Hypofractionated Radiation Therapy (Hypo-RT) for the Treatment of Localized Bladder Cancer
Abstract
BACKGROUND:
Various radiotherapeutic regimens are used in the treatment of bladder cancer.
OBJECTIVE:
We aimed to evaluate early toxicity and outcomes associated with hypofractionated radiation therapy (Hypo-RT), 55Gy in 20 fractions.
MATERIAL AND METHODS:
We identified 40 patients who received definitive Hypo-RT for localized bladder cancer. Most patients were men (62.5%), elderly (median age 82), had high Charlson Comorbidity Index score (median 7, range 4–9) and were nonsurgical candidates (80%). Sixty-eight percent had a macroscopically complete transurethral resection of bladder tumor (TURBT) and 33 patients (82.5%) received concurrent chemotherapy. Acute (< =3mo) and late (>3mo) toxicities were assessed according to CTCAE v4.0. Survival outcomes were estimated using the Kaplan-Meier method. Median follow up after Hypo-RT was 32 months (95% CI: 28–49 months).
RESULTS:
Overall rates of acute grade 2 genitourinary (GU) and gastrointestinal (GI) toxicities were 40% each, most commonly urinary frequency and diarrhea. Two cases of acute grade 3 GU/GI toxicity occurred. Late grade 2+ toxicity occurred in 3 patients (7.5%): 2 grade 2 GU and 1 grade 3 GI. Seventy-seven percent achieved a complete response (CR). Six patients (20%) developed disease recurrence at a median time of 9.1 months. The estimated 2-year DFS and 2-year DSS rate were 59% (95% CI, 45–78%) and 78% (95% CI, 65–93%), respectively. Receipt of concurrent chemotherapy (p = 0.003) and achieving a CR (p = 0.018) were univariably associated with improved DSS. Tis component was associated with worse DSS (p = 0.015).
CONCLUSION:
Hypo-RT had a favorable toxicity profile and encouraging cancer control outcomes in this mostly elderly and frail patient cohort.
ABBREVIATIONS
Hypo-RT | hypofractionated radiation therapy |
TURBT | transurethral resection of bladder tumor |
IRB | institutional review board |
PET | positron emission tomography |
CTV | clinical target volume |
PTV | planning target volume |
OARS | organs at risk |
IMRT | intensity-modulated radiation therapy |
CBCT | cone beam CT |
CTCAE | Common Terminology Criteria for Adverse Events |
PFS | progression-free survival |
DFS | disease-free survival |
OS | overall-survival |
DSS | disease-specific survival |
CR | complete response |
NED | no evidence or disease |
GI | gastrointestinal |
GU | genitourinary |
BACKGROUND
Bladder cancer is the sixth most common malignancy in the United States, and an estimated 82,000 cases will be diagnosed in 2023, predominantly in elderly men [1]. Over 16,000 patients will die of this disease, mainly due to distant metastases [1]. Multimodality bladder preservation therapy, consisting of maximal TURBT and concurrent chemoradiation is an important option for patients who are not optimal candidates for or decline radical cystectomy. While randomized trials comparing bladder preservation and radical cystectomy are lacking, survival outcomes have generally been comparable [2–4]. Most trials have reported complete response (CR) and bladder preservation rates ranging 70–80% with the best outcomes in patients who are lower stage disease and complete TURBT prior to chemoradiation [2, 5–18]. The addition of radiosensitizing chemotherapy, while important for locoregional control, has not been shown to impact survival [19, 20].
Various fractionation regimens have been explored in prospective trials and there is a lack of consensus on the optimal regimen or dose [6, 7, 11, 12, 18, 21]. With improved technical advancements, such as on-board imaging, more precise delivery of hypofractionated treatments is now feasible. Providing convenient definitive therapy is also important to improve patient access as elderly patients are less commonly offered curative treatment [22].
An emerging body of literature supports the use of hypofractionated schedules in bladder cancer [7, 8, 11, 12, 16, 18, 21]. Hypofractionated radiation therapy (Hypo-RT) is typically delivered over 3-4 weeks of daily treatment, compared with 6 and half weeks for conventional fractionation (CFRT). Both fractionation schemes are associated with similar disease control rates and acceptable rates of toxicity [2, 4, 6–8, 11, 12, 16, 21]. A recent meta-analysis found that patients treated to 55 Gy in 20 fractions had a lower risk of invasive locoregional recurrence than patients treated to 64 Gy in 32 fractions, with no excess risk of late grade 3-4 toxicity [8]. From the patients’ perspective, a shorter treatment course is more convenient, particularly in elderly populations with travel/mobility limitations.
Clinical trial participants may differ from real-world populations, owing to strict eligibility criteria [23]. This may render outcomes in such trials inapplicable to the general population of patients and particularly elderly and frail patients. In this study, we aim to evaluate early toxicity and outcomes associated with definitive Hypo-RT to 55 Gray (Gy) in 20 fractions for the treatment of localized bladder cancer in an unselected real-world patient cohort. We hypothesized that this treatment regimen would be well tolerated and result in high disease control rates.
MATERIALS AND METHODS
Patient Inclusion
With institutional review board (IRB) approval (17-068), we retrospectively reviewed the medical records of all patients with localized bladder cancer (cT1-4N0) treated with Hypo-RT to a dose of 55 Gy delivered over 20 fractions at our institution between 1/2016 and 12/2019 and had minimum follow up of 24 months. We identified 193 patients with bladder who received radiation therapy, of whom 40 patients received Hypo-RT. Pretreatment evaluation included a complete medical history and physical examination, complete blood count and metabolic panel, cystoscopy with biopsy/TURBT, and pelvic imaging (CT/MRI) as well as systemic evaluation including CT chest or positron emission tomography (PET). All pathologic specimens were reviewed at MSKCC. Patients with N1 or M1 disease, as well as those with small cell/ neuroendocrine carcinomas were excluded from this analysis.
Baseline patient and treatment characteristics are summarized in Table 1. Of the 40 patients identified, most patients were males (62.5%), elderly (median age 82 years, range 60–96 years), had a history of smoking (75%) and were not candidates for radical cystectomy (80%). The median Charlson Comorbidity Index was 7 (range 4–9). Five patients (12.5%) received neoadjuvant systemic treatment prior to chemoradiation. Non-cisplatin-eligible patients received carboplatin-based chemotherapy (n = 2). Most patients had T2 disease (67.5%) and pure urothelial carcinoma (70%). One patient with multiply recurrent high grade cT1 tumor who was not amenable to surgical management was included. The median time from diagnostic TURBT to Hypo-RT was 3.1 months (range 0.93–12.1 months.). Thirteen patients (13/40, 32.5%) required restaging TURBT prior to Hypo-RT, primarily due to prolonged time (>12 weeks) from diagnostic TURBT. The median time from last TURBT to start of Hypo-RT was 1.71 months (range 0.66–11.93 months). Sixty-eight percent had a macroscopically complete TURBT prior to chemoradiation. No patient had adjuvant systemic treatment. The median follow up time after Hypo-RT was 32 months (95% CI: 28–49 months).
Table 1
Patient and treatment characteristics
| Characteristic | |
| Sex, n (%) | |
| Female | 15 (37.5%) |
| Male | 25 (62.5%) |
| Age, median (range) | 82 years (60–96 years) |
| KPS, median (range) | 80 (50–100) |
| Hx of smoking, n (%) | 30 (70%) |
| Current smoking | 2 (5%) |
| Diabetes Mellitus, n (%) | 11 (27.5%) |
| COPD, n (%) | 6 (15%) |
| CAD, n (%) | 10 (25%) |
| Charlson Comorbidity Index, median (range) | 7 (4–9) |
| Surgical candidate, no n (%); yes n(%) | 32 (80%); 8 (20%) |
| Number or TURBTs, median (range) | 2 (0–3) |
| Time from TURBT to RT, median (range) | |
| All patients | 1.7 months (0.67–12.1 months) |
| Excluding patients who had neoadjuvant Tx | 1.7 months (0.67–5.2 months) |
| Quality of TURBT, n (%) | |
| *Incomplete | 12 (32.4%) |
| *Complete | 25 (67.6%) |
| Missing data for 3 patients. | |
| T stage, n (%) | |
| T1 | 1 (2.5%) |
| T2 | 27 (67.5%) |
| T3 | 9 (22.5%) |
| T4 | 3 (7.5%) |
| Histology, n (%) | |
| Pure urothelial | 29 (72.5%) |
| Urothelial with variant features | 10 (25%) |
| Pure variant | 1 (2.5%) |
| Tis Component, n (%) | 9 (22.5%) |
| LVI, n (%) | 8 (20%) |
| Multifocal tumor, n (%) | 17 (42.5%) |
| Hydronephrosis, n (%) | 4 (10%) |
| Unilateral | 3 (75%) |
| Bilateral | 1 (25%) |
| Percutaneous nephrostomy | 2 (50%) |
| Neoadjuvant systemic therapy, n (%) | 5 (12.5%) |
| Chemotherapy | 4 (80%) |
| Chemo-immunotherapy | 1 (20%) |
| Concurrent chemotherapy, n (%) | 33 (82.5%) |
| Planning method, n (%) | |
| IMRT | 10 (25%) |
| VMAT | 30 (75%) |
| D95% PTV, median (range) | 54.8 Gy (48.7–55.9 Gy) |
| Small bowel Dmax, median (range) | 55.0 Gy (0.98–59.0 Gy) |
| Small bowel D5cc, median (range) | 47.6 Gy (0.3–56.4 Gy) |
| Large bowel Dmax, median (range) | 57.0 Gy (8.9–59.2 Gy) |
| Large Bowel D5cc, median (range) | 54.4 Gy (4.2–57.5 Gy) |
| Rectum Dmax, median (range) | 56.9 Gy (29.0–59.1 Gy) |
| Rectum D5cc, median (range) | 52.4 Gy (11.9–57.6 Gy) |
| Bladder Dmax, median (range) | 58.1 Gy (56.3–60.0 Gy) |
| Right femur Dmax, median (range) | 37.7 Gy (12.6–58.2 Gy) |
| Left femur Dmax, median (range) | 37.2 Gy (22.7–57.00 Gy) |
*Valid percent.
Hypo-RT
In preparation for Hypo-RT, a CT simulation was performed in the supine position with an empty bladder and thermoplastic mold immobilization. Oral contrast was generally used to opacify small bowel. The clinical target volume (CTV) was defined as the whole bladder and proximal urethra in female patients and included the prostate in male patients. The planning target volume (PTV) was defined in most patients (97.5%) as a uniform expansion around the CTV of 1–1.5 cm around the bladder and 0.5 cm margin around the prostate. Pelvic lymph nodes were excluded. The prescribed dose to the PTV was 55 Gy in 20 fractions of 2.75Gy delivered daily, 5 days a week. One patient was treated to 44 Gy to the bladder and prostate with a simultaneous integrated boost to the high-risk region to 55 Gy. Organs at risk (OARs) included the rectum, large bowel, small bowel, and femoral heads. Planning goals and dose constraints are available in Table 2. IMRT or VMAT plans were generated using the Eclipse™ treatment planning system (Varian, Palo Alto, CA). All patients but one met the planning goal for the dose to 95% of the PTV (D95%). Image guidance consisted of daily pre-treatment orthogonal kVs and cone beam CTs (CBCT) with matching on the bladder.
Table 2
Planning goals and dose constraints
| Structure | Dose/Volume | Plan Dose | EQD2 Dose |
| Objective | |||
| PTV | D95% | > =4950 (90%) | |
| DMAX | < =110% | ||
| Small Bowel | DMAX | 5500(100%) | 6325 |
| D5cc or D05% | 5000 (91%) | 5500 | |
| Large Bowel | DMAX | 5940 (108%) | 7090 |
| D5cc or D05% | 5500 (100%) | 6325 | |
| Bladder | DMAX | No Hot Spots (110%) | 7290 |
| Rectum | DMAX | No Hot Spots (110%) | 7290 |
| D5cc | 6000 (109%) | 7200 | |
| Femurs | DMAX | 4500 (guideline) - 6050 (110%) if overlap with PTV | |
| Cauda | DMAX | 5000 (91%) | 5625 |
Chemotherapy
Five patients (12.5%) received neoadjuvant platinum-based chemotherapy (the median number of weekly cycles was 3.5, range 1–5). One patient also received atezolizumab with neoadjuvant chemotherapy. Overall, 33 patients (82.5%) were treated with concurrent gemcitabine chemotherapy, delivered twice weekly at a dose of 25mg/m2. No adjuvant chemotherapy was administered.
On- and post-treatment evaluation
Patients were evaluated weekly during Hypo-RT, then at each follow up timepoint with symptom assessment, physical examination and laboratory testing. Post treatment cystoscopy and CT imaging were generally pursued every 3 months for the first 2 years, and every 6 months thereafter. Treatment-related toxicity was assessed using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Acute toxicity was defined as any toxicity that occurred within 3 months post-treatment and late toxicity defined as any toxicity that occurred thereafter.
Statistical methods
Descriptive statistics were used to summarize patient characteristics. Progression-free survival (PFS), disease-free survival (DFS), disease-specific survival (DSS) and overall-survival (OS) were estimated using the Kaplan-Meier method. PFS was defined as the time from completion of Hypo-RT to first event of progression; local (bladder), regional (nodes), distant or death of any cause, whichever came first. DFS was defined as the time from completion of Hypo-RT to first event of progression; local (bladder), regional (nodes) or distant. DSS was defined as the time from completion of Hypo-RT to bladder cancer-related death. Death with no evidence of disease (NED) was censored. OS was defined as the time from completion of Hypo-RT to death of any cause. The date of last follow up was used in patients without events for all endpoints. A clinical complete response (cCR) to Hypo-RT was defined as no evidence of measurable disease on CT/MR imaging with or without a negative post treatment cystoscopy and negative cytology. Tumor recurrence or progression were determined by imaging and/or pathology. Cox proportional hazards regression was used to assess associations between predictor variables and outcomes. Complete response status was a time-dependent covariable. Statistical significance was defined using a two-sided test with an alpha of 0.05. All analyses were conducted using R version 4.2.2.
RESULTS
Treatment-associated toxicity
Most patients completed the entire course of Hypo-RT (90%, 36/40) and concurrent chemotherapy (76%, 25/33). Four patients (10%) did not complete the prescribed Hypo-RT course. One patient developed clostridium difficile-related diarrhea at 49.5 Gy and declined further treatment. A second patient was admitted for a urinary tract infection and declining functional status at 38.5 Gy. A third patient had worsening of preexisting hematuria and clot retention requiring continuous bladder irrigation at 44 Gy, later complicated by anemia-related atrial fibrillation. A fourth patient was admitted for diarrhea at 24.75 Gy, later complicated by a pulmonary embolism.
Ten patients (10/33, 30.3%) had chemotherapy dose reduction, interruption or termination during the course of treatment. The most common reason was hematologic toxicity (7/10, 70%), due to thrombocytopenia [grade 1 (n = 3); grade 2, (n = 2)] or anemia [grade 2 (n = 1)] or neutropenia [grade 2, (n = 10]. Three patients had treatment interruption for GI toxicities, including diarrhea and abdominal pain.
Toxicities are summarized in Table 3. Acute grade 2 GI and GU toxicities were each observed in 40% of patients. Two acute grade 3 toxicities were noted: grade 3 diarrhea (n = 1) and grade 3 hematuria (n = 1). No acute grade 4 or higher toxicity was observed. The most common GU toxicity was increased urinary frequency (all grades, 25/40 patients, 62.5%; grade 2, 13/40 patients, 32.5%). The most common GI toxicity was diarrhea (all grades, 21/40 patients, 52.5%; grade 2, 12/40 patients, 30%; grade 3, 1 patient 2.5%). The proportion of acute GI toxicity (grade ≥1) was found to be significantly higher in patients exceeding the point max dose to the small bowel (Dmax), compared with those meeting dose constraints (63% vs 37%, P = 0.041), but not with exceeding the small bowel D5cc (52% vs 48%, P = 0.1). No significant associations were found between acute GI toxicity and doses to the large bowel or rectum.
Table 3
Genitourinary & gastrointestinal toxicity
| Acute | Late | ||||
| Proportion | Fraction | Proportion | Fraction | ||
| Any GU | Grade 1 | 45% | 18/40 | 2.5% | 1/40 |
| Toxicity | Grade 2 | 40% | 16/40 | 5% | 2/40 |
| Grade 3 | 2.5% | 1/40 | 0% | 0/40 | |
| Grade 4-5 | 0% | 0/40 | 0% | 0/40 | |
| Any GI | Grade 1 | 25% | 10/40 | 0% | 0/40 |
| Toxicity | Grade 2 | 40% | 16/40 | 0% | 0/40 |
| Grade 3 | 2.5% | 1/40 | 2.5% | 1/40 | |
| Grade 4-5 | 0% | 0/40 | 0% | 0/40 | |
Of 38 patients (95%) who had follow up greater than 3 months after the completion of Hypo-RT, late toxicity occurred in 4 patients (10.5%). These included: grade 1 diarrhea (n = 1), grade 2 GU toxicity (nocturia, urgency and incontinence) (n = 2) and grade 3 radiation proctitis at 12 months managed endoscopically and required blood transfusions (n = 1). No patients had late grade 4 toxicity.
Treatment outcomes
Excluding one patient who had died without any post treatment assessment, 30 of 39 patients (77%) achieved a cCR at first post treatment assessment. cCR was determined using both imaging and cystoscopy in 25 patients and by imaging and cytology alone in 5 patients unable/unwilling to undergo cystoscopy. Patients who underwent a complete vs. incomplete TURBT had a higher proportion of cCR, although this difference was not statistically significant (80% vs 64%; P = 0.4).
At last follow up, 16/40 (40%) patients were alive with no evidence or disease (NED) and had a median follow up time of 31 months (95% CI: 25–39). Six patients (6/40, 15%) died of causes other than bladder cancer while NED. Six patients (6/40, 15%) were alive with active malignancy and 9 patients (9/40, 22.5%) had died of bladder cancer.
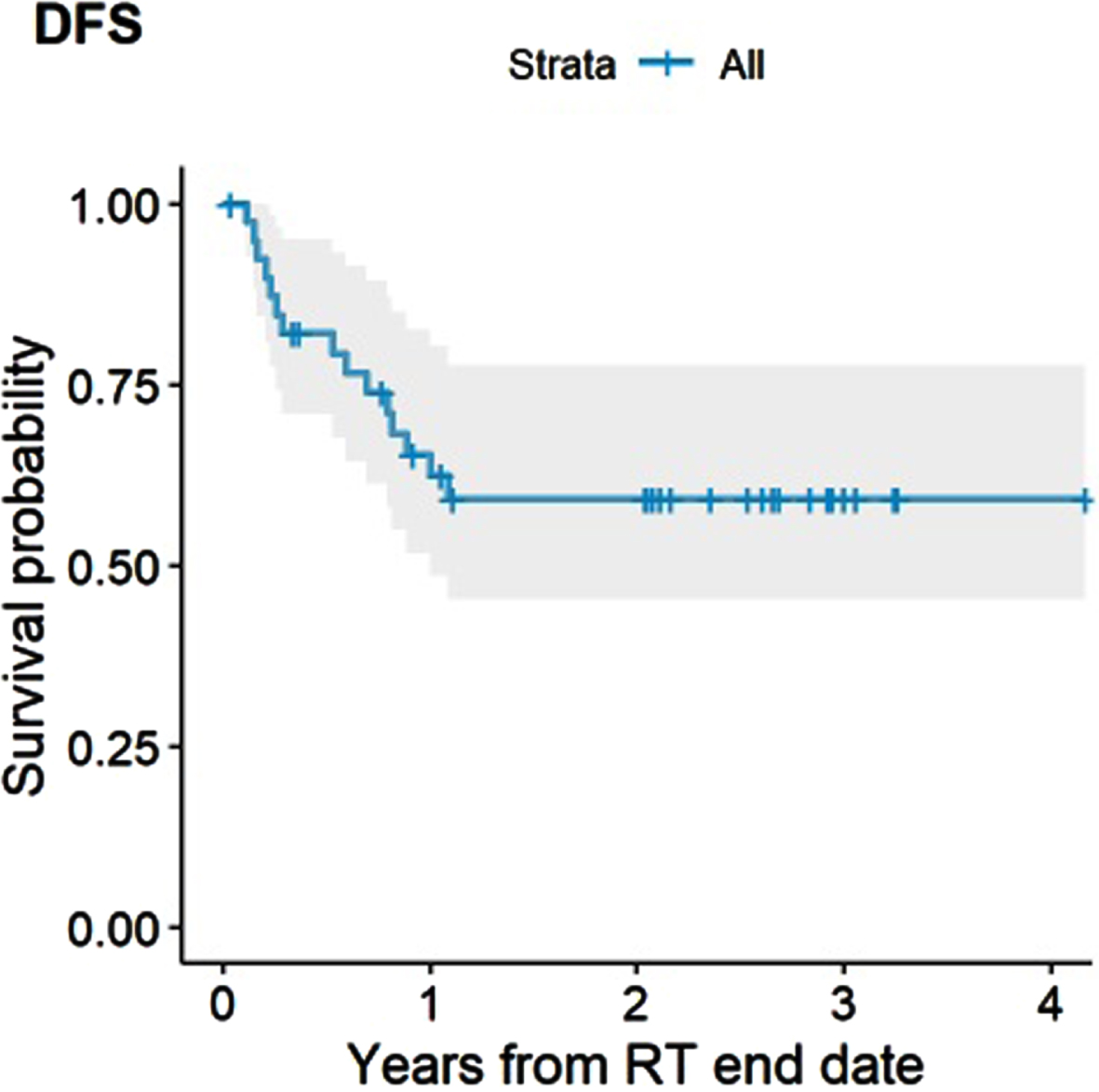
The estimated 2- and 3-year DFS rate for the entire cohort were both 59% (95% CI, 45–78%) (Fig. 1). No factors were significantly associated with DFS, including receipt of concurrent chemotherapy (HR 0.33, 95% CI, 0.1–1.09, P = 0.068) and achieving a CR (HR 0.33, 95% CI, 0.09–1.14, P = 0.08) or presence of hydronephrosis (HR 3.27, 95% CI, 0.91–11.7, P = 0.068). There were also no significant differences in PFS by completeness of TURBT, time from completion of TURBT to start of Hypo-RT, T stage, histology (urothelial vs mixed) and disease focality (unifocal vs multifocal).
Fig. 1
Disease-free Survival (DFS).
Patterns of failure
The most common first site for treatment failure was the bladder (9/40, 22.5%). Regional nodal recurrence alone (n = 2), simultaneous regional and distant recurrence (n = 2) and distant recurrence only (n = 2) were the first site of failure in 6/40 patients (15%).
Of 30 patients who achieved a cCR at first post-treatment assessment, 6 (20%) recurred at a median time of 9.1 months (range 6.4–13.1 months) without accounting for follow up time or competing risks. Three (50%) developed in-bladder failure, and 3 (50%) developed distant±regional failure. Of the 3 patients who developed in-bladder recurrence, 2 were non-invasive and underwent salvage TURBT. One patient who underwent salvage TURBT for non-invasive in-bladder recurrence is without evidence of disease at 49 months post salvage TURBT and one developed distant metastases at 12 months after salvage TURBT. One patient developed muscle invasive in-bladder recurrence and underwent salvage radical cystectomy, but developed distant disease at 2 months post salvage cystectomy.
Of the 10 patients who did had not achieve confirmed cCR at first assessment, 4 patients had local only disease, 2 had local disease and distant failure,3 patients had regional or distant failure only and one patient had not had any post-treatment assessment and died 4 months from completion of RT
Survival outcomes
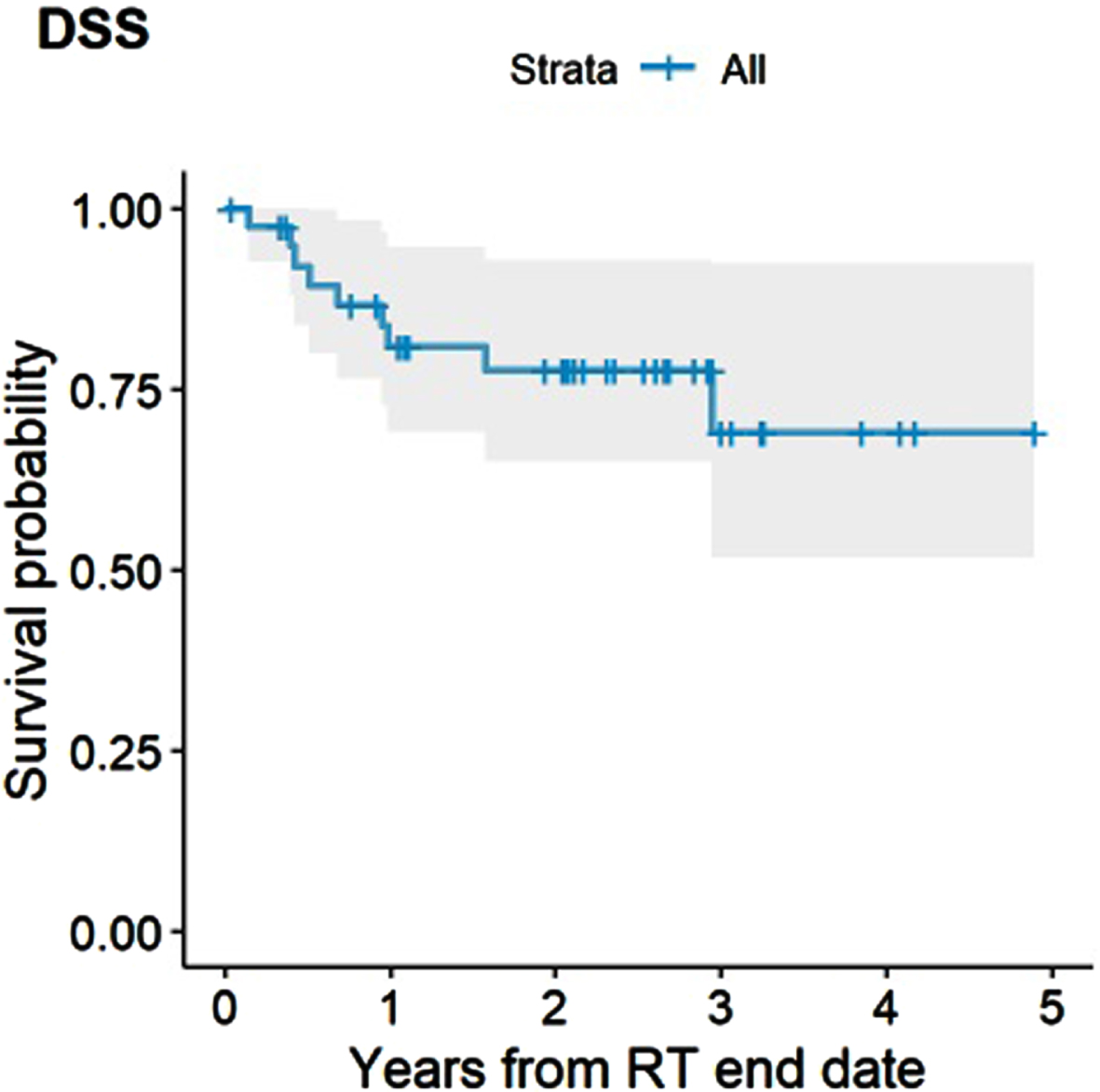
The median OS for the entire cohort was 35 months (95% CI, 35 months - not reached). The estimated 2-and 3-year OS rate were 69% (95% CI, 56% –85%) and 46% (95% CI, 29% –72%), respectively (Fig. 2). Receipt of concurrent chemotherapy (HR 0.05, 95% CI, 0.01–0.21, #iPt##/it#<0.001) and achieving a cCR (HR 0.32, 95% CI, 0.11–0.95, P = 0.039) were associated with improved OS, whereas presence of a Tis component at baseline was associated with worse OS (HR 3.26, 1.23–8.64, P = 0.018). Only concurrent chemotherapy persisted as a significantly associated with improved OS in multivariable analysis (HR 0.07, 0.02–0.27, P < 0.001). There was no significant difference in OS by age at diagnosis (≤75 vs >75), completeness of TURBT (complete vs incomplete), time from completion of TURBT to start of Hypo-RT, T stage (T1-2 vs T3-4), histology (urothelial vs mixed) and disease focality (unifocal vs multifocal). The estimated 2-and 3-year DSS rate were 78% (95% CI, 65% –93%) and 69% (95% CI, 52% –92%), respectively (Fig. 3). Receipt of concurrent chemotherapy (HR 0.09, 95% CI, 0.02–0.45, P = 0.003) and achieving a cCR (HR 0.17, 95% CI, 0.04–0.74, P = 0.018) were associated with improved DSS, whereas a Tis component was associated with worse DSS (HR 5.19, 1.38–19.6, P = 0.015). There was no significant difference in DSS by age at diagnosis (≤75 vs >75), completeness of TURBT, time from completion of TURBT to start of Hypo-RT, T stage, histology, disease focality, presence of LVI or hydronephrosis. Multivariable analysis was not performed for DSS due to a low number of events.
Fig. 2
Overall Survival (OS).
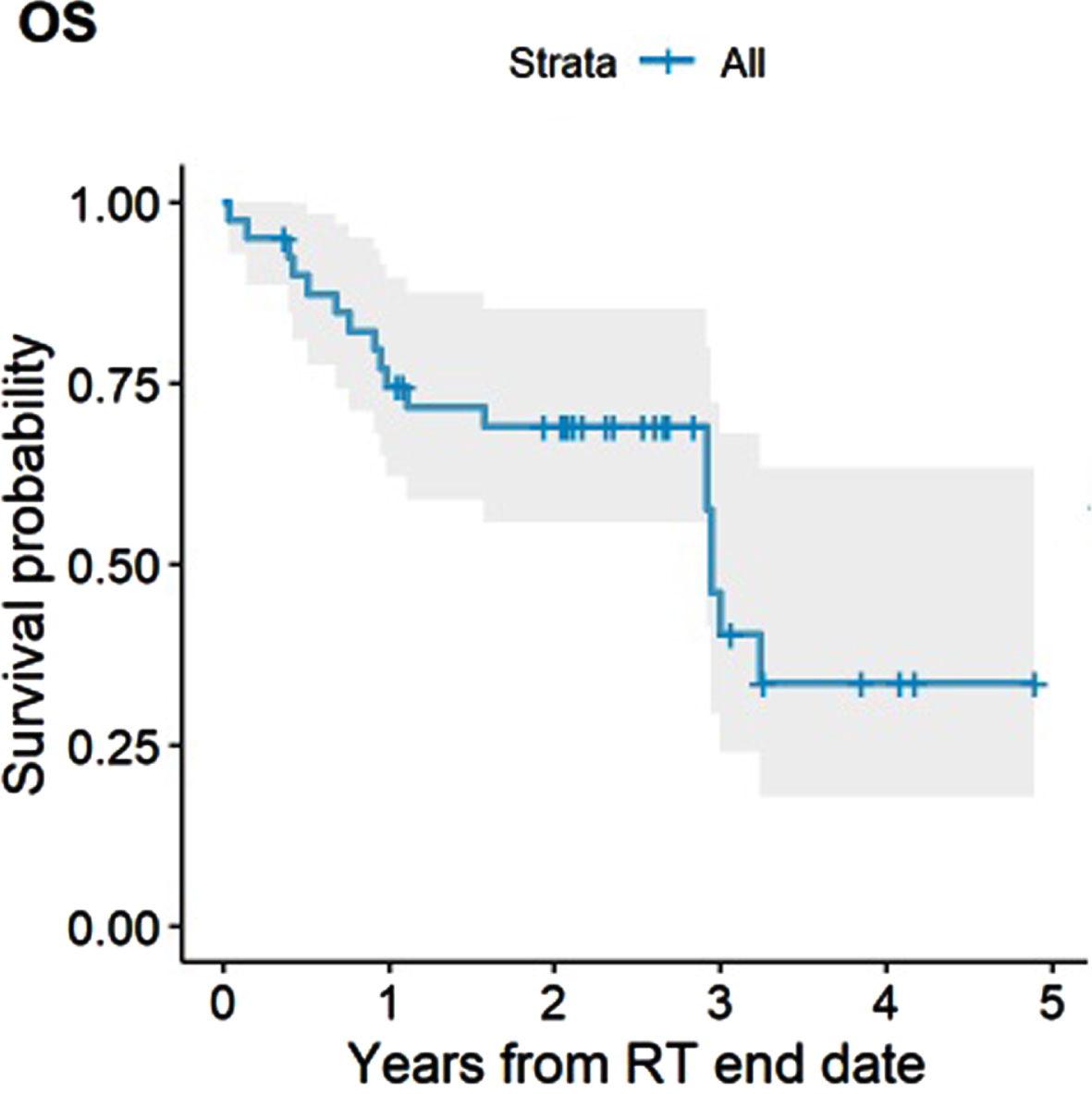
Fig. 3
Disease-specific Survival (DSS).
DISCUSSION
Our results demonstrate that Hypo-RT for localized bladder cancer, mostly delivered with concurrent chemotherapy, had a low rate of severe early and late toxicity. Early efficacy outcomes are encouraging with a CR rate of 75% and 55% of patients being NED at last follow up and a high 3-year DSS rate of 69%. The body of evidence on Hypo-RT for localized bladder cancer has been steadily growing and our results contribute to this data in a real-world cohort.
Elderly patients may be less likely to be treated aggressively with a curative intent [22]. Clearly, physicians’ and patients’ concerns for increased toxicity, compromised ability to complete the treatment course, as well as of competing risks for mortality, affect treatment selection for these patients [24]. In this study, 90% of patients completed Hypo-RT course. Of the four patients who did not complete treatment, only one had suspected radiation-associated toxicity demonstrating favorable tolerability of Hypo-RT in this age group.
Acute toxicity has been reported in variable rates with Hypo-RT and our results are in line with previous reports. In a phase II trial, Mohamed et al. reported 80.6% grade 1-2 and 19.4% grade 3 cystitis, 84% grade 1-2 and 16% grade 3 proctitis and 29% grade 1-2 and 3% grade 3 diarrhea [11]. Cowan et al reported rates of 40–55% and 50–70% grade 2-3 GU and GI toxicity at end of RT, respectively [16]. BC2001 found worse health-related quality of life (HRQOL) at the end of treatment with Hypo-RT, with no excess treatment interruptions. At 6 months post-treatment there was no difference in HRQOL [25]. The BCON and BC2001 joint analysis had not reported on acute toxicity due to differences in data collection between the trials [8]. A retrospective report of Hypo-RT with concurrent gemcitabine in an elderly cohort reported acute grade 2 GU and GI toxicity of 29% each and grade 3 GU and GI toxicity of 4% each [7]. We have documented acute grade 2 GU and GI toxicities in 40% each and acute grade 3 toxicity in 5%. Severe acute toxicity was minimal in this elderly frail cohort. We found a significant association between acute GI toxicity and exceeding the small bowel dose. Strictly adhering to dose constraints, and specifically small bowel Dmax may improve treatment tolerability and completion rates. About a third of patients treated with concurrent gemcitabine in our cohort did require some treatment modification, including interruption, dose reduction or omission of chemotherapy. This seemingly higher rate may be attributed to the older median age and associated medical comorbidities. Hypo-RT also appears to be durably well tolerated given our low rate of late grade 3 GU and GI toxicity. This is also consistent with prior Hypo-RT series with rates of grade 3 toxicity <6% overall [7, 8, 11]. As the median time to late toxicity in prior series may range from 16-–31 months, additional follow up or our patient cohort is critical to capture late toxicity events. The relatively low rate of severe toxicity in our study may be explained by differences in pelvic lymph node coverage, the use of modern radiotherapy techniques and by the fact that not all patients received concurrent chemotherapy. Nodal irradiation had not been standardized in bladder cancer and there is significant heterogeneity in its inclusion and extent of coverage. In general, the risk for nodal recurrence as the only site of failure is low, [2, 16, 17] and including the pelvic nodes in the field may be associated with higher toxicity rates [17]. Previous reports have suggested superiority of IMRT compared with 3-dimentional conformal radiotherapy in shaping the dose away from OARs while not compromising coverage of the PTV [26–28]. It is possible that IMRT and VMAT based planning implemented in all patients in this cohort, as well as image-guidance used daily may have contributed. As previously stated, the median follow up is relatively short and additional late toxicity may appear with longer follow up.
Our unselected patients’ outcomes compare favorably with previously published data. In a trial investigating reduction in treatment volume with Hypo-RT, Cowan et al. reported a 75% CR rate with whole bladder irradiation to 52.5 Gy in 20 fractions. The 5-year OS and cystectomy-free survival rate were 58% and 47%, respectively [16]. In a prospective phase II trial reported by Mohamed et al., 31 patients treated to 52.5 Gy in 20 fractions with concurrent weekly gemcitabine achieved an 80% radiological and pathological CR rate. At 2-years, OS was 94.4% and DFS was 72.6%.% [11]. Choudhury et al. reported results from a phase II trial of 50 patients treated to 52.5 Gy in 20 fractions with concurrent weekly gemcitabine with a CR rate of 88% and 3-year cancer-specific survival (CSS) and OS were 82% and 75%, repsectively [14]. Turgeon et al reported on an elderly cohort (median age of 79 years) of 24 patients treated to 50 Gy in 20 fractions with concurrent weekly gemcitabine. They reported an endoscopic CR rate of 83%. The 3-year OS and CSS rates were 61% and 71%, respectively [7 7].
Our cohort is unique in its elderly population (median age 82 years), compared with a median age of 74 years in both BC2001 and BCON, 66 years in the RTOG pooled analysis and 67 in Hypo-RT cohorts [2, 8, 14, 16, 19]. Despite the older age of our cohort, that 1/3 of patients had incomplete TURBT prior to therapy, and 20% did not receive concurrent chemotherapy, we still noted a 77% CR rate in this population.
Our reported estimated 2-year DFS of 59% seems comparable to an approximate rate of 60% reported by Turgeon et al. [7]. It is lower compared with the 2-year DFS or 72.6% in the publication by Mohamed et al. [11]. This difference can possible be explained by differences in definition of DFS used in the Mohamed study (diagnosis to last follow up) as well as inclusion of significant bilharziasis-related disease.
Reflective of this cohort’s high median Charlson comorbidity index (7), approximately 20% of patients died of causes unrelated to bladder cancer, causing OS and PFS to potentially underestimate this regimen’s effectiveness. Our reported DSS of 78% and 69% at 2- and 3-years, respectively, is similar to a 3-year CSS of 71% reported by Turgeon et al. for Hypo-RT with concomitant gemcitabine in an elderly cohort (median age, 79 years) and lower compared with 3-year DSS of 82% reported by Choudhury et al. in a cohort of younger, likely healthier patients (median age, 67 years).) [7, 14 14].
Overall, 20% of patients developed distant metastases which is reflective of the micrometastatic burden of disease likely present at initial diagnosis. While a similar rate has also been published by Choudhury et al. and Cowan et al. 6,8,18 rates as high as 35% have also been reported [2, 15]. Further efforts to identify and address micrometastatic spread are necessary to improve outcomes in this regard.
There are several strengths and limitations of our study which should be considered. Strengths include the consistency of treatment planning and delivery considerations for Hypo-RT as a single institution study. The main limitations of this study relate to its retrospective design relatively small cohort and short follow up which limit our ability to project longer-term toxicity and outcomes.
CONCLUSION
Hypo-RT for the treatment of localized bladder cancer had a favorable toxicity profile and encouraging cancer control outcomes in this unselected, mostly elderly and frail patient cohort. Hypo-RT should be a consideration for localized bladder cancer in elderly patients unable or unwilling to undergo radical cystectomy and have challenges to access treatment.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Assaf Moore – conception; performance of work; interpretation of data; writing the article. Had access to the data.
Stephanie M. Lobaugh – interpretation of data; writing the article. Had access to the data.
Zhigang Zhang – interpretation of data; writing the article. Had access to the data.
Jonathan E. Rosenberg – writing the article.
Gopa Iyer – writing the article.
Min Yuen Teo – writing the article.
Bernard Bochner – writing the article.
Timothy Donahue – writing the article.
David Aramburu Nunez – interpretation of data; had access to the data.
Alexandra Dreyfuss – interpretation of data; had access to the data.
Daniel Gorovets – writing the article.
Michael J. Zelefsky – writing the article.
Marisa A. Kollmeier – conception; performance of work; interpretation of data; writing the article. Had access to the data.
CONFLICTS OF INTEREST
Jonathan E. Rosenberg and Bernard Bochner are Editorial Board Members of this journal, but were not involved in the peer-review process nor had access to any information regarding its peer-review.
Assaf Moore, Stephanie M. Lobaugh, Zhigang Zhang, Gopa Iyer, Min Yuen Teo, Timothy Donahue, David Aramburu Nunez, Alexandra Dreyfuss, Daniel Gorovets, Michael J. Zelefsky and Marisa A. Kollmeier report no perceived conflicts of interest relevant to the current work.
REFERENCES
[1] | Siegel RL , Miller KD , Wagle NS , Jemal A . Cancer statistics, 2023. CA Cancer J Clin. (2023) ;73: (1):17–48. doi: 10.3322/caac.21763. PMID: 36633525. |
[2] | Mak RH , Hunt D , Shipley WU , et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. Journal of clinical oncology: Official journal of the American Society of Clinical Oncology. (2014) ;32: (34):3801–9. doi: 10.1200/JCO.2014.57.5548 |
[3] | Royce TJ , Feldman AS , Mossanen M , et al. Comparative Effectiveness of Bladder-preserving Tri-modality Therapy Versus Radical Cystectomy for Muscle-invasive Bladder Cancer. Clin Genitourin Cancer. (2019) ;17: (1):23–31.e3. doi: 10.1016/j.clgc.2018.09.023 |
[4] | García-Perdomo HA , Montes-Cardona CE , Guacheta M , Castillo DF , Reis LO . Muscle-invasive bladder cancer organ-preserving therapy: Systematic review and meta-analysis. World J Urol. (2008) ;36: (12):1997–2008. doi: 10.1007/s00345-018-2384-6 |
[5] | Oh KS , Soto DE , Smith DC , Montie JE , Lee CT , Sandler HM . Combined-modality therapy with gemcitabine and radiation therapy as a bladder preservation strategy: Long-term results of a phase I trial. Int J Radiat Oncol Biol Phys. (2009) ;74: (2):511–7. doi: 10.1016/j.ijrobp.2008.08.021 |
[6] | James ND , Hussain SA , Hall E , et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. New England Journal of Medicine. (2012) ;366: (16):1477–88. doi: 10.1056/NEJMoa1106106 |
[7] | Turgeon GA , Souhami L , Cury FL , et al. Hypofractionated intensity modulated radiation therapy in combined modality treatment for bladder preservation in elderly patients with invasive bladder cancer. Int J Radiat Oncol Biol Phys. (2014) ;88: (2):326–31. doi: 10.1016/j.ijrobp.2013.11.005 |
[8] | Choudhury A , Porta N , Hall E , et al. Hypofractionated radiotherapy in locally advanced bladder cancer: An individual patient data meta-analysis of the BCand BCON trials. The Lancet Oncology. (2021) ;22: (2):246–55. doi: 10.1016/S1470-2045(20)30607-0 |
[9] | Atasoy BM , Dane F , Alsan Cetin I , et al. Concurrent chemoradiotherapy with low dose weekly gemcitabine in medically inoperable muscle-invasive bladder cancer patients. Clinical and Translational Oncology. (2014) ;16: (1):91–5. doi: 10.1007/s12094-013-1047-8 |
[10] | Rödel C , Grabenbauer GG , Kühn R , et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: Long-term results. Journal of clinical oncology: Official journal of the American Society of Clinical Oncology. (2002) ;20: (14):3061–71. doi: 10.1200/JCO.2002.11.027 |
[11] | Mohamed HAH , Salem MA , Elnaggar MS , Gabr A , Abdelrheem AM . Trimodalities for bladder cancer in elderly: Transurethral resection, hypofractionated radiotherapy and gemcitabine. Cancer Radiotherapie: Journal de la Societe Francaise de Radiotherapie Oncologique. (2018) ;22: (3):236–40. doi: 10.1016/j.canrad.2017.09.013 |
[12] | Hammer L , Laufer M , Dotan Z , et al. Accelerated hypofractionated radiation therapy for elderly frail bladder cancer patients unfit for surgery or chemotherapy. Am J Clin Oncol. (2019) ;42: (2):179–83. doi: 10.1097/COC.0000000000000491 |
[13] | Coen JJ , Zhang P , Saylor PJ , et al. Bladder preservation with twice-a-day radiation plus fluorouracil/cisplatin or once daily radiation plus gemcitabine for muscle-invasive bladder cancer: NRG/RTOG -A randomized phase II trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2019) ;37: (1):44–51. doi: 10.1200/JCO.18.00537 |
[14] | Choudhury A , Swindell R , Logue JP , et al. Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2011) ;29: (6):733–8. doi: 10.1200/JCO.2010.31.5721 |
[15] | Giacalone NJ , Shipley WU , Clayman RH , et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: An updated analysis of the massachusetts general hospital experience. Eur Urol. (2017) ;71: (6):952–60. doi: 10.1016/j.eururo.2016.12.020 |
[16] | Cowan RA , McBain CA , Ryder WDJ , et al. Radiotherapy for muscle-invasive carcinoma of the bladder: Results of a randomized trial comparing conventional whole bladder with dose-escalated partial bladder radiotherapy. International Journal of Radiation Oncology Biology Physics. (2004) ;59: (1):197–207. doi: 10.1016/j.ijrobp.2003.10.018 |
[17] | Tunio MA , Hashmi A , Qayyum A , Mohsin R , Zaeem A . Whole-pelvis or bladder-only chemoradiation for lymph node-negative invasive bladder cancer: Single-institution experience. Int J Radiat Oncol Biol Phys. (2012) ;82: (3):e457–62. doi: 10.1016/j.ijrobp.2011.05.051 |
[18] | Hoskin PJ , Rojas AM , Bentzen SM , Saunders MI . Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2010) ;28: (33):4912–8. doi: 10.1200/JCO.2010.28.4950 |
[19] | Huddart RA , Hall E , Hussain SA , et al. Randomized noninferiority trial of reduced high-dose volume versus standard volume radiation therapy for muscle-invasive bladder cancer: Results of the BCtrial (CRUK/01/004). Int J Radiat Oncol Biol Phys. (2013) ;87: (2):261–9. doi: 10.1016/j.ijrobp.2013.06.2044 |
[20] | Coppin CM , Gospodarowicz MK , James K , et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (1996) ;14: (11):2901–7. doi: 10.1200/JCO.1996.14.11.2901 |
[21] | Méry B , Falk AT , Assouline A , et al. Hypofractionated radiationtherapy for treatment of bladder carcinoma in patients aged 90 yearsand more: A new paradigm to be explored? Int Urol Nephrol. (2015) ;47: (7):1129–34. doi: 10.1007/s11255-015-0999-8 |
[22] | Gray PJ , Fedewa SA , Shipley WU , et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: Results from the National Cancer Data Base. Eur Urol. (2013) ;63: (5):823–89. doi: 10.1016/j.eururo.2012.11.015 |
[23] | Averitt AJ , Weng C , Ryan P , Perotte A . Translating evidence into practice: Eligibility criteria fail to eliminate clinically significant differences between real-world and study populations. npj Digital Medicine. (2020) ;3: (1):67. doi: 10.1038/s41746-020-0277-8 |
[24] | Noon AP , Albertsen PC , Thomas F , Rosario DJ , Catto JWF . Competing mortality in patients diagnosed with bladder cancer: Evidence of undertreatment in the elderly and female patients. British Journal of Cancer. (2013) ;108: (7):1534–40. doi: 10.1038/bjc.2013.106 |
[25] | Huddart RA , Hall E , Lewis R , et al. Patient-reported quality of life outcomes in patients treated for muscle-invasive bladder cancer with radiotherapy± chemotherapy in the BCphase III randomised controlled trial. European Urology. (2020) ;77: (2):260–8. doi: https://doi.org/10.1016/j.eururo.2019.11.001 |
[26] | Van Rooijen DC , Van De Kamer JB , Hulshof M , Koning CCE , Bel A . Improving bladder cancer treatment with radiotherapy using separate intensity modulated radiotherapy plans for boost and elective fields. Journal of Medical Imaging and Radiation Oncology. (2010) ;54: (3):256–63. doi: https://doi.org/10.1111/j.1754-9485.2010.02169.x |
[27] | Hsieh CH , Chung SD , Chan PH , et al. Intensity modulated radiotherapy for elderly bladder cancer patients. Radiation Oncology. (2011) ;6: (1):75. doi: 10.1186/1748-717X-6-75 |
[28] | Sherry AD , Stewart A , Luo G , Kirschner AN . Intensity-modulated radiotherapy is superior to three-dimensional conformal radiotherapy in the trimodality management of muscle-invasive bladder cancer with daily cone beam computed tomography optimization. Journal of Radiation Oncology. (2019) ;8: (4):395–403. doi: 10.1007/s13566-019-00411-0 |




