A Procedural Checklist for Transurethral Resection of Bladder Tumors (TURBT) Enhances Operative Dictation and Assesses Surgeon Accuracy of Tumor Characteristic Predictions
Abstract
BACKGROUND:
A lack of standardization is pervasive in procedural application and reporting templates for TURBT with the use of a surgical checklist proposed as a means for quality improvement.
OBJECTIVE:
To introduce a TURBT checklist to assess surgeon prediction accuracy and the impact of standardized documentation on quality of resection and oncologic outcomes
METHODS:
Nine critical elements of a high-quality TURBT identified by literature review were incorporated into a prospectively implemented checklist for operative reports. The checklist included both visualized and predicted tumor characteristics. A retrospective single-institution analysis compared quality of dictation pre- and post-checklist implementation. Surgeon predictions were compared to final pathology reports to determine rates of concordance. Kaplan-Meier curves examined the association of checklist use with recurrence free survival (RFS).
RESULTS:
333 operative reports were included in this analysis, of which 107 (32.1%) were completed pre-checklist implementation. The average number of critical elements reported was 8.69 with checklist use compared to 4.99 without (p < 0.001). There was no significant difference in RFS between the pre- and post-checklist cohorts (log-rank test p = 0.53). Surgeons were least and most accurate in predicting low grade tumor (43.5%) and absence of muscle invasion (96.6%), respectively.
CONCLUSIONS:
Incorporation of a TURBT surgical checklist improves operative dictation and quality of reporting but did not directly impact RFS. With quality of initial resection a proven correlate to recurrence rates, checklist implementation to improve surgical performance and long-term oncologic outcomes reveals an interesting area of exploration highlighting the need for more standardized methodology when performing these procedures.
INTRODUCTION AND OBJECTIVE
Bladder cancer is the 10th most commonly diagnosed cancer worldwide with data projecting an increasing trajectory in recent years. Over 70–80% of those diagnosed with bladder cancer are found to have noninvasive disease (NMIBC), for which standard of care remains transurethral resection of bladder tumor (TURBT) [1, 2]. Despite generally low mortality rates for NMIBC, rates of intravesical recurrence may be up to 60% with multiple recurrences not uncommonly occurring throughout a lifetime [3]. As such, patients with NMIBC are often subject to repeat TURBTs as part of their disease course with increasing consequence of morbidity and overall healthcare expenditure [4].
Quality of initial TURBT has been shown to have significant impact on tumor recurrence rates, ranging from 1–43% [2]. Moreso, characteristics such as resection completeness and detrusor muscle presence in specimen have been demonstrated to have notable influence on these rates [5]. In the first study of its kind, Anderson et al noted that the use of an intra-operative surgical checklist improved reporting of critical procedural elements in a real-time setting [6]. Subsequent studies have reported similar results, with use of the checklist thought to increase awareness of existing deficiencies in operative documentation [7].
At the time of initial diagnosis, a TURBT obtains pathologic specimen to confirm presence of carcinoma as well as evaluate depth of invasion for staging. Overall management decisions are heavily influenced by surgeon perception of intra-operative pathologic features, making the ability to accurately predict tumor characteristics paramount. During a primary encounter, surgeon prediction of tumor features directs intraoperative method of specimen harvesting ranging from cold cup biopsy to wide resection with deep muscle sampling, with the risk of bladder injury incrementally increasing with each swipe. Re-staging TURBTs are recommended for cases in which detrusor muscle was absent in specimen, initial resection was thought to be incomplete, or a T1 tumor was detected [1]. Additionally, the need for immediate post-operative intravesical chemotherapy is primarily determined by intra-operative prediction of disease stratification as low risk [8]. In cases of extensive tumor involvement, intra-operative goals of maximal resection may be weighed against potentially devastating outcomes such as bladder perforation and risk of bleeding if muscle-invasion is suspected with potential immediate cystectomy a consideration. Finally, surgeon perception of tumor characteristics is critical to post-procedure counseling of presumptive disease course—which may unfold expeditiously pending final pathology.
At present, a lack of standardization is pervasive in both procedural application as well as reporting templates for TURBT with current literature proposing the use of a surgical checklist as means towards quality improvement with potential to enhance oncologic outcomes. Small analyses have shown that surgical checklists are feasible and may improve reporting of critical procedural elements [9–11]. However, as existing data is both sparse and variable, we sought out to create and implement a surgical checklist to validate its utility in improving quality of TURBT and general reporting, as well as assess its impact on recurrence-free survival (RFS). Utilizing this checklist, we further aimed to evaluate surgeon aptitude in identifying a variety of tumor features during TURBT.
METHODS
In accordance with our Institutional Review Board (#2023-15147), a literature review was performed consisting of primary studies investigating key TURBT elements that are highly correlated with cancer-specific and safety end goals. These key elements were selected based on current guidelines and expert opinions, including characteristics needed to denote disease risk (tumor size, quantity, characteristics, presence of CIS), disease staging, quality of resection (visualization of detrusor muscle in resection bed) and post-operative complications. Nine critical elements of a high-quality TURBT identified by literature review were incorporated into a prospectively implemented checklist for operative reports. The checklist included tumor status, use of blue light, and exam under anesthesia findings, as well visualized tumor characteristics such as status, number, size, location, and appearance. Surgeon predictions of grade, stage, presence of muscle in specimen, muscle invasion, and presence of carcinoma-in-situ (CIS) were also included. These elements were then compiled into user-friendly checklist that was integrated into the EPIC electronic medical record (EMR) to be utilized voluntarily when dictating operative reports. All patients treated for NMIBC with TURBT were included in the study. Patients with upper tract disease were excluded.
Starting 4/5/2021, surgeons were encouraged to use the checklist when documenting TURBTs.
This retrospective study was approved by our Institutional Review Board. Patients who had received TURBTs between 5/29/2019 and 8/24/2022 were identified by our billing department using the following CPT codes: 52204, 52214, 52224, 52234, 52235, and 52240. Date of TURBT was used to categorize patients as pre-checklist or post-checklist based on date of checklist implementation. Retrospective review of electronic medical records (EMR) was conducted to confirm receipt of TURBT, collect demographic and clinical data, and assess use of checklist.
We abstracted the following demographic data for each patient: age, sex, BMI, and tumor incidence (new, restaging, recurrent). Operative notes were used to identify the attending surgeon, predicted tumor grade (benign, low, high), predicted tumor stage (benign, Ta, Tis, T1, T2/T3), checklist use (yes or no), and documentation of individual checklist items (yes or no). Pathology reports were used to determine pathologic tumor grade, pathologic tumor stage, presence of carcinoma in situ (CIS), and presence of muscle in biopsy sample. Checklist predictions were compared to final pathology reports to determine overall prediction accuracy. The results were further stratified by provide practice volume (<6 TURBTs/year, 6–11 TURBTs/year, 12–17 TURBTS/year, and 18+ TURBTs/year) to identify the impact of experience on prediction accuracy.
Descriptive analysis of all categorical variables was performed using Pearson’s chi-square tests. Comparison of column proportions between the pre- and post-checklist cohorts was performed using two-sided z-tests. Comparison of checklist prediction accuracy between various provider practice volume levels using Fisher’s exact tests. Kaplan-Meier curves were used to represent the association of TURBT checklist use on recurrence free survival in patients. Survival analysis was limited to patients with NMIBC for whom at least 2 years of follow-up data was available. The log-rank rest was used to assess for significant differences in recurrence-free survival. Multivariate cox-regression models were created to identify variables associated with tumor recurrence. Time to recurrence was defined as the number of days from initial TURBT to subsequent pathology confirmed tumor recurrence. For patients with no pathology confirmed recurrence, follow-up was censored to the date of their most recent negative cystoscopy. Patients who had no follow-up visits after initial TURBT were excluded from the survival analysis. All analyses were performed using SPSS Version 27 (IBM, Chicago, Illinois). All tests were two-sided, and P-value was set at <0.05 to determine statistical significance.
RESULTS
Pre and post checklist documentation
Between 5/29/2019 and 8/24/2022, a total of 451 TURBTs (107 pre-checklist vs. 344 post-checklist) were performed. In the post-checklist implementation era, the compliance rate was 50.1%, with 226 TURBTs utilizing the checklist thus meeting inclusion criteria for further analysis in this study (Table 1). Between the two cohorts, we found no significant difference between patient age, gender, tumor incidence, pathologic grade, pathologic stage, or completeness of resection. There was a significant difference in biopsy method (p < 0.001); specifically between the percentage of bipolar loop (57.9% pre-checklist vs. 82.7% post-checklist, p < 0.001) and monopolar loop biopsies (12.1% pre-checklist vs. 1.3% post-checklist, p < 0.001).
Table 1
Comparison of cohort characteristics and checklist elements between pre- and post-checklist TURBTs
| Pre-Checklist Implementation (%) | Post-Checklist Implementation (%) | P-Value | |
| Number of Cases | 107 | 226 | |
| Mean Age at Surgery | 71.8 | 70.2 | 0.22 |
| Gender | |||
| Male | 77 (72.0) | 153 (67.7) | 0.43 |
| Female | 30 (28.0) | 73 (33.3) | 0.43 |
| Tumor Incidence | |||
| New | 36 (33.6) | 82 (36.3) | 0.64 |
| Recurrent | 63 (58.9) | 113 (50.0) | 0.13 |
| Restaging | 8 (7.5) | 31 (13.7) | 0.10 |
| Pathologic Tumor Stage | |||
| T0 | 28 (26.1) | 48 (21.2) | 0.42 |
| Ta | 41 (38.3) | 94 (41.6) | 0.57 |
| Tis | 5 (4.7) | 12 (5.3) | 0.81 |
| T1 | 18 (16.8) | 47 (20.8) | 0.39 |
| T2/3 | 15 (14.0) | 25 (11.1) | 0.44 |
| Pathologic Tumor Grade | |||
| Benign | 33 (30.8) | 54 (24.9) | 0.18 |
| Low | 23 (21.5) | 42 (18.6) | 0.53 |
| High | 51 (47.7) | 130 (57.5) | 0.09 |
| Biopsy Method | |||
| Cold Cup | 16 (15.0) | 34 (15.0) | 0.98 |
| Bipolar Loop | 62 (57.9) | 187 (82.7) | <0.001 |
| Monopolar Loop | 13 (12.1) | 3 (1.3) | <0.001 |
| Both | 0 (0.0) | 2 (0.9) | – |
| Not Documented | 12 (11.2) | 0 | – |
| Completeness of Resection | |||
| Bladder Biopsy | 18 (16.8) | 23 (10.2) | 0.09 |
| Complete Resection | 63 (58.9) | 141 (62.4) | 0.54 |
| Complete Resection w/ Likely Residual Tumor | 20 (18.7) | 62 (27.4) | 0.08 |
| Subcomplete Resection | 5 (4.7) | 0 (0.0) | – |
| Undocumented | 1 (0.9) | 0 (0.0) | – |
| Documentation of checklist Items | |||
| Tumor Size | 69 (64.5) | 219 (96.9) | <0.001 |
| Tumor Location | 105 (98.1) | 220 (97.3) | 0.66 |
| Number of Tumor Lesions | 70 (65.4) | 210 (92.9) | <0.001 |
| Blue Light Use | 25 (23.4) | 226 (100.0) | <0.001 |
| Tumor Characteristics (e.g. flat, sessile, nodular, etc.) | 66 (61.7) | 219 (96.9) | <0.001 |
| Resection Completeness | 68 (63.6) | 225 (99.6) | <0.001 |
| Biopsy Method | 91 (85.0) | 226 (100.0) | <0.001 |
| Visualization of Muscle | 35 (32.7) | 194 (85.8) | <0.001 |
| EUA | 5 (4.7) | 225 (99.6) | <0.001 |
The post-checklist cohort had an increase in the average number of key TURBT elements documented (4.99 pre-checklist vs. 8.69 post-checklist, p < 0.001) (Table 1). When comparing the pre-checklist vs. post-checklist cohorts, we also observed a significant increase in the percentage of notes that documented tumor size (65% vs. 97%, p < 0.001), number of tumor lesions (65% vs. 93%, p < 0.001), blue light use (23% vs. 100%, p < 0.001), tumor characteristics (62% vs. 97%, p < 0.001), resection completeness (64% vs. 99.6%, p < 0.001), biopsy method (85% vs. 100%, p < 0.001), visualization of muscle (33% vs. 86%, p < 0.001), and EUA (4.7% vs. 100%, p < 0.001). There was no significant difference in documentation of tumor location (98% vs. 97%, p = 0.66), and presence of muscle in specimen (61% vs. 63%, p = 0.71).
Surgeon prediction accuracy
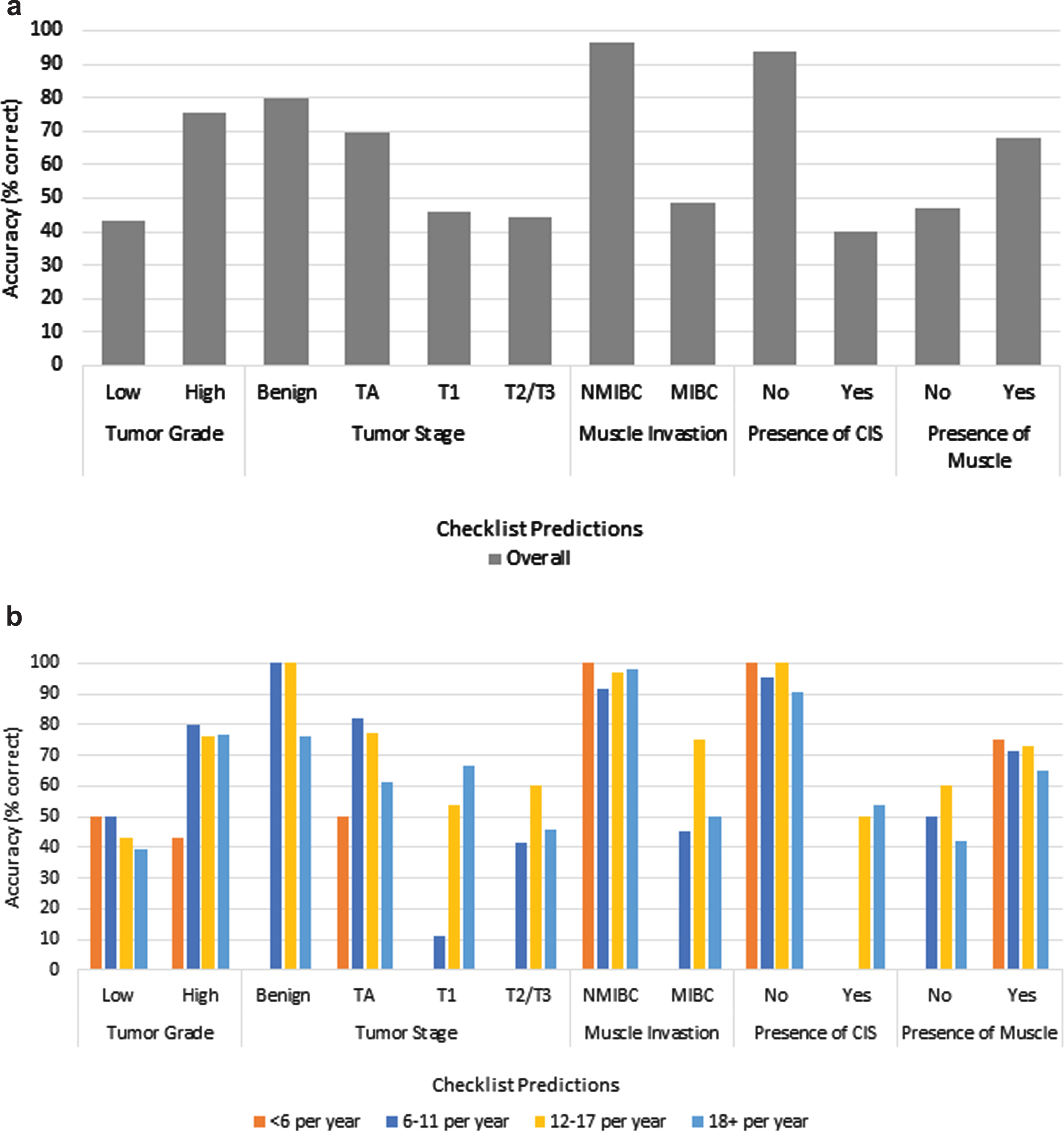
TURBT operative checklist predictions for 226 cases were compared to pathology reports to determine surgeon prediction accuracy. TURBTs were performed by 15 different surgeons with 3 performing <6 TURBTs per year, 6 performing 6–11, 3 performed 12–18, and 1 surgeon performing 18+ per year. Rates of accuracy for predicting tumor characteristics ranged widely (Fig. 1). Overall, surgeons were least accurate in predicting low grade tumor (43.5%) and most accurate in predicting absence of muscle invasion (96.6%). Increased surgical volume was only significantly associated with an increased ability to accurately identify T1 tumors (18+ vs. 12–18 vs. 6–11 vs. <6:66.7% vs. 53.8% vs. 11.1% vs. 0%, P = 0.01). Surgeons overall accurately predicted the absence of CIS (93.9%) and benign pathology (80.0%).
Fig. 1
a) Overall surgeon checklist accuracy. b) Comparison of surgeon checklist accuracy based on practice volume.
Survival analysis
A subset of 61 patients (47 no-checklist, 14 yes-checklist) met inclusion criteria for survival analysis. Two-year recurrence free survival was 51.1% and 21.4% in the no-checklist and yes-checklist groups, respectively. Compared to patients who had the checklist used during their initial TURBT, no-checklist patients had a longer median survival time (Fig. 2), however, the difference was not statistically significant (462 days vs. 301 days, log-rank P = 0.09). Multivariable cox-regression model controlled for age at diagnosis and sex revealed no significant difference in recurrence based on tumor incidence, checklist use, initial tumor grade, initial tumor stage, or provider practice volume (Table 2). Surgeon experience did not have a statistically significant effect on recurrence rates.
Fig. 2
Kaplan-Meier curve comparing the effect of checklist use (no vs. yes) at diagnosis on time to tumor recurrence for new patients.
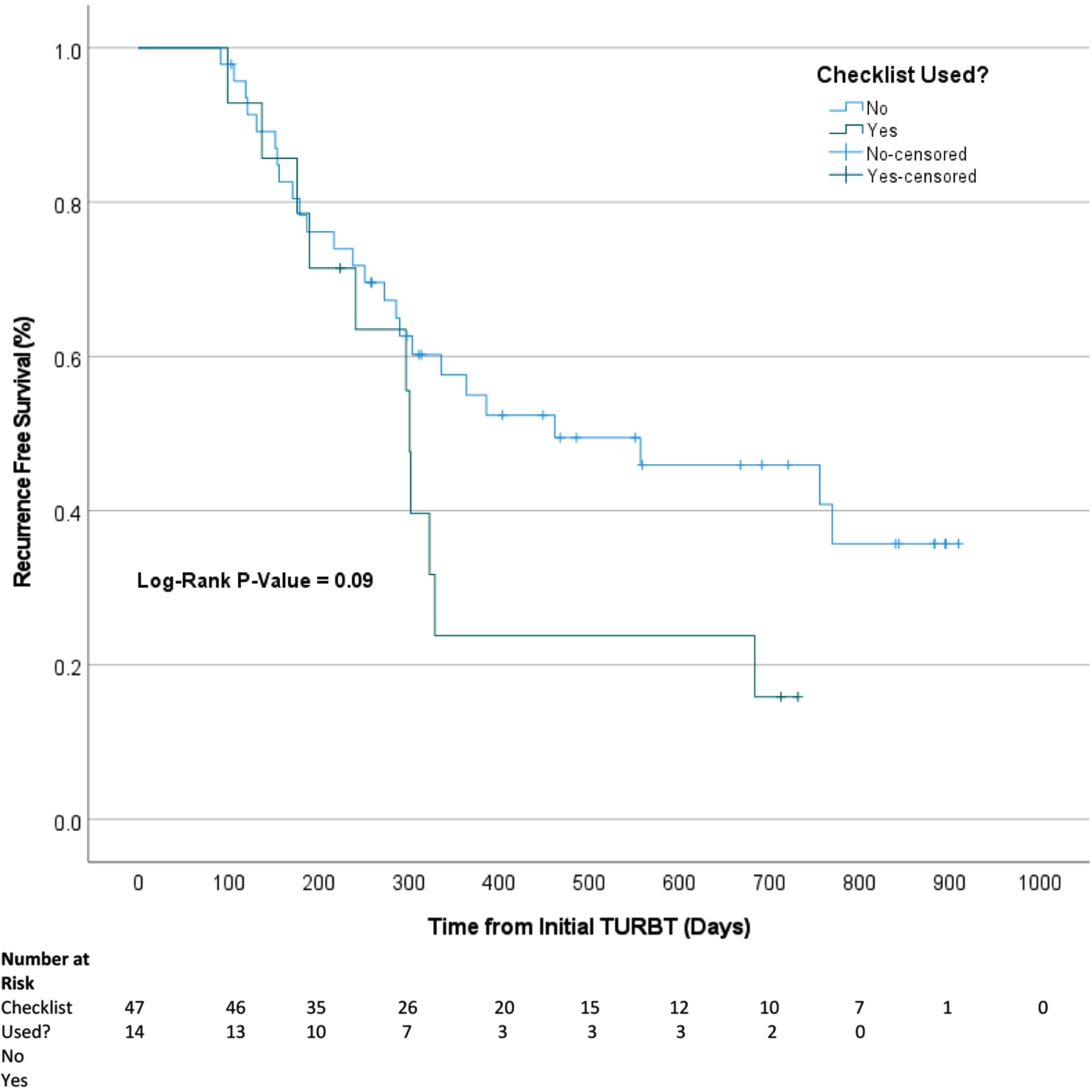
Table 2
Multivariate cox-regression model for tumor recurrence
| HR [95% CI] | P-Value | ||
| Sex | Male (N = 44) | Ref | |
| Female (N = 17) | 1.34 [0.66–2.74] | 0.42 | |
| Tumor Incidence | New (N = 25) | Ref | |
| Recurrent (N = 36) | 1.86 [0.92–3.77] | 0.08 | |
| Checklist Used | No (N = 47) | Ref | |
| Yes (N = 14) | 1.92 [0.93–3.99] | 0.08 | |
| Initial Tumor Grade | Low (N = 23) | Ref | |
| High (N = 38) | 1.05 [0.51–2.13] | 0.90 | |
| Initial Tumor Stage | Ta (N = 40) | Ref | |
| T1 (N = 15) | 1.42 [0.63–3.20] | 0.40 | |
| Tis (N = 6) | 0.77 [0.23–2.57] | 0.67 | |
| Provider Practice Volume | 6 –11 per year (N = 26) | Ref | |
| 12 –18 (N = 12) | 0.75 [0.30–1.97] | 0.58 | |
| 18+ per year (N = 23) | 0.92 [0.44–1.93] | 0.82 |
Model controlled for sex and age at diagnosis. HR = Hazard ratio, CI = Confidence interval, Ref = Reference.
DISCUSSION
The main facets of TURBT are obtaining histologic diagnosis, performing pathologic staging, and conducting a complete resection for either curative intent or optimization of future therapy. Albeit generally common and presumed to be routine procedure, quality of resection is widely variable and is dependent on surgeon experience. Poletajew et al cited a flat learning curve with 100 cases of experience the absolute minimum to obtain acceptable oncological and surgical outcomes [12]. In a striking study by Adiyat et al. 70% of patients were noted to have an initial incomplete resection, with about a third found to have macroscopic residual tumor at prior resection site [9]. The importance of performing a high-quality TURBT has been highlighted throughout the literature in reducing recurrence rates of NMIBC, with a wide range of variation in recurrence rates postulated to be secondary to differences in surgeon skill and experience [10]. As such, quality control and improvement has been an important area of burgeoning interest [11, 13]. Optimization of surgical technique is thought to improve quality of initial resection and overall care in patients undergoing TURBT, with the implementation of a surgical checklist previously utilized for this measure [11, 14]. Pan and Soloway compiled a comprehensive checklist incorporating pre-, intra-, and post-operative critical elements to enhance the performance of TURBT, although not tested in clinical practice [15].
Completeness of resection and presence of detrusor muscle in specimen are considered to be key prognostics of a high-quality TURBT. Interestingly, our study found that the utilization of checklist did not affect the presence of detrusor muscle in specimen. In a similar study, an increase in documentation from 5 to 8 items in the checklist was associated with a 2.9% increase in the probability of muscle presence in specimen, albeit without any statistical significance [6]. Consistent differences pre- and post- intervention in presence of muscle have not been identified across the current breadth of literature [16, 17]. This may be attributed to the specific attention this entity receives by surgeons intra-operatively as its reporting is critical to initial staging. Consideration must also be had regarding whether muscle was intentionally not obtained, as its absence has been shown to have a negligible role on recurrence rates for patients with low-grade Ta disease, a vast majority of those diagnosed with bladder cancer [18].
Our findings also revealed no significant difference in RFS with use of checklist during initial diagnostic TURBT, echoing prior findings by Kikuchi et al. [19]. Like Kikuchi et al., we also observed that tumor incidence, grade, and stage did not significantly affect recurrence. However, Suarez-Ibarrola et al were the first to report surgical checklist implementation to be independently associated with improved RFS, positing that enhancing surgical standardization and operative reporting can indeed improve oncologic outcomes. Notably, the improvement in RFS was not associated with presence of muscle in specimen, as no difference was identified with checklist use in this regard [20]. A more recent similar study identified a direct correlation between checklist use and RFS, suggesting a potential therapeutic benefit [21]. Moreso, the number of reported items in utilization of the checklist has been independently associated with significant improvement in RFS [22]. Although use of a surgical checklist has been demonstrated to improve oncologic outcomes, its success at increasing muscle in specimen remains to be proven, with Suh and Ku calling for improvements to correlate justification of quality of resection [23].
Of note, exam under anesthesia (EUA) had the starkest improvement in documentation from 4.7% to 99.6% with checklist implementation, highlighting significant underreporting albeit a guidelines-recommended critical element of evaluation. This finding was consistent with previous literature reporting similar drastic increases between pre- and post-checklist implementation [6]. This under-reporting raises the question of whether EUAs are simply not being documented, or may be potentially overlooked and not performed at all.
In this single-center analysis, urologists were noted to be highly accurate in predicting non-muscle invasive disease during TURBTs. The ability to identify high grade tumor, Ta disease, and the presence of muscle in specimen was also fairly accurate, albeit with increased margin of error. As such, preliminary risk assessment and stratification may be reliably performed to discern next best steps in management. The overall quality of TURBT is closely tied to surgeon perception of completeness of resection and presence of muscle in specimen, with significant bearings on patient disease course and overall prognosis. This study found that surgeons completing the checklist at our institution were 68% accurate in predicting the presence of muscle in specimen, with no significant difference between the low- and high-volume surgeon cohorts. Our findings are in concordance with Vale et al. citing 75% accuracy in prediction of TURBT resection depth into detrusor muscle, with the authors also reporting good but limited predictive ability in the detection of NMIBC and high-grade disease [24]. Adequate surgeon accuracy in distinguishing between benign and malignant lesions has previously been described, with more specific characteristics such as stage and grade shown to not be as well-predicted [8, 25]. In a study primarily investigating newly diagnosed patients, higher rates of accuracy in prediction of low grade (93.4%) and muscle-invasive disease (85.2%) were found [26].
A recent prospective cohort study found significant differences found between senior and junior surgeons regarding the presence of muscle in initial resection [27]. Moreso, 36.2% of patients operated on by experienced surgeons had residual tumor at re-TURBT when compared to 47.3% in those operated on by junior surgeons [27]. When looking specifically at resident involvement, trainees were found to be less likely to obtain muscle in specimen [28].
Quality of TURBT and its link to the predictive ability of surgeons not only dictates initial evaluation but also has implications in management of subsequent recurrences. Herr et al reported accurate predictions of tumor stage and grade in 93% of patients with Ta grade 1 tumors. The authors concluded that when identified on office cystoscopy, these tumors are amenable and safe for outpatient fulguration, avoiding unnecessary return to the operating room for TURBT and its associated morbidity, mortality, and healthcare expenditure [29].
This study found the highest rates of accuracy in prediction of NMIBC and absence of CIS, with lowest rates for low grade tumor and presence of CIS. The only significant difference between low- and high-volume surgeons was the ability to discern T1 disease. Overall, general predictive rates were highly variable with lower accuracy than anticipated. However, few considerations may be able to account for the differences encountered. Prediction accuracy for presence of MIBC falling short of 50% may be attributable to highly aggressive, bulky tumors that invade and replace normal muscle tissue. In these select cases with a lack of histologic identification of muscularis propria, patients can still be assumed to have MIBC based on clinical staging in the form of imaging and intra-operative assessment. Additionally, resident involvement in operative procedure was not standardized or stratified. As such, we are unable to identify the impact of surgeon experience on perception of tumor characteristics. Further analysis must be conducted to delineate how differences in surgeon perception of tumor characteristics and overall quality of TURBT may vary across multiple levels of training.
Our study shows that a checklist improves reporting of almost every key element, however, our methods are not without limitations. The retrospective nature of our assessment is susceptible to potential for underreporting in the form of inconsistent documentation. The analysis primarily relied on operative notes and pathology reports documented by various surgeons using multiple formats and templates. As such, it is possible that key elements could have been performed or considered but may not have been clearly delineated in the chart. By creating a structured approach with use of the checklist, our goal is to eliminate such tendencies moving forward. Additionally, checklist use was voluntary, requiring dictating surgeons to input a smartphrase while completing the operative note, lending to inconsistent usage across surgeons of varying experience levels. Limited by our electronic medical record (EMR), post-operative instillations as well as complication rates were not extractable data points systematically associated with surgery and thus are unable to be assessed. Furthermore, surveillance cystoscopy schedule was not uniform. Some patients who are surveilled more frequently may have recurrences captured earlier than their contemporaries who are surveilled less often. We were unable to conduct comprehensive subgroup analyses due to limited sample size and lack of long-term follow-up for part of our cohort with inclusion criteria requiring 2 years of follow-up. When assigning surgeons into expertise tiers, we chose average annual case volumes with arbitrary cutoffs because to our knowledge, there is no validated system of surgical experience categorization for TURBTs.
CONCLUSION
Utilization of a TURBT surgical checklist improves quality of reporting and serves as an effective tool to assess surgeon prediction of tumor characteristics. We did not identify an association of checklist use with surrogates of resection quality including presence of detrusor in specimen and RFS. Future efforts to improve quality of NMIBC care should include characterizing the potential benefits of implementing the standardized use of a TURBT checklist.
ACKNOWLEDGMENTS
This study was supported by the National Cancer Institute NCI LRP Award #2L30CA220768-03A1.
FUNDING
This study was supported by the National Cancer Institute NCI LRP Award #2L30CA220768-03A1.
AUTHOR CONTRIBUTIONS
Priya Dave, MD: conception, performance of work, interpretation of data, writing the article; had access to the data.
Rutul D. Patel, DO: performance of work, interpretation of data, writing the article; had access to the data.
Kush Desai, BA: performance of work, interpretation of data, writing the article; had access to the data.
Jonathan Davila, MD: conception, performance of work, interpretation of data, writing the article; had access to the data.
Alex Sankin, MD: conception, performance of work, interpretation of data, writing the article; had access to the data.
CONFLICT OF INTEREST
Priya Dave, MD has no conflicts of interest to report.
Rutul D. Patel, DO has no conflicts of interest to report.
Kush Desai, BA has no conflicts of interest to report.
Jonathan Davila, MD has no conflicts of interest to report.
Alex Sankin, MD has no conflicts of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
[1] | Babjuk M , et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - update, European Urology (2019) ;76: (5):639–57. |
[2] | Siegel RL , Miller KD , Jemal A Cancer statistics CA Cancer J Clin (2015) ;65: (1):5–29. |
[3] | Sylvester RJ Natural history, recurrence, and progression in superficial bladder cancer, Scientific World Journal (2006) ;6: :2617–25. |
[4] | Avritscher EB , et al. Clinical model of lifetime cost of treating bladder cancer and associated complications, Urology (2006) ;68: (3):549–53. |
[5] | Brausi M , et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: A combined analysis of seven EORTC studies, Eur Urol (2002) ;41: (5):523–31. |
[6] | Anderson C , et al. A 10-item checklist improves reporting of critical procedural elements during transurethral resection of bladder tumor, J Urol (2016) ;196: (4):1014–20. |
[7] | Guerero DN , et al. Improving the quality of transurethral resection of bladder tumour (TURBT) operative notes following the european association of urology guidelines: A completed audit loop study, Cureus (2022) ;14: (10):e30131. |
[8] | Cina SJ , et al. Correlation of cystoscopic impression with histologic diagnosis of biopsy specimens of the bladder, Hum Pathol (2001) ;32: (6):630–7. |
[9] | Adiyat KT , et al. "Complete transurethral resection of bladder tumor": Are the guidelines being followed? Urology (2010) ;75: (2):365–7. |
[10] | Brausi M , et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Grou J Urol (2011) ;186: (6):2158–67. |
[11] | Herr HW , Donat SM Quality control in transurethral resection of bladder tumours, BJU Int (2008) ;102: (9 Pt B):1242–6. |
[12] | Poletajew S , et al. The learning curve for transurethral resection of bladder tumour: How many is enough to be independent, safe and effective surgeon? J Surg Educ (2020) ;77: (4):978–85. |
[13] | Montgomery JS , Miller DC , Weizer AZ Quality indicators in the management of bladder cancer, J Natl Compr Canc Netw (2013) ;11: (4):492–500. |
[14] | Mostafid H , Brausi M Measuring and improving the quality of transurethral resection for bladder tumour (TURBT), BJU Int (2012) ;109: (11):1579–82. |
[15] | Pan D , Soloway MS The importance of transurethral resection in managing patients with urothelial cancer in the bladder: Proposal for a transurethral resection of bladder tumor checklist, Eur Urol (2012) ;61: (6):1199–203. |
[16] | Chamie K , et al. Quality of diagnostic staging in patients with bladder cancer: A process-outcomes link, Cancer (2015) ;121: (3):379–85. |
[17] | Mariappan P , Zachou A , Grigor KM Detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience, Eur Urol (2010) ;57: (5):843–9. |
[18] | Mastroianni R , et al. MP72-03 assessing the impact of absence of detrusor muscle in TA-LG urothelial carcinoma of the bladder on recurrence free survival, Journal of Urology (2020) ;203: (Supplement 4):e1073–4. |
[19] | Kikuchi H , et al. Quality improvement in managing patients with non-muscle-invasive bladder cancer by introducing a surgical checklist for transurethral resection of bladder tumor, PLoS One (2022) ;17: (10):e0276816. |
[20] | Suarez-Ibarrola R , et al. Surgical checklist impact on recurrence-free survival of patients with non-muscle-invasive bladder cancer undergoing transurethral resection of bladder tumour, BJU Int (2019) ;123: (4):646–50. |
[21] | Taoka R , et al. Use of surgical checklist during transurethral resection increases detrusor muscle collection rate and improves recurrence-free survival in patients with non-muscle-invasive bladder cancer, Int J Urol (2021) ;28: (7):727–32. |
[22] | Soria F , et al. MP08-10 the introduction of a surgical checklist for the transurethral resection of the bladder improves recurrence-free survival in non-muscle invasive bladder cancer patients, Journal of Urology (2018) ;199: (4S):e99–e99. |
[23] | Suh J , Ku JH To achieve the best performance of transurethral resection of bladder tumor: Implication of surgical checklist, Transl Androl Urol (2019) ;8: (Suppl 1):S85–7. |
[24] | Vale L , et al. , The value of surgeon’s perception during transurethral resection of bladder tumors: Can we trust in our eyes and experience to predict grade and staging? Porto Biomed J (2022) ;7: (4):e179. |
[25] | Mitropoulos D , et al. Accuracy of cystoscopy in predicting histologic features of bladder lesions, J Endourol (2005) ;19: (7):861–4. |
[26] | Mariappan P , et al. , Predicting grade and stage at cystoscopy in newly presenting bladder cancers-a prospective double-blind clinical study, Urology (2017) ;109: :134–9. |
[27] | Ali MH , et al. Role of surgeon experience in the outcome of transurethral resection of bladder tumors, Urol Ann (2020) ;12: (4):341–6. |
[28] | Bos D , et al. Impact of resident involvement in endoscopic bladder cancer surgery on pathological outcomes, Scand J Urol (2016) ;50: (3):234–8. |
[29] | Herr HW , Donat SM , Dalbagni G Correlation of cystoscopy with histology of recurrent papillary tumors of the bladder, Journal of Urology (2002) ;168: (3):978–80. |




