The Effect of Aerobic Exercise on Physical and Cognitive Outcomes in a Small Cohort of Outpatients with Schizophrenia
Abstract
Background:
Schizophrenia (SCZ) is a severe, chronic illness characterized by psychotic symptoms and impairments in many cognitive domains. Dysregulation of brain derived neurotrophic factor (BDNF) is associated with the cognitive impairments seen in patients with SCZ. Given the growing literature supporting a positive effect of aerobic exercise on cognition in other populations, we hypothesized that a structured aerobic exercise program would improve cognitive and functional outcomes in subjects with SCZ, potentially mediated by increases in BDNF.
Methods:
The study was a small randomized parallel group clinical trial of subjects with SCZ comparing 12 weeks of aerobic exercise (AE) against control (CON) stretching and balance training. At Baseline, Week 12, and Week 20 we collected serum samples for analysis of brain derived neurotrophic factor (BDNF), and assessed functional, physical, and cognitive outcomes. Linear regression models were used to compare change scores between timepoints.
Results:
We randomized 21 subjects to AE and 17 to CON; however, only 9 AE and 6 CON completed their programs. Subjects in both groups were slower at the 400 m walk in Week 12 compared to Baseline, but the AE group had significantly less slowing than the CON group (B = –28.32, p = 0.011). Between Week 12 and Week 20, the AE group had a significantly greater change score on the Composite and Visual Learning Domain of the MATRICS Consensus Cognitive Battery (B = 5.11, p = 0.03; B = 13.96, p = 0.006).
Conclusion:
These results indicate that participation in a structured aerobic exercise paradigm may modestly blunt physical function decline and enhance cognitive function in individuals with SCZ.
Abbreviations
AE | aerobic exercise |
BMI | body mass index |
BDNF | brain-derived neurotrophic factor |
HR | heart rate |
MCCB | Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery |
PANSS | positive and negative syndrome scale |
SCZ | Schizophrenia |
SLOF | Specific Levels of Functioning |
UPSA-2 | U. California San Diego Performance-Based skills assessment |
INTRODUCTION
Schizophrenia (SCZ) is a severe, chronic psychotic illness characterized by negative (anhedonia, flat affect, poor motivation) and positive symptoms (hallucinations, delusions, disorganization). It is a pervasive and persistent disease affecting approximately 1% of the world’s population, 50–70% of whom have symptoms throughout the course of their life [1, 2]. Furthermore, there is a significant cost associated with SCZ: $62.7 billion in the US in 2002, that can be attributed to not only the treatment of acute symptoms, but also the long-term care and assistance these patients require [3, 4]. Fortunately, some of these symptoms can be reduced by medication, but cognitive impairment is resistant to improvement.
Individuals diagnosed with SCZ tend to have global neurocognitive impairments that impact their perceptual-motor function, language, learning and memory, executive function, complex attention, and social cognition [5]. These neurocognitive impairments are thought to be related to structural and functional abnormalities within the brain. One such area that has been extensively investigated is the hippocampus [6–9]. Patients with SCZ have reduced volume, disrupted neuronal cytoarchitecture, reduced N-acetyl-aspartate indicating reduced neuronal integrity and disrupted glutamatergic and nicotinic neurotransmission in their hippocampus [10–12]. Furthermore, multiple studies have found that enlarged ventricles, poor white matter integrity, abnormal myelination of tracts between different regions of the brain, smaller thalamus and temporal lobes, enlarged caudate nucleus, and cerebral asymmetries all correlated with cognitive impairments in SCZ [13–17]. One proposed method of improving the neurocognitive impairments seen in SCZ is through the upregulation of brain-derived neurotrophic factor (BDNF).
BDNF is a neurotrophin known to promote neurogenesis, synaptogenesis, and brain plasticity through many different mechanisms [18, 19]. BDNF’s support of neurogenesis is through the enhancement of cell survivability in neuronal stem and progenitor cells [20]. Further, BDNF promotes the maturation and stabilization of neurotransmitter release which is necessary for synaptic plasticity [19]. BDNF’s role in synaptic plasticity allows for long-term potentiation and long-term depression, the primary mechanism of learning and memory [21, 22]. One method of improving neuroplasticity, as previously seen in animal models, is to endogenously upregulate BDNF through aerobic exercise (AE) leading to an increase in BDNF mRNA, new neurons in the hippocampus and an increase in neuronal spinal density [23–29].
AE in humans has been seen to contribute to an increase in neurotropic factors, increased hippocampal volume, and increases in VO2 max which highly correlated with improved memory [30–34]. Another study also found that the medial temporal lobe function in young adults was enhanced by AE. Their finding was attributed to increased concentrations of BDNF in serum and improved cognitive function on a face-name matching task [35]. Since a reduction of BDNF is associated with altered brain development, failures in neuroplasticity, synaptic dysconnectivity, and molecular abnormalities in patients with SCZ [36–38] several investigators have looked at the effect of AE intervention in participants with SCZ [9, 29, 39–43]. One study with a primary focus on full body AE found that serum BDNF increased almost six times as much in the AE intervention group in comparison to the control group [44]. Other studies, such as that by Oertel- Knochel et al. (2014), have analyzed the effects of AE on neurocognitive functioning in SCZ using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB). In their study, they administered five of the eight domains and found that a stronger effect occurred in the treatment group, with a significant improvement in processing speed, working memory, and visual learning [45]. Moreover, the meta-analysis conducted by Dauwan et al. (2016) found that exercise was able to reduce symptoms of schizophrenia (positive, negative, and general) and improve quality of life.
The neurocognitive deficits seen in SCZ are accompanied by a decreased ability to perform critical everyday functional skills including social and occupational function, maintaining a residence, managing medications, and ability to tend to basic self-care needs [46, 47]. Other symptoms that arise from these cognitive deficits are problems with planning, abstract thinking, and problem solving [48, 49]. Since BDNF dysregulation is a potential contributor to cognitive impairment in patients with SCZ, we conducted a small trial of AE vs. a control condition in SCZ and assessed cognitive and physical function change as well as a change in BDNF.
METHODS
Cohort description
The study was a randomized parallel group clinical trial comparing 12 weeks of AE training against a control [50] exercise condition which consisted of stretching and balance training. Retention of possible physical and cognitive change was assessed at a 20-week post baseline visit. At completion of the baseline visit, 38 subjects were randomized. All subjects were recruited from the Atlanta Veterans Affairs Medical Center or the surrounding community in accord with the Emory University Institutional Review Board and Atlanta VA Research and Development committee guidelines.
Inclusion into the study required that the individual have a diagnosis of SCZ, be between the ages of 18 and 70 years old, be on a steady dose of outpatient psychiatric medication for at least 30 days, be compliant with out-patient follow-up (at least 75% of outpatient appointments within the last year), have a stable living arrangement and access to transportation, and live a sedentary lifestyle (less than 20 minutes/day of regular physical activity) for the last month as measured by the Godin exercise questionnaire [51, 52]. Participants were excluded if they had an active substance use disorder within the previous 30 days or had more than two psychiatric admissions within the previous 6 months. Subjects were also excluded if they had a known HIV infection or AIDS, history of a traumatic brain injury, seizure disorder, known history of dementia, clinical history of mild cognitive impairment, Parkinson’s or other clinically significant neurological disease, an unstable medical condition that would interfere with fitness training, or a significant hearing or visual impairment. Subjects were randomized using a random number generator with odd values being controls and even values being AE. Timeline of study can be seen in Fig. 1.
Fig. 1
Timeline of study for a participant. Each visit consisted of: a blood draw and exercise, cognitive, and functional testing. Each experimental condition was an exercise class (aerobic exercise or stretching and balance) 3×/week for approximately an hour.

Exercise paradigms
The AE program consisted of exercise on a stationary bicycle ergometer 3 times a week. Exercise intensity began at low levels (50% of maximal heart rate reserve) calculated utilizing the Karvonen method [53]. The target exercise heart rate (HR) was calculated by subtracting the person’s age from 220. Resting HR was then subtracted from this number. The result was then multiplied by the target percent (50% for example) and the product was added back to resting HR to provide the target exercise session HR [53]. The Karvonan method has been demonstrated to correlate with measured maximal heart rate [54]. Intensity of exercise was increased by 5% every week (as tolerated by the participant) to a maximum of 80% of maximal heart rate.
Exercise time progressed from an initial 20 minutes per session to a maximum of 45 minutes by increasing 5 minutes each week. To further assess the quality of the participant’s exercise, we calculated a percent time in target HR zone. This is the number of heart-rate acquisitions that were above the 50% threshold divided by the number of total acquisitions. This value was then averaged across all sessions of the individual to find the average amount of time a participant was spending in a minimum light aerobic exercise zone [55]. The stretching and balance training consisted of progressive whole body stretching and toning exercises performed for the same amount of time as the AE condition. Both interventions focused on small group exercise with at most 5 participants at a time. Activities were led by at least 1 qualified instructor with a degree in exercise physiology and/or Personal Training certification through ACSM. To monitor effort during the training session, patients were outfitted with a chest strap FT7 heartrate monitor.
Participants could monitor their heart rate at will through a paired watch and study staff recorded heart rate every 5 minutes. If heart rate signal was lost during a session, study staff would manually obtain a participant’s heart rate using radial pulse for a 15 second duration and multiplying by 4.
To measure the effects of the exercise program on the individuals, we also had them complete the 400 m walk test and a test of VO2 max. The 400 m walk is a measure of physical functional ability and can serve as a predictor of morbidity and future disability. It has primarily been used in well-functioning older adults to estimate cardiorespiratory fitness [56, 57], functional status [58], and general health [59, 60]. The estimate maximal oxygen uptake (VO2max) prior to and after interventions was obtained from participants at baseline, week 12 and week 20. It utilized the YMCA submaximal fitness test, performed on a Monark 828e (upright) or RC4 (recumbent) cycle ergometer (Vansbro, Sweden). The YMCA test uses an extrapolation method in which heart rate workload values are obtained at 2–4 points during stages of increasing resistance and extrapolated to predict workload at the estimated maximum heart rate (e.g., 220-age). VO2 max is then calculated from the predicted maximum workload. Prior to beginning the test, the participants completed a 2 min warm-up consisting of pedaling without load so that they could adapt to the ergometer for the first minute and then begin the paradigm pedaling with a 0.5 kg.m load during the second minute. A progressive load is applied to the braking of the cycle. If a participant failed to reach the 9 min mark during the testing session then the first two data points are used to project the VO2 max [55, 61].
Blood sample collection
At each assessment timepoint (Baseline, Week 12, Week 20) serum samples were collected for analysis of brain derived neurotropic factor (BDNF) immediately prior (within 10 minutes) to the exercise testing that would occur during that visit. The serum samples were collected in SST tubes (BD 367820), inverted 5 times, and allowed to clot for an hour. They were then spun down for 15 minutes at 3000 rcf. The collected serum was diluted at a ratio of 1:150 for quantification of a mature BDNF concentration (pg/mL) using Biogenesis ELISA kits (#BEK-2211-2P) per manufacturer’s instructions. The individual conducting the ELISAs was blinded to the treatment of the individual.
Cognitive and functional assessments
Cognition and functional abilities were assessed at each timepoint (Baseline, 12 Weeks, 20 Weeks) the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB), the U. California San Diego Performance-Based skills assessment (UPSA-2), and the Specific Levels of Functioning (SLOF) scale. The MCCB is a consensus battery of neurocognitive tests assessing seven key cognitive domains relevant to SCZ: processing speed, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition [62]. The UPSA-2 is a measure of functional ability that can detect immediate improvements in the ability to carry out functionally meaningful tasks [63]. The SLOF is a rating scale designed to assess everyday functioning and behaviors of individuals with SCZ [64]. The positive and negative syndrome scale (PANSS), a standardized scale for measuring prevalence of positive and negative syndromes in SCZ was also administered at each timepoint to assess SCZ symptomatology [65]. All cognitive and functional assessments were completed by an assessor blinded to the treatment group.
Statistical methods
The analyses were conducted in two phases. The first phase was conducted on those subjects who completed baseline assessments and randomization. Means and standard deviations were determined for each outcome variable for the two subject groups. Medication class was recorded; however, chlorpromazine equivalents were not included in analyses given a lack of information regarding dosage. The two groups were compared by means of student’s t-tests or Chi square analyses on continuous or dichotomous variables as appropriate to determine any initial differences between the treatment groups. Logistic regressions including age, race, sex, group, and body mass index (BMI) were completed to determine whether any variables were associated with dropping out versus completion of the study. In the second phase of analysis, given the longitudinal nature of the study, we calculated change scores (Week 12 minus Baseline and Week 20 minus Week 12) to determine the differences between time points for those who completed 12 weeks of treatment. Change scores were then compared in t-tests between groups to determine any significant differences without the addition of covariates. To assess the effect of covariates on our outcome measures we completed backward linear regression models with change scores as the dependent variables and with age, race, sex, BMI, and treatment group as predictors. In order to test for associations of BDNF change with change in other outcome measures, BDNF change scores were added to the regression models. To ensure that a difference in baseline was not impacting our results we added the baseline value of an outcome measure as a covariate to all significant models as suggested by Twisk et al. (2018) [66]. Only variables significant at a p-value less than 0.1 were included in the final models. Outliers were considered values greater than 2.5 standard deviations from the mean and, if found, were dropped from the model. Statisticians were not unblinded due to previous involvement with the study in an advisory capacity. All statistics were completed using SAS (Cary, NC).
RESULTS
Baseline cohort results
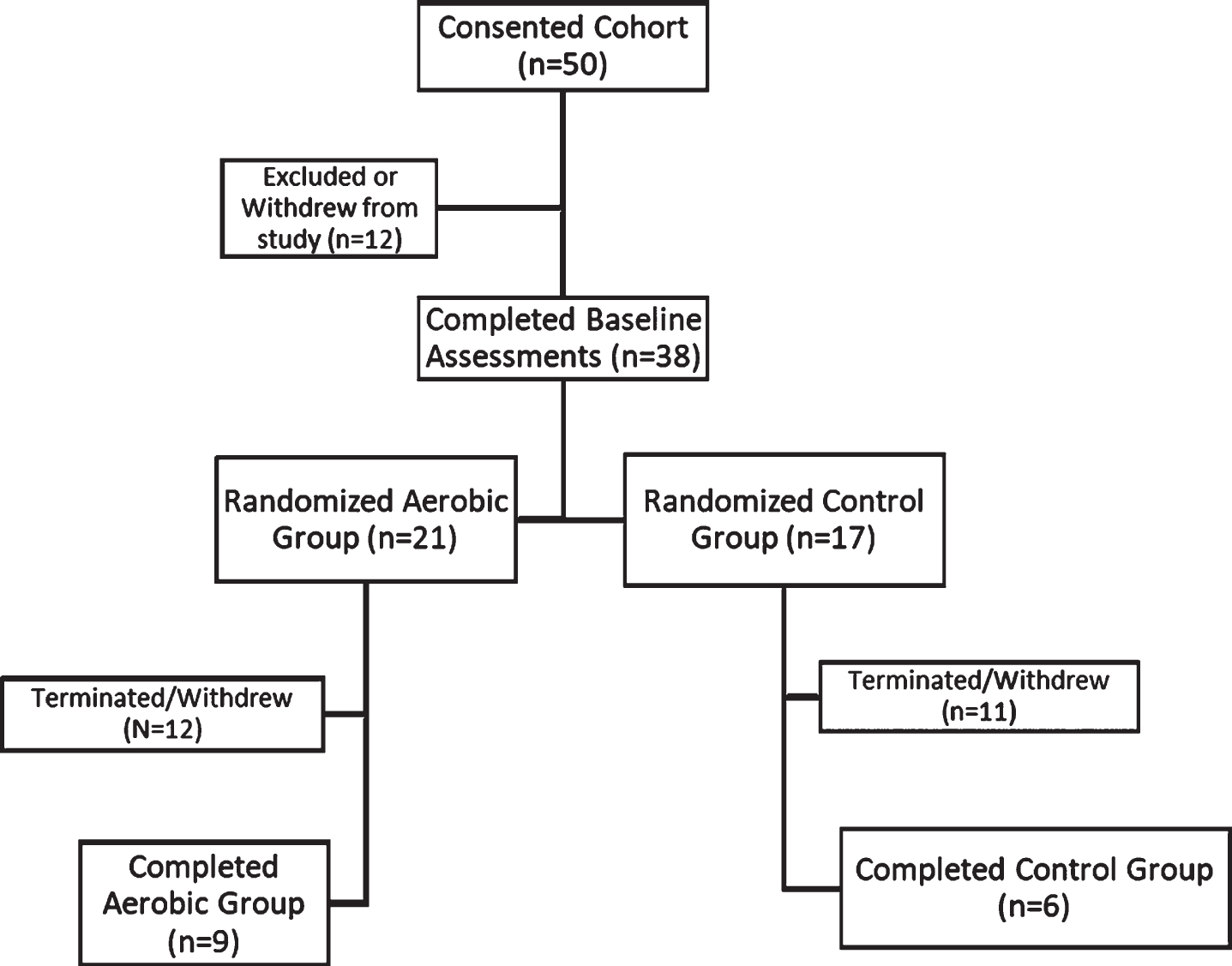
We initially consented 50 patients with SCZ to participate in the study. After those who were excluded for not meeting inclusion criteria or withdrew prior to randomization, we concluded with 38 patients who we randomized into the AE or CON groups (Fig. 2). Of those 38 patients, 21 were randomized to the AE group and 17 were randomized to the CON group.
Fig. 2
Flow chart of subject participation in the study.
Baseline population demographics, symptoms, functional assessments, physical function assessments, and cognitive scores for the entire cohort are presented in Table 1. There was no significant difference between groups in baseline demographics or outcome measures with the exception of a significantly higher score in the AE group for the Reasoning and Problem Solving domain of the MCCB (t = 2.28, p = 0.029). We also assessed whether there were any correlations among BDNF and either aerobic, cognitive, or functional outcomes at baseline. There were no significant associations even when adjusting for demographics.
Table 1
Demographics, physical function and cognitive function, all subjects at Baseline
| Variables Group | Aerobic Exercise | Control Condition | ||||||
| N | Mean/Count | Std Dev/% | N | Mean/Count | Std Dev/% | T-test/Chi Sq | p-value | |
| Age | 21 | 52.52 | 6.66 | 17 | 53.18 | 10.27 | –0.24 | 0.814 |
| BMI | 21 | 31.11 | 6.87 | 17 | 29.31 | 4.00 | 0.96 | 0.344 |
| Sex* | ||||||||
| Male | 21 | 16.00 | 76.20 | 17 | 15.00 | 88.23 | 0.427 | |
| Race* | ||||||||
| African American | 21 | 19.00 | 90.50 | 17 | 17.00 | 100.00 | 0.492 | |
| Medication* | ||||||||
| Atypical Antipsychotics | 21 | 14.00 | 66.67 | 17 | 13.00 | 76.47 | 0.721 | |
| Typical Antipsychotics | 21 | 1.00 | 4.76 | 17 | 2.00 | 11.76 | 0.577 | |
| Both (Atypical and Typical) | 21 | 2.00 | 9.52 | 17 | 1.00 | 5.88 | 1.000 | |
| No Antipsychotics | 21 | 4.00 | 19.05 | 17 | 1.00 | 5.88 | 0.355 | |
| Antidepressants | 21 | 6.00 | 28.57 | 17 | 4.00 | 23.53 | 1.000 | |
| PANSS | ||||||||
| Negative Symptoms | 21 | 18.71 | 6.50 | 17 | 17.00 | 4.76 | 0.91 | 0.371 |
| Positive Symptoms | 21 | 15.29 | 3.80 | 17 | 14.53 | 2.85 | 0.68 | 0.501 |
| Grand Total | 21 | 62.38 | 12.79 | 17 | 59.94 | 8.99 | 0.66 | 0.511 |
| SLOF | ||||||||
| Composite | 21 | 176.00 | 28.30 | 17 | 186.00 | 14.80 | –1.31 | 0.198 |
| UPSA | ||||||||
| Composite | 21 | 60.70 | 15.10 | 17 | 61.40 | 9.86 | –0.17 | 0.862 |
| Exercise | ||||||||
| Time 400 m (s) | 21 | 359.00 | 58.00 | 16 | 345.00 | 57.20 | 0.71 | 0.483 |
| VO2 max (mL/kg/min) | 17 | 28.00 | 10.30 | 12 | 30.50 | 6.69 | –0.73 | 0.473 |
| MATRICS | ||||||||
| Composite | 21 | 26.10 | 12.48 | 17 | 25.47 | 9.87 | 0.17 | 0.868 |
| Domains | ||||||||
| Attention/Vigilance | 21 | 33.67 | 11.35 | 17 | 34.88 | 11.86 | –0.32 | 0.750 |
| Reasoning and Problem Solving | 21 | 43.81 | 8.27 | 17 | 38.76 | 4.25 | 2.28 | 0.029 |
| Social Cognition | 21 | 30.81 | 11.04 | 17 | 34.41 | 14.20 | –0.88 | 0.385 |
| Speed of Processing | 21 | 32.38 | 13.30 | 17 | 34.94 | 15.80 | –0.54 | 0.591 |
| Visual Learning | 21 | 35.62 | 12.48 | 17 | 30.24 | 9.72 | 1.46 | 0.154 |
| Verbal Learning | 21 | 36.71 | 5.41 | 17 | 36.29 | 11.16 | 0.14 | 0.888 |
| Working Memory | 21 | 34.57 | 14.67 | 17 | 34.94 | 9.63 | –0.09 | 0.929 |
| BDNF Concentration (pg/mL) | 21 | 208.89 | 70.07 | 17 | 215.91 | 123.14 | –0.22 | 0.826 |
*Denotes use of Fisher Exact test given small sample size.
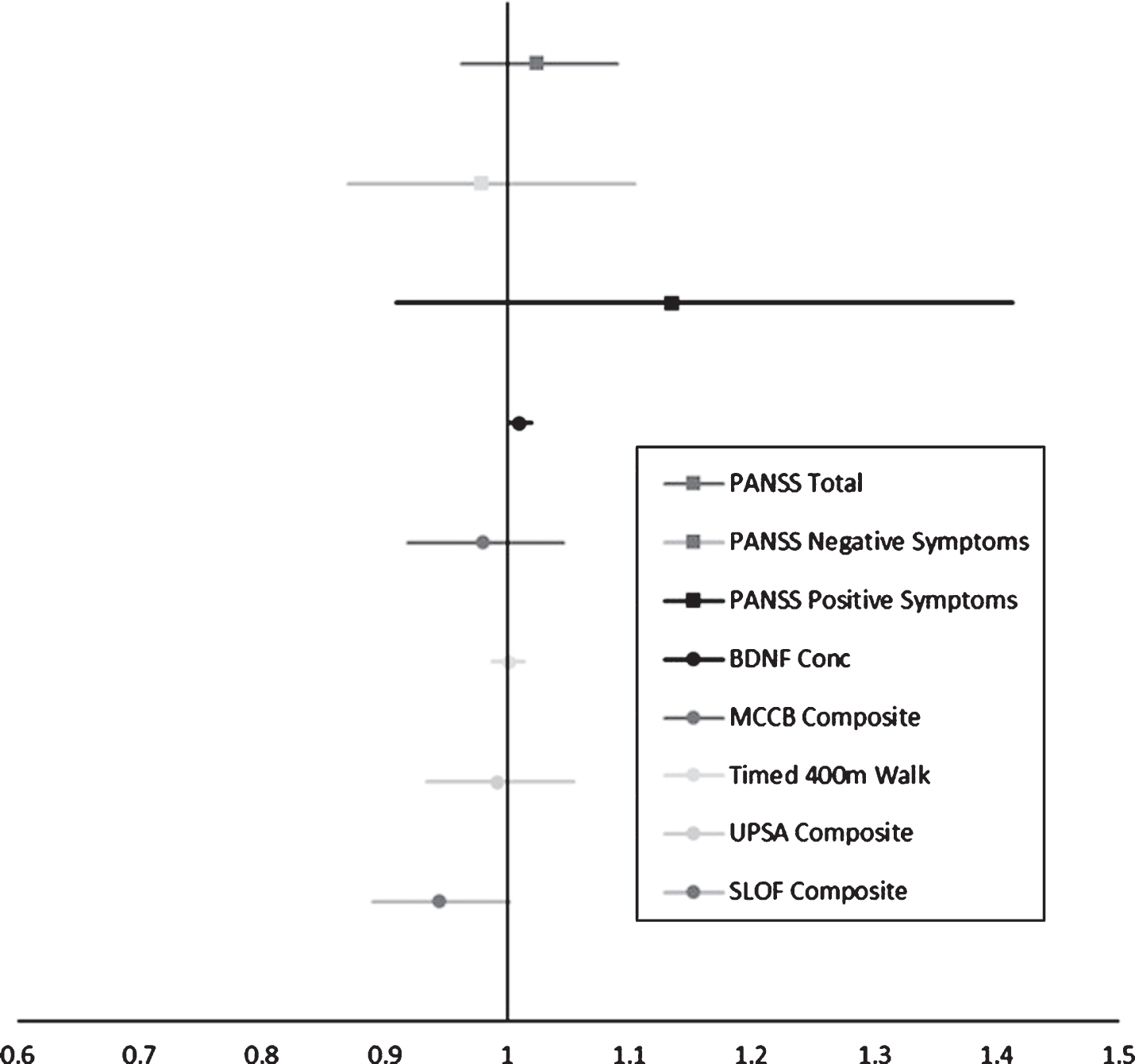
There was significant attrition in both groups. Of those who met primary inclusion criteria, 47, we found that 17 participants who dropped out of the study decided to stop showing up for appointments and were subsequently lost to follow up. There was also a significant proportion of patients, 9, who abruptly decided that they no longer wanted to participate in the study. Further, there were 3 individuals who we were forced to terminate their participation in the study for not complying with study procedures, 2 who withdrew due to transportation issues, and 1 who withdrew due to significant physical sequelae from the exercise paradigm (Fig. 2). To determine whether there were any factors that contributed to dropout, we conducted a logistic regression on the likelihood of dropping out during the study. We found that the likelihood of dropping out was greater for those with a higher concentration of serum BDNF at baseline (OR = 1.01 (CI: 1.001,1.02), p = 0.035). We also found a trend level association such that worse baseline performance on the SLOF predicted a greater likelihood of dropping out of the study (OR = 0.94 (CI: 0.89,1.002), p = 0.057). We also found that while the positive domain of the PANSS was not significant, the hallucinations item was significantly associated with dropping out of the study (OR = 2.186 (CI: 1.099,4.349), p = 0.026). The odds ratios and respective 95% confidence intervals on the likelihood of dropping out of the study attributed to performance on a given outcome measure or symptom domain can be seen in Fig. 3.
Fig. 3
Odds Ratios for likelihood of dropping out of the study using logistic regression. An OR > 1 indicates greater likelihood of dropping out. BDNF was significant (OR = 1.01 (CI: 1.001,1.02), p = 0.035) and SLOF Composite was at a strong trend level (OR = 0.94 (CI: 0.89,1.002), p = 0.057).
Completed cohort results
The final cohort of subjects who completed 12 weeks of treatment included nine in the AE group and six in the CON group. Demographics and treatment characteristics for this group are shown in Table 2. The subjects’ percent compliance in attending treatment sessions was not different between the groups. As intended, time spent in the target heart rate zone was significantly greater in the AE than the CON group (p = 0.008).
Table 2
Baseline demographics for subjects who completed 12 weeks of treatment
| Variables | Aerobic Exercise | Control Condition | ||||||
| N | Mean/Count | Std Dev/% | N | Mean/Count | Std Dev/% | T-test/Chi Sq | P-value | |
| Age | 9 | 53.44 | 7.14 | 6 | 52.67 | 10.07 | 0.16 | 0.874 |
| Total Minutes Exercised | 9 | 1241.61 | 18.19 | 6 | 1258.21 | 59.49 | –0.66 | 0.534 |
| % Time in Target Heart Rate Zone | 9 | 38.56 | 30.95 | 6 | 2.59 | 2.45 | 3.47 | 0.008 |
| % Attendance Compliance | 9 | 90.30 | 5.52 | 6 | 81.29 | 14.35 | 1.47 | 0.192 |
| Sex | ||||||||
| Male | 9 | 7.00 | 77.78 | 6 | 5.00 | 83.33 | 0.47 | 1.000 |
| Race | ||||||||
| African American | 9 | 8.00 | 88.89 | 6 | 6.00 | 100.00 | 0.60 | 1.000 |
| Medication* | ||||||||
| Atypical Antipsychotics | 9 | 7.00 | 77.78 | 6 | 4.00 | 66.67 | 1.000 | |
| Typical Antipsychotics | 9 | 0.00 | 0.00 | 6 | 1.00 | 16.67 | 0.400 | |
| Both (Atypical and Typical) | 9 | 1.00 | 11.11 | 6 | 0.00 | 0.00 | 1.000 | |
| No Antipsychotics | 9 | 1.00 | 11.11 | 6 | 1.00 | 16.67 | 1.000 | |
| Antidepressants | 9 | 2.00 | 22.22 | 6 | 2.00 | 33.33 | 1.000 | |
*Denotes use of Fisher Exact test given small sample size.
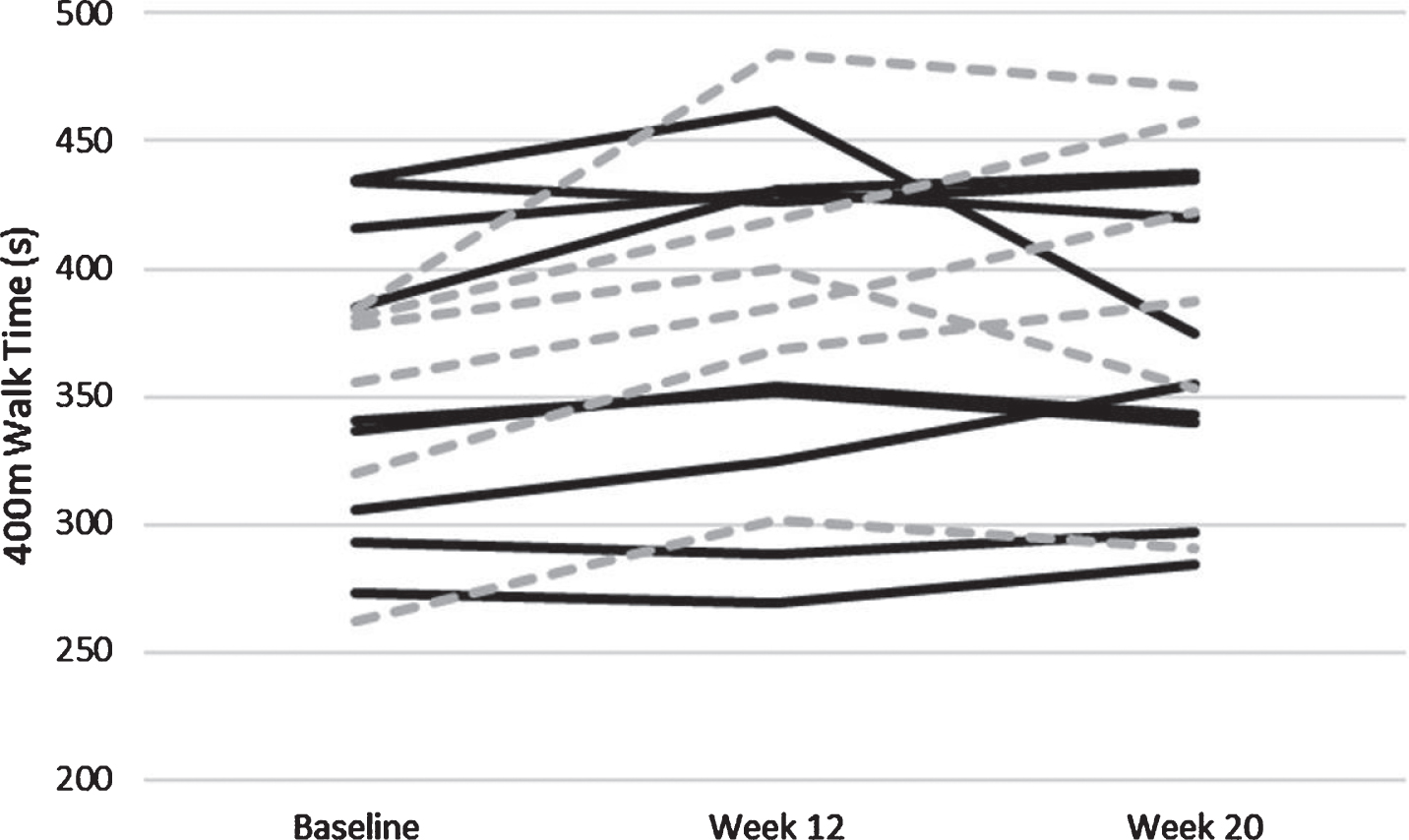
For the second phase of analyses, change scores for functional assessments, cognitive performance, and physical function were computed for Week 12 minus Baseline and for Week 20 minus Week 12. These variables are shown in Tables 3 and 4 respectively. We conducted linear regressions on these change scores that included age, race, sex, BMI, and the baseline value of the outcome measure as additional variables in each model. Regarding the 400 m walk, subjects in both groups were slower at Week 12 than at Baseline, but the AE group had significantly less slowing than the CON group (B = –28.32, p = 0.011; Fig. 4). For other outcome measures there were no significant differences between change scores at Week 12 for the two treatment groups.
Table 3
Change scores Week 12 minus Baseline
| Variables | Aerobic Exercise | Control Condition | ||||||
| N | Mean | Std Dev | N | Mean | Std Dev | T-test | P-value | |
| BMI | 9 | 0.77 | 1.63 | 6 | 0.10 | 1.80 | 0.75 | 0.466 |
| PANSS | ||||||||
| Negative Symptoms | 9 | –6.67 | 5.05 | 6 | –2.50 | 2.81 | –1.83 | 0.091 |
| Positive Symptoms | 9 | –2.78 | 3.67 | 6 | –4.33 | 2.50 | 0.90 | 0.383 |
| Grand Total | 9 | –14.44 | 10.36 | 6 | –10.33 | 10.75 | –0.74 | 0.471 |
| SLOF Composite | 9 | 3.78 | 9.23 | 6 | 0.00 | 14.41 | 0.62 | 0.544 |
| UPSA Composite | 9 | 3.00 | 8.92 | 6 | –3.67 | 8.66 | 1.43 | 0.175 |
| Exercise | ||||||||
| Timed 400 m Walk (s) | 9 | 13.00 | 17.31 | 6 | 46.17 | 27.87 | –2.86 | 0.013 |
| VO2 max (mL/kg/min) | 6 | 1.53 | 7.98 | 4 | –1.26 | 5.86 | 0.60 | 0.567 |
| MATRICS | ||||||||
| Composite | 9 | 2.78 | 6.83 | 6 | 1.50 | 5.68 | 0.38 | 0.712 |
| Attention/Vigilance | 9 | 2.89 | 11.48 | 6 | 1.00 | 5.14 | 0.43 | 0.673 |
| Reasoning and | 9 | –2.56 | 5.27 | 6 | 2.17 | 2.48 | –2.33 | 0.038 |
| Problem Solving | ||||||||
| Social Cognition | 9 | 5.00 | 17.35 | 6 | –1.00 | 10.06 | 0.76 | 0.461 |
| Speed of Processing | 9 | 0.00 | 9.27 | 6 | –0.83 | 7.44 | 0.18 | 0.857 |
| Visual Learning | 9 | 4.33 | 10.10 | 6 | 11.17 | 10.28 | –1.27 | 0.225 |
| Verbal Learning | 9 | –1.33 | 5.52 | 6 | –6.17 | 13.20 | 0.85 | 0.428 |
| Working Memory | 9 | 3.67 | 8.28 | 6 | 0.83 | 6.01 | 0.72 | 0.486 |
| BDNF Concentration | 9 | 6.42 | 67.36 | 6 | 44.28 | 173.11 | –0.51 | 0.628 |
Table 4
Change scores Week 20 minus Week 12
| Variables | Aerobic Exercise | Control Condition | ||||||
| N | Mean | Std Dev | N | Mean | Std Dev | T-test | P-value | |
| BMI | 9 | 0.18 | 0.97 | 6 | –0.08 | 0.51 | 0.60 | 0.557 |
| PANSS | ||||||||
| Negative Symptoms | 9 | –0.67 | 4.64 | 6 | 0.17 | 5.34 | –0.32 | 0.753 |
| Positive Symptoms | 9 | –0.78 | 2.82 | 6 | –0.17 | 2.32 | –0.44 | 0.667 |
| Grand Total | 9 | –1.78 | 9.82 | 6 | –0.33 | 10.73 | –0.27 | 0.792 |
| SLOF Composite | 9 | –2.89 | 6.39 | 6 | –2.83 | 8.59 | –0.01 | 0.989 |
| UPSA Composite | 9 | 3.67 | 7.48 | 6 | 3.17 | 3.97 | 0.15 | 0.884 |
| Exercise | ||||||||
| Timed 400 m Walk (s) | 9 | –5.67 | 33.48 | 6 | 4.00 | 33.63 | –0.55 | 0.594 |
| VO2 max (mL/kg/min) | 5 | 0.97 | 1.60 | 4 | 1.77 | 3.21 | –0.49 | 0.637 |
| MATRICS | ||||||||
| Composite | 9 | 1.78 | 4.66 | 6 | –3.33 | 2.50 | 2.44 | 0.030 |
| Attention/Vigilance | 9 | 2.44 | 4.98 | 6 | 1.33 | 5.65 | 0.40 | 0.694 |
| Reasoning and | 9 | 0.33 | 4.09 | 6 | –0.67 | 4.59 | 0.44 | 0.666 |
| Problem Solving | ||||||||
| Social Cognition | 9 | –2.22 | 13.58 | 6 | –2.67 | 7.00 | 0.07 | 0.943 |
| Speed of Processing | 9 | 0.56 | 9.49 | 6 | –2.67 | 5.39 | 0.75 | 0.467 |
| Visual Learning | 9 | 6.67 | 10.54 | 6 | –4.00 | 4.94 | 2.63 | 0.022 |
| Verbal Learning | 9 | –0.78 | 5.29 | 6 | –2.00 | 5.25 | 0.44 | 0.667 |
| Working Memory | 9 | 0.89 | 5.95 | 6 | –4.17 | 5.81 | 1.63 | 0.128 |
| BDNF Concentration (pg/mL) | 9 | 38.11 | 47.95 | 6 | –6.09 | 137.44 | 0.76 | 0.478 |
Fig. 4
Individually plotted time course of 400 m walk time in seconds. Individuals who were apart of the Aerobic Exercise paradigm are solid black lines, and those apart of the control paradigm are hashed grey lines.
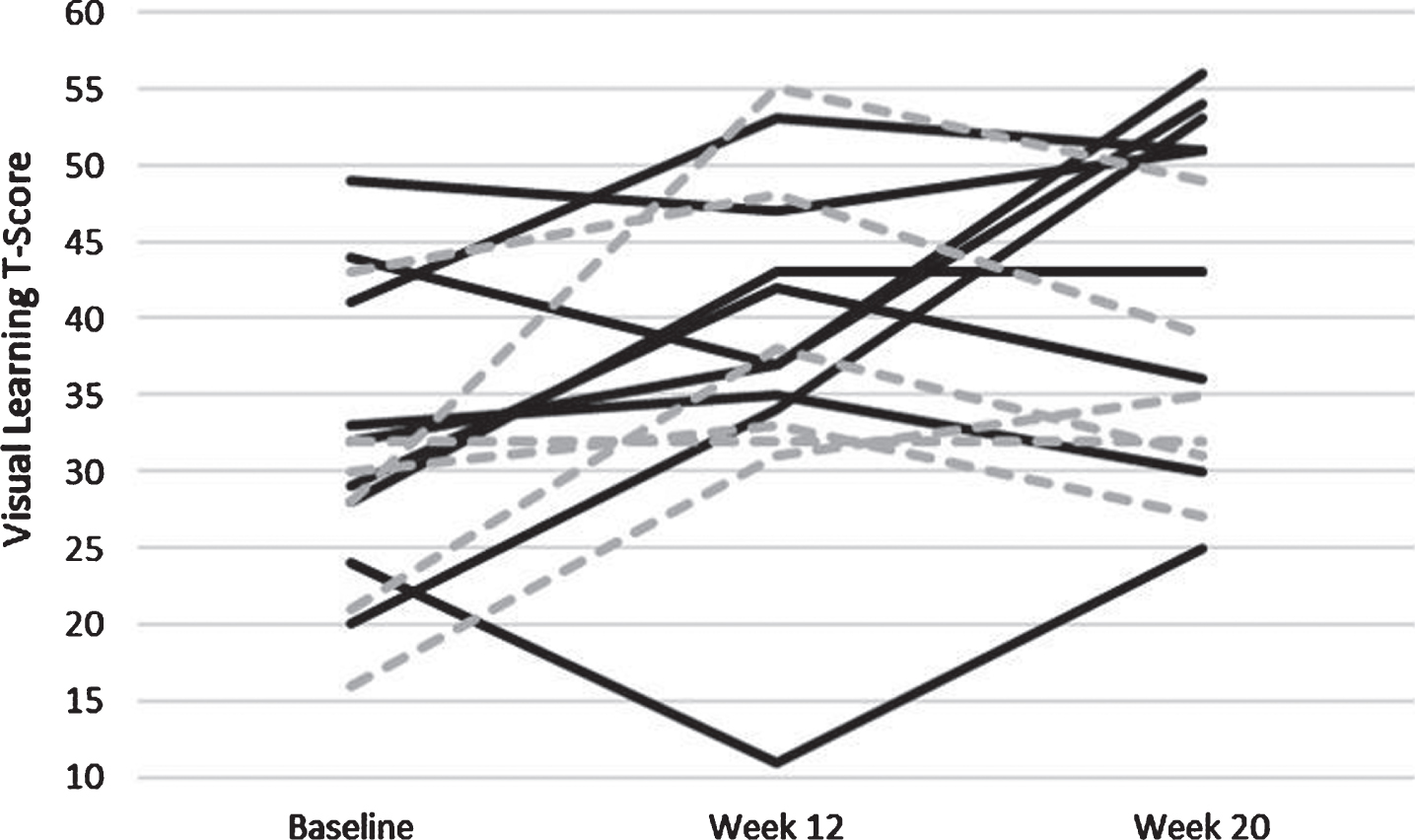
In similarly structured linear regression models with change scores for Week 20 minus Week 12 as the dependent variables, subjects in the AE group improved in the MCCB Composite score from Week 12 to Week 20 whereas those in the CON group worsened: this difference was significant (B = 5.11, p = 0.03; Fig. 5). Similarly, for the Visual Learning domain of the MCCB, subjects in the AE group showed significantly greater improvement compared to CON subjects (B = 13.96, p = 0.006; Fig. 6). Change scores for other outcome measures did not differ significantly between the two treatment groups.
Fig. 5
Individually plotted time course of T-Scores from the Composite Score of the MATRICS. Individuals who were apart of the Aerobic Exercise paradigm are solid black lines, and those apart of the control paradigm are hashed grey lines.
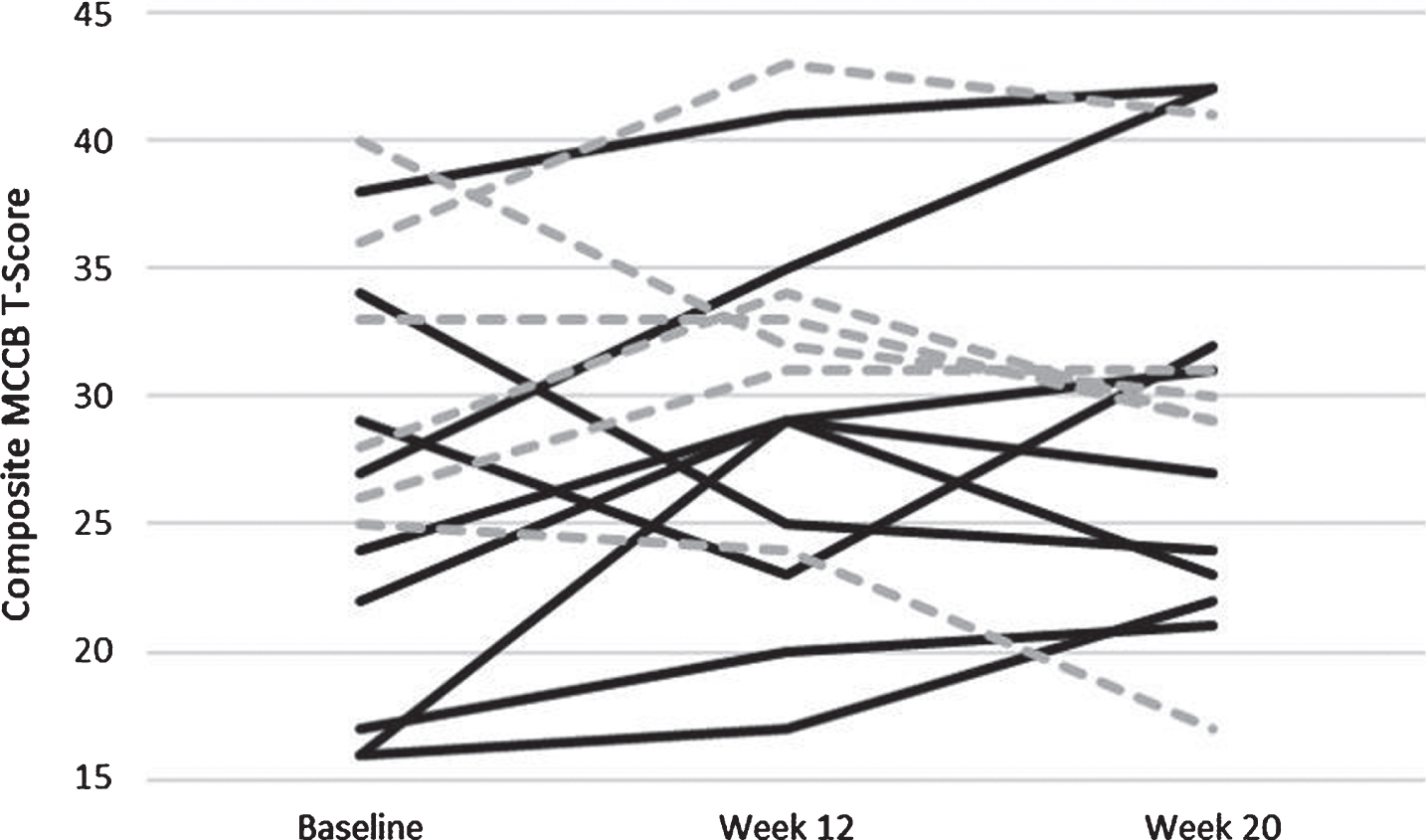
Fig. 6
Individually plotted time course of T-Scores from the Visual Learning Domain of the MATRICS. Individuals who were apart of the Aerobic Exercise paradigm are solid black lines, and those apart of the control paradigm are hashed grey lines.
In regressions that included change in BDNF as an additional variable, increase in BDNF concentration between Baseline and Week 12 was associated with a slower 400 m walk time in both groups (B = 0.093, p = 0.049). Increase in BDNF from Week 12 to Week 20 was associated with a significant increase in the Reasoning and Problem Solving module (B = 0.02, p = 0.008) and a trending increase in functional scores as measured by the SLOF (B = 0.04, p = 0.069). Change in BDNF was not significantly associated with change in other outcome measures in either group.
DISCUSSION
We report effects on cognitive, functional, and physical measures following 12 weeks of AE training compared to a CON condition consisting of balance and stretching training in patients with SCZ in an outpatient treatment setting. The results herein give a preliminary indication of improvement in cognition and a slowing of mobility disability with AE compared to the CON condition in SCZ subjects.
To our knowledge this study is one of the first studies to show an improvement in cognition in SCZ following completion of a 12-week exercise treatment. Interestingly, during the time after the treatment period (Week 12 to Week 20), individuals in the AE group also significantly improved on the MCCB Composite and on the Visual Learning domain. It is possible that this post-treatment improvement could be attributed to improved exercise habits developed by the AE subjects during the course of the treatment that persisted through the post-treatment period. However, without objective measures of physical activity during the post treatment follow up it is difficult to interpret this finding. This finding also fits with a study conducted by Oertel-Knöchel et al. (2014) that found an improvement in the visual learning and other domains of the MCCB after an exercise intervention [45].
In terms of positive and negative symptoms, both treatment groups had a decrease in PANSS scores over the treatment period, although the decrease was not significantly different between the groups. This finding is consistent with prior studies that reported a decrease in symptoms attributed to participation and the associated interactions of group exercise [44, 45, 67].
Contrary to our hypothesis of improved cardiovascular fitness following the intervention in the AE group, VO2 max showed no change. While not surprising for the CON group, this finding is not consistent with previous implementation of the prescribed AE intervention [55, 68–70]. Because the completers in the AE group were compliant with the treatment (as measured by time in HR zone during the 12-week intervention) this null finding may be due to a blunted cardiovascular response to exercise in SCZ from the disease process itself and/or a side effect of medication(s) [71]. However, more research is needed on this issue. Time to complete a 400m walk was slower in both groups at Week 12 when compared to Baseline, but the AE group had statistically less slowing than the CON group. This modest but significant benefit of AE over CON condition may be related to a decreased trajectory towards mobility dysfunction of the AE group as the 400 m walk is primarily used to assess mobility [72]. Despite not “improving” on the 400 m, blunting the decline with AE is an important finding as walking capacity and related muscular fitness are indeed impaired in patients with schizophrenia [71].
It is important to highlight the substantial rate of dropouts in both groups. This high dropout rate may be attributed to the symptoms of SCZ which were quite prominent in both groups and could have decreased their ability to complete the research study. In our initial cohort we found a trend level significance of dropping out associated with worse performance on the SLOF. This makes sense in that lower scores on the SLOF indicate lower functional capabilities such that those subjects would be less likely to complete an exercise program with considerable demands such as those included in our protocol. Indeed, a prior study reported that SLOF scores significantly correlated with increased functional capacity, as indicated by UPSA-B scores, in a cohort of SCZ subjects [73]. Given our current findings, the SLOF could be used as a clinical tool to determine whether exercise would be an appropriate and feasible intervention for an individual patient. A higher level of baseline functioning would indicate a greater level of independence and self-sufficiency that could lend itself to participation and completion of treatment in this modality.
While many studies do not analyze the rate of dropout, we considered these data to be relevant to the feasibility of exercise programs in future research and as clinical interventions. A recent meta-analysis conducted by Vancampfort et al. (2016) found that approximately 74.2% of patients would remain in a study that was conducted similar to ours (aerobic exercise group and active control group). Based on their findings we would expect that having a professional who was supervising the sessions would help improve retention; however, it clearly did not have an effect on our population since our retention rate was 39% [74]. Kimhy et al. (2015) conducted a somewhat similar exercise study in which the retention rate was 79% [44]. Subjects in their study were considerably younger than ours (approximately 37±10 years old vs. 53±8 years old), which may contribute to the differential retention. These authors believed their retention was due to use of an active-play video game that provided exercise training in a more interesting and fun manner than the use of traditional exercise equipment. In consideration of their success with retention compared to the significant attrition we experienced, we speculate that greater retention in exercise training could be achieved through a direct to consumer approach with technology- based and/or home-based exercise programs. This would potentially allow for exercise to be more engaging or completed on an individual’s own timeline in their home, thereby eliminating problems with travel experienced by many of our subjects. On the other hand, such an approach would cut out the human interaction that comes with participation in an exercise class. Our CON condition and AE condition had identical contact time with study staff. This human factor and increased interaction with staff, as well as other participants, may have contributed to the decrease in negative symptoms we saw in both treatment groups. To this point, considerable literature indicates that structured supervised activity in the form of psychosocial interventions can improve course in general and negative symptoms particular in SCZ [75, 76]. It could have been the nonspecific beneficial effects of regular contact with our research staff that improved negative symptoms in both of our subject groups. Even though our study suffered from a poor retention rate, it does not outweigh the potential benefit of an exercise program with regular contact with exercise professionals to patients with schizophrenia.
At Week 12 we found that those with a slower 400 m walk time had higher BDNF concentrations across both treatment groups. This finding is puzzling yet is somewhat in accord with a prior study showing that BDNF was higher in younger subjects who completed low resistance exercise (similar to that found in our CON group) when compared to those completing high resistance training [77, 78]. We also found that there was a significant increase in the Reasoning and Problem Solving domain associated with an increase in BDNF between Week 12 and Week 20. This finding aligns with a previously conducted meta-analysis by Ahmed et al. (2015) which found that higher levels of BDNF expression correlated with improved scores on reasoning and problem-solving tasks [79]. Another interesting association with BDNF was demonstrated by the finding that a higher BDNF at baseline resulted in a greater likelihood of dropping out during the 12-week intervention. We speculate that this finding could be attributed to the medication the subjects were on, since antipsychotic medications, both first-generation and second-generation, have effects on peripheral BDNF concentrations [80]. However, medication alone is not a likely explanation for our findings since there was no significant difference in medication between those dropping out and those who completed the study.
There are significant limitations to this study, most notably the small sample size of completers. The high rate of attrition could be attributed to the symptoms of SCZ such as paranoia and avolition that may have impacted our subjects’ motivation to complete the exercise program [81]. Regarding limitations on our BDNF results, we were unable to measure brain BDNF directly. Despite the knowledge that cerebrospinal fluid BDNF is reduced in those with SCZ and that BDNF crosses the blood brain barrier [50, 82, 83] the correlation between peripheral and central BDNF levels is still unknown and as such makes it hard to discern the effect of AE on BDNF concentrations in nervous tissue. Another limitation of the study is that we did not assess the Val66Met polymorphism of BDNF. This polymorphism has been implicated in impaired learning and memory in individuals both with and without SCZ [84–86]. This polymorphism has also been seen to effect BDNF secreted in response to physical activity, and therefore could be a significant confounder in this study [84]. Another limitation is that there are other micromolecules that have been implicated in the association between exercise and BDNF, such as insulin-like growth factor 1 (IGF-1) and tropomyosin receptor kinase B (TrkB), which we have not analyzed that could significantly impact the results [87].
The homogeneity amongst our subjects is both a limitation and strength of this study. On one hand it reduces the generalizability of the study. On the other hand, it highlights the significant differences found in this small pilot study because they cannot be attributed to differences in the demographics of the two treatment groups. A strength of this study is the use of a positive control intervention consisting of exercise classes of identical time and identical staff contact as per our previously published methods [55, 68–70]. This allows us to discern whether any improvements can be attributed specifically to the AE or could be more generally attributed to participation in a structured program. Another strength of this study is our use of validated measures of physical, cognitive, and everyday functioning that would allow for easy reproducibility.
In summary, these results indicate that participation in a structured aerobic exercise paradigm may modestly enhance cognitive function and blunt physical function decline in individuals with SCZ. This study provides preliminary evidence that aerobic exercise can lead to a cognitive and functional benefit in this population. Future studies should expand upon ours with a larger sample size and increased neurotrophic, metabolomic, and immunologic data to better understand the effects of exercise in a psychiatrically diverse population.
DISCLOSURES
Infrastructure support was provided by the Office of Research and Development, the Mental Health Service Lines, and the Center of Visual and Neurocognitive Rehabilitation at the Atlanta Veterans Affairs Medical Center, Decatur, GA. Additional infrastructure support was provided by the Department of Psychiatry and Behavioral Sciences of the Emory University School of Medicine, Atlanta, GA. E.D. has received research support for work unrelated to this project from Auspex Pharmaceuticals, Inc. and Teva Pharmaceuticals, Inc. Other authors have nothing to disclose. E.D. is a full-time attending psychiatrist in the Mental Health Service Line at the Atlanta Veterans Affairs Medical Center, Decatur, GA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs.
CONTRIBUTORS
J.N. and E.D. designed the study and obtained funding to conduct the study. Data were collected by A.A., K.M., N.T., B.C., M.F., and M.C. J.B. provided clinical lab support for analysis of samples. Analysis of exercise data were designed by N.M., J.N. and E.D. The data were analyzed and interpreted by N.M., J.N., and E.D. The manuscript was written by N.M., M.B. and E.D. All authors reviewed and approved the final manuscript.
ACKNOWLEDGMENTS
Supported by a Veterans Affairs Rehabilitation Research and Development SPiRE Grant (1 I21 RX001897-01 to E.D.), a Veterans Affairs Merit grant (I01RX002825 to J.N.), and a Center of Excellence Grant to the Center for Visual and Neurocognitive Rehabilitation (CVNR) of the Atlanta Veterans Affairs Healthcare System (VARR&D C9246C).
REFERENCES
[1] | McGrath J , Saha S , Chant D , Welham J . Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol Rev. (2008) ;30: :67–76. |
[2] | Harvey PD , Davidson M . Schizophrenia: Course over the lifetime. Neuropsychopharmacology: Fifth Generation of Progress. 2002:641-55. |
[3] | Wiersma D , Wanderling J , Dragomirecka E , Ganev K , Harrison G , An Der Heiden W , et al. Social disability in schizophrenia: Its development and prediction over 15 years in incidence cohorts in six European centres. Psychol Med. (2000) ;30: (5):1155–67. |
[4] | Wu EQ , Birnbaum HG , Shi L , Ball DE , Kessler RC , Moulis M , et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. (2005) ;66: (9):1122–9. |
[5] | Sachdev PS , Blacker D , Blazer DG , Ganguli M , Jeste DV , Paulsen JS , et al. Classifying neurocognitive disorders: The DSM-5 approach. Nat Rev Neurol. (2014) ;10: (11):634–42. |
[6] | Boyer P , Phillips JL , Rousseau FL , Ilivitsky S . Hippocampal abnormalities and memory deficits: New evidence of a strong pathophysiological link in schizophrenia. Brain Res Rev. (2007) ;54: (1):92–112. |
[7] | Pajonk FG , Wobrock T , Gruber O , Scherk H , Berner D , Kaizl I , et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. (2010) ;67: (2):133–43. |
[8] | Weinberger DR . Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry. (1999) ;45: (4):395–402. |
[9] | van der Stouwe ECD , van Busschbach JT , de Vries B , Cahn W , Aleman A , Pijnenborg GHM . Neural correlates of exercise training in individuals with schizophrenia and in healthy individuals: A systematic review. Neuroimage Clin. (2018) ;19: :287–301. |
[10] | Leonard S , Adams C , Breese CR , Adler LE , Bickford P , Byerley W , et al. Nicotinic receptor function in schizophrenia. Schizophr Bull. (1996) ;22: (3):431–45. |
[11] | Benes FM , McSparren J , Bird ED , SanGiovanni JP , Vincent SL . Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. (1991) ;48: (11):996–1001. |
[12] | Tamminga CA , Southcott S , Sacco C , Wagner AD , Ghose S . Glutamate dysfunction in hippocampus: Relevance of dentate gyrus and CA3 signaling. Schizophr Bull. (2012) ;38: (5):927–35. |
[13] | Antonova E , Sharma T , Morris R , Kumari V . The relationship between brain structure and neurocognition in schizophrenia: A selective review. Schizophr Res. (2004) ;70: (2-3):117–45. |
[14] | Buckley PF . Neuroimaging of schizophrenia: Structural abnormalities and pathophysiological implications. Neuropsychiatr Dis Treat. (2005) ;1: (3):193–204. |
[15] | Fitzsimmons J , Kubicki M , Shenton ME . Review of functional and anatomical brain connectivity findings in schizophrenia. Curr Opin Psychiatry. (2013) ;26: (2):172–87. |
[16] | Reid MA , White DM , Kraguljac NV , Lahti AC . A combined diffusion tensor imaging and magnetic resonance spectroscopy study of patients with schizophrenia. Schizophr Res. (2016) ;170: (2-3):341–50. |
[17] | Casanova MF , Rothberg B . Shape distortion of the hippocampus: A possible explanation of the pyramidal cell disarray reported in schizophrenia. Schizophr Res. (2002) ;55: (1-2):19–24. |
[18] | Cotman CW , Berchtold NC . Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. (2002) ;25: (6):295–301. |
[19] | Nieto R , Kukuljan M , Silva H . BDNF and schizophrenia: From neurodevelopment to neuronal plasticity, learning, and memory. Front Psychiatry. (2013) ;4: :45. |
[20] | Numakawa T , Odaka H , Adachi N . Actions of Brain-Derived Neurotrophic Factor and Glucocorticoid Stress in Neurogenesis. Int J Mol Sci. (2017) ;18: (11). |
[21] | Aicardi G , Argilli E , Cappello S , Santi S , Riccio M , Thoenen H , et al. Induction of long- term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. (2004) ;101: (44):15788–92. |
[22] | Poo MM . Neurotrophins as synaptic modulators. Nat Rev Neurosci. (2001) ;2: (1):24–32. |
[23] | Neeper SA , Gomez-Pinilla F , Choi J , Cotman C . Exercise and brain neurotrophins. Nature. (1995) ;373: (6510):109. |
[24] | Neeper SA , Gomez-Pinilla F , Choi J , Cotman CW . Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. (1996) ;726: (1-2):49–56. |
[25] | Nokia MS , Lensu S , Ahtiainen JP , Johansson PP , Koch LG , Britton SL , et al. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol. (2016) ;594: (7):1855–73. |
[26] | Stranahan AM , Khalil D , Gould E . Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. (2007) ;17: (11):1017–22. |
[27] | van Praag H . Neurogenesis and exercise: Past and future directions. Neuromolecular Med. (2008) ;10: (2):128–40. |
[28] | van Praag H , Shubert T , Zhao C , Gage FH . Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. (2005) ;25: (38):8680–5. |
[29] | Liu PZ , Nusslock R . Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front Neurosci. (2018) ;12: :52. |
[30] | Erickson KI , Voss MW , Prakash RS , Basak C , Szabo A , Chaddock L , et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. (2011) ;108: (7):3017–22. |
[31] | Szuhany KL , Bugatti M , Otto MW . A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. (2015) ;60: :56–64. |
[32] | Firth J , Stubbs B , Vancampfort D , Schuch F , Lagopoulos J , Rosenbaum S , et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. (2018) ;166: :230–8. |
[33] | Erickson KI , Leckie RL , Weinstein AM . Physical activity, fitness, and gray matter volume. Neurobiol Aging. (2014) ;35: (Suppl 2):S20–8. |
[34] | Kramer AF , Erickson KI . Effects of physical activity on cognition, well-being, and brain: Human interventions. Alzheimers Dement. (2007) ;3: (2 Suppl):S45–51. |
[35] | Griffin EW , Mullally S , Foley C , Warmington SA , O’Mara SM , Kelly AM . Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. (2011) ;104: (5):934–41. |
[36] | Buckley PF , Mahadik S , Pillai A , Terry A Jr. . Neurotrophins and schizophrenia. Schizophr Res. (2007) ;94: (1-3):1–11. |
[37] | Durany N , Thome J . Neurotrophic factors and the pathophysiology of schizophrenic psychoses. Eur Psychiatry. (2004) ;19: (6):326–37. |
[38] | Shoval G , Weizman A . The possible role of neurotrophins in the pathogenesis and therapy of schizophrenia. Eur Neuropsychopharmacol. (2005) ;15: (3):319–29. |
[39] | Dauwan M , Begemann MJ , Heringa SM , Sommer IE . Exercise Improves Clinical Symptoms, Quality of Life, Global Functioning, and Depression in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr Bull. (2016) ;42: (3):588–99. |
[40] | Dauwan M , Begemann MJH , Slot MIE , Lee EHM , Scheltens P , Sommer IEC . Scheltens P, Sommer IEC. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: A transdiagnostic systematic review and meta-analysis of randomized controlled trials. J Neurol. 2019. |
[41] | Firth J , Stubbs B , Rosenbaum S , Vancampfort D , Malchow B , Schuch F , et al. Aerobic Exercise Improves Cognitive Functioning in People With Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr Bull. (2017) ;43: (3):546–56. |
[42] | Falkai P , Malchow B , Schmitt A . Aerobic exercise and its effects on cognition in schizophrenia. Curr Opin Psychiatry. (2017) ;30: (3):171–5. |
[43] | Lin J , Geng X , Lee EH , Chan SK , Chang WC , Hui CL , et al. Yoga reduces the brain’s amplitude of low-frequency fluctuations in patients with early psychosis results of a randomized controlled trial. Schizophr Res. (2017) ;184: :141–2. |
[44] | Kimhy D , Vakhrusheva J , Bartels MN , Armstrong HF , Ballon JS , Khan S , et al. The Impact of Aerobic Exercise on Brain-Derived Neurotrophic Factor and Neurocognition in Individuals With Schizophrenia: A Single-Blind, Randomized Clinical Trial. Schizophr Bull. (2015) ;41: (4):859–68. |
[45] | Oertel-Knochel V , Mehler P , Thiel C , Steinbrecher K , Malchow B , Tesky V , et al. Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. (2014) ;264: (7):589–604. |
[46] | Harvey PD , Green MF , Keefe RS , Velligan DI . Cognitive functioning in schizophrenia: A consensus statement on its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry. (2004) ;65: (3):361–72. |
[47] | Rimes RR , de Souza Moura AM , Lamego MK , de Sa Filho AS , Manochio J , Paes F , et al. Effects of Exercise on Physical and Mental Health, and Cognitive and Brain Functions in Schizophrenia: Clinical and Experimental Evidence. CNS Neurol Disord Drug Targets. (2015) ;14: (10):1244–54. |
[48] | Heinrichs RW , Zakzanis KK . Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. (1998) ;12: (3):426–45. |
[49] | Tripathi A , Kar SK , Shukla R . Cognitive Deficits in Schizophrenia: Understanding the Biological Correlates and Remediation Strategies. Clin Psychopharmacol Neurosci. (2018) ;16: (1):7–17. |
[50] | Vasic N , Connemann BJ , Wolf RC , Tumani H , Brettschneider J . Cerebrospinal fluid biomarker candidates of schizophrenia: Where do we stand? Eur Arch Psychiatry Clin Neurosci. (2012) ;262: (5):375–91. |
[51] | Godin G , Shephard RJ . A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. (1985) ;10: (3):141–6. |
[52] | Gionet NJ , Godin G . Self-reported exercise behavior of employees: A validity study. J Occup Med. (1989) ;31: (12):969–73. |
[53] | Karvonen MJ , Kentala E , Mustala O . The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. (1957) ;35: (3):307–15. |
[54] | Camarda SR , Tebexreni AS , Pafaro CN , Sasai FB , Tambeiro VL , Juliano Y , et al. Comparison of maximal heart rate using the prediction equations proposed by Karvonen and Tanaka. Arq Bras Cardiol. (2008) ;91: (5):311–4. |
[55] | McGregor KM , Crosson B , Krishnamurthy LC , Krishnamurthy V , Hortman K , Gopinath K , et al. Effects of a 12-Week Aerobic Spin Intervention on Resting State Networks in Previously Sedentary Older Adults. Front Psychol. (2018) ;9: :2376. |
[56] | Simonsick EM , Fan E , Fleg JL . Estimating cardiorespiratory fitness in well-functioning older adults: Treadmill validation of the long distance corridor walk. J Am Geriatr Soc. (2006) ;54: (1):127–32. |
[57] | Simonsick EM , Montgomery PS , Newman AB , Bauer DC , Harris T . Measuring fitness in healthy older adults: The Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. (2001) ;49: (11):1544–8. |
[58] | Simonsick EM , Newman AB , Nevitt MC , Kritchevsky SB , Ferrucci L , Guralnik JM , et al. Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. (2001) ;56: (10):M644–9. |
[59] | Newman AB , Haggerty CL , Kritchevsky SB , Nevitt MC , Simonsick EM , Health ABCCRG . Walking performance and cardiovascular response: Associations with age and morbidity–the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. (2003) ;58: (8):715–20. |
[60] | Newman AB , Simonsick EM , Naydeck BL , Boudreau RM , Kritchevsky SB , Nevitt MC , et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. (2006) ;295: (17):2018–26. |
[61] | Nocera JR , Mammino K , Kommula Y , Wharton W , Crosson B , McGregor KM . Effects of Combined Aerobic Exercise and Cognitive Training on Verbal Fluency in Older Adults. Gerontol Geriatr Med. (2020) ;6: :2333721419896884. |
[62] | Keefe RS , Vinogradov S , Medalia A , Silverstein SM , Bell MD , Dickinson D , et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. (2011) ;37: (5):1057–65. |
[63] | Patterson TL , Goldman S , McKibbin CL , Hughs T , Jeste DV . UCSD Performance-Based Skills Assessment: Development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. (2001) ;27: (2):235–45. |
[64] | Schneider LC , Struening EL . SLOF: A behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr. (1983) ;19: (3):9–21. |
[65] | Kay SR , Fiszbein A , Opler LA . The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) ;13: (2):261–76. |
[66] | Twisk J , Boesman L , Hoekstra T , Rijnhart J , Welten M , Heymans M . Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun. (2018) ;10: :80–5. |
[67] | Scheewe TW , Backx FJ , Takken T , Jorg F , van Strater AC , Kroes AG , et al. Exercise therapy improves mental and physical health in schizophrenia: A randomised controlled trial. Acta Psychiatr Scand. (2013) ;127: (6):464–73. |
[68] | McGregor KM , Crosson B , Mammino K , Omar J , Garcia PS , Nocera JR . Influences of 12- Week Physical Activity Interventions on TMS Measures of Cortical Network Inhibition and Upper Extremity Motor Performance in Older Adults-A Feasibility Study. Front Aging Neurosci. (2017) ;9: :422. |
[69] | Nocera J , Crosson B , Mammino K , McGregor KM . Changes in Cortical Activation Patterns in Language Areas following an Aerobic Exercise Intervention in Older Adults. Neural Plast. (2017) ;2017: :6340302. |
[70] | Nocera JR , McGregor KM , Hass CJ , Crosson B . Spin exercise improves semantic fluency in previously sedentary older adults. J Aging Phys Act. (2015) ;23: (1):90–4. |
[71] | Vancampfort D , Probst M , De Herdt A , Corredeira RM , Carraro A , De Wachter D , et al. An impaired health related muscular fitness contributes to a reduced walking capacity in patients with schizophrenia: A cross-sectional study. BMC Psychiatry. (2013) ;13: :5. |
[72] | Fielding RA , Rejeski WJ , Blair S , Church T , Espeland MA , Gill TM , et al. The Lifestyle Interventions and Independence for Elders Study: Design and methods. J Gerontol A Biol Sci Med Sci. (2011) ;66: (11):1226–37. |
[73] | Cardenas V , Abel S , Bowie CR , Tiznado D , Depp CA , Patterson TL , et al. When functional capacity and real-world functioning converge: The role of self-efficacy. Schizophr Bull. (2013) ;39: (4):908–16. |
[74] | Vancampfort D , Rosenbaum S , Schuch FB , Ward PB , Probst M , Stubbs B . Prevalence and predictors of treatment dropout from physical activity interventions in schizophrenia: A meta- analysis. Gen Hosp Psychiatry. (2016) ;39: :15–23. |
[75] | Dixon LB , Dickerson F , Bellack AS , Bennett M , Dickinson D , Goldberg RW , et al. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull. (2010) ;36: (1):48–70. |
[76] | Elis O , Caponigro JM , Kring AM . Psychosocial treatments for negative symptoms in schizophrenia: Current practices and future directions. Clin Psychol Rev. (2013) ;33: (8):914–28. |
[77] | Forti LN , Van Roie E , Njemini R , Coudyzer W , Beyer I , Delecluse C , et al. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp Gerontol. (2015) ;70: :144–9. |
[78] | Forti LN , Van Roie E , Njemini R , Coudyzer W , Beyer I , Delecluse C , et al. Effects of resistance training at different loads on inflammatory markers in young adults. Eur J Appl Physiol. (2017) ;117: (3):511–9. |
[79] | Ahmed AO , Mantini AM , Fridberg DJ , Buckley PF . Brain-derived neurotrophic factor (BDNF) and neurocognitive deficits in people with schizophrenia: A meta-analysis. Psychiatry Res. (2015) ;226: (1):1–13. |
[80] | Favalli G , Li J , Belmonte-de-Abreu P , Wong AH , Daskalakis ZJ . The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res. (2012) ;46: (1):1–11. |
[81] | Mittal VA , Vargas T , Osborne KJ , Dean D , Gupta T , Ristanovic I , et al. Exercise Treatments for Psychosis: A Review. Curr Treat Options Psychiatry. (2017) ;4: (2):152–66. |
[82] | Nurjono M , Lee J , Chong SA . A Review of Brain-derived Neurotrophic Factor as a Candidate Biomarker in Schizophrenia. Clin Psychopharmacol Neurosci. (2012) ;10: (2):61–70. |
[83] | Pan W , Banks WA , Fasold MB , Bluth J , Kastin AJ . Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. (1998) ;37: (12):1553–61. |
[84] | Egan MF , Kojima M , Callicott JH , Goldberg TE , Kolachana BS , Bertolino A , et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. (2003) ;112: (2):257–69. |
[85] | Goldberg TE , Iudicello J , Russo C , Elvevag B , Straub R , Egan MF , et al. BDNF Val66Met polymorphism significantly affects d’ in verbal recognition memory at short and long delays. Biol Psychol. (2008) ;77: (1):20–4. |
[86] | Ho BC , Milev P , O’Leary DS , Librant A , Andreasen NC , Wassink TH . Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. (2006) ;63: (7):731–40. |
[87] | Vakhrusheva J , Marino B , Stroup TS , Kimhy D . Aerobic Exercise in People with Schizophrenia: Neural and Neurocognitive Benefits. Curr Behav Neurosci Rep. (2016) ;3: (2):165. |



