New Intravesical Agents for BCG-Unresponsive High-Risk Non-Muscle Invasive Bladder Cancer
Abstract
BACKGROUND:
With the exception of the FDA-approved valrubicin and pembrolizumab, there are no standard second-line treaments for BCG-unresponsive high-risk non-muscle invasive bladder cancer (NMIBC).
OBJECTIVES:
To provide a systematic review of the novel intravesically administered therapeutic agents for the salvage treatment of BCG-unresponsive NMIBC.
METHODS:
Online search of the PubMed, EMBASE and Web of Science databases was performed. The endpoints of this review were to evaluate the efficacy of the agents in terms of complete response rates (CR) and durability of CR, overall survival, recurrence-free survival and cancer-specific survival and to report on their toxicity profile. A search on Clinicaltrials.gov was performed to identify ongoing clinical trials.
RESULTS:
14 studies were included in this review. The critical clinical need for the development of an effective, safe and durable intravesical drug for the salvage treatment of high-risk NMIBC seems to be met mainly by intravesical gene therapy; in fact, data support the FDA-approved nadofaragene firadenovec as a potentially important therapeutic advancement in this context. Promising results are also being obtained by the combination of N-803/BCG and by innovative drug delivery systems.
CONCLUSIONS:
Considering the plethora of novel intravesical treatments that have completed phase II evaluation, one can reasonably expect that clinicians will soon have at their disposal new agents and treatment options for BCG-unresponsive NMIBC. In the near future, it will be up to the urologist to identify, for each specific patient, the right agent to use, based on safety, results and cost-effectiveness.
INTRODUCTION
Up to 80% of bladder cancers (BCa) are non-muscle invasive at first diagnosis [1]. Although transurethral resection of the bladder (TURB) by itself can eradicate a papillary Ta or T1 tumour completely, these tumours commonly recur and can progress to muscle-invasive BCa (MIBC). It is therefore necessary to consider adjuvant instillation therapy or, in specific cases with a very high risk of progression, immediate radical cystectomy (RC) [1].
Adjuvant treatment with intravesical bacillus Calmette–Guérin (BCG) is recommended for high-risk (HR) non-muscle invasive bladder cancer (NMIBC) and for intermediate-risk NMIBC, especially after mitomycin C failure [2]. However, up to 50% of the patients fail to maintain a response within 5 years of BCG treatment [3]. BCG failure is generally defined as any high grade (HG) disease occurring during or after BCG therapy, under which several categories have been defined by the U.S. Food and Drug Administration (FDA). For example, NMIBC may not respond at all (BCG refractory) or may relapse after initial response (BCG relapsing). All BCG refractory tumours and those who develop T1/Ta HG recurrence within 6 months of completion of adequate BCG exposure, or develop carcinoma in situ (CIS) within 1 year of completion of adequate BCG exposure are defined as BCG-unresponsive. Furthermore, some patients have to discontinue BCG because of side effects (BCG intolerance).
For patients with NMIBC in whom BCG therapy has failed RC may be the best treatment; however, it is associated with Clavien grade III/IV complication rate of almost 14% in the robotic and 19% in the open RC group, associated with a 2% risk of mortality [4]. Furthermore, RC can lead to substantial loss in health related quality of life (QoL) from adapting to urinary diversion, including altered body image and loss of sexual function [5].
In order to overcome the morbidity of RC or for patients unfit or unwilling to undergo major surgery, several agents have been tested as second-line treatment for patients who do not respond, or have recurring disease, after BCG. Repeat-BCG therapy seems appropriate for non-HG and even for some HG recurrent tumours (namely those relapsing beyond one year after BCG exposure (cases which do not meet the criteria of BCG-unresponsive disease)) [6]. Di Lorenzo et al. [7] reported on 40 patients receiving BCG reinduction: 87.5% of patients failed to respond to BCG reinduction at one year; one patient died of systemic disease, 37.5% of the patients had disease progression and had to undergo cystectomy and 40% underwent radiation therapy plus systemic chemotherapy after one year.
Intravesical valrubicin has been approved by FDA for use in patients with CIS in whom BCG therapy had failed. However, it has demonstrated suboptimal efficacy. The 3-mo CR rate was 18%. Kaplan-Meier analysis indicated a probability of disease-free status of 22% at 6 months, 10% at 1 year, and 4% at 2 years. Probability of cystectomy was 12% at 6 months, 24% at 1 year, and 30% at 2 years [8].
Promising results from early-phase trials have been reported for intravesical taxane [9–10] and gemcitabine [7, 11].
Pembrolizumab, an immune checkpoint inhibitor was approved in patients with BCG-unresponsive, high-risk NMIBC. The single-arm KEYNOTE-057 trial included 96 patients with CIS with or without high-grade Ta or T1 cancer and documented a CR rate of 41% (95% CI 31–51), with a median response duration of 16.2 months (0-longer than 30.4 months) and 19% durable CR rate at 12 months. However, the drug is associated with systemic immune-related side-effects and the indication does not include patients with high grade Ta or T1 tumors [12].
Overall, based on the available data, there are scarce therapeutic modalities in case of BCG-failure and it is not really clear, which subgroup of patients who do not respond, or have recurring disease, after BCG would benefit more from a specific new agent. However, there is emerging literature reporting prospective data on novel intravesical therapeutic agents.
The objective of this paper is to provide a systematic review of these agents focusing on their efficacy and toxicity profiles.
MATERIALS AND METHODS
Search strategy
Two authors (ADA, GC) performed an independent online search of the PubMed, EMBASE and Web of Science databases according the PRISMA checklist. All the available clinical trials evaluating intravesical therapies to treat recurring NMIBC after previous intravesical BCG were evaluated.
The following keywords were used for the search: “bacillus Calmette-Guerin” or “BCG,” “non-muscle invasive bladder cancer” or “NMIBC” or “urothelial carcinoma,” “BCG-refractory bladder cancer” or “BCG failure” or “BCG unresponsive” and “intravesical therapy” were used for publication years 2000–2023.
Studies that were not in the English language, included MIBC patient populations, lacked a post-treatment evaluation of response, referred to “traditional” intravesical chemotherapeutics agents or those on systemic immunotherapy for NMIBC were excluded.
Initial evaluation was based on the title and abstract of the article. Pre-selected outcomes were subjected to full-text review. Eligible publications were subjected to full-text assessment, including a manual search of the reference lists. A standardized data collection template containing the trial’s background information was used by a single reviewer (GC) to collect the data from each report. The obtained data were then confirmed by the other authors.
Finally, a search on Clinicaltrials.gov was performed in order to identify ongoing clinical trials on these new intravesical agents.
Data extraction
Data were extracted independently by two authors (ADA, GC) and reviewed by a third author (GG). The extracted data included: year of publication, source of the data, time period of the study, total number of patients, study design, BCG maintenance schedule, cancer stage (carcinoma in situ (CIS) with or without papillary disease versus papillary disease), complete response (CR) rates for 3, 6, 12, and 24 months post-treatment evaluation, progression rates, RC rates, and toxicity profiles.
Primary endpoints
The primary endpoint of this systematic review was to evaluate the efficacy of the novel intravesical agents in terms of CR and durability of CR, overall survival (OS), recurrence-free survival (RFS), progression-free survival (PFS) and cancer-specific survival (CSS). The secondary endpoint was to report on the toxicity profile of these agents. Finally, the ongoing clinical trials of these new intravesical agents are reported.
RESULTS
Search results
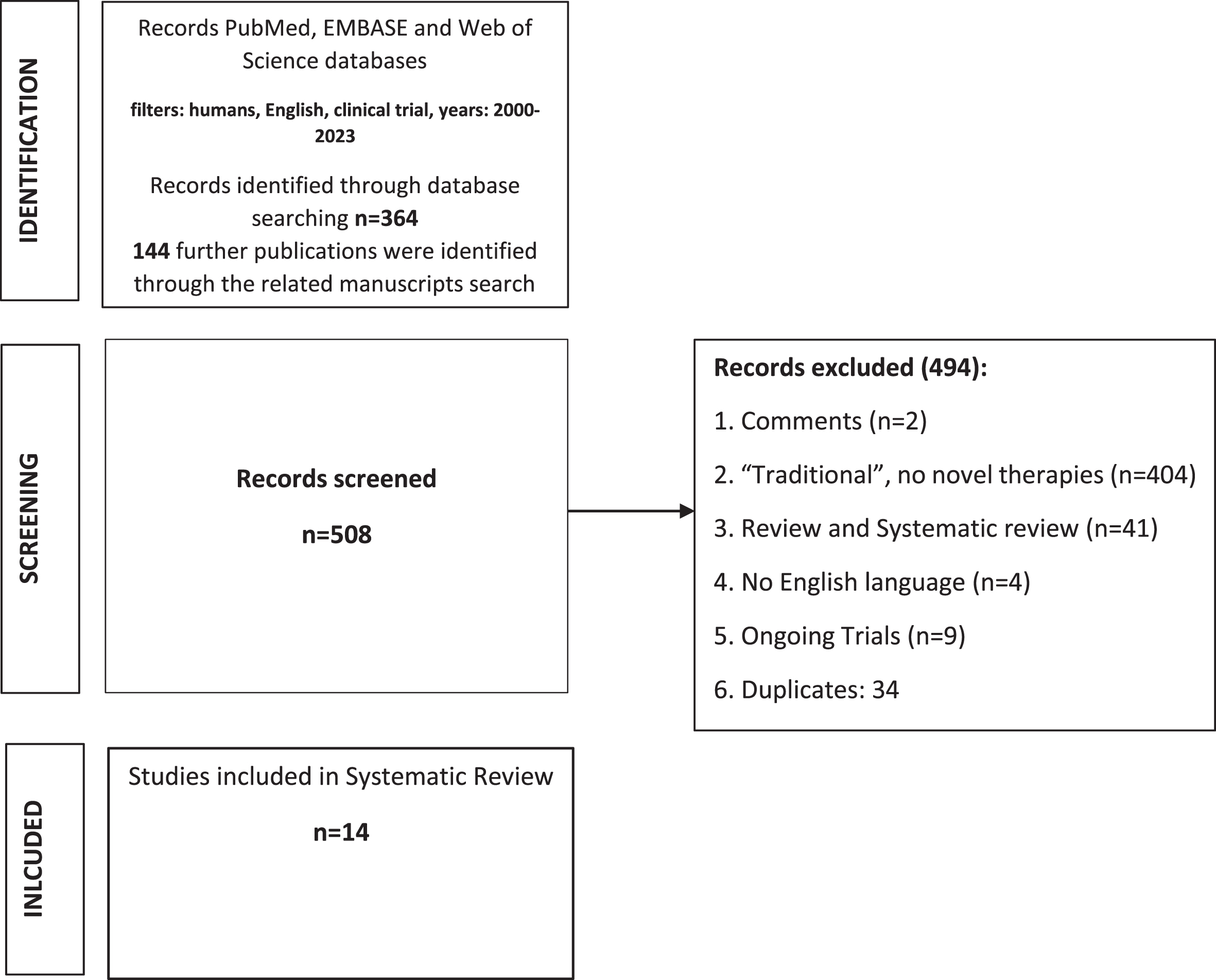
The initial online search of electronic databases yielded 364 publications that were evaluated by title with or without assessment of the abstract. 144 further publications were identified through the related manuscripts search, for a total of 508 papers. Following the application of the exclusion criteria, 14 studies were included in this review [13–26]. The selection process is explained in the PRISMA flow diagram in Fig. 1. Six ongoing trials have been also identified.
Fig. 1
PRISMA flowchart.
Novel intravesical treatments
The main novel agents for the salvage intravesical treatment of NMIBC after BCG-failure that have been identified through the search of literature, as well as their mechanism of action, are summarized in Table 1 [27–37]. Efficacy and toxicity profiles are summarized in Table 2.
Table 1
Main novel agents for the salvage intravesical treatment of NMIBC after BCG-failure
| Nadofaragene Firadenovec (rAd-IFNa/Syn3) (A viral-vector-based gene therapy that induces endogenous production of local IFNa) | Replication deficient recombinant viral-vector-based gene therapy that provides a copy of the human interferon alfa-2b gene to urothelial cells. Syn3 is a polyamide surfactant that promotes viral transduction of the urothelium. Their combination in rAd-IFNa/Syn3 causes tumor regression by triggering local rather than systemic production of interferon alfa-2b; in this way the agent provides the potential for high, sustained levels of IFNa by inducing endogenous production that may enhance efficacy [27–30]. |
| CG0070 (selective oncolytic adenovirus kills tumor cells through local GM-CSF synthesis) | Cretostimogene Grenadenorepvec (CG0070) is a cancer-selective oncolytic adenovirus engineered to preferentially replicate in tumor cells. It destroys tumors by selective replication in Rb-pathway defective tumor cells, and kills tumor cells through immunogenic cell death and local GM-CSF synthesis [31]. |
| Paclitaxel-hyaluronic acid (Originated from the chemical conjugation of paclitaxel with HA higher cytotoxic activity, up to 800-fold, compared to paclitaxel alone) | Oncofid-P-B is a novel compound under development by Fidia Farmaceutici S.p.A. that originates from the chemical conjugation of paclitaxel with HA, leading to several physico-chemical improvements over paclitaxel alone, such as water solubility and bladder muco-adhesive properties [19]. Moreover, Oncofid-P-B acts by a unique mechanism of action which leads to a significant increase of paclitaxel activity in superficial bladder tumors due to the specific binding of the HA mojety to CD44, its natural receptor, over-expressed on the surface of several tumors, including bladder carcinoma [32]. The higher cytotoxic activity, up to 800-fold, of Oncofid-P-B in comparison to paclitaxel alone has been documented in human bladder cancer lines overexpressing CD44 [32]. |
| Nab-paclitaxel (is a novel agent in the taxane family modified with the addition of albumin particles to increase solubility and facilitate drug delivery) | Taxanes inhibit microtubule depolymerization and disrupt the mitotic spindle, thereby blocking cell division and inducing apoptosis [33].Nab-paclitaxel is a novel agent in the taxane family which has been modified with the addition of albumin particles to form nanoparticles to increase solubility and facilitate drug delivery to tumor cells via biological interactions with albumin receptors that mediate drug transport across epithelial cells. It has greater water solubility and an accelerated drug transport method into tumor cells accross epithelial cells [34, 35]. |
| VPM1002BC (A modified mycobacterium BCG) | Modified mycobacterium BCG for the treatment of NMIBC. The genetic modifications are expected to result in better immunogenicity and less side effects [23, 24]. |
| Vicinium (Oportuzumab Monatox, VB4-845) (A fusion protein inhibits protein synthesis) | Vicinium is a fusion protein consisting of an Epithelial Cell Adhesion Molecule (EpCAM)-specific antibody fragment fused to Pseudomonas Exotoxin A, a potent inhibitor of protein synthesis [25]. |
| N-803 (mutated IL-15 based immunostimulatory fusion protein compound) | IL-15 is a proinflammatory cytokine critical for the generation, maintenance and homeostasis of T cell responses. It can boost CD4+ and CD8+ T cell and NK proliferation, activation and function. The IL-15 superagonist N-803 has been developed as a way to enhance the innate and cellular immune responses to HIV and consequently the antiviral activity [36]. It has also demonstrated antitumor activity across a spectrum of diseases, including BCa [37]. In the latter case, the compound shows a promising antitumor activity in combination to BCG, but a low efficacy when administered alone. |
Table 2
Summary of study design, oncologic efficacy and adverse events of the novel salvage intravesical treatments for NMIBC after BCG-failure
| Study | Agent | Participants No. | Study Design | Maintenance therapy | Cancer Stage | 3/6-Mo efficacy | 12-Mo efficacy | ≥24-Mo efficacy | Cystectomy, % | Toxicities/adverse events |
| Dinney et al (2013) | rAdeIFN-a/Syn3 | 17 | Phase I | 29% received maintenance | 35% CIS, 24% CIS/Ta, 6% CIS/T1, 35% Ta | 41% 3-mo CR | 29% CR | 23.5% CR | 59 | Urgency 88% Headache 54% Fatigue 47% Nausea 35% |
| Shore et al (2017) | rAdeIFN-a/Syn3 | 40 | Phase II | 3 treatments at mo 4, 7, and 10 | 52.5% CIS, 10% CIS/Ta, 12.5% CIS/T1, 10% Ta, 15% T1 | 42.5% 6-mo HG RF | 35% HG RF | 35 | Urgency 40% Dysuria 40% Fatigue 32.5% Hematuria 25% | |
| Boorjian et al (2021) | rAdeIFN-a/Syn3 | 151 | Phase III | 3 treatments at mo 3, 6, and 9 | 52% CIS, 13 CIS/Ta, 3% CIS/T1, 22% Ta,10% T1 | 59.6% 3-mo CR47.7% 6-mo HG RF | 30.5% HG RF | 26 | Fatigue 20% Bladder spasm 15% Urgency 14% | |
| Burke et al (2012) | CG0070 | 35 | Phase II | 63% received scheduled multidose treatment | 23% CIS, 6% CIS/Ta, 20% CIS/T1, 43% Ta, 9% T1 | 48.6 % 3-mo CR | Dysuria 71.4% Hematuria 43% Urinary frequency 43% Urgency 34% | |||
| Packiam et al (2018) | CG0070 | 45 | Phase II Interim results for patients with BCG-unresponsive NMIBC who refused cystectomy | 24 pure CIS, 8 CIS + Ta, 4 CIS + T1, 6 Ta, 3 T1 | 47% 6-mo CR | Urinary bladder spasms 36% Hematuria 28% Dysuria 25% Urgency 22% | ||||
| McKiernan et al (2011) | Nab-Paclitaxel | 18 | Prospective Phase I | Induction: 6 x weekly instillation of nab-paclitaxel with starting dose 150 mg to 500mg | 28% 6-wk CR | Dysuria 28% | ||||
| McKiernan et al (2014) | Nab-paclitaxel | 28 | Phase II | 6 treatments in 6 mo | 46% CIS, 7% CIS/Ta, 18% CIS/T1, 14% Ta, 14% T1 | 35.7% 6-wk CR | 35.7% CR | 30.6% RF | 26 | Grade 1 14% Grade 2 18% |
| Robins et al. (2017) | Nab-paclitaxel | 28 | Phase II | 500mg/100mL of nab-paclitaxel in 6 weekly intravesical instillations | Recurrent CIS, Ta, and T1 urothelial carcinoma | 36% 6-wk CR | 36% CR | 18% CR at 3 yrs | 39 | NR |
| Bassi et al. (2011) | Paclitaxel-hyaluronic acid | 16 | Phase I | No | Not specified | 60% CR at 1 week after the last instillation | 39% overall | |||
| Hurle et al (2022) | Paclitaxel-hyaluronic acid | 20 | Phase I | 12 monthly instillations | CIS±Ta-T1 | 65% 3mo CR60% 6 mo CR | 40% CR | 35 | 15% drug-related AEs | |
| Rentsch et al. (2022) | VPM1002BC | 6 | Phase I | No | CIS Ta-T1 | NR | NR | NR | NR | Anemia 33% Alguria 33% |
| Rentsch et al. (2022) | VPM1002BC | 42 | Phase I/II | Six weekly intravesical instillations followed by maintenance for 1 yr (3 instillations at 3, 6, and 12 mo after the first instillation) | T1 7 (17.5) T1/CIS 5 (12.5) Ta 6 (15.0) Ta/CIS 5 (12.5) CIS 17 (42.5)-HG urothelial carcinoma 40 (100.0) | RFS rate in the bladder was 49.3% at 60wks after trial registration (95% confidence interval [CI] 32.1–64.4%) | RFS 47.4% (95% CI 30.4–62.6% ] at 2 yr RFS 43.7% (95% CI 26.9–59.4%) at 3 years after trial registration. | 37.5 | Frequency/urgency 16.7% Genitourinary infection 33% | |
| Kowalski et al. (2012) | Vicinium | 46 | Phase II study | Up to 3 maintenance cycles of 3 weekly administrations every 3 months. | CIS±Ta-T1 | 40% 3-mo CR26.7% 6-mo CR | 15.6% CR | Renal+urinary disorders 63% | ||
| Chamie K et al (2023) | N-803+BCG | 82 cohort A | Phase II/III | BCG plus N-803 weekly for 6 consecutive weeks (initial induction treatment period). After the first disease assessment, eligible patients will receive either a 3-week maintenance course or a 6-week re-induction course (second treatment period) at Month 3. Eligible patients will continue to receive maintenance treatment in the third treatment period at Months 6, 9, 12, and 18. | CIS±Ta-T1 | (cohort A) 55% 3mo CR 56% 6mo CR | 45% 12mo CR | Dysuria Pollakiuria Haematuria |
Nadofaragene firadenovec (rAd-IFNa/Syn3)
Three studies [13–15] have evaluated the safety and preliminary efficacy of this agent that was FDA-approved for adult patients with HR BCG-unresponsive NMIBC with CIS with or without papillary tumors on December 2022. Dinney et al. [13] enrolled 17 patients with recurrent NMIBC after BCG and administered a single treatment of rAd-IFNa/Syn3. The authors did not encounter any dose limiting toxicity; urgency was the most common adverse event (88%), followed by headache (59%), fatigue (47%) and nausea (35%). High and prolonged dose related urine IFNa and Syn3 levels were measured, proving the efficacy of the gene transfer. The 12-mo CR rate was 29%.
Shore et al. [14] evaluated the efficacy of this treatment in terms of 12-mo HG RFS in a phase 2 study. 43 patients with HG BCG-refractory or relapsed NMIBC were randomly assigned to receive 1011 viral particles (vp) or 3×1011 vp/ml. The overall 12mo HG RFS was 35%. Interestingly, of the 14 patients who were recurrence free at 12 months, 10/14 (71%) had an anti-adenovirus antibody response (defined as 4 times the predose titer) compared with 11/25 (44%) who experienced recurrence.
Recently, Boorjian et al. [15] presented the results of a phase 3, multicenter, open-label, repeat-dose single-arm trial on the efficacy of rAd-IFNa/Syn3 in the management of BCG-unresponsive BCa. Eligible patients received a single intravesical 75 ml dose of rAd-IFNa/Syn3 (3×1011 viral particles/mL). Repeat dosing at 3, 6 and 9 months was done in the absence of HG recurrence.
The 3-mo CR rate for CIS was 53.4% (55/103 patients; 95% CI 43,3–63,3) and 25 of 103 patients (24,3%) remained HG recurrence-free at 12 months. Median duration of CR was 10 months. 73 of 103 patients (71%) developed recurrent HG NMIBC and five (5%) progressed to MIBC (≥pT2).
In the HG Ta or T1 cohort, 35 of 48 patients (72,9%; 95% CI 58,2–84,7) were HG recurrence-free at month 3 and 21 patients (43,8%; 95% CI 29,5–58,8) were recurrence-free at month 12. Thus, 21 of 35 (60%) patients with HG Ta or T1 NMIBC who were HG recurrence-free at 3 months maintained that status at 12 months. Median duration of HG RFS was 12.35 months.
Concerning the entire studied population, HG RFS at 1 year was 30.5%. Progression to muscle invasion was observed in 5% of patients in the CIS cohort and 6% of patients in the HG Ta/T1. 40/151 patients (26%) underwent RC by the 12th month. Median time to cystectomy for the CIS cohort was 9 months (IQR 4.9–11.0) and 8 months (5.8–13.1) for the HG Ta/T1 cohort. Patients who achieved a CR had a significantly longer median time to RC compared with those who did not (11.4 months, IQR 7.7–14.9) versus 6.4 months, 4.2–10.6; p = 0,043). The authors further confirmed the safety profile of nadofaragene firadenovec, with only three patients stopping treatment due to an adverse event, no treatment-related deaths, and no pattern of immune-related adverse events noted. The dosing schedule of nadofaragene firadenovec (one intravesical treatment every 3 months) was demonstrated to be convenient for both patients and physicians.
CG0070 (monotherapy and combination with checkpoint inhibitors)
Monotherapy activity of CG0070 in NMIBC after BCG failure has been established in the studies V0046 [16] and BOND-002 [17].
In the first phase 1 trial (V0046) 35 patients with high-risk, BCG-failure NMIBC (CIS-containing or papillary) received single or multiple (every 28 days x 3 or weekly x 6) intravesical infusions of CG0070 at 1 of 4 dose levels (1×1012, 3×1012, 1×1013 or 3×1013 viral particles). Response to treatment was based on cystoscopic assessment and biopsy or urine cytology. CG0070 demonstrated a CR rate of 48.6% at 3 months and met the safety/efficacy outcomes, with the target dose and schedule identified for a subsequent phase 2 study [16].
Next, CG0070 monotherapy was evaluated in a phase 2 trial (BOND-002) of 45 patients with BCG-failure NMIBC (CIS containing) [17]. In the interim analysis, the 6-mo CR was achieved in 47% of patients.
The phase 3 trial NCT04452591 (BOND-003) is ongoing [38]. The objective of this trial is to evaluate the activity of intravesical administration of CG0070 in patients with tissue pathology confirmed NMIBC who have BCG unresponsive disease, with either CIS with or without Ta/T1 disease. The estimated enrollement is 110 patients while the study completion date is July 2025.
A further therapeutic modality for BCG-unresponsive NMIBC is represented by the combination of CG0070 with an immune checkpoint inhibitor. CORE1 trial (phase 2) is a single arm study of CG0070 combined with pembrolizumab in patients with NMIBC unresponsive to BCG. All patients received CG0070 induction weekly (1×1012 vp/mL) for 6 weeks, followed by a second induction course weekly for 3 weeks in responders and 6 weeks in non-responders. All responders subsequently received a maintenance course weekly for 3 weeks. Patients concurrently received pembrolizumab every 6 weeks (as opposed to the usual 3 weeks) at a dose of 400 mg through year 2. Impressively, an overall CR rate of 85% was observed that was longitudinally maintained with 6-, 9-, and 12-month CR rates of 82%, 81%, and 68%, respectively. Adverse events were predominantly transient, grade 1-2 and local (genitourinary) [39].
Paclitaxel-hyaluronic acid
Bassi et al. [18] provided the first phase 1 study on paclitaxel-hyaluronic acid bioconjugate (ONCOFID-P-B™) given by intravesical instillation to 16 patients with BCG-refractory CIS. The solution was administered for 6 consecutive weeks. At 1 week after the last instillation CR rate was 60%. No dose limiting toxicity occurred at any drug level evaluated. A total of 11 adverse events were reported by 7 patients.
In another phase 1 trial, Hurle et al. [19] evaluated the safety and efficacy of Oncofid P-B in 20 patients with CIS±Ta-T1, unresponsive or intolerant to BCG, and unwilling or unfit for cystectomy. The drug was administered by intravesical instillation for 12 consecutive weeks (intensive phase) followed, in CR patients, by 12 monthly instillations (maintenance phase). The CR was defined as a negative cystoscopy, negative biopsy of the urothelium and negative cytology. At the end of the intensive phase, 15 of the 20 enrolled patients (75%), achieved the CR. Patients still in CR after 3, 6, 9 and 12 months of maintenance phase were 13 (65%), 12 (60%), 9 (45%) and 8 (40%), respectively. Only seven (5 mild and 2 moderate) drug-related adverse events were reported in three patients.
Nanoparticle albumin-bound paclitaxel
In 2011 McKiernan et al. [20] provided the first phase 1 trial to evaluate the dose limiting toxicity and maximum deliverable dose of intravesical nab-paclitaxel. A total of 18 patients (13 men, 5 women) were enrolled in the study. Grade 1 local toxicities were experienced by 10 (56%) patients with dysuria being the most common, and no grade 2, 3 or 4 drug related local toxicities were encountered. 6-wk CR rate was 28%.
McKiernan et al. [21], assessed the efficacy of the drug in patients with recurrent CIS, T1 and Ta urothelial carcinoma in whom at least 1 prior regimen of intravesical BCG failed. Patients received 500 mg/100 ml nab-paclitaxel administered in 6 weekly intravesical instillations plus monthly maintenance treatments for 6 months for complete responders. A total of 28 patients were enrolled in the study. 6-wk CR rate was 35.7%. At 1 year all of these responses remained durable after maintenance therapy. At a mean followup of 21 months (range 5–47) 19/28 (67.8%) patients retained their bladders without progression or distant metastases. Only grade 1-2 adverse events were noted in 9 of 28 (32.1%) patients.
Robins et al. [22] reported the long-term follow-up results of the trial reported in [14]. The CR rate was 36% at 6-wk assessment. Three-year RFS rate was 18% while the five-year OS and CSS rates were 56% and 91%, respectively. RC-free survival was 61%.
VPM1002BC
Rentsch et al. [23] reported on patient safety of the first intravesical application of VPM1002BC in humans. Six patients with BCG failure received a treatment of 6 weekly instillations with VPM1002BC. No DLT (dose limiting toxicity) occurred during the DLT-period. No grade≥3 adverse events occurred. Excretion of VPM1002BC in the urine was limited to less than 24 hours.
Rentch et al. also provided the first phase 1-2 trial reporting on the efficacy, safety, tolerability and quality of life of intravesical VPM1002BC for the treatment of NMIBC recurrence after conventional BCG therapy [24]. Patients were scheduled for standard treatment of six weekly instillations with VPM1002BC followed by maintenance for 1 yr. Treatment was stopped in cases of recurrence. The RFS rate in the bladder was 49.3% at 60wks after trial registration (95% confidence interval [CI] 32.1–64.4%) and remained at 47.4% (95% CI 30.4–62.6% ] at 2 yr and 43.7% (95% CI 26.9–59.4%) at 3 years after trial registration. Treatment-related adverse events were mainly of grade 1-2. No grade≥4 adverse events occurred.
Vicinium (Oportuzumab Monatox, VB4-845)
In Phase 1 and 2 studies, intravesical Vicinium demonstrated good safety profile and meaningful clinical activity in BCG-unresponsive high-grade NMIBC. In the study of Kowalski et al. [25] 46 patients received 1 induction cycle of 6 (cohort 1) or 12 (cohort 2) weekly intravesical oportuzumab monatox instillations of 30 mg, followed by up to 3 maintenance cycles of 3 weekly administrations every 3 months. 3-mo CR rate was 41% in cohort 1 and 39% in cohort 2; the overall CR rate was 44%. For complete responders, median time to recurrence was 274 and 408 days in cohorts 1 and 2, respectively. The most common adverse events were mild to moderate reversible bladder symptoms.
Moreover, the single-arm multicenter registrational phase III VISTA trial (NCT02449239) has been completed and presented as a congress abstract [40]. The CR rate of the evaluable CIS patients (n = 89) at 3 months was 40%. Of the 3-month CIS responders, 52% remained disease-free for 12 months. The recurrence-free rates of the evaluable papillary patients (n = 38) at 3, 12 and 24 months were 71, 50 and 37%, respectively. Overall, the rate of RC was 10% (6 of 63) for the 3-month responders. Vicinium was well tolerated with 52% of patients experiencing treatment-related adverse events, the majority being grade 1–2.
The FDA denied the biologic license application in August, 2021 for Vicinium for the treatment of BCG-unresponsive NMIBC.
A phase I study completed accrual testing the combination of Vicinium with Durvalumab in high-grade NMIBC pretreated with BCG and the results are pending [41].
ALT-803 (N-803)
In a recent open-label, multicenter study [26], patients with BCG-unresponsive bladder CIS with or without Ta/T1 papillary disease were treated with intravesical N-803 plus BCG (cohort A) or N-803 alone (cohort C). Patients with BCG-unresponsive HG Ta/T1 papillary NMIBC also received N-803 plus BCG (cohort B). In cohort A, at the 3-, 6-, and 12-month response assessments, CR rates were 55% (45 of 82 patients; 95% CI = 43.5% to 65.9%), 56% (46 of 82 patients; 95% CI = 44.7% to 67.0%), and 45% (37 of 82 patients; 95% CI = 34.1% to 56.5%), respectively. At 24 months in patients with CR, the Kaplan–Meier estimated probability of avoiding cystectomy and of DSS was 89.2% and 100%, respectively. The efficacy population in cohort B consisted of 72 evaluable patients with a median follow-up duration of 20.7 months (range, 2.9 to 37.1 months). DFS rates at 12, 18, and 24 months were 55.4% (95% CI = 42.0% to 66.8%), 51.1% (95% CI = 37.6% to 63.1%), and 48.3% (95% CI = 34.5% to 60.7%), respectively, by Kaplan–Meier analysis. The cystectomy rate was 7% (5 of 72 patients). Concerning cohort C, CR at 3 months was achieved in only 2 (20%) of 10 patients who were administered N-803 alone. Six patients underwent reinduction. Only one patient (10%) maintained a CR at 6 months. On the basis of protocol-defined stopping rules, cohort C was discontinued for futility and enrollement was stopped. The most frequently reported adverse events for patients who received BCG plus N-803 (cohorts A and B, n = 161) were those expected for intravesical instillation of BCG and included dysuria, pollakiuria, and haematuria. The FDA issued a complete response letter on May 11, 2023 regarding the biologic license application.
Other agents
a) TAR-200.
The GemRIS device, developed by Taris Biomedical, is a drug delivery system designed for prolonged intravesical drug delivery. The device consists of a 5-cm semipermeable silicone tube that functions as an osmotic pump and slowly releases dissolving gemcitabine tablets [42]. Pharmacokinetically, 60–70% of the drug load is delivered over 2 wk, compared to the 2-h conventional dwell time for intravesical drugs [43].
The GemRIS device is inserted via an 18 F urethral catheter and self-coils into a pretzel shape intravesically [44]. A phase 1 marker lesion trial among 12 NMIBC patients was recently conducted. In one of two arms, patients received the device on day 0 for 7 d, a second device on day 21 for 7 d, followed by TURB on day 28. The second arm investigated two 21-d courses of the device without a holiday between treatment courses and TURB on day 42. Patients were followed for 2 yr for safety, tollerability and RFS. Results from this trial have not yet been reported [45].
In AUA 2023 the first results from the randomized trial SunRISe-1 in patients with BCG-unresponsive HR NMIBC (CIS patients (+/–papillary disease) who did not receive a RC) receiving TAR-200 in combination with Cetrelimab, TAR-200 alone, or Cetrelimab alone were presented. From an efficacy standpoint, 73% of patients in the TAR-200 arm achieved a CR while the CR rate in the cetrelimab arm was 38%. Overall, most AEs in the TAR-200 group were grade≤2. 9% of patients discontinued TAR-200 and 4% cetrelimab due to treatment-related adverse events [46].
b) Intravesical immune therapy.
One in vivo study in a mouse model demonstrated a similar efficacy between intravesical administration of PD-1 inhibitor and systemic anti-PD-1 drug [47]. Two further small studies of intravesical Pembrolizumab (NCT 02808143) and Durvalumab (NCT03759496) in patients with HG BCG-unresponsive NMIBC are recruiting [48].
c) TARA-002.
TARA-002 is a lyophilized biological preparation for instillation containing cells of Streptococcus pyogenes (Group A, type 3) su strain treated with benzylpenicillin. The safety and toxicity profile of TARA-002 in adults with HG NMIBC is currently being evaluated by the ongoing phase 1a/b ADVANCED-1 trial [49]. In the month of July 2023, the new TARA-002-101-Ph1b/2 (ADVANCED-2) trial has been posted. This trial is an open-label Phase 1b/2 dose expansion study planned to investigate the safety and anti-tumor activity of intravesical treatment of high-grade CIS NMIBC (±Ta/T1) with intravesical instillation of TARA-002 in adult patients. Participants will be enrolled into one of 2 cohorts:
Cohort A:
• Participants with CIS (±Ta/T1) who are unable to obtain intravesical BCG, or
• Participants with CIS (±Ta/T1) who have not received intravesical BCG for 24 months prior to CIS diagnosis
Cohort B:
• Participants with CIS (±Ta/T1) who are BCG unresponsive after completion of adequate BCG therapy (minimum 5/6 doses induction and 2/3 doses maintenance or 2/6 doses reinduction)
The study plans to enroll 102 patients and to be completed in May 2026 [50].
Discussion
BCG is the standard therapy after TURB for high-risk NMIBC. However, post-BCG recurrence/progression occurs frequently and noncystectomy options are limited.
The only US FDA-approved bladder-sparing treatment options for BCG-unresponsive high-risk NMIBC are intravesical thiotepa (which is not used anymore due to its lack of efficacy), intravesical valrubicin, characterised by questionable efficacy, intravenous pembrolizumab [48, 51] and nadofaragene.
Several other treatments have been evaluated without definitive outcomes. Further BCG therapy is not recommended. Mycobacterium phlei cell wall-nucleic acid complex (MCNA), although promising in preliminary reports of efficacy [52] did not receive FDA approval as the results were not confirmed by a phase 3 trial [48]. Cytotoxic intravesical chemotherapic agents, alone or in combination, have also been adopted for the management of NMIBC after BCG-failure. The use of intravesical gemcitabine and doxetaxel has been evaluated by some studies that, although promising lack recent updates [7, 9]. Concerning these two drugs, there is more recent interest on their sequential administration that although supported by retrospective studies [53], lacks prospective validation.
Device-assisted instillation of mitomycin c, through thermo-chemotherapy or electromotive drug administration have also provided interesting results [48], that warrant further clinical investigation.
Widespread adoption of these agents has been hampered by small sample sizes, heterogeneous cohorts, lack of long-term results and of solid prospective validation.
Currently, no established and effective intravesical therapies are available for those patients whose tumors recur after BCG. Thus, according to the EAU guidelines [54], treatments other than RC in this setting must be considered oncologically inferior to RC. Nevertheless, many innovative agents are in the pipeline, and hopefully will be ready to be used soon in a routine setting. Overall, these emerging bladder-sparing treatments appear to have 12-month efficacy ranging between 29–60%, combined with a favourable toxicity profile, characterised mainly by low-grade adverse events and no treatment-related deaths.
In particular, the phase 3 trial reported efficacy and the manageable adverse event profile may render the nadofaragene firadenovec the new gold standard for patients with BCG-unresponsive NMIBC [55], especially for those unwilling to undergo or unfit for RC. This agent showed first-of-its-kind efficacy for gene therapy, with a 3 mo CR rate of nearly 60% in all enrolled and treated patients. At 12 mo, 30.5% of all patients were free from HG recurrence. The results compare favorably to pembrolizumab. 94.7% of patients did not progress to muscle invasion during the study, and of those who did, salvage cystectomy was feasible.
Phase 1 trials have been published for paclitaxel-hyaluronic acid (Oncofid-P-B™). Only a phase II “marker lesion” study on the ablative activity of the Oncofid-P-B has been published. The paper reports a CR rate of 45% after the induction cycle with a very good tolerability and safety profile. However, the paper is not followed by a full-text publication yet [56].
Phase 1-2 trials for intravesical nanoparticle albumin bound paclitaxel demonstrated a 12 mo CR rate of 36% and 18% at 3 year and RC-free survival was 61% accompanied by only grade 1 local toxicities [22]. To our knowledge, no phase 3 studies are available on these agents.
Concerning the recombinant mycobacterium BCG VPM1002BC, both phase 1 and 2 trials have been conducted already. At 1 year after starting treatment, almost half of the patients remained recurrence-free. The 3-yr outcome of 43.7% of the patients remaining without recurrence is promising. Again, no phase 3 trials have been conducted yet.
Phase 1 and 2 trials have also documented an adequate safety profile and promising activity for intravesical CG0070 (a GM-CSF expressing oncolytic adenovirus) while the phase 3 trial NCT04452591 (BOND-003) is ongoing [38].
Similarly, the final analysis of the results of the phase 3 VISTA trial (NCT02449239) on Vicinium, that are promising but only published as a congress abstract, is pending until final publication is available.
FDA-approved pembrolizumab and nadofaragene represent the most robust and promising advances made in the last 20 years for patients with HR NMIBC who do not respond, or have recurring disease, after BCG. Considering the options that are currently under scrutiny, with multiple promising local and systemic agents that are currently in phase I-III trials, one can reasonably expect that clinicians will soon have at their disposal new agents and treatment options for salvage therapy after BCG failure.
Future directions
The inherent properties of the bladder pose the biggest obstacle to developing effective intravesical treatments for NMIBC. Current research is now focusing on methods to improve the delivery of intravesical therapies, through the development of innovative intravesical drug delivery devices [45], nanocarriers that are used to optimize targeted drug delivery, reverse thermosensitive hydrogels that increase the dwell time of intravesical drugs as well as (magnetic) mucoadhesives [42].
Conclusions
The critical clinical need for the development of an effective, safe and durable intravesical drug for the salvage treatment of high-risk NMIBC seems to be met mainly by gene therapy. In this context, data support nadofaragene firadenovec as a potentially important therapeutic advancement for a historically difficult-to-treat disease. N803/BCG and innovative drug delivery systems seem promising as well. Considering the plethora of novel intravesical treatment that are currently emerging, it will be up to the urologist and future research to identify, for each specific patient, the right agent to use, based on safety, results and cost-effectiveness.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
A.D.A. conception and design; analysis and interpretation of data; writing the article; critical revision of the manuscript for important intellectual content.
M.K. interpretation of data; critical revision of the manuscript for important intellectual content.
G.C. acquisition of data; material support; drafting of the tables; critical revision of the manuscript for important intellectual content.
O.F. critical revision of the manuscript for important intellectual content.
F.H. critical revision of the manuscript for important intellectual content.
M.R. critical revision of the manuscript for important intellectual content.
A.G. critical revision of the manuscript for important intellectual content.
P.G. interpretation of data; critical revision of the manuscript for important intellectual content.
G.G: conception; performance of work; interpretation of data; critical revision of the manuscript for important intellectual content.
ETHICAL CONSIDERATIONS
This study, as a literature review is exempt from any requirement for Institutional Review Board approval. No human or animal research was involved in the elaboration of this manuscript.
CONFLICT OF INTEREST
A.D.A., M.K., G.C., O.F., F.H., M.R., A.G., P.G. have no conflicts of interest concerning the manuscript. G.G. is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and/or its supplementary material.
REFERENCES
[1] | van Rhijn BWG , Burger M , Lotan Y , Solsona E , Stief CG , Sylvester RJ , Witjes JA , Zlotta AR . Recurrence and progression of disease in non–muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. (2009) ;56: :430–42. |
[2] | Pignot G , Baboudjian M , Lebacle C , Chamouni A , Lechevallier E , Irani J , Tillou X , Waeckel T , Monges A , Doisy L , Walz J , Gravis G , Mourey E , Duperron C , Masson-Lecomte A , Efficacy of hyperthermic intravesical chemotherapy (HIVEC) in patients with non-muscle invasive bladder cancer after BCG failure. World J Urol. 2023 Feb 22. doi: 10.1007/s00345-023-04332-z |
[3] | Witjes JA . Management of BCG failures in superficial bladder cancer: a review. Eur Urol. (2006) ;49: (5):790–7. |
[4] | Catto JWF , Khetrapal P , Ricciardi F , Ambler G , Williams NR , Al-Hammouri T , Khan MS , Thurairaja R , Nair R , Feber A , Dixon S , Nathan S , Briggs T , Sridhar A , Ahmad I , Bhatt J , Charlesworth P , Blick C , Cumberbatch MG , Hussain SA , Kotwal S , Koupparis A , McGrath J , Noon AP , Rowe E , Vasdev N , Hanchanale V , Hagan D , Brew-Graves C , Kelly JD ; iROC Study Team. Effect of robot-assisted radical cystectomy with intracorporeal urinary diversion vs open radical cystectomy on 90-day morbidity and mortality among patients with bladder cancer: a randomized clinical trial. JAMA. (2022) ;327: :2092–103. |
[5] | Yang LS , Shan BL , Shan LL , Chin P , Murray S , Ahmadi N , Saxena A . A systematic review and metaanalysis of quality of life outcomes after radical cystectomy for bladder cancer. Surg Oncol. (2016) ;25: :281–97. |
[6] | Gallagher BL , Joudi FN , Maymí JL , O’Donnell MA . Impact of previous bacille Calmette-Guérin failure pattern on subsequent response to bacille Calmette-Guérin plus interferon intravesical therapy. Urology. (2008) ;71: (2):297–301. |
[7] | Di Lorenzo G , Perdona S , Damiano R , Faiella A , Cantiello F , Pignata S , Ascierto P , Simeone E , De Sio M , Autorino R . Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer. Cancer. (2010) ;116: (8):1893–900. |
[8] | Dinney CP , Greenberg RE , Steinberg GD . Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol. (2013) ;31: (8):1635–42. |
[9] | Barlow LJ , McKiernan JM , Benson MC . The novel use of intravesical docetaxel for the treatment of non-muscle invasive bladder cancer refractory to BCG therapy: a single institution experience. World J Urol. (2009) ;27: (3):331–5. |
[10] | Barlow LJ , McKiernan JM , Benson MC . Long-term survival outcomes with intravesical docetaxel for recurrent non muscle invasive bladder cancer after previous Bacillus Calmette–Guérin Therapy. J Urol. (2013) ;189: (3):834–9. |
[11] | Skinner EC , Goldman B , Sakr WA , Petrylak DP , Lenz H-J , Lee CT , Wilson SS , Benson M , Lerner SP , Tangen CM , Thompson IM . SWOG Sphase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical Bacillus Calmette–Guérin. J Urol. (2013) ;190: (4):1200–4. |
[12] | Balar AV , Kamat AM , Kulkarni GS , Uchio EM , Boormans JL , Roumiguié M , Krieger LEM , Singer EA , Bajorin DF , Grivas P , Seo HK , Nishiyama H , Konety BR , Li H , Nam K , Kapadia E , Frenkl T , de Wit R . Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. (2021) ;22: (7):919–30. |
[13] | Dinney CP , Fisher MB , Navai N , O’Donnell MA , Cutler D , Abraham A , Young S , Hutchins B , Caceres M , Kishnani N , Sode G , Cullen C , Zhang G , Grossman HB , Kamat AM , Gonzales M , Kincaid M , Ainslie N , Maneval DC , Wszolek MF , Benedict WF . Phase I trial of intravesical recombinant adenovirus mediated interferon-α2b formulated in Syn3 for Bacillus Calmette-Guérin failures in nonmuscle invasive bladder cancer. J Urol. (2013) ;190: (3):850–6. |
[14] | Shore ND , Boorjian SA , Canter DJ , Ogan K , Karsh LI , Downs TM , Gomella LG , Kamat AM , Lotan Y , Svatek RS , Bivalacqua TJ , Grubb RL 3rd, Krupski TL , Lerner SP , Woods ME , Inman BA , Milowsky MI , Boyd A , Treasure FP , Gregory G , Sawutz DG , Yla-Herttuala S , Parker NR , Dinney CPN . Intravesical rAd-IFNα/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J Clin Oncol. (2017) ;35: (30):3410–6. |
[15] | Boorjian SA , Alemozaffar M , Konety BR , Shore ND , Gomella LG , Kamat AM , Bivalacqua TJ , Montgomery JS , Lerner SP , Busby JE , Poch M , Crispen PL , Steinberg GD , Schuckman AK , Downs TM , Svatek RS , Mashni J Jr, Lane BR , Guzzo TJ , Bratslavsky G , Karsh LI , Woods ME , Brown G , Canter D , Luchey A , Lotan Y , Krupski T , Inman BA , Williams MB , Cookson MS , Keegan KA , Andriole GL Jr, Sankin AI , Boyd A , O’Donnell MA , Sawutz D , Philipson R , Coll R , Narayan VM , Treasure FP , Yla-Herttuala S , Parker NR , Dinney CPN . Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. (2021) ;22: (1):107–17. |
[16] | Burke JM , Lamm DL , Meng MV , Nemunaitis JJ , Stephenson JJ , Arseneau JC , Aimi J , Lerner S , Yeung AW , Kazarian T , Maslyar DJ , McKiernan JM . A first in human phase 1 study of CGa GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol. (2012) ;188: (6):2391–7. |
[17] | Packiam VT , Lamm DL , Barocas DA , Trainer A , Fand B , Davis RL 3rd, Clark W , Kroeger M , Dumbadze I , Chamie K , Kader AK , Curran D , Gutheil J , Kuan A , Yeung AW , Steinberg GD . An open label, single-arm, phase II multicenter study of the safety and efficacy of CGoncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol. (2018) ;36: (10):440–7. |
[18] | Bassi PF , Volpe A , D’Agostino D , Palermo G , Renier D , Franchini S , Rosato A , Racioppi M . Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette-Guérin refractory carcinoma in situ of the bladder: results of a phase I study. J Urol. (2011) ;185: (2):445–9. |
[19] | Hurle R , Guazzoni G , Colombo P , Santoro A , De Cobelli O , Trapani ED , Nohales G , Carlos L , Duran-Merino R , Lazzeri M . Oncofid-P-B: a novel treatment for BCG unresponsive carcinoma in situ (CIS) of the bladder: Results of a prospective European Multicentre study at 15 months from treatment start. Urol Oncol. (2022) ;40: (1):11.e9–11.e15. |
[20] | McKiernan JM , Barlow LJ , Laudano MA , Mann MJ , Petrylak DP , Benson MC . A phase I trial of intravesical nanoparticle albumin-bound paclitaxel in the treatment of bacillus Calmette-Guérin refractory nonmuscle invasive bladder cancer. J Urol. (2011) ;186: (2):448–51. |
[21] | McKiernan JM , Holder DD , Ghandour RA , Barlow LJ , Ahn JJ , Kates M , Badalato GM , Roychoudhury A , Decastro GJ , Benson MC . Phase II trial of intravesical nanoparticle albumin bound paclitaxel for the treatment of nonmuscle invasive urothelial carcinoma of the bladder after bacillus Calmette-Guérin treatment failure. J Urol. (2014) ;192: (6):1633–8. |
[22] | Robins DJ , Sui W , Matulay JT , Ghandour R , Anderson CB , DeCastro GJ , McKiernan JM . Long-term Survival Outcomes With Intravesical Nanoparticle Albumin-bound Paclitaxel for Recurrent Non-muscle-invasive Bladder Cancer After Previous Bacillus Calmette-Guérin Therapy. Urology. (2017) ;103: :149–53. |
[23] | Rentsch CA , Bosshard P , Mayor G , Rieken M , Püschel H , Wirth G , Cathomas R , Parzmair GP , Grode L , Eisele B , Sharma H , Gupta M , Gairola S , Shaligram U , Goldenberger D , Spertini F , Audran R , Enoiu M , Berardi S , Hayoz S , Wicki A . Results of the phase I open label clinical trial SAKK 06/14 assessing safety of intravesical instillation of VPMBC, a recombinant mycobacterium Bacillus Calmette Guérin (BCG), in patients with non-muscle invasive bladder cancer and previous failure of conventional BCG therapy. Oncoimmunology. (2020) ;9: (1):1748981. |
[24] | Rentsch CA , Thalmann GN , Lucca I , Kwiatkowski M , Wirth GJ , Strebel RT , Engeler D , Pedrazzini A , Hüttenbrink C , Schultze-Seemann W , Torpai R , Bubendorf L , Wicki A , Roth B , Bosshard P , Püschel H , Boll DT , Hefermehl L , Roghmann F , Gierth M , Ribi K , Schäfer S , Hayoz S . A Phase 1/2 Single-arm Clinical Trial of Recombinant Bacillus Calmette-Guérin (BCG) VPMBC Immunotherapy in Non-muscle-invasive Bladder Cancer Recurrence After Conventional BCG Therapy: SAKK 06/14. Eur Urol Oncol. (2022) ;5: (2):195–202. |
[25] | Kowalski M , Guindon J , Brazas L , Moore C , Entwistle J , Cizeau J , Jewett MAS , MacDonald GC . A phase II study of oportuzumab monatox: an immunotoxin therapy for patients with noninvasive urothelial carcinoma in situ previously treated with Bacillus Calmette–Guérin. J Urol. (2012) ;188: (5):1712–8. |
[26] | Chamie K , Chang SS , Kramolowsky E , Gonzalgo ML , Agarwal PK , Bassett JC , Bjurlin M , Cher ML , Clark W , Cowan BE , David R . IL-15 superagonist NAI in BCG-unresponsive non–muscle-invasive bladder cancer. NEJM Evidence. (2022) ;2: (1):EVIDoa2200167. |
[27] | Benedict WF , Tao Z , Kim CS , Zhang X , Zhou JH , Adam L , McConkey DJ , Papageorgiou A , Munsell M , Philopena J , Engler H , Demers W , Maneval DC , Dinney CP , Connor RJ . Intravesical Ad-IFN alpha causes marked regression of human bladder cancer growing orthotopically in nude miceand overcomes resistance to IFN alpha protein. Mol Ther. (2004) ;10: :525–32. |
[28] | Tao Z , Connor RJ , Ashoori F , Dinney CP , Munsell M , Philopena JA , Benedict WF . Efficacy of a single intravesical treatment with Ad-IFN/Syn 3 is dependent on dose and urine IFN concentration obtained: Implications for clinical investigation. Cancer Gene Ther. (2006) ;13: :125–30. |
[29] | Connor RJ , Anderson JM , Machemer T , Maneval DC , Engler H . Sustained intravesical interferon protein exposure is achieved using an adenoviral-mediated gene delivery system: A study in rats evaluating dosing regimens. Urology. (2005) ;66: :224–9. |
[30] | Yamashita M , Rosser CJ , Zhou JH , Zhang XQ , Connor RJ , Engler H , Maneval DC , Karashima T , Czerniak BA , Dinney CPN , Benedict WF . Syn3 provides high levels of intravesical adenoviral-mediated gene transfer for gene therapy of genetically altered urothelium and superficial bladder cancer. Cancer Gene Ther. (2002) ;9: :687–91. |
[31] | Ramesh N , Ge Y , Ennist DL , Zhu M , Mina M , Ganesh S , Reddy PS , Yu DC . CGa conditionally replicating granulocyte macrophage colony-stimulating factor–armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res. (2006) ;12: :305–13. |
[32] | Rosato A , Banzato A , De Luca G , Renier D , Bettella F , Pagano C , Esposito G , Zanovello P , Bassi P . HYTAD1-p a new paclitaxel-hyaluronic acid hydrosoluble bioconjugate for treatment of superficial bladder cancer. Urol Oncol. (2006) ;24: :207–15. |
[33] | de Wit R , Kruit WH , Stoter G , e Boer M , Kerger J , Verweij J . Docetaxel (Taxotere): an active agent in metastatic urothelial cancer; results of a phase II study in nonchemotherapy-pretreated patients.. Br J Cancer. (1998) ;78: :1342. |
[34] | Sparreboom A , Scripture CD , Trieu V , Williams PJ , De T , Yang A , Beals B , Figg WD , Hawkins M , Desai N . Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol). Clin Cancer Res. (2005) ;11: :4136. |
[35] | Gradishar WJ , Tjulandin S , Davidson N , Shaw H , Desai N , Bhar P , Hawkins M , O’Shaughnessy J . Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oilbased paclitaxel in women with breast cancer. J Clin Oncol. (2005) ;23: :7794. |
[36] | Harwood O , O’Connor S . Therapeutic Potential of IL-15 and N-803 in HIV/SIV Infection. Viruses. (2021) ;13: (9):1750. |
[37] | Chen W , Liu N , Yuan Y , Zhu M , Hu X , Hu W , Wang S , Wang C , Huang B , Xing D . ALT-803 in the treatment of non-muscle-invasive bladder cancer: Preclinical and clinical evidence and translational potential. Front Immunol. (2022) ;13: :1040669. |
[38] | https://classic.clinicaltrials.gov/ct2/show/NCT04452591?term=bond+003&draw=2&rank=1, accessed on 12.07. 2023. |
[39] | https://classic.clinicaltrials.gov/ct2/show/NCT04387461?term=Core+001&draw=2&rank=1, accessed on 12.07. 2023. |
[40] | Shore N , Beach M , O’Donnell M , Jewett MAS , Kulkarni GS , Dickstein R , Wolk F , Dunshee C , Belkoff L , Dillon RL , Cizeau J , Kassouf W . Phase 3 results of Vicinium in BCG-unresponsive non-muscle invasive bladder cancer. J Urol. (2020) ;203: (4S, Supplement):PD03–02 e 72. |
[41] | https://classic.clinicaltrials.gov/ct2/show/NCT03258593, accessed on 12.07.2023. |
[42] | [42] Douglass L , Schoenberg M . The future of intravesical drug delivery for non-muscle invasive bladder cancer. Bladder Cancer. (2016) ;2: :285–92. |
[43] | Nickel JC , Jain P , Shore N , Anderson J , Giesing D , Lee H , Kim G , Daniel K , White S , Larrivee-Elkins C , Lekstrom-Himes J , Cima M . Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: safety and efficacy of a new drug delivery device. Sci Transl Med. (2012) ;4: :143ra100. |
[44] | Tan WS , Kelly JD . Intravesical device-assisted therapies for non- muscle-invasive bladder cancer. Nat Rev Urol. (2018) ;15: :667. |
[45] | Grimberg DC , Shat A , Inam BA . Overview of Taris Gem-RIS, a novel drug delivery system for bladder cancer. Eur Urol focus. 2020. |
[46] | |
[47] | Rajkumar A , Wang J , Neuzil KE , Kirschner AN , Chang SS . Intravesical anti-PD-1 immune checkpoint inhibition in urothelial bladder cancer in a mouse model. J Clin Oncol. (2020) ;38: (6_suppl):537–7. |
[48] | Lebacle C , Loriot Y , Irani J . BCG-unresponsive high-grade non-muscle invasive bladder cancer: what does the practicing urologist need to know? World J Urol. (2021) ;39: (11):4037–46. |
[49] | https://classic.clinicaltrials.gov/ct2/show/NCT05085977, accessed on 12.07.2023. |
[50] | https://www.clinicaltrials.gov/study/NCT05951179?term=tara-002&rank=1, accessed on 01-08-2023. |
[51] | Bree KK , Brooks NA , Kamat AM . Current Therapy and Emerging Intravesical Agents to Treat Non-Muscle Invasive Bladder Cancer. Hematol Oncol Clin North Am. (2021) ;35: (3):513–29. |
[52] | Li R , Amrhein J , Cohen Z , Champagne M , Kamat A . Efficacy of mycobacterium phlei cell wall-nucleic acid complex (MCNA) in BCG-unresponsive patients. Bladder Cancer. (2017) ;3: (1):65–71. |
[53] | Steinberg RL , Thomas LJ , Brooks N , Mott SL , Vitale A , Crump T , Rao MY , Daniels MJ , Wang J , Nagaraju S , DeWolf WC , Lamm DL , Kates M , Hyndman ME , Kamat AM , Bivalacqua TJ , Nepple KG , O’Donnell MA . Multi-institution evaluation of sequential gemcitabine and docetaxel as rescue therapy for nonmuscle invasive bladder cancer. J Urol. (2020) ;203: (5):902–9. |
[54] | European Urology guidelines. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Non-muscle-Invasive-Bladder-Cancer-2023_2023-03-10-101110jued.pdf |
[55] | Kulkarni GS . Nadofaragene firadenovec: a new gold standard for BCG-unresponsive bladder cancer? Lancet Oncol. (2021) ;22: (1):8–9. doi: 10.1016/S1470-2045(20)30586-6. |
[56] | Bassi PF , Racioppi M , Palermo G , Selli C , Battaglia M , Gontero P , Simeone C , Eisendhardt A , Vom Dorp F , Neisius A , Arjona MF , Castro Diaz D , Ramirez Backhaus M , Moreno Sierra J , Llorente C , Nohales Taurines G , Giordan N , Marinello A . Oncofid-P-B (paclitaxel-hyaluronic acid) in the intravesical therapy of patients affected by primary or recurrent Ta G1-G2 papillary cancer of the bladder. A phase II marker lesion study. European Urology Supplements. (1056) ;17: (2):e1056–e1057. |




