Effectiveness of Prolonged Antibiotic Prophylaxis in Radical Cystectomy: Preliminary Analysis of the MACS Randomized Clinical Trial
Abstract
BACKGROUND:
Standard 24-hour antibiotic prophylaxis is widely employed to minimize the risk of infection complications within 30 days following radical cystectomy. However, a considerable variety of protocols and drug combinations don’t prevent a high complication rate, ranging from 37 to 67%. This paper presents the interim analysis of the MACS clinical trial, comparing antibiotic prophylaxis regimens by duration.
OBJECTIVE:
To evaluate the rate of infection complications within 30 days following radical cystectomy by comparing standard 24-hour antibiotic prophylaxis (Group A) with a prolonged 120-hour regimen (Group B).
METHODS:
Patients were randomized in a 1 : 1 ratio. The primary endpoint was the evaluation of the frequency of infection complications. The secondary endpoints were the rate of re-administrating antibiotics and the dynamics of the inflammation biomarker.
RESULTS:
A total of 78 patients (85.0% of the sample size) were enrolled (Group A: 40 and Group B: 38). The baseline and perioperative features were balanced between groups. The overall complication rate was higher in Group A (65.0% vs. 41.1%, p = 0.043). The infection complication rate was 2.7 times higher in the standard antibiotic prophylaxis group: 37.5% compared to 18.4% cases in Group B (p = 0.041), and upper urinary tract infection was more frequent in Group A (22.5% vs. 2.6%). The prolonged antibiotic prophylaxis reduced the overall frequency of infection complications compared with standard 24-hour prophylaxis (RR = 0.12; 95% CI 0.02–0.88; p = 0.037).
CONCLUSIONS:
In this interim analysis, the administration of prolonged antibiotic prophylaxis over 120 hours appears to be safe and feasible, demonstrating a reduction in the total number of complications, particularly infection complications.
INTRODUCTION
Radical cystectomy (RC), employing various methods of urinary diversion, is the primary approach for treating very high-risk non-muscle invasive bladder cancer (NMIBC), as well as non-metastatic muscle-invasive bladder cancer (MIBC) [1, 2]. However, this operation is frequently associated with a substantial incidence of postoperative complications, notably urinary tract infections (UTIs) and surgical site infections, with reported rates up to 67% [3–4]. According to several authors, it may depend on the variant of urinary diversion. The UTI/septicaemia was the most common complication following RC, with an incidence of 90.4 per 1,000 person-years, and it depended on the types of urinary diversions. There was a higher risk of UTI among patients who had a continent cutaneous reservoir (HR: 1.11, 95% CI: 0.94–1.30) or an orthotopic neobladder (HR: 1.21, 95% CI: 1.05–1.39) compared to those with an ileal conduit [5]. In other meta-analysis, the incidence of UTI was significantly lower in patients with an ileal conduit compared to those with an orthotopic neobladder (26.1% vs. 30.4%, respectively; OR: 0.67, 95% CI: 0.58–0.77, p < 0.01) [6]. Consequently, the prevention of healthcare-associated infections emerges as a pivotal focus within internal control measures aimed at ensuring the quality and safety of medical activities.
Antibiotic prophylaxis (AP) is a prominent approach in the management of antimicrobial therapy within hospital environments, with the goal of mitigating the risk of postoperative or systemic infection by employing systemic antibiotics during the perioperative period [7]. In alignment with clinical recommendations and principles of evidence-based medicine, the initial antibiotic dose should be administered 30–60 minutes before the commencement of the surgical procedure, and the time window for antibiotic administration is confined to 24 hours following the RC (standard AP) [7].
On the other hand, the choice of AP often depends on the surgeon’s preferences, bacterial culture, or local practices. The Kransnow et al. (2017) investigated AP protocols in 8,351 patients undergoing RC across 353 hospitals in the United States. It was reported that 579 unique antibiotic regimens were prescribed on the day of the operation. Furthermore, only 15.0% of patients received AP in accordance with recommendations, while 51.0% of patients were administered 2 or more antibiotics [8]. In a retrospective analysis by Ross et al. (2021), after controlling for BMI, ASA, and type of urinary diversion this study demonstrated that increased length of antibiotic prophylaxis was not associated with the frequency of ICs (RR = 0.99, 95% CI 0.50–1.99) in their cohort [9].
Atduev et al. (2019) demonstrated a shift in the paradigm of RC from “conditionally contaminated” to “highly contaminated” surgery through pre- and post-operative intra-abdominal bacterial cultures: intra-abdominal lavage fluid at the end of RC revealed various types of microorganisms in 54.4% of cases. In a factor analysis aimed at identifying predictors of any infectious complication, intraoperative contamination of the abdominal cavity was found to be a significant factor (OR 3.4; 95% CI: 1.742–6.637; p < 0.001). Moreover, in one group of patients receiving carbapenems (OR = 0.306), complications of grades III–V developed three times less frequently than in the groups of patients receiving protected penicillins (OR = 1) and third-generation fluoroquinolones (OR = 0.932). Therefore, the prescription of antibiotics should be regarded not as prevention, but as therapy and carried out with broad-spectrum antibacterial drugs, in accordance with the principles of rational antibiotic therapy [10].
The obtained data cast doubt on the contemporary choice of antimicrobial agents (AMAs), the regimen, and the duration of AMA administration limited to only 24 hours after RC. The justification for using the type of AP in RC is to reduce the frequency of ICs, with the secondary goal being the reduction of unwarranted and excessive antibiotic use. Despite published clinical recommendations for general surgical procedures, clinical practice varies within one country and even within urological facilities in one city [7]. The above facts emphasize the necessity of prospective research on the effectiveness of AP regimens in RC. Therefore, the aim of this study is to assess the risk of developing ICs depending on the length of the AP regimen.
METHODS
Trial design
The Multicentral Preventive Antibiotics with Cystectomy within Enhanced Recovery after Surgery (MACS) is a prospective, randomized, multicenter phase III study assessing the efficacy of the standard 24-hour AP regimen (Group A) compared to the prolonged regimen over 120 hours (Group B) as a measure for reducing the incidence of postoperative ICs within 30 days following RC. The study was registered at ClinicalTrials.gov (NCT05392634).The enrolled patients were randomly assigned in a 1 : 1 ratio to receive different types of AP. Group A received standard AP where either a broad-spectrum semi-synthetic penicillin (Amoxicillin/clavulanic acid 1.2 g) or a second-generation cephalosporin antibiotic (Cefuroxime 1.5 g) was administered parenterally 30 minutes before incision. In cases of surgery duration over 4 hours or intraoperative blood loss exceeding ≥1.5 liters, an additional dose of the antibiotic was administered. The duration of perioperative AP in this group was no more than 24 hours, and extension of therapy was not allowed. In Group B, prolonged AP utilized the synthetic antibiotic Meropenem (1 g every 8 hours for 5 days). This prolonged regimen was based on a previous retrospective study which showed carbapenem course was associated with lower infectious complication rates [10]. It was not allowed antibiotics prior to the post-op ureteral stent removal.
Eligibility criteria for participants
Participants of both genders > 18 years old with morphologically confirmed diagnoses of very high-risk NMIBC or non-metastatic MIBC, an Eastern Cooperative Oncology Group (ECOG) performance status 0–1, and an intent-to-treat RC with one of the appropriate variants of urinary diversion (heterotopic Mainz-I, Bricker derivation, or orthotopic J- or U-pouch) were enrolled in the study. Patients were ineligible if they had severe or uncontrolled concomitant chronic diseases, demonstrated individual intolerance to the antibacterial drugs specified in the study protocol, or exhibited two or more criteria of systemic inflammatory response syndrome at the time of hospitalization (temperature ≥38 °C; heart rate ≥90/min; respiratory rate < 20/min; blood leukocytes > 12 · 109/L; inadequate bone marrow, liver, or kidney function, as revealed by laboratory tests taken 10–14 days before the operation), creatinine clearance > 50 ml/min. Written informed consent was obtained from all patients before randomization. All research centers were to use the enhanced recovery after surgery protocol. RC was performed by laparoscopic approach with intracorporeal urinary diversion.
Outcome measures
The selection of primary and secondary endpoints was in accordance with the recommendations of the Combatting Bacterial Resistance in Europe (COMBACTE) project [11].
The primary endpoint was an assessment of the frequency of ICs within 30 days following RC comparing the standard 24-hour AP to the prolonged 120-hour regimen (event rate). Secondary endpoints were: the dynamics of biomarkers of systemic inflammatory response: C-reactive protein (CRP), systemic immune-inflammatory index (SII); the frequency of re-administration of any antibiotics during hospitalization (antibiotic re-administration rate).
Reporting and grading of all complications after RC was in accordance with EAU Guidelines Panel Assessment and Recommendations (Clavien-Dindo system preferred) [12]. Data on complications were systematically gathered by a designated data manager from each research center, irrespective of the surgeon’s diagnosis, within the 30 days following RC. Infection-related complications, which were the focus of clinical interest, included a symptomatic UTIs, sepsis, and surgical site infections. CRP levels (mg/L) were also measured on the 1st, 3rd, and 9th day after RC. The systemic immune-inflammatory index (SII) was determined on the 1st and 14th day after RC using the formula: platelet count · 109/L×(neutrophil count · 109/L ÷ lymphocyte count · 109/L). In both groups, urine culture was collected intraoperatively before skin incision with bladder catheterization in sterile conditions (sample 1), after stent/catheter removal (sample 2).
Statistical analysis
The planned sample size of 92 patients was estimated using an inequality test for 2 independent proportions (“Sample Size Calculators” UCSF CTSI, Kohn MA, Senyak J.). Type I error was 5%, and type II error was 20% [13, 14]. This preliminary analysis (85% out of the sample size) was conducted in accordance with the research schedule and requirements of the foundation. Data collection from research centers and the randomization process (block type) were performed using the “REDCap aggregator 12.2.3” (Vanderbilt University, USA). Patients were stratified based on two criteria: the administration of neoadjuvant therapy (platinum-containing chemotherapy, immunotherapy, targeted therapy; absence of therapy) and the type of urinary diversion (orthotopic; heterotopic derivation). All analyses were performed in accordance with the intention-to-treat principle.
Quantitative indicators were assessed for normal distribution using the Shapiro-Wilk test or the Kolmogorov-Smirnov test; comparison was performed using the t-test. Categorical data were described with absolute values and percentages for comparison using Pearson’s chi-square test and Fisher’s exact test. Statistical analysis was conducted using StatTech v. 4.0.5, Russia. The P value < 0.05 was considered significant.
RESULTS
Trial population
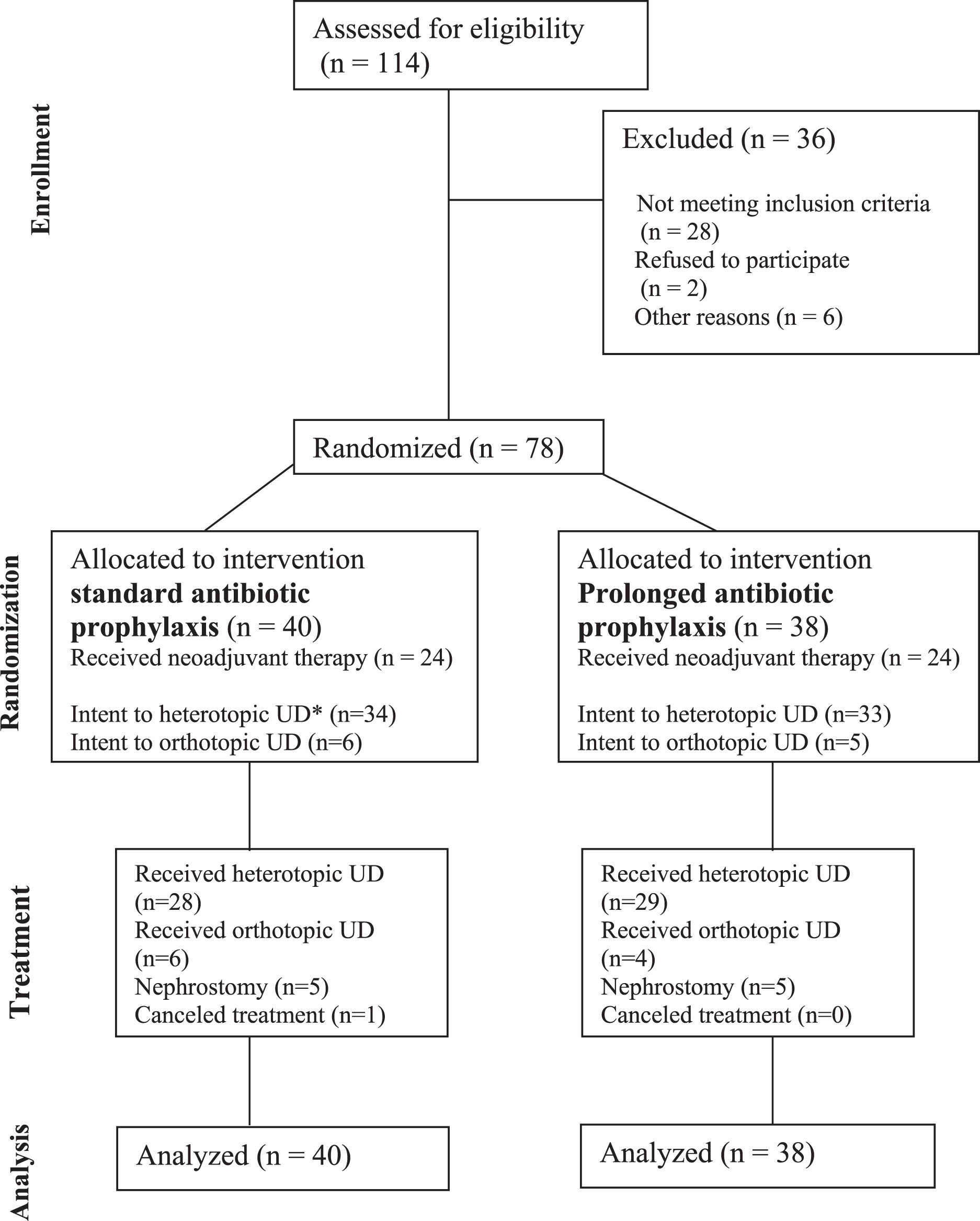
From May 2022 to December 2023, 78 patients underwent randomization (85% out of the sample size) and were assigned to receive RC with urinary diversion with AP. Forty patients were included in Group A (standard AP), and 38 patients in Group B (prolonged AP). The patient enrollment, randomization, follow-up, and reasons for exclusion are shown in Fig. 1.
Fig. 1
Study design.
The study groups were well-balanced in terms of gender, BMI, prior neoadjuvant treatment, the TNM stage, and the presence of previously established drains (nephrostomy drainage, epicystostomy). However, it was observed that Group B had a higher prevalence of older patients, with an average age of 67.4±8.2 (64.7–70.1) years, compared to 62.8±8.8 (59.9–65.6) years in Group A (p = 0.020) (see Table 1). Neoadjuvant therapy was performed in 48/78 cases (61.5%): platinum-based chemotherapy was predominantly prescribed in 46/78 (59.0%) of cases, and 2/78 (2.5%) patients received a PD-1 inhibitor antibody.
Table 1
Baseline characteristics of included patients
| Features | Standard AP (group A) n = 40 | Prolonged AP (group B) n = 38 | P value | |
| Local centers | Center #1, abs. (%) | 31 (77,5) | 29 (76,3) | 0,888 |
| Center #2, abs. (%) | 5 (12,5) | 4 (10,5) | ||
| Center #3, abs. (%) | 4 (10,0) | 5 (13,2) | ||
| Male gender, abs. (%) | 37 (92,5) | 35 (92,1) | 1,0 | |
| Age M±SD (95% CI), years | 62,77±8,80 (59,96–65,59) | 67,37±8,2 (64,67 + 70,06) | 0,020 | |
| BMI M±SD (95% CI) | 27,35±4,56 (25,89–28,81) | 26,56±(25,26–27,87) | 0,422 | |
| ECOG status, abs. (%) | ||||
| 0 | 34 (85,0) | 32 (84,2) | 1,0 | |
| 1 | 6 (15,0) | 6(15,8%) | ||
| Availability of drainage**, abs. (%) | 7 (17,5) | 7 (18,4) | 1,0 | |
| Previous treatment | Neoadjuvant therapy, abs. (%) | 24 (60,0) | 24 (63,2) | 0,774 |
| – GC***, abs. (%) | 21 (52,5) | 23 (60,5) | 0,475 | |
| – MVAC***, abs. (%) | 2 (5,0) | 0 (0,0) | 0,494 | |
| – immunotherapy***, abs. (%) | 1 (2,5) | 1 (2,6) | 1,0 | |
| – treatment at the stage of muscle-invasive cancer (BCG) abs. (%) | 2 (5,0) | 2 (5,3) | 1,0 | |
| Urinary diversion (intent-to-treat) | ||||
| – heterotopic, abs. (%) | 34 (85,0) | 33 (86,8) | 1,0 | |
| – orthotopic, abs. (%) | 6 (15,0) | 5 (13,2%) | ||
| Histological variant | ||||
| – pure type, abs. (%) | 36 (90,0) | 36 (94,7) | 0,676 | |
| – variant histology, abs. (%) | 4 (10,0) | 2 (5,3) | ||
| cT | Ta/is, abs. (%) | 4 (10,0) | 3 (7,9) | 0,890 |
| T1, abs. (%) | 5 (12,5) | 7 (18,4) | ||
| T2, abs. (%) | 14 (35,0) | 15 (39,5) | ||
| T3, abs. (%) | 14 (35,0) | 10 (26,3) | ||
| T4, abs. (%) | 3 (7,5) | 3 (7,9) | ||
| CN | CN0, abs. (%) | 36 (90,0) | 33 (86,8) | 0,734 |
| cN1, abs. (%) | 4 (10,0) | 5 (13,2) | ||
| cM | cM0, abs. (%) | 40 (100,0) | 37 (97,4) | 0,487 |
| cM1, abs. (%) | 0 (0,0) | 1 (2,6) |
**-presence of installed drainages (nephrostomy) due to obstructed urine outflow; ***GC regimen gemcitabine + cisplatin, MVAC-methotrexate, vinblastine, doxorubicin, cisplatin; Immunotherapy in clinical trial; The data was present for all variables in all patients.
Most surgeries were performed using the minimally invasive laparoscopic approach: in Group A in 39/40 (97.8%) and in Group B in 36/38 (94.7%) cases (p = 0.610). According to fast-track principles, the drainage of the abdominal cavity or pelvis was used only in every fourth patient (Table 2). The groups were balanced with respect to the planned type of urinary diversion (intent-to-treat): 34/40 (85.0%) in Group A and 33/38 (86.8%) in Group B (p = 1.0) were planning heterotopic urinary diversion. When assessing postoperative indicators (as treated), no statistically significant differences were found (p = 0.796). It is noteworthy that 10 patients (12.8%) underwent surgery without a reconstructive-plastic component, involving the placement of nephrostomy drainage (5/40 cases in Group A and 5/38 cases in Group B), and in 1 (1.3%) case in Group A, the treatment volume was changed to external beam radiotherapy due to technical difficulties. Surgical treatment in the standard AP group was supplemented with pelvic lymphadenectomy in 100% of cases, while in the prolonged AP group, lymphadenectomy was not performed in one case. The average number of removed lymph nodes did not differ between the groups (p = 0.066). Other characteristics are detailed in Table 2.
Table 2
Perioperative features of included patients
| Features | Standard AP (group A) n = 40 | Prolonged AP (group B) n = 38 | P value |
| Characteristics of the surgical stage of treatment | |||
| Laparoscopic access, abs. (%) | 39 (97,5) | 36 (94,7) | 0,610 |
| Bloodloss M±SD (95% CI), ml | 347,75±306,30 (249,79–445,71) | 402,37±300,83 (303,49–501,25) | 0,430 |
| Duration of operation M±SD (95% CI), minutes | 307,75±102,60 (268,94–334,56) | 304,47±101,62 (271,07–337,88) | 0,907 |
| Drainage of the abdominal cavity or pelvis, abs. (%) | 9 (23,1) | 9 (24,3) | 1,0 |
| Lymphnode dissection, abs. (%) | 40 (100,0) | 37 (97,4) | 0,487 |
| Standard, abs. (%) | 6 (15,0) | 3 (8,1) | 0,490 |
| Extended, abs. (%) | 21 (52,5) | 18 (48,6) | |
| Super extended, abs. (%) | 13 (32,5) | 16 (43,2) | |
| Number of lymph nodes removed, M±SD (95% CI) | 19,07±8,16 (16,46–21,69) | 22,51±8,0 (19,85–25,18) | 0,066 |
| Number of positive lymph nodes, M±SD (95% CI) | 1,38±2,97 (0,43–2,32) | 1,79±3,93 (0,50–3,08) | 0,600 |
| Urinary diversion (as treated) | |||
| Heterotopic, abs. (%) | 28 (70,0) | 29 (76,3) | 0,796 |
| Orthotopic, abs. (%) | 6 (15,0) | 4 (10,5) | |
| Other types*, abs. (%) | 6 (15,0) | 5 (13,2) | |
| Pathological characteristics | |||
| Histological variant | |||
| – pure type, abs. (%) | 36 (90,0) | 34 (89,5) | 1,0 |
| – variant histology, abs. (%) | 4 (10,0) | 4 (10,5) | |
| Incidental prostate cancer, abs. (%) | 17 (42,5) | 19 (50,0) | 0,507 |
| Positive surgical margin**, abs. (%) | 4 (10,0) | 2 (5,3) | 0,676 |
| ypT0, abs. (%) | 6 (15,0) | 11 (28,9) | 0,031 |
| pT1, abs. (%) | 13 (32,5) | 4 (10,5) | |
| pT2a, pT2b, abs. (%) | 3 (7,5) | 10 (26,3) | |
| pT3a, abs. (%) | 3 (7,5) | 4 (10,5) | |
| pT3b, abs. (%) | 8 (20,0) | 7 (18,4) | |
| pT4a, abs. (%) | 4 (10,0) | 3 (7,9) | |
| pT4b, abs. (%) | 3 (7,5) | 0 (0,0) | |
| pN0, abs. (%) | 29 (72,5) | 29 (76,3) | 0,914 |
| pN1, abs. (%) | 4 (10,0) | 5 (13,2) | |
| pN2, abs. (%) | 5 (12,5) | 4 (10,5) | |
| pN3, abs. (%) | 2 (5,0) | 1 (2,6) | |
*Other – operation was not performed (1 case) or the operation was completed by removing the ureterocutaneostomy or applying a nephrostomy drainage; positive surgical margin of the ureters, urethra, or invasion into the pelvic wall; The data was present for all variables in all patients.
Primary outcome
The total number of complications in the standard AP group was 65.0% vs. 42.1% into prolonged AP group (p = 0.043). The relative risk (RR) of developing any adverse event was lower in the prolonged AP group by 35.0%; however, no statistical significance of the influence of the AP duration was detected (RR = 0.65, 95% CI 0.42–1.00, p = 0.05).
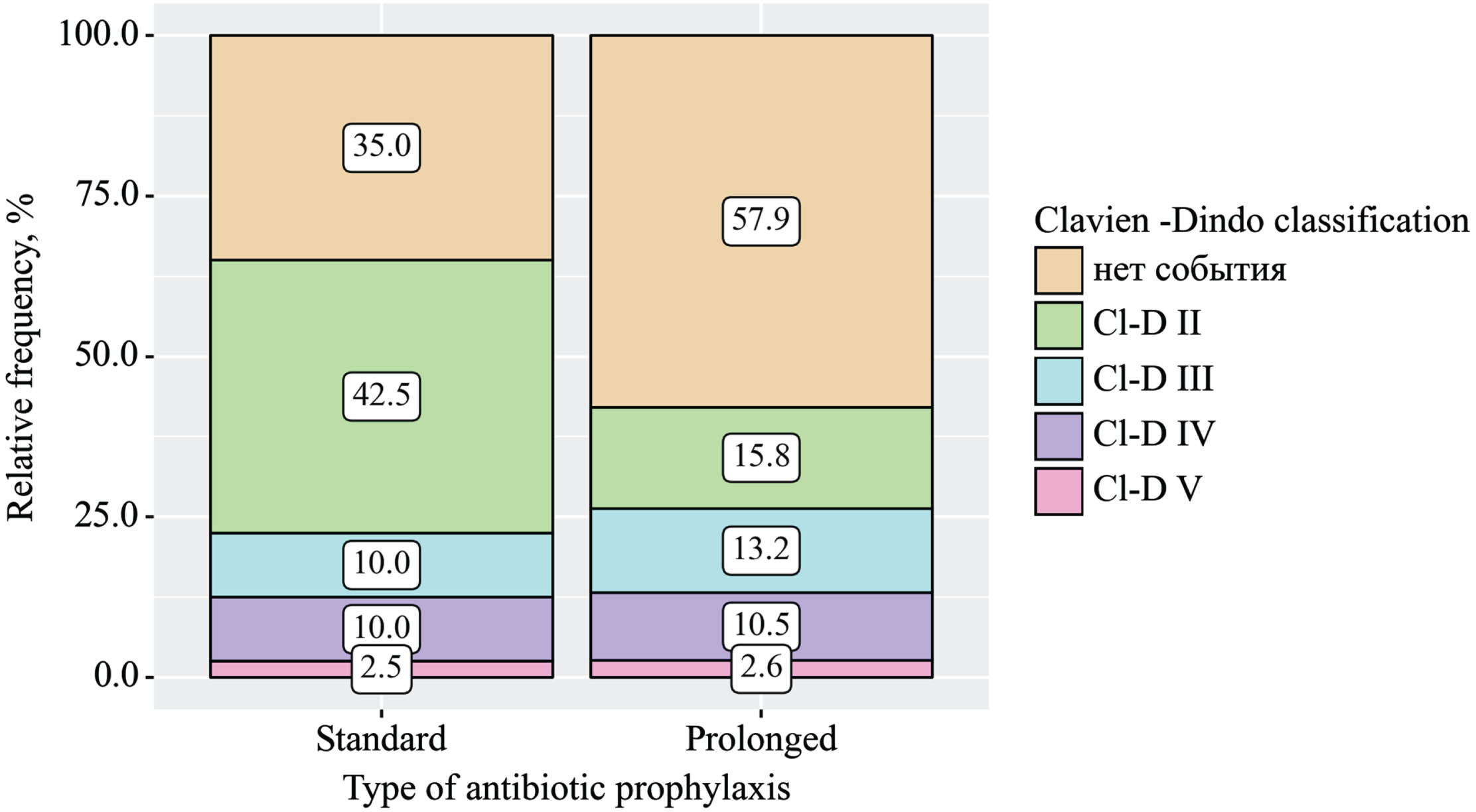
The frequency of ICs was 2.7 times higher in the standard AP group, with 15 cases (37.5%), compared to 7 cases (18.4%) in the prolonged AP group (Pearson’s chi-square test p = 0.041). When assessing the impact of the AP duration on the frequency of infectious complications, a significant deviation in favor of Group B was found (RR = 0.30; 95% CI 0.15–0.60; p < 0.001). The severity of complications according to the Clavien-Dindo classification did not differ between the groups (p = 0.134) (Table 3).
Table 3
Complications in the period 30 days after radical cystectomy
| Features | Antibiotic prophylaxis | p | ||
| Standard | Prolonged | |||
| Complications in the period 30 days after radical cystectomy (any types), abs. (%) | No events | 14 (35,0) | 22 (57,9) | 0,043 |
| Events | 26 (65,0) | 16 (42,1) | ||
| Events of clinical interest, abs. (%) | No | 25 (62,5) | 31 (81,6) | 0,041 |
| Yes | 15 (37,5) | 7 (18,4) | ||
| Clavien-Dindo classification, abs. (%) | No events | 14 (35,0) | 22 (57,9) | 0,131 |
| Cl-D II | 17 (42,5) | 6 (15,8) | ||
| Cl-D III | 4 (10,0) | 5 (13,2) | ||
| Cl-D IV | 4 (10,0) | 4 (10,5) | ||
| Cl-D V | 1 (2,5) | 1 (2,6) | ||
The data was present for all variables in all patients.
Postoperative complications varied between groups, p < 0.001 (Fig. 2A) but according to the Clavien-Dindo system, differences were not revealed (p = 0.131) (Fig. 2B). Among events of clinical interest, symptomatic UTIs were more frequently identified in the standard AP group – in 22.5% of cases (9/40), and wound infection in 7.5% (3/40). In the prolonged group, UTIs were identified in only 2.6% (1/38) of cases, and no cases of wound infection were reported. At the same time, there were 2 cases (5.3%) of subcutaneous incisional hernia in the prolonged group. When assessing the impact of the AP duration on the UTI frequency, a significant deviation in favor of prolonged AP was found (RR = 0.12; 95% CI 0.02–0.88; p = 0.037). Among other complications, different frequencies of therapeutic complications such as thrombosis, lung infarction pneumonia, and COVID-19 infection were observed. In the standard AP group, only 7/40 cases (17.5%) were identified, but no significant influence of AP on the risk of developing this type of complication was found (RR = 0.07, 95% CI 0.01–1.19; p = 0.065).
Fig. 2A
Variants of complications within 30 days after radical cystectomy depending on the type of antibiotic prophylaxis (p < 0.001) (Pyelonephritis as symptomatic UTI; internal disease- other non-infectious complications (for example, heart attack, pulmonary embolism)).
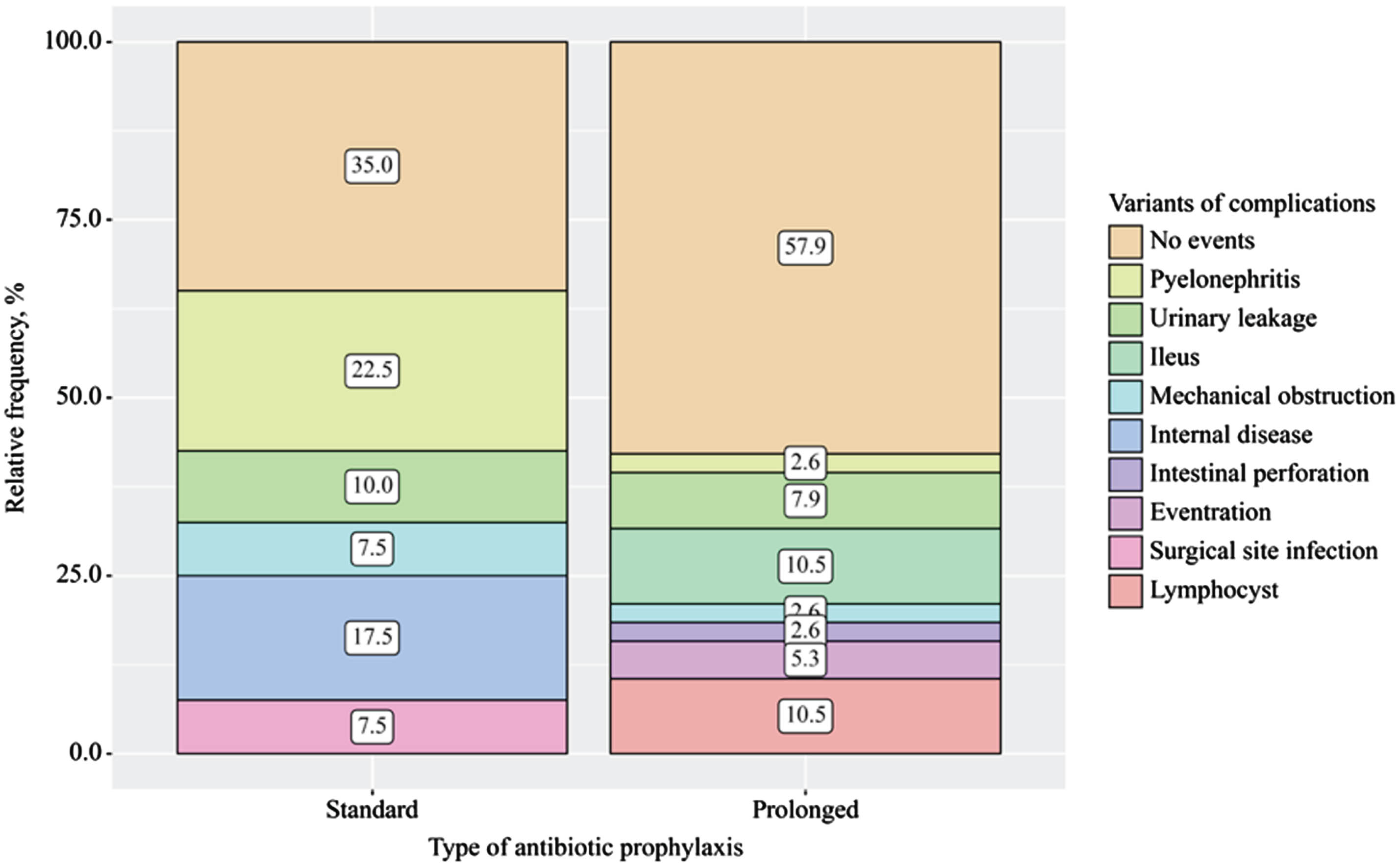
Fig. 2B
Reporting all complications after radical cystectomy in accordance with EAU Guidelines Panel Assessment and Recommendations (Clavien-Dindo system).
Reoperation was required for 17 patients. Indications for surgery included wound dehiscence (2/2.6%), wound infection (3/3.4%), anastomotic leak (1/1.3%), intestinal obstruction (4/5.2%), and uretero-ileal anastomotic leak (6/7.8%). The 30-day mortality did not differ between the groups (p = 1.0).
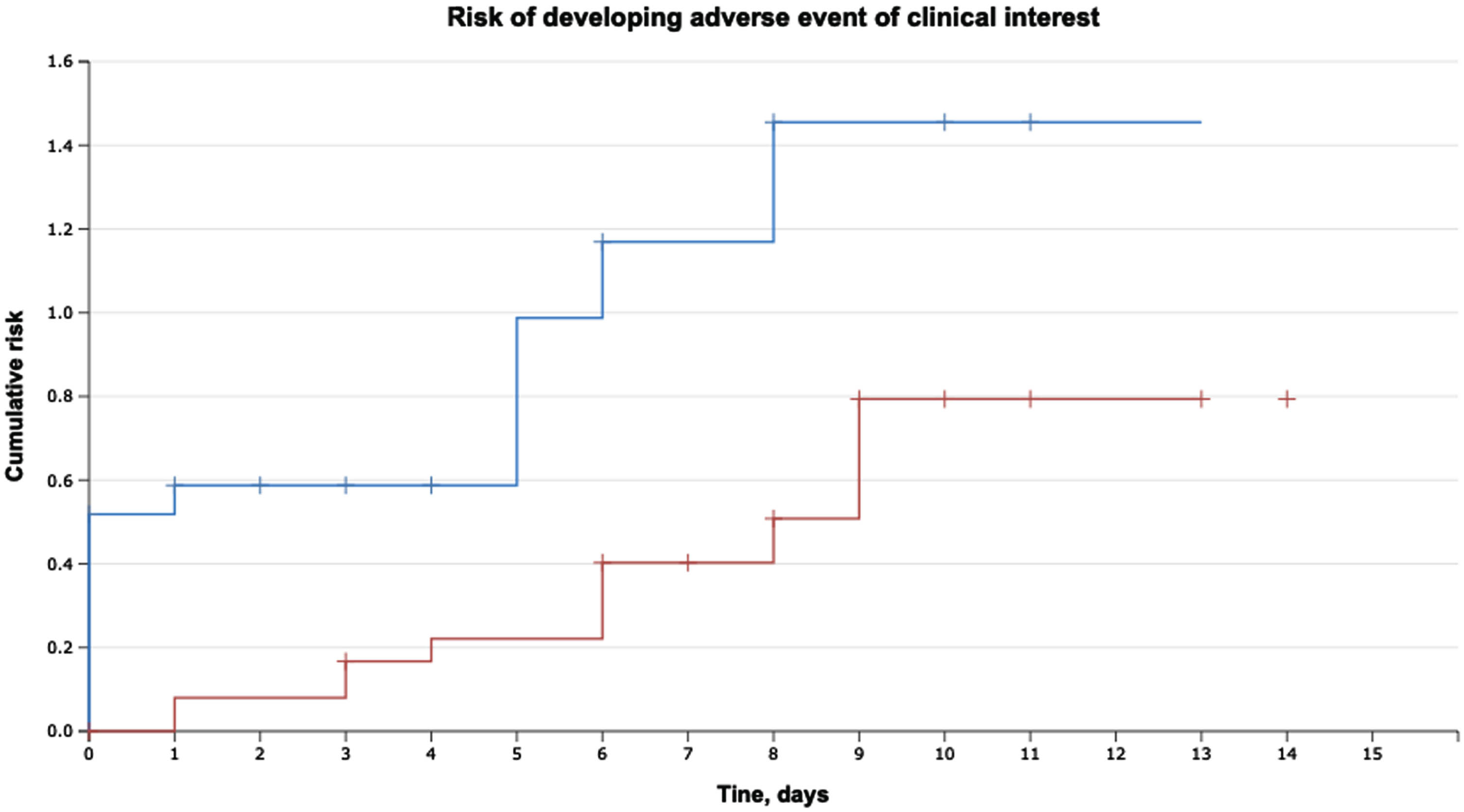
The median time to the development of any complication was 1.0 (0.0–6.0) days. The evaluation of the time to the development of events of clinical interest (ICs) within 30 days following RC showed that the hazard ratio was 2.5 when the ratio of median lengths for standard group and prolonged AP groups was 4.5/9.4 days (HR = 2.5; 95% CI 0.21–0.75; p = 0.002) (see Fig. 3).
Fig. 3
Time to the development of events of clinical interest (event-free survival) within 30 days following RCwith urinary diversion (p = 0.002).
Secondary outcomes
The antibiotic re-administration rate within 30 days following RC in both groups showed that additional antibiotic therapy was required in 45.0% of cases (18/40) in the standard AP group, while in the prolonged AP group, it was needed in only 21.1% of cases (8/38). The use of the prolonged regimen for 120 hours reduced the risk of additional administration of another antibiotic by 54.0% (RR = 0.46, 95% CI 0.23–0.94; p = 0.034).
When analyzing the level of CRP on the 1st, 3rd, and 9th day after RC, a significant deviation from the upper limit of the norm (>5 mg/l) was noted in all cases. At the same time, an increase in CRP up to 100.57 (73.99–132.25) mg/l was observed from the first day for the category of patients who subsequently developed any complication (p < 0.001). The greatest increase from day 1 in CRP was observed in the group of patients with subsequent development of pyelonephritis 108.10 (79.64–124) mg/l and incisional hernia 115.42 (79.64–124.0) mg/l (p = 0.007). The level of CRP elevation depending on the AP group is shown in Table 4. It was found that the level of CRP was lower in the prolonged AP group on the 1st and 3rd days (p = 0.020 and p = 0.026), and no difference was found on day 9 (p = 0.352).
Table 4
Results of 30-day postoperative follow-up
| Features | Standard AP (group A) n = 40 | Prolonged AP (group B) n = 38 | P value |
| Length of hospitalization, Me (Q1–Q3), days | 19 (13.00–21.2) | 17 (13.25–21.75) | 0.802 |
| 30-days mortality, abs. (%) | 1 (2.5) | 1 (2.6) | 1.0 |
| Removal of ureteral stents, M±SD (95% CI), days | 9.3±6.76 (7.14–11.46) | 10.68 (7.92–13.44) | 0.424 |
| Removal of the urethral catheter*, M±SD (95% CI), days | 21.80±12.68 (6.06–37.54) | 20.20±7.92 10.37–30.03 | 0.817 |
| Re-administration antibiotics rate, abs. (%) | 18 (45.0) | 8 (21.1) | 0.025 |
| SII*** index 1-st day after RC, Me (Q1–Q3) | 531.05 (292.25–1311.97) | 557.50 (295.73–1115.53) | 0.791 |
| SII index 14-th day after RC, Me (Q1–Q3) | 1139.40 (865.80–1637.88) | 983.10 (608.12–1596.03) | 0.219 |
| CRP**** 1st day, Me (Q1–Q3), mg/l | 89.6 (74.58–119.3) | 54.05 (39.6–99.11) | 0.020 |
| CRP 3ds day, Me (Q1–Q3), mg/l | 172.25 (136.44–278.68) | 143.74 (97.75–219.47) | 0.026 |
| CRP 9th day, Me (Q1–Q3), mg/l | 60.05 (34.65–77.36) | 47.31 (18.01–103.55) | 0.352 |
| Time to development of an event of clinical interest M±SD (95% CI), days | 4.44±0.76 (2.9–5.9) | 9.4±0.99 (7.5–11.4) | p=0.002 HR = 0.39 95% CI (0.21–0.75) |
| Positive bacterial culture, sample 1, abs. (%) | 15 (37.5) | 12 (31.6) | 0.583 |
| Positive bacterial culture, sample 2, abs. (%) | 20 (50.0) | 11 (28.9) | 0.048 HR=0.57 95% CI (0.32–1.04, p = 0.067) |
*Urethral catheter was used only for orthotopic urinary diversion, n = 10; **the drug was prescribed on demand due to the development of an undesirable event (complication). ***SII-systemic immune inflammation index; ****CRP-C-reactive protein.
Considering the diagnostic significance of CRP on the 1st and 3rd days, the probability of developing complications depending on the CRP level was assessed. The prediction of complication development was based on the criterion of reaching a CRP level on the first day ≥89.6 mg/l, with sensitivity and specificity of the model being 61.9% and 80.6%, respectively (p < 0.001). The ROC analysis of the probability of complications based on the CRP level on the 1st day and the type of AP revealed a significant relationship (p = 0.020). In the context of using prolonged AP, a CRP level ≥77.0 mg/l on the 1st day following RC served as an indicator for the future development of ICs. The model demonstrated a sensitivity of 68.4% and a specificity of 70.0%.
The systemic immune inflammation index was also assessed on the 1st and 14th days following RC (Table 4). No significant differences of SII index were revealed between the 1st and 14th day depending on the AP group (p = 0.791 and p = 0.219, respectively). However, the highest level of SII was recorded on the 1st day in cases of future incisional hernia – 1858.65 (1440.9–2276.3) and wound infection – 1327.30 (765.1–2830.1). When assessing the probability of developing complications within 30 days after RC based on the SII measured on the 1st day, ROC analysis was 0.542±0.066 (95% CI 0.414–0.671), which did not turn out to be statistically significant (p = 0.521).
According to the results of bacterial cultures, positive sample 1 was in 35.0% (27/78) of screened patients (see Table 4), both gram-positive and gram-negative. There were no significant differences in the frequency of bacterial infection of the urinary tract before surgery between the groups (p = 0.583); positive sample 2 in the standard AP group was in 50.0% of the data was present for all variables in all patients.
Cases (20/40), and in the prolonged therapy group, in 28.9%. The relative risk of significant bacterial growth in sample 2 was lower by 43.0% in the group using carbapenems for 120 hours (RR = 0.57, 95% CI 0.32–1.04, p = 0.067), but not statistically significant. There were no cases of Cl. Defficile.
DISCUSSION
This prospective randomized trial was based on previous observation study Atduev et al. (2019) dedicated to evaluating the role of bacterial contamination (BC) of the abdominal cavity during RC. BC was emerged as a significant predictor of postoperative ileus and infectious complications (OR 3.4; 95% CI: 1.742–6.637, p < 0.001). The findings demonstrate that RC is considered a contaminated and “dirty” procedure, and in an experiment (n = 32), the effectiveness of carbapenems in reducing the frequency of complications of grades 3–4 was demonstrated (not a single case was reported). Meanwhile, in the early postoperative period, in the groups receiving protected penicillin (40.2%) and third-generation fluoroquinolones (32.2%), the need for antibiotic change due to clinical ineffectiveness was quite common [10].
In this prospective multicenter randomized study, it was observed that despite the administration of prophylactic antibiotics before RC with urinary diversion, patients experienced ICs. The prolonged 120-hour regimen of AP resulted in a 70.0% reduction in the risk of ICs, including UTIs and surgical site infections (RR = 0.30; CI 95% 0.15–0.60; p < 0.001). The ICs hazard ratios indicate 2.5-fold faster time to event of clinical interest in the standard AP group (p = 0.002). The analysis of the inflammatory marker revealed that the CRP level can be considered an effective diagnostic tool for the preliminary assessment of the risk of clinically significant complications, but SSI levels after surgery cannot be used as markers for predicting the development of complications. These data have direct practical applications.
After intestinal urinary diversion, a key challenge is the risk of postoperative infections. In long-term studies on RC outcomes, UTIs were common within the first 3 months post-surgery [15, 16]. Recent research by Kim et al. (2018) compared ICs rate in 185 cases RC in a prolonged AP group (25-day use of 3-staged multiple antibiotics) versus results of 102 patients in standard 24-hour group (use of cefotetan). The rates of short UTIs within 60 days were similar (28.6% vs. 28.4%, p = 0.969), and bacteremia rates were comparable (p = 0.415). According to this retrospective research, short-term AP effectively prevents UTIs and does not require the use of a combination of three or more antibacterial drugs [17].
In a study by Numau et al. (2020), comparing intraoperative-only vs. prolonged antibiotic duration (median 3 days), the overall IC rate was 44%. There was no significant difference between AP groups (25.0% vs. 34.0%, p = 0.23). All ICs were linked to the lower estimated glomerular filtration rate (eGFR) (p = 0.023) [18]. Lower eGFR correlated with a higher IC rate, likely associated with tumor advance, urinary outflow obstruction, nephrostomic drainage, and chronic bacteriuria, though these factors were not individually analyzed.
The effectiveness of AP is even less studied among elderly patients (>65 years old), despite 90% of NMIBC being identified in patients over 55 years old. According to the systematic review by Ahmed (2017), prolonged AP demonstrated a 24% reduction in the risk of recurrent UTIs for elderly female patients (RR = 0.76; 95% CI 0.61–0.95). Importantly, no statistically significant increase in the risk of adverse events was noted (RR 1.52; 95% CI 0.76–3.03) [19]. The medium age in this study was 65.01±8.7 (37–82) years. On the contrary, a decrease in age below the average was associated with an increased risk of a clinical event occurring: RR 0.91 (95% CI: 0.85–0.98; p = 0.010). This fact underscores the necessity of determining indications or contraindications for the length of AP in the elderly patient population.
Among systemic inflammation parameters, baseline CRP was independently associated with 30-day clinically significant bacterial infections in some large trials. According to our results, a CRP level ≥89.6 mg/l on the first day had a prognostic role for ICs (p < 0.001). Given that CRP is already widely available in clinical routine, it could be rapidly used as a prognostic marker, which represents levels of systemic inflammation. We recommend that serum CRP can be quickly integrated into surgical complications risk assessment of RC to optimize monitoring strategies.
This study has several limitations. A major flaw of this study is that Group A and Group B vary both by the duration of antibiotic administration and by type of antibiotic. Therefore, it cannot be concluded that prolonged administration of antibiotics (and not simply the type of antibiotic) improves infectious complications. Furthermore, this trial was not large enough to demonstrate the clinical benefit of prophylactic antibiotics in terms of preventing ICs after RC in patients with urinary diversion, but not enough to demonstrate the preventive benefits in subgroup analysis. In 14.1% of patients, the stated volume of urinary diversion was not performed, which could subsequently distort the results of surgical treatment and reduce the expected incidence of complications. Although patients with previously installed drainages (nephrostomy drainage, epicystostomy) were included at the same frequency, the impact of this factor on the incidence of ICs was not investigated. Additionally, the microbiome in urine samples from these patients and the microbiome in the case of the development of an IC were not studied. Nevertheless, since all registered patients underwent RC with urinary diversion at large clinical centers, allowing for standardized interventions, the potential impact of surgical experience on the incidence of complications was not taken into account. It is worth noting that in one center, which contributed 60% of the enrolled patients, the surgeon’s experience exceeded 500 cystectomies over 10 years, a factor that could potentially introduce a bias to the results.
Conclusion
In this preliminary analysis, the administration of prolonged (over 120 hours) instead of standard (24 hours) antibiotic prophylaxis appears to be safe and feasible, demonstrating a reduction in the total number of complications, particularly infection complications. Our results conflict with the existing guidelines suggesting AP with semi-synthetic penicillin or second-generation cephalosporin antibiotics, by the way, impact of additional factors, such as an early postoperative recovery program (ERAS) program, was not assessed. Contrarily, hospitals involved in this study focused on implementing such a program. This circumstance represents an intriguing prospect, prompting the initiation of a meta-analysis to investigate also the effect of the ERAS principles on the incidence of infection complications.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The Foundation for Cancer Research Support (“RakFond”), Moscow, Russian Federation.
AUTHOR CONTRIBUTIONS
N.A.K., G.T.Yu., B.M.V.: conception and design. B.A.M., N.A.K., G.T.Yu. administrative support. B.M.V., T.N.I., R.S.A.: provision of patients. B.M.V., T.N.I., R.S.A.: collection and assembly of data. B.M.V. data analysis and interpretation. All authors: manuscript writing. All authors: Final approval of the manuscript.
CONFLICTS OF INTEREST
All antibiotics used in this trial were provided by hospitals, and this trial was designed and conducted independently of any pharmaceutical company.
B.M.V., B.A.M., G.T.Yu., T.N.I., R.S.A. and N.A.K. have no conflicts of interest to report.
ETHICAL CONSIDERATIONS
This study was approved by The Local Ethics Committee of the FSBI “NMRC of oncology N.N. Petrov” MH of the Russian Federation (No. 1/129 on April 28, 2022). All patients gave their written informed consent.
Trial registration in clinical trials: NCT05392634.
DATA AVAILABILITY
The trial protocol and deidentified participant data collected for this trial are available from the corresponding author B.M.V. on reasonable request.
REFERENCES
[1] | Witjes JA , Bruins HM , Cathomas R , Compérat EM , Cowan NC , Gakis G , Hernández V , Linares Espinós E , Lorch A , Neuzillet Y , Rouanne M , Thalmann GN , Veskimäe E , Ribal MJ , van der Heijden AG . European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol. (2021) ;79: (1):82–104–10.1016/j.eururo.2020.03.055. Epub 2020 Apr 29. PMID: 32360052. |
[2] | Babjuk M , Burger M , Capoun O , Cohen D , Compérat EM , Dominguez Escrig JL , Gontero P , Liedberg F , Masson-Lecomte A , Mostafid AH , Palou J , van Rhijn BWG , Rouprêt M , Shariat SF , Seisen T , Soukup V , Sylvester RJ . European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). . Eur Urol. (2022) ;81: (1):75–94.10.1016/j.eururo.2021.08.010. |
[3] | Lawrentschuk N , Colombo R , Hakenberg OW , Lerner SP , Månsson W , Sagalowsky A , Wirth MP . Prevention and management of complications following radical cystectomy for bladder cancer. Eur Urol. (2010) ;57: (6):983–1001.10.1016/j.eururo.2010.02.024. |
[4] | Nosov AK , Reva SA , Dzhalilov IB , Petrov SB . Radical cystectomy for bladder cancer: Comparison of early surgical complications during laparoscopic, open-access and videoassisted surgery. Cancer Urology. (2015) ;11: (3):71–8 (In Russ.)]. https://doi.org/10.17650/1726-9776-2015-11-3-71-78. |
[5] | van Hemelrijck M , Thorstenson A , Smith P , Adolfsson J , Akre O . Risk of in-hospital complications after radical cystectomy for urinary bladder carcinoma: Population-based follow-up study of 7608 patients. BJU Int. (2013) ;112: (8):1113–20.10.1111/bju.12239. Epub 2013 Jul 26. |
[6] | Browne E , Lawrentschuk N , Jack GS , Davis NF . A systematic review and meta-analysis of the long-term outcomes of ileal conduit and orthotopic neobladder urinary diversion. Can Urol Assoc J. (2021) ;15: (1):E48–57.10.5489/cuaj.6466. |
[7] | Lightner DJ , Wymer K , Sanchez J , Kavoussi L . Best Practice Statement on Urologic Procedures and Antimicrobial Prophylaxis. J Urol. (2020) ;203: (2):351–6.10.1097/JU.0000000000000509. |
[8] | Krasnow RE , Mossanen M , Koo S , Kubiak DW , Preston MA , Chung BI , Kibel AS , Chang SL . Prophylactic Antibiotics and Postoperative Complications of Radical Cystectomy: A Population Based Analysis in the United States. J Urol. (2017) ;198: (2):297–304.10.1016/j.juro.2017.02.3340. |
[9] | Ross JPJ , Breau RH , Vigil H , Hickling DR , Angel JB , Mallick R , Cagiannos I , Morash C , Lavallée LT . Association between radical cystectomy prophylactic antimicrobial regimen and postoperative infection. Can Urol Assoc J. (2021) ;15: (12):E644–51.10.5489/cuaj.7158. |
[10] | Atduev VA , Gasrataliev VE , Ledyaev DS , Shevelev IS , Belsky VA . Predictors of postoperative complications of radical cystectomy. Oncology, Journal im. P.A. Herzen. (2019) ;(T. 8 (5)):348–57 (In Russ.)]. |
[11] | Timsit JF , de Kraker MEA , Sommer H , Weiss E , Bettiol E , Wolkewitz M , Nikolakopoulos S , Wilson D , Harbarth S . COMBACTE-NET consortium. Appropriate endpoints for evaluation of new antibiotic therapies for severe infections: A perspective from COMBACTE’s STAT-Net. Intensive Care Med. (2017) ;43: (7):1002–12.10.1007/s00134-017-4802-4. |
[12] | Mitropoulos D , Artibani W , Graefen M , Remzi M , Rouprêt M , Truss M . European Association of Urology Guidelines Panel. Reporting and grading of complications after urologic surgical procedures: An ad hoc EAU guidelines panel assessment and recommendations. Eur Urol. (2012) ;61: (2):341–9.10.1016/j.eururo.2011.10.033. |
[13] | Hulley SB , Cummings SR , Browner WS , Grady DG , Newman TB . Designing Clinical Research: An Epidemiologic Approach (2nd ed.). Philadelphia: Lippincott, Williams & Wilkins. (2013) . p. 75. |
[14] | Fleiss JL , Tytun A , Ury HK . A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics. (1980) ;36: (2):343–6. |
[15] | Hautmann RE , de Petriconi RC , Volkmer BG . 25 years of experience with 1,000 neobladders: Long-term complications. J Urol. (2011) ;185: , 2207–12. |
[16] | Mano R , Baniel J , Goldberg H , et al.. Urinary tract infections in patients with orthotopic neobladder. Urol Oncol. (2014) ;32: :e9–14. |
[17] | Kim CJ , Kim KH , Song W , Lee DH , Choi HJ . Impact of a change in duration of prophylactic antibiotics on infectious complications after radical cystectomy with a neobladder. Medicine (Baltimore). (2018) ;97: (47):e13196.10.1097/MD.0000000000013196. |
[18] | Numao N , Fujiwara R , Uehara S , Yasuoka S , Fujiwara M , Komai Y , Yuasa T , Yamamoto S , Fukui I , Yonese J . Intraoperative Only versus Extended Duration Use of Antimicrobial Prophylaxis for Infectious Complications in Radical Cystectomy with Intestinal Urinary Diversion. Urol Int. (2020) ;104: (11-12):954–9.10.1159/000509881. |
[19] | Ahmed H , Davies F , Francis N , Farewell D , Butler C , Paranjothy S . Long-term antibiotics for prevention of recurrent urinary tract infection in older adults: Systematic review and meta-analysis of randomised trials. BMJ Open. (2017) ;7: (5):e015233.10.1136/bmjopen-2016-015233. |




