The Benefits of Exercise on Structural and Functional Plasticity in the Rodent Hippocampus of Different Disease Models
Abstract
In this review, the benefits of physical exercise on structural and functional plasticity in the hippocampus are discussed. The evidence is clear that voluntary exercise in rats and mice can lead to increases in hippocampal neurogenesis and enhanced synaptic plasticity which ultimately result in improved performance in hippocampal-dependent tasks. Furthermore, in models of neurological disorders, including fetal alcohol spectrum disorders, traumatic brain injury, stroke, and neurodegenerative disorders including Alzheimer’s, Parkinson’s and Huntington’s disease exercise can also elicit beneficial effects on hippocampal function. Ultimately this review highlights the multiple benefits of exercise on hippocampal function in both the healthy and the diseased brain.
ABBREVIATIONS
AD | Alzheimer’s disease |
BDNF | brain derived neurotrophic factor |
CA | Cornu Ammonis |
CFC | contextual fear conditioning |
DGC | dentate granule cell |
DG | dentate gyrus |
EC | entorhinal cortex |
fMRI | functional magnetic resonance imaging |
GABA | gamma amino butyric acid |
GCL | granule cell layer |
HD | Huntington disease |
LPP | lateral perforant path |
LTD | long-term depression |
LTP | long-term potentiation |
MPP | medial perforant path |
MWM | Morris water maze |
NMDA | N-methyl D-aspartate |
PD | Parkinson’s disease |
PND | postnatal day |
RAM | radial arm maze |
TBI | traumatic brain injury |
INTRODUCTION
The hippocampus is a bilateral structure that is found in the medial temporal lobe of the mammalian brain and is considered part of the limbic system. The hippocampus consists of the dentate gyrus (DG), the Cornu Ammonis (CA) 1 and CA3 regions (also known as the hippocampus proper) and the subiculum (reviewed by [1]). The hippocampus has been the focus of intensive research due to its important role in learning and memory, particularly that associated with declarative (i.e., explicit) and spatial memory [1].
The hippocampus displays both structural and functional plasticity into adulthood. This plasticity can be positively manipulated by interventions such as physical exercise. In this review we summarize the benefits of voluntary physical exercise on hippocampal neurogenesis (which is referred to structural plasticity in this review), and function (which is referred to synaptic plasticity and behavioral improvement in this review) in rodents. We also review the potential benefits of physical exercise on hippocampal plasticity in rodent models of neurological disorders, including traumatic brain injury, stroke and neurodevelopmental and neurodegenerative disorders. For purposes of simplicity, we have focused on studies utilizing rats and mice and where voluntary physical exercise paradigms have been employed. Finally, we have limited our review to studies examining adult animals, except for the section on neurodegenerative diseases. While exercise has also been shown to be beneficial in juvenile and aging animals [2–7], these studies are outside the scope of this review.
The hippocampus
Information flow in the hippocampus
Information flow in the hippocampus is generally unidirectional and is known as the trisynaptic circuit [8] (Fig. 1). The first connection in the trisynaptic circuit originates from Layer II of the entorhinal cortex (EC) and projects to the DG via a group of fibres known as the perforant path. The fibres synapse on the dendrites of the dentate granule cells (DGCs) located in the molecular cell layer. There are two subdivisions of the perforant path: medial (MPP) and lateral (LPP). The MPP originates in the medial EC and projects to the middle one-third of the molecular cell layer, whereas the LPP originates in the lateral EC and projects to the outermost third of the molecular cell layer. Both the MPP and LPP provide excitatory input onto the DGCs but are physiologically distinct and have different short-term and long-term plasticity properties [9–11]. The MPP and LPP are the major inputs of cortical information into the hippocampus and the DGCs play an important role in processing and filtering information before it is sent to other regions of the hippocampus via the trisynaptic circuit [8]. From the DG, DGC axons project onto pyramidal cells in the CA3 region of the hippocampus (Fig. 1). This pathway is unique in that the bundles of axons are unmyelinated and are therefore known as the mossy fibres. The third component of the trisynaptic circuit is the CA3 to CA1 projection known as the Schaffer Collaterals. Pyramidal cells of the CA3 send axons into the stratum radiatum and stratum oriens of the CA1 and innervate apical and basal dendrites of the CA1 pyramidal cells [1]. The CA1 then projects information to the subiculum and both the CA1 and the subiculum project fibres back to Layers V and VI of the EC to complete the cortical information loop (Fig. 1). It should be noted that the CA1 also receives projections from the EC and this pathway is known as the temporoammonic pathway [1]. These projections arise from layers III and V of the EC. This pathway may play a more prominent role in stress and anxiety related behaviours as opposed to spatial memory [12] (which is discussed below (Section 1.1.2)).
The role of the hippocampus in spatial memory
In the 1950 s, Dr. William Scoville performed a bilateral limbic surgery on patient Henry Molasion (commonly known as H.M.), giving the first real insight into the function of the hippocampus [13]. In order to treat H.M.’s recurrent seizures, the hippocampus, amygdala, collateral sulcus, perihinal cortex, EC and the medial mammillary nucleus, were removed [14]. Following surgery, H.M was afflicted with severe memory deficits; he lacked the ability to form and retain long-term memories of new facts (semantic memory) and events (episodic memory). This was the first case where a strong link was made between the hippocampus and explicit (i.e. intentional and conscious) memory.
Numerous studies have now refined the role of the hippocampus in the formation and maintenance of explicit spatial memory [15, 16]. In the 1980’s, Dr Richard Morris created the Morris water maze (MWM) test with the purpose of training rodents to learn the spatial location of a hidden platform submerged in cloudy water as a test for hippocampal function [17]. He observed that hippocampal lesions significantly impaired performance in this task [17].
Other studies have identified place cells in the hippocampus, which has led to the cognitive map theory of hippocampal function [18, 19]. These are cells that become active when a rodent is in a specific location in a specific environment [18] and such cells have also been detected in humans [20]. The presence of place cells within the hippocampus provides evidence that this structure organizes and stores stimuli with respect to a spatial framework (i.e. a cognitive map) and may be used for spatial navigation [21, 22]. Indeed, recent studies have suggested that the hippocampal formation acts as a network that encodes events and their contexts, with each distinct region playing a specific but complementary role in the processing of spatial information.
Within this scenario, the DG is believed to play a unique role in spatial pattern separation (reviewed by [23]). Spatial pattern separation is the enhancement of contrast between two similar spatial patterns or events. At the neuronal level this is thought to occur either through change in neuronal firing rate or through the firing of different neuronal sets [24, 25]. The DGCs display sparse firing characteristics and there are very few connections between DGCs and CA3 pyramidal cells [26], which make the DG optimally structured for spatial pattern separation. Furthermore, the existence of populations of DGCs that fire only when a rodent is in a given environment indicate that the DG contains place fields that are important for pattern separation [27–29].
The CA3 region has been implicated in spatial pattern completion – the capacity to retrieve previously stored information when presented with partial or incomplete inputs [30]. Evidence for this comes from studies where mice that lacked N-methyl-D-aspartate (NMDA) receptor expression in the CA3 were trained on the MWM. While these mice were able to perform the task as well as control mice, if the external environment was altered by removing a subset of the external cues so the mice only had an incomplete set of visual cues to recall the location of the platform, deficits in performance were observed [31]. These results indicate that the ability to use partial/incomplete spatial input was compromised and implicate the CA3 in spatial pattern completion.
The CA1 region of the hippocampus has been implicated in the processing of the temporal order of spatial patterns or events. This function is important to ensure that spatial events separated by time are encoded with minimum overlap [32]. The CA1 region also plays a major role in spatial memory (see Section 3.2.1) and contextual fear conditioning (see Section 3.2.2) and is the most widely studied area of the hippocampus [1]. Ablating or impairing the function of the CA1 region leads to deficits in delayed spatial discrimination [33], Morris Water Maze performance [34], temporal ordering tasks [35], and contextual fear conditioning [36] in rodents and spatial memory impairments in monkeys [37].
Together, the three major regions of the hippocampus – the DG, CA3 and CA1 encode spatial memory, but each region has its own distinct role within this function. The DG is associated with pattern separation, the CA3 with spatial pattern completion, and the CA1 with temporal order processing. The three regions work in concert to encode spatial memory.
Structural and functional plasticity in the hippocampus
The hippocampus is a dynamic structure that displays both structural and functional plasticity. Structural plasticity can occur through the process of adult hippocampal neurogenesis which occurs only in the DG sub-region of the hippocampus [38, 39]. Through this process new neurons are produced and integrated into existing CNS circuitry throughout the life span. Progenitor cells create neuroblasts in the subgranular zone of the DG that migrate into the granule cell layer (GCL) and differentiate into DGCs (reviewed by [40]). These new neurons incorporate themselves into existing neuronal circuits and display action potentials and functional synaptic inputs similar to DG granule cells formed during the developmental period (reviewed by [41]).
Functional, or synaptic, plasticity in the hippocampus is the process by which neurons alter their ability to communicate with one another. Enhancing synaptic efficacy, also known as long-term potentiation (LTP), is largely dependent upon kinase activation and protein synthesis, and serves as the primary biological mechanism for understanding how learning and memory processes operate in the brain [42, 43]. Long-term depression (LTD) is the opposite of LTP and is characterized by a decrease in synaptic efficacy [43, 44]. This process has been linked to forgetting.
PHYSICAL EXERCISE-INDUCED ENHANCEMENTS IN STRUCTURAL PLASTICITY IN THE HIPPOCAMPUS
The beneficial effects of physical exercise on adult hippocampal neurogenesis were first reported by van Praag et al., (1999) showing that voluntary wheel running promotes hippocampal neurogenesis in adult mice [45]. A positive correlation between running distance and levels of hippocampal neurogenesis has been repeatedly demonstrated in mice [46–48]. Notably, enhancement of neurogenesis by running can be affected by variables including genetic background [48, 49], age [4, 50], form of running (forced vs. voluntary; 39, 45–47), housing environment (single or group housing; [54], and duration (days to months) of exercise training [7, 47, 55]. Despite the variability among different studies, exercise-induced enhancements in hippocampal neurogenesis have been consistently reported in mice and rats in the literature [4, 5, 45–49, 52, 56–58, 54, 59] (Table 1).
The pioneer study by van Praag and colleagues (1999) indicated that long-term voluntary wheel running for 2 to 4 months significantly increased the process of neuronal survival in female adult C57 mice and concurrently enhanced synaptic plasticity and learning and memory performance in the MWM [45]. Further studies by this group have also found significant increases in neuronal differentiation and survival following 45 days voluntary wheel running in both young and aged C57B/L male mice [5].
Recent studies have revealed that physical exercise exerts its promoting effects on cells at different stages of neurogenesis. In adult male and female C57B/L mice, voluntary running increases the number of proliferating cells with a peak occurring after 3-days of short-term running, followed by a return to basal levels after 32 [60] or 35 days of running [61]. Conversely, significant increases in differentiating neuronal cells can only be observed after 10 days of running [7]. These data indicate that voluntary running-induced cell proliferation occurs only in the initial period of running, and longer periods of running mainly promote neuronal differentiation and survival rate of newborn neurons.
Adult neurogenesis is derived from self-renewing neural stem cells (type-1 cells, radial glial-like stem cells: nestin and GFAP positive), which can give rise to intermediate progenitor cells (type-2a cells: nestin positive, GFAP negative) that undergo amplification and acquire neural cell fate to neural progenitors (type 2b cells: nestin and doublecortin positive). These neural progenitors will then differentiate into migrating neuroblasts (type-3 cells: nestin negative, doublecortin positive) that exit cell cycle to become post-mitotic neurons (reviewed by [62]). It is elusive whether running induces adult neurogenesis by influencing proliferation or survival of distinct populations of precursor cells or both. Suh and colleagues (2007) reported that voluntary wheel running activated proliferation of early lineage radial glial-like stem cells [63], which is echoed by the findings of Bednarczyk et al., [64] showing an increase in the number of early lineage stems cells which are Ki67 (a endogenous marker for proliferating cells) and GFAP positive. Similar results were also reported by Lugert et al., (2010) who showed that running activates quiescent radial precursor cells to enter into the cell cycle [65]. In female adult C57BL/1 mice, voluntary wheel running for 7 days induces a transient increase in proliferative precursor cells as well as a reduced portion of DCX positive type-2b/3 cells that re-entered S-phase [61], suggesting that running induces neurogenesis not only by enhancing cell proliferation, but also by promoting cell cycle exit of progenitor cells. Farioli-Vecchioli and colleagues have shown that 12-days of running shortens the cell cycle of NeuroD1-positive progenitor cells (type-2b and -3) in wild-type mice, and also appears to shorten the length of the S-phase as well as the length of the whole cell cycle of both GFAP and Sox2 positive neural stem cells and NeuroD1-positive progenitors in Btg1-null mice that lack the anti-proliferative gene Btg1 [66]. Conversely, Fischer and colleagues only observed a trend towards a shorter cell cycle length in mice showing increased cell proliferation following 5- days of running, and concluded that the effect of running on progenitor cell proliferation was not caused by shortening of cell cycle length [67]. The contradictory results reported by Fischer et al., [67] and Farioli-Vecchioliet al., [66] may be due to the fact that the cell cycle length was examined on the entire proliferating population vs. specific progenitor cell populations. Furthermore, the duration of running (5 days vs 12 days) could have impacted these results. Taken together, running may exert its pro-neurogenic effect by cell cycle shortening on specific subpopulations of neural stem cells and its effect may depend on duration of running.
Prolonged wheel running also increases the number of neuroblasts and mature neurons in the DG [64]. Of note, the presence of a running wheel itself is a form of environmental enrichment which can promote the processes of adult neurogenesis, because both locked wheel and running wheel mice show increases in proliferating cells as indicated by significant increases in co-labeled Ki67 and GFAP positive cells [68]. Importantly, Bednarczyk and colleagues reported that increased number of neuroblasts (DCX positive cells) and newborn neurons (co-labeled with BrdU and NeuN) were only observed in running mice, but not in the mice with locked wheels, indicating that running is needed to increase maturation and survival of post-mitotic neuroblasts. Taken together, there appear to be running-independent and -dependent stages of adult hippocampal neurogenesis [68].
Environmental enrichment that includes supplying cages with toys, tunnels and running wheels induces robust increases in cell proliferation and neurogenesis [69]. However, a recent study has indicated that the running wheel is the critical stimulus for inducing neurogenesis in an enriched environment [70]. When mice are exposed sequentially to the running and then the environment enrichment, there is an additive increase in neurogenesis [71]. Increasing the pool of proliferating precursor cells by running together with an appropriate survival-promoting stimulus such as environmental enrichment follows the idea that the combination of exercise and environmental enrichment may potentially lead to more adult neurogenesis which may benefit hippocampal function.
PHYSICAL EXERCISE-INDUCED ENHANCEMENTS IN FUNCTIONAL PLASTICITY IN THE HIPPOCAMPUS
Synaptic plasticity
Synaptic plasticity refers to changes in neural communication as a result of experience, and can be measured throughout the brain. Two forms of synaptic plasticity have been assessed in the hippocampus, LTP, commonly associated with memory formation, and LTD, which has more recently been associated with memory clearance or forgetting.
The effects of exercise on synaptic plasticity in the hippocampus have been understudied (reviewed by [72]) despite the fact that exercise can reliably enhance performance on a range of hippocampal behaviors (see Section 3.2). The effects of exercise on in vitro hippocampal synaptic plasticity were first shown in female mice in 1999 [45], where one week of voluntary wheel running resulted in greater LTP in the DG. These findings were replicated both in vivo and in vitro in male rats where voluntary and forced running enhanced LTP in the DG [59, 73, 74] and CA3 [75]. In the DG, exercise may enhance LTP by lowering the induction threshold for LTP, though the mechanism for this change in induction threshold is unknown [73].
Interestingly, in van Praag’s seminal study of female mice, there was no effect of wheel running on CA1 LTP [45]. Conversely, forced exercise in sleep-deprived rats overcame impairments in in vivo late-phase LTP in the CA1 to the level of non-sleep deprived rats [76]. Interestingly, in this study forced exercise had no effect on CA1 LTP in healthy rats, suggesting that exercise-mediated enhancements of LTP in the CA1 are only evident in models where CA1 LTP is already depleted such as in sleep deprivation, aging and neurodegenerative disease [76–78] (see section 4.4). While these regional differences between DG and CA1 LTP were originally thought to be a direct result of increased neurogenesis following exercise, this group later showed that new neurons display enhanced LTP induction and only develop normal synaptic responses when they are 4-5 weeks old [79].
Bidirectional plasticity
More recently, studies of the effects of exercise on bidirectional plasticity have emerged. Vasuta et al., [80] studied exercise-induced changes in in vitro synaptic plasticity in the mouse DG, and the contributions of different NMDAR-subunits on these physiological changes. The effects of voluntary wheel running on DG LTP were replicated, however wheel running had no effect on DG LTD. LTP in the DG is generally NMDAR-dependent [73], however until 2007, the physiological contributions of the different NMDAR subunits on exercise-mediated plasticity were unknown. In this study, selective NR2A or NR2B blockade abolished DG LTP in non-exercised mice. In exercised animals, however, only NR2A blockade abolished DG LTP, and NR2B blockade significantly reduced, but did not block DG LTP. Conversely, neither exercise nor selective blockade of NR2A or NR2B subunits had an effect on DG LTD in sedentary animals, but NR2A blockade abolished LTD while NR2B blockade left LTD intact in exercised mice. Interestingly, while exercise increases NR2B mRNA specifically in the DG region, it does not affect NR1 or NR2A subunit mRNA nor either of these subunits in the CA1 region [73]. Together, these data indicate a unique role for the NR2A subunit in both LTP and LTD in the DG that emerges following exercise.
A great disparity exists in the study of hippocampal physiology following exercise in non-diseased animals. The exercise-induced changes in NMDAR-subunit composition in the hippocampus discussed here are likely just one component of the benefits of exercise, as others have reported increases in dendritic complexity [81] and neurotrophic factors [82] to name a few, which all have implications on synaptic plasticity in the hippocampus [83, 84]. Indeed, there are many mechanisms that may underlie the exercise-induced changes (or lack thereof) in synaptic plasticity in different sub-regions of the hippocampus (many of which are reviewed in this issue) however more work must be done in order to fully understand how each of these putative mechanisms contributes to exercise-induced hippocampal plasticity.
Behavioral plasticity
The effect of voluntary wheel running on hippocampal-dependent behaviors has been extensively studied in both healthy and diseased rodents. Here, we will summarize the most commonly used behaviors in the study of exercise in healthy animals.
Morris water maze
The Morris water maze task (MWM) is a classic hippocampal behavioral task that has been modified in order to assess different aspects and types of memory such as working memory, long-term memory acquisition, and retrieval. Fundamentally, in this task, animals are placed in a pool of opaque water, and must use distal visual cues in order to locate a hidden platform where they may escape the water. Following multiple learning trials over multiple days, the swim path or latency to reach the platform can be measured as an indication of spatial memory acquisition. The platform can be removed for a probe trial where the time spent searching the trained platform location can be assessed to measure reference memory.
The MWM is the most studied behavioral task in exercise literature. Runners acquire the MWM task more readily than non-runners, where by the third day of training, runners take a more direct path and take less time to reach the platform than non-runners [45]. At the time of the probe trial, runners spend a significantly greater proportion of time in the correct quadrant, than incorrect quadrants, and the improvements in performance in runners were paralleled by increases in hippocampal neurogenesis. The rapid acquisition of the MWM task by runners has been extensively replicated [46, 85], and this acquisition by runners may be more rapid than mice housed in enriched environments [70], though together, environmental enrichment and exercise might have additive effects on behavioral improvement in the MWM [71]. Curiously, when hippocampal neurogenesis is disrupted by irradiation, running-induced improvements in performance in the MWM are no longer evident [86].
Fear conditioning
In contextual fear conditioning (CFC) tasks, an animal is conditioned to fear a context by providing a tone followed by a footshock. After a pre-determined delay, the animal is returned to the fearful context without the footshock, and the freezing response to either the context or the tone in that context is measured. This behavioral task requires the activity of the amygdala, hippocampus, as well as frontal and cingulate cortices (reviewed by [87]).
CFC has been commonly used to assess learning and memory improvements following exercise. Following 30 days of voluntary wheel running, male rats exhibited more freezing in the fearful context when returned to that context 24 hours after footshock training [88]. This evidence of increased freezing to the fearful context has been reported in other groups following exercise [89, 90]. In a study by Burghardt et al., [89] male rats that ran voluntarily froze more than rats that were in the locked control condition, indicating that the voluntary aspect of exercise plays a role in this type of learning. There also appears to be a sensitive period during which voluntary exercise has an effect on this fear conditioning, where running between footshock training and testing has no effect on freezing times, while running before training results in increased freezing to the fearful context [90]. Unlike spatial memories in the MWM, the exercise-mediated behavioral improvements in CFC are not dependent on intact hippocampal neurogenesis [86].
Arm mazes
The radial arm maze (RAM) is a spatial memory task that is sensitive to hippocampal damage. This task can be modified for a multitude of memory types and has classically been used to assess working memory. For this version of the task, the animal can exit a central zone and visit a multitude of arms (from 8–48) in order to collect a food or water reward located at the end of each rewarded arm. The animal must remember which arms it has already visited in order to not visit the same arm again.
Female rats that underwent voluntary wheel running required fewer trials to reach criterion than their non-runner counterparts [91]. Similarly, in male rats, runners made fewer reference errors and more correct choices than non-runners [92].
The Y maze is another spatial memory task where animals must make a choice to pick either the left or right arm in order to receive a reward at the end of one arm, which is hidden from the view of the animal. As in the MWM, runner mice showed rapid acquisition of the Y-maze task, reaching over 80% correct arm choices by the second trial during the first training session [93]. Once non-runners had learned the Y-maze task, and were allowed 14 days of voluntary running, they had better memory retention than mice that did not run at all. In this same task, when, after the 14-day running (or non-running) delay, the reward location was reversed, those mice that ran acquired the reversal learning task more rapidly [93].
Novel object recognition
Novel object recognition is another classic hippocampal-dependent task (reviewed by [94]), which has been used to study different types of memory. In its most simple version, animals are allowed to freely explore an environment containing two identical objects followed by a pre-determined interval before being returned to the environment for a test phase. In the test phase, one of the familiar objects is replaced with a novel object, which the animal will spend more time investigating if it remembers the familiar object. This task is known to require the intact function of both the hippocampus and the perirhinal cortex in rats [95, 96].
Voluntary wheel running also has been shown to improve novel object recognition in mice [97] and rats [77, 98] where improved performance was correlated with increases in BDNF expression in the perirhinal cortex [98]. There also appears to be a temporal relationship between the time that exercise stops and the behavioral test begins, where testing immediately after exercise produces the greatest improvements in object recognition and BDNF expression in the hippocampus and perirhinal cortex [99].
Learning and forgetting of hippocampus-dependent memories
New avenues of study have sought to understand the relationship between neurogenesis and forgetting (see [100] for a review). Feng et al., (2001) demonstrated that impaired neurogenesis causes increased memory retention in the CFC task, suggesting that hippocampal neurogenesis may play a role in memory clearance [101]. Similarly, when hippocampal neurogenesis is ablated, working memory was improved in the RAM [102]. This ablation of hippocampal neurogenesis slows the decay of the hippocampal dependency of remote CFC memories, whereas in controls, remote CFC memories become less hippocampal dependent over time. Running increases the rate at which these CFC memories become hippocampal independent without affecting the expression of the memory, an effect that is blocked by irradiation [103]. Most recently Akers et al., (2014) have shown a clear link between neurogenesis and forgetting where running induced forgetting of a CFC task in adult mice. Using guinea pigs and degus, species that both have low levels of postnatal neurogenesis, they showed intact memory retention except when neurogenesis was stimulated by memantine [104]. Together these studies begin to suggest a role for neurogenesis and memory retention and clearance in the hippocampus.
EFFECTS OF EXERCISE IN NEUROLOGICAL DISORDERS
Fetal alcohol spectrum disorders
Ethanol can cause significant damage to the fetus [105, 106] and can result in fetal alcohol spectrum disorders (FASD) with the extent of damage caused by ethanol varying due to the timing, frequency and volume of ethanol consumed, as well as the genetics and metabolism of the mother.
FASD can be modelled in rodents using a variety of methodologies which are outlined in recent reviews from our laboratory [107, 108]. The most commonly used methods are the liquid diet model where a pregnant dam receives a liquid diet containing 36% (v/v) ethanol throughout pregnancy, and the gavage model where ethanol is administered via oralintubation.
FASD and structural plasticity
FASD can lead to a variety of structural deficits in the hippocampus including decreases in adult hippocampal neurogenesis [109–113]. The effect of exercise has been examined in a variety of models of FASD to determine whether beneficial effects on neurogenesis and neuron structure occur [111, 114, 115].
Redila et al., (2006) were the first to show that decreases in cell proliferation that occur with prenatal ethanol exposure can be rescued in rats with one week of exercise [115]. Using a different model of FASD, where animals were exposed to ethanol during the third trimester equivalent (corresponding to PND1-10 in the rat), Helfer et al., (2009) found that 12 days of voluntary running wheel exercise during adolescence increased proliferation of cells in the DG, but long-term survival of these neurons was not increased [111]. It is important to note that in this study, baseline levels of proliferation and neurogenesis were comparable between the control and the ethanol-exposed animals, which differs from the study byRedila et al. (2006).
The effect of exercise on neurogenesis was also examined using the gavage model where ethanol was administered throughout all three trimester equivalents [114]. Ethanol exposure reduced cell proliferation and increased early neuronal maturation, but did not affect cell survival [114]. When ethanol exposed animals were given access to voluntary running wheels for 12 days beginning at PND 48, increases in cell proliferation and maturation were observed which indicates that despite deficits caused by ethanol exposure, the brain still has the capacity to respond to the beneficial effects of exercise [114]. Remarkably, increases in the proliferation marker Ki67 were actually much greater in ethanol exposed animals than in control animals following exercise. This may be linked to the fact that ethanol exposed animals ran significantly more than controls, which could indicate a hyperactive phenotype in these animals [114].
These varied results between studies are probably due to the different models of ethanol exposure employed. However, results from Helfer et al., (2009) and Boehme et al., (2011) suggest that while exercise may be able to stimulate proliferation and differentiation, additional therapies may need to be utilized to ensure that the new neurons are able to survive and integrate into functional networks of the hippocampus. For example, Hamilton et al., (2014) combined exercise followed by environmental enrichment. Using BrdU labeling, the authors found that ethanol-exposed animals had lower rates of cell survival than control animals. However, in ethanol-exposed animals who were given voluntary access to a running wheel (started at PND 30 for 12 days) followed by access to an enriched environment (from PND 42 – 72), the levels of cell survival were comparable to those of control animals [110]. These results indicate that both exercise and enrichment may be needed in order to enhance cell proliferation as well as cellsurvival.
FASD and synaptic plasticity
Ethanol exposure causes long lasting deficits in synaptic plasticity in the DG – in particular, LTP is decreased in adolescent and adult male offspring [116–121]. Interestingly, in females, LTP in the DG is increased in adolescent animals, and there are no differences in LTP between control and ethanol-exposed offspring in adulthood [117,121–123]. Only one study has examined the effect of exercise on synaptic plasticity in the DG of animals exposed to ethanol during development. Only males were examined in this study, but the results clearly indicate that access to a running wheel from PND 28 – 60 (32 days), was enough to completely reverse the deficits in LTP observed in the ethanol-exposed offspring [118].
FASD and behaviour
Learning and memory and executive function are impaired in individuals with FASD [124–127]. Functional brain abnormalities can be examined in human populations using functional MRI (fMRI) technology. fMRI has been used to examine spatial working memory in adults and children with FASD, and impairments compared to control subjects are commonly observed [128, 129]. fMRI has also indicated that subjects with FASD exhibit abnormal patterns of brain activation during tasks involving verbal learning [130], verbal working memory [131], and visual working memory [132].
There is a multitude of data indicating that learning and memory, particularly spatial, reference, and working memory, are impaired in animals that were exposed to ethanol during development. Deficits in the MWM where animals show impairments in spatial acquisition, reference and place memory are observed in animals when ethanol exposure occurs both in the prenatal [118, 133–141] and postnatal [142–148] periods.
Only two studies to date have examined the effects of exercise on behaviour in the MWM in animals with FASD. Christie et al., (2005) examined the effects of voluntary wheel running from PND 54 – 64 in animals where the liquid diet model of ethanol administration was used. In this study, the two trial version of the MWM was conducted over a five day period, and both reference and working memory were assessed. Animals exposed to ethanol displayed significant deficits in both reference and working memory when compared to control groups; however, this deficit was completely reversed in the animals that were allowed access to a running wheel [118]. Similar findings were obtained by Thomas et al., (2008) using the postnatal gavage model of ethanol exposure (3rd trimester equivalent). In this study, animals were allowed access to a running wheel from PND 21 – 51 and then from PND 52 – 57 they were tested in the MWM (4 trials a day, plus a probe trial on the final day). Ethanol-exposed animals that did not exercise had significant deficits in performance, however, these deficits were greatly reduced in the animals allowed access to a running wheel [147]. Performance in the probe trial was not affected by ethanol-exposure; however, exercise also increased performance in this task in both ethanol-exposed and control animals.
The effect of exercise on other hippocampal behaviours has also been examined using the gavage model of FASD. Schreiber et al., (2012) have shown that neonatal ethanol exposure causes significant deficits in CFC tasks [149]. Interestingly, if ethanol-exposed animals were given access to a running wheel from PND 30 – 42 and then environmental enrichment from PND 42 – 72, these deficits in behaviour were not overcome [149]. In contrast, a similar study from the same laboratory showed that exercise could benefit other forms of contextual fear conditioning [110]. Ethanol-exposure causes deficits in trace eyeblink conditioning [150] and a variant of contextual fear conditioning called the context pre-exposure facilitation effect [112, 151]. Hamilton et al., (2014) examined the effects of exercise (PND 30 – 42) followed by environmental enrichment (PND 42 – 72) on these behaviours following ethanol-exposure from PND 4 – 9. They determined that the combination of exercise and enrichment was able to overcome the deficits induced by neonatal ethanol exposure [110].
Other behavioural phenotypes which may be mediated through the hippocampus and are common with FASD include increased depression and anxiety [152–155]. Brocardo et al., (2012) showed that animals exposed to ethanol throughout all three trimester equivalents (i.e. GD 1–21 plus PND 4–10) using the gavage model have increased depression-like and anxiety-like behaviours. These effects were present in both male and female offspring. A cohort of ethanol-exposed animals was given access to a running wheel for 12 days from PND 48 – 60 and at PND 60 was tested for anxiety and depression-like behaviours. Exercise did not rescue anxiety-like behaviours measured using the elevated plus maze in male or female offspring [156]. Ethanol-exposed females showed increased depressive-like behaviour in the forced swim test when compared to control groups. This effect was not rescued by exercise [156]. In males, a similar increase in depression-like behaviours was observed following ethanol-exposure, however, unlike in females, this effect could be rescued by exercise [156]. This study suggests that gender may play a role in response to exercise-elicited antidepressant effect in ethanol-exposed rats.
Summary
FASD causes a variety of perturbations in hippocampal structure and function which translate into deficits in hippocampal behaviour. Reductions in neurogenesis are commonly observed which may underlie the deficits in synaptic plasticity and hippocampal behaviour.
Although limited, there are a handful of studies which have examined the benefits of exercise in FASD and the results from these studies provide evidence that exercise may be able to overcome these structural and functional deficits in the hippocampus, to a point where ethanol-exposed animals can no longer be distinguished from control animals. These improvements also appear to translate into positive behavioural changes suggesting that exercise may be beneficial in overcoming some of the learning and memory deficits associated with FASD. However, it is important to note that exercise may not be beneficial for all behavioural phenotypes exhibited in animals exposed to ethanol during the perinatal period [149, 156]. Furthermore, studies like those of Brocardo et al., (2012) emphasize the importance of examining both males and females, because sex-specific differences in the effects of ethanol, and the benefits of exercise, may be observed.
Traumatic brain injury
Traumatic brain injury (TBI) can occur when the head hits, or is hit by an object. Up to 1.7 million incident or recurrent TBIs occur annually in the US, most commonly caused by falls, vehicular accidents, assault, and sports injuries [157]. Direct mechanical insult to brain tissue caused by the initial impact, skull fracture, or penetrating injury, along with the stretching and shearing of cell processes and vasculature, result in a cascade of pathophysiological processes throughout the brain that can cause immediate and persistent symptoms. Impaired learning and memory are common symptoms of TBI, regardless of the type or severity of injury. Despite its central, and therefore relatively protected location, changes in hippocampal volume have been reported in humans and rodents after TBI [158–161], suggesting it is susceptible to damage by secondary injury mechanisms, which likely contribute to persistent learning and memory impairment resulting from TBI (reviewed by [162]). It is an important goal of experimental animal models of TBI to identify mechanisms underlying TBI symptoms like learning and memory impairment, and to identify interventions that can treat them (for a review of animal models of TBI see [163]). Owing to the heterogeneity of this type of injury, there has been great difficulty in finding effective treatments. The potential to promote structural and functional plasticity in the hippocampus makes exercise an attractive means of rescuing cognitive deficits arising from TBI.
Structural and functional plasticity in TBI
There is a paucity of information available on how exercise directly affects hippocampal structure and functional plasticity following TBI; however emerging evidence shows that initiating moderate exercise after a short recovery period may elevate levels of plasticity-promoting proteins and reduce cognitive deficits after experimental TBI in rodents. These effects are consistently mediated by elevations in the neurotrophin BDNF.
An important focus of this research is to establish a timeline to determine when exercise becomes restorative after TBI, as there is evidence that intense physical and cognitive exertion in the acute recovery phase may exacerbate TBI pathology and increase symptom severity [164]. Due to the heterogeneity in exercise paradigms between studies there is controversy over the optimal time to initiate an exercise intervention, but the majority of literature suggests that delaying the onset of exercise is beneficial. In a mouse model of TBI, MWM performance impairments were rescued, CREB and BDNF expression were elevated, and hippocampal neurogenesis was increased by seven days of voluntary wheel running initiated 5 weeks, but not 1 week post-injury [165]. In rats, however, seven days of voluntary wheel running initiated three days after TBI elevated hippocampal BDNF levels, and ameliorated the TBI-induced increase in hippocampal myelin-associated glycoprotein (MAG) and Nogo-A, which are proteins that inhibit axonal growth [166]. Another group found that adult male rats showed reduced deficits in acquisition of spatial reference memory the MWM and elevated hippocampal BDNF when given access to a running wheel 14–20 days after mild TBI [167, 168]. However, when running wheel access was provided 0–6 days post-injury MWM impairments were increased, and BDNF levels were not changed [168]. In these paradigms, administration of a BDNF inactivator prior to exercise attenuated enhancements in MWM performance [167] and elevations in plasticity related proteins [166].
Injury severity may dictate when it is best to begin an exercise intervention after TBI. In adult male rats, exercise-induced elevations in BDNF were seen when running wheel access was provided at 14–20 days after a mild TBI, but when a moderate TBI was induced, BDNF was only elevated when access to the running wheel was withheld until 30–36 days post-injury [169]. Interestingly, the same group also found that hippocampal BDNF was elevated after voluntary running 0–7 days after moderate TBI, though they noted that injured animals ran significantly less than controls [170]. This highlights an important challenge in this area of research, as injured animals are more likely to abstain from voluntary running, making it difficult to control for exercise duration and exertion. It is possible that in paradigms where voluntary exercise is initiated immediately after TBI, injured rats abstained from exercise during the initial period where they were more vulnerable to sustain further damage.
Summary
The available literature collectively suggests that delayed exercise after TBI can reduce learning and memory impairment, and elevate plasticity-related proteins in the hippocampus. However, there is a paucity of information on precisely how elevated neurotrophins affect structural plasticity and improve behavioural recovery after brain injury. Insight in this matter could be gained through histological and electrophysiological analysis of hippocampal tissue in order to determine whether these exercise-mediate changes correlate with changes in structural and functional plasticity.
Stroke
Ischemic and hemorrhagic stroke occur when blood circulation to a certain area of the brain is restricted due to vessel blockade or rupture, respectively. This produces metabolic distress, tissue damage and cell death in the affected area. Stroke is an extremely prevalent condition, affecting up to 800 000 Americans annually [171]. Depending on which area of the brain is affected, symptomology can include debilitating and persistent impairment to cognitive, motor, visual, auditory, and speech function. When the affected area includes the hippocampus, learning and memory can be severely impaired (reviewed by [172]). Stroke often results in motor deficits, and the potential for exercise to induce plasticity in sensory and motor cortices, and improve functional recovery in motor tasks has been well studied [173–175]. There is evidence that exercise can augment hippocampal plasticity after stroke as well. However, the information available on the therapeutic effects of voluntary exercise is extremely limited, likely due to difficulty implementing voluntary exercise paradigms in motor-impaired animals. Where voluntary exercise paradigms have been used, outcome measures primarily focuses on exercise mediated elevation in hippocampal plasticity-related proteins, indicating there is a need for further research directly measuring how exercise affects structural and functional plasticity in the hippocampus.
Structural and functional plasticity in stroke
Motor deficits resulting from stroke complicate the application of an exercise intervention. This is an important consideration, as controls may exercise more vigorously or longer duration in voluntary exercise paradigms, but forced exercise may not produce equivalent results. For this reason, information on how voluntary exercise affects structural and functional plasticity in the hippocampus is extremely limited. In a mouse model of ischemic stroke, voluntary wheel running, but not forced swimming, rescued memory impairments in the MWM. This was accompanied by elevated CREB phosphorylation, and increased survival of adult generated neurons in the hippocampus [176]. Another mouse model found that 42 days of voluntary wheel running was sufficient to increase hippocampal neurogenesis and reduce learning impairments in the MWM after ischemia [177].
There is evidence that voluntary running elevates hippocampal BDNF after ischemic stroke, but more research is needed to determine if this effect translates into changes in hippocampal plasticity or cognitive outcome [174, 175].
Summary
Physical activity has the potential to improve stroke outcome by augmenting hippocampal plasticity, but there is a lack of information on how exercise-mediated improvements in cognition and changes in plasticity related proteins correlate with structural and functional changes in the hippocampus. Ongoing research should attempt to establish how exercise-mediated behavioral recovery and neurotrophin expression correlate with neurogenic changes and electrophysiological analysis of hippocampal tissue.
Neurodegenerative disorders
Neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD), constitute a heterogeneous group of disorders that commonly display a characteristic progressive loss of structure and/or function of populations of neurons and glia in the CNS [178]. This neuronal degeneration primarily affects specific neuronal populations, which include cortical and hippocampal neurons in AD [179], dopaminergic neurons in PD [180], and striatal gamma-aminobutyric acid (GABA)ergic neurons in HD (reviewed by [181]). Clinically, these diseases are predominantly adult onset and show chronic progression of cognitive deficits.
Animal models of neurodegenerative diseases provide valuable tools in assessing therapeutic strategies for these disorders. Bearing in mind the well-defined benefits of physical exercise on cognitive function, it is reasonable to consider that exercise may in fact be used as a strategy to prevent or impair the cognitive decline associated with age-related neurodegenerative diseases. In this section, we review the influence of voluntary exercise on structural and functional plasticity in adult-aged rodent models of AD, PD, and HD.
Alzheimer’s disease
AD, the leading cause of dementia in the elderly, is an age-related, irreversible neurodegenerative disorder clinically characterized by progressive memory loss and multiple cognitive deficits (reviewed by [182]). Important neuropathological hallmarks of AD in the brain include the gradual accumulation of extracellular plaque depositions composed of amyloid-beta (Aβ) peptide, the formation of neurofibrillary tangles (NFT) consisting of hyperphosphorylated tau protein, and substantial neuronal death [183, 184]. The etiology of AD is not yet known, however two competing hypotheses exist. In the Amyloid hypothesis, Aβ plaques are considered to be the causal component in AD pathogenesis [185]. The second theory, the Presenilin hypothesis, is based on loss-of-function presenilin mutations that lead to neurodegeneration and memory impairment in AD [186]. Despite this controversy, the hippocampus is known to be particularly vulnerable to AD, and is one of the first regions to be affected [187].
AD and structural plasticity
Studies of adult hippocampal neurogenesis in a variety of available transgenic rodent models of AD (reviewed by [188]) have generated contradictory results [156, 189]. Decreases in neurogenesis were reported in transgenic or knock-in mice carrying the PDAPP mutation [190], the Swedish mutation in the APP gene [191–194], PS1 gene mutations [193–196], as well as in double-transgenic mice for APP and PS1 [193, 194]. APP, PS1, and tau protein triple-transgenic mice also show deficits in neurogenesis [197]. In contrast, transgenic mice that express APP with the Swedish, Dutch, and London mutations [198], or with the Swedish and Indiana mutations [199, 200] were found to have increased hippocampal neurogenesis. Discrepancies in the literature are likely based on a variety of factors such as transgenic model used, stage of disease progression, and difference in protocols used to evaluate neurogenesis.
A successful treatment for AD has yet to be developed. A putative lifestyle treatment is exercise, which has been shown to promote neurogenesis in rodent models of AD. APPswe-PS1ΔE9 mice provided 10 weeks of voluntary wheel running showed enhanced levels of hippocampal neurogenesis. Moreover, running significantly prevented neuronal cell loss in the DG and CA3 hippocampal subregions, but did not affect the CA1 [201]. In the Tg2576 AD model, voluntary runners were found to have greater hippocampal volume compared to sedentary littermates following 16 weeks of access to wheel running [202]. An age-dependent effect of voluntary wheel running was found in the APP23 AD mouse model, where 10 days of exercise induced an increase in adult hippocampal neurogenesis in 18 month-old animals, but had no effect in the 6 month-old cohort [203]. These findings suggest the ability of the AD brain to upregulate cell proliferation and neuronal differentiation in response to voluntary physical exercise.
AD and functional plasticity
García-Mesa et al. (2011) used electrophysiology to investigate synaptic function and plasticity in 3xTg-AD mice that were subjected to 6-month wheel running treatment. LTP could not be produced in the 3xTg-AD animals, while exercise was found to only weakly protect the impairment of LTP induction at the CA1-medial prefrontal cortex synapse [204]. Considering the limited evidence available that examines the role of exercise on synaptic plasticity in AD, the remainder of this section focuses primarily on hippocampal behaviour. Various transgenic mouse models of AD show deficits in several hippocampal-dependent learning and memory tasks, such as object recognition, spatial learning, and fear conditioning (reviewed by [188, 205]).
Clinical studies with AD patients indicate that physical and cognitive activities are associated with reduced risk of disease [206–208]. Similarly, rodent models of AD that undergo voluntary exercise display encouraging results. Using a Tg2576 mouse model, Yuede and colleagues (2009) found that voluntary runners (16 weeks of wheel running) displayed greater investigation latencies of novel objects in a recognition memory paradigm in comparison to sedentary controls, or forced exercise animals [202]. Transgenic animals containing the ɛ4 allele of the APOE gene exhibit cognitive impairment on the hippocampus-dependent radial-arm water maze task. Following 6 weeks of free access to wheel-running, ɛ4 mice displayed improvements in this task compared to their sedentary counterparts [209]. The TgCRND8 model of AD also benefits from voluntary exercise; 5-months of voluntary running decreased Aβ plaques in the hippocampus and enhanced hippocampal-dependent learning in the MWM [210]. Another AD model, THY-Tau22, displays progressive learning and memory alterations [211, 212]. In response to a 9-month voluntary exercise period, memory alterations were prevented in the THY-Tau22 mice as assessed by a two-trial Y-maze task in which running animals spent more time in the novel arm [213]. Cognitive deterioration as well as behavioural and psychological symptoms of dementia (BPSD)-like behaviours, such as anxiety and the startle response, were ameliorated by exercise in triple transgenic (3xTg) AD mice with access to running wheel. Of the different experimental cohorts studied, the best neuroprotection was identified in 7 month-old 3xTg-AD mice that had undergone exercise treatment for 6 months [204]. APPswe/PS1ΔE9 mice display spatial memory impairments in the MWM. In response to 10 weeks of voluntary-wheel running, this cognitive decline is significantly decreased in comparison to sedentary controls [201].
In contrast to the above-mentioned studies, TgCRND8 mice with access to running wheel for a 10 week period displayed no improvements in cognitive or neuropathological parameters in comparison to non-runner controls. Despite this, spontaneous stereotypic behaviours of TgCRND8 mice [214], such as repetitive bouts of jumping, climbing, or bar-chewing, were significantly reduced in the wheel-running transgenics [215]. Furthermore, APP23 mice that ran for 8.5 months did not show enhancements in spatial learning, or hippocampal neurogenesis. Interestingly, environmental enrichment lacking a running wheel improved MWM performance and increased hippocampal neurogenesis [216]. This is an important distinction, as other studies report enhanced cellular plasticity in TgCRND8 [217] and improved cognition in PS1/PDAPP [218] AD models in response to “enrichment” that includes running wheels.
Summary
Although voluntary exercise is the focus of the present review, AD-related exercise research is by no means limited to this paradigm. Treadmill and forced exercise experimental designs are also commonly used in this field. Similar to the majority of voluntary running studies, they show the importance of exercise in preventing the cognitive decline associated with AD [219, 220], repressing neuronal cell death [221], enhancing neurogenesis [222], improving hippocampal LTP [219], and improving cognitive function [223–226]. Trends in current literature suggest that exercise may help to prevent the cognitive decline associated with AD and delay the onset of symptoms. Interestingly, these benefits appear to require relatively long periods of exercise. While cognitive improvements in AD studies require voluntary access to running wheels for 10 weeks up to 9 months, healthy animals or FASD model animals for example, typically undergo exercise paradigms ranging from 7 days to 2 months (see previous sections).
Parkinson’s disease
PD is characterized by the progressive loss of dopamingeric neurons in the substantia nigra that projects to the striatum of the basal ganglia. Physical symptoms of this disorder including motor dysfunction such as rigidity; tremor and bradykinesina are accompanied by non-motor symptoms such as cognitive deficits and depressive disorders. Volumetric magnetic resonance imaging studies have suggested a progressive hippocampal volume loss in PD patients [227]. Intracellular accumulation of α-synuclein is the pathological feature of Parkinson’s disease and mutations in the gene encoding for this protein is known to be linked to this disease [228]. Abnormalities in hippocampal plasticity have been observed in α-synuclein-related transgenic animal models [229, 230] as well as lesion models of dopamine depletion using either the1-methyl-4-phenyl-1,2,3,6-tetrhydropyridine (MPTP) or 6-hydroxydomine (6-OHDA) [231–233], which leads to death of dopamingeric neurons and subsequent depletion of dopamine in the dorsal striatum.
PD and structural plasticity
Altered structural plasticity including cell proliferation, differentiation, and survival of adult-born neurons, as well as decreased dendritic complexity and spine formation has been related to the pathogenesis of PD (reviewed by [234]). Decreased hippocampal neurogenesis is altered in 6-OHDA and MPTP–induced animal models of PD [235, 236]. Similarly, decreased cell proliferation and neuronal survival are also found in transgenic mice expressing mutant α-synuclein [237, 238], which echo the decreased hippocampal cell proliferation observed in postmortem brain from PD patients [235].
There are currently no studies examining the effects of voluntary wheel running on structural plasticity in PD. However, in the 6-OHDA- rat model of PD, a regime of 4-weeks of treadmill exercise (30 min/day, 5 days/week) increased cell proliferation and the migration of neural stem cells towards the lesion site accompanied by up-regulated BDNF and glial cell-derived neurotrophic factor (GDNF) in the striatum [239]. Exercise training also enhances survival and integration of transplanted cells in animal model of PD (reviewed by [240]).
PD and functional plasticity
Impaired synaptic plasticity in hippocampal sub-regions has been reported in animal models of PD. Decreased LTP and enhanced LTD were found in the CA1 region in mice injected with MPTP [233], while decreased LTP and impairment of long-term recognition memory was found in the DG of mice with bilateral 6-hydroxydopamine (6-OHDA) lesions [231]. The impaired LTD in the DG may be linked to disrupted dopaminergic and noradrenergic transmission in the DG [232] and decreased NR2A/NR2B subunit ratio in NMDA receptors [241].
Although clinical studies have demonstrated that physical training improved motor function as well as cognitive performance in PD patients [242, 243], currently there are no studies reporting changes of hippocampal synaptic plasticity following physical training in animal models of PD. Thus, it is unclear whether exercise-induced beneficial effects on cognitive performance on PD patients are at least partly mediated by enhanced hippocampal neurogenesis or synaptic plasticity.
Studies examining the potential beneficial effects of physical exercise on PD are primarily focused on behavioral recovery on motor skill performance [244–248]. In the MPTP mouse model, improvement in motor function is likely linked to increased dopamine transmission, enhanced expression of dopamine receptors or increased glutamate receptor expression in the dorsal striatum following exercise [249, 250].
Summary
In summary, there are very few studies examining the benefits of voluntary wheel running on structural and functional plasticity in the hippocampus. This may be due to the etiology of the disease and the difficulties in motor function that are observed. However, evidence from human studies does suggest that voluntary exercise, potentially in pre-symptomatic patients may be beneficial for hippocampal function.
Huntington’s disease
HD is an autosomal dominant neurodegenerative disease caused by a characteristic cytosine-adenine-guanine (CAG) repeat expansion within the HD gene, which results in an expansion of poly-glutamine residues in the huntingtin protein. Under normal conditions, there are 6 – 35 CAG repeats, whereas HD patients may have up to 250 repeats [251]. As a result, mutant huntingtin aggregates into insoluble ubiquitinated neuronal intranuclear inclusions (NIIs) [252]. This mutation primarily causes neurodegeneration of medium spiny neurons in the caudate nucleus, as well as deterioration of certain populations of striatal interneurons [253] and cortical atrophy in other brain regions as the disease progresses (reviewed by [169]).
The characteristic clinical symptom of HD is progressive involuntary choreatic movements followed by later onset bradykinesia and rigidity [254]. In addition to motor disturbances, cognitive impairment and psychiatric symptoms are associated with the progression of the disease, which is invariably fatal 15 – 20 years after onset (reviewed by [242]).
HD and structural plasticity
In contrast to the contradictory results accompanying neurogenesis in AD, there is an apparent consensus that HD reduces adult hippocampal neurogenesis. Lazic and colleagues (2004) were the first to identify this using the R6/1 transgenic model of HD [256]. Additional research of R6/1 [257–259] and R6/2 mice [260–263], as well as a transgenic rat model [264] have extended upon our understanding of adult neurogenesis deficits in the hippocampus of HD rodent models.
To investigate the potential of physical exercise as therapeutic strategy in HD, various studies have analyzed the modulation of neurogenesis after voluntary running in a variety of HD models. Those that specifically investigated adult animals, show that running enhances adult hippocampal neurogenesis, but only in wildtype controls and not in R6/1 HD mice [265, 266].
HD and functional plasticity
Sedentary R6/1 HD mice develop phenotypic rear-paw clasping by 16 weeks of age. Additionally, they display abnormal rearing behavior as well as deficits in motor coordination and spatial working memory. Following 10 weeks of access to a running wheel, the rear-paw clasping onset is delayed, there are normalized levels of rearing behavior and cognitive impairments are ameliorated. Despite this, motor coordination is not rescued [267]. Voluntary exercise during a 4 week period was found to have anti-depressive effects in R6/1 transgenic mice that showed reduced immobility in forced-swim test [265].
In the current review, we focus on adult animals. However, characteristic motor dysfunction is an unavoidable confound with rodent HD models since they are known to run less than wildtype littermates. Considering the progressive nature of HD, several studies have used juvenile animals to investigate the impact of exercise prior to overt symptoms. In 5 week old R6/2 HD mice, a 4 week period of voluntary wheel-running did not rescue the observed reduction in adult hippocampal neurogenesis (60% of wildtype level) [268]. More recently, Potter and collaborators (2010) established that exercise was not beneficial in N171-82Q transgenics, and that it may in fact be detrimental to the vulnerable HD nervous system [269]. Conversely, others have reported that wheel running delays onset of motor deficits [270], improves gait, motor coordination, and slows progression of cognitive dysfunction [271] in juvenile R6/1 HD mice.
Summary
There is a paucity of data examining the effects of exercise on hippocampal plasticity in HD. Taken together, the effectiveness of exercise as an environmental therapy for HD is currently transgenic model-dependent with age-related discrepancies in the literature.
DISCUSSION AND CONCLUSIONS
There is a multitude of evidence that suggests that physical exercise can lead to significant benefits in hippocampal structure and function in rodents, which ultimately results in behavioral benefits. As highlighted in the various sections there are some gaps in the literature that need to be filled, but the majority of the evidence suggests that exercise can be beneficial not only in healthy adult animals (Table 1), but also in disease models, including developmental disorders, mood disorders, traumatic brain injury and neurodegeneration (Tables 2–4). How exercise leads to these substantial benefits in hippocampal function is still under debate. Exercise increases blood vasculature and enhances angiogenesis in the brain and boosts levels of neurotrophins, including brain derived neurotrophic factor (BDNF) [272, 273]. These increases are observed with short or long periods of running, and appear to remain upregulated for a period of time after exercise has ceased [73, 82, 272, 274]. BDNF can increase the production of cell survival genes and proteins important for synaptic plasticity and neurogenesis which may explain the benefits observed in synaptic plasticity and learning and memory [275, 276]. Other factors to consider include enhancements in dendritic complexity and spine density as well as glial cell function. The many potential mechanisms through which exercise can boost hippocampal function are discussed in great detail in recent reviews [277–279].
This review focussed primarily on rodent models of disease, but it is important to note that many of the benefits of exercise found in rodents are also observed in humans. Many human studies have found significant benefits of exercise, not only in healthy individuals but also in individuals with neuropsychiatric and neurodegenerative disorders. It is difficult to assess neurogenesis and synaptic plasticity in humans, however, there is ample evidence to suggest that exercise can enhance learning and memory and other aspects of hippocampal function (reviewed by [277]). Exercise has been shown to enhance hippocampal learning and memory tasks in children [280, 281], adolescents, [282], adults [283] and the elderly [284, 285]. In fact, many studies have investigated whether exercise can reduce or prevent age-related cognitive decline. Studies have indicated that exercise can boost gray matter volume in the hippocampus of aged individuals [286] which leads to enhanced memory [284], and may delay the onset of dementia or Alzheimer’s disease (reviewed by [287,288]).
In conclusion, exercise interventions can be extremely beneficial for hippocampal structure and function. The benefits of exercise on hippocampal neurogenesis, synaptic plasticity and behaviour have been extensively studied and have been summarized in this review. The mechanisms responsible for these beneficial effects appear to be vast and diverse, but many effects appear to converge on enhancing BDNF in the brain. Importantly, the benefits of physical exercise are also observed in humans, indicating that exercise interventions should be considered at any age in order to enhance hippocampal learning and memory and delay cognitive decline.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare regarding this manuscript.
REFERENCES
1 | Anderson P, Morris R, Amaral D, Bliss T, O’Keefe J. The Hippocampus Book [Internet]. Oxford University Press. 2007. 872 p. Available from: http://books.google.com/books/about/The_hippocampus_book.html?id=zg6oyF1DziQC |
2 | Kitamura T, Mishina M, Sugiyama H. Dietary restriction increases hippocampal neurogenesis by molecular mechanisms independent of NMDA receptors. Neurosci Lett [Internet]. 2005/12/17 ed. 2006;393(2-3):94-6. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list uids=16356642 |
3 | Helfer JL, Goodlett CR, Greenough WT, Klintsova AY. The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Res [Internet]. 2009/08/04 ed. 2009;1294:1-11. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list uids=19647724 |
4 | Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol [Inter- net]. 2012 Jun [cited 2015 Mar 19];72(6):943-52. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3485396&tool=pmcentrez&rendertype=abstract |
5 | Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci [Internet]. 2005 Sep 21 [cited 2014 Jul 14];25(38):8680-5. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1360197&tool=pmcentrez&rendertype=abstract |
6 | Kannangara TS, Lucero MJ, Gil-Mohapel J, Drapala RJ, Simpson JM, Christie BR, et al. Running reduces stress and enhances cell genesis in aged mice. Neurobiol Aging [Internet]. 2011 Dec [cited 2015 Mar 19];32(12):2279-86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20106549 |
7 | Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents agerelated decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging [Internet]. 2006 Oct [cited 2015 Mar 19];27(10):1505-13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16271278 |
8 | Andersen P, Bliss TVP, Skrede KK(1971) Lamellar organization of hippocampal excitatory pathwaysExp Brain Res13: 222238 |
9 | McNaughton BL(1980) Evidence for two physiologically distinct perforant pathways to the fascia dentataBrain Res199: 119 |
10 | Colino A, Malenka RC(1993) Mechanisms underlying induction of long-term potentiation in rat medial and lateral perforant paths in vitro J Neurophysiol69: 11501159 |
11 | Bramham CR, Milgram NW, Srebro B. Activation of AP5sensitive NMDA Receptors is Not Required to Induce LTP of Synaptic Transmission in the Lateral Perforant Path. Eur J Neurosci [Internet]. 1991;3:1300-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12106227 |
12 | Kallarackal AJ, Kvarta MD, Cammarata E, Jaberi L, Cai X, Bailey AM, et al. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J Neurosci [Internet]. 2013 Oct 2 [cited 2015 Jul 7];33(40):15669-74. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3787493&tool=pmcentrez&rendertype=abstract |
13 | SCOVILLEWB, MILNER B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry [Internet]. 1957 Feb;20(1):11-21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/13406589 |
14 | Corkin S, Amaral DG, González RG, Johnson KA, Hyman BTH(1997) M.’s medial temporal lobe lesion: findings from magnetic resonance imagingThe Journal of neuroscience: The Official Journal of the Society for Neuroscience39643979 |
15 | Vargha-Khadem F(1997) Differential effects of early hippocampal pathology on episodic and semantic memoryScience376380 |
16 | O’Reilly RC, Norman KA(2002) Hippocampal and neocortical contributions to memory: Advances in the complementary learning systems frameworkTrends in Cognitive Sciences505510 |
17 | Morris RG, Garrud P, Rawlins JN, O’Keefe J(1982) Place navigation impaired in rats with hippocampal lesionsNature297: 681683 |
18 | O–Keefe J,Dostrovsky J. The hippocampus as a spatialmap. Preliminary evidence fromunit activity in the freely-moving rat. Brain Res [Internet]. 1971 Nov;34(1):171-5. Available http://www.ncbi.nlm.nih.gov/pubmed/5124915 |
19 | O’Keefe J, Conway DH(1978) Hippocampal place units in the freely moving rat: Why they fire where they fireExperimental Brain Research31: 4573590 |
20 | Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL(2003) Cellular networks underlying human spatial navigationNature425: 184188 |
21 | Taube JS, Muller RU, Ranck JB(1990) Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulationsJ Neurosci10: 436447 |
22 | Fyhn M, Molden S, Witter MP, Moser EI, Moser M-B(2004) Spatial representation in the entorhinal cortexScience305: 12581264 |
23 | Kesner RP(2013) An analysis of the dentate gyrus functionBehavioural Brain Research17 |
24 | Leutgeb JK, Leutgeb S, Moser M-B, Moser EI(2007) Pattern separation in the dentate gyrus and CA3 of the hippocampusScience315: 961966 |
25 | Treves A, Tashiro A, Witter ME, Moser EI(2008) What is the mammalian dentate gyrus good for?Neuroscience11551172 |
26 | Galimberti I, Gogolla N, Alberi S, Santos AF, Muller D, Caroni P(2006) Long-term rearrangements of hippocampal mossy fiber terminal connectivity in the adult regulated by experienceNeuron50: 749763 |
27 | Muller RU, Kubie JL(1987) The effects of changes in the environment on the spatial firing of hippocampal complex-spike cellsJ Neurosci7: 19511968 |
28 | Eichenbaum H, Wiener SI, Shapiro ML, Cohen NJ(1989) The organization of spatial coding in the hippocampus: A study of neural ensemble activityJ Neurosci9: 27642775 |
29 | Jung MW, McNaughton BL(1993) Spatial selectivity of unit activity in the hippocampal granular layerHippocampus3: 165182 |
30 | Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem [Internet]. 2007 Nov [cited 2015 Mar 19];14(11):771-81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18007020 |
31 | Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD(2002) Requirement for hippocampal CA3 NMDA receptors in associative memory recallScience297: 211218 |
32 | Kesner RP, Lee I, Gilbert P(2004) A behavioral assessment of hippocampal function based on a subregional analysisRev Neurosci15: 333351 |
33 | Volpe BT, Davis HP, Towle A, Dunlap WPLoss of hippocampal CA1 pyramidal neurons correlates with memory impairment in rats with ischemic or neurotoxin lesions |
34 | Olsen GM, Scheel-Krüger J, Møller A, Jensen LHRelation of spatial learning of rats in the Morris water maze task to the number of viable CA1 neurons following four-vessel occlusion |
35 | Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus [Internet]. 2001 Jan [cited 2015 Jun 21];11(6):626-36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11811656 |
36 | Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus [Internet]. 2004 Jan [cited 2015 Jun 2];14(3):301-10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15132429 |
37 | Ridley RM, Timothy CJ, MacLean CJ, Baker HF. Conditional learning and memory impairments following neurotoxic lesion of the CA1 field of the hippocampus. Neuroscience [Internet]. 1995 Jul [cited 2015 Jun 30];67(2):263-75. Available from: http://www.sciencedirect.com/science/article/pii/030645229500063O |
38 | Altman J, Das GD(1967) Postnatal neurogenesis in the guinea-pigNature214: 509310981101 |
39 | Ehninger D, Ehninger D, Kempermann G, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res [Internet]. 2008;331(1):243-50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17938969 |
40 | Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus [Internet]. 2006 Jan [cited 2013 Aug 15];16(3):199-207. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16411231 |
41 | Van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH(2002) Functional neurogenesis in the adult hippocampusNature415: 687510301034 |
42 | Bliss TVP, Lomo T(1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the preforant pathJ Physiol232: 331356 |
43 | Bliss TVP, Collingridge GL(1993) A synaptic model of memory: Long-term potentiation in the hippocampusNature361: 73139 |
44 | Christie BR(1996) Long-term depression (LTD) in the hippocampusHippocampus6: 112 |
45 | Van Praag H, Christie BR, Sejnowski TJ, Gage FH(1999) Running enhances neurogenesis, learning, and long-term potentiation in miceProc Natl Acad Sci96: 231342713431 |
46 | Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T(2003) Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel runningBehav Neurosci117: 510061016 |
47 | Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: Circadian and dose-dependent effects. J Neurosci Res [Internet]. 2004;76(2):216-22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15048919 |
48 | Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav [Inter1561net]. 2011;10(3):345-53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21223504 |
49 | Merritt JR, Rhodes JS. Mouse genetic differences in voluntary wheel running, adult hippocampal neurogenesis and learning on the multi-strain-adapted plus water maze. Behav Brain Res [Internet]. 2015;280:62-71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25435316 |
50 | Kim YP, Kim H, Shin MS, Chang HK, Jang MH, Shin MC, et al. Age-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Neurosci Lett [Internet]. 2004;355(1-2):152-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14729257 |
51 | Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res [Internet]. 2006;1104(1):64-72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16824490 |
52 | Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience [Internet]. 2008;156(3):456-65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18721864 |
53 | Li H, Liang A, Guan F, Fan R, Chi L, Yang B. Regular treadmill running improves spatial learning and memory performance in young mice through increased hippocampal neurogenesis and decreased stress. Brain Res [Internet]. 2013;1531:1-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23916669 |
54 | Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci [Internet]. 2006;9(4):526-33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16531997 |
55 | Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus [Internet]. 2009;19(10):898-906.Available from: http://www.ncbi.nlm.nih.gov/pubmed/19156854 |
56 | Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adultmouse dentate gyrus. Nat Neurosci [Internet]. 1999 Mar [cited 2011 Aug 23];2(3):266-70. Available from: http://dx.doi.org/10.1038/6368 |
57 | Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci [Internet]. 2003;17(10):2042-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12786970 |
58 | Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci U S A [Internet]. 2014;111(44):15810-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25331877 |
59 | Patten AR, Sickmann H, Hryciw BN, Kucharsky T, Parton R, Kernick A(2013) Long-term exercise is needed to enhance synaptic plasticity in the hippocampusLearn Mem20: 11642647 |
60 | Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol [Internet]. 2003;467(4):455-63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14624480 |
61 | Brandt MD, Maass A, Kempermann G, Storch A. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neuroge nesis. Eur J Neurosci [Internet]. 2010;32(8):1256-64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20950279 |
62 | Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M(2010) Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and agingCell Stem Cell6: 5445456 |
63 | Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell [Internet]. 2007;1(5):515-28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18371391 |
64 | Bednarczyk MR, Aumont A, Decary S, Bergeron R, Fernandes KJ. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus [Internet]. 2009;19(10):913-27. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19405143 |
65 | Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell [Internet]. 2010;6(5):445-56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20452319 |
66 | Farioli-Vecchioli S, Mattera A, Micheli L, Ceccarelli M, Leonardi L, Saraulli D, et al. Running rescues defective adult neurogenesis by shortening the length of the cell cycle of neural stem and progenitor cells. Stem Cells [Internet]. 2014 Jul [cited 2015 Jul 2];32(7):1968-82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24604711 |
67 | Fischer TJ, Walker TL, Overall RW, Brandt MD, Kempermann G. Acute effects of wheel running on adult hippocampal precursor cells in mice are not caused by changes in cell cycle length or S phase length. Front Neurosci [Internet]. 2014;8:314. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25339861 |
68 | Bednarczyk MR, Hacker LC, Fortin-Nunez S, Aumont A, Bergeron R, Fernandes KJ. Distinct stages of adult hippocampal neurogenesis are regulated by running and the running environment. Hippocampus [Internet]. 2011;21(12):1334-47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20623741 |
69 | Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature [Internet]. 1997;386(6624):493-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9087407 |
70 | Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS(2012) Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J miceNeuroscience219: 6271 |
71 | Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G(2009) Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in miceFront Neurosci3: 50 |
72 | Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK(2008) Exercising our brains: How physical activity impacts synaptic plasticity in the dentate gyrusNeuroMolecular Medicine4758 |
73 | Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR(2004) Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo Neuroscience124: 17179 |
74 | Kim S-E, Ko I-G, Kim B-K, Shin M-S, Cho S, KimC-J (2010) Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampusExp Gerontol45: 5357365 |
75 | O’Callaghan RM, Ohle R, Kelly AM(2007) The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learningBehav Brain Res176: 2362366 |
76 | Zagaar M, Dao A, Levine A, Alhaider I, Alkadhi K(2013) Regular exercise prevents sleep deprivation associated impairment of long-term memory and synaptic plasticity in the CA1 area of the hippocampusSlee36: 5751761 |
77 | Kumar A, Rani A, Tchigranova O, Lee W-H, Foster TC(2012) Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiologyNeurobiol Aging33: 4828.e1828.e17 |
78 | Dao AT, Zagaar MA, Levine AT, Salim S, Eriksen JL, Alkadhi KA(2013) Treadmill exercise prevents learning and memory impairment in Alzheimer’s disease-like pathologyCurr Alzheimer Res10: 5507515 |
79 | Van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH(2002) Functional neurogenesis in the adult hippocampusNature415: 10301034 |
80 | Burgess N, Barry C, Keefe JO, Al BET, O’Keefe J(2007) An oscillatory interference model of grid cell firingHippocampus000: 13 |
81 | Eadie BD, Redila VA, Christie BR(2005) Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine densityJ Comp Neurol486: 13947 |
82 | Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW(1996) Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brainBrain Res726: 1-24956 |
83 | Korte M, Kang H, Bonhoeffer T, Schuman E(1998) A role for BDNF in the late-phase of hippocampal long-term potentiationNeuropharmacology37: 4-5553559 |
84 | Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T(1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factorProc Natl Acad Sci92: 1988568860 |
85 | Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW(2004) The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exerciseNeurosci Lett363: 14348 |
86 | Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS(2008) Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J miceNeuroscience155: 410481058 |
87 | P C, NR R, KE B. Cued and Contextual Fear Conditioning for Rodents [Internet]. CRC Press, Boca Raton (FL); 2009 [cited 2015 May 1]. Available from: http://europepmc.org/abstract/med/21204331 |
88 | Baruch DE, Swain RA, Helmstetter FJ(2004) Effects of Exercise on Pavlovian Fear ConditioningBehav Neurosci118: 511231127 |
89 | Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J(2006) Alterations in fear conditioning and amygdalar activation following chronic wheel running in ratsPharmacol Biochem Behav84: 2306312 |
90 | Greenwood BN, Strong PV, Foley TE, Fleshner M(2009) A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioningHippocampus19: 109881001 |
91 | Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK(2000) Exercise influences spatial learning in the radial arm mazePhysiol Behav70: 5425429 |
92 | Schweitzer NB, Alessio HM, Berry SD, Roeske K, Hagerman AE(2006) Exercise-induced changes in cardiac gene expression and its relation to spatial maze performanceNeurochem Int48: 1916 |
93 | Van der Borght K, Havekes R, Bos T, Eggen BJL, Van der Zee EA(2007) Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampal neurogenesisBehav Neurosci121: 2324334 |
94 | Antunes M, Biala G(2012) The novel object recognition memory: Neurobiology, test procedure, and its modificationsCognitive Processing93110 |
95 | Aggleton JP, Albasser MM, Aggleton DJ, Poirier GL, Pearce JM(2010) Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognitionBehav Neurosci124: 15568 |
96 | Clark RE, Zola SM, Squire LR(2000) Impaired recognition memory in rats after damage to the hippocampusJ Neurosci20: 2388538860 |
97 | Lafenêtre P, Leske O, Ma-Högemeie Z, Haghikia A, Bichler Z, Wahle P(2010) Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesisFront Behav Neurosci3: 34 |
98 | Hopkins ME, Bucci DJ(2010) BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memoryNeurobiol Learn Mem94: 2278284 |
99 | Hopkins ME, Nitecki R, Bucci DJ(2011) Physical exercise during adolescence versus adulthood: Differential effects on object recognition memory and brain-derived neurotrophic factor levelsNeuroscience194: 8494 |
100 | Yau S, Li A, So K(2015) Involvement of Adult Hippocampal Neurogenesis in Learning and ForgettingNeural Plast501 |
101 | Feng R, Rampon C, Tang Y-P, Shrom D, Jin J, Kyin M, et al. Deficient Neurogenesis in Forebrain-Specific Presenilin-1 Knockout Mice Is Associated with Reduced Clearance of Hippocampal Memory Traces. Neuron [Internet]. 2001 Dec [cited 2015 May 15];32(5):911-26. Available from: http://www.sciencedirect.com/science/article/pii/S0896627301005232 |
102 | Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew M V, et al. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A [Internet]. 2007 Mar 13 [cited 2015 May 5];104(11):4642-6. Available from: http://www.pnas.org/content/104/11/4642.full |
103 | Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, et al. Adult Neurogenesis Modulates the Hippocampus-Dependent Period of Associative Fear Memory. Cell [Internet]. 2009 Nov [cited 2015 Mar 13];139(4):814-27. Available from:http://www.sciencedirect.com/science/article/pii/S0092867409013099 |
104 | Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang H-LL, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science [Internet]. 2014 May 9 [cited 2015 May 25];344(6184):598-602. Available from: http://www.sciencemag.org/content/344/6184/598.abstract |
105 | Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet [Internet]. 1973/11/03 ed. 1973;302(7836):999-1001. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=4127281 |
106 | Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA [Internet]. 2003;290(22):2996-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14665662 |
107 | Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: Insights from different rodent models. Brain Res Rev [Internet]. 2010/05/18 ed. 2010;64(2):283-303. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20471420 |
108 | Patten AR, Fontaine CJ, Christie BR. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front Pediatr [Internet]. 2014 Jan [cited 2015 Feb 10];2:93. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4153370&tool=pmcentrez&rendertype=abstract |
109 | Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, et al. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus [Internet]. 2006;16(3):305-11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16425237 |
110 | Hamilton GF, Jablonski SA, Schiffino FL, St Cry SA, Stanton ME, Klintsova AY(2014) Exercise and environment as an intervention for neonatal alcohol effects on hippocampal adult neurogenesis and learningNeuroscience265: 274290 |
111 | Helfer JL, Goodlett CR, Greenough WT, Klintsova AY(2009) The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurtBrain Res1294: 111 |
112 | Hamilton GF, Murawski NJ, St.cyr SA, Jablonski SA, Schiffino FL, Stanton ME(2011) Neonatal alcohol exposure disrupts hippocampal neurogenesis and contextual fear conditioning in adult ratsBrain Res1412: 88101 |
113 | Hamilton GF, Boschen KE, Goodlett CR, Greenough WT, Klintsova AY(2012) Housing in Environmental Complexity Following Wheel Running Augments Survival of Newly Generated Hippocampal Neurons in a Rat Model of Binge Alcohol Exposure During the Third Trimester EquivalentAlcohol Clin Exp Res36: 11961204 |
114 | Boehme F, Gil-Mohapel J, Cox A, Patten A, Giles E, Brocardo PS(2011) Voluntary exercise induces adult hippocampal neurogenesis and BDNF expression in a rodent model of fetal alcohol spectrum disordersEur J Neurosci33: 17991811 |
115 | Redila VA, Christie BR(2006) Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrusNeuroscience137: 12991307 |
116 | Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus [Internet]. 1997;7(2):232-8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9136052 |
117 | Titterness AK, Christie BR. Prenatal ethanol exposure enhances NMDAR-dependent long-term potentiation in the adolescent female dentate gyrus. Hippocampus [Internet]. 2010/11/17 ed. 2012;22(1):69-81. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21080406 |
118 | Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V(2005) Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male ratsEur J Neurosci21: 17191726 |
119 | Varaschin RK, Akers KG, Rosenberg MJ, Hamilton DA, Savage DD. Effects of the cognition-enhancing agent ABT-239 on fetal ethanol-induced deficits in dentate gyrus synaptic plasticity. J Pharmacol Exp Ther [Internet]. 2010/03/24 ed. 2010;334(1):191-8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20308329 |
120 | Brady ML, Diaz MR, Iuso A, Everett JC, Valenzuela CF, Caldwell KK(2013) Moderate prenatal alcohol exposure reduces plasticity and alters NMDA receptor subunit composition in the dentate gyrusJ Neurosci33: 10621067 |
121 | Sickmann HM, Patten AR, Morch K, Sawchuk S, Zhang C, Parton R, et al. Prenatal ethanol exposure has sex-specific effects on hippocampal long-term potentiation. Hippocampus [Internet]. 2013/09/03 ed. 2013; Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23996604 |
122 | Patten AR, Sickmann HM, Dyer RA, Innis SM, Christie BR(2013) Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposureNeurosci Lett551: 711 |
123 | Patten AR, Brocardo PS, Sakiyama C, Wortman RC, Noonan A, Gil-Mohapel J, et al. Impairments in hippocampal synaptic plasticity following prenatal ethanol exposure are dependent on glutathione levels. Hippocampus [Internet]. 2013/09/03 ed. 2013; Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23996467 |
124 | Streissguth AP, Sampson PD, Barr HM. Neurobehavioral dose-response effects of prenatal alcohol exposure in humans from infancy to adulthood. Ann N Y Acad Sci [Internet]. 1989/01/01 ed. 1989;562:145-58. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2742272 |
125 | Uecker A, Nadel L. Spatial locations gone awry: Object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia [Internet]. 1996/03/01 ed. 1996;34(3):209-23. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8868278 |
126 | Mattson SN, Riley EP. Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. J Int Neuropsychol Soc [Internet]. 1999;5:462-71. Available from: <Go to ISI>://WOS:000081714500008 |
127 | Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD(2003) Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water taskBehav Brain Res143: 8594 |
128 | Malisza KL, Allman AA, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: A functional magnetic resonance imaging study. Pediatr Res [Internet]. 2005;58:1150-7. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16306185 |
129 | Spadoni AD, Bazinet AD, Fryer SL, Tapert SF, Mattson SN, Riley EP(2009) BOLD response during spatial working memory in youth with heavy prenatal alcohol exposureAlcohol Clin Exp Res33: 20672076 |
130 | Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ(2007) Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposureNeuroreport18: 635639 |
131 | O’Hare ED, Lu LH, Houston SM, Bookheimer SY, Mattson SN, O’Connor MJ(2009) Altered frontal-parietal functioning during verbal working memory in children and adolescents with heavy prenatal alcohol exposureHum Brain Ma30: 32003208 |
132 | Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE(2009) Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disordersAlcohol Clin Exp Res33: 16711689 |
133 | Blanchard BA, Riley EP, Hannigan JH. Deficits on a spatial navigation task following prenatal exposure to ethanol. Neurotoxicol Teratol [Internet]. 1987;9:253-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3627089 |
134 | Gianoulakis C(1990) Rats exposed prenatally to alcohol exhibit impairment in spatial navigation testBehav Brain Res36: 217228 |
135 | Westergren S, Rydenhag B, Bassen M, Archer T, Conradi NG(1996) Effects of Prenatal Alcohol Exposure on Activity and Learning in Sprague Dawley RatsPharmacolBiochemBehav55: 515520 |
136 | Kim CK, Kalynchuk LE, Kornecook TJ, Mumby DG, Dadgar NA, Pinel JP(1997) Object-recognition and spatial learning and memory in rats prenatally exposed to ethanolBehav Neurosci111: 985995 |
137 | Matthews DB, Simson PE(1998) Prenatal exposure to ethanol disrupts spatial memory: Effect of the training-testing delay periodPhysiol Behav64: 6367 |
138 | Gabriel KI, Johnston S, Weinberg J(2002) Prenatal ethanol exposure and spatial navigation: Effects of postnatal handling and agingDev Psychobiol40: 345357 |
139 | Richardson DP, Byrnes ML, Brien JF, Reynolds JN, Dringenberg HC(2002) Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pigEur J Neurosci16: 15931598 |
140 | Iqbal U, Dringenberg HC, Brien JF, Reynolds JN(2004) Chronic prenatal ethanol exposure alters hippocampal GABA(A) receptors and impairs spatial learning in the guinea pigBehav Brain Res150: 117125 |
141 | Incerti M, Vink J, Roberson R, Wood L, Abebe D, Spong CY(2010) Reversal of alcohol-induced learning deficits in the young adult in a model of fetal alcohol syndromeObstet Gynecol115: 350356 |
142 | Goodlett CR, Peterson SD(1995) Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal ratsNeurobiol Learn Mem64: 265275 |
143 | Pauli J, Wilce P, Bedi KS(1995) Spatial learning ability of rats following acute exposure to alcohol during early postnatal lifePhysiol Behav58: 10131020 |
144 | Tomlinson D, Wilce P, Bedi KS(1998) Spatial learning ability of rats following differing levels of exposure to alcohol during early postnatal lifePhysiol Behav63: 205211 |
145 | Johnson TB, Goodlett CR(2002) Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male ratsAlcohol Clin Exp Res26: 8393 |
146 | Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T(2004) Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adultsNeurobiol Dis17: 3403414 |
147 | Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci [Internet]. 2008/12/03 ed. 2008;122(6):1264-73. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19045946 |
148 | Thomas JD, Idrus NM, Monk BR, Dominguez HD(2010) Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in ratsBirth Defects Res A Clin Mol Teratol88: 827837 |
149 | Schreiber WB, St.Cyr SA, Jablonski SA, Hunt PS, Klintsova AY, Stanton ME(2013) Effects of exercise and environmental complexity on deficits in trace and contextual fear conditioning produced by neonatal alcohol exposure in ratsDev Psychobiol55: 483495 |
150 | Murawski NJ, Jablonski SA, Brown KL, Stanton ME(2013) Effects of neonatal alcohol dose and exposure window on long delay and trace eyeblink conditioning in juvenile ratsBehav Brain Res236: 307318 |
151 | Murawski NJ, Stanton ME(2010) Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4-9Behav Brain Res212: 133142 |
152 | Caldwell KK, Sheema S, Paz RD, Samudio-Ruiz SL, Laughlin MH, Spence NE(2008) Fetal alcohol spectrum disorder-associated depression: Evidence for reductions in the levels of brain-derived neurotrophic factor in a mouse modelPharmacol Biochem Behav90: 614624 |
153 | Famy C, Streissguth AP, Unis AS(1998) Mental illness in adults with fetal alcohol syndrome or fetal alcohol effectsAm J Psychiatry155: 552554 |
154 | O’Connor MJ, Kasari C(2000) Prenatal alcohol exposure and depressive features in childrenAlcohol Clin Exp Res24: 10841092 |
155 | Roebuck TM, Mattson SN, Riley EP(1999) Behavioral and psychosocial profiles of alcohol-exposed childrenAlcohol Clin Exp Res23: 10701076 |
156 | Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR(2012) Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exerciseNeuropharmacology62: 16071618 |
157 | Faul M, Xu L, Wald M, Coronado V(2010) Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006Atlanta, GA |
158 | Beauchamp MH, Ditchfield M, Maller JJ, Catroppa C, Godfrey C, Rosenfeld J V(2011) Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injuryInt J Dev Neurosci29: 2137143 |
159 | Monti JM, Voss MW, Pence A, McAuley E, Kramer AF, Cohen NJ(2013) History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in lifeFront Aging Neurosci5: August19 |
160 | Tate DF, Bigler ED(2000) Fornix and hippocampal atrophy in traumatic brain injuryLearn Mem7: 442446 |
161 | Hicks RR, Smith DH, Lowenstein DH, Saint Marie R, McIntosh TK(1993) Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampusJ Neurotrauma10: 4405414 |
162 | Vakil E(2005) The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: A selective reviewJournal of Clinical and Experimental Neuropsychology |
163 | Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci [Internet]. 2013;14(2):128-42. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3951995&tool=pmcentrez&rendertype=abstract |
164 | Majerske CW, Mihalik JP, Ren D, Collins MW, Reddy CC, Lovell MR(2008) Concussion in sports: Postconcussive activity levels, symptoms, and neurocognitive performanceJ Athl Train43: 3265274 |
165 | Piao CS, Stoica BA, Wu J, Sabirzhanov B, Zhao Z, Cabatbat R(2013) Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injuryNeurobiol Dis54: 252263 |
166 | Chytrova G, Ying Z, Gomez-Pinilla F(2007) Exercise normalizes levels of MAG and Nogo-A growth inhibitors after brain traumaEur J Neurosci27: October 2007111 |
167 | Griesbach GS, Hovda DA, Gomez-Pinilla F(2009) Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activationBrain Res1288: 6105115 |
168 | Griesbach GS, Hovda D a, Molteni R, Wu a, Gomez-Pinilla F(2004) Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor upregulation and recovery of functionNeuroscience125: 1129139 |
169 | Griesbach GS, Gómez-Pinilla F, Hovda D a(2007) Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependentJ Neurotrauma24: 711611171 |
170 | Griesbach GS, Hovda D a, Gomez-Pinilla F, Sutton RL(2008) Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in ratsNeuroscience. IBRO154: 2530540 |
171 | Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: A report from the American Heart Association. [Internet]. Circulation. 2014. e28-e292 p. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24352519 |
172 | Lim C, Alexander MP(2009) Stroke and episodic memory disordersNeuropsychologia47: 30453058 |
173 | Marin R, Williams A, Hale S, Burge B, Mense M, Bauman R(2003) The effect of voluntary exercise exposure on histological and neurobehavioral outcomes after ischemic brain injury in the ratPhysiol Behav80: 2-3167175 |
174 | Ke Z, Yip SP, Li L, Zheng XX, Tong KY(2011) The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: A rat brain ischemia modelPLoS One6: 2111 |
175 | Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D(2005) Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemiaNeuroscience136: 49911001 |
176 | Chun XL, Jiang J, Qi GZ, Xin JZ, Wang W, Zhi JZ(2007) Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from strokeJ Neurosci Res85: 816371646 |
177 | Geibig CS, Keiner S, Redecker C(2012) Functional recruitment of newborn hippocampal neurons after experimental strokeNeurobiol Dis46: 2431439 |
178 | Patten DA, Germain M, Kelly MA, Slack RS(2010) Reactive oxygen species: Stuck in the middle of neurodegenerationJ Alzheimers Dis20: Suppl 2S357S367 |
179 | Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M(1998) Genetic dissection of Alzheimer’s disease and related dementias: Amyloid and its relationship to tauNat Neurosci1: 5355358 |
180 | Shulman JM, De Jager PL, Feany MB(2011) Parkinson’s disease: Genetics and pathogenesisAnnu Rev Pathol6: 193222 |
181 | Vonsattel JP, DiFiglia M(1998) Huntington diseaseJ Neuropathol Exp Neurol57: 5369384 |
182 | Perl DPNeuropathology of Alzheimer’s diseaseMt Sinai J Med77: 13242 |
183 | Hardy J, Selkoe DJ(2002) The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeuticsScience297: 5580353356 |
184 | Selkoe DJ(2001) Alzheimer’s Disease: Genes, Proteins, and TherapyPhysiol Rev81: 2741766 |
185 | Hardy JA, Higgins GA(1992) Alzheimer’s disease: The amyloid cascade hypothesisScience256: 5054184185 |
186 | Shen J, Kelleher RJ. The presenilin hypothesis of Alzheimer's disease: Evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A [Internet]. 2007 Jan 9 [cited 2015 Jun 1];104(2):403-9. Available from: http://www.pnas.org/content/104/2/403.short |
187 | Braak H, Braak E(1997) Staging of Alzheimer-related cortical destructionInt Psychogeriatr9: Suppl 1257261discussion 269-72 |
188 | Dodart J-C, May P(2005) Overview on rodent models of Alzheimer’s diseaseCurr Protoc NeurosciNov; Chapter 9:Unit 9.22 |
189 | Mu Y, Gage FH(2011) Adult hippocampal neurogenesis and its role in Alzheimer’s diseaseMol Neurodegener6: 85 |
190 | Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ(2006) Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s diseaseJ Comp Neurol495: 17083 |
191 | Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP(2002) Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s diseaseJ Neurochem83: 615091524 |
192 | Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG(2004) Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stressNeuroscience127: 3601609 |
193 | Verret L, Jankowsky JL, Xu GM, Borchelt DR, Rampon C(2007) Alzheimer’s-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesisJ Neurosci27: 2567716780 |
194 | Zhang C, McNeil E, Dressler L, Siman R(2007) Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s diseaseExp Neurol204: 17787 |
195 | Wen PH, Hof PR, Chen X, Gluck K, Austin G, Younkin SG(2004) The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult miceExp Neurol188: 2224237 |
196 | Chevallier NL, Soriano S, Kang DE, Masliah E, Hu G, Koo EH(2005) Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutationAm J Pathol167: 1151159 |
197 | Rodríguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM(2008) Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s diseasePLoS One3: 8e2935 |
198 | Kolecki R, Lafauci G, Rubenstein R, Mazur-Kolecka B, Kaczmarski W, Frackowiak J(2008) The effect of amyloidosis-beta and ageing on proliferation of neuronal progenitor cells in APP-transgenic mouse hippocampus and in cultureActa Neuropathol116: 4419424 |
199 | Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE(2004) Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw,Ind) miceProc Natl Acad Sci U S A101: 361336313367 |
200 | López-Toledano MA, Shelanski ML(2007) Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind)J Alzheimers Dis12: 3229240 |
201 | Tapia-Rojas C, Aranguiz F, Varela-Nallar L, Inestrosa NC(2015) Voluntary running attenuates memory loss, decreases neuropathological changes and induces neurogenesis in a mouse model of Alzheimer’s diseaseBrain Pathol |
202 | Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM(2009) Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tgmouse model of Alzheimer’s diseaseNeurobiol Dis Elsevier Inc35: 3426432 |
203 | Mirochnic S, Wolf S, Staufenbiel M, Kempermann G(2009) Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer diseaseHippocampus19: 1010081018 |
204 | García-Mesa Y, López-Ramos JC, Gimènez-Llort L, Revilla S, Guerra R, Gruart A(2011) Physical exercise protects against Alzheimer’s disease in 3xTg-AD miceJ Alzheimer’s Dis24: 421454 |
205 | Ashe KHLearning and memory in transgenic mice modeling Alzheimer’s diseaseLearn Mem8: 6301308 |
206 | Friedland RP, Fritsch T, Smyth KA, Koss E, Lerner AJ, Chen CH(2001) Patients with Alzheimer’s disease have reduced activities in midlife compared with healthy control-group membersProc Natl Acad Sci U S A98: 634403445 |
207 | Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y(2009) Physical Activity, Diet, and Risk of Alzheimer DiseaseJAMA302: 6627 |
208 | Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA(2002) Participation in cognitively stimulating activities and risk of incident Alzheimer diseaseJAMA287: 6742748 |
209 | Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW(2009) Exercise improves cognition and hippocampal plasticity in APOE epsilon4 miceAlzheimers Dement5: 4287294 |
210 | Adlard P a, Perreau VM, Pop V, Cotman CW(2005) Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s diseaseJ Neurosci25: 1742174221 |
211 | Schindowski K, Bretteville A, Leroy K, Bègard S, Brion J-P, Hamdane M(2006) Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficitsAm J Pathol169: 2599616 |
212 | Van der Jeugd A, Ahmed T, Burnouf S, Belarbi K, Hamdame M, Grosjean M-E(2011) Hippocampal tauopathy in tau transgenic mice coincides with impaired hippocampus-dependent learning and memory, and attenuated late-phase long-term depression of synaptic transmissionNeurobiol Learn Mem95: 3296304 |
213 | Belarbi K, Burnouf S, Fernandez-Gomez F-J, Laurent C, Lestavel S, Figeac M(2011) Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathologyNeurobiol Dis43: 248694Elsevier Inc |
214 | Ambrèe O, Touma C, Görtz N, Keyvani K, Paulus W, Palme R(2006) Activity changes and marked stereotypic behavior precede A β pathology in TgCRND8 Alzheimer miceNeurobiol Aging27: 7955964 |
215 | Richter H, Ambrèe O, Lewejohann L, Herring A, Keyvani K, Paulus W(2008) Wheel-running in a transgenic mouse model of Alzheimer’s disease: Protection or symptom?Behav Brain Res190: 7484 |
216 | Wolf S a, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M(2006) Cognitive and Physical Activity Differently Modulate Disease Progression in the Amyloid Precursor Protein (APP)-23 Model of Alzheimer’s DiseaseBiol Psychiatry60: Mdc13141323 |
217 | Herring A, Ambrèe O, Tomm M, Habermann H, Sachser N, Paulus W(2009) Environmental enrichment enhances cellular plasticity in transgenic mice with Alzheimer-like pathologyExp Neurol216: 1184192Elsevier Inc |
218 | Costa D a, Cracchiolo JR, Bachstetter AD, Hughes TF, Bales KR, Paul SM(2007) Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanismsNeurobiol Aging28: 831844 |
219 | Liu HL, Zhao G, Cai K, Zhao HH, Shi L De(2011) Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiationBehav Brain Res218: 2308314Elsevier B.V |
220 | Liu HL, Zhao G, Zhang H, Shi L De(2013) Long-term treadmill exercise inhibits the progression of Alzheimer’s disease-like neuropathology in the hippocampus of APP/PS1 transgenic miceBehav Brain Res256: 261272Elsevier B.V |
221 | Um H-S, Kang E-B, Koo J-H, Kim H-T, Jin-Lee , Kim E-J(2011) Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s diseaseNeurosci Res69: 2161173Elsevier Ireland Ltd and Japan Neuroscience Society |
222 | Kim B-K, Shin M-S, Kim C-J, Baek S-B, Ko Y-C, Kim Y-P(2014) Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease ratsJ Exerc Rehabil10: 128 |
223 | Ke HC, Huang HJ, Liang KC, Hsieh-Li HM(2011) Selective improvement of cognitive function in adult and aged APP/PS1 transgenic mice by continuous non-shock treadmill exerciseBrain Res1403: 111Elsevier B.V |
224 | Hoveida R, Alaei H, Oryan S, Parivar K, Reisi P(2011) Treadmill running improves spatial memory in an animal model of Alzheimer’s diseaseBehav Brain Res216: 1270274Elsevier B.V |
225 | Kang EB, Cho JY(2014) Effects of treadmill exercise on brain insulin signaling and β -amyloid in intracerebroventricular streptozotocin induced-memory impairment in rats18: 18996 |
226 | Choi DH, Kwon IS, Koo JH, Jang YC, Kang EB, Byun JE(2014) The effect of treadmill exercise on inflammatory responses in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type18: 2225233 |
227 | Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson's disease is associated with hippocampal atrophy. Mov Disord [Internet]. 2003;18(7):784-90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12815657 |
228 | Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron [Internet]. 2010;66(5):646-61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20547124 |
229 | Steidl J V, Gomez-Isla T, Mariash A, Ashe KH, Boland LM. Altered short-term hippocampal synaptic plasticity in mutant alpha-synuclein transgenic mice. Neuroreport [Internet]. 2003;14(2):219-23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12598733 |
230 | Gureviciene I, Gurevicius K, Tanila H. Aging and alpha-synuclein affect synaptic plasticity in the dentate gyrus. J Neural Transm [Internet]. 2009;116(1):13-22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19002552 |
231 | Bonito-Oliva A, Pignatelli M, Spigolon G, Yoshitake T, Seiler S, Longo F, et al. Cognitive impairment and dentate gyrus synaptic dysfunction in experimental parkinsonism. Biol Psychiatry [Internet]. 2014;75(9):701-10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23541633 |
232 | Pendolino V, Bagetta V, Ghiglieri V, Sgobio C, Morelli E, Poggini S, et al. l-DOPA reverses the impairment of Dentate Gyrus LTD in experimental parkinsonism via beta-adrenergic receptors. Exp Neurol [Internet]. 2014;261:377-85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25058044 |
233 | Zhu G, Li J, He L, Wang X, Hong X. MPTP-induced changes in hippocampal synaptic plasticity and memory are prevented by memantine through the BDNF-TrkB pathway. Br J Pharmacol [Internet]. 2015; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25560396 |
234 | Regensburger M, Prots I, Winner B. Adult hippocampal neurogenesis in Parkinson's disease: Impact on neuronal survival and plasticity. Neural Plast [Internet]. 2014 Jan [cited 2015 Mar 2];2014:454696. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4106176&tool=pmcentrez&rendertype=abstract |
235 | Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci [Internet]. 2004;7(7):726-35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15195095 |
236 | Lesemann A, Reinel C, Huhnchen P, Pilhatsch M, Hellweg R, Klaissle P, et al. MPTP-induced hippocampal effects on serotonin, dopamine, neurotrophins, adult neurogenesis and depression-like behavior are partially influenced by fluoxetine in adult mice. Brain Res [Internet]. 2012;1457:51-69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22520437 |
237 | Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, et al. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci [Internet]. 2008;28(16):4250-60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18417705 |
238 | Nuber S, Petrasch-Parwez E, Winner B, Winkler J, von Horsten S, Schmidt T, et al. Neurodegeneration and motor dysfunction in a conditional model of Parkinson's disease. J Neurosci [Internet]. 2008;28(10):2471-84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18322092 |
239 | Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, et al. Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res [Internet]. 2010;1310:200-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19900418 |
240 | Döbrössy MD, Nikkhah G(2012) Role of experience, training, and plasticity in the functional efficacy of striatal transplantsProg Brain Res200: 303328 |
241 | Costa C, Sgobio C, Siliquini S, Tozzi A, Tantucci M, Ghiglieri V, et al. Mechanisms underlying the impairment of hippocampal long-term potentiation and memory in experimental Parkinson's disease. Brain [Internet]. 2012;135(Pt 6):1884-99. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22561640 |
242 | Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, Yip J, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch Phys Med Rehabil [Internet]. 2008;89(7):1221-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18534554 |
243 | Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol [Internet]. 2013;12(7):716-26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23769598 |
244 | Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience [Internet]. 2003;119(3):899-911. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12809709 |
245 | Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res [Internet]. 2004;77(3):378-90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15248294 |
246 | Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci [Internet]. 2007;27(20):5291-300. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17507552 |
247 | Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson's disease with severe neurodegeneration. BMC Neurosci [Internet]. 2009;10:6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19154608 |
248 | Smith BA, Goldberg NR, Meshul CK. Effects of treadmill exercise on behavioral recovery and neural changes in the substantia nigra and striatum of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse. Brain Res [Internet]. 2011;1386:70-80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21315689 |
249 | Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci [Internet]. 2007;30(5):211-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17367873 |
250 | Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci [Internet]. 2009;12(3):333-41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19198605 |
251 | (1993) The Huntington’s Disease Collaborative Research GrouA novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomesThe Huntington’s Disease Collaborative Research Grou.Cell72: 6971983 |
252 | DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP(1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brainScience277: 533419901993 |
253 | Li S-H, Li X-J(2004) Huntingtin and its role in neuronal degenerationNeuroscientist10: 5467475 |
254 | Myers RH, Vonsattel JP, Stevens TJ, Cupples LA, Richardson EP, Martin JB(1988) Clinical and neuropathologic assessment of severity in Huntington’s diseaseNeurology38: 3341347 |
255 | Gil JM, Rego AC(2008) Mechanisms of neurodegeneration in Huntington’s diseaseEur J Neurosci27: 1128032820 |
256 | Lazic SE, Grote H, Armstrong RJE, Blakemore C, Hannan AJ, van Dellen A(2004) Decreased hippocampal cell proliferation in R6/1 Huntington’s miceNeuroreport15: 5811813 |
257 | Grote HE, Bull ND, Howard ML, van Dellen A, Blakemore C, Bartlett PF(2005) Cognitive disorders and neurogenesis deficits in Huntington’s disease mice are rescued by fluoxetineEur J Neurosci22: 820812088 |
258 | Lazic SE, Grote HE, Blakemore C, Hannan AJ, van Dellen A, Phillips W(2006) Neurogenesis in the R6/1 transgenic mouse model of Huntington’s disease: Effects of environmental enrichmentEur J Neurosci23: 718291838 |
259 | Walker TL, Turnbull GW, Mackay EW, Hannan AJ, Bartlett PF(2011) The Latent Stem Cell Population Is Retained in the Hippocampus of Transgenic Huntington’s Disease Mice but Not Wild-Type MicePLoS OneZheng J6: 3e18153 |
260 | Gil J, Mohapel P, Araújo IM, Popovic N, Li J-Y, Brundin P(2005) Reduced hippocampal neurogenesis in R6/2 transgenic Huntington’s disease miceNeurobiol Dis20: 3744751 |
261 | Peng Q, Masuda N, Jiang M, Li Q, Zhao M, Ross CA(2008) The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington’s disease mouse modelExp Neurol210: 1154163 |
262 | Phillips W, Morton AJ, Barker RA(2005) Abnormalities of Neurogenesis in the R6/2 Mouse Model of Huntington’s Disease Are Attributable to the In Vivo MicroenvironmentJ Neurosci25: 501156411576 |
263 | Gil JMAC, Leist M, Popovic N, Brundin P, Petersèn A(2004) Asialoerythropoietin is not effective in the R6/2 line of Huntington’s disease miceBMC Neurosci5: 17 |
264 | Kandasamy M, Couillard-Despres S, Raber KA, Stephan M, Lehner B, Winner B(2010) Stem Cell Quiescence in the Hippocampal Neurogenic Niche Is Associated With Elevated Transforming Growth Factor-β Signaling in an Animal Model of Huntington DiseaseJ Neuropathol Exp Neurol69: 7717728 |
265 | Renoir T, Pang TY, Zajac MS, Chan G, Du X, Leang L(2012) Treatment of depressive-like behaviour in Huntington’s disease mice by chronic sertraline and exerciseBr J Pharmacol165: 513751389 |
266 | Ransome MI, Hannan AJ(2013) Impaired basal and running-induced hippocampal neurogenesis coincides with reduced Akt signaling in adult R6/1 HD miceMol Cell Neurosci54: 93107 |
267 | Pang TYC, Stam NC, Nithianantharajah J, Howard ML, Hannan a J(2006) Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in huntington’s disease transgenic miceNeuroscience141: 569584 |
268 | Kohl Z, Kandasamy M, Winner B, Aigner R, Gross C, Couillard-Despres S(1155) Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington’s diseaseBrain Res2433 |
269 | Potter MC, Yuan C, Ottenritter C, Mughal M, van Praag H(2010) Exercise is not beneficial and may accelerate symptom onset in a mouse model of Huntington’s diseasePLoS Curr117 |
270 | Van Dellen A, Cordery PM, Spires TL, Blakemore C, Hannan AJ(2008) Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregate density in a mouse model of Huntington’s diseaseBMC Neurosci9: 34 |
271 | Harrison DJ, Busse M, Openshaw R, Rosser AE, Dunnett SB, Brooks SP(2013) Exercise attenuates neuropathology and has greater benefit on cognitive than motor deficits in the R6/1 Huntington’s disease mouse modelExp Neurol248: 457469Elsevier Inc |
272 | Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature [Internet]. 1995 Jan 12 [cited 2011 Dec 4];373(6510):109. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7816089 |
273 | Van Der Borght K, Kóbor-Nyakas DÉ, Klauke K, Eggen BJL, Nyakas C, Van Der Zee EA(2009) Physical exercise leads to rapid adaptations in hippocampal vasculature: Temporal dynamics and relationship to cell proliferation and neurogenesisHippocampus19: 10928936 |
274 | Molteni R, Ying Z, Gómez-Pinilla F(2002) Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarrayEur J Neurosci16: 611071116 |
275 | Lipsky RH, Marini AM(2007) Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticityAnnals of the New York Academy of Sciences130143 |
276 | Cowansage KK, LeDoux JE, Monfils M-H(2010) Brain-derived neurotrophic factor: A dynamic gatekeeper of neural plasticityCurr Mol Pharmacol3: 11229 |
277 | Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci [Internet]. 2013 Oct [cited 2014 Jul 15];17(10):525-44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24029446 |
278 | Hötting K, Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev [Internet]. 2013 Nov [cited 2014 Aug 21];37(9 Pt B):2243-57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23623982 |
279 | Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR(2002) Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticityJ Neurophysiol88: 521872195 |
280 | Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res [Internet]. 2010 Oct 28 [cited 2011 Aug 25];1358:172-83. Available from: http://dx.doi.org/10.1016/j.brainres.2010.08.049 |
281 | Monti JM, Hillman CH, Cohen NJ(2012) Aerobic fitness enhances relational memory in preadolescent children: The FITKids randomized control trialHippocampus22: 918761882 |
282 | Herting MM, Nagel BJ(2012) Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescentsBehav Brain Res233: 2517525 |
283 | Stroth S, Hille K, Spitzer M, Reinhardt R(2009) Aerobic endurance exercise benefits memory and affect in young adultsNeuropsychol Rehabil19: 2223243 |
284 | Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A [Internet]. 2011 Feb 15 [cited 2014 Jul 11];108(7):3017-22. Available from: http://www.pnas.org/content/108/7/3017.abstract |
285 | Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: Interactions between exercise, depression, and BDNF. Neuroscientist [Internet]. 2012 Feb 1 [cited 2015 Mar 2];18(1):82-97. Available from: http://nro.sagepub.com/content/18/1/82.short |
286 | Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging [Internet]. 2014 Sep [cited 2015 Mar 7];35 Suppl 2:S20-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24952993 |
287 | Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer's disease. Arch Med Res [Internet]. 2012 Nov [cited 2015 Jun 9];43(8):615-21. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3567914&tool=pmcentrez&rendertype=abstract |
288 | Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis [Internet]. 2013 Sep [cited 2015 Mar 4];57:47-55. Available from: http://www.sciencedirect.com/science/article/pii/S0969996112002264 |
Figures and Tables
Fig.1
A simplified hippocampal circuitry illustrating trisynaptic and monosynaptic circuits. The basic circuitry of the hippocampus is commonly termed the trisynaptic circuit. Layer II of the entorhinal cortex provides input to the granule cells of the dentate gyrus via the medial (light blue) and lateral (purple) perforant paths. The dentate granule cells project to pyramidal cells of the CA3 via the mossy fibre pathway (green). CA3 pyramidal neurons project to the CA1 via schaffer collaterals (pink). The CA1 pyramidal cells project to both the subiculum and to layers V and VI of the entorhinal cortex. Abbreviations: Cornu Ammonis (CA); Dentate Gyrus (DG); Entorhinal Cortex (EC); Lateral Perforant Path (LPP); Medial Perforant Path (LPP); Mossy Fibres (MF); Schaffer Collaterals (SC); Subiculum (S).
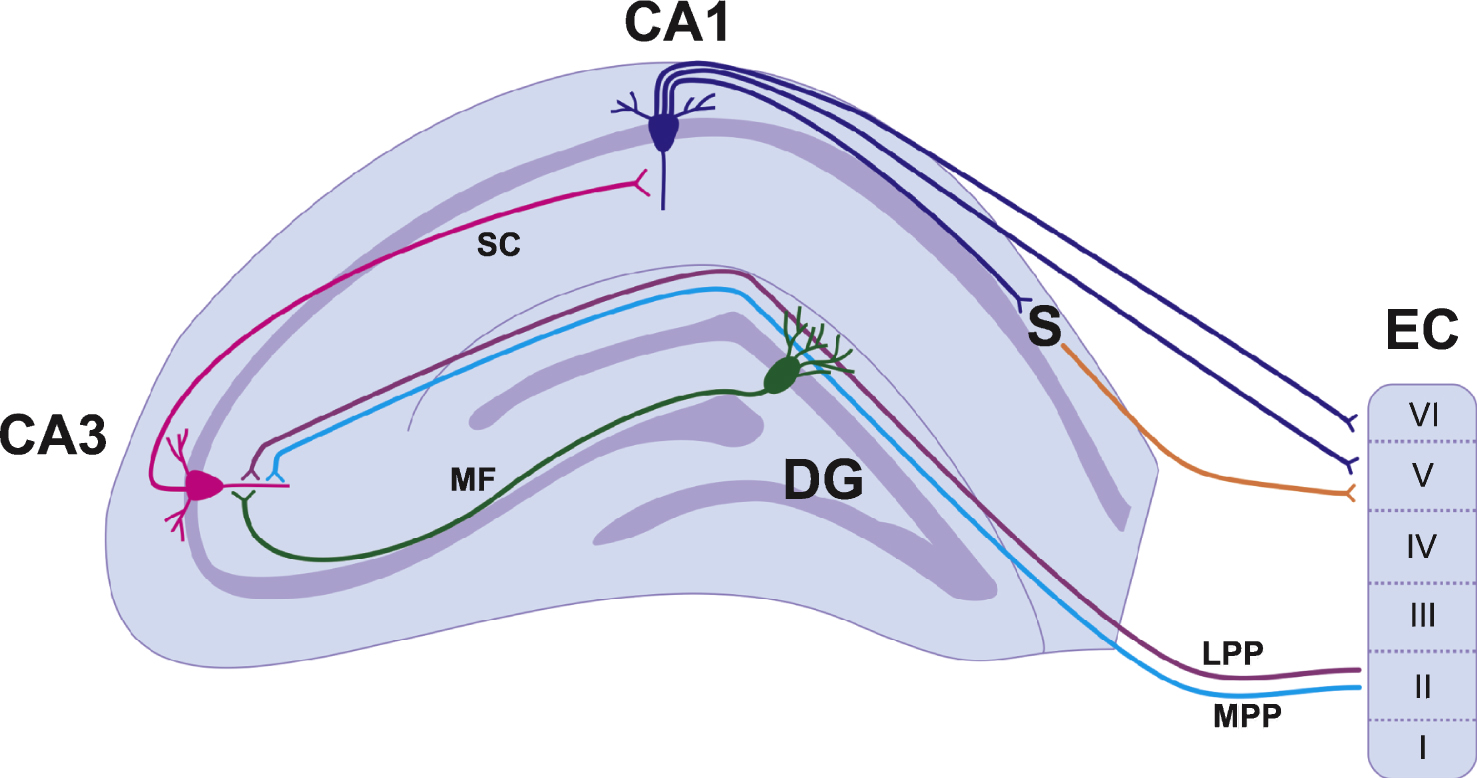
Table 1
Effect of voluntary running on hippocampal plasticity in healthy rodents
| Hippocampal neurogenesis | |||||
| Animal age | Species | Running period | Markers examined | Effect of running | Reference |
| 3 months old | Mouse | 12 days | BrdU | Increased cell | van Praag et al., |
| (Proliferation and | proliferation, | 1999 | |||
| Survival) | cell survival and | ||||
| NeuN | net neurogenesis | ||||
| (neurogenesis) | |||||
| 3 months old | Mouse | 30 days | BrdU (survival) | Increased cell | van Praag et al., |
| BrdU/NeuN | survival and | 1999 | |||
| (neuronal | neuronal | ||||
| differentiation) | differentiation | ||||
| 3 months old | Mouse | 45 days | BrdU (survival) | Increased cell | van Praag et al., 2005 |
| or 19 months old | survival and | ||||
| neuronal differentiation | |||||
| BrdU/NeuN/S100β | |||||
| triple labeling | |||||
| 6–8 weeks old | Mouse selectively | 40 days | BrdU (survival) | Increased cell | Rhodes et al., 2003 |
| bred for high | proliferation, | ||||
| levels of running | survival and | ||||
| BrdU/Neuro/ | neuronal | ||||
| S100β triple | differentiation | ||||
| labeling | |||||
| 6 weeks old | Mouse | 12 days or 19 days | BrdU (survival) | Increased cell | Synder et al., 2009 |
| PCNA (cell | proliferation | ||||
| proliferation) | after 14 days | ||||
| running, | |||||
| Increased cell | |||||
| survival in after | |||||
| both 14 days | |||||
| and 19 days of | |||||
| running | |||||
| 3 months old | Mouse | 12 days | BrdU(Proliferation | Increased cell | Brown et al., 2003 |
| and survival) | proliferation and | ||||
| survival | |||||
| 8-9 weeks old | Mouse | 14 days | BrdU, Ki67 (cell | Increased cell | Yau et al., 2014 |
| proliferation) | proliferation, | ||||
| DCX | neuronal | ||||
| (neurogenesis) | differentiation | ||||
| BrdU/DCX | and net | ||||
| (neuronal | neurogenesis | ||||
| differentiation) | |||||
| 8 weeks old | Mouse | 24 hours, 3 | BrdU, IdU, CldU | Increased cell | Brandt et al., 2010 |
| days, 7 days or | proliferation at | ||||
| 35 days | 24 hours, 3 days | ||||
| and 7 days | |||||
| (peak at 3 | |||||
| days). Return to | |||||
| baseline at 35 | |||||
| days | |||||
| 12 weeks old | Transgenic | 4 weeks | BrdU and nestin- | Specific | Kronenberg et al., 2003 |
| mouse | GFP positive cells | increase in type | |||
| expressing | 2b transient | ||||
| GFP driven by | amplifying | ||||
| nestin gene | progenitor cells | ||||
| promotor | |||||
| 74 days at end | Mouse | 3, 10 or 32 days | BrdU, Ki67, | Increased cell | Kronenberg et al., 2006 |
| of experiment | DCX, calretinin | proliferation at 3 | |||
| days and 10 | |||||
| days (peak at 3 | |||||
| days). Returned | |||||
| to baseline at 35 | |||||
| days. DCX and | |||||
| Calretinin | |||||
| increased at 10 | |||||
| days and 32 days. | |||||
| 2 months old | Mouse | 12 days | BrdU and PCNA | Increased cell | Kannangara et al., 2009 |
| (cell proliferation) | proliferation | ||||
| 6–8 weeks old | Rat | 7 days or 28 days | Brdu (cell | Increased cell | Yau et al., 2012 |
| proliferation) | proliferation and | ||||
| BrdU/DCX | differentiation | ||||
| (neuronal | following 28 | ||||
| differentiation) | days of running | ||||
| 6–8 weeks old | Rat | 14 days | BrdU (cell | Increased cell | Yau et al., 2011 |
| proliferation) | proliferation and | ||||
| BrdU/DCX co- | neuronal | ||||
| labeling (Neuronal | differentiation | ||||
| differentiation) | |||||
| 2 months old | Rat | 3, 7, 14, 28, 56 days | Ki 67 (cell | Increased cell | Patten et al., 2013 |
| proliferation) | proliferation | ||||
| NeuroD (neuronal | after 3,7 or 28 | ||||
| differentiation) | days of running | ||||
| Increased | |||||
| neuronal | |||||
| differentiation | |||||
| after 14 or 28 | |||||
| days of running |
| Hippocampal synaptic plasticity | |||||
| Animal age | Species | Running period | Area(s) analyzed | Effect of running | Reference |
| 3 months old | Mouse | 30 days | DG and CA1 | Enhanced LTP | Van Praag et al., 1999 |
| In vitro | in the DG, but | ||||
| not CA1 | |||||
| 2 months old | Rat | 3, 7, 14, 28, 56 days | DG | Enhanced LTP | Patten et al., 2013 |
| In vivo | following 56 | ||||
| days of running | |||||
| Adult (exact | Rat | 7+ days | DG | Enhanced STP | Farmer et al., 2004 |
| age not | In vivo | and LTP, | |||
| specified) | reduced | ||||
| threshold for | |||||
| LTP | |||||
| 3–5 weeks old | Mouse | 7–10 days | DG | Enhanced LTP, | Vasuta et al., 2007 |
| In vitro | no effect on | ||||
| LTD, both | |||||
| abolished by | |||||
| NR2A blockade |
| Hippocampal dependent behavior | |||||
| Animal age | Species | Running period | Behavioral test | Effect of running | Reference |
| 3 months old | Mouse | 30 days | MWM | Rapid | Van Praag et al., 1999 |
| acquisition | |||||
| (day 3), better | |||||
| memory in | |||||
| probe test | |||||
| 2 months old | Rat | 3 weeks | MWM | Rapid | Adlard et al., 2004 |
| acquisition | |||||
| (day 2) | |||||
| 6–8 weeks old | Mouse | 34 days | MWM | Rapid | Rhodes et al., 2003 |
| acquisition in | |||||
| control runners | |||||
| but not | |||||
| selectively-bred | |||||
| runners | |||||
| 3 months old | Rat | 1 week | MWM | CAMKII | Vaynman et al., 2007 |
| blockade | |||||
| improves | |||||
| acquisition in | |||||
| runners but | |||||
| impairs memory | |||||
| on probe test | |||||
| ∼7–9 weeks old | Rat | 30 days | Contextual fear | Increased | Baruch et al., 2004 |
| conditioning | freezing 24hr | ||||
| later | |||||
| ∼4–6 weeks old | Rat | 8 weeks | Contextual fear | Increased | Burghardt et al., 2006 |
| conditioning | freezing 24hr | ||||
| later, most | |||||
| freezing in rats | |||||
| that ran the | |||||
| most | |||||
| Adult (exact | Rat | 6 weeks | Contextual fear | Increased | Greenwood et al., 2009 |
| age not | conditioning | freezing when | |||
| specified) | running | ||||
| occurred before | |||||
| conditioning but | |||||
| no effect when | |||||
| running | |||||
| occurred | |||||
| between | |||||
| conditioning and | |||||
| testing | |||||
| 2 months old | Mouse | 3 weeks | Contextual fear | Reduced fear | Akers et al., 2014 |
| conditioning | memory in | ||||
| runners | |||||
| 6–8 weeks old | Mouse | 5 weeks | Contextual fear | No effect on | Kitamura et al., 2009 |
| conditioning | memory | ||||
| expression, | |||||
| speed up decay | |||||
| rate of | |||||
| hippocampal | |||||
| dependency of | |||||
| memory | |||||
| 5 months old | Rat | 7 weeks | Radial arm maze | Fewer trials to | Anderson et al., 2000 |
| criterion | |||||
| 10 weeks old | Mouse | 14 days | Y-Maze | Rapid | Van der Borght et al., |
| acquisition by | 2006 | ||||
| trial 2, after | |||||
| learning in non- | |||||
| runners, 14 | |||||
| days of running | |||||
| improved | |||||
| memory | |||||
| retention |
Abbreviations: BrdU, bromodeoxyuridine; CA, Cornu Ammonis; DG, dentate gyrus; DCX, doublecortin; MWM, Morris Water Maze; PCNA, proliferating cell nuclear antigen.
Table 2
Effects of voluntary running on adult hippocampal neurogenesis in animal models of neurological disorders
| Disease model | Animal age/ Model | Running period | Markers examined | Effect of running | Reference |
| FASD | 7 weeks old/ | 7 days | BrdU | Rescued deficit | Redila et al., 2006 |
| Rat | (proliferation | in proliferation | |||
| and survival) | and survival | ||||
| 4 weeks old/ | 12 days | BrdU | Increased | Helfer et al., 2009 | |
| Rat | (proliferation | proliferation, no | |||
| and survival) | effect on | ||||
| survival | |||||
| 7 weeks old/ | 12 days | Ki67, BrdU, | Increased | Boehme et al., 2011 | |
| Rat | NeuroD | proliferation | |||
| and neuronal | |||||
| differentiation | |||||
| 4 weeks old/ | 12 days (followed by | BrdU (survival) | Rescued deficit | Hamilton et al., 2014 | |
| Rat | 30 days enrichment) | in cell survival | |||
| TBI | Not reported | ||||
| Stroke | Adult/ Mouse/ | 42 days (beginning day | BrdU (Proliferaion) | Increased | Geibig et al., 2012 |
| of infarct) | proliferation | ||||
| photothrombotic | relative to | ||||
| cortical infarct | sedentary | ||||
| stroke group | |||||
| Adult/ Mouse/ | 42 days (beginning 7 | BrdU (Proliferation) | No change in | Chun et al., 2007 | |
| days after MCAO) | proliferation | ||||
| MCAO | |||||
| Adult/ Mouse/ | 42 days (beginning 7 | BrdU (Survival) | Increased | Chun et al., 2007 | |
| days after MCAO) | |||||
| MCAO | survival relative | ||||
| to sedentary | |||||
| stroke | |||||
| AD | 5 months old/ | 10 weeks | Nissl staining; | Prevented | Tapia-Rojas et al., 2015 |
| Mouse/ | BrdU (proliferation | neuronal loss; | |||
| APPswe/PS1 | and survival) | Increased | |||
| ΔE 9 | proliferation | ||||
| and neuronal | |||||
| differentiation | |||||
| 5 months old/ | 16 weeks | Nissl staining | Greater | Yuede et al., 2009 | |
| Mouse/ | hippocampal | ||||
| Tg2576 | volume | ||||
| 6 and 18 months | 10 days | BrdU, DCX, NeuN | Increased | Mirochnic et al., 2009 | |
| old / Mouse/ | proliferation | ||||
| APP23 | and neuronal | ||||
| differentiation | |||||
| in 18 month-old | |||||
| mice only | |||||
| PD | Not reported | ||||
| HD | 8–12 weeks old/ | 4 weeks | BrdU | No effect | Renoir et al., 2012 |
| Mouse/ | (proliferation | ||||
| R6/1 | and survival) | ||||
| 3 months old/ | 8 days | Ki67, BrdU, | No effect | Ransome and Hannan, 2013 | |
| Mouse/ | NeuN | ||||
| R6/1 |
Abbreviations: AD, Alzheimer’s Disease; BrdU, bromodeoxyuridine; DCX, doublecortin; FASD, fetal alcohol spectrum disorder; HD, Huntington’s Disease; MCAO, intraluminal middle cerebral artery occlusion; PD, Parkinson’s Disease; TBI, traumatic brain injury.
Table 3
Effects of voluntary running on hippocampal synaptic plasticity in animal models of neurological disorders
| Animal model | Animal age | Running period | Area analyzed | Effect of running | Reference |
| FASD | 8 weeks old/ Rat | 10 days | DG | Rescued deficit in LTP | Christie et al., 2005 |
| TBI | Not reported | ||||
| Stroke | Not reported | ||||
| AD | 7 months old/ Mouse/ | ||||
| 3xTg-AD | 6 months | CA1 | Rescued deficit in LTP | García-Mesa et al., 2011 | |
| PD | Not reported | ||||
| HD | Not reported |
Abbreviations: AD, Alzheimer’s disease; CA, Cornu Ammonis; DG, dentate gyrus; FASD, fetal alcohol spectrum disorders; HD, Huntington’s disease; LTP, long-term potentiation; PD, Parkinson’s disease; TBI, traumatic brain injury.
Table 4
Effects of voluntary running on hippocampal dependent behaviours in animal models of neurological disorders
| Animal model | Animal age/ Model | Running period | Behaviour examined | Effect of running | Reference |
| FASD | 8 weeks old/ | 10 days | MWM | Deficits in | Christie et al., 2005 |
| Rat | reference and | ||||
| working | |||||
| memory were | |||||
| reversed | |||||
| 3 weeks old/ | 30 days | MWM | Rescued | Thomas et al., 2008 | |
| Rat | deficits in | ||||
| reference | |||||
| memory | |||||
| 4 weeks old/ | 12 days | Contextual fear | No effect | Schreiber et al., 2012 | |
| Rat | (followed by | conditioning | |||
| 30 days | |||||
| enrichment) | |||||
| 4 weeks old/ | 12 days | Context pre- | Rescued | Hamilton et al., 2014 | |
| Rat | (followed by | exposure | deficits | ||
| 30 days | facilitation | ||||
| enrichment) | |||||
| TBI | Adult/ Rat/ | 7 days | MWM | Reduced | Grace et al., 2009 |
| Lateral FPI | (starting 14 | deficits in | |||
| days post | acquisition of | ||||
| injury) | spatial memory | ||||
| Adult/ Mouse/ | 4 weeks | MWM | Reduced | Piao et al., 2013 | |
| Left parietal | (starting 1 or | impairment in | |||
| CCI | 5 weeks post | acquisition of | |||
| injury) | spatial memory, | ||||
| reference | |||||
| memory, | |||||
| reversal | |||||
| memory | |||||
| acquisition, and | |||||
| reversal | |||||
| reference | |||||
| memory after 5 | |||||
| week delay in | |||||
| exercise onset | |||||
| No change after | |||||
| 1-week delay in | |||||
| exercise onset | |||||
| Adult/ Rat/ | 7 days | MWM | Increased | Griesbach et al., 2004 | |
| Lateral FPI | (starting day | impairment in | |||
| of injury) | acquisition of | ||||
| spatial memory | |||||
| Adult/ Rat/ | 7 days | MWM | Decreased | Griesbach et al., 2004 | |
| Lateral FPI | (starting 14 | impairment in | |||
| days post injury) | acquisition of | ||||
| spatial memory | |||||
| after delayed | |||||
| exercise | |||||
| initiation | |||||
| Stroke | Adult/ Mouse/ | 42 days | MWM | Increased rate | Geibig et al., 2012 |
| photothrombotic | (beginning | of spatial | |||
| cortical infarct | day of infarct) | memory | |||
| acquisition. No | |||||
| change in | |||||
| reference | |||||
| memory | |||||
| Adult/ Mouse/ MCAO | 42 days | MWM | Rescued | Chun et al., 2007 | |
| (beginning 7 | impairment in | ||||
| days after | acquisition of | ||||
| MCAO) | spatial memory | ||||
| AD | 5 months old/ | 16 weeks | Novel object recognition | Reduced | Yuede et al., 2009 |
| Mouse/ Tg2576 | decline in | ||||
| recognition | |||||
| memory | |||||
| 10–12 months | 6 weeks | Radial-arm water maze | Improved | Nichol et al., 2009 | |
| old/ Mouse/ | performance | ||||
| APOE ɛ4 | (less errors) | ||||
| 1 month old/ | 5 months | MWM | Enhanced | Adlard et al., 2005 | |
| Mouse/ | learning rate | ||||
| TgCRND8 | |||||
| 3 months old/ | 10 weeks | One-trial object | No effect | Richter et al., 2008 | |
| recognition paradigm, | |||||
| Barnes maze | |||||
| Mouse/ | |||||
| TgCRND8 | |||||
| 3 months old/ | 9 months | Two-trial Y-maze | Prevented | Belbarbi et al., 2011 | |
| Mouse/ | development of | ||||
| THY-Tau22 | memory | ||||
| alterations | |||||
| 4 and 7 months | 1 month and | MWM; Startle Response; | Rescued | García-Mesa et al., 2011 | |
| Dark and Light | |||||
| box test | |||||
| old/ Mouse/ | 6 months | retention | |||
| 3xTg-AD | deficiency; | ||||
| reduced BPSD- | |||||
| like behaviours | |||||
| 5 months old/ | 10 weeks | MWM | Prevented | Tapia-Rojas et al., 2015 | |
| Mouse/ | impairment in | ||||
| APPswe- | spatial acuity | ||||
| PS1ΔE9 | and memory | ||||
| acquisition | |||||
| PD | Not reported | ||||
| HD | 10 weeks old/ | 10 weeks | T-maze; Y-maze | Rear-paw | Pang et al., 2006 |
| Mouse/ R6/1 | clasping onset | ||||
| delayed; | |||||
| rescued deficit | |||||
| in spatial | |||||
| working | |||||
| memory | |||||
| 8–12 weeks old/ | 4 weeks | Forced-swim test | Anti-depressive | Renoir et al., 2012 | |
| Mouse/ R6/1 | effects | ||||
| (reduced | |||||
| immobility) |
Abbreviations: AD, Alzheimer’s disease; CCI, controlled cortical impact; FASD, fetal alcohol spectrum disorder; FPI, fluid percussion injury; HD, Huntington’s disease; MCAO, middle cerebral artery occlusion; MWM, Morris Water Maze; PD, Parkinson’s disease; TBI, traumatic brain injury.




