Challenging Cases in Urothelial Cancer: Case 31: LG Ta Bladder Cancer
Bladder cancer is one of the most common cancers. It is also one of the most expensive cancers in large part because the prevalence is high and following the initial diagnosis and treatment patients are likely to develop another bladder tumor. These so called “recurrences” are most often the result of prior or current carcinogen exposure. The most common carcinogens are those present in cigarettes, which accounts for 50–65% of bladder cancers.
Although urologists often indicate that these subsequent bladder tumors are a “recurrence”, they are more likely new tumors. Alternative explanations are implantation of floating viable tumor cells on the altered bladder surface after a transurethral resection (TUR BT) or an incomplete resection. In these scenarios subsequent bladder cancers would appropriately be termed a recurrence of the prior cancer.
Following a TUR BT patients undergo surveillance cystoscopy at regular intervals. These monitoring visits and the treatment of new tumors account for much of the high cost of bladder cancer treatment.
If patients develop a new or recurrent tumor, the standard approach has traditionally been to schedule the patient for a formal TUR BT in an operating room. Each of these visits require medical clearance followed by a TUR BT using general or spinal anesthesia. The two cases presented here raise the question whether this is always required. In particular, If the subsequent tumors, i.e., recurrences, are small and appear to be low grade and confined to the urothelium (Ta) is a formal TUR BT always required?
Papillary bladder tumors have been traditionally graded from 1 (low) to 3 (high). Several years ago, the WHO and ISUP changed the grading system in part because many pathologists labelled the majority of tumors grade 2. The pathologist now diagnose bladder tumors low or high grade. With the new system grade 1 tumors and some of those previously diagnosed grade 2 are low-grade. The remainder of the grade 2 and all the grade 3 bladder cancers are diagnosed high - grade.
The most common bladder cancers are confined to the urothelial surface, (Ta). The chance of a subsequent tumor, i.e., “recurrence”, following an initial TUR BT of low-grade Ta tumors is 30–70% depending on the size and number of tumors. Despite the risk of a “recurrence” the chance that the patients with a low-grade Ta tumor develop a subsequent bladder cancer that invades the bladder wall, i.e., the lamina propria or muscularis propria, is very low. Consequently, patients who have only low-grade Ta (LG Ta) bladder cancer rarely die of bladder cancer.
To summarize, the great majority of men and women who present with low grade Ta bladder cancer are at risk of a subsequent tumor but infrequently develop a potentially lethal phenotype which results in death from bladder cancer.
Patients with a high-grade papillary bladder tumor confined to the urothelium (high grade Ta) are at higher risk of developing another tumor following initial diagnosis and at higher risk of a “recurrence” which invades the basement membrane than patients with low grade Ta. However, their risk of a lethal tumor is still relatively low, although higher than patients with low grade bladder cancer.
To complicate the discussion I believe, and have reported, that there has been an increase in the grade distribution of what appears to be papillary Ta bladder tumors by some pathologists. Over the past few decades, we found that our pathologists more often diagnose papillary Ta tumors high grade when the endoscopic appearance suggests the tumor is low grade. Pathologists may be concerned that they are under grading. If there is any atypia they are more likely to diagnose the tumor as high grade even if the majority of the specimen is grade 1–2.
The great majority of men and women who die of bladder cancer have a high-grade muscle invasive urothelial cancer at their initial diagnosis. Another group of patients who are at increased risk of dying of bladder cancer are those with a high-grade cancer which invades the lamina propria (T1).
With this introduction I present two common clinical scenarios of patients with low grade Ta bladder cancer. The urology literature concentrates on the management of bladder cancer patients who are at high risk of progression and death from bladder cancer. This is true for the majority of cases published under this heading of Challenging Cases in this journal.
The purpose of these two cases is to address the management for both initial and “recurrent” papillary bladder tumors which appear by endoscopy to be low grade Ta.
In the discussion of these two cases I use the term TUR BT in the generic sense indicating an endoscopic approach to remove bladder tumors while the patient receives other than topical anesthesia, e.g., general or spinal. Removal of the tumor can be accomplished by cold cup excision followed by cautery, traditional monopolar or bipolar loop resection, vaporization with a rollerball electrode, or with a laser - transurethral laser ablation (TULA). A standard loop TUR BT can be piecemeal or en bloc. The latter technique (as well as the cold cup biopsy) is designed to provide a specimen without cautery artifact.
Importantly, urologists are quite accurate at identifying the grade of a papillary tumor particularly when it is low grade at the time of endoscopy.
The first patient is a 75-year-old man who presented with gross hematuria. He has moderate lower urinary tract symptoms.
A CT scan of the abdomen and pelvis was normal. Office flexible cystoscopy revealed a moderately enlarged prostate and two small papillary bladder tumors, less than 1 cm. They appeared to be low grade Ta. The rest of the bladder was normal. I would have entertained office cautery if this was not his first bladder tumor or he were frail. He underwent a TUR BT. I used the cold cup to remove the tumors and a button bipolar electrode to cauterize the area where the tumors were located (Figs. 1–3) I did not attempt to obtain muscle. The pathology was low grade, non –invasive papillary urothelial carcinoma (LG Ta). There was no muscle in the specimen (Fig. 4).
The second patient is a 76-year-old healthy woman whose initial diagnosis of bladder cancer was in 2014 after she presented with gross hematuria. She quit smoking after a 30-pack year history. A renal ultrasound was normal.
In 2014 office cystoscopy revealed papillary LG Ta appearing tumors located at the left hemitrigone. The initial TUR BT consisted of cold cup biopsies followed by coagulation with a ball electrode. The pathology report was papillary low-grade noninvasive bladder cancer (LG Ta). There was no invasion and no muscle in the specimen.
She had office cystoscopic monitoring every 4–6 months through July 2020. On each occasion there was only very small areas of low grade appearing papillary tumor confined to the left hemitrigone. There were no changes and active surveillance was continued without any new tumors or growth of the existing small papillary areas until 2020.
A larger area of LG Ta appearing tumor was observed at the left trigone in July 2020. She was taken to the operating room and, once again, I used cold cup resection and cautery to remove the tumors. The tumors were low grade Ta. There was no muscle in the specimen.
In August 2021 another tumor was identified. She was taken to the operating room and cold cup excision followed by cautery was used. The pathology was high grade (grade 2) according to the WHO/ISUP grading system and there was, for the first and only time, microscopic invasion into the lamina propria, i.e., HG T1a. Muscle was present and not involved.
Following the guidelines after a diagnosis of a HG T1 tumor a repeat TUR BT was performed using a resectoscope loop at the area of previous resection (no obvious tumor was seen). The pathologist reported a few strands of LG Ta tumor and necrotic muscle. There was no invasion. She received six weeks of intravesical BCG.
In December 2021 she underwent a TUR BT with loop resection for what appeared to be LG Ta tumor. On this occasion the tumors were located on the left lateral wall and anterior near the air bubble. The pathology was low grade Ta urothelial cancer. There was no muscle in the specimen. This was followed by intravesical gemcitabine, weekly for 6 weeks.
The next TUR BT was in June 2023. The procedure was cold cup tumor removal followed by cautery with the button bipolar electrode. The pathology was low- and high-grade (grade 2) urothelial cancer with no invasion, Ta.
The most recent event was in December 2023. She presented with gross hematuria. The tumor was anterior and about 4 cm. A loop TUR BT with the bipolar resectoscope was used (Figs. 5, 6). The pathology was low grade Ta urothelial carcinoma (Fig. 7). There was no lamina propria or muscle in the specimen.
My approach is to minimize trauma to the bladder when tumors appear to be papillary LG Ta. If papillary “recurrences” are small, I prefer office cautery or active surveillance. This avoids an expensive procedure, is more time efficient for the patient and the urologist and eliminates the potential morbidity of an anesthetic. It also minimizes damage to the bladder. If, as in one of the several tumor resections in the second case, I underestimate the stage of the tumor I can perform a repeat TUR BT. This would not differ if an initial loop TUR BT revealed a HG T1 tumor. The approach would still be a repeat TUR BT.
I realize there are differences of opinion about the extent of an initial and subsequent tumor resection for what appears to be low grade or even high-grade non-muscle invasive bladder cancer (NMIBC). I therefore have asked others to critique my management of these two patients. I have arbitrarily selected urologists from different geographic areas who I know have an interest in this topic. I have asked them to reply specifically to the following questions.
As in my case 1, after an initial diagnostic cystoscopy of what appears to be papillary low grade bladder cancer do you routinely perform a TUR BT using loop resection? Is it necessary to obtain muscularis propria in the specimen?
Referring to case 2, I want them to indicate how they manage patients with “recurrent” small papillary low grade appearing tumor(s) at the time of surveillance cystoscopy. Do they consider active surveillance if the tumors are few and small? Do they use office cautery? If the size and number require a TUR BT under anesthesia what is their technique and objective in terms of depth of resection?
Case 1
Fig. 1-2
Two papillary low grade Ta appearing tumors.
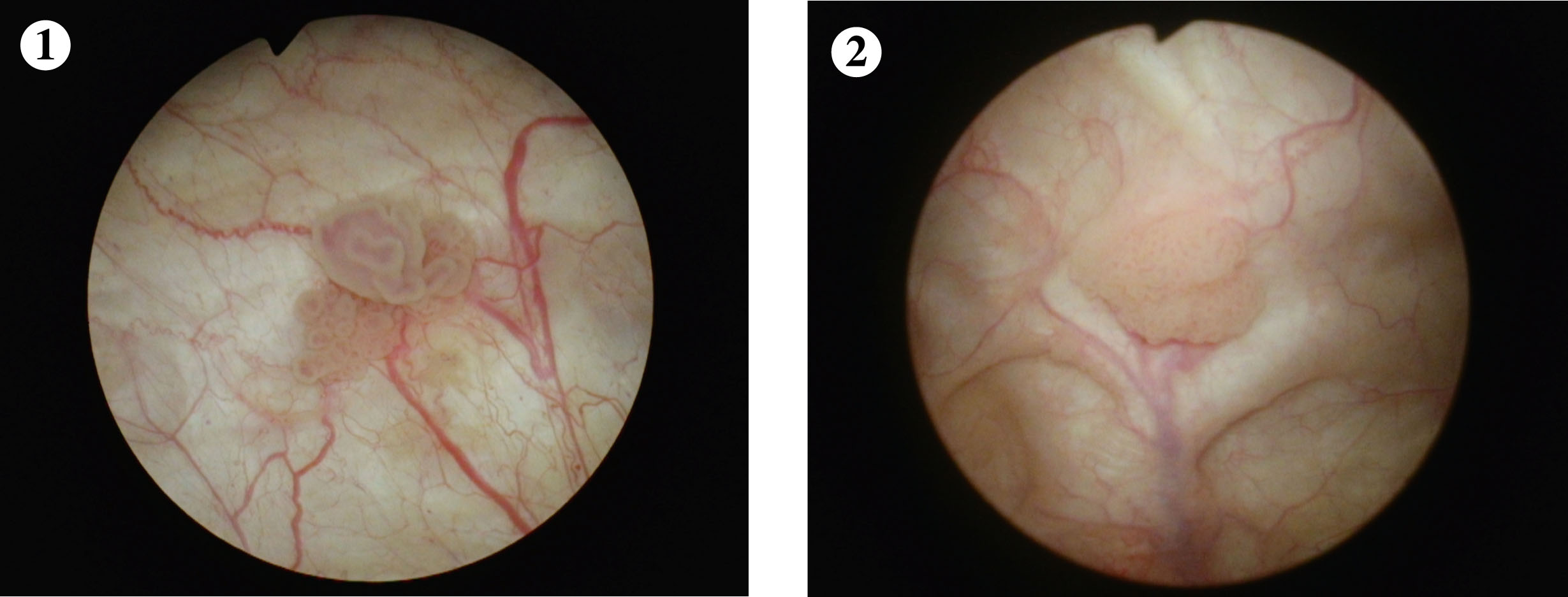
Fig. 3
Cold cup biopsy of apparent low grade Ta papillary bladder tumor.
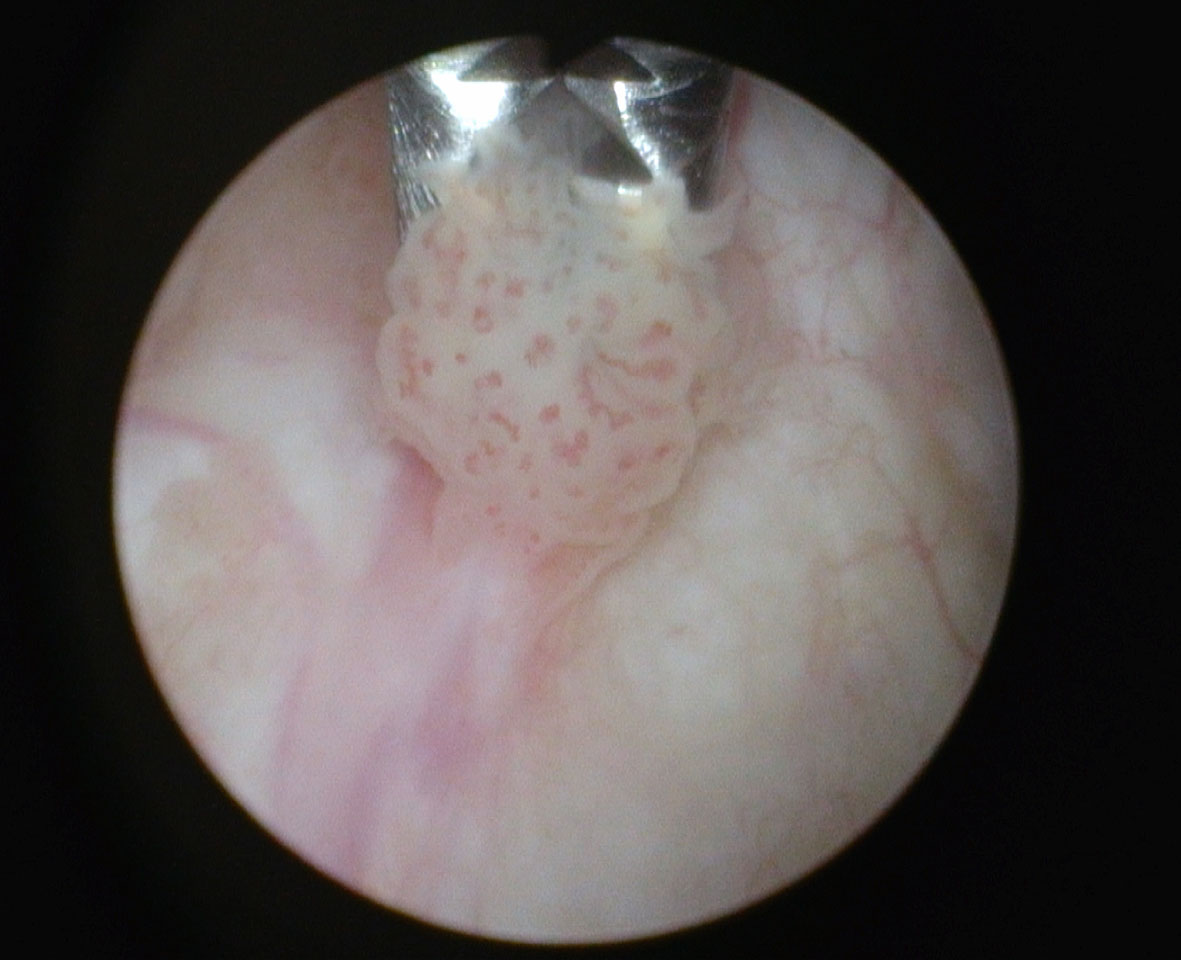
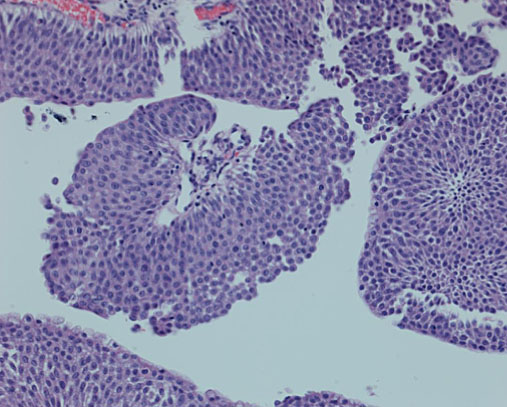
Fig. 4
Low grade non invasive papillary urothelial tumor.
Case 2
Fig. 5
4 cm anterior papillary low grade appearing tumor.
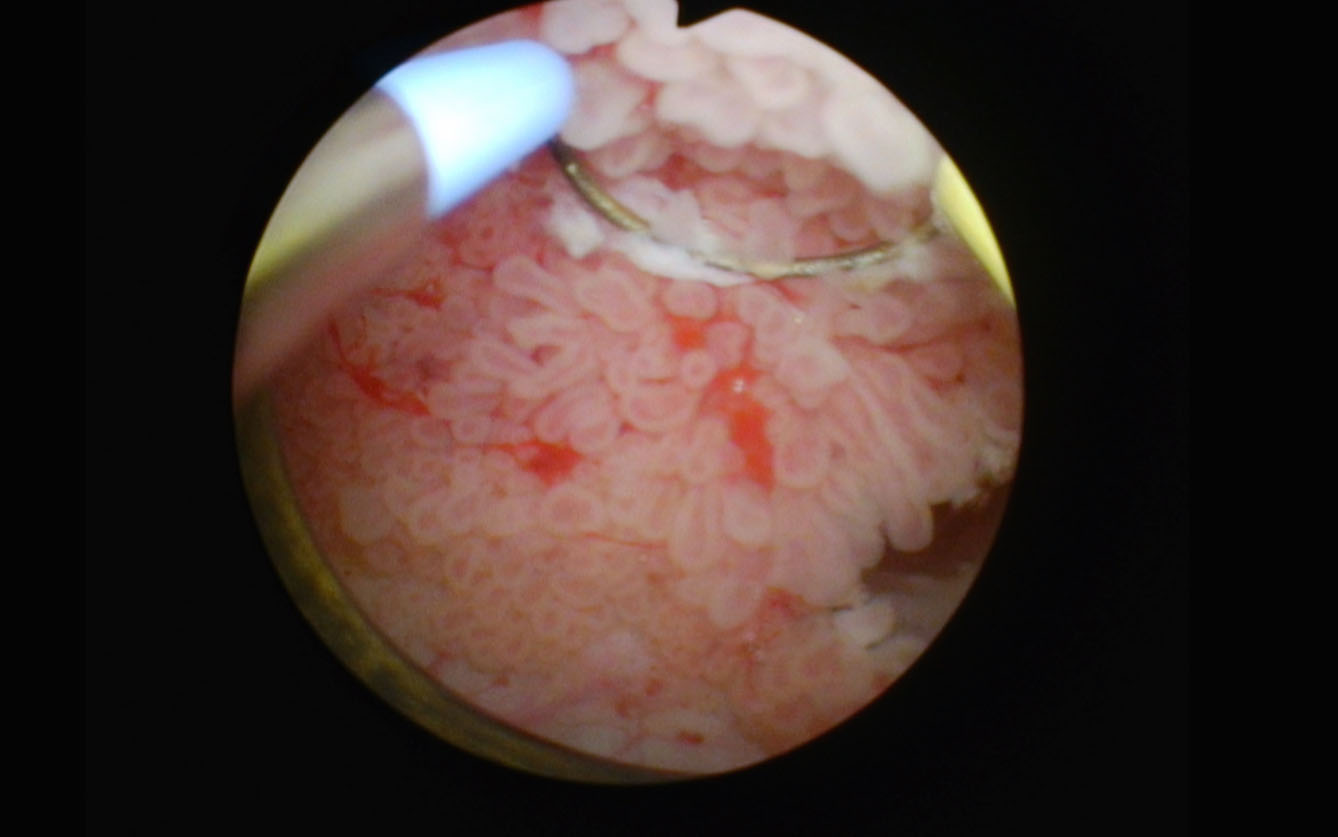
Fig. 6
Base of tumor post TUR BT.
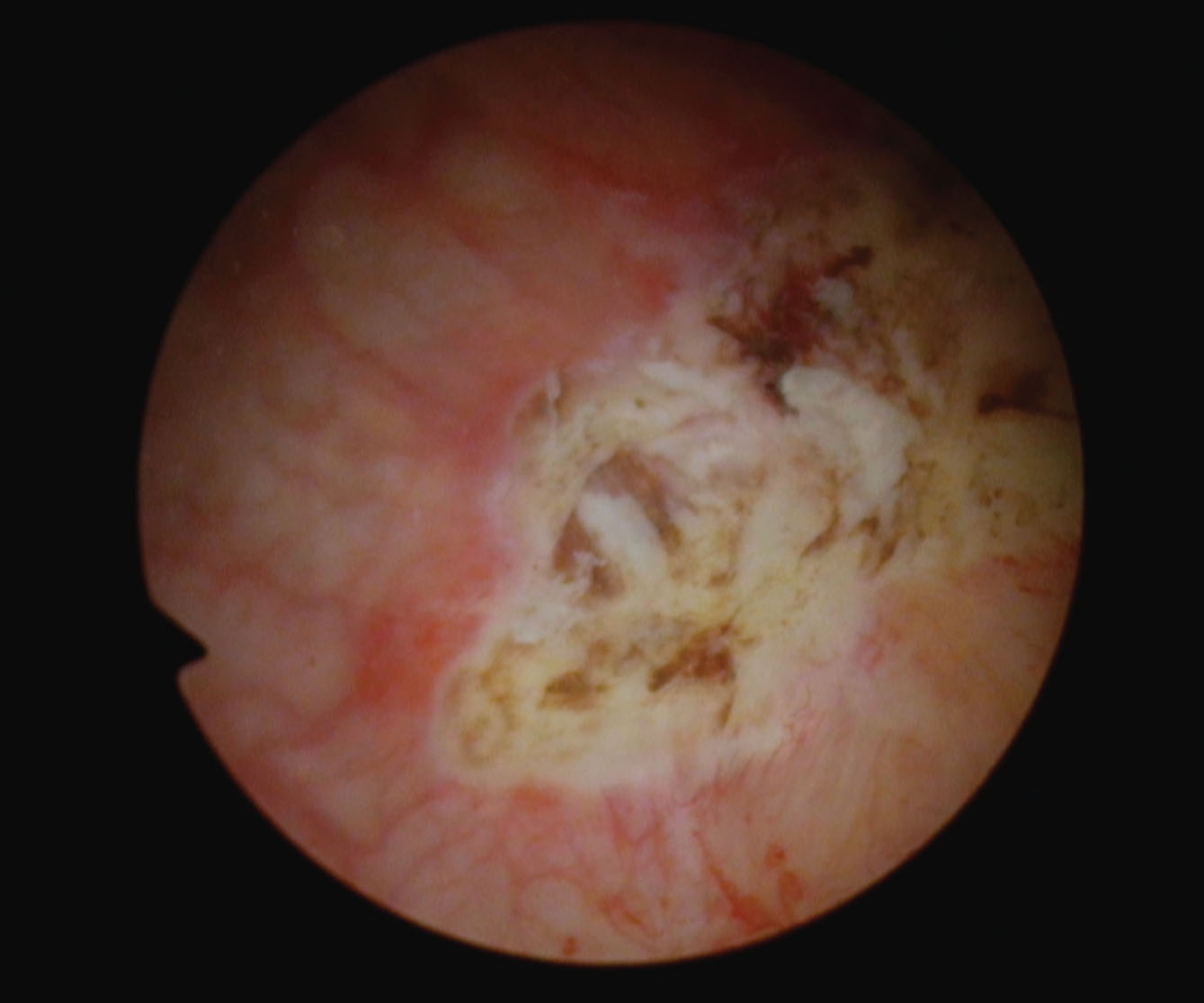
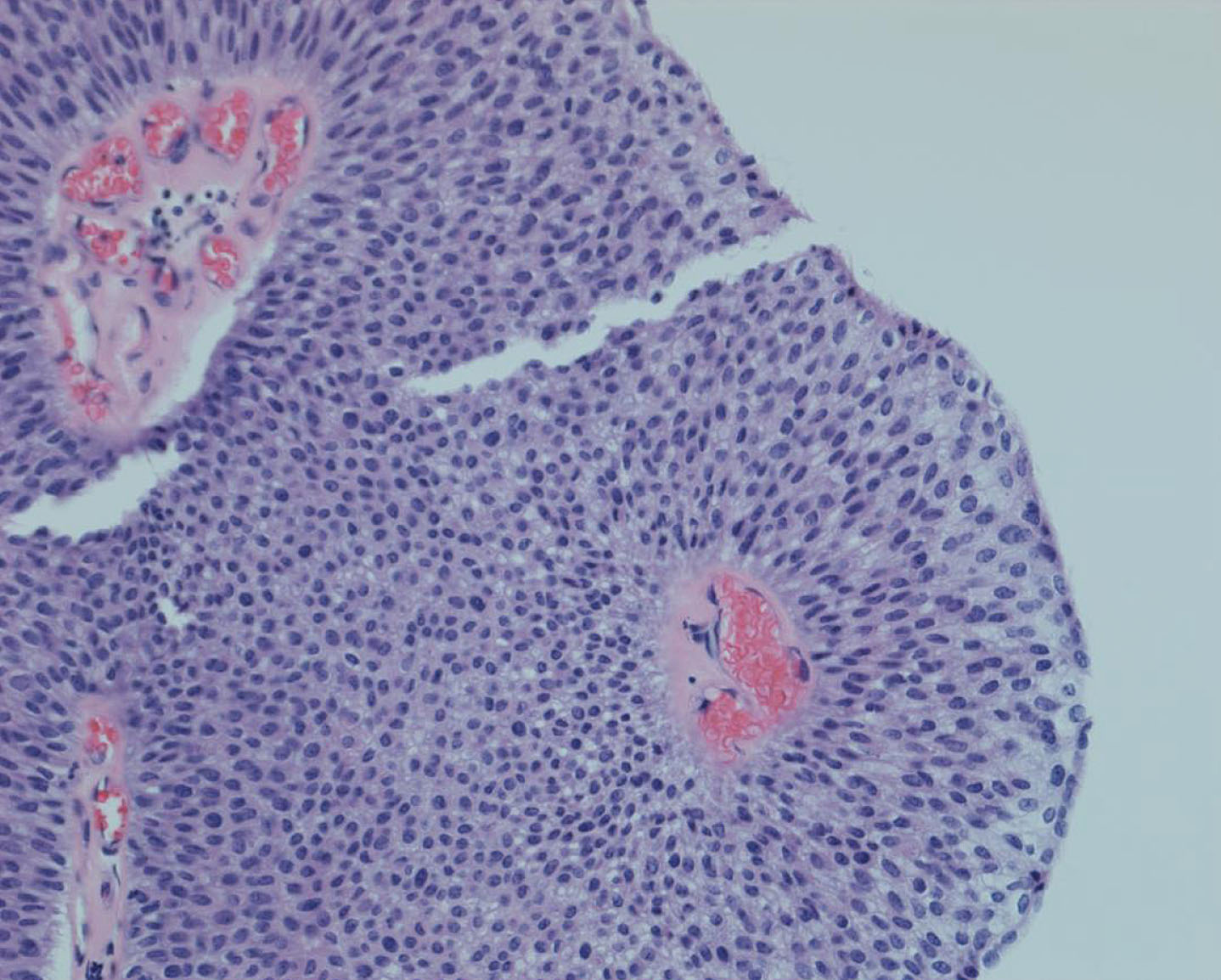
Fig. 7
Low grade non invasive papillary urothelial tumor.
Responses to Case of LG Ta Bladder Cancer
Harry Herr.
Attending Surgeon, Memorial Sloan Kettering Cancer Center, New York
Initial tumors are handled by a thorough TUR BT in the operating room.
For LG Ta I do not use adjuvant intravesical chemotherapy.
Recurrent tumors are managed based on observation. If they appear to be small and LG Ta they are cauterized with a bugbee electrode in the office. A formal TUR BT using a resectoscope loop is used if the patient has a large tumor or too many tumors to tolerate office cauterization. All cystoscopies whether outpatient or in the operating room use both white light and narrow band imaging as the CROES study showed a reduction in recurrences of low risk bladder tumors.
Patients are followed every 6 months for three years and then annually.
We have evaluated our results in 423 patients over two decades using outpatient fulguration when possible, with occasional TUR BT when required by size or number. One third are taken care of by outpatient management alone! Selected intermediate risk patients are included in this analysis.
With a median follow up of 17 years the cause specific survival rate is 99%. One percent died of bladder cancer. Nine percent progressed to high grade Ta or T1. Only one patient had a MIBC.
Rodolfo Hurle
Instituto Clinico Humanitos, Milan, Italy
For initial diagnosis, I always prefer a formal TUR BT using the bipolar resectoscope. If the tumor appears low grade I refrain from performing a deep resection. Muscle is not required.
For the patient with recurrent LG Ta I agree with your approach. I do not obtain muscle if a TUR BT is required.
I use BCG even with LG Ta and prefer maintenance.
We maintain an active surveillance protocol for patients with a history of LG Ta with suspected LG Ta recurrence.
Yair Lotan
University of Texas Southwestern Medical Center, Dallas, Texas
The initial patient resection is in the operating room using both blue and white light. I use postoperative intravesical chemotherapy.
In managing recurrent tumors in patients with prior low-grade Ta, I use office cautery if less than 1 cm. I biopsy the tumor and then use cautery. I may elect post procedure intravesical gemcitabine.
U. Bansal
Memorial Hospital, Hollywood, Florida
With an initial diagnosis of bladder cancer I perform a TUR BT in the hospital and try to obtain at least lamina propria in the specimen. If the tumor is small I employ the cold cup followed by cautery. In general muscle is often in the specimen. If the tumor is larger I will perform a standard loop TUR BT and regardless of the grade try to obtain muscle in the specimen. I generally try to have a separate specimen labeled “deep” resection to aid the pathologist.
For small LG Ta appearing recurrences I use office cautery.
I do not use active surveillance as I prefer cautery for small tumors.
I prefer to label all tumors separately based on location. If tumors are small, I will not attempt to obtain muscle. I perform a deep resection on the largest or highest-grade appearing lesion. For this lesion I will obtain a specimen labelled deeper biopsy.
Overall, if a tumor is small and appears to be LG Ta, I prefer to have at least lamina propria. If tumors are greater then 1–2 cm even if papillary, I will obtain muscle.
Michael O’Donnell
University of Iowa, Iowa City, Iowa
I initiate the investigation of all patients suspected of having bladder cancer with office cystoscopy.
I will consider biopsy in the office if tumors are small, and the patient is willing to tolerate some discomfort.
Case 1. For an initial TUR BT in a patient with what appears to have LG Ta bladder cancer I try initially to obtain some tissue without cautery with a cold cup biopsy to aid the pathologist. If the tumor can completely be excised with the cold cup that is fine and I cauterize the base with a Bugbee electrode. If the tumor appears to be Ta there is no need to obtain muscularis propria. Most specimens will have lamina propria.
If the tumor is more than 1.5 cm I will usually use the bipolar resectoscope. I do not employ an en bloc technique. I use a thulium laser when the tumor is difficult to reach. I initially try to obtain a biopsy with the flexible endoscope.
I typically use Cysview for all formal TUR BTs under anesthesia because of the ability to see peripheral margins and the occasional identification of small tumors which may be missed with white light.
Case 2. I do not use active surveillance routinely for small LG Ta tumors but I tell the patient there is no urgency to remove these lesions but try to do so in 2–3 months.
If I see small LG Ta appearing tumors in the office during surveillance I will use office cautery and may obtain a biopsy just to determine the grade. In this patient with a prior HG T1a tumor I would more likely obtain a biopsy and perform a bladder wash for cytology.
For the patient with recurrent LG Ta only and very small (<5 mm) I will cauterize without a biopsy.
I do not use a laser in the office because the logistics are difficult.
In the patient with small recurrent multiple LG Ta appearing tumors I will often biopsy a few of the tumors and then use the monopolar resectoscope with the ball electrode and sometimes with the cutting current and vaporize the other tumors. I usually then instill a single dose of gemcitabine.
I do not see a need to obtain muscle in the specimen for resection of tumors which appear to be LG Ta. Often there is some lamina propria in specimens which is sufficient. If there is invasion this will prompt a repeat TUR BT anyway. In such cases I may obtain a cold cup of the base.
Added points: Urologists are not as good as identifying grade and stage, e.g. LG Ta, as you might believe. In a couple of studies about 30% of presumed LG Ta were incorrect.
About 15–20% of LG Ta tumors followed up to 20 years will show grade/stage progression.
High grade tumors tend to stay high grade after a TUR BT alone. However, if they receive BCG or chemotherapy 4–7% will be LG Ta and stay that way.
Seth Lerner
Baylor College of Medicine, Houston, Texas
Case 1. I would manage patient exactly as you did.
Case 2. I would prescribe intravesical chemotherapy after management of the recurrences using mitomycin C
James Catto
University of Sheffield, Sheffield, England
I try to minimize trauma to the bladder. The objective is to adequately sample the cancer for grade and stage. If this is with cold cup –great. If not, then loop TUR BT. I try to obtain muscle but I do not consider it necessary. If the pathology is LG Ta and no muscle I would not perform a re TUR BT.
Regarding active surveillance I often use it.
We use laser ablation rather than cautery for in office removal of small LG Ta bladder tumors.
Paramananthan Mariappan
Edinburgh, United Kingdom
For newly diagnosed bladder tumor as in patient described in case 1, I would always biopsy and cauterize the area. Obtaining muscle does not significantly impact the outcome. Given the small size a TUR BT as opposed to the cold cup biopsy might produce cautery artifact which might impact the pathologist’s ability to accurately determine the grade and stage. I usually instill intravesical mitomycin C following the procedure.
If the tumor is Ta I like to distinguish whether it is grade 1 or 2 as the risk of recurrence and progression in grade or stage is higher with grade 2. This will also impact our surveillance frequency.
On initial diagnosis of a papillary LG Ta appearing tumor an ultrasound is sufficient to evaluate the upper urinary tract.
Case 2. I emphasize smoking cessation for all patients with bladder cancer, if they are still smoking.
I monitor some patients with active surveillance. I obtain urine for cytology at least yearly for patients undergoing active surveillance. If patients have multiple subsequent tumors I obtain a CT scan to ensure there are no upper tract tumors.
This patient is unusual as she had one instance of HG T1a. In our Scottish series we saw progression from intermediate risk to high grade Ta/T1 in 6% of 536 patients. 8 of 31 were HG T1. I agree with the use of BCG for this patient and would have suggested maintenance.
When patients have frequent recurrences I use photodynamic endoscopy to identify small lesions/tumor.
I utilize active surveillance for LG (G1) Ta bladder cancer after discussion with the patient.
I have used office cautery and are currently acquiring the laser for TULA of small low grade papillary tumors.
When patients require a TUR BT I prefer cold cup and cautery for tumors less than 1 cm and if tumors are 1 cm or larger I prefer an en bloc resection with at least lamina propria. If tumors are large I try to obtain muscularis propria unless the bladder wall is thin.
Hugh Mostafid
Royal Surrey County Hospital, Guildford, England
In general I would mange these two patients in a similar fashion.
On an initial diagnosis I would always biopsy the tumor. I would utilize cold cup biopsy for small tumors as I would want to document the grade and stage.
For follow up, if tumors appear to be low grade Ta I employ both active surveillance and office cautery after discussion with the patient. I use transurethral laser ablation (TULA).
If a patient with apparent LG Ta tumor requires a TUR BT, I do not try to obtain muscularis propria as the risk of under staging is low and a deeper resection may increase the risk of a complication.
I use mitomycin C for patients with multiple recurrences of LG Ta.
Peter Black
University of British Columbia, Vancouver, British Columbia, Canada
Case 1. I always perform a formal biopsy/resection for the initial diagnosis.
I do not try to obtain muscle if the tumor appears to be Ta, regardless of grade. I agree with concept of utilizing cold cup when possible. If I perform a TUR BT I prefer en bloc and there is usually muscle in the specimen.
I routinely utilize office biopsy for indeterminant lesions when monitoring patients. I utilize office cautery for papillary LG Ta recurrences in patient with prior LG Ta.
The second patient had one tumor which was HG T1a and thus would prefer histology on all subsequent tumors but, if small, I would perform office biopsy and cautery.
I generally prefer office cautery to active surveillance for “recurrent” small, papillary tumors.
Fred Witjes
Radboud University Medical Center, Nijmegen, The Netherlands
I usually remove any initial tumor. I use cold cup when possible, to avoid trauma to the bladder.
During follow up I alternate urinary marker, e.g., cytology, with cystoscopy. For subsequent LG Ta tumors I use active surveillance, office coagulation, or if large or multiple a TUR.
Arthur Sagalowsky
UT Southwestern Medical Center, Dallas, Texas
Case 1 is a patient with a tumor which appears to be low grade low stage and thus low potential to be high grade or invasive and thus little chance of progression. Complete resection of the tumor which maximizes accurate histopathology is paramount. I prefer a TUR BT with a loop with a stutter step technique lifting the tumor intact to minimize cautery artifact. If the tumor is small, I use a cold cup to remove the tumor whenever the cautery cut might destroy the specimen. I try to obtain lamina propria with either technique. Insisting on muscularis propria on such a low-risk tumor seems unnecessary and exposes the patient to a higher risk of bleeding or perforation. If the pathology returns as high grade or invasive a repeat TUR BT can be performed. This is not likely.
I take random biopsies in all initial cases to check for dysplasia or carcinoma in situ which cannot be excluded by visual appearance alone. I would not use intravesical chemotherapy on this patient with two small LG Ta tumors.
Case 2. The multiple recurrences would have prompted me to utilize intravesical gemcitabine and to reimage the upper urinary tract. I agree with the 6 week course of BCG after the single instance of T1.
I am concerned about the recent finding of the 4 cm Ta tumor in the anterior wall and would thus consider additional intravesical chemotherapy.
Mauricio Brausi
Department of Urology, AUSL, Modena, Italy
Case 1. I always use cold cup biopsy to remove the tumor to avoid bladder wall perforation. If needed, I can obtain muscle with the cold cup forceps. I cauterize the base.
I do not prescribe post operative single dose chemotherapy.
Case 2. For patients with small recurrent low grade tumors, I use active surveillance if patients are elderly or have significant co morbidity or if they are on an anticoagulation medication.
In younger patients with multiple recurrent LG Ta bladder tumors, I prefer a TUR BT and look for CIS. After the second recurrence I prescribe BCG.
Johannes Breyer
University of Regensburg, Regensburg, Germany
Case 1. Patients with hematuria have an office-based cystoscopy first. I do not biopsy tumors in the office.
I will attempt an en bloc resection if possible, if not a loop TUR BT. I would attempt to obtain muscle.
Case 2. If on follow up cystoscopy there are low grade Ta appearing tumors I consider active surveillance in selected cases, e.g., frail or elderly patient.
I do not use office cautery or laser removal in the office. If the recurrent tumors appear to be low grade and Ta muscle is not mandatory as part of the TUR BT.
Ashish Kamat
MD Anderson Hospital, Houston, Texas
Case 1. In patients with hematuria, I usually perform office cystoscopy unless there is a large tumor on imaging which clearly requires a TUR BT.
Office biopsy is reserved for recurrent tumors or when an area on cystoscopy is suspicious but is not an obvious tumor.
I use blue light as well as white light for all initial tumor resections. If tumor(s) are small I use cold cup. If size permits, I use en bloc resection if not a bipolar loop resection. If I feel confident a tumor is low grade I do not obtain muscularis propria.
Case 2. When a patient has multiple LG Ta recurrent bladder tumors, I occasionally use active surveillance but order cytology and want it to be negative. I do not want to miss a high-grade tumor or CIS.
I will often use office biopsy. If I am convinced a recurrent tumor is LG Ta I will cauterize without a biopsy.
I have not used a laser in the office.
If LG recurrent tumors are small and appear Ta I prefer cold cup and cautery to avoid cautery artifact. As with case 1 I do not obtain muscle if tumors appear to be LG Ta.
Alex Zlotta
University of Toronto, Toronto, Ontario, Canada
Case 1. I perform office cystoscopy during the initial evaluation for a patient with hematuria. If I identify a tumor, I rarely perform a biopsy in the office.
Occasionally I will cauterize a small low grade Ta appearing bladder tumor in the office but rarely if the initial tumor. This would happen only if there was a real concern about anesthesia. I send urine for cytology and use narrow band imaging.
If the bladder tumor is small, LG Ta appearing, and on a thin pedicle I will use a loop without cautery and scrape the tumor off the urothelium. This is like a cold cup biopsy. I cauterize the bed. Muscle is not required.
As in case 1, if the tumor is a little larger and appears LG Ta I prefer an en bloc with the loop using the stutter technique and low power. The goal is to minimize damage to the specimen. If obvious low grade and Ta no muscle is needed. If in doubt, I obtain muscle.
Case 2. Active surveillance for LG Ta appearing recurrences is useful and I employ this often. I send urine for cytology as a guide. Very often I begin with active surveillance and may use office cautery if the tumors are stable in size and number.
I do not use office biopsy since if cytology is negative for high grade cancer and the tumors appear LG Ta this is unnecessary.
I do not use a laser in the office but use office cautery regularly for LG Ta recurrent tumor.
I do not try to obtain muscle when a TUR BT is required for LG Ta appearing tumors. It places patients at risk with no benefit for patients with this entity which can be managed with surveillance or minimally invasive therapy.
Makarand Khochikar
Cancer Hospital of Miraj, Sangli Maharashtra, India
Case 1. I firmly believe, the first assessment is the best chance to get things right. I perform cystoscopy and TURBT with deep muscle biopsy. I do not perform random biopsies to avoid the trauma related to this. I use narrow band imaging before and after the resection to ensure that I’ve removed all tumors.
The only time I perform an office biopsy is when patients who have had a prior LG Ta tumor and are coming in for a follow up cystoscopy and I find small recurrences or new tumors. In that case I perform cold biopsies and cauterize the base. Once again, I use the NBI with the flexible Olympus cystoscope. If there are suspicious areas, I do not perform office bladder biopsies but bring the patients to the operating room for a formal TUR BT. I will also obtain urine for cytology in these cases.
When I perform a formal TUR BT for tumors less than 2.5 cm, I perform an en bloc resection with cautery and I attempt to obtain muscularis propria in the biopsy.
If tumors are greater than 2.5 cm I will perform a standard TUR BT and obtain muscular propria.
Case 2. For recurrent LG Ta appearing tumors, I generally do not use active surveillance, but I perform biopsy with office cautery.
If patients have significant comorbidities or on anticoagulation, I will use active surveillance with proper counseling so the patient understands the concept of active surveillance. I also will obtain urine cytology periodically. If cytology is positive then the lesion may be high-grade and I would encourage the patient to have a standard resection.
On occasion I do perform office biopsy with a cold cup. I prefer to have a biopsy prior to office cautery.
I do not use the laser to remove tumors in the office.
When removing small low-grade Ta appearing tumors in the operating room, I use cold cut biopsy and cauterize the base.
If I take a patient to the operating room for low-grade Ta appearing tumors and they are small I do not take deep muscle but if they are larger, I prefer obtaining muscle.
One concern I have is that if the biopsy report says no muscle is included in the biopsy one cannot comment on muscle invasion and patients and relatives and possibly other urologists might feel that you have performed an incomplete procedure despite knowing the expertise of the urologist. Therefore, if the patient goes for a second opinion they may be told they need additional investigation or a restaging TURBT in order to have a proper evaluation.
Juan Palou Redorta
Fundacio Puigvert, Barcelona, Spain
Case 1. The first TUR BT is a most important step in the management of the patient with bladder cancer. It is not infrequent to have a high grade tumor even though the tumor appears to be low grade and Ta. Therefore, it is better to have muscle in the specimen during the initial resection. If the patient had a negative cytology and a papillary tumor then muscle may not be needed.
Case 2. I would not have performed follow up cystoscopy so often. If the patient had a solitary low grade Ta tumor there should be cystoscopy at three months and then at one year. The NICE guidelines are to discontinue after one year if no subsequent tumor.
If the patient had a large new tumor, I would offer adjuvant intravesical chemotherapy.
After the HG T1a tumor I agree with BCG.
After the most recent TUR BT she could receive BCG or electromotive chemotherapy with mitomycin C with or without BCG.
In general, I prefer a TUR BT with loop resection for multifocal tumors.
I consider active surveillance for some patients with LG Ta small tumors but then cystoscopy every 6–12 months do reduce cost.
In elderly or frail patients with LG Ta I do nothing unless they have hematuria.
I use office cautery for LG Ta small papillary tumor recurrences.
In summary, if tumors are small and appear LG Ta and cytology is negative an aggressive TUR BT is not necessary. If there is any doubt about the grade and stage or a positive cytology it is better to have muscle in the specimen.
Robert Contieri
Humanitas Hospital, Milan, Italy
Case 1. For the initial diagnosis of a bladder tumor, I perform a transurethral resection in the operating room. If, as in case 1, the tumor is small and appears low-grade, and there is a urinary cytology which is negative for high grade urothelial cancer, I perform a “superficial” resection with no attempt at including muscle in the specimen.
Case 2. In the case of the patient with a prior history of low-grade Ta bladder cancer and a small recurrence I recommend active surveillance until the number of tumors or the size increases. When this occurs, I bring the patient to the operating room for a TURBT and utilize the en bloc method when possible. If my impression is that the tumor is low-grade Ta and the cytology is negative for high grade cancer, I will not perform a deep resection. I’m not concerned about including muscle. If a patient develops a high-grade tumor, I no longer offer active surveillance but prefer to perform a TURBT or cold cup biopsy and cauterization depending on the size and appearance of the tumor. In patients with HG Ta I prescribe BCG. In such patients I do not use active surveillance in the future but rely on a TUR BT for subsequent tumors.
Michael Stockle
University Hospital, Saarland Homborg, Germany
Case 1. When a patient sees me with gross hematuria the first diagnostic procedure is a renal and bladder ultrasound and urinary cytology. Ultrasound has a high sensitivity to detect larger bladder tumors and cytology will detect a high-grade bladder tumor. When I combine both modalities only small low-grade tumors remain undetected.
For many patients with suspicious findings by ultrasound or cytology I avoid outpatient cystoscopy and schedule them for a transurethral resection under anesthesia. I perform less outpatient cystoscopy than urologists in the United States because of the easy availability of ultrasound in our German system.
I do not perform bladder biopsies in the office. For patients with small papillary LG tumors, I utilize active surveillance. After almost 4 decades in urology, I’ve not been disappointed using this strategy.
When I identify small low-grade Ta appearing tumors who are not on active surveillance, I perform cold cup followed by cauterization. I feel that endoscopic evaluation is very reliable, and I avoid being more invasive. I never understood why some feel that a proper TUR BT for an obvious non invasive bladder cancer should require obtaining muscle in the specimen. I perform loop resection in most cases using an end bloc technique if there is a large tumor with a narrow base.
After a TUR BT of larger or multifocal bladder cancer I routinely recommend a second resection six weeks later or after a six-week course of BCG. It is surprising how many of these patients still have tumor after six weeks despite a “complete” resection having been performed. The majority of patients who are initially operated on for Ta bladder cancer remain with Ta tumor.
I almost never perform biopsy of a tumor in the office setting. I also do not use office cautery, but this is a concept I will consider in the future for small papillary recurrences. I do not use the laser.
Case Summary
Case 1. A man presented with two small papillary bladder tumors which appear to be low grade and confined to the urothelial surface, LG Ta.
Most of the responders performed an office evaluation which included a flexible cystoscopy and all of them removed the tumors in the operating room. The majority performed either cold cup biopsy followed by cautery or an en bloc resection to minimize cautery artifact and provide an optimal specimen for the pathologist. A few of the urologists try to obtain muscle in the specimen during the initial tumor resection.
Some emphasize the use of urinary cytology to support the visual information regarding the presence of cancer and the tumor grade. A few indicate the use of narrow band imaging or cysview to aid in a complete survey of the bladder.
Case 2. A patient with a long history of LG Ta bladder cancers. Only one of several tumor events had a tumor which had minimal lamina propria invasion.
Once again, most of the responders prefer a minimally invasive approach. Almost all of them use active surveillance or office cautery for patients with small LG Ta appearing tumor "recurrences". Most use a bugbee electrode. Two use a laser. Some perform a biopsy in the office but the majority do not.
I encourage the readers to read the individual responses as there are some important caveats in the management of LG Ta bladder cancer.
I am most appreciative of all of those who took the time to respond to my questions on the management of this common bladder tumor. It indicates that they are confidant they can predict the biological behavior of papillary low grade appearing tumors at the time of endoscopy and minimize the impact of their treatment in terms of extent of resection and in many cases avoid a standard TUR BT.
AUTHOR CONTRIBUTIONS
MSS contributed to the work conception, performance, analysis of data and writing; NAA contributed to performance and analysis of data.
INFORMED CONSENT
Written informed consent for publication was obtained from the patients.
ETHICAL CONSIDERATIONS
The author has institutional review board approval for reviewing bladder cancer cases for outcome (MHS.2020.026).
CONFLICT OF INTEREST
MSS and NAA have nothing to disclose.




