The Influence of Aerobic Exercise on Hippocampal Integrity and Function: Preliminary Findings of a Multi-Modal Imaging Analysis
Abstract
Aerobic exercise (AE) interventions represent promising therapeutic approaches in disorders that compromise hippocampal integrity, but a more comprehensive account of the neural mechanisms stimulated by AE in the human brain is needed. We conducted a longitudinal pilot-study to assess the impact of a 12-week AE intervention on hippocampal structure and function in 10 healthy, human participants (50% females; 25–59 years). Using a novel combination of multimodal MRI techniques, we found significant increases in left hippocampal volume, Cornu Ammonis subfield area 1, NAA concentration and immediate verbal recall performance. Our preliminary findings highlight the utility of a multimodal approach in assessing hippocampal integrity.
The hippocampus is a region that harbors a considerable degree of neuroplasticity, such that it is able to undergo changes to its structure and function in response to intrinsic and extrinsic influences. This makes it particularly susceptible to neurodegenerative influences, including ageing and disorders such as Schizophrenia and Alzheimer’s Disease; but also quite receptive to neuroprotective influences, such as those induced by aerobic exercise (AE) [1]. Therefore, it is possible that AE could be a useful clinical tool in the treatment of disorders that have a particularly detrimental impact on the hippocampus.
Existing literature provides strong support for the notion of AE inducing positive hippocampal neuroplastic changes. Research using rodents has consistently demonstrated that AE can increase the rate of synaptic plasticity, neurogenesis, angiogenesis, and the circulation of important neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) in the hippocampus [2]. Furthermore, several longitudinal studies using healthy human participants have demonstrated AE interventions to be associated with various macro-scale indicators of plasticity, such as growth in hippocampal volume [3–6] and improvements in white-matter integrity [7]. However, given the highly invasive nature of the techniques typically used to measure molecular or cellular changes in the brain, replicating micro-scale findings in the human brain remains a challenge. Furthering the use of techniques that translate findings in the animal literature to human studies will contribute to a comprehensive understanding of how AE affects the human brain and will be important in determining its clinical utility.
Prior human research has primarily investigated the impact of AE using only a single neuroimaging modality (e.g. structural magnetic resonance imaging; sMRI), with only a small number of longitudinal studies measuring multiple components of hippocampal integrity [4–6, 8]. Using multiple imaging techniques provides a more comprehensive account of the changes that occur in response to an intervention. For example, a recent study by Wagner [9] found AE to be associated with a 2% decrease in hippocampal volume, which contradicts the majority of the literature showing AE to increase or have no effect on hippocampal volume [10, 11]. However, this study also employed MR-spectroscopy (MRS) and showed that AE had no impact on levels of N-acetylaspartate (NAA), a metabolite that is only found in neurons, considered a marker of neuronal health [12] and generally linked to AE-induced hippocampal growth [8, 13]. This finding allowed the authors to conclude that the decrease in hippocampal volume is unlikely to be driven by a loss of neurons (as NAA did not decrease), but more likely to be driven by changes in glial cells. This demonstrates the additional detail that can be attained using multimodal imaging, allowing a more informed debate as to how AE impacts the hippocampus.
The current study employed multimodal MRI to track changes in human hippocampal integrity following a 12-week AE intervention. Previous studies have validated this intervention length, with several trials eliciting changes in hippocampal gray-matter integrity after 12-weeks or less of AE training [5, 6]. Extending upon previous studies, we assessed (1) hippocampal volume and its constituent subfield (i.e. cornu ammonis, CA1-3 and dentate gyrus) volumes to identify both global and subfield-specific volumetric changes (T1/T2-weighted sMRI); (2) fractional anisotropy (FA) of the fimbria and hippocampal portion of the cingulum bundle to characterize hippocampal white matter microstructure integrity (diffusion tensor imaging; DTI); (3) N-acetylaspartate (NAA) levels to construct an in vivo biochemical profile informative of neuronal viability (MRS); (4) memory performance (hippocampus-dependent memory tasks); and (5) aerobic fitness (maximum volume of oxygen consumption; VO2max). It was hypothesised that following the 12-week AE intervention, we would see indications of improved hippocampal integrity. Specifically, we anticipated significant increases in hippocampal volume, primarily within the dentate gyrus, increased NAA concentration, and increased FA values within the fimbria and hippocampal portion of the cingulum bundle. We hypothesised that these changes would be associated with increases in VO2max.
Ethics approval was obtained from the Monash University Human Research Ethics Committee (MUHREC). A total of 14 participants were recruited, of which four withdrew for reasons of injury or not being able to attend the follow-up assessment. Data from 10 participants was therefore used in the final statistical analysis (50% female, 25 to 59 years, Mdn = 39, M = 41 years, SD = 10.93). Subjects were required to self-report a low to moderate pre-intervention fitness level as measured by the the International Physical Activity Questionnaire (IPAQ) (range 33 – 2940 MET minutes/week, M = 967.10, SD = 850.43), report no contraindications to aerobic exercise, nor any history of substance abuse, neurological disease, infarcts, or psychiatric illness.
The intervention was conducted over 12-weeks during which participants were instructed to complete three or more sessions of aerobic exercise per week. The duration (excluding warm-up and cool-down) of individual sessions was increased incrementally over the course of the 12-weeks, from 30 minutes (week 1–4), to 40 minutes (week 5–8), to 50 minutes (week 9–12). Two different exercise modalities were alternated between sessions: a continuous session performed at a heart rate equivalent of 70% VO2max and an interval session alternating between 80% for 3 minutes (work phase) and 60% for 3 minutes (rest phase). The higher-intensity interval modality was included to elicit training adaptation to enhance VO2max in the subsequent post-intervention re-test [14]. Participants could complete sessions in supervised group exercise classes, which were conducted on a cycle ergometer, or in their own time, which could include cycling, running or walking. Exercise engagement outside of supervised group exercise classes was monitored remotely using a Garmin VivoFit. All participants met the requirements of the exercise program.
Within three days pre- and post-intervention, participants underwent a comprehensive assessment. The components of this assessment were as follows:
Cognitive assessment: This included two measures of hippocampus-dependent memory function; the Rey Auditory Verbal Learning Task (RAVLT) and the Spatial Pattern Separation Task (SPST) [15]. Alternate versions of the RAVLT were used for the pre- and post-intervention assessments.
Physical assessment: Maximal oxygen uptake (VO2max) was estimated by means of a continuous incremental exercise test to volitional exhaustion on a motorized treadmill, during which gas (O2 and CO2) exchange was continuously monitored (breath-by-breath analysis). Two of the following criteria needed to be met to achieve VO2max: (1) a plateau in VO2 irrespective of increases in external work, (2) maximal respiratory exchange ratio of ≥1.10 and (3) a heart rate within 10 beats per minute of age-predicted heart rate max.
MRI assessment: The MRI sessions were conducted using a 3T Siemens Skyra, including a T1-weighted sMRI (TR/TE/TI = 2300/3/950 ms, 1.0×1.0×1.0 mm3), a high-resolution T2-weighted sMRI (TR/TE = 3990/21 ms, 0.4×0.4×2.6 mm, 24 A/P slices without gap) for hippocampal subfield segmentation [16], single voxel 1H-MRS at left hippocampus (TR/TE = 2000/30 ms, 256 averages, 20×15×30 mm) and the diffusion-tensor imaging (DTI, β-value = 3000 s/mm, 60 direction, 2.5×2.5×2.5 mm, TR/TE = 8800/110 ms). To assess the hippocampal volume, Automatic Segmentation of Hippocampal Subfields (ASHS) was used to segment hippocampus into eight subfield regions using the default template provided [16]: cornu ammonis (CA) 1, 2 and 3, dentate gyrus (DG) and subiculum. Total hippocampal volume was defined as sum of CA1, CA2, CA3, DG and subiculum regions, then corrected for ICV (intracranial volume, generated by ASHS) using a similar method as Erickson et al. [3]. For MRS analysis, LC-model (version 6.3-1H) was used to quantify the concentration of total N-acetylaspartate (NAA) and creatine (Cr), after quality control (Cramer-Rao lower bound <20%, signal-to-noise ratios >10). Metabolite peaks were measured within the left hippocampus, as this is the lateralization which is evidenced to display the greater degree of exercise induced neuroplasticity [8, 11]. The relative amplitude, i.e., NAA/Cr was used in this report for further analysis [12]. We followed the same pipeline reported in Yücel et al. [17] to assess hippocampal white matter integrity, which is defined by the mean of fractional anisotropy (FA) in two regions of interest: the fimbria and the hippocampal portion of the cingulum bundle defined by JHU-ICBM atlas.
We compared mean scores from pre- to post-exercise intervention for all assessments. As a result of the small sample size (N = 10), the criteria were not met for parametric tests. A Wilcoxon signed rank test was conducted using an alpha level of 0.05. Effect sizes were reported as r2 values (proportion of variance explained), where small = 0.01, medium = 0.09 and large = 0.25.
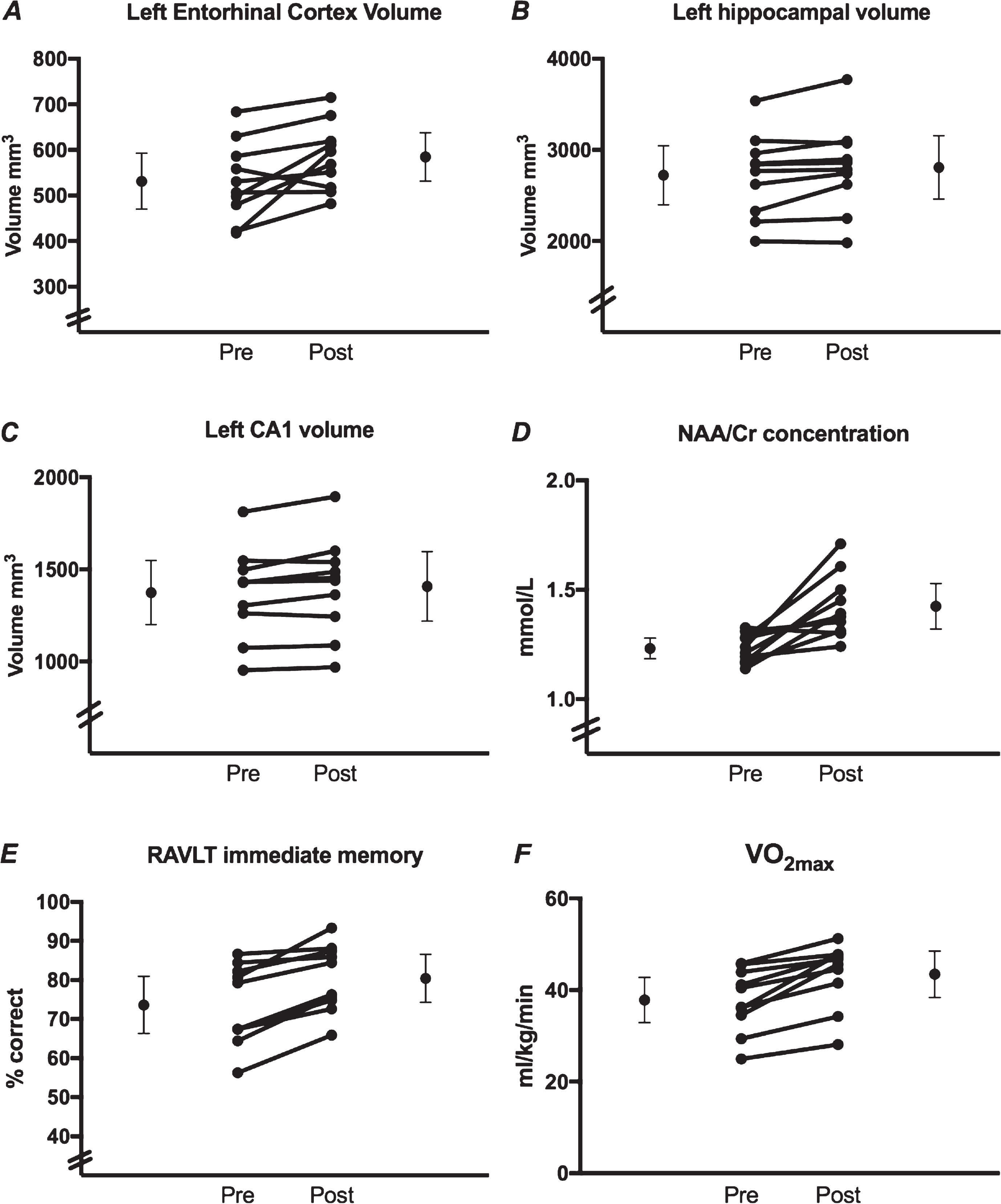
From pre- to post-intervention, the results (Table 1) indicated statistically significant increases in left Entorhinal Cortex (ERC) volume of 10.01% (Fig. 1A), left hippocampal volume of 3.15% (Fig. 1B), left CA1 volume of 2.45% (Fig. 1C), NAA/Cr concentration of 16.26% (Fig. 1D), 6.38% improvement on immediate verbal memory performance (as measured by the RAVLT) (Fig. 1E), and a 15% increase in VO2max (Fig. 1F). There were no significant differences in any other outcome measures.
Table 1
Means (SD), Wilcoxon signed-rank Z values and effect sizes (r2) for measures of hippocampal integrity and function, and cardiorespiratory fitness pre- and post- 12-week exercise intervention
| Pre-intervention | Post-intervention | Z | r2 | |
| Hippocampal integrity (MRI) | ||||
| (L) Entorhinal cortex | 531.45 (85.71) | 584.66 (74.16) | 2.29* | 0.42 |
| (R) | 612.26 (40.43) | 593.78 (75.79) | – | |
| (L) Total Hippocampal Volume | 2722.95 (453.65) | 2808.82 (486.20) | 2.29* | 0.26 |
| (R) | 2836.18 (406.07) | 2813.55 (437.55) | 0.46 | – |
| (L) DG volume | 844.96 (123.93) | 843.06 (145.35) | 0.36 | – |
| (R) | 890.80 (129.12) | 892.68 (145.17) | 0.15 | – |
| (L) CA volume | 1441.10 (253.33) | 1478.26 (275.62) | 1.89 | – |
| (R) | 1471.84 (239.06) | 1446.45 (234.42) | 1.68 | – |
| (L) CA1 volume | 1373.68 (243.25) | 1407.38 (263.98) | 2.19* | 0.24 |
| (R) | 1383.00 (217.03) | 1361.18 (222.45) | 1.68 | – |
| (L) CA2 volume | 19.62 (7.04) | 19.91 (7.44) | 0.05 | – |
| (R) | 24.02 (6.30) | 24.33 (4.42) | 1.07 | – |
| (L) CA3 volume | 47.80 (9.33) | 50.97 (11.22) | 0.97 | – |
| (R) | 64.82 (31.14) | 60.95 (18.27) | 0.56 | – |
| (L) Subiculum volume | 346.52 (114.02) | 381.67 (83.71) | 1.78 | – |
| (R) | 378.43 (63.80) | 385.59 (70.56) | 0.76 | – |
| NAA/Cr (mmol/L) | 1.23 (0.07) | 1.43 (0.15) | 2.70** | 0.37 |
| FA | 0.388 (0.03) | 0.391 (0.03) | 1.17 | |
| Hippocampal function (RAVLT) | ||||
| SPST (% correct) | 91.1 (6.39) | 91.4 (6.02) | 0.46 | – |
| Immediate memory (% correct) | 71.05 (8.49) | 77.43 (8.79) | 2.81** | 0.40 |
| Delayed memory (% correct) | 76 (23.14) | 82.67 (16.98) | 1.45 | – |
| Physical fitness | ||||
| VO2max ml/kg/min | 37.83 (6.94) | 43.47 (7.10) | 2.83** | 0.40 |
**p≤0.01, *p≤0.05; n = 10; All volumes are ICV-corrected and in mm3; L = Left, R = Right, DG = dentate gyrus, CA = Cornu Ammonis, NAA/Cr = N-acetyl-aspartate and creatine ratio, FA = fractional anisotrophy for the fimbria tract and hippocampal portion of the cingulum tract, SPST = spatial pattern separation task score, VO2max (ml/kg/min) = maximum volume of oxygen uptake in milliliters per kilogram per minute, RAVLT = Rey Auditory Verbal Learning Task, MRI = Magnetic Resonance Imaging.
Fig.1
Individual data points pre- and post- 12-week exercise intervention for outcome variables showing statistically significant change. The means and 95% confidence intervals are represented by the outer circles. The inner circles show individual subject data.
Post hoc correlation tests were conducted between degree of change in aerobic fitness level, and all cognitive and neurobiological variables that showed significant changes following intervention. As performed in Erickson et al. [3], variables were converted into a percentage change. Due to deviations from normal distribution, non-parametric Spearman correlations were conducted. We found no significant association between improvements in fitness and indicators of hippocampal integrity.
Employing a novel multi-modal imaging sequence, we found preliminary evidence to support structural changes in medial temporal lobe (MTL) regions following a 12-week AE intervention. We identified a volumetric increase in the left hippocampus, similar to that of previous work demonstrating unilateral increases in left hippocampal volume following an AE intervention [11]. Upon segmenting the hippocampi into its constituent subfields, we found that the hippocampal growth was driven by a significant volumetric increase in the CA1 region. This is in contrast to pre-clinical studies that have shown volume increases primarily in the DG, the site where neurogenesis occurs [18], as well as human studies which found pronounced increases in the hippocampal head where the DG in located [3, 6]. This may mean the CA1 volume increase was driven by another component of neuroplasticity, such as angiogenesis, synaptic plasticity, or glial cell density. For example, other preclinical studies have demonstrated increased dendritic spine density in region CA1 following long-term running [19]. Interestingly, the same study also observed changes in dendritic morphology in the ERC, which may help to account for the left ERC volumetric increase elicited in the current study.
While micro-scale neuroplastic changes are relatively hard to detect in humans in vivo, we used MRS to assess NAA. Hippocampal NAA levels increased by ≈16%, suggesting AE is also associated with improvements in neuronal integrity, and supports the idea that changes to neuronal morphology may be contributing to volumetric changes [8]. Comparison of pre- and post-intervention FA values indicated that there were no significant changes to white matter microstructure within the fimbria and hippocampal proportion of the cingulum bundle. Prior research investigating the relationship between AE and local white matter integrity has been inconsistent [7] and this study is the first to examine hippocampus specific fiber tracts within this context. It is therefore difficult to conclude if this finding represents the true impact of AE of hippocampal white matter integrity or if changes were not detectable because of our small sample size.
While aerobic fitness was significantly improved in the participants, this was not directly correlated with changes in hippocampal integrity or function. This contrasts with the results of previous studies that have demonstrated hippocampal volume [3], NAA concentration [13] and hippocampus-dependent memory performance [3] are positively associated with higher aerobic fitness in healthy individuals. This discrepancy may be the result of some individuals displaying a greater change in their aerobic fitness than others, despite completing similar levels of AE engagement. Previous research has suggested that individuals vary in the extent to which they respond to AE interventions and this can influence the relationship between aerobic fitness (as assessed here via VO2max) and brain-related changes [20]. Our findings may have been more susceptible to this variability given the relatively small sample size.
The findings of this study should be considered preliminary and interpreted in light of certain limitations. Most notable being the small sample size and absence of a control group. A randomized-controlled trial of these exercise parameters with a larger, well-powered sample size is required to validate these findings. Despite this, our study has highlighted the merit of using a multimodal assessment of hippocampal integrity within a longitudinal design. Employing multiple imaging techniques, such as the simultaneous use of MRI, MRS and DTI as well as using subfield segmentation, will allow future research to build a more comprehensive measure of hippocampal integrity, that may account for AE’s impact on hippocampal structure and function. Work of this nature will help to bridge the gap between animal and human research by corroborating neuroplastic changes that occur on the macroscopic and microscopic level, as well as providing a more robust model for using AE to treat various disorders in humans.
CONFLICT OF INTEREST
Ms. Den Ouden, Mr. Kandola, Dr. Suo, Mr. Hendrikse, Dr. Costa, Prof. Watt, Dr. Lorenzetti, Ms. Chye, Mr Parkes, Ms. Sabaroedin and Prof. Yücel declare that they have no conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank staff from Heath at Work Occupational Health Service, 4/75 Lorimer Street, South Wharf, VIC 3006, for assisting with participant recruitment. This research was supported by funding from Monash University and the David Winston Turner Endowment Fund. Professor Murat Yücel (#APP1117188) and Professor Matthew Watt (#APP1077703) are supported by Fellowships from the National Health and Medical Research Council of Australia.
REFERENCES
[1] | Bartsch T , Wulff P . The hippocampus in aging and disease: From plasticity to vulnerability. Neuroscience. (2015) ;309: :1–16. doi:10.1016/j.neuroscience.2015.07.084 |
[2] | Voss MW , Vivar C , Kramer AF , et al. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. (2013) ;17: :525–44. doi:10.1016/j.tics.2013.08.001 |
[3] | Erickson KI , Voss MW , Prakash RS , et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. (2011) ;108: :3017–22. doi:10.1073/pnas.1015950108 |
[4] | Kleemeyer MM , Kühn S , Prindle J , et al. Changes in fitness are associated with changes in hippocampal microstructure and hippocampal volume among older adults. Neuroimage. (2016) ;131: :155–61. doi:10.1016/j.neuroimage.2015.11.026 |
[5] | Maass A , Düzel S , Goerke M , et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. (2015) ;20: :585–93. doi:10.1038/m2014.114 |
[6] | Thomas AG , Dennis A , Rawlings NB , et al. Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. Neuroimage. (2016) ;131: :162–70. doi:10.1016/j.neuroimage.2015.10.090 |
[7] | Sexton C , Betts JF , Demnitz N , et al. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage. (2016) ;131: :81–90. doi:10.1016/j.neuroimage.2015.09.071 |
[8] | Pajonk FG , Wobrock T , Gruber O , et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. (2010) ;67: :133–43. doi:10.1001/archgenpsychiatry.2009.193 |
[9] | Wagner G , Herbsleb M , de la Cruz F , et al. Hippocampal Structure, Metabolism, and Inflammatory Response after a 6-Week Intense Aerobic Exercise in Healthy Young Adults: A Controlled Trial. J Cereb Blood Flow Metab. (2015) ;35: 1570–8. doi:10.1038/jcbfm.2015.125 |
[10] | Kandola A , Hendrikse J , Lucassen PJ , et al. Aerobic exercise as a tool to improve hippocampal plasticity and function in humans: Practical implications for mental health treatment. Front Hum Neurosci. (2016) ;10: :373. doi:10.3389/fnhum.2016.00373 |
[11] | Firth J , Stubbs B , Vancampfort D , et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. (2018) ;166: :230–8. doi:10.1016/j.neuroimage.2017.11.007 |
[12] | Mostert JP , Blaauw Y , Koch MW , et al. Reproducibility over a 1-month period of 1H-MR spectroscopic imaging NAA/Cr ratios in clinically stable multiple sclerosis patients. Eur Radiol. (2008) ;18: :1736–40. doi:10.1007/s00330-008-0925-x |
[13] | Erickson KI , Weinstein AM , Sutton BP , et al. Beyond vascularization: Aerobic fitness is associated with N-acetylaspartate and working memory. Brain Behav. (2012) ;2: :32–41. doi:10.1002/brb3.30 |
[14] | Gormley SE , Swain DP , High R , et al. Effect of intensity of aerobic training on VO2max. Med Sci Sports Exerc. (2018) ;40: :1336–43. doi:10.1249/MSS.0b013e31816c4839 |
[15] | Holden HM , Gilbert PE . Less efficient pattern separation may contribute to age-related spatial memory deficits. Front Aging Neurosci. (2012) ;4: :9. doi:10.3389/fnagi.2012.00009 |
[16] | Yushkevich PA , Wang H , Pluta J , et al. Nearly automatic segmentation of hippocampal subfields in in vivo focal T2-weighted MRI. Neuroimage. (2010) ;53: :1208–24. doi:10.1016/j.neuroimage.2010.06.040 |
[17] | Yücel M , Lorenzetti V , Suo C , et al. Hippocampal harms, protection and recovery following regular cannabis use. Transl Psychiatry. (2016) ;6: :e710. doi:10.1038/t2015.201 |
[18] | Eadie BD , Redila VA , Christie BR . Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. (2005) ;486: :39–47. doi:10.1002/cne.20493 |
[19] | Stranahan AM , Khalil D , Gould E . Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. (2007) ;17: :1017–22. doi:10.1002/hipo.20348 |
[20] | Déry N , Pilgrim M , Gibala M , et al. Adult hippocampal neurogenesis reduces memory interference in humans: Opposing effects of aerobic exercise and depression. Front Neurosci. (2013) ;7: :66. doi:10.3389/fnins.2013.00066 |



