Comparative Analysis of Very Reduced vs Full Dose BCG Treatment for High-Risk Non-Muscle Invasive Bladder Cancer: A Contemporary Experience from Chile
Abstract
BACKGROUND:
Adjuvant bacillus Calmette-Guérin (BCG) is recommended for high-risk (HR) non-muscle invasive bladder cancer (NMIBC), but BCG shortages have led to exploration of reduced-dose regimens and shortened maintenance durations out of necessity, with limited data on treatment efficacy in Latin America.
OBJECTIVE:
Oncological outcomes of HR-NMIBC patients treated with reduced (RD,1/4th dose) vs full dose (FD) BCG instillations of Danish Strain 1331 BCG.
METHODS:
We performed a retrospective study of HR-NMIBC patients treated with BCG between 2003 and 2022 at our center in Santiago Chile. We stratified patients according to either RD (1/4th dose) or FD BCG. Univariate and multivariable Cox regression models were used to predict recurrence. Kaplan-Meier method was used to calculate survival estimates.
RESULTS:
Of a total of 200 patients, 116 (58%) had RD and 84 (42%) FD BCG. Median follow-up was 57 months (IQR: 29–100). Patients who received FD BCG had a lower risk of recurrence (HR: 0.41, 95% CI 0.22–0.74) and high-grade (HG)-recurrence (HR: 0.30, 95% CI 0.15–0.61; p = 0.001). More patients in the RD vs FD group progressed to MIBC (10/84 vs 2/116; p = 0.18). Additionally, patients were less likely to stop BCG treatment in the RD group compared to the FD group due to toxicity (5% vs 11%, p = 0.14).
CONCLUSIONS:
A 1/4th dose of Danish Strain 1331 BCG treatment was associated with worse recurrence free rate and HG-recurrence rate in our cohort. Patients with RD had lower discontinuation treatment rates due to a reduced toxicity profile. These findings would suggest that RD BCG would compromise oncological outcomes in HR-NMIBC patients.
INTRODUCTION
Bladder cancer (BC) is the 10th most common cancer globally, with an age-standardized (ASR) incidence rate of 5.6 per 100,000 [1]. In Chile, BC has an ASR of 4.6 per 100,000; however, certain regions within the country have experienced a significantly increased incidence and mortality due to arsenic exposure, a known risk factor for BC [1, 2].
Bacillus Calmette-Guérin (BCG) was introduced as an adjuvant intravesical immunotherapy for the treatment of non-muscle invasive bladder cancer (NMIBC) over 50 years ago and has since remained the gold standard for high-risk NMIBC [3]. However, the urology community constantly faces shortages of BCG supply due to increasing demand and difficulties in its production. The American Urological Association (AUA) proposed a solution for high-risk patients, recommending a reduced dose (RD) of 1/2 to 1/3rd when the full BCG dose is unavailable [4]. However, the European Association of Urology (EAU) expressed reservations about reducing the BCG dose and instead suggested shortening the duration of BCG maintenance from 3 years to 1 year [5].
In Chile, the use of a 1/4th dose of BCG became common practice following a change in BCG manufacturer in 2003. The new BCG product was packaged in 4 vials, unlike the previous that was packed in 3 vials (Connaught). Regardless of that, a strong trend to use reduced BCG doses had established itself in Chile, in part due to national experiences showing acceptable responses [6]. However, those series were not limited to high-risk NMBIC, raising concerns about their real efficacy. In this study, we assessed the oncologic outcomes of patients receiving a 1/4th dose of Danish Strain 1331 BCG compared to a full dose of BCG in high-grade NMIBC patients.
MATERIAL AND METHODS
Data and population
This study was approved by the institutional review board of the Clínica Alemana, Santiago, Chile (IRB Approval 2020-90). We performed a retrospective single-institution study of NMIBC patients who were treated with adjuvant intravesical BCG therapy from January 2003 to March 2022.
The BCG strain used was Danish Strain 1331 (AJ Vaccines, Denmark). The cohort was stratified according to BCG dose: RD BCG (1/4th dose) and full dose (FD) BCG. The RD BCG group was defined as patients who were treated with one vial of 30 mg of Danish strain BCG resulting in a 1/4th dose of BCG. The FD BCG group of patients was treated with 120 mg of Danish strain BCG using 4 vials of 30 mg.
The clinical stage was determined by the highest histopathologic grade or stage on initial transurethral resection of bladder tumor (TURBT) or restaging TURBT and based on the American Joint Committee on Cancer (AJCC) Tumor-Node-Metastases (TNM) classification [7]. Tumor grade was based on the WHO/International Society of Urological Pathology (ISUP) 2016 grading system [8]. High risk NMIBC was defined according to the EAU guidelines up to 2021, which included any high grade, any T1, or any CIS. The RD patients were treated before 2017 and FD patients on or after 2017. Surveillance schedules were followed according to the bladder cancer European Association of Urology (EAU) guidelines. All surgical specimens were assessed by a single genitourinary pathologist at our institution.
Outcomes
The primary endpoint was recurrence-free rate (any recurrence). This was calculated as the number of months from index TURBT prior to BCG initiation and date of pathologic proven disease recurrence or last available follow-up. Secondary endpoints were high-grade (HG)-recurrence free rate, progression and overall survival (OS). Progression was defined as histological confirmed muscle-invasive bladder cancer (MIBC).
Statistics
Descriptive statistics were performed using the Kruskal–Wallis test for continuous variables and the Pearson’s chi-square test for categorical variables. Frequencies were reported for categorical variables and medians and interquartile ranges (IQR) for continuous variables. Kaplan-Meier method was used to estimate the time to recurrence, HG recurrence, progression and OS stratified by BCG dose. Patients who did not develop an event were censored at the time of death or at the date of the last follow-up. Median follow-up was determined using the reverse Kaplan-Meier method. The log-rank test was used to compare survival functions between the two cohorts. Univariate and multivariate Cox regression models were used to identify predictors of recurrence, high-grade recurrence, and death. Variables statistically significant on univariate analysis were included in the multivariable analysis. Statistical significance was set with a p-value <0.05. The data were analyzed using Stata/SE, version 14.1 (Stata Corp. LP, College Station, TX, USA). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
RESULTS
A total of 200 NMIBC patients, of whom 116 (58%) received RD BCG and 84 (42%) received FD BCG, were analyzed. Patient demographics and descriptive statistics are reported in Table 1. The median follow-up of patients alive was 57 months (IQR 29–100). The median age of the cohort was 71 years (IQR: 62–78). There were no significant differences in age, sex, smoking status, grade, stage, or presence of concomitant CIS, re-TUR, total BCG instillations or cystectomy performed between the two groups; however, there were significantly more patients with recurrent tumors at presentation in the FD compared to the RD group (21% vs 10%, p = 0.022). A total of 29 patients died from any cause during the follow-up, 25 in the RD group and 4 in the FD group. Patients were less likely to stop BCG treatment in the RD group compared to the FD group due to toxicity although there was no significant difference (5% vs 11%, p = 0.14).
Fig. 1
Kaplan Meier curve stratified by BCG dose. A. recurrence free rate, p = 0.0007. B. HG-recurrence free rate, p = 0.0006.
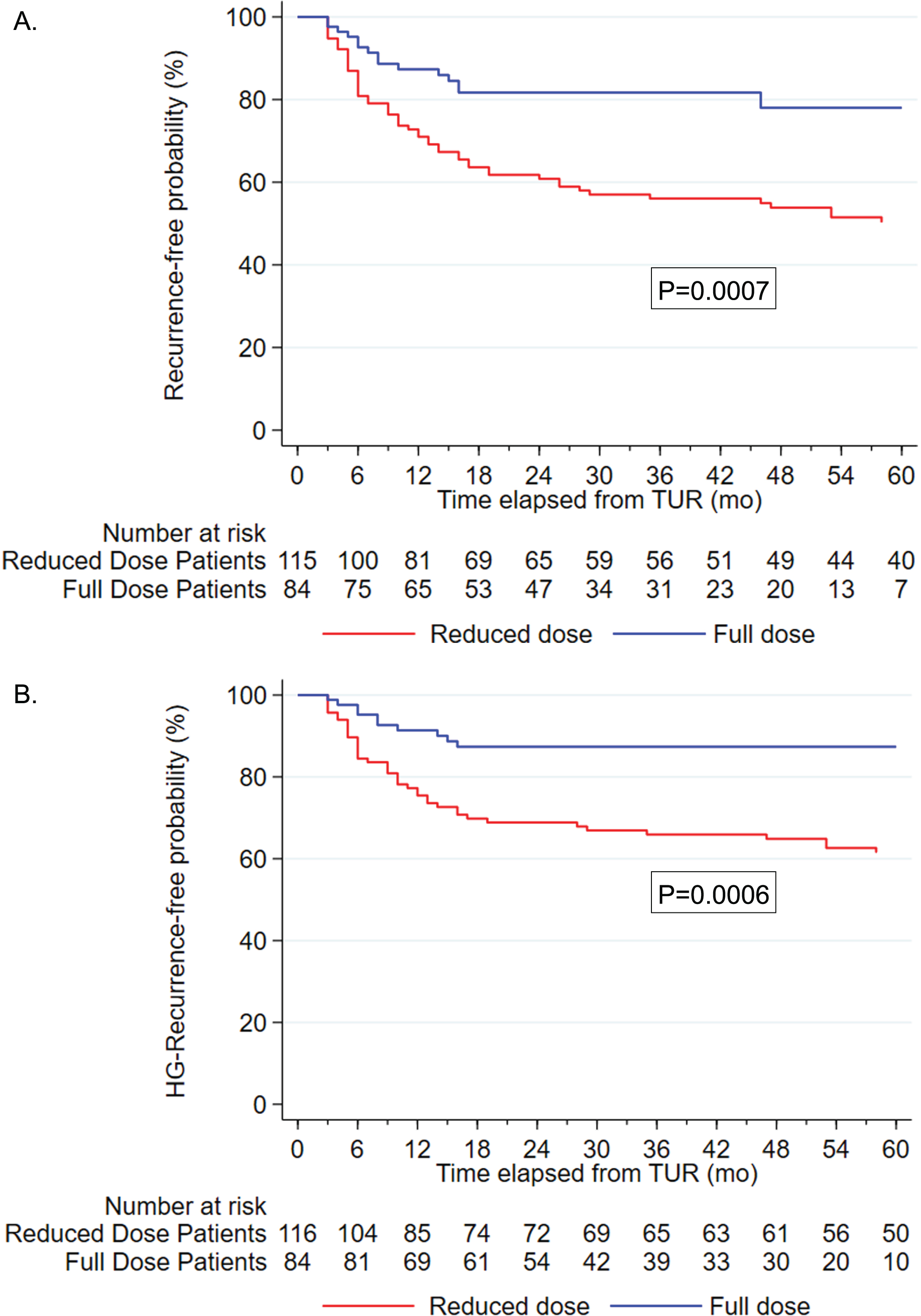
Table 1
Patient demographics
| Total (n = 200) | Reduced Dose | Full Dose | p-value | |
| (n = 116) | (n = 84) | |||
| Age at surgery, years (IQR) | 71 (62, 78) | 70 (62, 80) | 72 (62, 77) | 0.6 |
| Sex, n (%) | ||||
| Male | 158 (79%) | 90 (78%) | 68 (81%) | 0.6 |
| Smoking status, n (%) (N = 128) | ||||
| Non-smoker | 20 (15%) | 14 (21%) | 6 (9.4%) | 0.062 |
| Former smoker | 84 (64%) | 37 (54%) | 47 (73%) | |
| Active smoker | 28 (21%) | 17 (25%) | 11 (17%) | |
| Primary vs Recurrent tumor, n (%) (N = 193) | ||||
| Primary | 168 (85%) | 102 (90%) | 66 (79%) | 0.022 |
| Recurrent | 29 (15%) | 11 (10%) | 18 (21%) | |
| Clinical T Stage, n (%) | ||||
| Ta | 77 (38%) | 44 (38%) | 33 (39%) | 0.9 |
| CIS | 16 (8.0%) | 10 (8.6%) | 6 (7.1%) | |
| T1 | 107 (54%) | 62 (53%) | 45 (54%) | |
| Concomitant CIS, n (%) | ||||
| Absent | 140 (70%) | 83 (72%) | 57 (68%) | 0.6 |
| Present | 60 (30%) | 33 (28%) | 27 (32%) | |
| Re-TUR performed, n (%) | ||||
| No | 131 (66%) | 79 (68%) | 52 (62%) | 0.4 |
| Yes | 69 (34%) | 37 (32%) | 32 (38%) | |
| Total BCG instillations, n (%) | 14 (9, 18) | 12 (6, 18) | 15 (9, 19) | 0.085 |
| Stop due to toxicity, n (%) | ||||
| No | 185 (92%) | 110 (95%) | 75 (89%) | 0.14 |
| Yes | 15 (7.5%) | 6 (5.2%) | 9 (11%) | |
| Cystectomy, n (%) | ||||
| No | 181 (90%) | 102 (88%) | 79 (94%) | 0.15 |
| Yes | 19 (10%) | 14 (12%) | 5 (6.0%) |
Abbreviations: IQR stands for Interquartile range, RD stands for reduced dose of BCG (1/4th dose), FD stands for full dose BCG. Data values are presented as n (%) unless otherwise indicated. Percentages may not add up to 100 because of rounding. P-values were determined by chi-square test or Kruskal–Wallis test where indicated.
Recurrence
Of 72 patients that developed recurrence 57 were in the RD and 15 in the FD group. The median time to recurrence was 46 months (IQR: 24, 78). In the RD group, the recurrence-free rate at 12 and 36 months was 71% (95% CI: 62% –78%) and 56% (95% CI: 46% –64%) respectively. In the FD group, the recurrence-free rate was 87% (95% CI: 78% –93%) and 82% (95% CI: 71% –89%) at 12- and 36-months, respectively (Supplemental Table 1).
There were a total of 55 HG recurrences; 45 in the RD and 10 in the FD group. There were significant differences in the recurrence free rate (p = 0.0007) and the HG-recurrence free rate (p = 0.0006) between the two groups (Fig. 1A & 1B). FD BCG was associated with a lower risk of recurrence (Hazard ratio [HR] 0.37, 95% CI 0.21–0.67; p = 0.001), (Table 2A). Likewise, FD BCG (FD HR 0.30, 95% CI 0.15–0.61; p = 0.001) was associated with a significantly lower HG recurrence risk following multivariable analysis (Table 2B).
Table 2
Univariate and multivariate logistic regression analysis for: A) recurrence, B) high-grade recurrence and, C) overall survival
| A. | |||||||
| Variable | Recurrence | ||||||
| Univariate analysis | Multivariable analysis | ||||||
| HR | (95% CI) | p-value | HR | (95% CI) | p-value | ||
| BCG Dose | |||||||
| Reduced | ref | ref | |||||
| Full | 0.39 | 0.22,0.69 | 0.001 | 0.37 | 0.21,0.67 | 0.001 | |
| Age | |||||||
| 1.05 | 1.02,1.07 | <0.001 | 1.04 | 1.01,1.06 | 0.002 | ||
| Primary vs recurrent | |||||||
| Primary | ref | ref | |||||
| Recurrent | 1.93 | 1.09,3.43 | 0.025 | 1.81 | 0.99,3.32 | 0.054 | |
| B. | |||||||
| Variable | High-Grade Recurrence | ||||||
| Univariate analysis | Multivariable analysis | ||||||
| HR | (95% CI) | p-value | HR | (95% CI) | p-value | ||
| BCG Dose | |||||||
| Reduced | ref | ref | |||||
| Full | 0.32 | 0.16,0.64 | 0.001 | 0.30 | 0.15,0.61 | 0.001 | |
| Age | |||||||
| 1.04 | 1.02,1.07 | 0.001 | 1.04 | 1.01,1.06 | 0.007 | ||
| Primary vs recurrent | |||||||
| Primary | ref | ref | |||||
| Recurrent | 1.95 | 1.04,3.77 | 0.037 | 1.85 | 0.94,3.65 | 0.074 | |
| C. | |||||||
| Variable | Overall Survival | ||||||
| Univariate analysis | Multivariable analysis | ||||||
| HR | (95% CI) | p-value | HR | (95% CI) | p-value | ||
| BCG Dose | |||||||
| Reduced | ref | ref | |||||
| Full | 0.41 | 0.14,1.23 | 0.113 | 0.48 | 0.16,1.43 | 0.189 | |
| Age | |||||||
| 1.09 | 1.05,1.14 | <0.001 | 1.09 | 1.05,1.13 | <0.001 | ||
Progression
Twelve patients progressed to ≥pT2 or N+ or M+ disease; of which two patients (2/84) were treated with FD BCG and the remaining ten patients (10/116) with RD BGC. A Kaplan-Meier analysis did not show a significant difference between the two groups, p = 0.18.
Overall survival
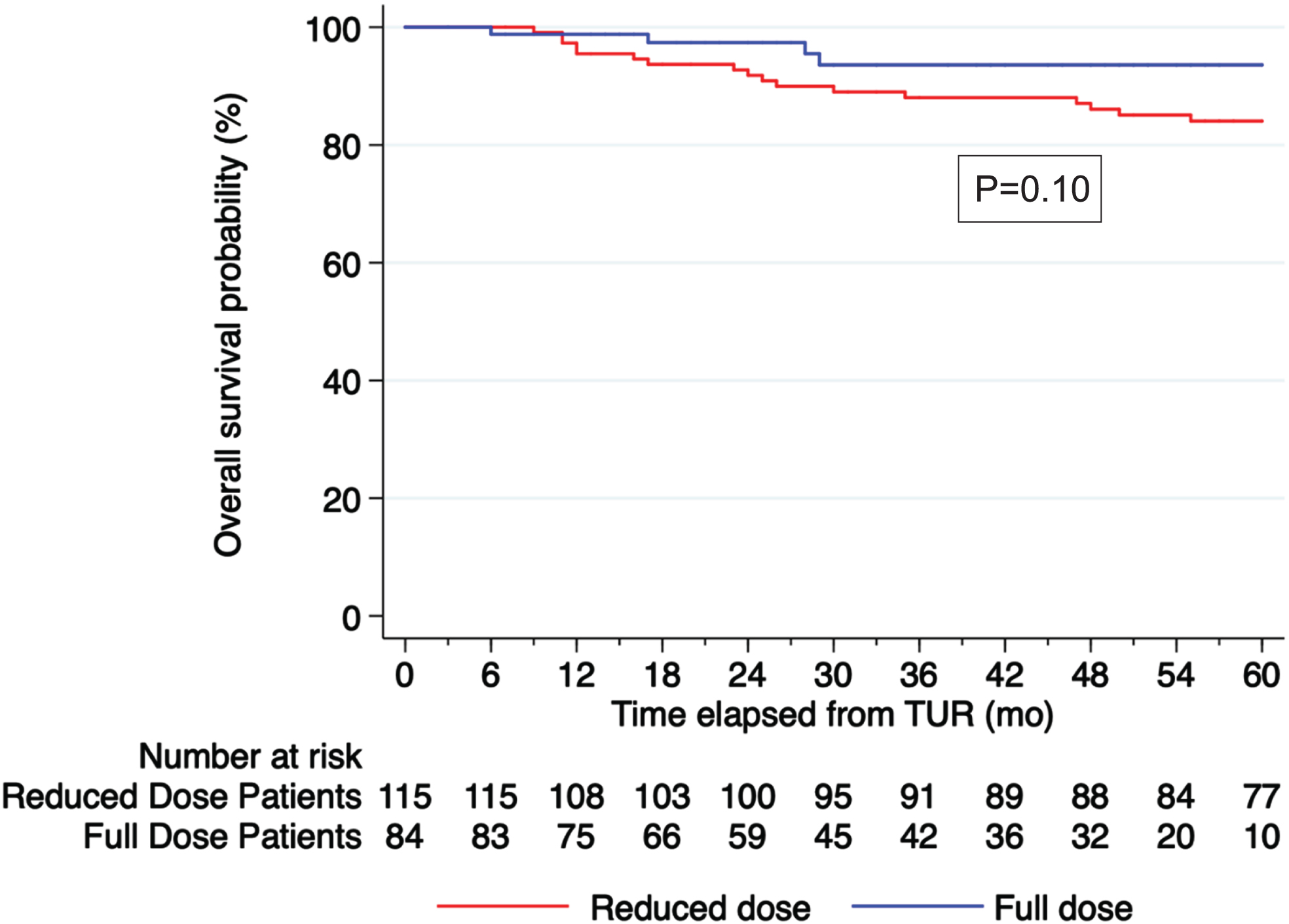
At 36 months, the OS rate was 90% for the whole cohort (95% CI: 85% –94%). There was no significant difference in OS on Kaplan-Meier analysis, p = 0.10 (Fig. 2).
On multivariate analysis, increasing age (HR 1.09, 95% CI 1.05–1.13, p < 0.001) was associated with a higher risk of death while FD BCG was not significantly associated with a lower risk of death (FD BCG HR 0.48, 95% CI 0.16–1.43 p = 0.189) (Table 2A).
Fig. 2
Kaplan Meier curve for OS stratified by BCG dose, p = 0.10.
DISCUSSION
This study showed that in a Chilean patient population treated with Danish strain BCG, 1/4th dose was associated with worse recurrence free rate and HG-recurrence free rate.
In contrast to other studies that utilized a 1/3rd reduced dose of BCG, which reported no significant difference in oncological outcomes, our findings are consistent with the study by Ojea et al. which examined a 1/6th dose of Connaught strain BCG and demonstrated a decrease in efficacy [9–12]. These findings suggest that a minimum dose of BCG is required for similar oncologic effects may be 1/3 dose and 1/4th dose may not be sufficient.
A recent meta-analysis of 17 studies comprising 3,757 patients and multiple BCG strains showed increased recurrence rates with different regimens of RD BCG (1/3,1/2 and 2/3) (OR 1.19, 95% CI 1.03, 1.36; p = 0.02). However, this was no longer seen when maintenance therapy was administered (OR 1.07, 95% CI 0.96–1.29; p = 0.17) [13]. This was also confirmed by the NIMBUS trial where fewer instillations were associated with a higher risk of recurrence [14]. Furthermore, as previously reported, primary vs recurrent tumor was an independent predictor of further recurrence [15].
In addition, a randomized study by Agrawal et al. using the Danish strain of BCG alone found no significant difference in recurrence between 1/3rd, 2/3rds, and full dose BCG (20% vs. 25% vs. 20% respectively; p > 0.05) [16]. However, a difference in local toxicity was observed (30% vs. 41.7% vs. 70%, respectively; p < 0.01). Differing from our study, their study did not include patients with CIS. It is plausible that a further reduction from a 1/3rd dose of the Danish strain BCG to a 1/4th dose might result in a higher incidence of recurrences, as seen in our study. Alternatively, these differences could be attributed to the distinct characteristics of the population studied, who might have been subjected to diverse environmental risk factors, such as smoking and arsenic exposure. To establish conclusive evidence of outcomes between 1/4th dose and full dose BCG, larger randomized trials will be necessary.
We found a greater proportion of discontinuation of therapy in the FD group due to toxicity, albeit not statistically significant, likely due to the small cohort size. While the study by Oddens et al, did not observe any significant difference in toxicity between the 1/3 dose of BCG and the full dose of BCG [9], a recent study that compared the side effects and tolerability of full dose and reduced dose BCG using patient surveys during treatment found significant differences in fatigue (53% vs 43%, p = 0.0003) and incomplete dwell time (13% vs 5%, p < 0.0001) with FD compared to RD BCG [17]. Other studies have also shown significantly more pronounced bladder symptoms in patients receiving a FD compared to RD BCG treatment [11, 15, 18–20].
Our work should be interpreted in the context of its several limitations including its retrospective nature, single institution study, small cohort of patients, lack of comorbidity data and case selection bias. In addition, the strain of BCG used in Chile is different to the strain used in the US and Europe. However, a meta-analysis of clinical trials examining nine different BCG strains found no clear evidence to support the superiority of any specific BCG strain in reducing disease recurrence [21]. Furthermore, we do not have complete information available about tumor size and, number of tumors, and prior recurrence rate which may affect the outcomes of the groups depending on the distribution among the groups. However, this is the first study evaluating the efficacy of RD BCG in Chile, where this practice continues to be upheld in certain medical centers. The results from the study have the potential to impact local clinical practice.
CONCLUSIONS
Our results show that in our patient population in Chile, a 1/4th dose of Danish strain BCG does not provide equivalent oncological control compared to FD BCG. While optimizing resources and minimizing adverse effects is important, careful consideration should be given to ensure sufficient BCG dosage for optimal outcomes.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Valentina Grajales- conception, performance and interpretation of data.
Roberto Contieri- performance of data.
Wei Shen Tan- interpretation of data.
Marta Flores- interpretation of data.
Marcela Schultz- interpretation of data.
Rodrigo Pinochet- interpretation of data.
Alberto Bustamante- interpretation of data.
Ashish M. Kamat- conception, interpretation of data.
Mario I. Fernández- conception, interpretation of data.
CONFLICT OF INTEREST
Valentina Grajales has no conflict of interest to report.
Roberto Contieri has no conflict of interest to report.
Wei Shen Tan was a consultant to Combat Medical.
Marta Flores has no conflict of interest to report.
Marcela Schultz has no conflict of interest to report.
Rodrigo Pinochet has no conflict of interest to report.
Alberto Bustamante has no conflict of interest to report.
Ashish M. Kamat is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Ashish M. Kamat is a consultant or advisory board member for Abbott Molecular, Arquer Diagnostics, ArTara Therapeutics, Asieris Pharmaceuticals, AstraZeneca, BioClin Therapeutics, Bristol Myers Squibb, Cepheid, Cold Genesys, Eisai, Engene, Ferring Pharmaceuticals, FerGene, Imagine Pharma, Janssen, MDxHealth, Medac, Merck, Pfizer, Photocure, ProTara Therapeutics, Roviant Sciences, Seattle Genetics, Sessen Bio, Theralase Technologies, TMC Innovation, and US Biotest; has received grants and/or research support from Adolor Corporation, Bristol Myers Squibb, FKD Industries, Heat Biologics, Merck, Photocure, SWOG/NIH, Specialized Programs of Research Excellence (SPORE), and AIBCCR; and holds the patent for Cytokine Predictors of Response to Intravesical Therapy (CyPRIT) jointly with UT MD Anderson Cancer Center.
Mario I. Fernández is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Mario I. Fernández is a consultant or advisory board member for Grünenthal.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-230047.
REFERENCES
[1] | Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer.; [Available from: https://gco.iarc.fr/today. |
[2] | Fernández MI , López JF , Vivaldi B , Coz F Long-term impact of arsenic in drinking water on bladder cancer health care and mortality rates 20 years after end of exposure, J Urol (2012) ;187: (3):856–61. |
[3] | Lobo N , Brooks NA , Zlotta AR , Cirillo JD , Boorjian S , Black PC , et al. 100 years of Bacillus Calmette-Guérin immunotherapy: From cattle to COVID-19, Nat Rev Urol (2021) ;18: (10):611–22. |
[4] | Association AU. BCG Shortage Info. Important Message About the BCG Shortage2020 05/2023. Available from: https://www.auanet.org/about-us/bcg-shortage-info. |
[5] | Babjuk MB , Compérat M , Palou E , van Rhijn JR , Rouprêt B , Shariat M , Sylvester S , Zigeuner R , Gontero R , Mostafid PH Statement concerning the shortage of BCG vaccine from the EAU Guidelines Panel on Non-muscle-invasive Bladder Cancer06/2023. Available from: https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer/publications-appendices. |
[6] | Rivera P , Orio M , Hinostroza J , Venegas P , Pastor P , Gorena M , et al. Our experience with 1 mg BCG vaccine instillation in T1 stage cancer of the bladder, Actas Urol Es (1999) ;23: (9):757–62. |
[7] | Amin ME , Greene SB , Byrd FL , Brookland DR , Washington RK , Gershenwald MK , Compton JE , Hess CC , Sullivan KR , Jessup DC , Brierley M , Gaspar JD , Schilsky LE , Balch RL , Winchester CM , Asare DP , Madera EA , Gress M , Meyer DMLR AJCC Cancer Staging Manual. 8th ed. Switzerland: Springer Cham; 2017. |
[8] | Humphrey PA , Moch H , Cubilla AL , Ulbright TM , Reuter VE The WHO classification of tumours of the urinary system and male genital organs-part B: Prostate and bladder tumours, Eur Urol (2016) ;70: (1):106–19. |
[9] | Oddens J , Brausi M , Sylvester R , Bono A , van de Beek C , van Andel G , et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: One-third dose versus full dose and 1 year versus 3 years of maintenance, Eur Urol (2013) ;63: (3):462–72. |
[10] | Lobo N , Bree KK , Hensley PJ , Nogueras-Gonzalez GM , Abraham P , Navai N , et al. Reduced-dose bacillus Calmette-Guérin (BCG) in an era of BCG shortage: Real-world experience from a tertiary cancer centre, BJU Int (2022) ;130: (3):323–30. |
[11] | Kumar A , Dubey D , Bansal P , Mandhani A , Naik S Urinary interleukin-8 predicts the response of standard and low dose intravesical bacillus Calmette-Guerin (modified Danish strain) for superficial bladder cancer, J Urol (2002) ;168: (5):2232–5. |
[12] | Ojea A , Nogueira JL , Solsona E , Flores N , Gómez JM , Molina JR , et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: Low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (135 mg) versus mitomycin C, Eur Urol (2007) ;52: (5):1398–406. |
[13] | Verri P , Baboudjian M , Diana P , Gallioli A , Territo A , Gaya JM , et al. Reduced- vs full-dose BCG in bladder cancer: A systematic review and meta-analysis, Actas Urol Esp (Engl Ed) (2023) ;47: (1):4–14. |
[14] | Grimm MO , van der Heijden AG , Colombel M , Muilwijk T , Martínez-Piñeiro L , Babjuk MM , et al. Treatment of high-grade non-muscle-invasive bladder carcinoma by standard number and dose of BCG instillations versus reduced number and standard dose of BCG instillations: Results of the european association of urology research foundation randomised phase III clinical trial “NIMBUS", Eur Urol (2020) ;78: (5):690–8. |
[15] | Martínez-Piñeiro JA , Flores N , Isorna S , Solsona E , Sebastián JL , Pertusa C , et al. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guérin with a reduced dose of 27 mg in superficial bladder cancer, BJU Int (2002) ;89: (7):671–80. |
[16] | Agrawal MS , Agrawal M , Bansal S , Agarwal M , Lavania P , Goyal J The safety and efficacy of different doses of bacillus Calmette Guérin in superficial bladder transitional cell carcinoma, Urology (2007) ;70: (6):1075–8. |
[17] | Kuperus JM , Busman RD , Kuipers SK , Broekhuizen HT , Noyes SL , Brede CM , et al. Comparison of side effects and tolerability between intravesical bacillus calmette-guerin, reduced-dose BCG and gemcitabine for non-muscle invasive bladder cancer, Urology (2021) ;156: , 191–8. |
[18] | McElree IM , Steinberg RL , Mott SL , O’Donnell MA , Packiam VT Comparison of sequential intravesical gemcitabine and docetaxel vs bacillus calmette-Guérin for the treatment of patients with high-risk non-muscle-invasive bladder cancer, JAMA Netw Open (2023) ;6: (2):e230849. |
[19] | Chamie K , Chang SS , Kramolowsky E , Gonzalgo ML , Agarwal PK , Bassett JC , et al. IL-15 superagonist NAI in BCG-unresponsive non–muscle-invasive bladder cancer, NEJM Evidence (2023) ;2: (1):EVIDoa2200167. |
[20] | Rosser CJ , Tikhonenkov S , Nix JW , Chan OTM , Ianculescu I , Reddy S , et al. Safety, tolerability, and long-term clinical outcomes of an IL-15 analogue (N-803) admixed with bacillus calmette-guérin (BCG) for the treatment of bladder cancer, Oncoimmunology (2021) ;10: (1):1912885. |
[21] | Boehm BE , Cornell JE , Wang H , Mukherjee N , Oppenheimer JS , Svatek RS Efficacy of bacillus calmette-Guérin strains for treatment of nonmuscle invasive bladder cancer: A systematic review and network meta-analysis, J Urol (2017) ;198: (3):503–10. |




