Electromotive Drug Administration Chemotherapy with Mitomycin C Versus Bacillus Calmette-Guerin for the Treatment of Non-Muscle Invasive Bladder Cancer
Abstract
BACKGROUND:
Devices that increase the penetrance of intravesical chemotherapeutics are emerging as alternatives to classical Bacillus Calmette Guérin (BCG) treatment.
OBJECTIVE:
To compare the efficacy of mitomycin C applied with the electromotive drug delivery device (MMC-EMDA) versus BCG in patients with intermediate and high-risk non-muscle-invasive bladder cancer (NMIBC) without carcinoma in situ (CIS).
METHODS:
Prospective non-randomized study in which 47 patients received MMC-EMDA (40 mg of MMC diluted in 50 mg of distilled water at 20 mA for 30 min. Regimen of 6 weekly and then 6 monthly instillations) and 48 patients received BCG (50 mg of OncoCITE® diluted in 50 ml of normal saline for 60 min. Regimen of 6 weekly instillations and then 3 weekly instillations at months 3, 6 and 12). The primary endpoint was the recurrence-free rate (RFR) at 24 months. Secondary endpoints were time to recurrence and progression-free rate (PFR) at 24 months follow-up.
RESULTS:
Baseline patient assessment and mean follow-up time were similar in both groups (MMC-EMDA group: 26.4 months; BCG group: 28.4 months (p = 0.44)). The RFR at 24 months was 80.9% for the MMC-EMDA group and 77.1% for the BCG group (p = 0.969). The mean time to recurrence was 12.5 months in the MMC-EMDA group and 14 months in the BCG group (p = 0.681). At 24 months, PFR was 97.9% in the MMC-EMDA group and 93.8% in the BCG group (p = 0.419).
CONCLUSIONS:
No differences were found between MMC-EMDA and BCG treatments in patients with high-risk and intermediate-risk NMIBC without CIS.
INTRODUCTION
Non-muscle invasive bladder cancer (NMIBC) is characterized by a high risk of recurrence and progression. In order to reduce tumor recurrence and progression, adjuvant intravesical therapies are recommended after transurethral resection of the bladder tumors (TURBT). The standard treatment for patients at high risk of progression is immunotherapy with Bacillus Calmette Guérin (BCG) in an induction and maintenance regimen. However, a high percentage of these patients experience recurrence or progression despite having receiving such treatment [1, 2]. In addition, BCG treatment is associated with a high percentage of local and systemic adverse events [3].
As an alternative to immunotherapy, the use of chemotherapy has been proposed. The most commonly used molecule is mitomycin C (MMC) which has the disadvantage of having a high molecular weight (334KDa), meaning its effect on the deeper regions of the bladder wall may not be optimal [4]. All of the above has led to the development of new devices that increase the penetration of chemotherapeutic agents in the bladder wall with the aim of increasing the alternatives to classical BCG immunotherapy treatment [5].
The electromotive drug administration (EMDA) device improves MMC penetration into the bladder wall [6–8]. This is achieved through a generator that emits a controlled electric current from an anode, consisting of a specific 16 Fr catheter that also introduces the drug inside the bladder, to a cathode placed in the hypogastrium. The combination of chemotherapy with the local electrical current causes a directional and accelerated movement of the ionized drug toward the tissue. The electrokinetic phenomena of electroosmosis, iontophoresis, and electroporation are added to this [9].
Intravesical chemotherapy of MMC enhanced with EMDA (MMC-EMDA) as an adjuvant treatment has been studied in two clinical trials that establish that MMC-EMDA is an effective alternative to BCG. Di Stasi, in 2003 [10], applied it to a cohort of patients with carcinoma in situ (CIS). Later, the same author applied MMC-EMDA alternating with BCG instillations in patients with T1 urothelial carcinoma [11]. For this reason, the most suitable patient profile for this therapy, and the most optimal administration regimen still need to be clarified.
This study aims to compare adjuvant intravesical chemotherapy treatment with MMC enhanced with the EMDA device versus BCG treatment in patients with intermediate and high risk NMIBC without CIS. The primary objective is to evaluate the recurrence-free rate (RFR) at 24 months. The secondary objectives are to evaluate the time to recurrence, the progression free rate (PFR), and the safety of the application of chemotherapy with the EMDA device.
MATERIALS AND METHODS
Non-randomized study in patients receiving MMC-EDMA or BCG for NMIBC.
Each patient chose their assignment to either group after receiving information about each therapeutic method and its adverse events.
Enrollment commenced in 2019 and continued through 2021. The Local Ethics Committee approved the study. All patients signed an informed consent prior to the start of treatment.
Inclusion criteria: Patients older than 18 years with histologically confirmed urothelial carcinoma and classified in intermediate and high-risk groups. Primary or recurrent bladder cancer without treatment with BCG or MMC-EMDA in the previous 2 years. World Health Organization performance status <2. Adequate bone marrow reserve, normal renal and hepatic function, and bladder capacity greater than 150 mL.
Exclusion criteria: History of T2 urothelial carcinoma, previous history or concomitance of CIS. Treatment with chemotherapy or pelvic radiotherapy during the last three months.
Pregnant/lactating women. Known allergy to MMC or BCG. Active BCG infection or history of BCG sepsis.
Initial assessment: Urinary cytology, cystoscopy, and resection of all visible bladder tumors were performed in all cases. In addition, random biopsies of the bladder mucosa were also taken in cases of: non-papillary tumor, history of high-grade tumor, previous positive cytology. When tumor resection was incomplete or no muscle was present in the specimen, a second TURBT was performed 2 to 4 weeks apart. Before starting treatment, pathology of the upper urinary tract was excluded by Computed Tomography Urography (CTU), and urinary tract infection ruled out by urine culture.
Intervention and treatment schedule
Treatment with MMC-EMDA or BCG began 4–6 weeks after TURBT.
Patients in the study group received 40 mg of MMC diluted in 50 mg of distilled water applied using the EMDA device (Physionizer® 30, manufactured by Physion®, Medolla, Italy) at 20 mA for 30 minutes [12]. The administration protocol consisted of an induction of 6 weekly instillations and maintenance of 6 monthly instillations.
Patients in the control group received instillations with BCG (50 mg OncoCITE® diluted in 50 ml of normal saline) for 60 minutes. The protocol consists of 6 weekly induction instillations continued with maintenance therapy of 3 weekly instillations at months 3, 6 and 12.
In case of toxicity, delay of treatment was allowed. However, dose reduction was not allowed.
Follow up
The patient follow-up protocol consisted of cytology and cystoscopy with biopsies of all visible tumors every 3 months. When cytology was positive, random biopsies of bladder mucosa were also taken. CTU was performed at 6, 12 and 24 months.
Patients leave the study if they withdraw informed consent or do not complete the first six months of follow-up.
Variables
At baseline, patient demographics and tumor characteristics were recorded: size, number, stage, WHO grade 2004, risk group, previous recurrence and previous treatments. During the follow-up, the following was recorded: Recurrence: histologically confirmed detection of a bladder cancer of a lower or similar grade. Progression: histologically confirmed detection of higher-grade bladder cancer, CIS or muscle- invasive cancer, or detection of metastases or local disease progression (T3 -T4) on radiological study. Adverse events (AE): assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Grade 1 or 2 toxicities are treated symptomatically. Treatment is discontinued when an allergy or toxicity grade >3 occurs or when an AE occurs that causes treatment to be delayed for more than two weeks. We define time to recurrence as the time elapsed from the first surgical intervention to the next one in which recurrence is detected. Similarly, time to progression is the time elapsed from the first surgery to the next surgery or imaging test in which progression is confirmed.
Statistical analysis
Data were collected prospectively. Categorical and continuous variables were analyzed using chi-square, Fisher’s exact, and Student’s t-tests. The Kaplan-Meier method was used to assess time-to-event outcomes and curves were constructed for each arm of the study. A comparison was estimated using a long-rank test. All tests were bilateral and a p value <0.05 was considered statistically significant. Statistical analyses were performed with SPSS V23.0 (Armonk, NY: IBM Corp.).
RESULTS
One hundred and six patients met the inclusion criteria and agreed to participate in the study. Before the choice of treatment, seven patients were excluded. Forty-eight patients opted to receive treatment with MMC-EMDA but one patient did not go on to initiate treatment. Fifty-one patients opted for standard BCG therapy. Three patients were lost before the start of treatment. Fig. 1.
Fig. 1
Patient flowchart.
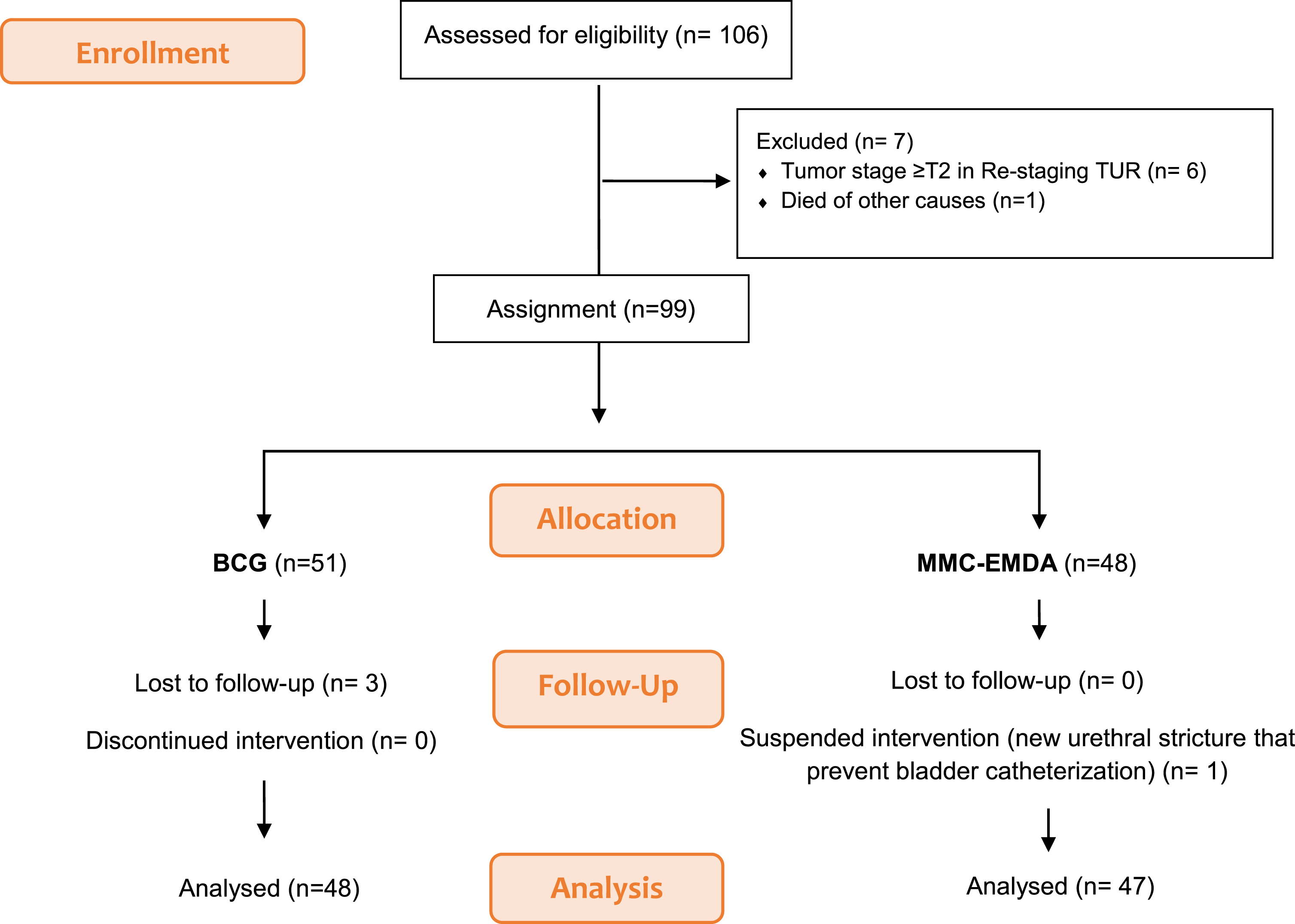
Finally, 47 patients in the MMC-EMDA group and 48 patients in the BCG group were considered for analysis. Fig. 1.
The descriptive analysis of the demographic and clinical characteristics of the patients at the start of the study is shown in Table 1. Both groups were homogeneous with no significant differences between the groups.
Table 1
Patient and tumor characteristics at baseline
| Variable | MMC-EMDA | BCG | p |
| n = 47 | n = 48 | ||
| Age, mean (±SD) | 69,8±11 | 72,1±11 | 0,326 |
| Gender, n (%) | 0,758 | ||
| Male | 37 (78.7%) | 39 (81,3%) | |
| Female | 10 (21.3%) | 9 (18,8%) | |
| Group of risk of EAU | 0,349 | ||
| Intermediate | 28 (59,6%) | 24 (50%) | |
| High | 19 (40,4%) | 24 (50%) | |
| Tumor size, n (%) | 0,614 | ||
| <3 cm | 29 (61,7%) | 32 (66,7%) | |
| ≥3 cm | 18 (38,3%) | 16 (33,3%) | |
| Tumor stage, n (%) | 0,344 | ||
| Ta | 29 (61,7%) | 25 (52,1%) | |
| T1 | 18 (38,3%) | 23 (47,9%) | |
| Tumor grade WHO 2004, n (%) | 0,354 | ||
| Low gade | 20 (42,6%) | 16 (33,3%) | |
| High grade | 27 (57,4%) | 32 (66,7%) | |
| Number of tumors, n (%) | 0,225 | ||
| 1 | 22 (46,8%) | 29 (60,4%) | |
| 2–7 | 21 (44,7%) | 18 (37,5%) | |
| ≥8 | 4 (8,5%) | 1 (2,1%) | |
| Previous recurrence, n (%) | 0,123 | ||
| None | 29 (61,7%) | 37 (77,1%) | |
| Yes | 18 (38,3%) | 11 (29,1%) |
*EAU: European Association of Urology.
In the MMC-EMDA group, the mean age of the patients was 69.8 years, and 78.7% were male. In the BCG group, the mean age of the patients was 72.1 years and 81.3% were male. Most cases were primary tumors (MMC-EMDA: 61.7% BCG: 77.1% p = 0.123), smaller than 3 cm (MMC-EMDA: 61.7% BCG: 66.7% p = 0.614), with a similar distribution among the risk groups (MM-EMDA: Intermediate risk: 59.6%, High risk: 40.4%. BCG: Intermediate risk: 50%, High risk: 50% p = 0,349).
The mean follow-up of patients was 26.4 months (standard deviation (SD) of 12.8 months) in the MMC-EMDA group and 28.4 months (SD 9.14 months) in the BCG group (p = 0.44).
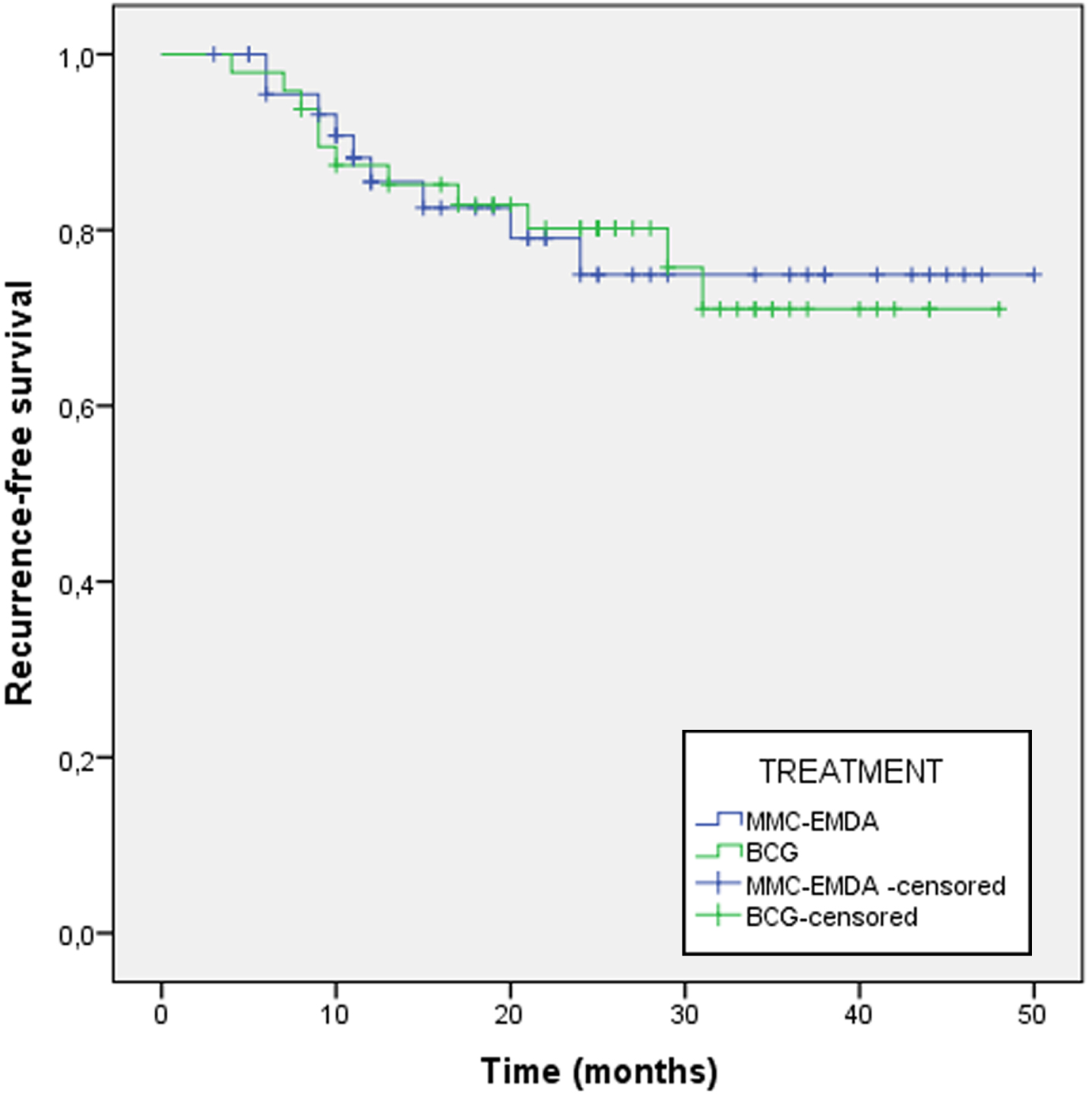
The 24-month RFR was 80.9% for MMC-EMDA and 77.1% for BCG (p = 0.969) (Fig. 2). The mean time to recurrence was 12.5 months for MMC-EMDA and 14 months for BCG (p = 0.681).
Fig. 2
Recurrence-free survival recurrence at 24 months follow-up between MMC-EMDA group and BCG group.
The PFR at 24 months was 97.9% for MMC-EMDA and 93.8% for BCG (p = 0.419). There was one progression in the MMC-EMDA group due to the appearance of CIS at 5 months. In the BCG group there were 3 progressions by passage to T2 in a mean time of 12.6 months.
We performed a multivariate analysis adjusted for age, sex, number of tumors, size, stage and tumor grade, we found that the treatment applied did not influence the prognosis of recurrence with a hazard ratio 0.702 (95% CI 0.255–1.938) p = 0.495.
Regarding treatment safety (Table 2), in the MMC-EMDA group, the percentage of planned instillations received was 75%. The AEs reported were mild in 14 patients (29.8%), moderate in 5 (10.6%) and one patient (2.1%) presented a severe AE. In total, 10 patients (21.3%) in this group discontinued treatment. Eight due to AEs: 3 due to bladder spasms, 3 due to urinary tract infection, 1 due to general malaise and 1 due to a severe adverse reaction due to a previously unknown drug allergy.
Table 2
Adverse events reported by patients
| Adverse events reported | MMC-EMDA | BCG | p |
| n = 47 | n = 48 | ||
| None n, (%) | 24 (51,1%) | 23 (47,9%) | 0,556 |
| Grade 1 n, (%) | 14 (29,8%) | 19 (40,4%) | |
| Dysuria, n | 1 | 6 | |
| Bladder spams, n | 5 | 2 | |
| Skin Burns, n | 5 | 0 | |
| Urinary tract infection, n | 2 | 6 | |
| General malaise, n | 1 | 5 | |
| Grade 2 n, (%) | 5 (10,6%) | 5 (10,6%) | |
| Urinary tract infection, n | 1 | 1 | |
| Skin Burns, n | 2 | 0 | |
| Bladder spams, n | 2 | 0 | |
| Hematuria, n | 0 | 1 | |
| Fever, n | 0 | 3 | |
| Grade 3 n, (%) | 0 (0%) | 0 (0%) | |
| Grade 4 n, (%) | 1 (2,1%) | 0 (0%) | |
| MMC Allergy, n | 1 | 0 | |
| Grade 5 n, (%) | 0 (0%) | 0 (0%) |
The percentage of planned instillations received in the BCG arm was 87.3%, 19 patients (40.4%) had mild and 5 (10.6%) moderate AEs. Eight patients (17.4%) of this group discontinued treatment. Six as a result of AEs: 2 due to urinary tract infections, 3 due to dysuria, and 1 patient due to fever.
During the study, three patients from the EMDA group (6.4%) and three patients from the BCG group (6.3%) died (p = 0.979). All of them died from causes unrelated to their neoplastic disease or treatments.
DISCUSSION
We present the results of a prospective study comparing adjuvant treatment with MMC-EMDA versus BCG in patients with intermediate and high-risk NMIBC, selecting those patients without CIS. Our results suggest that, in terms of prevention of recurrences, using MMC-EMDA in the induction and maintenance regimen presents results, comparable to treatment with BCG induction and maintenance for one year. Moreover, it shows an adequate safety profile. Similar to previously published studies, where the adverse events of MMC applied with EMDA show similar rates to those of passive MMC administration (local symptoms such as cystitis, hematuria, and urinary frequency were observed in 25% of the passive MMC group and 26% of the EMDA group, p = 0.47) [7]. However, it appears that suprapubic pain and urethral burning are more common in the former group [6].
The application of MMC-EMDA as adjuvant treatment was evaluated by Di Stasi et al. [10], who conducted a clinical trial involving patients with high-risk NMIBC with CIS. They included 3 treatment groups, passive MMC, MMC-EMDA and BCG in a similar schedule and had a mean follow-up of 43 months, and observed an RFR for MMC of 15%, compared to an RFR of 47% in the MMC-EMDA and BCG group (p = 0.092). More recent is the work of Zazzara et al. [12] comparing treatment with MMC-EMDA and BCG. They retrospectively analyzed a subgroup of paired patients and found no difference in terms of recurrence and progression. Our study, also found no significant differences when comparing treatment with MMC-EMDA and BCG.
Other devices also aim to increase the penetration of intravesical chemotherapeutics, but they do so through heat; this is called chemohyperthermia (CHT). Arends et al. [13], in 2016, published their clinical trial in which they applied chemohyperthermia (CHT) using radiofrequency-induced thermochemotherapy (RITE) through a device called Synergo SB- TS 101. They randomized 190 patients with high- and intermediate-risk NMIBC to receive MMC boosted with Synergo or BCG. After 24 months of follow-up, they obtained an RFR of 78.1% for CHT versus 64.1% for BCG (p = 0.08).
Guerrero Ramos et al. [14] conducted a clinical trial in which 50 patients with high-risk NMIBC without CIS were randomized to receive treatment with BCG or MMC-enhanced with conduction CHT applied by the combat device (HIVEC). Their results found a similar RFR at 24 months between both treatments (RFR 86.5% for HIVEC and 71.8% for BCG (p = 0.184)). Our results are close fairly similar, (RFR 80.9% for MMC-EMDA and 77.1% for BCG (p = 0.969). This could be because we have selected similar patient profiles, excluding patients with CIS.
Studies in patients who do not respond to BCG also show better results in the absence of CIS. Di Gianfrancesco et al. [15] found RFR in patients with CIS of 12.5% and 57.1% in patients without CIS, all treated with MMC-EMDA (p < 0.05). A similar pattern seems to occur with the RITE device. Tan et al. [16] had to prematurely end their clinical trial because patients with previous BCG failure and CIS treated with RITE had a significantly lower disease- free survival compared to the control group treated with institutional standard second-line therapy (HR 2.06; p = 0.01).
Therefore, we believe that the patient profile and the most appropriate administration protocol are yet to be elucidated. In our study, our aim was to demonstrate how, in the selection of intermediate and high-risk NMIBC patients without CIS, the administration of MMC-EMDA is an effective alternative to BCG. Based on the results, the clinical application of these therapies could be in patients who wish to avoid BCG or even as a replacement for such treatment when it is not available. As a response to the shortage of BCG, various associations have recommended administering MMC to patients with intermediate-risk NMIBC. However, replacing BCG with MMC may lead to reduced effectiveness in terms of preventing tumor recurrence and progression. Consequently, alternative approaches have emerged to mitigate these disparities. However, randomized studies are needed to address these questions.
The main limitation of this study is that it is not a randomized study, However, the analysis of variables indicates that the groups are comparable, despite some observed differences. Nevertheless, our limited sample size prevents us from conducting a stratified analysis. Furthermore, this study does not allow us to answer how much of the treatment effect comes from the device, as we did not consider the possibility of introducing a MMC-passive group given that we included high-risk patients. Another weakness is that follow-up time does not allow us to achieve median survival. Future studies of other cohorts are needed to validate our results, and the treatment regimen.
The MMC applied with the EMDA device effective in preventing relapses similar to the BCG in the profile of patients with high and intermediate risk NMIBC without CIS at 24 months of follow-up.
ACKNOWLEDGMENTS
We acknowledge the Doctorate Program in Clinical Medicine and Public Health of the University of Granada, in which a thesis is being carried out regarding these devices.
FUNDING
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. The authors had access to the data.
Material preparation, data collection and analysis were performed by Manolo Pareja Vilchez, M Teresa Melgarejo Segura, Ana Morales Martínez and Yaiza Yáñez Castillo.
The first draft of the manuscript was written by Miguel Angel Arrabal Polo and Francisco Gutiérrez Tejero. Revisions were made by Miguel Arrabal Martín. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
ETHICAL CONSIDERATIONS
Ethical approval: The study obtained the approval of the Local Ethics Committee before experiment was started (Approved 27/7/2018. Registration: IRB number 0992-N-18).
Consent to participate: Written informed consent was obtained from the parents.
The authors affirm that human research participants provided informed consent for publication of the manuscript and images.
CONFLICT OF INTEREST
María Teresa Melgarejo Segura, Ana Morales Martínez, Yaiza Yáñez Castillo, Miguel Ángel Arrabal Polo, Francisco Gutiérrez Tejero, Manuel Pareja Vílchez and Miguel Arrabal Martín have no relevant financial or non-financial interests to disclose.
REFERENCES
[1] | EAU-Guidelines-on-Non-Muscle-Invasive-Bladder-Cancer-2022.pdf [Internet]. [cited 2022 Jul 28]. Available from: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Non-Muscle-Invasive-Bladder-Cancer-2022.pdf |
[2] | Cambier S , Sylvester RJ , Collette L , Gontero P , Brausi MA , van Andel G , et al. EORTC nomograms and risk groups for predicting recurrence,progression, and disease-specific and overall survival innon-muscle-invasive stage Ta-T1 urothelial bladder cancer patientstreated with 1–3 years of maintenance bacilluscalmette-Guérin. Eur Urol. (2016) ;69: (1):60–9. |
[3] | Brausi M , Oddens J , Sylvester R , Bono A , van de Beek C , van Andel G , et al. Side effects of Bacillus Calmette-Guérin (BCG) in thetreatment of intermediate- and high-risk Ta, T1 papillary carcinomaof the bladder: Results of the EORTC genito-urinary cancers grouprandomised phase 3 study comparing one-third dose with full dose and1 year with 3 years of maintenance BCG. Eur Urol. (2014) ;65: (1):69–76. |
[4] | Dalton JT , Wientjes MG , Badalament RA , Drago JR , Au JL . Pharmacokinetics of intravesical mitomycin C in superficial bladder cancer patients. Cancer Res. (1991) ;51: (19):5144–52. |
[5] | Tan WS , Kelly JD . Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat Rev Urol. (2018) ;15: (11):667–85. |
[6] | Colombo R , Brausi M , Da Pozzo L , Salonia A , Montorsi F , Scattoni V , et al. Thermo-chemotherapy and electromotive drug administration of mitomycin C in superficial bladder cancer eradication. a pilot study on marker lesion. Eur Urol. (2001) ;39: (1):95–100. |
[7] | Di Stasi SM , Valenti M , Verri C , Liberati E , Giurioli A , Leprini G , et al. Electromotive instillation of mitomycin immediately before transurethral resection for patients with primary urothelial non-muscle invasive bladder cancer: A randomised controlled trial. Lancet Oncol. (2011) ;12: (9):871–9. |
[8] | Racioppi M , Di Gianfrancesco L , Ragonese M , Palermo G , Sacco E , Bassi PF . ElectroMotive drug administration (EMDA) of Mitomycin C as first-line salvage therapy in high risk ‘BCG failure’ non muscle invasive bladder cancer: 3-years follow-up outcomes. BMC Cancer. (2018) ;18: (1):1224. |
[9] | Kos B , Vásquez JL , Miklavcič D , Hermann GGG , Gehl J . Investigation of the mechanisms of action behind Electromotive DrugAdministration (EMDA). Peer J. (2016) ;4: , e2309. |
[10] | Di Stasi SM , Giannantoni A , Stephen RL , Capelli G , Navarra P , Massoud R , et al. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: A prospective randomized study. J Urol. (2003) ;170: (3):777–82. |
[11] | Di Stasi SM , Giannantoni A , Giurioli A , Valenti M , Zampa G , Storti L , et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: A randomised controlled trial. Lancet Oncol. (2006) ;7: (1):43–51. |
[12] | Zazzara M , Nazaraj A , Scarcia M , Cardo G , Carando R , Ludovico GM . Electromotive drug administration of mitomycin C (EMDA/MMC) versus intravesical immunotherapy with bacillus calmette-Guérin (BCG) in intermediate and high risk non muscle invasive bladder cancer. Urol Int. 2021;1-8. |
[13] | Arends TJH , Nativ O , Maffezzini M , de Cobelli O , Canepa G , Verweij F , et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus calmette-Guérin for adjuvant treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol. (2016) ;69: (6):1046–52. |
[14] | Guerrero-Ramos F , González-Padilla DA , González-Díaz A , de la Rosa-Kehrmann F , Rodríguez-Antolín A , Inman BA , et al. Recirculating hyperthermic intravesical chemotherapy withmitomycin C (HIVEC) versus BCG in high-risk non-muscle-invasivebladder cancer: Results of the HIVEC-HR randomized clinical trial. World J Urol (2022) . |
[15] | Di Gianfrancesco L , Ragonese M , Palermo G , Sacco E , Bassi P , Racioppi M . Second-Line conservative device-assisted intravesical treatment in selected patients with recurrent high-risk non-muscle-invasive bladder cancer. Clin Genitourin Cancer. (2021) ;19: (2):e100–19. |
[16] | Tan WS , Panchal A , Buckley L , Devall AJ , Loubière LS , Pope AM , et al. Radiofrequency-induced thermo-chemotherapy effect versus asecond course of bacillus calmette-guérin or institutionalstandard in patients with recurrence of non-muscle-invasive bladdercancer following induction or maintenance bacilluscalmette-Guérin therapy (HYMN): A phase III, open-label,randomised controlled trial. Eur Urol. (2019) ;75: (1):63–71. |




