Control of the Cell Cycle in Adult Neurogenesis and its Relation with Physical Exercise
Abstract
In the adult brain the neurogenesis is mainly restricted to two neurogenic regions: newly generated neurons arise at the subventricular zone (SVZ) of the lateral ventricle and at the subgranular zone of the hippocampal subregion named the dentate gyrus. The hippocampus is involved in learning and memory paradigms and the generation of new hippocampal neurons has been hypothesized to be a pivotal form of plasticity involved in the process. Moreover the dysregulation of hippocampal adult neurogenesis has been recognized and could anticipate several varieties of brain disease such as Alzheimer disease, epilepsy and depression. Over the last few decades numerous intrinsic, epigenetic and environmental factors have been revealed to deeply influence the process of adult neurogenesis, although the underlying mechanisms remain largely unknown. Growing evidence indicates that physical exercise represents one of the main extrinsic factor able to profoundly increase hippocampal adult neurogenesis, by altering neurochemistry and function of newly generated neurons. The present review surveys how neurogenesis can be modulated by cell cycle kinetics and highlights the putative role of the cell cycle length as a key component of the beneficial effect of running for hippocampal adult neurogenesis, both in physiological conditions and in the presence of defective neurogenesis.
INTRODUCTION
Since 1962 when Altman suggested the presence of newly generated neurons in the adult brain, huge progress has been made in understanding the cellular and molecular processes that govern the proliferation, differentiation and integration of new neurons in the preexisting memory circuits. Adult neurogenesis has been clearly identified and confirmed in two brain regions: the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus of the hippocampal formation [1]. Newborn neurons in the SVZ migrate through the rostral migratory stream where they become granule and periglomerular neurons in the olfactory bulb. Cells born in the adult SGZ migrate into the deep layer of the dentate gyrus and fully differentiate into excitatory dentate granule cells [2]. Adult neurogenesis is an highly dynamic process that can be finely modulated both negatively by stress and aging and positively, by means of physical activity, environment enrichment, and learning. Among these factors numerous studies have pointed out that voluntary physical activity represent the most potent inducer of adult neurogenesis [3–5]. However the running-induced increase of proliferation is region-specific, occurring exclusively in the hippocampus while it does not enhance SVZneurogenesis [6].
In this review we first summarize the recent discoveries describing the cell cycle components and mechanisms which underlie proliferation of the neural precursors cells in the adult neurogenic niches. Then we focus on new data regarding changes in the cell cycle kinetics and lengthening observed in the two neurogenic niches. Finally we will analyze how the length of the cell cycle might have a major role in triggering the increased proliferation following running.
PROTEINS AND REGULATORS OF THE CELL CYCLE
The cell cycle is a process that leads to the duplication of the cell into two daughter cells. This mechanism consist of a series of events starting from a quiescent state (G0) followed by the entry into the first phase (G1) during which the cell prepares for DNA replication (S phase). Later the cell enters in a second gap in which it continues to grow (G2), followed by the mitosis (M). This complex mechanism is finely orchestrated by the highly coordinated action of two main classes of molecules: the cyclin-dependent kinases (Cdks) and their regulatory partners, i.e., the cyclins [7]. In the G1 phase cyclin D forms a complex with Cdk4/6 to phosphorylate the retinoblastoma protein pRB, inducing the E2f transcription factor to trigger the expression of several genes involved in the cell cycle progression [8, 9]. Another complex, Cdk2-cyclinE, further phosphorylates Rb activating the transcription of genes essential for the DNA replication phase entry [10, 11]. Later, the interaction between Cdk2 and cyclin A regulates S/G2 transition [12]. Finally, Cdk1 and cyclin B complex (being also denominated “mitosis promoting factor”) controls the onset of mitosis and regulates the structural steps during the cell division [13]. The cell cycle is negatively regulated at any specific checkpoint by two major classes of non enzymatic cyclin-dependent kinase inhibitors (CDKIs) that directly interact with already formed cyclin-CDK complexes: the INK4 and the CDK-inhibitory protein (CIP)/kinase-inhibitor protein (KIP) families [14, 15]. The CIP/KIP inhibitors have a broad CDK preference and have been implicated in cell cycle withdrawal and quiescence [16]. The INK4 family includes four proteins (p16Ink4a, p15Ink4b, p18Ink4c and p19Ink4d) that play a pivotal role in preventing the formation of cyclin/Cdk4-6 complexes [17]. The CIP/KIP family comprises three proteins in mammals (p21Cip1, p27Kip1 and p57Kip2) which have a broader range of Cdk inhibitory activity compared to the Ink4 inhibitors and contain characteristic motifs that enable them to interfere with cyclin E/CDK2 or cyclin A/CDK2 to block or slow down the cell cycle progression [18, 19].
ROLE OF THE CELL CYCLE MACHINERY IN ADULT NEUROGENESIS
The finding that the production of new neuronscontinues throughout life in the adult mammalian brain has generated enormous interest and substantial advances in the field of neuroscience. During the adult neurogenesis occurring in SVZ and dentate gyrus neuroblasts are continuously produced and migrate to reach their targeted circuits where they fully differentiate into mature neurons and integrate into the preexisting network [20]. Despite the common origin from neural stem cells, the neuroblasts originated in SVZ and in the dentate gyrus display highly different proliferative properties and differentiation fate, which in turn give rise to mature neurons with profoundly different morphological and electrophysiological characteristics (see below). Consequently, the cell cycle machinery in the two different adult neurogenic niches presents a substantial diversity as regards the molecular mediators involved and replicative kinetics of the proliferating cell types.
In recent years, numerous studies have revealed an important role of the molecules of the cell cycle in the regulation of quiescence and expansion of stem cells and of differentiation of neural progenitors in the adult neurogenic niches [21, 22]. We should consider that the amount of new neurons generated depends on three components: i) the division mode; an asymmetric (neurogenic) division of a neural stem cell maintains the original pool, by generating one replica of the mother cell and one differentiating cell, while a symmetric neurogenic division generates two new neurons at the expense of the pool of mother cells; ii) the length of cell cycle; iii) the fraction of cells that exit the cycle and enters quiescence or differentiates; this key decision occurs at the G1 phase [23]. A decrease of the cell cycle length, in stem/progenitor cells, by accelerating the division rate, may be a primary inducer of the generation of a greater number of neurons in consequence of an expansion of the progenitor cells, but it requires to be accompanied by the appropriate mode of division leading to exit from cell cycle and to differentiation (e.g., subsequent rounds of symmetric proliferative and then neurogenic divisions).
CELL CYCLE REGULATION IN THE ADULT DENTATE GYRUS
The adult neurogenesis in adult dentate gyrus has been divided into different developmental stages in which quiescent neural stem cells enter into the cell cycle (type 1 NSCs) and give rise to postmitotic neurons through three consecutive stages of intermediate progenitor cells (type-2ab) and neuroblasts (type-3), which migrate within the granule layers, locally maturing into glutamatergic granule neurons, and functionally integrating into pre-existing neuronal circuitry [24].
Through the use of genetically modified mouse models it has been demonstrated that a dysregulation of the cell cycle represents one of the main causes of profound changes in the homeostasis of adult neurogenesis often related to disorders of the central nervous system. One of the most recent hypotheses proposed in the recent years is the so called “cell cycle length hypothesis”, according to which the lengthening of the G1 phase, caused by the down regulation of Cdk6, plays a predominant role in the premature exit from the cell cycle and differentiation [25, 26]. In fact Cdk6 -/- mice presented a two-fold decrease of the number of proliferating cells, and the progenitor cells exited the cell cycle showing a lengthned G1 phase with reduced production of new neurons. In contrast no change was observed in Cdk4 -/- cells [25]. Conversely, overexpression of Cdk4-cyclinD1 complex induces the expansion of the pool of stem cells at the expense of differentiation, thereby inhibiting neurogenesis in the adult hippocampus [27]. These data suggest that the proliferation of adult neural stem cells is strictly dependent on the presence of specific CDK proteins, whose activity regulates their proliferative fate.
Moreover, the cell cycle progression, and thus the action of CDKs, may be modulated by histone deacetylases (HDACs). In fact, the inhibition of HDACs activity has been shown to decrease the proliferation of neural stem cells and promote neuronal differentiation [28]. Interestingly, a recent paper showed that HDAC3, which is highly expressed in the brain [29], appears to be specifically required for the proliferation of adult NSCs in the dentate gyrus through the regulation of CDK1 levels [29]. Indeed the authors demonstrated that in the NSCs HDAC3 stabilizes CDK1 to promote normal cell cycle progression, whereas in NSCs lacking HDAC3, Cdk1 is degraded through the ubiquitin-proteasome pathway, resulting in G2/M phase progression defects [30].
A completely different role has instead been identified for Cdk5, which is abundantly expressed in neural tissues where, unlike the other CDKs, is predominantly activated in post-mitotic cells, either in embryo or in the neurogenic regions of the adult brain. Cdk5 is involved in various cellular events not directly related to cell cycle, such as regulation of several steps in neuronal migration and differentiation by controlling microtubule and actin cytoskeletal organization in the cerebellum and neocortex, and regulation of synaptic plasticity, all these actions essentially through phosphorylation of different protein substrates (for review see [31]). Two different papers have shown that lack of Cdk5 induces a strong decrease in the migration, dendritic extension and survival of newborn neurons in the adult dentate gyrus of the hippocampus, thus implying a role of Cdk5 in their maturation [32, 33].
Numerous studies have shown that cyclin D2 is the most critical cyclin in the adult neurogenic niches. In fact, it has been shown that its ablation causes a nearly complete blockage of adult neurogenesis in the adult dentate gyrus, indicating that cyclin D1 is not able to compensate for the lack of cyclin D2 [34–36]. Furthermore, the few cells generated within the adult dentate gyrus of cyclin D2-knockout mice belong to the astroglial lineage [34]. Moreover, another report showed that in cyclin D1 knockout dentate gyrus occurred a significant decrease of BrdU +cells [37]. Altogether, this may suggest that cyclin D2 in dentate gyrus is required for the commitment of neural stem cells to the neuronal differentiation, while cyclin D1 for astrocytic differentiation.
The role of the Cip/Kip inhibitors family in the nervous system has been revealed by several recent studies that have highlighted their activity during neural development and in vitro models. The study of the p21Cip1 knockout mice has led to quite discordant data regarding its function in the maintenance of quiescence and in the regulation of the proliferation of adult neural stem cells. It has been described that the deletion of the p21Cip1gene causes an increase in proliferation of stem/progenitor cells in the dentate gyrus of 2-month-old mice [38, 39], though the mechanisms involved in p21Cip1-dependent regulation of self-renewal are not understood. In an other studies this increase of proliferation does not occur unless after stroke [40].
p27Kip1 has been extensively investigated in neural development and adult neurogenesis [41]. A recent study shows that p27Kip1 represents an important regulator of proliferation of immature neuron and is one of the main mediators in the maintenance of hippocampal stem cell quiescence and reservoir, by mediating the molecular program that keeps adult stem cells out of the cell cycle [42]. This action is exerted by p27Kip1 through its N-terminal domain, likely through CDK inhibition [42].
Finally, a recent study demonstrates that p57Kip2 is expressed in quiescent radial NSCs, but not in rapidly dividing progenitors. Deletion of cdkn1c (p57Kip2 gene) in adult NSCs abrogates their quiescence and activates their proliferation, leading to excessive reduction of NSCs and neurogenesis in the aged brain [43]. Moreover it has been shown that the anti-depressant action of the glucocorticoid receptor on differentiation and proliferation of hippocampal progenitor cells is mediated by the expression of p57 Kip2, suggesting a different role of this inhibitor in adult neurogenesis [44].
CELL CYCLE REGULATION IN THE ADULT SUBVENTRICULAR ZONE
In the adult rodent SVZ, neuroblast are continuously produced and migrate rostrally in the form of cell aggregates called “chains”, along an highly restricted route termed the rostral migratory stream (RMS) [45, 46] towards the olfactory bulb where they finally maturate into GABA-ergic local interneurons [47]. The new neurons in the SVZ are generated by quiescent radial glia-like cells (type B cells; [48]), which give rise to rapidly proliferating transient amplifying cells, expressing transcription factors of the Dlx family (type C cells; [49]). These type C cells in turn generate migrating neuroblast which exit the cell cycle and approach the rostral migratory stream (type A cells; [50]).
A study carried out in the postnatal SVZ of Cdk5 knockout mice revealed that deletion of this gene causes severe impairment in migrating neuroblasts of the adult SVZ, suggesting that Cdk5 plays a pivotal role in the architecture and orientation of the neuroblast chain in the SVZ [51].
Concerning the role of the cyclins in the SVZ neurogenesis, a recent paper has shown that the absence of the antiproliferative gene PC3/Tis21 induces an increment of both cyclins D1 and D2 in the adult SVZ associated with a sharp increase in the proliferation of newborn stem cells. This suggests that both cyclins may play an important role in the regulation of proliferation in the SVZ [52]. Indeed, previous work has shown that cyclin D1 certainly plays a role in the proliferation of SVZ cells, since primary cultures of SVZ neural cells from cyclin D1-knockout mice showed a significant decrease of BrdU incorporation accompanied by accumulation in G0/G1 [37]; however, cyclin D1-knockout SVZ neural cells were prevented from differentiating into astrocytes with no effect in the differentiation into neurons [37]. This would suggest that cyclin D1 in SVZ is more necessary for the commitment of SVZ neural stem cells to astrocytic differentiation.
Moreover, while p21Cip1 deletion causes an increment of the proliferation rate of neurospheres isolated from young adult mice (2 months), the opposite situation occurs in old mice (16 months) in vitro and in vivo, leading to subsequent exhaustion of the NSC pool [39]. This suggest that p21Cip1 has a central role in the maintainance of NSCs throughout the entire lifespan of the organism, as proposed in the hematopoietic stem cells [53]. More recent studies suggest that p21Cip1 maintains the self-renewal of NSCs by repressing Sox2 [52] or Bmp2 [55]. As Bmp2 induces premature terminal differentiation of multipotent NSCs into non-neurogenic astrocytes, this would imply thatthe actions of p21Cip1 are not limited to the control of cell cycle.
It has been demonstrated that p27Kip1 deletion alters the normal histogenesis of the adult SVZ by increasing the number of transit-amplifying progenitors in homeostatic or ischemic conditions, suggesting an important function of this cyclin-dependent kinase inhibitor in the restriction of neural precursor regeneration mainly at a late stage after cerebral ischemia [56, 57].
Concerning the Ink4 inhibitors of the CDKs, it has been shown that deletion of p16Ink4a increases the self-renewal of SVZ stem cells [58] and the generation of new neurons in old mice (15–19 months; [59]). Moreover, p16Ink4a expression becomes detectable only during aging, suggesting that p16Ink4a causes the loss of the replicative ability (senescence) in aging SVZ stem cells; an alternative interpretation could be that p16Ink4a preserves the pool of SVZ stem cells during aging by maintaining their quiescence.
Figures 1 and 2 summarize the molecular controls involved in the regulation of the adult neurogenic niches, either in terms of cell cycle regulation (Fig. 1) or in terms of effects on proliferation and differentiation of neural cells (Fig. 2).
CELL CYCLE KINETICS IN ADULT NEUROGENESIS
Accumulating evidence reveals that the cell cycle kinetics plays a very important role in the regulation of neurogenesis [60]. In fact, it has been demonstrated that during development the modulation of cell cycle is instructive for the switch between proliferation and asymmetric division, with small variations in the length of the G1 phases inducing profound changes in the proliferative kinetic of the neural stem cell pool. Only recently attention has been focused on the identification of the cell cycle parameters in adult neurogenic niches in physiological and pathological conditions. In fact one of the most fascinating challenges of neuroregenerative medicine is represented by the induction of endogenous neurogenesis as an attempt to recover after brain damage or during neurodegeneration. In this context, the manipulation of the cell cycle kinetics could be a pivotal tool to achieve an efficient expansion of the neural stem cell pool, as a converging result of increased recruitment from quiescence and proliferation. In the adult dentate gyrus the newly generated progenitor cells divide once a day with a total length of the cycle of about 22–24 hours [61–64]. A detailed study showed that the cell cycle length differs significantly within the pool of adult hippocampal stem/progenitor cells; namely, mitotic GFAP + radial glia-like cells and NeuroD1 + neuronal progenitors divide significantly faster than amplifying neural progenitor cells by accelerating their S-phase [62]. Moreover, analysis of the dynamics of adult progenitor cell proliferation in the adult sub ventricular zone (SVZ) reveals that the actively dividing neural stem cells (B cells) have a shorter cell cycle length (18 hours) when compared to the other proliferating cell types residing in the SVZ (C and A cells), displaying similar cell cycle kinetics (17–22 hours) [65]. The modulation of the cell cycle length in the SVZ was particularly analyzed during the neurogenic response triggered by stroke, the cerebrovascular damage that causes permanent brain damage and represents the second leading cause of mortality worldwide. Indeed this pathological stimulus causes a shortening of the S phase (from 5 to 2 hours), and consequently of the entire cell cycle (from 19 to 12 hours), which in turn induces a substantial increase in the symmetric neuron generating division, and a subsequent decrease in the cell cycle exit [66–68]. These events represent a key mechanism by which a significant number of new neurons migrate to the brain regions damaged by the ischemic insult [69]. Most of these new neurons die once arrived in the damaged site and are not integrated into preexisting circuits, although this increase of post-stroke endogenous neurogenesis sets the stage for the creation of therapeutic strategies to promote brain function following ischemic injury.
RELATION BETWEEN THE CELL CYCLE AND DIFFERENTIATION IN NEUROGENESIS
The cell cycle and differentiation of neural stem cells are correlated processes. This point is relevant to the possibility of efficiently triggering the generation of new neurons by means of a neurogenic stimulus, such as physical exercise, or, for instance, the treatment with chemical substances impacting on 5HT pathways.
As mentioned above, it has been proposed that the duration of the cell cycle regulates differentiation, in particular a lengthening of the G1 phase may trigger neural differentiation [26, 70]. While it is undeniable that the inhibition of the cell cycle is sufficient to trigger differentiation of neural stem cells, as observed recently for instance in double CDK2/CDK4 mutants [71], this occurs evidently only in a differentiation-permissive environment. In fact, for instance, the amplification of neural stem cells triggered by cyclin D1 is not sufficient to generate new neurons, unless the proliferative stimulus ceases after amplification, in order to allow the process of differentiation [27]. We have to be aware that complex and distinguishable molecular interactions between the cell cycle and differentiation take place.
PC3/Tis21 is a gene paradigmatic for the interplay between proliferation and differentiation. It is a transcriptional cofactor physiologically expressed in neural progenitor cells of different areas of the adult murine brain, such the hippocampus, the SVZ and the cerebellum, where it induces them to exit the cell cycle and to differentiate [52, 72–74]. PC3/Tis21 is a negative regulator of the cell cycle, possibly through the binding of PC3/Tis21 protein to the promoter sequences of cyclin D1, and/or thanks to its ability to form complexes with histone deacetylases or histone modifying factors, such as the methyltransferase Prmt1 ([77] for detail). In fact, overexpression of PC3/Tis21 in dentate gyrus progenitor cells, driven by the nestin promoter in a conditional transgenic mouse, shows that the number of cells incorporating BrdU, after a short pulse, is significantly reduced [74]. Conversely, the knockout of PC3/Tis21 induces an increase of the number of stem and progenitor cells incorporating BrdU in the dentate gyrus and also in the SVZ [52, 75]. Furthermore, the length of the G1/S phase appears to decrease in PC3/Tis21 knockout progenitor cells; in fact in these cells we observe an increase of the ratio (inversely proportional to the length of the cell cycle) between progenitor cells in S-phase (BrdU+) and actively dividing (Ki67+) [75].
Moreover, as observed in adult progenitor cells of the dentate gyrus, SVZ and cerebellum, PC3/Tis21 is endowed also with a pro-differentiative action, cell-intrinsic and independent of the antiproliferative effect. Such a pro-differentiative effect was proposed by the group of Gerd Kempermann, observing that PC3/Tis21 is selectively expressed in postmitotic dentate gyrus neurons (stage 5; [76], and was demonstrated by us showing that stage 5 early postmitotic dentate gyrus neurons lacking PC3/Tis21 accumulate, unable to terminally differentiate into stage 6 although they have already exited the cell cycle [75]. Similarly, in the adult SVZ, the knockout of PC3/Tis21 leads not only to an increase of the proliferation of stem cells but also to evident impairment of the terminal differentiation of neuroblasts (A cells) [52]; (Fig. 2). A key point is that PC3/Tis21 is necessary for the differentiation of cells that are already post-mitotic, indicating that the two processes, arrest of proliferation with exit from the cell cycle and terminal differentiation, are, at least in adult neurons, separate processes. Notably, it is impossible to enforce differentiation in PC3/Tis21-null progenitor cells of the dentate gyrus by simply applying a neurogenic proliferative stimulus (such as fluoxetine, using a protocol that allows a stimulus-free period after treatment in order to allow differentiation; see [27], L. Micheli and M. Ceccarelli, unpublished data). In PC3/Tis21-null stem cells of the SVZ the defect of terminal differentiation can be rescued only through specific molecules that regulate differentiation, i.e., by treating cells with BMP4 or by silencing Inhibitor of Differentiation 3 (Id3) [52]. This suggests that terminal differentiation in neural stem cells is controlled by PC3/Tis21 through these molecules; in particular, PC3/Tis21 has been shown to negatively regulate the transcription of Id3 by associating to its promoter [75, 77]. The fact that differentiation of neural progenitor cells cannot be rescued by a proliferative stimulus such as fluoxetine (or, presumably, as physical exercise) further indicates a clear separation between the two processes of proliferation and terminal differentiation.
Furthermore, a dual role, with distinct effects on the proliferation and differentiation of neural stem cells, is observed for several known anti-proliferative genes, which display a direct differentiative action. These include Rb [78], Geminin, which exerts its dual role through control of chromatin regulators [79], or even the positive regulator of proliferation cyclin D1; this latter has recently been shown to exert in vivo a transcriptional function in mouse development, in addition to its well-established control of cell cycle, by associating with cohorts of gene promoter sequences, including regulators of neural differentiation such as NeuroD1 [80]. Another relevant example of cell cycle-independent action concerns p27Kip1, which promotes neuronal differentiation in the mouse cerebral cortex by stabilizing the proneural Neurogenin2 protein, an activity carried by the N-terminal half of the protein [81]. This action may be working also in differentiated neurons of the adult hippocampus, where p27Kip1 is expressed ([42]; Fig. 2), also given that Neurogenin2 plays an important role in the differentiation steps of adult hippocampal neurogenesis [82].
PHYSICAL EXERCISE AND CELL CYCLE KINETICS IN ADULT NEUROGENESIS
In rodents, voluntary exercise robustly enhances cell proliferation and the number of newly generated neurons in the dentate gyrus of the hippocampus [3, 4]. Moreover recent data show that exercise is able to induce neurogenesis in the hypothalamus and ependymal lining of the third ventricle, leading to recovery of homeostatic functions in the adult brain after brain injury [83].
Many other studies have stated that voluntary exercise is able to facilitate both structural and functional plasticity in the adult dentate gyrus, enhancing cell proliferation and neurogenesis [3, 84] and synaptic plasticity [84–86]. This provides an improvement in several specific hippocampus-dependent behavioural task such as Morris Water Maze and pattern separation [87–89], even though new neurons produced in response to exercise do not contribute significantly to the synaptic process until well after they are mature [90]. Moreover, physical activity is able to delay or in part prevent symptoms of neurodegenerative disease in mouse models [91–93], to restore neurogenesis in a mouse model of HIV [94] and to lead to faster recovery or less severe learning deficits after brain injury [95–97]. Table 1 summarizes the main studies describing the factors that alter the homeostasis of the adult neurogenesis through imbalance of cell cycle, and the known effects of physical exercise.
All this experimental evidence raises interesting questions regarding the molecular and cellular mechanisms involved in mediating the beneficial effects of exercise. One of the most promising candidates in this sense is the neurotrophin BDNF. It has been shown that deletion of the gene encoding TrkB, the high affinity receptor for BDNF, prevents the exercise-induced enhancement of neurogenesis and LTP [98, 99], while intracerebral infusion of BDNF increases hippocampal adult neurogenesis, by mimicking exercise-induced changes in learning [100, 101]. Another study shows that the bone morphogenetic protein (BMP), a negative regulator of adult neurogenesis, plays a central role in mediating the effects of exercise on both neurogenesis and hippocampus-dependent learning and memory [102]. Indeed, running reduces levels of hippocampal BMP, and transgenic mice with reduced BMP signaling exhibit remarkable gains in hippocampal cognitive performance and neurogenesis, mirroring the effects of physical exercise; on the contrary, overexpression of BMP4 prevents the running-dependent increase of cell proliferation and generation of new neurons (Dcx+) [102]. This fits with the notion that radial stem cell quiescence in the adult hippocampus is maintained via BMP/BMPR1A signaling [103] and suggests that BMP plays a direct role in checking the running-induced proliferation of hippocampal progenitor cells.
Another molecule responsible for the the voluntary running-induced increase of hippocampal progenitor proliferation appears to be the Wnt signaling inhibitor Secreted frizzled-related protein 3 (Sfrp3) [104]. In fact, interestingly, the deletion of Sfrp3 activates quiescent radial neural stem cells, and the reduction of Sfrp3 turns out to be essential for the activity-induced adult neural progenitor proliferation.
Moreover, a very interesting study indicate that adipocyte-secreted adiponectin (AND) plays a pivotal role in mediating the effects of exercise on dentate gyrus neurogenesis, likely by activation of the adiponectin receptor (ADNR1)/AMP-activated protein kinase (AMPK) signalin pathways [105].
In terms of cellular mechanisms involved in the exercise-dependent activation of hippocampal neurogenesis, recently the Bartlett laboratory has shown that microglia have a direct regulatory effect on the activity of adult hippocampal progenitors in response to voluntary exercise. Using transgenic models labeling microglia cells, they demonstrate that the exercise-induced increase in neural precursor cell proliferation is mediated via endogenous microglia; notably, depletion of microglia from hippocampal neurosphere cultures annulled the positive effect of voluntary exercise on neural precursor cells [106].
As pointed out above, the length of the cell cycle may play an important role in modulating the increased proliferation of new stem/progenitor cells in the neurogenic niches of the adult mouse.
In this regard, a recent paper has shown that the running triggers the proliferation of neural progenitors in the hippocampal dentate gyrus, by selectively shortening their S phase (from 12.9 to 10.2 hours) and consequently cell cycle length (from 24.9 to 22.0 hours) [63]. The importance of a running-mediated cell cycle modulation is supported by the observation that physical activity is also able to reactivate the plasticity of neural stem cells when the cell cycle inhibitory control is missing, with profound implications for the long-term modulation of adult neurogenesis. Indeed, in the mice ablated of the antiproliferative gene Btg1, where a postnatal hyperproliferation is followed by a gradual depletion of the pool of stem cells in the dentate gyrus with a consequent decreased neurogenesis, 12 days of running significantly increase cell proliferation and long-term neurogenesis in the adult hippocampal dentate gyrus, by triggering the recruitment of quiescent neural stem cells and reactivating their hyperproliferation and expansion. The phenomena described above are probably dependent on the running-induced shortening of S-phase and of the cell cycle of neural stem and progenitor cells. A possibility is that the acceleration of the cell cycle stabilizes the expansion of the neural stem cells. In contrast, in the Btg1 wild-type mice running provokes the shortening of S-phase and cell cycle only of committed progenitor cells, resulting in a transient proneurogenic effect. These data indicate that the replicative potentiality of the neural stem cells is not limited with aging and that after physical exercise the deprived stem cells pool is still ready to be reactivated when the inhibitory cell cycle control exerted by Btg1 is missing, still highlighting the key role of Btg1 in maintaining the quiescence of adult NSC [63]. This new concept should represents a prerequisite to build a more general cellular model able to trigger the mechanisms of adult neurogenesis, and in particular to increase the functional reserve of stem cells, which could, in turn, provide a favorable endogenous microenvironment for the process of neural regeneration after brain injury.
As for the cellular mechanisms underlying the stem cells recovery in Btg1-null mice by running, it is worth noting that the process of hyperproliferation observed in the stem/progenitor cells of the dentate gyrus (and SVZ) after ablation of the Btg1 gene is detectable only in the early postnatal period, while in adult mice the proliferating progenitor cells decrease in number and show a tendency to exit the cycle and become quiescent. There is in fact a higher number of adult Btg1-null stem/progenitor cells, compared to wild-type, ceasing to cycle after entering the S-phase, detected as BrdU +Ki67 −; the opposite occurs in the early postnatal Btg1-null stem/progenitor cells [107]. Accordingly, in Btg1-null adult dentate gyrus stem and progenitor cells the length of the S phase increases considerably [63]. The increased exit from cell cycle observed in adult Btg1-null dentate gyrus is concomitant with a several-fold increase of stem cells expressing p21Cip1 or p53 and is followed within a few days by apoptosis [107]. Therefore, this process consists of a progressive age-dependent shift towards quiescence and loss of proliferative capability, followed by decrease of the stem cell pool. Notably, the same events have been observed after ablation of other inhibitors of proliferation, such as p21Cip1 [39], p57Kip2 [43], or of the Notch effector RBPJ [108]. In particular, it has been shown that the reduction of p57Kip2 protein in the nucleus of radial NSCs contributes to the activation of NSCs in response to physical exercise [43].
At the origin of the age-dependent decrease of the proliferative capability of Btg1 knockout stem cells could be an increase of the cell cycle inhibitors p21 and p53. This may represent a cellular attempt to contain the initial burst of proliferation observed at an early age - consequence of the loss of the proliferation inhibitor Btg1 – by driving the stem/progenitor cells to quiescence and thus preventing, at least for some cells, apoptosis, an event normally occurring when a negative regulator of cell cycle is suppressed [109]. This would explain why the pool of stem cells is never fully depleted in the Btg1-null adult dentate gyrus, but can recover its proliferative capability when stimulated by physical exercise. The molecular mechanisms by which Btg1, as a transcriptional cofactor, regulates the cell cycle molecules in neural stem cells still needs to be clarified. However, it has been shown that Btg1 associates with Prmt1 and binds the transcriptional element Caf1/CNOT7, which have both been implicated in growth arrest [110, 111].
More recently another report showed that 5 days of voluntary physical exercise does not induce a significant change in cell cycle kinetics of stem/progenitor cells of the dentate gyrus, despite a strong increase of proliferation of newborn neurons in dentate gyrus of runners mice [64]. The authors conclude that small cell cycle alterations in cell cycle length after running may represent only a consequence and not the causal regulating factor of the neural precursor expansion in the dentate gyrus.
These conflicting data may reflect different experimental paradigms (12 days vs 5 days of run as well as different housing conditions of mice during voluntary running), and open interesting perspectives on the molecular pathways involved in the positive regulation of proliferation exerted by running in the neuralprogenitors.
CONCLUSION
It is well established that the beneficial effects of the running exercise are the result of a multitude of independent factors including modifications in synaptic plasticity, spine density, neurotrophins and microenvironment in the neurogenic niches, that as a whole might be able to mediate the positive effects on learning and memory, the decrease of the risk of neurodegenerative diseases and the delay of the intellectual decline associated with aging. The discovery of the involvement of the cell cycle machinery in the proneurogenic effect of physical activity opens new interesting scenarios in understanding the cellular and molecular mechanisms underlying the effects of the run on the brain. Moreover it provides a first causal relationship between the proneurogenic effect of physical exercise and cell cycle kinetics. Further studies are needed to identify the cell cycle components involved in the running-dependent S-phase shortening as well as to check whether a fine modulation of the cell cycle may mirror the effects of exercise in the neurogenic niches of adult brain. A promising example is provided by the discovery that in the absence of an antiproliferative inhibition, the physical activity can reactivate the pool of quiescent stem cells and cause their expansion, an event which is not occurring under physiological conditions. These results could provide new pivotal information in modulating the dynamics of the recruitment and proliferation of adult neural stem cells and suggests that the “run-activated neural stem cells” could harness the potential to regenerate the neuronal population lost due to aging, neurological and neurodegenerativedisorders.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
This work was supported by grants from the Italian Ministry of Economy and Finance to CNR (Project FaReBio; to F.Tirone) and from the Italian Ministry of University and Research (Project EBRI-CNR to F.Tirone).
REFERENCES
1 | Kempermann G, Gage FH(1999) New nerve cells for the adult brainSci Am280: 54853 |
2 | Cameron HA, Wooley CS, McEwan BS, Gould E(1993) Differentiation of newly born neurons and glia in the dentate gyrus of the adult ratNeuroscience56: 2337344 |
3 | van Praag H, Kempermann G, Gage FH(1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrusNat Neurosci2: 3266270 |
4 | Olson AK, Eadie BB, Ernst C, Christie BR(2006) Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathwaysHippocampus16: 3250260 |
5 | Fabel K, Kempermann G(2008) Physical activity and the regulation of neurogenesis in the adult and aging brainNeuromolecular Med10: 25966 |
6 | Brown J, Cooper-Kuhn CM, Kempermann G, van Praag H, Winkler J, Gage FH, Kuhn H(2003) Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesisEur J Neurosci17: 1020422046 |
7 | Malumbres M, Barbacid M(2005) Mammalian cyclin-dependent kinasesTrends Biochem Sci30: 11630641 |
8 | Lundberg AS, Weinberg RA(2008) Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexesMol Cell Biol18: 2753761 |
9 | Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC(1999) Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cell move through G1Cell98: 6859869 |
10 | Morgan DO(1997) Cyclin-dependent kinases: engines, clocks, andmicroprocessorsAnnu Rev Cell Dev Biol13: 261291 |
11 | Adams PD(2001) Regulation of the retinoblastoma tumor suppressor protein by cyclin/cdksBiochem Biophys Acta1471: 3M123M133 |
12 | Dirks PB, Rutka JT(1997) Current concepts in neuro-oncology: The cell cycle-a reviewNeurosurgery40: 510001013 |
13 | Nigg EA(2001) Mitotic kinases as regulators of cell division and itscheckpointsNat Rev Mol Cell Biol2: 12132 |
14 | Sherr CJ, Roberts JM(1995) Inhibitors of mammalian G1 cyclin-dependent kinaseGenes Dev9: 1011491163 |
15 | Copani A, Nicoletti F(2005) Cell cycle mechanism and neuronal cell deathNew YorkEd. Kluwer Academic/Plenum Publishers |
16 | Besson A, Dowdy SF, Roberts JM(2008) CDK inhibitors: cell cycle regulators and beyondDev Cell14: 2159169 |
17 | Carnero A, Hudson JD, Price CM, Beach DH(2000) p16INK4A and p19ARF act in overlapping pathways in cellular immortalizationNat Cell Biol2: 3148155 |
18 | Malumbres M, Barbacid M(2009) Cell cycle, CDKs and cancer: a changing paradigmNat Rev Cancer9: 3153166 |
19 | Satyanarayana A, Kaldis P(2009) Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanismOncogene28: 3329252939 |
20 | Zhao C, Deng W, Gage FH(2008) Cell. Mechanisms and functional implications of adult neurogenesisCell132: 4645660 |
21 | Beukelars P, Vandenbosch R, Caron N, Nguyen L, Moonen G, Malgrange B(2012) Cycling or not cycling: Cell cycleregulatory molecules and adult neurogenesisCell Mol Life Sci69: 914931503 |
22 | Patricio P, Mateus-Pinheiro A, Sousa N, Pinto L(2013) Re-cycling paradigms: Cell cycle regulation in adulthippocampalneurogenesis and implications for depressionMol Neurobiol48: 18496 |
23 | Götz M, Huttner WB(2005) The cell biology of neurogenesisNat Rev Mol Cell Biol10777788 |
24 | Kempermann G, Jessberger S, Steiner B, Kronenberg G(2004) Milestones of neural development in the adult hippocampusTrends in Neurosci27: 8447452 |
25 | Beukelaers P, Vandenbosch R, Caron N, Nguyen L, Belachew S, Moonen G, Kiyokawa H, Barbacid M, Santamaria D, Malgrange B(2011) Cdk6-dependent regulation of G(1) length controls adult neurogenesisStem Cells29: 4713724 |
26 | Calegari F, Huttner WB(2003) An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesisJ Cell Sci116: Pt 2449474955 |
27 | Artegiani B, Lindemann D, Calegari F(2011) Overexpression of cdk4 and cyclinD1 triggers greater expansion of neuralstem cells in the adult mouse brainJ Exp Med.208: 5937948 |
28 | Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH(2004) Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cellsProc Natl Acad Sci U S A101: 471665916664 |
29 | Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ(2007) Distribution of histone deacetylases 1-11 in the rat brainJ Mol Neurosci31: 14758 |
30 | Jiang Y, Hsieh J(2014) HDAC3 controls gap 2/mitosis progression in adult neural stem/progenitor cells by regulating CDK1 levels PNAS111: 371354113546 |
31 | Kawuachi T(2014) Cdk5 regulates multiple cellular events in neural development, function and diseaseDev Growth Differ56: 5335348 |
32 | Jessberger S, Aigner S, Clemenson GDJr, Toni N, Lie DC, Karalay O, Overall R, Kempermann G, Gage FH(2008) Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampusPlos Biol6: 1e272 |
33 | Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, Bibb JA, Eisch AJ(2008) Cdk5 is essential for adult hippocampal neurogenesisProc Natl Acad Sci USA105: 471856718571 |
34 | Kowalczyk A, Filipkowsky RK, Rylski M, Wilczynsky GM, Konopacky FA, Jaworsky J, Cyemerych MA, Sicinsky P, Kaczmarek L(2004) The critical role of cyclin D2 in adult neurogenesisJ Cell Biol167: 2209213 |
35 | Glickstein SB, Monaghan JA, Koeller HB, Jones TK, Ross ME(2009) Cyclin D2 is critical for intermediate progenitor cell proliferation in the embryonic cortexJ Neurosci29: 3096149624 |
36 | Jaholkowski P, Kiryk A, Jedynak P, Ben Abdallah NM, Knapska E, Kowalczyk A, Piechal A, Blecharz-Klin K, Figiel I, Lioudyno V, Widy-Tyszkiewicz E, Wilczynski GM, Lipp HP, Kaczmarek L, Filipkowski RK(2009) New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learningLearn Mem16: 7439451 |
37 | Ma J, Yu Z, Qu W, Tang Y, Zhan Y, Ding C, Wang W, Xie M(2010) Proliferation and differentiation of neural stem cells are selectively regulated by knockout of cyclin D1J Mol Neurosci42: 13543 |
38 | Pechnick RN, Zonis S, Wawrowsky K, Pourmorady J, Chesnokova V(2008) p21Cip1 restricts neuronal proliferation inthe subgranular zone of the dentate gyrus of the hippocampusProcNatl Acad Sci U S A105: 413581363 |
39 | Kippin TE, Martens DJ, van der Kooy D(2005) p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacityGenes Dev19: 6756767 |
40 | Qiu J, Takagi Y, Harada J, Rodrigues N, Moskowitz MA, Scadden DT, Cheng T(2004) Regenerative response in ischemic brain restricted by p21cip1/waf1J Exp Med199: 7937945 |
41 | Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT(2000) Stem cell repopulation efficiency but not pool size is governed by p27(kip1)Nat Med6: 1112351240 |
42 | Andreu Z, Khan MA, González-Gómez P, Negueruela S, Hortigüela R, San Emeterio J, Ferrón SR, Martínez G, Vidal A, Fariñas I, Lie DC, Mira H(2015) The cyclin-dependent kinase inhibitor p27 kip1 regulates radial stem cell quiescence and neurogenesis in the adult hippocampusStem Cells33: 1219229 |
43 | Furutachi S, Matsumoto A, Nakayama KI, Gotoh Y(2013) p57 controls adultneural stem cell quiescence and modulates the pace of lifelongneurogenesisEMBO J32: 7970981 |
44 | Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Price J, Pariante CM(2011) Antidepressantsincrease human hippocampal neurogenesis by activating the glucocorticoid receptorMol Psychiatry16: 7738750 |
45 | Lledo PM, Alonso M, Grubb MS(2006) Adult neurogenesis and functional plasticity in neuronal circuitsNat Rev Neurosci7: 3179193 |
46 | Lois C, Alvarez-Buylla A(1994) Long-distance neuronal migration in adult mammalian brainScience264: 11451148 |
47 | Lepousez G, Nissant A, Lledo PM(2015) Adult Neurogenesis and the Future of the Rejuvenanting Brain CircuitsNeuron86: 2387401 |
48 | Alvarez-Buylla A, Lim DA(2004) For the long run: maintaining germinal niches in the adult brainNeuron41: 5683686 |
49 | Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A(2002) EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cellsNeuron36: 610211034 |
50 | Sequerra EB(2014) Subventricular zone progenitors in time and space: generating neuronal diversityFront Cell Neurosci8: 434 |
51 | Hirota Y, Oshima T, Kaneko N, Ikedo M, Iwasato T, Kulkarni AB, Mikoshiba K, Okano H, Sawamoto K(2007) Cyclin-dependent kinase 5 is required for control of neuroblast migration in the post-natal subventricular zoneJ Neurosci27: 71282912838 |
52 | Farioli-Vecchioli S, Ceccarelli M, Saraulli D, Micheli L, Cannas S, D’Alessandro F, Scardigli R, Leonardi L, Cinà I, Costanzi M, Mattera A, Cestari V, Tirone F(2014) Tis21 is required for adult neurogenesis in thesubventricular zone and for olfactory behavior regulating cyclins, BMP4, Hes1/5 and IdsFront Cell Neurosci8: 98 |
53 | Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT(2000) Hematopoietic stem cell quiescence maintained byp21cip1/waf1Science287: 545918041808 |
54 | Marqués-Torrejón , Marqués-Torrejón MÁ, Porlan E, Banito A, Gómez-Ibarlucea E, Lopez-Contreras AJ, Fernández-Capetillo O, Vidal A, Gil J, Torres J, Fariñas I(2013) Cyclin-dependent kinase inhibitor p21 controls adult neural stemcell expansion by regulating Sox2 gene expressionCell Stem Cell12: 188100 |
55 | Porlan E, Morante-Redolat JM, Marqués-Torrejón MÁ, Andreu-Agulló C, Carneiro C, Gómez-Ibarlucea E, Soto A, Vidal A, Ferrón SR, Fariñas I(2013) Transcriptionalrepression of Bmp2 by p21(Waf1/Cip1) links quiescence to neuralstem cell maintenanceNat Neurosci16: 1115671575 |
56 | Doetsch F, Verdugo JM, Caille I, Alvarez-Buylla A, Chao MV, Casaccia-Bonnefil P(2002) Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesisJ Neurosci22: 622552264 |
57 | Qiu J, Takagi Y, Harada J, Topalkara K, Wang Y, Sims JR, Zheng G, Huang P, Ling Y, Scadden DT, Moskowitz MA, Cheng T(2009) p27Kip1 constrains proliferation of neural progenitor cells in adult brain under homeostatic and ischemic conditionsStem Cells27: 4920927 |
58 | Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R(2005) Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathwaysGenes Dev19: 1214321437 |
59 | Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ(2006) Increasingp16INK4a expression decreases forebrain progenitors and neurogenesis during ageingNature443: 7110448452 |
60 | Bragado Alonso S, Schulze-Steikow M, Calegari F(2014) Cell cycle activity of neural precursors in the diseased mammalian brainFront Neurosci27: 839 |
61 | Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G(2011) Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampusCell Stem Cell8: 5566579 |
62 | Brandt MD, Hübner M, Storch A(2012) Brief report: Adult hippocampal precursor cells shorten S-phase and total cell cycle length during neuronal differentiationStem Cells30: 1228432847 |
63 | Farioli-Vecchioli S, Mattera A, Micheli L, Ceccarelli M, Leonardi L, Saraulli D, Costanzi M, Cestari V, Rouault JP, Tirone F(2014) Running rescues defective adult neurogenesis by shortening the length of the cell cycle of neural stem and progenitor cellsStem Cells32: 719681982 |
64 | Fischer TJ, Walker TL, Overall RW, Brandt MD, Kempermann G(2014) Acute effects of wheel running on adult hippocampal precursor cells in mice are not caused by changes in cell cycle length or S phase lengthFront Neurosci8: 314 |
65 | Ponti G, Obernier K, Guinto C, Jose L, Bonfanti L, Alvarez-Buylla A(2013) Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult miceProc Natl Acad Sci USA.110: 11E1045E1054 |
66 | Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M(2004) Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult ratJ Neurosci24: 2558105815 |
67 | Zhang RL, Zhang ZG, Lu M, Wang Y, Yang JJ, Chopp M(2006) Reduction ofthe cell cycle length by decreasing G1 phase and cell cyclereentry expand neuronal progenitor cells inthe subventricular zone of adult rat after strokeJ Cereb BloodFlow Metab26: 6857863 |
68 | Zhang RL, Zhang ZG, Roberts C, LeTourneau Y, Lu M, Zhang L, Wang Y, Chopp M(2008) Lengthening the G(1) phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after strokeJ Cereb Blood Flow Metab28: 3602611 |
69 | Christie KJ, Turnley AM(2013) Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brainFront Cell Neurosci6: 70 |
70 | Calegari F, Haubensak W, Haffner C, Huttner WB(2005) Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain developmentJ Neurosci25: 2865336538 |
71 | Lim S, Kaldis P(2012) Loss of Cdk2 and Cdk4 induces a switch from proliferation to differentiation in neural stem cellsStem Cells30: 715091520 |
72 | Canzoniere D, Farioli-Vecchioli S, Conti F, Ciotti MT, Tata AM, Augusti-Tocco G, Mattei E, Lakshmana MK, Krizhanovsky V, Reeves SA, Giovannoni R, Castano F, Servadio A, Ben-Arie N, Tirone F(2004) Dual control of neurogenesis by PC3 through cell cycle inhibition and induction of Math1J Neurosci24: 1333553369 |
73 | Farioli-Vecchioli S, Tanori M, Micheli L, Mancuso M, Leonardi L, Saran A, Ciotti MT, Ferretti E, Gulino A, Pazzaglia S, Tirone F(2007) Inhibition of medulloblastoma tumorigenesis by the antiproliferative and pro-differentiative gene PC3FASEB J21: 922152225 |
74 | Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cinà I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F(2008) The timing of differentiation of adult hippocampal neurons is crucial for spatial memoryPLoS Biol6: 10e246 |
75 | Farioli-Vecchioli S, Saraulli D, Costanzi M, Leonardi L, Ciná I, Micheli L, Nutini M, Longone P, Oh SP, Cestari V, Tirone F(2009) Impaired terminal differentiation of hippocampal granule neuronsand defective contextual memory in PC3/Tis21 knockout micePLoSOne4: 12e8339 |
76 | Attardo A, Fabel K, Krebs J, Haubensak W, Huttner WB, Kempermann G(2010) Tis21 expression marks not only populations of neurogenicprecursor cells but also new postmitotic neurons in adulthippocampal neurogenesisCereb Cortex20: 2304314 |
77 | Micheli L, Ceccarelli M, Farioli-Vecchioli S, Tirone F(2015) Control ofthe normal and pathological development of neural stem andprogenitor cells by the PC3/Tis21/Btg2 and Btg1 genesJ CellPhysiol230: 1228812890 |
78 | Chen D, Opavsky R, Pacal M, Tanimoto N, Wenzel P, Seeliger MW, Leone G, Bremner R(2007) Rb-mediated neuronal differentiation through cell-cycle-independent regulation of E2f3aPLoS Biol5: 7e179 |
79 | Seo S, Kroll KL(2006) Geminins double life: chromatin connections thatregulate transcription at the transition from proliferation todifferentiationCell Cycle5: 4374379 |
80 | Bienvenu F, Jirawatnotai S, Elias JE, Meyer CA, Mizeracka K, Marson A, Frampton GM, Cole MF, Odom DT, Odajima J, Geng Y, Zagozdzon A, Jecrois M, Young RA, Liu XS, Cepko CL, Gygi SP, Sicinski P(2010) Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screenNature463: 7279374378 |
81 | Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, Philpott A, Roberts JM, Guillemot F(2006) p27kip1independently promotes neuronal differentiation andmigration in the cerebral cortexGenes Dev20: 1115111524 |
82 | Roybon L, Hjalt T, Stott S, Guillemot F, Li JY, Brundin P(2009) Neurogenin2 directs granule neuroblast production andamplification while NeuroD1 specifies neuronal fate duringhippocampal neurogenesisPLoS One4: 3e4779 |
83 | Niwa A, Nishibori M, Hamasaki S, Kobori T, Liu K, Wake H, Mori S, Yoshino T, Takahashi H(2015) Voluntary exercise induces neurogenesis inthe hypothalamus and ependymal lining of the third ventricleBrain Struct FunctJan 30. [Epub ahead of print] PubMed PMID: 25633473 |
84 | van Praag H, Christie BR, Sejnowsky TJ, Gage FH(1999) Running enhancesneurogenesis, learning, and long term potentiation in miceProcNatl Acad Sci USA96: 231342713431 |
85 | Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR(2004) Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo Neuroscience124: 17179 |
86 | Titterness AK, Wiebe E, Kwasnica A, Keyes G, Christie BR(2011) Voluntary exercise does not enhance long-term potentiation in the adolescent female dentate gyrusNeuroscience183: 2531 |
87 | Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ(2010) Running enhances spatial pattern separation in miceProc Natl Acad Sci U S A107: 523672372 |
88 | Hopkins ME, Nitecki R, Bucci DJ(2011) Physical exercise during adolescence versus adulthood:differential effects onobject recognition memory and brain-derived neurotrophic factor levelsNeuroscience194: 8494 |
89 | Kohman RA, Clark PJ, Deyoung EK, Bhattacharya TK, Venghaus CE, Rhodes JS(2012) Voluntary wheel running enhances contextual but not trace fear conditioningBehav Brain Res226: 117 |
90 | Patten AR, Sickmann H, Hryciw BN, Kucharsky T, Parton R, Kernick A, Christie BR(2013) Long-term exercise is needed to enhance synaptic plasticity in the hippocampusLearn Mem20: 11642647 |
91 | Adlard PA, Perreau VM, Cotman CW(2005) The exercise-induced expression of BDNF within the hippocampus varies across life-spanNeurobiol Aging26: 4511520 |
92 | Kaspar BK, Frost LM, Christian L, Umapathi P, Gage FH(2005) Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosisAnn Neurol57: 5649655 |
93 | Hoveida R, Alaei H, Oryan S, Parivar K, Reisi P(2011) Treadmill running improves spatial memory in an animal model of Alzheimer’s diseaseBehav Brain Res216: 1270274 |
94 | Lee MH, Wang T, Jang MH, Steiner J, Haughey N, Ming GL, Song H, Nath A, Venkatesan A(2011) Rescue of adult hippocampal neurogenesis in a mouse model of HIV neurologic diseaseNeurobiol Dis41: 3678687 |
95 | Gobbo OL, O’Mara SM(2005) Exercise, but not environmental enrichment, improves learning after kainic acid-induced hippocampal neurodegeneration in association with an increase in brain-derived neurotrophic factorBehav Brain Res159: 12126 |
96 | Carro E, Trejo JL, Busiguina S, Torres-Aleman I(2001) Circulatinginsulin-like growth factor I mediates theprotective effects of physical exercise against brain insults ofdifferent etiology and anatomyJ Neurosci21: 1556785684 |
97 | Wong-Goodrich SJ, Pfau ML, Flores CT, Fraser JA, Williams CL, Jones LW(2010) Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiationCancer Res70: 2293299338 |
98 | Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF(2008) TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatmentNeuron59: 3399412 |
99 | Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M(2008) Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behaviorProc Natl Acad Sci U S A105: 401557015575 |
100 | Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S(2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult ratsExp Neurol192: 2348356 |
101 | Griffin EW, Bechara RG, Birch AM, Kelly AM(2009) Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanismHippocampus19: 10973980 |
102 | Gobeske KT, Das S, Bonaguidi MA, Weiss C, Radulovic J, Disterhoft JF, Kessler JA(2009) BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in micePLoS One4: 10e7506 |
103 | Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortigüela R, Marqués-Torrejón MA, Nakashima K, Colak D, Götz M, Fariñas I, Gage FH(2010) Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampusCell Stem Cell7: 17889 |
104 | Jang MH, Bonaguidi MA, Kitabatake Y, Sun J, Song J, Kang E, Jun H, Zhong C, Su Y, Guo JU, Wang MX, Sailor KA, Kim JY, Gao Y, Christian KM, Ming GL, Song H(2013) Secreted frizzled-related protein 3regulates activity-dependent adult hippocampal neurogenesisCellStem Cell12: 2215223 |
105 | Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, Xu A, So KF(2014) Physical exercise-induced hippocampalneurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectinProc Natl Acad Sci U S A111: 441581015815 |
106 | Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF(2012) Microglia modulate hippocampal neural precursor activity in response to exercise and agingJ Neurosci32: 1964356443 |
107 | Farioli-Vecchioli S, Micheli L, Saraulli D, Ceccarelli M, Cannas S, Scardigli R, Leonardi L, Ciná I, Costanzi M, Ciotti MT, Moreira P, Rouault J-P, Cestari V, Tirone F(2012) Btg1 is required to maintain the pool of stem and progenitor cells of the dentate gyrus and subventricular zoneFront Neurosci6: 124 |
108 | Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R(2010) Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brainsJ Neurosci30: 934893498 |
109 | Lee EY, Hu N, Yuan SS, Cox LA, Bradley A, Lee WH, Herrup K(1994) Dual roles of the retinoblastoma protein in cell cycle regulation and neuron differentiationGenes Dev8: 1720082021 |
110 | Paik WK, Kim S, Lim IK(2014) Protein methylation and interaction with the antiproliferative gene, BTG2/TIS21/Pc3Yonsei Med J55: 2292303 |
111 | Bogdan JA, Adams-Burton C, Pedicord DL, Sukovich DA, Benfield PA, Corjay MH, Stoltenborg JK, Dicker IB(1998) Human carbon catabolite repressor protein (CCR4)-associative factor cloning, expression and characterization of its interaction with the B-cell translocation protein BTG1Biochem J336: Pt 2471481 |
112 | Wu X, Shi W, Zhao W, Shao B, Yuan Q, Li C, Zhang S, Sun B, Wu Q, Chen J(2102) Changes in Pirh2 and p27kip1 Expression Following Traumatic Brain Injury in Adult RatJ Mol Neurosci46: 1184191 |
113 | Varodayan FP, Zhu XJ, Cui XN, Porter BE(2009) Seizures increase cell proliferation in the dentate gyrus by shortening progenitor cell-cycle lengthEpilepsia50: 1226382647 |
114 | Schindowski K, Belarbi K, Bretteville A, Ando K, Buée L(2008) Neurogenesis and cell cycle-reactivated neuronal death during pathogenic tau aggregationGenes Brain Behav7Suppl 1: 92100 |
115 | Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, Nath AImpaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulationJ Neurovirol19: 5418431 |
Figures and Tables
Fig.1
Cell cycle regulation in the adult neurogenic niches, dentate gyrus and SVZ (B and C), in comparison with the canonical model of the cell cycle (A). The detailed description of the various regulatory steps and of the key molecules involved in cell cycle control is exhaustively described in the text. It is important to note that in the context of the two neurogenic niches the role of several key molecules in the control of cell cycle progression has yet to be identified.
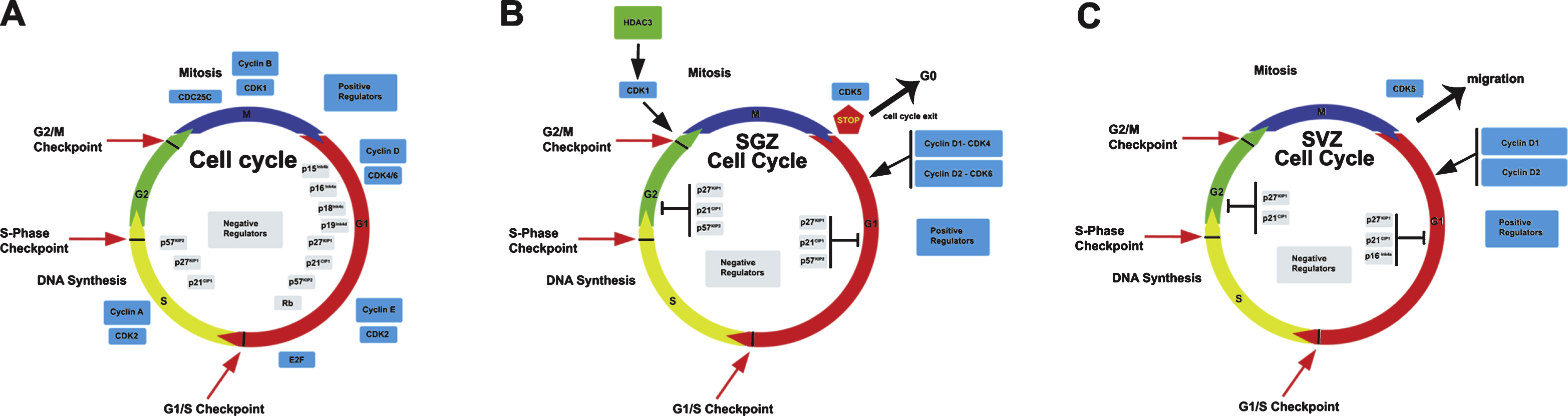
Fig.2
Scheme depicting the role of cell cycle-related molecules on the proliferation and differentiation of stem and progenitor cells in the adult dentate gyrus and SVZ. The data were obtained by knockout experiments performed in vitro and in vivo and are detailed in the text. Dentate gyrus: Cdk6 and cyclin D1/2 are required for the proliferation of stem cells, while p27Kip1 or p21Cip1 are required to maintain the quiescence of stem cells. Moreover, cyclin D1 is involved in astrocytic differentiation while cyclin D2, p27Kip1, p21Cip1 and Tis21 are involved in the commitment of neural stem cells to the neuronal differentiation. SVZ: Cdk6 and cyclin D1/2 appear to be required for the proliferation of stem cells, while p16Ink4a and Btg1 are necessary to maintain the quiescence of stem cells, in aging or in adult mouse, respectively. Cyclin D2, Cdk5 and Tis21 are involved or required for neuronal differentiation.
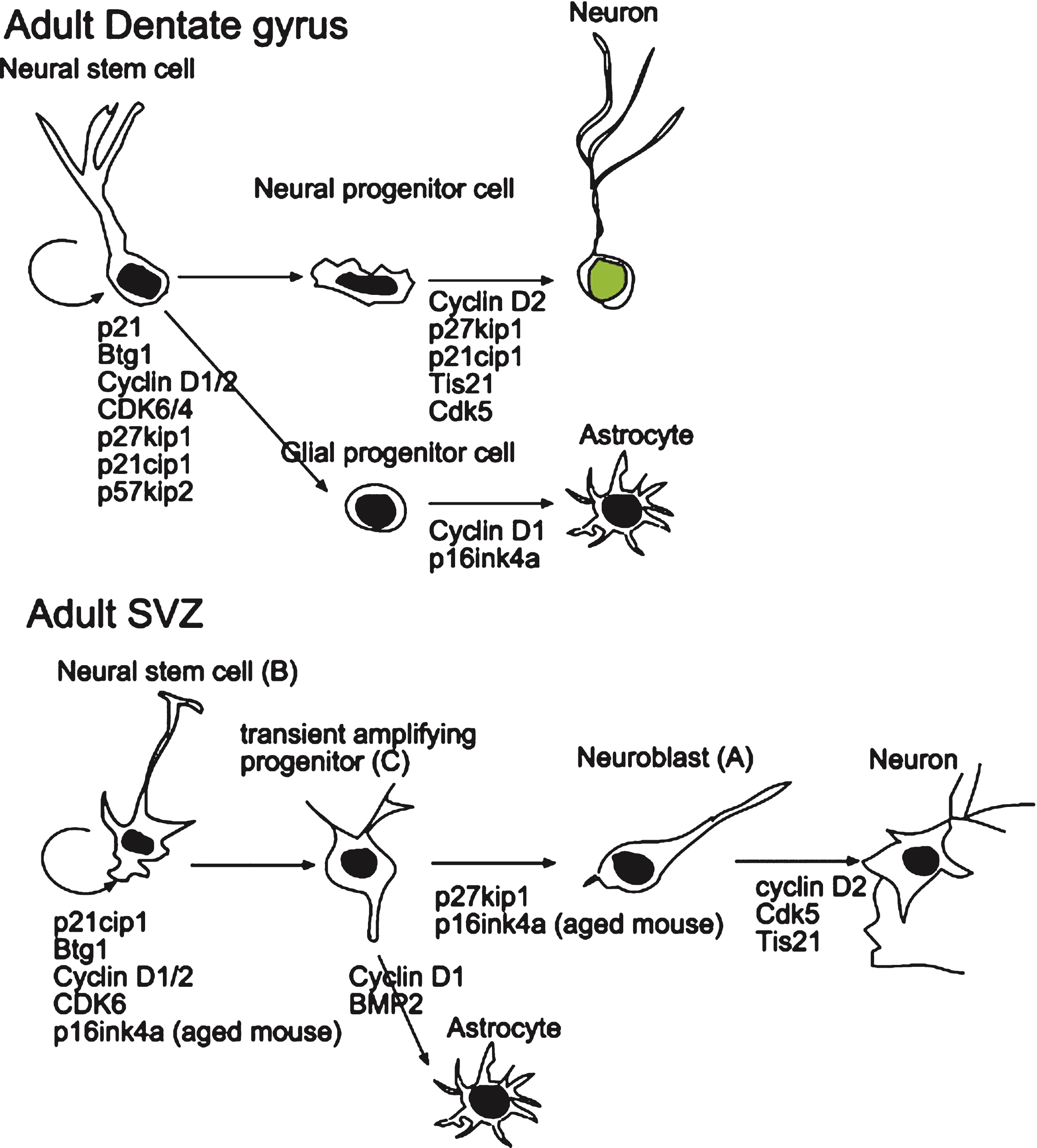
Table 1
Summary of the factors involved in the alterations of adult neurogenesis through a direct involvement of the cell cycle progression
| Experimental model | Neurogenesis in DG | Neurogenesis in SVZ | Cell cycle/molecular changes | Effect of running | Ref |
| Stroke | ↑ | ↑ | ↓ G1 phase and ↑ cell cycle reentry in SVZ⟶ expansion of progenitors after stroke; | ↑ neurogenesis ↑spatial memory ↑axon rigeneration | [67] |
| ↑ p27KIP1 and p21CIP1 in the DG and SVZ⟶ inhibition on neural regeneration after stroke; | [57, 40] | ||||
| Traumatic brain injury (TBI) | ↑ | ↑ | ↓ p27KIP1 level after TBI⟶ ↑ glia proliferation; | Not assessed | [112] |
| Epilepsy | ↑ | Not assessed | ↑ p27KIP1 ⟶ shortening cell cycle; ↑ progenitor proliferation | Not assessed | [113] |
| Alzheimer Disease | THY-TAU22 mice ↑ | Not assessed | ↑ cylin D1 ↑p21CIP1 ↑ p27KIP1 | Not assessed | [114] |
| HIV | ↓ | Not assessed | ↓ CDK5 hyperactivation | ↑ proliferation ↑arborization ↑BDNF | [115] |




