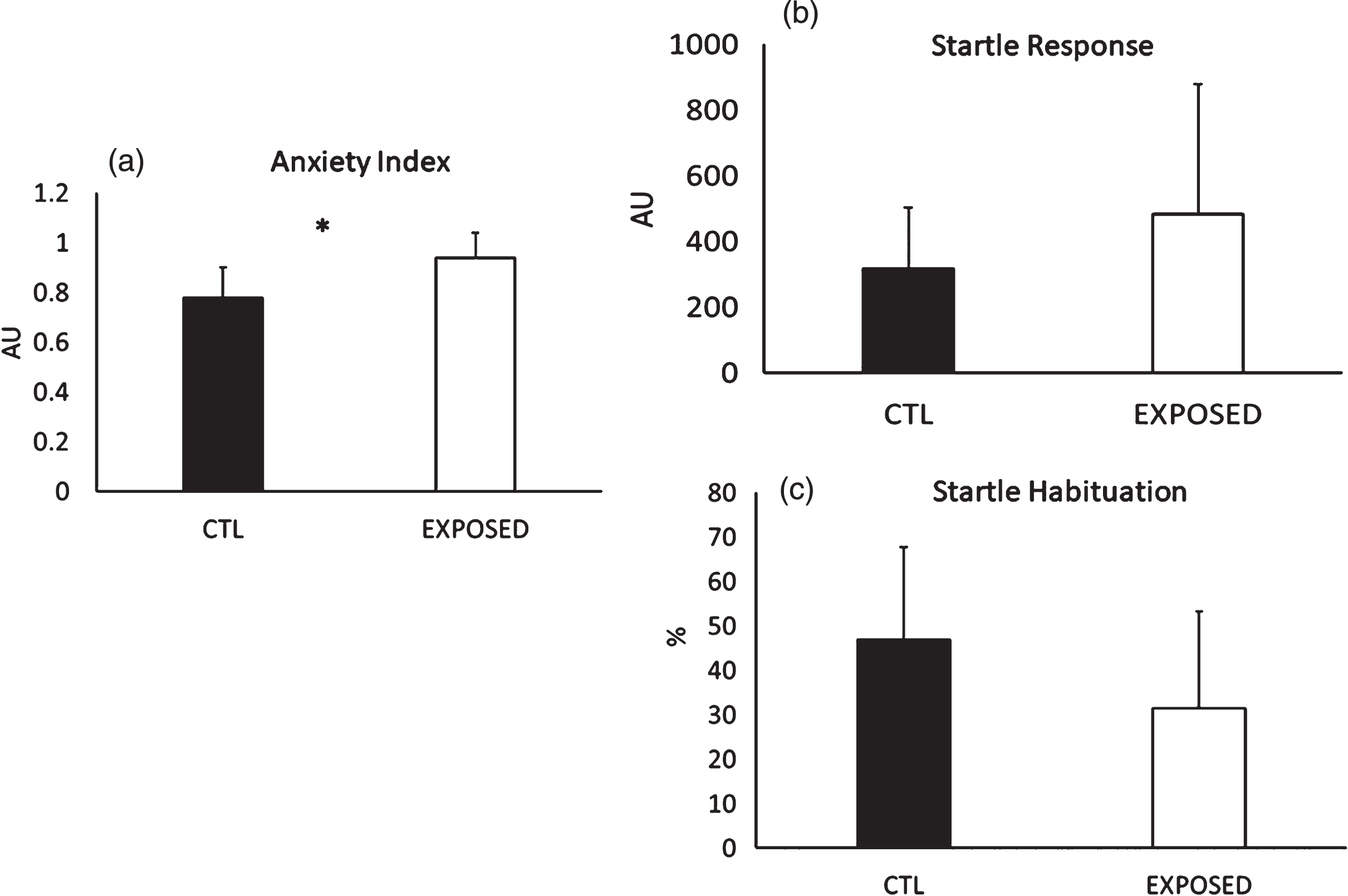
Animals exposed to the blast wave experienced a significantly greater increase in anxiety (p < 0.001) but no differences were noted in the startle response (p = 0.22), while a trend towards a lower startle habituation was noted (p = 0.06) (see Fig. 3a–c).
Fig. 3
(a) Group Comparisons on anxiety index, (b) startle response and (c) startle habituation. *=Significant difference between the groups. All data are reported as means±SEM.
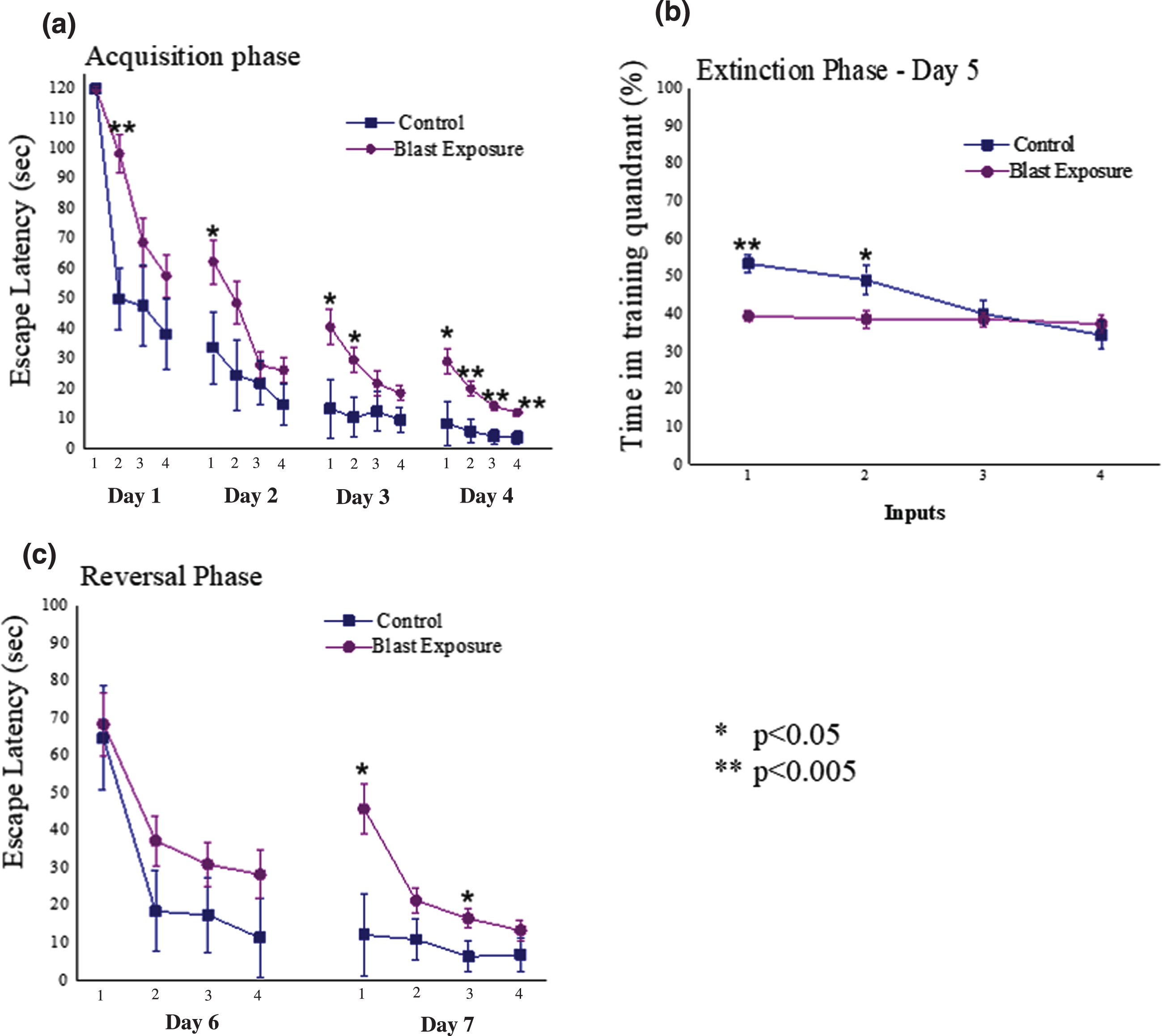
Average daily escape latency time for each group during the acquisition phase can be observed in Fig. 4a. Significant main effects for input (F (15,525) = 52.2, p < 0.0001) and groups (F (1,35) = 10.4, p < 0.003) were observed. In addition, a significant interaction was also noted between the groups (F (15,525) = 2.0, p < 0.015). Examination of the extinction trial (day 5, Fig. 4b) revealed significant main effects for input (F (7,245) = 12.6, p < 0.0001) and groups (F (1,35) = 6.7, p < 0.015), but no significant interaction (p > 0.05). Analysis of the reversal phase (days 6 and 7; Fig. 4c) revealed significant main effects for both input (F (3,105) = 8.4, p < 0.0001) and groups (F (1,35) = 4.3, p < 0.05), and a significant interaction (F (3,105) = 5.7, p < 0.0015). It appears that animals exposed to the low-pressure blast wave had a significantly impaired learning and spatial memory compared to unexposed animals.
Fig. 4
(a) Daily escape latency time for each group during the acquisition phase of the Morris Water Maze (MWM); (b) latency time achieved during extinction phase (day 5 of the MWM) (c) Daily latency times achieved during reversal phase (days 6 and 7 of the MWM); *=Significant difference between the groups. All data are reported as means±SEM. D1 = Day 1; D2 = Day 2; D3 = Day 3; D4 = Day 4; WMW = Morris water maze; *=p < 0.05; **=p < 0.005.
Comparison of locomotor skills between exposed and unexposed animals during the MWM revealed no significant differences over the acquisition (F(1,35) = 0.1, p = 0.7454), extinction (F(1,35) = 0.426, p = 0.518) and retrieval phases (F(1,35) = 0.03, p = 0.86) between the groups, or between days in each group, indicating no motor disturbances in any of the groups. The mean swimming speed±SEM over the acquisition, extinction and retrieval phases in the control group was: 22.1±0.51 cm s–1; 22.8±0.37 cm s–1 and 22.58±0.36 cm s–1, respectively and in the blast exposed group was: 21.94±0.24 cm s–1; 22.76±0.22 cm s–1 and 22.97±0.33 cm s–1, respectively. The average distance swam to the platform decreased over days, irrespective of group. During the acquisition phase, there was a significant difference in distance traveled (F (1, 35) = 9.9, p < 0.0035) between the control and blast exposed groups (770.72±73.3 cm and 1267.44±91.4 cm, respectively). During the reversal phase, a significant difference was also noted in distance traveled (F (1, 35) = 6.18, p < 0.02) between the control and blast exposed groups (428.96±66.49 cm and 742.0±72.16 cm, respectively).
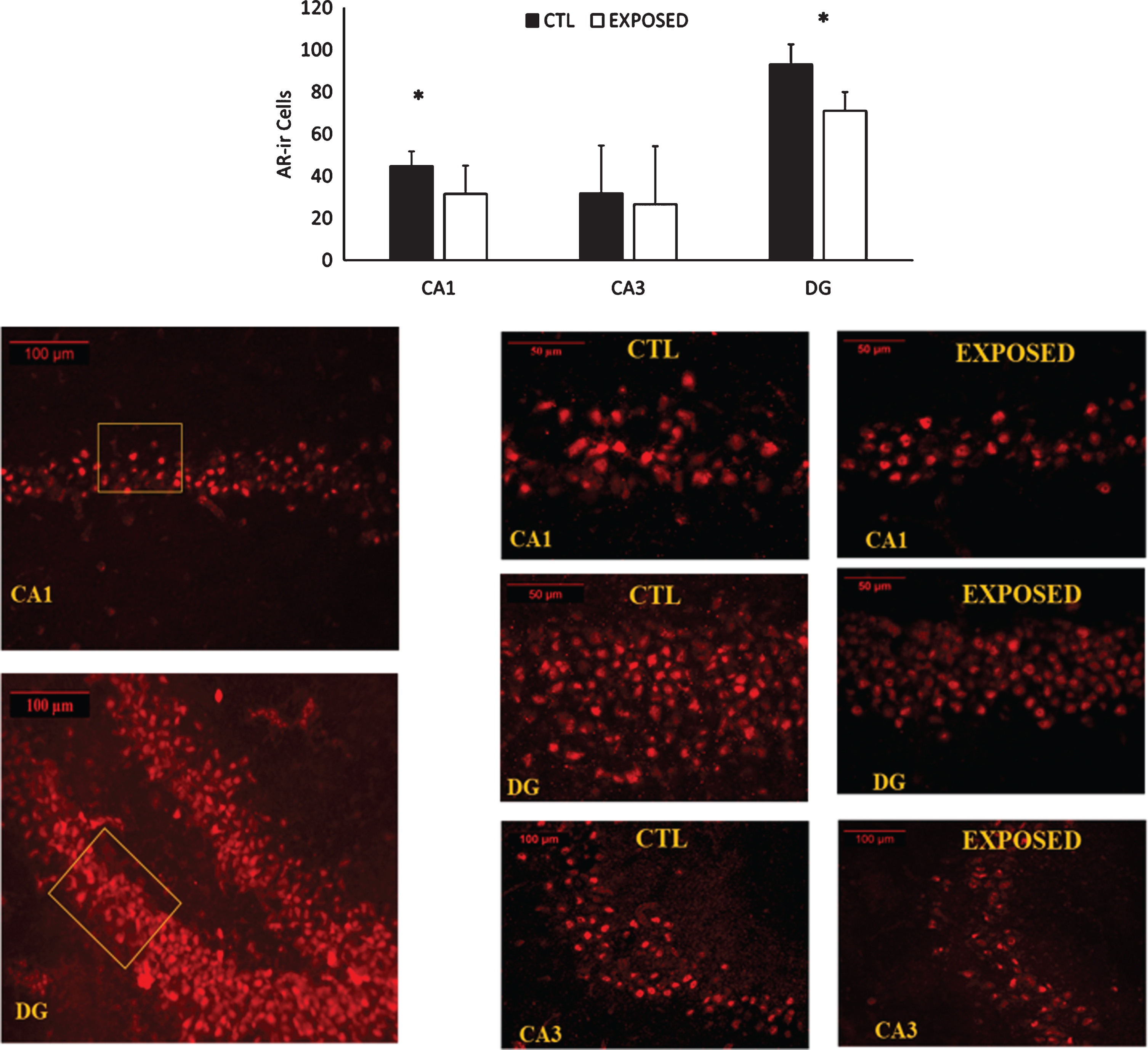
Changes in AR density in the different sections of the hippocampus are depicted in Fig. 5. Exposure to the low-pressure blast wave resulted in significant decreases in AR density in both CA1 (p = 0.006) and DG (p = 0.031) subregions of the hippocampus. A trend was approached (p = 0.11) towards a decrease in the CA3 subregion.
Fig. 5
AR expression in the CA1, CA3 and DG gyrus subregions of the hippocampus. Representative images of the immunofluorescent stains for androgen receptor expression appear in the lower half of the figure. About 30 sections were deployed from each brain. Seven representative sections of the hippocampus were chosen from each rat. Results are expressed as the average staining intensity and presented as the mean±standard error of the mean. *=Significant difference between the groups.
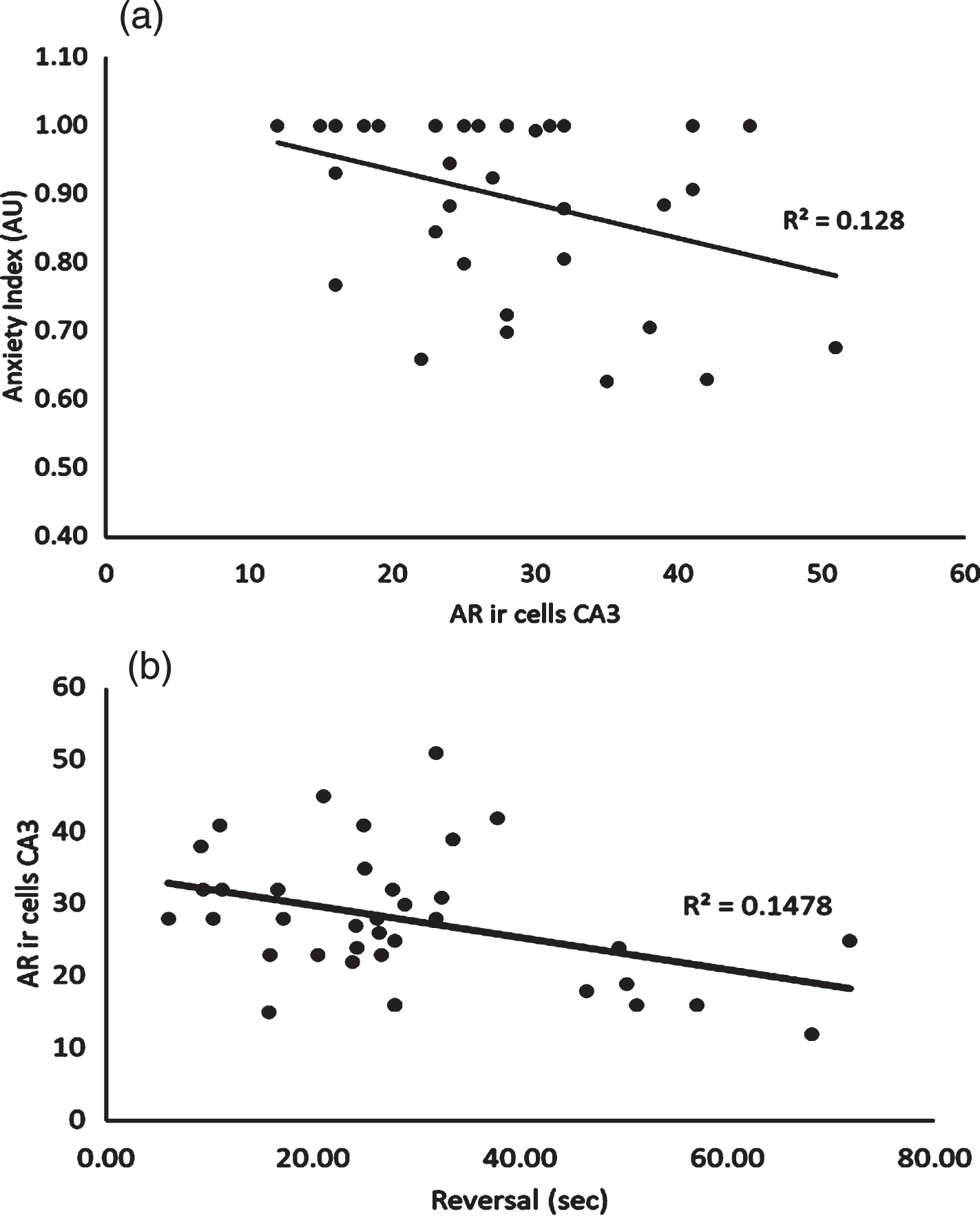
The relationship between AR density in the CA3 region of the hippocampus was significantly correlated to both anxiety index (r = –0.36, p = 0.031) and the reversal phase of the MWM (r = –0.38, p = 0.019) (see Fig. 6a and b, respectively). No other significant bivariate correlations were noted.
Fig. 6a
Relationship between Androgen Receptor Expression in the CA3 Subregion of the Hippocampus and Anxiety Index. 6b: Relationship between Androgen Receptor Expression in the CA3 Subregion of the Hippocampus and Average Latency Time Achieved during Reversal Phase of the Morris Water Maze.
DISCUSSION
Results of this study indicated that exposure to a low-pressure blast wave attenuated the pattern of AR density in the hippocampus, which was associated with a higher level of anxiety and impaired spatial memory. A single low intensity blast wave that was employed in this study has been suggested to model the traumatic experience associated with exposure to an improvised explosive device and/or other battlefield-related blast waves [20], and has been previously demonstrated to be an effective model in causing mTBI in rodents [20]. This present investigation appears to be the first study to demonstrate that exposure to a single low-pressure blast wave can alter androgen receptor density and affect behavior and cognitive function.
Our results are similar to those recently reported by Fenchel and colleagues [12], albeit using a different stressor. Animals exposed to a PSS experienced a significant decrease of AR density, which was associated with increases in anxiety. However, when testosterone was provided to the animal following PSS exposure, behavioral outcomes improved. The similarities between these studies is not surprising. A number of reports have indicated that symptoms associated with mTBI are at times difficult to distinguish clinically from PTSD [5–7, 29]. In both disorders patients often complain of fatigue, irritability and poor sleep, while impairments in concentration, attention, and memory are also noted [6, 7, 29]. The increased irritability and impaired memory observed in this study is consistent with the behavioral patterns associated with mTBI. The reduced AR in the hippocampus is likely part of the etiological mechanism associated with these behavioral changes [30, 31].
There have been a number of investigations focusing on the role of steroid hormones in modulating anxiety-like behavior. Testosterone, or its metabolite dihydrotestosterone (DHT), is thought to have direct actions through its receptor. However, it may also act indirectly through its aromatization into estradiol and subsequently activating the estrogen receptor (ER). Activation of both AR and ER have been shown to influence synaptic plasticity and neurogenesis [32–36]. Much of the evidence examining the role of steroid hormones and anxiety has focused on the ER [37], with fewer investigations examining the role of the AR. Estradiol has been suggested to have a greater role in synaptic plasticity in younger animals (4–8 weeks), with a reduced role in mature animals (>12 weeks) [36]. It is likely that as testosterone production increases during puberty it takes on a greater role in the behavioral response. Considering we examined 4-month-old rats, the focus on the AR appears to be appropriate. There have been several studies that support the direct role that the AR has on behavioral changes. Chen and colleagues [38] demonstrated that the interaction of testosterone with its receptor in mice exerted an anxiolytic effect, but when the receptor was mutated an increase in anxiety-like behavior was noted. In addition, other investigations have reported that AR insensitivity was also associated with elevated anxiety levels [39]. Others have shown that gonadectomized male rats experienced depressive and anxiety-like behavior which was reversed when these animals were implanted with testosterone [32].
Memory impairments demonstrated in this study are also consistent from exposure to a low-pressure blast wave [21–23], and symptomatic of mTBI [7]. The reduction in of the pattern of AR density following exposure is also thought to be part of the etiology associated with impaired memory. Picot and colleagues [40] using a AR mutation model in the CA1 subregion in mice, reported significant impairments to visual memory and novel object recognition. Others have reported significant memory impairment associated with a decreased of the AR, which was reversed when testosterone replacement was provided [12].
One mechanism that links changes in AR density in the hippocampus and neuronal health and synaptic plasticity may be through testosterone’s role in maintaining BDNF levels. Although not reported in this study, a recent investigation using a low-pressure blast wave reported a significant attenuation of BDNF levels in the hippocampus [21]. Decrease in AR density has been demonstrated to have a direct effect on BDNF levels. Li and colleagues [31] appear to have been the first research group to demonstrate that gonadectomized male rats experienced a significant reduction in BDNF levels, which was reversed upon testosterone replacement. Recently, Hung and colleagues [41] indicated that a reduction in AR expression following a chronic mild stress was also associated with a reduction in BDNF levels in the CA1 area in mice. Thus, decreases in AR density may increase anxiety and impair memory through its role on neurotrophin levels.
There are several limitations to this study. BDNF levels was not reported, but in similar stress exposures has been shown to be significantly impaired [21]. Circulating testosterone concentrations was not examined, whether exposure to the low-pressure blast wave directly reduced AR density, or whether it was the result of an attenuation in circulating testosterone concentrations is not known. Previous research has demonstrated a positive association between brain AR density and circulating testosterone concentrations [42]. Finally, whether testosterone replacement therapy either prior to or immediately following exposure can maintain AR content following exposure to this single blast wave exposure is not known but should be further examined. In conclusion, the results of this study demonstrate that exposure to a low-pressure blast wave resulted in a decrease of the pattern of AR density in the hippocampus, which is associated with significant behavioral and cognitive changes.
FUNDING
The authors report no funding.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
REFERENCES
[1] | Fortes MB , Diment BC , Greeves JP , Casey A , Izard R , Walsh NP . Effects of a daily mixed nutritional supplement on physical performance, body composition, and circulating anabolic hormones during 8 weeks of arduous military training. Appl Physiol Nutr Metab. (2011) ;36: :967–75. |
[2] | Nindl BC , Barnes BR , Alemany JA , Frykman PN , Shippee RL , Friedl KE . Physiological consequences of U. S. Army Ranger training. Med Sci Sports Exerc. (2007) ;39: :1380–7. |
[3] | Nindl BC , Castellani JW , Warr BJ , Sharp MA , Henning PC , Spiering BA , et al. Physiological Employment Standards III: physiological challenges and consequences encountered during international military deployments. Eur J Appl Physiol. (2013) ;113: :2655–72. |
[4] | Hyatt KS , Davis LL , Barroso J . Finding the New Normal: Accepting Changes After Combat-Related Mild Traumatic Brain Injury. J Nurs Scholarsh. (2015) ;47: :300–9. |
[5] | Pape TL , High WM Jr, St Andre J , Evans C , Smith B , Shandera-Ochsner AL , et al. Diagnostic accuracy studies in mild traumatic brain injury: a systematic review and descriptive analysis of published evidence. Pm R. (2013) ;5: :856–81. |
[6] | Bryant R , O’Donnell M , Creamer M , McFarlane A , Clark C , Silove D . The psychiatric sequelae of traumatic injury. Am J Psychiatry. (2010) ;167: :312–20. |
[7] | Elder GA , Dorr NP , De Gasperi R , Gama Sosa MA , Shaughness MC , Maudlin-Jeronimo E , et al. Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J Neurotrauma. (2012) ;29: :2564–75. |
[8] | Dretsch MN , Williams K , Emmerich T , Crynen G , Ait-Ghezala G , Chaytow H , et al. Brain-derived neurotropic factor polymorphisms, traumatic stress, mild traumatic brain injury, and combat exposure contribute to postdeployment traumatic stress. Brain Behav. (2016) ;6: :e00392. |
[9] | Saldanha CJ , Duncan KA , Walters BJ . Neuroprotective actions of brain aromatase. Front Neuroendocrinol. (2009) ;30: :106–18. |
[10] | Kimonides VG , Spillantini MG , Sofroniew MV , Fawcett JW , Herbert J . Dehydroepiandrosterone antagonizes the neurotoxic effects of corticosterone and translocation of stress-activated protein kinase 3 in hippocampal primary cultures. Neuroscience. (1999) ;89: :429–36. |
[11] | Taylor MK , Laurent HK , Larson GE , Rauh MJ , Hiller Lauby MD , Granger DA . Salivary nerve growth factor response to intense stress: effect of sex and body mass index. Psychoneuroendocrinology. (2014) ;43: :90–4. |
[12] | Fenchel D , Levkovitz Y , Vainer E , Kaplan Z , Zohar J , Cohen H . Beyond the HPA-axis: The role of the gonadal steroid hormone receptors in modulating stress-related responses in an animal model of PTSD. Eur Neuropsychopharmacol. (2015) ;25: :944–57. |
[13] | Cohen H , Zohar J . An Animal Model of Posttraumatic Stress Disorder: The Use of Cut-Off Behavioral Criteria. Annals of the New York Academy of Sciences. (2004) ;1032: :167–78. |
[14] | Delhez M , Hansenne M , Legros JJ . Testostérone et dépression chez l’homme de plus de 50 ans. Andropause et psychopathologie: bilan symptomatique mineur [Testosterone and depression in men aged over 50 years. Andropause and psychopathology: minimal systemic work-up]. Ann Endocrinol (Paris). (2003) ;64: (2):162–9. |
[15] | MacLusky NJ , Hajszan T , Prange-Kiel J , Leranth C . Androgen modulation of hippocampal synaptic plasticity. Neuroscience. (2006) ;138: (3):957–65. doi: 10.1016/j.neuroscience.2005.12.054 |
[16] | Zhang P . Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog.Neurobiol. (2009) ;89: :134–52. |
[17] | Edinger KL , Frye CA . Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behav.Neurosci. (2004) ;118: :1352–64. |
[18] | Frye CA , Edinger KL , Seliga AM , Wawrzycki JM . 5alpha-reduced androgens may have actions in the hippocampus to enhance cognitive performance of male rats. Psychoneuroendocrinology. (2004) ;29: (8):1019–27. doi: 10.1016/j.psyneuen.2003.10.004 |
[19] | Janowsky JS , Chavez B , Orwoll E . Sex steroids modify working memory. J Cogn Neurosci. (2000) ;12: (3):407–14. doi: 10.1162/089892900562228 |
[20] | Dolan S , Martindale S , Robinson J , Kimbrel NA , Meyer EC , Kruse MI . et al. Neuropsychological sequelae of PTSD and TBI following war deployment among OEF/OIF veterans. Neuropsychol Rev. (2012) ;22: :21–34. |
[21] | Hoffman JR , Zuckerman A , Ram O , Sadot O , Stout JR , Ostfeld I , et al. Behavioral and Inflammatory Response in Animals Exposed to a Low-Pressure Blast Wave and Supplemented with β-Alanine. Amino Acids. (2017) ;49: ,871–86. |
[22] | Zuckerman A , Ram O , Ifergane G , Matar MA , Sagi R , Ostfeld I , et al. Controlled Low-Pressure Blast Wave Exposure Causes Distinct Behavioral and Morphological Responses Modelling Mild Traumatic Brain Injury, Post-Traumatic Stress Disorder, and Comorbid Mild Traumatic Brain Injury-Post-Traumatic Stress Disorder. J. Neurotrauma. (2017) ;34: :145–64. |
[23] | Zuckerman A , Ram O , Ifergane G , Matar MA , Kaplan Z , Hoffman JR , et al. Role of Endogenous and Exogenous Corticosterone on Behavioral and Cognitive Responses to Low-Pressure Blast Wave Exposure. J Neurotrauma. (2019) ;36: :380–94. |
[24] | Feldman Z , Gurevitch B , Artru AA , Oppenheim A , Shohami E , Reichenthal E , et al. (1996). Effect of magnesium given 1 hour after head trauma on brain edema and neurological outcome. J Neurosurg. (1996) ;85: :131–7. |
[25] | Morris R . Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. (1984) ;11: :47–60. |
[26] | File SE , Zangrossi H Jr , Sanders FL , Mabbutt PS . Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol Behav. (1993) ;54: :1109–11. |
[27] | Paxinos G , Watson C . A stereotaxic atlas of the rat brain. New York: Academic. 1988. |
[28] | Cohen J . Statistical power analysis for the behavioral sciences. 2nd. 1988. |
[29] | Ojo JO , Greenberg MB , Leary P , Mouzon B , Bachmeier C , Mullan M , et al. Neurobehavioral, neuropathological and biochemical profiles in a novel mouse model of co-morbid post-traumatic stress disorder and mild traumatic brain injury. Front Behav Neurosci. (2014) ;8: :213. |
[30] | Atwi S , McMahon D , Scharfman H , MacLusky NJ . Androgen Modulation of Hippocampal Structure and Function. Neuroscientist. (2016) ;22: :46–60. |
[31] | Li M , Masugi-Tokita M , Takanami K , Yamada S , Kawata M . Testosterone has sublayer-specific effects on dendritic spine maturation mediated by BDNF and PSD-95 in pyramidal neurons in the hippocampus CA1 area. Brain Res. (2012) ;1484: :76–84. |
[32] | Carrier N , Saland SK , Duclot F , He H , Mercer R , Kabbaj M . The Anxiolytic and Antidepressant-like Effects of Testosterone and Estrogen in Gonadectomized Male Rats. Biol Psychiatry. (2015) ;78: :259–69. |
[33] | Domonkos E , Hodosy J , Ostatníková D , Celec P . On the Role of Testosterone in Anxiety-Like Behavior Across Life in Experimental Rodents. Front Endocrinol (Lausanne). (2018) ;9: :441. |
[34] | Duarte-Guterman P , Lieblich SE , Wainwright SR , Chow C , Chaiton JA , Watson NV , et al. Androgens Enhance Adult Hippocampal Neurogenesis in Males but Not Females in an Age-Dependent Manner. Endocrinology. (2019) ;160: :2128–36. |
[35] | Hamson DK , Wainwright SR , Taylor JR , Jones BA , Watson NV , Galea LA . Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology. (2013) ;154: :3294–304. |
[36] | Ooishi Y , Kawato S , Hojo Y , Hatanaka Y , Higo S , Murakami G , et al. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J Steroid Biochem Mol Biol. (2012) ;131: :37–51. |
[37] | Mhaouty-Kodja S . Role of the androgen receptor in the central nervous system. Mol Cell Endocrinol. (2018) ;465: :103–12. |
[38] | Chen CV , Brummet JL , Lonstein JS , Jordan CL , Breedlove SM . New knockout model confirms a role for androgen receptors in regulating anxiety-like behaviors and HPA response in mice. Horm Behav. (2014) ;65: :211–8. |
[39] | Hamson DK , Jones BA , Csupity AS , Ali FM , Watson NV . Androgen insensitive male rats display increased anxiety-like behavior on the elevated plus maze. Behav Brain Res. (2014) ;259: :158–63. |
[40] | Picot M , Billard JM , Dombret C , Albac C , Karameh N , Daumas S , et al. Neural Androgen Receptor Deletion Impairs the Temporal Processing of Objects and Hippocampal CA1-Dependent Mechanisms. PLoS One. (2016) ;11: :e0148328. |
[41] | Hung YY , Huang YL , Chang C , Kang HY . Deficiency in Androgen Receptor Aggravates the Depressive-Like Behaviors in Chronic Mild Stress Model of Depression. Cells. (2019) ;8pii: :E1021. |
[42] | Perez-Pouchoulen M , Toledo R , Garcia LI , Perez-Estudillo CA , Coria-Avila GA , Hernandez ME , et al. Androgen receptors in Purkinje neurons are modulated by systemic testosterone and sexual training in a region-specific manner in the male rat. Physiol Behav. (2016) ;156: :191–8. |



