Impact of Adjuvant Gemcitabine Containing Chemotherapy Following Radical Nephroureterectomy for Patients with Upper Tract Urothelial Carcinoma: Results from a Propensity-Score Matched Cohort Study
Abstract
BACKGROUND:
The evidence regarding perioperative adjuvant chemotherapy and personalized surveillance strategies for upper tract urothelial carcinoma is limited.
OBJECTIVE:
To evaluate whether adjuvant gemcitabine containing chemotherapy affects the oncological outcomes of advanced upper tract urothelial carcinoma (UTUC).
METHODS:
The CROES-UTUC registry is an observational, international, multi-center study on patients diagnosed with UTUC. Patient and disease characteristics from 2380 patients with UTUC were collected, and finally 738 patients were included in this analysis. The primary outcome of this study was recurrence-free survival. Propensity score matching was performed. Kaplan-Meier and multivariate Cox regression analyses were performed by stratifying patients according to the treatment of adjuvant chemotherapy.
RESULTS:
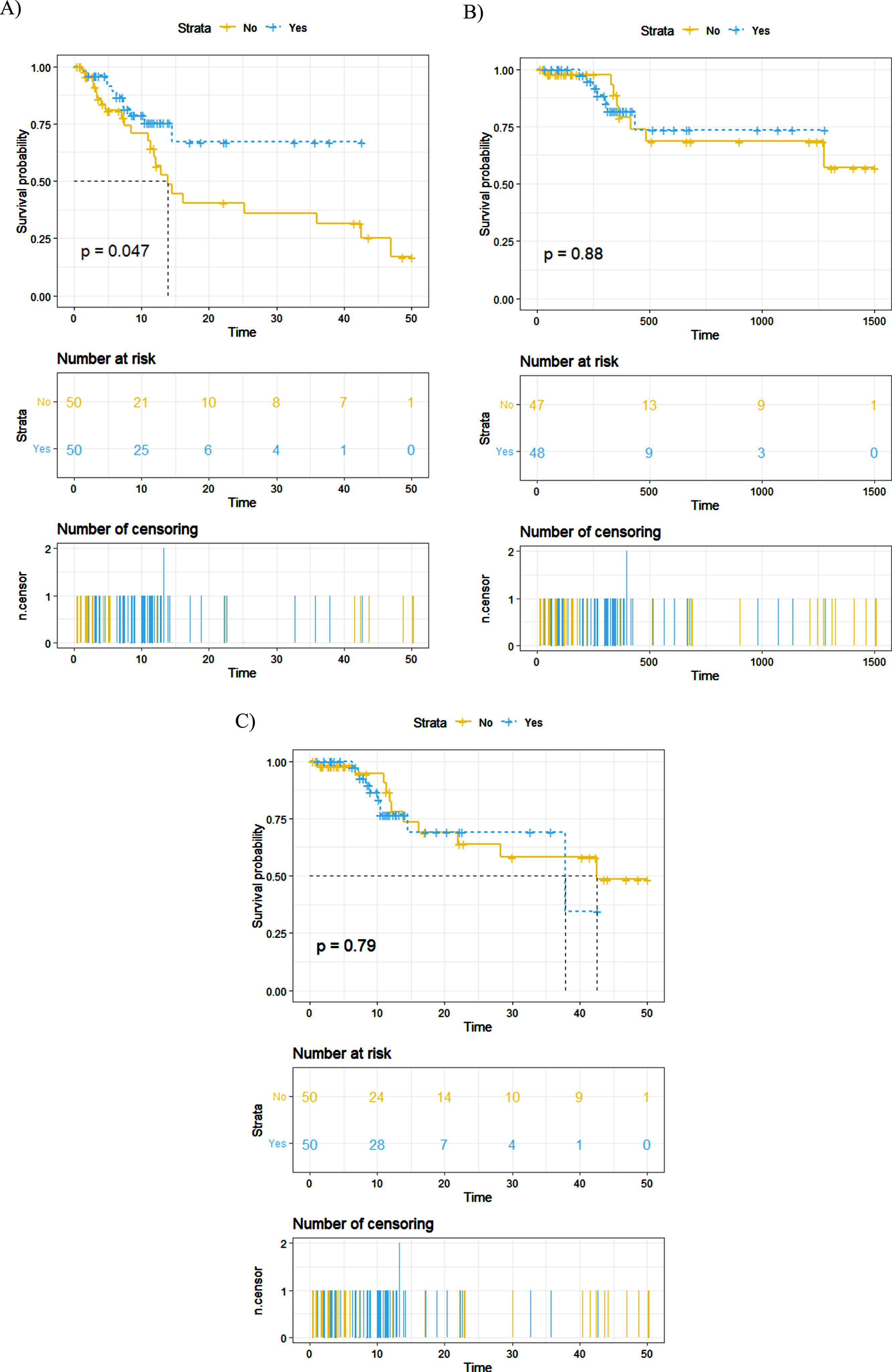
A total of 738 patients were included in this analysis, and 59 patients received adjuvant chemotherapy (AC), including 50 patients who received gemcitabine. A propensity score matching was performed, including 50 patients who received gemcitabine containing treatment and 50 patients without adjuvant chemotherapy. Disease recurrence occurred in 34.0% of patients. The recurrence rate in the AC group was 22.0%, which was significantly lower than the non-AC group (46.0%). Kaplan-Meier analyses also showed that AC was associated with a lower likelihood of tumor recurrence (p = 0.047). However, AC was not significantly associated with a higher overall survival (OS) (p = 0.908) and cancer-specific survival (CSS) (p = 0.979). Upon multivariate Cox regression analysis, AC was associated with a lower risk of tumor recurrence (HR = 0.297, p = 0.028).
CONCLUSION:
The present study confirms that adjuvant gemcitabine containing chemotherapy could decrease the risk of tumor recurrence in patients with locally advanced UTUC following nephroureterectomy. However, more studies are need to draw a clearer image of the value of this treatment method.
INTRODUCTION
Urothelial carcinomas (UCs) are the fourth most prevalent histologic subtype of cancer in developed countries [1], located in the lower (bladder and urethra) and/or the upper (pyelocaliceal cavities and ureter) urinary tract. Upper tract urothelial carcinoma (UTUC) is a rare cancer that only accounts for 5-10% of all urothelial malignancies. In western countries, its yearly incidence is around two cases per 100,000 people [2]. The standard treatment is radical nephroureterectomy (RNU) followed by surveillance for high risk UTUC [2]. However, the recurrence rate for patients with localized UTUC after initial treatment is around 30%, and the 5-year specific survival is < 50% for pT2/pT3 and < 10% for pT4 UTUC [3–6]. To improve the survival of patients with adverse clinical and/or pathologic features, multi-modal treatment management, such as adjuvant chemotherapy, may be considered.
Several studies reported that neoadjuvant chemotherapy could result in survival improvements and tumor downstaging in patients with urothelial carcinoma [7–10]. Also promising results are reported following platinum-based palliative chemotherapy for patients with UCs [11]. Although neoadjuvant chemotherapy is attractive because patients can be treated when they still have sufficient renal function, the absence of pathological specimen may cause an unreliable UTUC staging and potential overtreatment [12].
In contrast, with definitive pathology from RNU, patients can be selected for adjuvant chemotherapy (AC), thus reducing the risk of overtreatment. Previous studies did not provide sufficient evidence and even showed conflicting conclusions [13–15]. Birtle et al. reported that adjuvant platinum-based chemotherapy could significantly improve disease-free survival for patients in a randomized controlled trial, which may not be replicable in a real-world setting [16]. Also, little focus exists on the use of adjuvant gemcitabine containing chemotherapy [17]. In order to identify the efficacy of adjuvant gemcitabine containing chemotherapy, we used a real-world prospective global dataset to assess the effect of adjuvant gemcitabine containing chemotherapy on recurrence-free survival (RFS), cancer-specific survival (CSS) and overall survival (OS) for patients with UTUC.
PATIENTS AND METHODS
The CROES-UTUC registry is an international, observational, multi-center cohort study focusing on the management of patients suspected of UTUC [18]. Since the initiation of the registry in November 2014, 101 centres from 29 countries have joined the registry. The registry follows the recommendations of the Agency for Healthcare Research and Quality for the design and use of patient registries for scientific, clinical, and health policy purposes [19]. The study was registered with clinicaltrials.gov (NCT02281188) [20], and the study protocol was published before [18].
Patient selection
Consecutive patients aged≥18 years who had suspected UTUC undergoing radical nephroureterectomy were included. Patients who died within 30 days of RNU or without detailed information on the use of adjuvant chemotherapy were excluded. To meet the criteria for adjuvant chemotherapy, treatment must have started within 90 days following RNU. Patients with previous bladder cancer or other malignancies were not excluded. The study criteria were broad to provide comprehensive real-world data regarding the management and outcomes of patients with suspected UTUC.
Pathological evaluation
Surgical specimens were processed following standard pathological procedures and reviewed by genitourinary pathologists at each institution. Tumors were staged according to the 2009 American Joint Committee on Cancer/Union Internationale Contre le Cancer TNM classification and graded according to the 2004 World Health Organization classification [21].
Data collection and definition
Clinical data on baseline characteristics, risk factors, clinical assessment, intervention received, and survival outcomes were recorded [21]. Data from all participating centers were collected using an online Data Management System. The Data Management System was a web-based system located and maintained at the CROES Office. All urological patients were asked to report their medical history at the time of diagnosis. Patients who did not receive adjuvant chemotherapy after surgery were included in the observation group.
Follow-up Regimen
The primary outcome of this study was RFS. Disease recurrence was defined as tumour recurrence at site of surgery, regional lymph nodes or distant metastasis. Secondary we evaluated the CSS, and OS. For RFS, participants either had a first recurrence or were censored (no recurrence, deceased with no recurrence or lost to follow-up at the end of the study). Time to recurrence or censoring was calculated by taking the difference between the corresponding date of recurrence (when available) or date of follow-up and the date of the surgery.
Statistical analysis
Descriptive statistics were applied for patient, demographic and disease characteristics. We used the propensity score matching (PSM) to account for the conditional probability of treatment selection and to establish the marginal causal effects of the intervention. We selected the tumour stage and tumour grade to build the propensity score via the generalized boosted model (GBM) [22]. This machine-learning method has been shown to outperform simple logistic regression in the context of case-mix adjustment. Regarding this approach, we created a matched sample by matching treated and untreated subjects in a 1 : 1 ratio based on the logit of the propensity score and using calipers of width equal to 0.2 of the standard deviation of the logit of the propensity score. A greedy, nearest-neighbour matching algorithm was used to form pairs of treated and untreated subjects.
Associations between categorical variables were assessed using the chi-squared test.
The influence of AC on oncologic outcomes was analyzed using the Kaplan-Meier (K-M) analysis, and the significance was assessed by the log-rank test. Multivariate Cox regression analysis was conducted for traditional prognostic factors, including smoking status, pathological stage, and grade. A p-value of < 0.05 was statistically significant. All statistical analyses were performed using SPSS version 26 (IBM Corporation, Armonk, New York) and R [23]. Complete case analysis was performed in case of any missing data on the variables of interest.
RESULTS
Overview
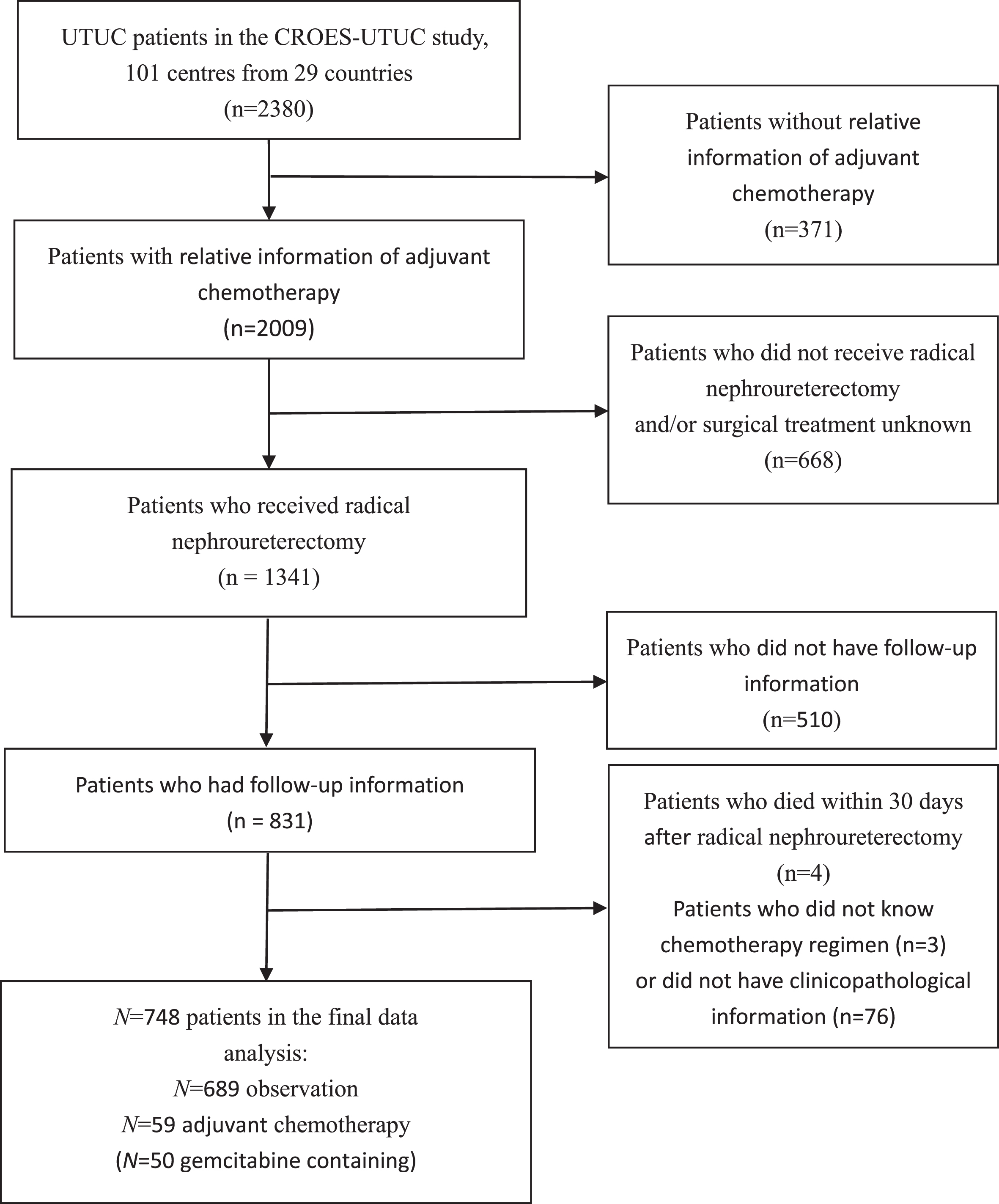
A total of 2380 patients were registered in the CROES-UTUC study (Supplementary 2). 748 Patients with UTUC were included in this study, consisting of 59 patients in the AC group and 689 patients in the observation group (Fig. 1). Of the patients who received AC, 84.7% (n = 50) were treated with gemcitabine containing AC. Supplementary 1 shows the distribution of baseline characteristics of the cohort, before PSM; 73.0% of patients were male, with a median age at diagnosis of 71.2 (41.0–94.0) years. 62.1% were smokers, including 27.5% of current smokers and 34.6% of ex-smokers. 34.6% of patients had≥2 Charlson Comorbidity Index (CCI). Regarding the pathological tumor stage, 44.0% had pTa/Tis/T1 disease and 56.0% had T2-4 tumor. And G3 disease was found in 60.6% of patients.
Fig. 1
Flowchart describing the selection of patients who underwent adjuvant chemotherapy versus observation after radical nephroureterectomy for upper tract urothelial carcinoma.
Baseline characteristics after PSM
PSM was performed in a 1 : 1 ratio. Baseline characteristics after PSM are shown in Table 1. Of the 100 patients in our cohort after PSM, 79% was male and the median age at diagnosis was 71.0 years (48.0-88.0). The estimated pre-operation glomerular filtration rate (eGFR) was 50 mL/min or higher in 92% of patients. Between adjuvant treated and untreated groups, no significant differences were noted for gender, smoking status, CCI, pre-operative eGFR, tumor location, and multifocality.
Table 1
Baseline characteristics between gemcitabine containing AC vs observation groups after propensity score matching. (*p < 0.05; **p < 0.01; and ***p < 0.001)
| Overall | Gemcitabine containing AC | Observation | P-value | |
| N = 100 | N = 50 | N = 50 | ||
| Age | – | |||
| Mean (SD) | 70.0 (9.2) | 67.7 (8.5) | 72.1 (9.4) | |
| Median | 71.0 | 69.0 | 73.0 | |
| [Min, Max] | [48.0,88.0] | [48.0,82.0] | [54.0,88.0] | |
| Gender | 0.806 | |||
| Female | 21 (21.0) | 10 (20.0) | 11 (22.0) | |
| Male | 79 (79.0) | 40 (80.0) | 39 (78.0) | |
| Smoking Status | 0.834 | |||
| Current smoker | 23 (23.0) | 10 (20.0) | 13 (26.0) | |
| Ex-smoker | 36 (36.0) | 20 (40.0) | 16 (32.0) | |
| Nonsmoker | 35 (35.0) | 17 (34.0) | 18 (36.0) | |
| Missing | 6 (6.0) | 3 (6.0) | 3 (6.0) | |
| CCI | 0.675 | |||
| 0-1 | 33 (33.0) | 16 (32.0) | 17 (34.0) | |
| ≥2 | 35 (35.0) | 16 (32.0) | 19 (38.0) | |
| Missing | 32 (32.0) | 18 (36.0) | 14 (28.0) | |
| Anticoagulant drugs | 0.202 | |||
| Yes | 22 (22.0%) | 9 (18.0%) | 13 (26.0%) | |
| No | 73 (73.0%) | 40 (80.0%) | 33 (66.0%) | |
| Missing | 5 (5.0%) | 1 (2.0%) | 4 (8.0%) | |
| PT Stage | 1 | |||
| Ta/Tis/T1 | 6 (6.0) | 3 (6.0) | 3 (6.0) | |
| T2 | 16 (16.0) | 8 (16.0) | 8 (16.0) | |
| T3 | 64 (64.0) | 32 (64.0) | 32 (64.0) | |
| T4 | 14 (14.0) | 7 (14.0) | 7 (14.0) | |
| Tumor Grade | 1 | |||
| G1 | 2 (2.0) | 1 (2.0) | 1 (2.0) | |
| G2 | 8 (8.0) | 4 (8.0) | 4 (8.0) | |
| G3 | 90 (90.0) | 45 (90.0) | 45 (90.0) | |
| Multifocality | 0.610 | |||
| Yes | 19 (19.0%) | 11 (22.0%) | 8 (16.0%) | |
| No | 73 (73.0%) | 36 (72.0%) | 37 (74.0%) | |
| Missing | 8 (8.0%) | 3 (6.0%) | 5 (10.0%) | |
| Site of tumor | 0.604 | |||
| Renal pelvis | 50 (50.0%) | 26 (52.0%) | 24 (48.0%) | |
| Ureter | 33 (33.0%) | 15 (30.0%) | 18 (36.0%) | |
| Both | 9 (9.0%) | 6 (12.0%) | 3 (6.0%) | |
| Missing data | 8 (8.0%) | 3 (6.0%) | 5 (10.0%) | |
| Pre-RNU eGFR, (ml/min/1.73m2) | 0.302 | |||
| ≤49 | 19 (19.0) | 9 (18.0) | 10 (20.0) | |
| ≥50 | 73 (73.0) | 39 (78.0) | 34 (68.0) | |
| Missing | 8 (8.0) | 2 (4.0) | 6 (12.0) | |
| Adverse events | 0.146 | |||
| Yes | 22 (22.0) | 14 (28.0) | 8 (16.0) | |
| No | 76 (76.0) | 36 (72.0) | 40 (80.0) | |
| Missing | 2 (2.0) | – | 2 (4.0) | |
| Chemotherapy regimen | – | |||
| Gemcitabine | 32 | – | ||
| Gemcitabine+Cisplatin | 14 | – | ||
| Gemcitabine+Carboplatin | 2 | – | ||
| Gemcitabine+BDCA | 1 | – | ||
| Gemcitabine+DTX | ||||
| +Cisplatin | 1 | – |
CCI: Charlson Comorbidity Index; eGFR: estimated glomerular filtration rate; AC: Adjuvant chemotherapy; RNU: radical nephroureterectomy.
Association of AC with oncological outcomes and adverse events
Regarding the pathological stage and grade, no difference exists between the AC and observation groups. Disease recurred in 34.0% (n = 34) of patients, of whom 11 (22.0%) in the AC group and 23 patients (46.0%) in the observation group. Fifteen patients ultimately died from UTUC. In order to study the impact of gemcitabine containing AC on oncological outcomes, we performed a KM analysis and found that patients who received AC had a significant higher RFS (p = 0.047, Figure. 2A) but no difference was seen between groups for CSS and OS (p = 0.88 and p = 0.79, respectively). In addition, we divided the 50 patients who received gemcitabine containing AC into two groups according to the number of AC cycles (1-3 VS.>3), but there was no significant difference in oncological outcomes. In order to decrease the influence of platinum, we also did a sensitivity analysis by comparing the RFS between the gemcitabine monotherapy, the platinum-containing chemotherapy, and the observation groups (Supplementary 3). No significant differences were found between the gemcitabine monotherapy and the platinum-containing chemotherapy, and the gemcitabine monotherapy showed a better oncological outcome than the observation group.
Fig. 2
Propensity score-matched comparison of (A) recurrence-free survival, (B) cancer-specific survival, and (C) overall survival between patients who did and did not receive Gemcitabine – based AC.
In the multivariate cox regression analyses, AC was associated with a lower risk of tumour recurrence (HR = 0.297, p = 0.028, Table 2), but it was not significantly associated with CSS (p = 0.979) and OS (p = 0.908). Besides that, the CCI score showed a trend with an increased risk of death from UTUC (HR = 4.497, p = 0.058) and overall death (HR = 5.071, p = 0.051). However, the traditional prognostic factors, such as tumor stage and grade, did not show a significant association. In addition, we further analyzed the safety profile of the AC group and observation group. By comparing the occurrence of post-operation adverse events, we found that although the AC group reported more adverse events (14 vs. 8), there did not exist a significant difference between the two groups (p = 0.146).
Table 2
Multivariate Cox’s regression analyses on recurrence-free survival, cancer-specific survival, and overall survival for UTUC patients after propensity score matching. (*p < 0.05; **p < 0.01; and ***p < 0.001)
| RFS | CSS | OS | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Smoke | 0.784 | 0.461 | 0.245 | |||
| Nonsmoker | Reference | Reference | Reference | |||
| Ex-smoker | 1.454 (0.490-4.315) | 0.500 | 0.292 (0.033-2.615) | 0.271 | 0.161 (0.019-1.366) | 0.094 |
| Current smoker | 1.301 (0.414-4.087) | 0.652 | 1.277 (0.279-5.837) | 0.753 | 0.863 (0.210-3.545) | 0.838 |
| CCI | 0.655 | 0.058 | 0.051 | |||
| 0-1 | Reference | Reference | Reference | |||
| ≥2 | 1.266 (0.450-3.563) | 4.497 (0.949-26.044) | 5.071 (0.994-25.858) | |||
| pT Stage | 0.806 | 0.567 | 0.956 | |||
| Ta/Tis/T1 | Reference | Reference | Reference | |||
| T2 | 0.973 (0.170-5.571) | 0.975 | 0.131 (0.001-11.862) | 0.376 | 0.832 (0.102-6.764) | 0.864 |
| T3 | 0.594 (0.108-3.265) | 0.549 | 0.266 (0.005-15.532) | 0.523 | 1.203 (0.183-7.888) | 0.847 |
| T4 | 0.560 (0.066-4.759) | 0.595 | 0.076 (0.001-6.433) | 0.255 | 0.820 (0.084-8.033) | 0.865 |
| Tumor Grade | 0.892 | 0.719 | 0.339 | |||
| G1-2 | Reference | Reference | Reference | |||
| G3 | 1.121 (0.216-5.807) | 2.038 (0.042-97.787) | 0.368 (0.047-2.858) | |||
| Gemcitabine containing AC | 0.028* | 0.979 | 0.908 | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.297 (0.100-0.877) | 1.019 (0.249-4.176) | 1.078 (0.301-3.855) | |||
CI=confidence interval; HR = hazard ratio; AC = Adjuvant chemotherapy; CCI: Charlson Comorbidity Index.
DISCUSSION
Our results showed that AC was significantly associated with a decreased risk of tumor recurrence. Unlike previous studies [24], our results did not show an OS and CSS benefit in patients who received AC versus observation. After adjusting for traditional prognostic factors in a multivariate cox regression analysis, AC remained a significant association with tumor recurrence.
In the literature, the relative effect on survival of adjuvant chemotherapy is still unclear in UTUC due to insufficient data and contradictory results [16, 17, 24]. Youssef et al. analyzed three studies in a systematic review and found that adjuvant systematic chemotherapy had a positive impact on survival in patients with UTUC [13]. A retrospective study including 3253 patients also reported that AC could improve the OS outcome of patients with pT3/pT4 and/or pN+UTUC [24]. However, a retrospective study from Yafi et al. investigated the efficacy of AC in 1029 patients and found limited efficacy of AC in patients with UTUC [25]. In addition, a multi-center study included 1544 patients and found no OS benefit for patients who received AC [17]. Besides, Wan et al. also reported that AC could decrease the risk of tumor recurrence but could not improve OS and CSS for pT3NanyM0 patients [26].
In the current study, we created a matched sample including 100 patients and confirmed the impact of adjuvant gemcitabine containing chemotherapy on tumor recurrence. Similar to Wan et al.’s study, our results did not show that AC was linked with OS or CSS. Although UTUC is relatively chemo-sensitive and AC might improve RFS, it could not guarantee an increase in CSS and OS [27]. OS and CSS are also influenced by several factors, such as limited expectancy of survival and subsequent lines of therapy [28]. In addition, our results also showed a similar occurrence of adverse events between AC and observation groups, dissimilar from previous studies [16, 29]. In contrast to the phase III POUT trial [16], most of our patients received gemcitabine monotherapy, but patients in POUT trial received combination chemotherapy; this is also a reflection than patients that we treat in real life could differ from what we see in a clinical trial setting. The present analysis does not support a direct benefit from AC to the study population. In a provocative manner the analysis implies rather a ‘cosmetic’ benefit over a real oncological benefit for this patient population. We therefore should question if one should recommend AC with gemcitabine to avoid potential morbidity when there is no real oncological benefit.
There are several inherent limitations to this study. First, the non-randomized nature of the study is suboptimal in evaluating the treatment effects of an intervention, whereas we tried to use PSM to decrease the bias. Second, clinical practices and operating decisions could vary across the participating centers. This introduces heterogeneity, and some results may be challenging to interpret. Third, the follow-up surveillance protocol was not standardized and was subject to the discretion of the participating centers. This might in turn affect the accuracy and precision of the follow-up survival data. Meanwhile, the sample amount is too limited to show more reliable results. In summary, although the registry is not devoid of limitations, its strength mainly relies on its design, based on a prospective registry conducted with a common protocol.
CONCLUSION
Based on the CROES-UTUC registry, we found that adjuvant gemcitabine containing chemotherapy decreased the risk of tumor recurrence, but it did not improve the OS and CSS in patients with UTUC following RNU in a real-world setting. More RCTs comparing adjuvant gemcitabine containing chemotherapy to observation are warranted to develop a clearer image of the value-based utility of AC in this patient population.
ACKNOWLEDGMENTS
We would like to thank Mrs Sonja van Rees - Vellinga (Executive Director CROES) for her collaboration and support.
FUNDING
This work was supported by an unrestricted educational grant from STORZ to the Clinical Research Office of the Endourology Society (CROES).
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design, and had access to the data. Zhao Hongda, Liu Kang, Chi-Fai Ng, Pilar Laguna, Paolo Gontero, Joyce Baard, Ozcan Yildiz: conception, performance of work and interpretation of data; Zhao Hongda: manuscript writing; Jeremy Yuen-Chun Teoh: reviewed the paper; Jean dela Rosette and Jeremy Yuen-Chun Teoh: the project development. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
Jeremy Yuen-Chun Teoh is an Editorial Board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Zhao Hongda, Liu Kang, Chi-Fai Ng, Jean de la Rosette, Pilar Laguna, Paolo Gontero, Joyce Baard and Ozcan Yildiz have no conflicts of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics. CA Cancer J Clin. (2019) ;69: :7–34. |
[2] | Roupret M , Babjuk M , Burger M , Capoun O , Cohen D , Comperat EM , et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: Update. European Urology. (2021) ;79: :62–79. |
[3] | Jeldres C , Sun M , Isbarn H , Lughezzani G , Budaus L , Alasker A , et al. A population-based assessment of perioperative mortality after nephroureterectomy for upper-tract urothelial carcinoma. Urology. (2010) ;75: :315–20. |
[4] | Lughezzani G , Burger M , Margulis V , Matin SF , Novara G , Roupret M , et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. (2012) ;62: :100–14. |
[5] | Lughezzani G , Jeldres C , Isbarn H , Sun M , Shariat SF , Alasker A , et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of patients. Eur J Cancer. (2009) ;45: :3291–7. |
[6] | Roupret M , Hupertan V , Seisen T , Colin P , Xylinas E , Yates DR , et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol. (2013) ;189: :1662–9. |
[7] | International Collaboration of T, Medical Research Council Advanced Bladder Cancer Working P, European Organisation for R, Treatment of Cancer Genito-Urinary Tract Cancer G, Australian Bladder Cancer Study G, National Cancer Institute of Canada Clinical Trials G, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 4 trial. J Clin Oncol. (2011) ;29: :2171–7. |
[8] | Loehrer PJ Sr , Einhorn LH , Elson PJ , Crawford ED , Kuebler P , Tannock I , et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. (1992) ;10: :1066–73. |
[9] | Sternberg CN , Skoneczna I , Kerst JM , Albers P , Fossa SD , Agerbaek M , et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+M0 urothelial carcinoma of the bladder (EORTC 4): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. (2015) ;16: :76–86. |
[10] | Coleman JA , Yip W , Wong NC , Sjoberg DD , Bochner BH , Dalbagni G , et al. Multicenter Phase II Clinical Trial of Gemcitabine and Cisplatin as Neoadjuvant Chemotherapy for Patients With High-Grade Upper Tract Urothelial Carcinoma. Journal of Clinical Oncology. (2023) :JCO. 2200763. |
[11] | Moschini M , Shariat SF , Roupret M , De Santis M , Bellmunt J , Sternberg CN , et al. Impact of Primary Tumor Location on Survival from the European Organization for the Research and Treatment of Cancer Advanced Urothelial Cancer Studies. J Urol. (2018) ;199: :1149–57. |
[12] | Chitale S , Mbakada R , Irving S , Burgess N . Nephroureterectomy for transitional cell carcinoma - the value of pre-operative histology. Ann R Coll Surg Engl. (2008) ;90: :45–50. |
[13] | Leow JJ , Martin-Doyle W , Fay AP , Choueiri TK , Chang SL , Bellmunt J . A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol. (2014) ;66: :529–41. |
[14] | Hellenthal NJ , Shariat SF , Margulis V , Karakiewicz PI , Roscigno M , Bolenz C , et al. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma: results from the Upper Tract Urothelial Carcinoma Collaboration. J Urol. (2009) ;182: :900–6. |
[15] | Necchi A , Lo Vullo S , Mariani L , Moschini M , Hendricksen K , Rink M , et al. Adjuvant chemotherapy after radical nephroureterectomy does not improve survival in patients with upper tract urothelial carcinoma: a joint study by the European Association of Urology-Young Academic Urologists and the Upper Tract Urothelial Carcinoma Collaboration. Bju International. (2018) ;121: :252–9. |
[16] | Birtle A , Johnson M , Chester J , Jones R , Dolling D , Bryan RT , et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet. (2020) ;395: :1268–77. |
[17] | Necchi A , Lo Vullo S , Mariani L , Moschini M , Hendricksen K , Rink M , et al. Adjuvant chemotherapy after radical nephroureterectomy does not improve survival in patients with upper tract urothelial carcinoma: a joint study by the European Association of Urology-Young Academic Urologists and the Upper Tract Urothelial Carcinoma Collaboration. BJU Int. (2018) ;121: :252–9. |
[18] | Baard J , Celebi M , de la Rosette J , Alcaraz A , Shariat S , Cormio L , et al. Evaluation of Patterns of Presentation, Practice, and Outcomes of Upper Tract Urothelial Cancer: Protocol for an Observational, International, Multicenter, Cohort Study by the Clinical Research Office of the Endourology Society. JMIR Res Protoc. (2020) ;9: :e15363–. |
[19] | Registries for Evaluating Patients Outcomes: A user’s Guide Internet. In AHRQ Methods for Effective Health Care. Rockville, MD: Agency for Healthcare Research and Quality (US); (2014) . |
[20] | Registry for Treatment of Upper Urinary Tract Tumours. NIH U.S. National Library of Medicine ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02281188. |
[21] | Baard J , Cormio L , Cavadas V , Alcaraz A , Shariat SF , de la Rosette J , et al. Contemporary patterns of presentation, diagnostics and management of upper tract urothelial cancer in 101 centres: the Clinical Research Office of the Endourological Society Global upper tract urothelial carcinoma registry. Curr Opin Urol. (2021) ;31: :354–62. |
[22] | McCaffrey DF , Griffin BA , Almirall D , Slaughter ME , Ramchand R , Burgette LF . A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. (2013) ;32: :3388–414. |
[23] | Team RC. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. (No Title). (2021) . |
[24] | Seisen T , Krasnow RE , Bellmunt J , Roupret M , Leow JJ , Lipsitz SR , et al. Effectiveness of Adjuvant Chemotherapy After Radical Nephroureterectomy for Locally Advanced and/or Positive Regional Lymph Node Upper Tract Urothelial Carcinoma. J Clin Oncol. (2017) ;35: :852–60. |
[25] | Yafi FA , Tanguay S , Rendon R , Jacobsen N , Fairey A , Izawa J , et al. Adjuvant chemotherapy for upper-tract urothelial carcinoma treated with nephroureterectomy: assessment of adequate renal function and influence on outcome. Urol Oncol. (2014) ;32: :31 e17–24. |
[26] | Song W , Jeong JY , Jeon HG , Seo SI , Jeon SS , Choi HY , et al. Impact of adjuvant chemotherapy on oncologic outcomes following radical nephroureterectomy for patients with pT3NanyM0 upper tract urothelial carcinoma: A retrospective cohort study. Int J Surg. (2019) ;66: :12–7. |
[27] | Biswas B , Ganguly S , Ghosh J , E P , Dabkara D . Adjuvant Chemotherapy for Upper Tract Urothelial Carcinoma: Is There Sufficient Evidence? J Clin Oncol (2017) ;35: :2095–6. |
[28] | Vassilakopoulou M , de la Motte Rouge T , Colin P , Ouzzane A , Khayat D , Dimopoulos MA , et al. Outcomes after adjuvant chemotherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a large multicenter collaborative study. Cancer. (2011) ;117: :5500–8. |
[29] | Schlack K , Boegemann M , Steinestel J , Schrader AJ , Krabbe LM . The safety and efficacy of gemcitabine for the treatment of bladder cancer. Expert Rev Anticancer Ther. (2016) ;16: :255–71. |




