Comparison of Robotic vs Open Cystectomy: A Systematic Review
Abstract
BACKGROUND:
The benefits of a robot-assisted radical cystectomy (RARC) compared to an open approach is still under debate. Initial data on RARC were from trials where urinary diversion was performed by an extracorporeal approach, which does not represent a completely minimally invasive procedure. There are now updated data for RARC with intracorporeal urinary diversion that add to the evidence profile of RARC.
OBJECTIVE:
To perform a systematic review and meta-analysis of the effectiveness of RARC compared with open radical cystectomy (ORC).
MATERIALS AND METHODS:
Multiple databases were searched up to May 2022. We included randomised trials in which patients underwent RARC and ORC. Oncological and safety outcomes were assessed.
RESULTS:
Seven trials of 907 participants were included. There were no differences seen in primary outcomes: disease progression [RR 0.98, 95% CI 0.78 to 1.23], major complications [RR 0.95, 95% CI 0.72 to 1.24] and quality of life [SMD 0.05, 95% CI -0.13 to 0.38]. RARC resulted in a decreased risk of perioperative blood transfusion [RR 0.57, 95% CI 0.43 to 0.76], wound complications [RR 0.34, 95% CI 0.21 to 0.55] and reduced length of hospital stay [MD -0.62 days, 95% CI -1.11 to -0.13]. However, there was an increased risk of developing a ureteric stricture [RR 4.21, 95% CI 1.07 to 16.53] in the RARC group and a prolonged operative time [MD 70.4 minutes, 95% CI 34.1 to 106.7]. The approach for urinary diversion did not impact outcomes.
CONCLUSION:
RARC is an oncologically safe procedure compared to ORC and provides the benefits of a minimally invasive approach. There was an increased risk of developing a ureteric stricture in patients undergoing RARC that warrants further investigation. There was no difference in oncological outcomes between approaches.
INTRODUCTION
There has been considerable uptake in robot-assisted radical cystectomy (RARC) over the last five years. In England, there has been an increase in the proportion of cystectomies being performed robotically from 11% in 2013 to 40% in 2019 [1]. These patterns have also been seen in the United States and other countries where there is access to robotic surgery [2]. At the early stages of adoption, although the extirpation part of the procedure was completed robotically, the diversion has often been performed through an open approach. Therefore, it could be argued that the maximal benefit of minimally invasive surgery was not being obtained with these ‘hybrid’ procedures [3]. Randomised controlled trials (RCTs) comparing RARC with extracorporeal diversion to open radical cystectomy (ORC) did not generally show any significant difference between the two approaches [4].
As surgical experience is gained, there is a growing trend to perform RARC with an intracorporeal urinary diversion and therefore, a complete robotic procedure. In theory, this should accentuate the benefits of minimally invasive surgery including decreased bleeding, decreased hospital stay and early recovery of bowel function [3]. High quality data in the form of randomised trials have, until recently, only been available for RARC with extra-corporeal diversion. However, the iROC trial published its results recently and provides us with level one evidence of RARC with intracorporeal diversion for days alive and out of hospital [5]. Although an intracorporeal diversion is hypothesised to provide the aforementioned benefits, it is important to assess whether there are any potential downsides. Retrospective studies have suggested that intracorporeal diversion significantly prolongs operation time [6] and could impact the quality of bowel and ureteric anastomoses due to technical difficulty [7, 8].
We aim to perform an updated systematic review and meta-analysis of open vs robotic cystectomy. Importantly, we compare outcomes of extra- and intracorporeal diversions within robotic procedures. We hypothesise that robotic cystectomy with intracorporeal diversion will further improve peri-operative outcomes such as blood loss and length of stay but at the cost of prolonged surgical time. We hypothesise that a smaller surgical incision for intracorporeal diversions should minimise wound complications. As bowel is not exposed to atmosphere, we also hypothesise that the incidence of paralytic ileus will be lower in the intracorporeal diversion group. However, there may be an increased risk of ureteric stricture with intracorporeal diversion due to the technical challenges with robotic anastomosis. We do not expect there to be differences in oncological outcomes between the surgical techniques.
METHODS
This systematic review and meta-analysis is based on the methodology of a previously published paper by our group and performed according to PRISMA guidelines [9]. The protocol was registered a priori in PROSPERO (CRD42018103678). The detailed methodology can be found in the aforementioned publication [9]. We performed an updated search of multiple databases including MEDLINE, EMBASE, ScienceDirect, Cochrane Libraries, HTA database and Web of Science up to 25th May, 2022 using the original search terms. We tracked citations of previously included papers and cross-checked references lists of eligible papers to ensure all relevant records were included. We had no restrictions on language nor date of publication.
We included all randomised trials comparing RARC to ORC. We excluded non-randomised studies. We did not place any restrictions on the extent of lymph node dissection, the type of diversion nor the approach. The indication for surgery had to be due to bladder cancer for patients to be included. We also excluded patients with metastatic disease undergoing a palliative cystectomy.
In accordance to the Cochrane Handbook of Systematic Reviews, abstract screening, full-text review and data extraction was performed by two separate authors independently with a third, senior author consulted to resolve any discrepancies [10].
The primary outcomes were the same as the previous paper:
•
• Disease progression: defined as radiological or pathological evidence of disease following radical cystectomy or death from bladder cancer
• Major complication (Clavien-Dindo grade≥3) within 90 days of surgery:
• Quality-of-life (QoL) at 90 days measured by a validated QoL instrument (e.g. Functional Assessment of Cancer Therapy (FACT))
We included all the secondary outcomes that were assessed in our previous paper:
•
• Positive surgical margin: the presence of cancer cells at the edge of the removed surgical specimen
• Peri-operative blood transfusion rate: the receipt of a blood transfusion intra-operatively or during the post-operative period up to 90 days
• Operative time: the duration of radical cystectomy and urinary diversion
• Length of hospital stay: the duration of index admission when radical cystectomy was performed
• Local recurrences: evidence of bladder cancer in the pelvic soft tissue and/or lymph nodes following radical cystectomy
Following expert consensus, we decided to perform additional in-depth complication analysis for this updated paper, especially focusing on outcomes that may be impacted by the mode of diversion (intra- or extracorporeal). These were all decided on a priori. Thus, the following outcomes were also assessed in this paper:
•
• Ureteric stricture: defined as a narrowing in the ureter at the level at the uretero-ileal anastomosis (with or without intervention) that had caused kidney obstruction as demonstrated by symptomatic, biochemical and/or radiological means
• Paralytic ileus: defined as a non-mechanical reduction in bowel motility
• Wound complications: defined as the occurrence of superficial/deep wound infection and/or wound dehiscence
• Thromboembolic events: defined as the occurrence of a deep vein thrombosis and/or pulmonary embolus
We performed subgroup analysis based on the modality of urinary diversion: extracorporeal vs intracorporeal. We also intended to perform subgroup analysis based on the extent of lymph node dissection but there was insufficient data available.
Statistical analysis was performed according to the recommendations of the Cochrane Handbook of Systematic Reviews [10]. We performed random effects models for all analyses with Mantel-Haenszel for dichotomous outcomes and inverse variance method for continuous outcomes. We used Version 2 of the Cochrane risk-of-bias tool for randomized trials to assess for bias in the included studies. We also created a Summary of Findings table according to GRADE guidance to assist interpretations of our findings [11].
RESULTS
The full PRISMA flow diagram is shown in Appendix 1. The search retrieved 1071 records after removal of duplicates of which 59 had their full texts reviewed. Seven studies were determined to meet the eligibility criteria and were included for analysis [5, 12–17]. The characteristics of these studies are shown in Table 1. Since our last paper on this topic, there were two new trials published. Importantly, both these studies performed intracorporeal diversion and are the first RCTs to do so [5, 17]. There were also updated results available for previously included studies [18–21]. The studies were undertaken in either the United States or United Kingdom, except Maibom et al which was conducted in Denmark. Only RAZOR and iROC were multi-institutional studies.
Table 1
Characteristics of included studies
| Urinary diversion method | Author | Year | Location | n | Institutions | Number of surgeons | Surgical experience | Ileal conduit diversion, n(%) | Neobladder diversion, n(%) |
| Extracorporeal | Bochner (12, 22) | 2015 | USA | 118 | Memorial Sloan Ketting, New York, USA | Four | >10 years post-fellowship | 50 (42) | 68 (58) |
| Khan(13, 18) | 2016 | UK | 60 | Guy’s and St Thomas’, London, United Kingdom | Each approach performed by a single surgeon | >150 ORC and > 110 RARC | 53 (90) | 7 (10) | |
| Nix(14) | 2010 | USA | 41 | University of North Carolina, Chapel Hill, USA | NR | >400 ORC and > 75 RARC | 28 (68) | 13 (32) | |
| Parekh(15, 38) | 2013 | USA | 47 | University of Miami, Miami, USA | One | NR | NR | NR | |
| Parekh(16, 19, 21) | 2018 | USA | 350 | Multiple | Twenty-six | >10 RCs | 265 (76) | 85 (24) | |
| Intracorporeal | Catto(5) | 2022 | UK | 338 | Multiple | NR | ≥30 RARCs | 301 (89) | 37 (11) |
| Maibom(17) | 2021 | Sweden | 50 | University of Copenhagen, Copenhagen, Denmark | Each approach performed by a single surgeon | NR | 50 (100) | 0 (0) |
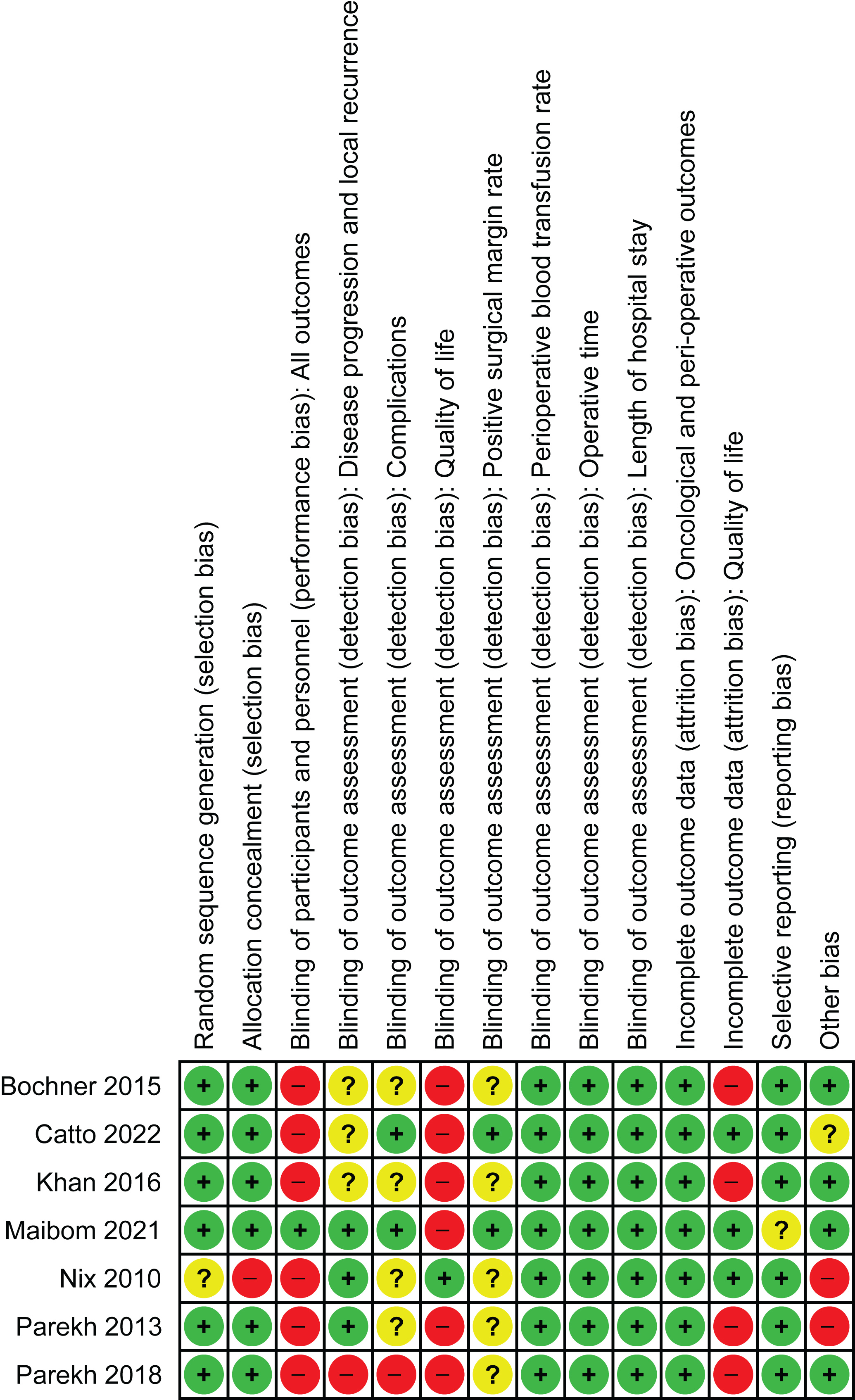
The risk of bias is depicted in Appendix 2. In summary, these were high-quality surgical trials. Only a single trial was able to overcome the challenge of double-blinding by separating the care teams who were involved in the actual procedure and were unblinded from those who were involved with care on the ward and were blinded [17].
The Summary of Findings table is shown in Table 2.
Table 2
GRADE Summary of findings
| Outcomes | No of participants (studies) Follow-up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with ORC | Risk difference with RARC | ||||
| Disease progression | 775 (4 RCTs) | ⊕⊕⊕ ○ Moderatea | RR 0.98 (0.78 to 1.23) | 265 per 1,000 | 5 fewer per 1,000 (58 fewer to 61 more) |
| Major complication | 827 (5 RCTs) | ⊕⊕⊕ ○ Moderatea | RR 0.95 (0.72 to 1.24) | 207 per 1,000 | 10 fewer per 1,000 (58 fewer to 50 more) |
| Quality of life | 511 (4 RCTs) | ⊕⊕ ○○ Lowb | – | – | SMD 0.05 higher (0.13 lower to 0.22 higher) |
| Positive surgical margin rate | 591 (6 RCTs) | ⊕⊕⊕ ○ Moderatec | RR 1.14 (0.58 to 2.24) | 51 per 1,000 | 7 more per 1,000 (21 fewer to 63 more) |
| Perioperative blood transfusion rate | 683 (4 RCTs) | ⊕⊕⊕ ⊕ High | RR 0.57 (0.43 to 0.76) | 288 per 1,000 | 124 fewer per 1,000 (164 fewer to 69 fewer) |
| Operative time | 907 (7 RCTs) | ⊕⊕⊕ ⊕ High | – | The mean operative time was 270 minutesd | MD 70.43 minutes higher (34.13 higher to 106.74 higher) |
| Length of hospital stay | 895 (7 RCTs) | ⊕⊕⊕ ○ Moderatec | – | The median length of hospital stay was 8 daysd | MD 0.62 days lower (1.11 lower to 0.13 lower) |
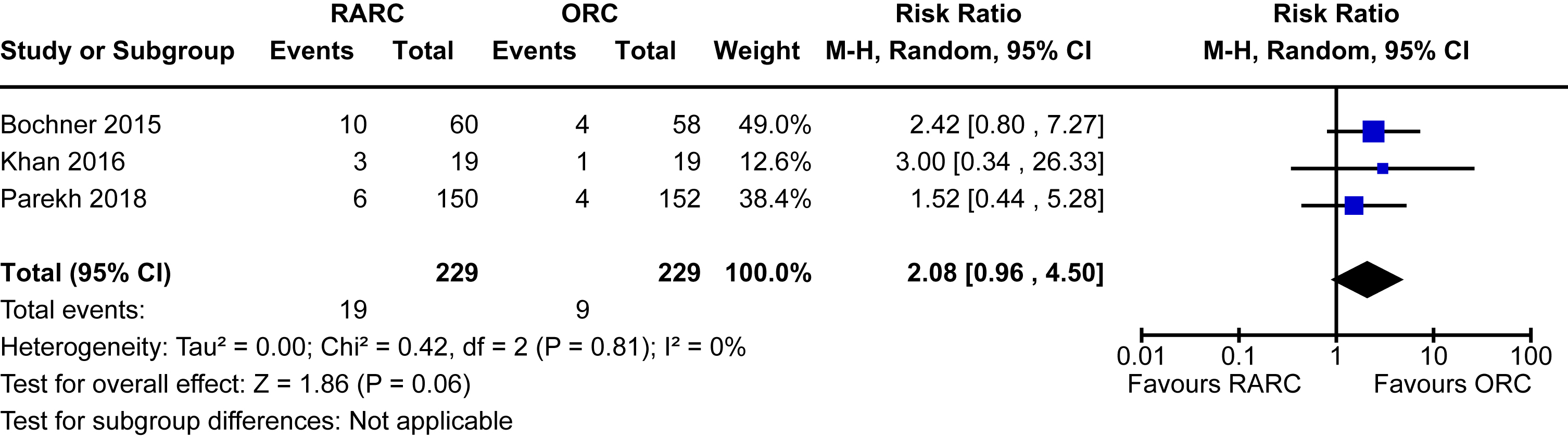
| Local recurrence | 458 (3 RCTs) | ⊕⊕ ○○ Lowa,c | RR 2.08 (0.96 to 4.50) | 39 per 1,000 | 42 more per 1,000 (2 fewer to 138 more) |
| Ureteric Stricture | 658 (3 RCTs) | ⊕⊕ ○○ Lowa,c | RR 4.21 (1.07 to 16.53) | 6 per 1,000 | 20 more per 1,000 (0 fewer to 95 more) |
| Thromboembolic Events | 867 (6 RCTs) | ⊕⊕ ○○ Lowa,c | RR 0.48 (0.20 to 1.11) | 77 per 1,000 | 40 fewer per 1,000 (61 fewer to 8 more) |
| Wound Complications | 827 (5 RCTs) | ⊕⊕⊕ ○ Moderatea | RR 0.34 (0.21 to 0.55) | 151 per 1,000 | 100 fewer per 1,000 (119 fewer to 68 fewer) |
| Ileus | 432 (4 RCTs) | ⊕⊕ ○○ Lowa,c | RR 0.97 (0.46 to 2.05) | 198 per 1,000 | 6 fewer per 1,000 (107 fewer to 208 more) |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). aDowngraded one level due to concerns regarding study limitations. bDowngraded two levels due to significant concerns regarding study limitations. cDowngraded one level due to wide confidence intervals. dEstimates obtained from iROC trial
CI: confidence interval; MD: mean difference; RR: risk ratio; SMD: standardised mean difference
| GRADE Working Group grades of evidence |
| High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. |
| Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. |
| Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. |
Disease progression
Four trials of 775 patients provided data on disease progression [5, 13, 16, 20]. The overall incidence of disease progression across the groups was 26% (events = 205). Bochner et al and Khan et al reported oncological outcomes up to median follow-up of 5 years [18, 20] but the other included studies reported outcomes at 2 years [5, 16]. There was no difference between patients undergoing RARC compared to ORC [RR 0.98, 95% CI 0.78 to 1.23]. There was no heterogeneity detected (I2 = 0%). There was also no difference seen based on the modality of diversion (p = 0.56). The forest plot is shown in Appendix 3. We graded the certainty of evidence as moderate after downgrading one level due to concerns about study limitations.
Major complications
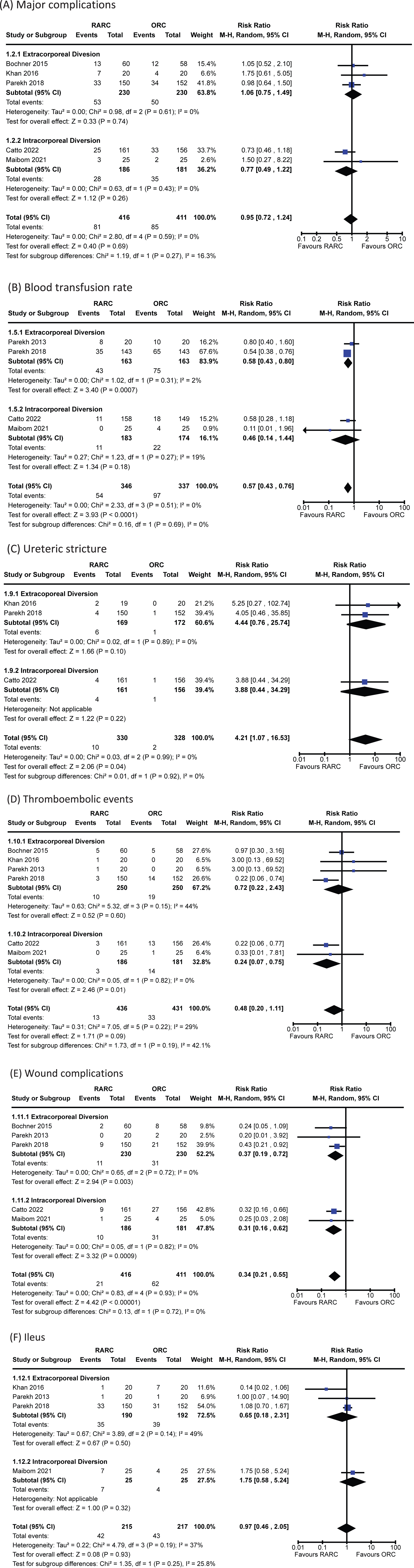
Data for 90-day Clavien-Dindo grade III-V complications was reported in five trials [5, 13, 16, 17, 20]. The incidence of major complications in patients undergoing RARC was 19% and 21% in the ORC group. There was no significant difference when comparing RARC vs ORC [RR 0.95, 95% CI 0.72 to 1.24]. No heterogeneity was detected in this analysis (I2 = 0%). There was no difference between extracorporeal and intracorporeal diversions (p = 0.27). The forest plot is seen in Fig. 1A. We graded the certainty of evidence as moderate after downgrading one level due to concerns about study limitations.
Fig. 1
Forest plots for: (A) major complications, (B) blood transfusion, (C) ureteric stricture, (D) thromboembolic events, (E) wound complications, (F) ileus. Legend: RARC = robot-assisted radical cystectomy; ORC = open radical cystectomy.
Quality of life
Four trials reported on quality of life [5, 15, 16, 22]. There was no significant difference between the reported quality of life measures between RARC and ORC [SMD 0.05, 95% CI -0.13 to 0.38]. No heterogeneity was detected (I2 = 0%). Again, no difference was seen between the modality of diversion (p = 0.38). The forest plot is shown in Appendix 4. We graded the certainty of evidence as low after downgrading two levels due to significant concerns about study limitations.
Positive surgical margin
The incidence of a positive surgical margin in the six studies which reported this outcome was 5% [13–17, 22]. There was no significant difference between RARC and ORC [RR 1.14, 95% CI 0.58 to 2.24]. There was no heterogeneity detected (I2 = 0%). There was no difference between extracorporeal and intracorporeal diversions (p = 0.88). The forest plot is shown in Appendix 5. We graded the certainty of evidence as moderate after downgrading one level due to concerns about imprecision and confidence intervals that crossed thresholds that would be clinically significant. It should be noted that we were unable to differentiate positive soft tissue margins from positive margins of urethra or ureters.
Perioperative blood transfusions
Data for this outcome was available in four trials of 683 patients [5, 15–17]. The incidence of blood transfusion in the RARC group was 16% and 29% in the ORC group. Thus, undergoing a RARC was associated with a significantly lower risk of requiring a perioperative blood transfusion compared to ORC [RR 0.57, 95% CI 0.43 to 0.76]. No heterogeneity was detected (I2 = 0%). No difference was seen between the modality of diversion (p = 0.69). The forest plot is seen in Fig. 1B. We graded the certainty of evidence as high.
Operative time
All included studies of 907 patients reported on operative time [5, 12–18, 20]. There was a significantly longer operative time in the group undergoing RARC compared to ORC [MD 70.4 minutes, 95% CI 34.1 to 106.7]. There was significant heterogeneity observed for this outcome (I2 = 95%). There was no difference between extracorporeal and intracorporeal diversions (p = 0.91). The forest plot is shown in Appendix 6. We graded the certainty of evidence as high.
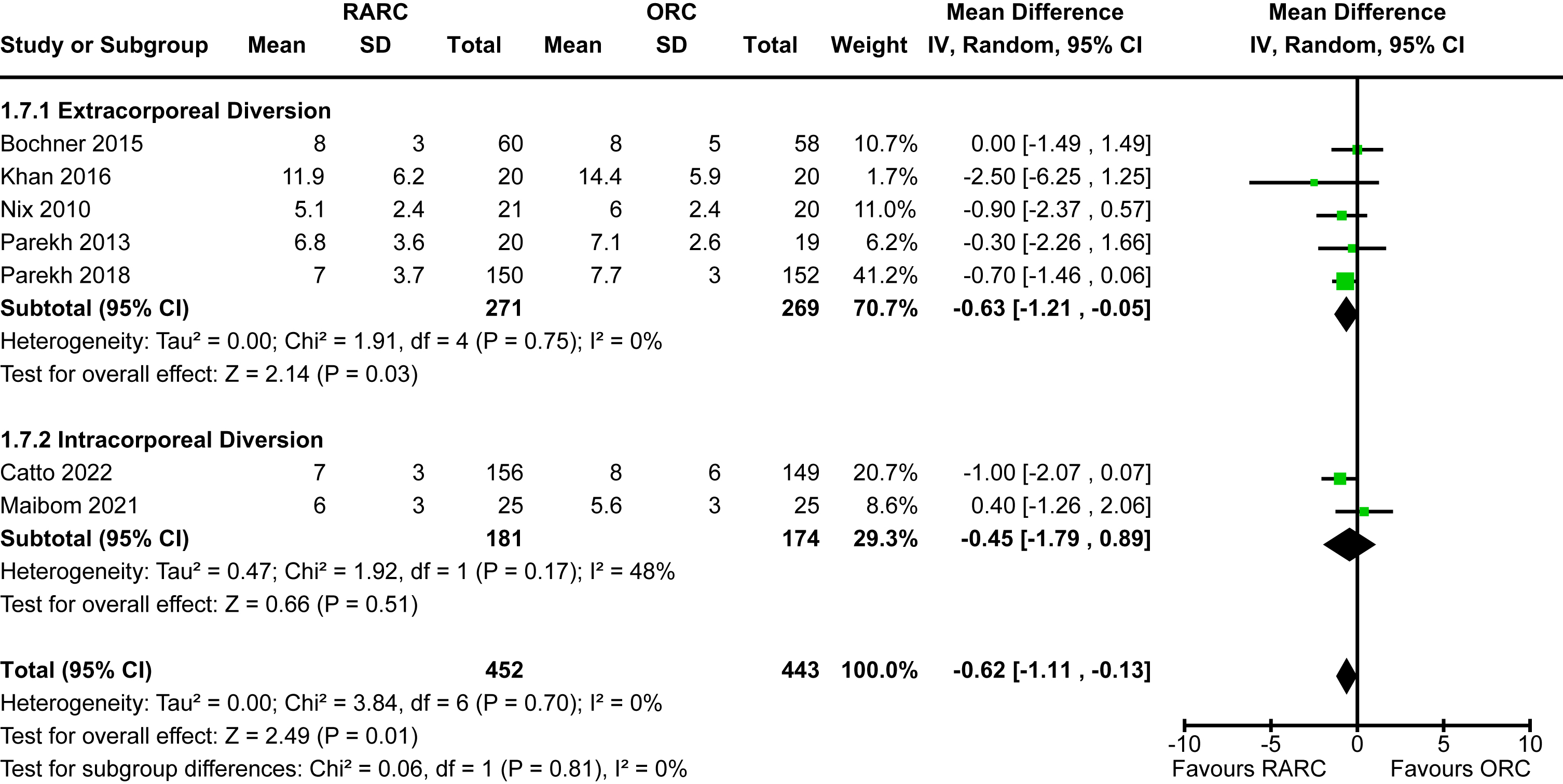
Length of hospital stay
Data for this outcome was available for all eligible studies [5, 12–18, 20]. The mean length of stay for studies that reported on extracorporeal diversion ranged from 5-12 days whereas the two studies that performed intracorporeal diversion ranged from 6-7 days. The mean length of stay for open cases was 6-14 days. There was a statistically significant reduction in length of hospital stay in the patients undergoing RARC compared to ORC [MD -0.62 days, 95% CI -1.11 to -0.13]. There was no heterogeneity detected (I2 = 0%). The mode of diversion had no impact on length of hospital stay (p = 0.81). The forest plot is shown in Appendix 7. We graded the certainty of evidence as moderate after downgrading one level due to concerns about imprecision and confidence intervals that crossed thresholds that would be clinically significant.
Local recurrence
Three trials of 458 patients reported on local recurrence defined as the evidence of bladder cancer in the pelvic soft tissue and/or lymph nodes following radical cystectomy [12, 13, 16]. There were only 29 events of local recurrence observed. The incidence was higher in the RARC group (8.3%) compared to the ORC group (3.9%). However, the difference between the groups was not statistically significant [RR 2.08, 95% CI 0.96 to 4.50]. There was no heterogeneity detected (I2 = 0%). Subgroup analysis based on the mode of diversion was unable to be conducted due to a lack of data for this outcome for patients undergoing intracorporeal diversion. The forest plot is shown in Appendix 8. We graded the certainty of evidence as low after downgrading one level for study limitations and a further level for imprecision.
Ureteric stricture
Three trials of 658 patients reported on the occurrence of ureteric stricture following radical cystectomy [5, 13, 16]. The overall incidence of ureteric strictures was 1.8%. There was an increased risk of developing a ureteric stricture in the robotic group [RR 4.21, 95% CI 1.07 to 16.53]. There was no heterogeneity observed (I2 = 0%). There was no difference in the incidence of strictures whether the diversion was performed extra- or intra-corporeally (p = 0.92). The forest plot is shown in Fig. 1C. We graded the certainty of evidence as low after downgrading one level for study limitations and a further level for imprecision.
Thromboembolic events
The incidence of thromboembolic events amongst the six studies with information on this outcome was 5.3% [5, 12, 13, 15–17]. There was no difference in risk between patients undergoing RARC and ORC [RR 0.48, 95% CI 0.20 to 1.11]. A small amount of heterogeneity was observed (I2 = 29%). It should be noted that both large, multi-institutional RCTs (RAZOR and iROC) showed a significantly decreased risk of thromboembolic events in the RARC arm – both reported an identical RR of 0.22 with similar confidence intervals. The mode of diversion had no impact on the risk of thromboembolic events (p = 0.19). The forest plot is shown in Fig. 1D. We graded the certainty of evidence as low after downgrading one level for study limitations and a further level for imprecision.
Wound complications
Five studies of 827 patients reported sufficient data to assess this outcome [5, 12, 15–17]. The incidence of wound complications was 10.0%. The was a significantly lower risk of experiencing a wound complication in the RARC group [RR 0.34, 95% CI 0.21 to 0.55]. No heterogeneity was detected (I2 = 0%). No difference was seen between the modality of diversion (p = 0.72). The forest plot is shown in Fig. 1E. We graded the certainty of evidence as moderate after downgrading one level due to concerns about study limitations.
Paralytic ileus
The incidence of a paralytic ileus amongst included patients was 19.8% [5, 13, 15–17]. There was no difference observed between the two groups [RR 0.97, 95% CI 0.46 to 2.05]. A small degree of heterogeneity was observed (I2 = 37%). There was no difference in the risk of ileus whether the diversion was performed extra- or intra-corporeally (p = 0.25). The forest plot is shown in Fig. 1F. We graded the certainty of evidence as low after downgrading one level for study limitations and a further level for imprecision.
DISCUSSION
This updated systematic review and meta-analysis of nearly 1,000 patients from high-level randomised trials outlines the benefits and drawbacks of RARC and ORC. Compared to our previously published paper, this study includes the results of two additional trials that were the first to perform intra-corporeal urinary diversions compared to the others that performed diversions extra-corporeally. The results of this study validate the safety of a robotic approach to radical cystectomy by showing equivalence between RARC and ORC for the primary outcomes of disease progression, major complications and quality of life. As hypothesised, due to the minimally invasive nature of RARC, there was a significantly lower need for blood transfusions, decreased risk of wound complications and a shorter length of stay in this group. We do acknowledge that the reduction in length of stay was only just over half a day, and this may not be clinically significant. On the other hand, RARC was associated with an increased operative time. There was also an increased risk of uretero-ileal stricture in the robotic group at limited follow-up. Concerningly, although failing to achieve statistical significance, there was a two-fold increase in the risk of local recurrence in patients undergoing a robotic cystectomy. There maybe several factors contributing to this, other than surgical approach, that we discuss below. The type of urinary diversion did not impact any of the outcomes assessed.
Overall, the findings from this study are in line with the primary studies and the wider literature. Our initial review also demonstrated that surgical approach for radical cystectomy did not have a significant impact on patient-important outcomes [9]. The results from other systematic reviews that also included non-randomised studies were consistent with these findings [23, 24]. Therefore, both RARC and ORC can be considered to be equivalent in the wider sense with the decision on approach based on surgeon experience/training, accessibility and health economics. It should be noted that minor differences have been observed between extra- and intra-corporeal diversion in some studies. We observed a trend towards lower major complication rates and decreased risk of thromboembolic events with intra-corporeal diversions but none of these achieved statistical significance likely due to insufficient statistical power. We hypothesise that the reduction in thromboembolic events are likely multifactorial and at least partly related to earlier mobilisation in the post-operative period with a minimally-invasive approach, especially with intra-corporeal diversion where the surgical incision is not large. Some of the benefits of RARC with intra-corporeal diversion are currently diluted by the increased operative time which in itself would increase the risk of thromboembolic events but as surgical time decreases with experience then we can expect the benefits on minimising thromboembolic events to increase. Analysis of the International Robotic Cystectomy Consortium did demonstrate a decreased risk of complications at 90 days in the intracorporeal group but most of the other outcomes were comparable between the techniques [25]. A multi-institutional, French study also reported no difference in peri-operative outcomes between the different modalities of diversion [26]. We hypothesised that there may be a reduced risk of wound complications with intra-corporeal diversion because of small incision lengths but this was not seen in our results. This maybe either because there is no true difference or that there was insufficient power.
As with most complex surgical procedures, surgical experience has been shown to have a significant impact on outcomes following RARC. Dell’Oglio et al in a cohort study demonstrated that as surgical experience in RARC with intracorporeal diversion increased, there was a lower incidence of Clavien-Dindo grade≥2 complications and a shorter operative time [27]. There was also a lower incidence of disease recurrence at 18 months with increasing experience. We hypothesise that differences, and potential shortcomings in training and experience, may have contributed to the observed absolute difference in local recurrence rates between RARC and ORC. It is plausible that sub-standard lymph node dissections in the RARC group could be a factor in this outcome, especially during the learning curve. Guru et al reported that average lymph node yield increased with experience from 13 to 23 [28]. Furthermore, there have been previous concerns that the robotic technique may negatively impact oncologic dissection and impact cancer cell dissemination through pneumoperitoneum [29]. However, a secondary analysis of the RAZOR trial reported that surgical approach did not affect patterns of recurrence [21]. This finding, albeit not statistically significant, warrants investigation in future studies to ensure oncological safety.
We did observe an increased risk of ureteric strictures in the group undergoing RARC. This has been seen in other non-randomised studies. Using Surveillance, Epidemiology, and End Results-Medicare data, RARC had a higher incidence of ureteric strictures at six months compared to ORC, 12% vs 7% [30]. Even with extended follow-up to 24 months, stricture rates were lower in patients undergoing ORC. Similarly, Reesink et al reported a 17% incidence of uretero-ileal strictures at three months in their single institution study. The RARC group had an increased risk of strictures compared to ORC (25% vs 13%, p < 0.01) [31]. Importantly, the first study showed that the incidence of strictures was inversely related to hospital volume of RARC [30]. Similarly, the second study showed that the incidence of strictures after RARC was highest in the first 12 months following introduction of the robot and then subsequently decreased [31]. Therefore, we suggest that the findings of increased stricture rate in our meta-analysis may again be driven by surgical experience rather than purely due to the cystectomy being performed robotically. Surgeons should still reflect on technical factors that may be contributing to this finding and consider advances such as the use of indocyanine green. Ahmadi and colleagues reported that checking distal ureter vascularity with indocyanine green led to a larger length of ureter being excised and no occurrences of strictures, compared to an 11% incidence of stricture in the group of patients for whom indocyanine green was not used [32]. The lack of haptic feedback with an intra-corporeal robotic approach may also be impacting the degree of tension placed on the anastomosis which may be contributing to the stricture rate.
There are limitations of this systematic review and meta-analysis that should be considered when interpreting its results. There are differences in surgical technique between and within studies and also in post-operative management that may have contributed to the results despite randomisation. Despite randomisation that is intended to balance measured and unmeasured confounders between intervention groups, there was not explicit pre-planned stratification based on these factors (e.g. receipt of neoadjuvant chemotherapy or VTE prophylaxis) and therefore imbalances may exist between the groups that could confound the results. The majority of patients received an ileal conduit as their urinary diversion and therefore results are mainly applicable to these patients and there maybe differences that were not detected in this meta-analysis in patients having a neobladder. Despite pooling the results from several studies, some outcomes may still be under-powered to detect a difference; for example, there was only a small proportion of high-risk patients included in the trials and hence this may not be sufficient to detect an effect on disease recurrence. Early recovery after surgery (ERAS) protocol has been widely adopted in high-volume, tertiary cystectomy centres such as those participating in the study and has been shown to greatly improve the outcomes after radical cystectomy and maybe masking some of the benefits of a robotic approach [33]. For example, randomised evidence has demonstrated that the use of a mu-opioid receptor antagonist reduced the incidence of post-operative ileus and hospital stay [34]. However, a single institution study comparing the impact of surgical approach after institution of an ERAS protocol demonstrated no difference in major complications or readmissions between ORC and RARC [35]. The studies were not powered to assess most of the secondary outcomes we tested and therefore there may be differences that we have not observed. For example, the findings regarding the increased risk of ureteric strictures and the signal toward a possible risk of increased recurrence in the RARC group warrant further investigation. However, the secondary analyses are only hypothesis generating and the results of such should be interpreted within that context. The findings of this meta-analysis may not be generalisable to the wider community given that many of these surgeons were high-volume, fellowship-trained, experts and their outcomes may not be replicated by less experienced surgeons given the complexity of radical cystectomy [36]. Furthermore, outcomes in a clinical trial have been consistently shown to be superior to those seen in the real-world [37]. Other relevant outcomes such as readmission rate and the need for secondary interventions were not assessed in our study and may provide important information in appraising the differences between surgical approaches for radical cystectomy. Likewise, we did not assess survival outcomes because we did not believe that surgical approach itself is a critical factor in determining overall survival outcomes when compared to other factors that have a bigger impact, such as disease stage and nodal involvement, but it is possible that there may be a difference in survival between robotic and open cystectomy that should be assessed in future studies.
CONCLUSIONS
Robot-assisted radical cystectomy and open cystectomy are comparable for primary outcomes of disease progression, major complications and quality of life. Robot-assisted radical cystectomy does offer the benefit of being minimally invasive, resulting in decreased risk of blood transfusions, wound infections and decreased length of hospital stay. However, open cystectomy was a shorter procedure and had a lower risk of ureteric stricture. There was no difference in outcomes based on whether urinary diversion was performed extra- or intra-corporeally. In addition to accessibility and surgical experience, patients should be counselled on these individual risks and benefits of each approach when making a clinical decision.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Conception: Sathianathen, Furrer, Thomas, Dundee, Corcoran, Weight, Konety, Nair, Lawrentschuk.
Performance of work: Sathianathen, Pan, Furrer.
Interpretation of data: Thomas, Dundee, Corcoran, Weight, Konety, Nair, Lawrentschuk.
Writing the article: Sathianathen, Pan, Furrer, Thomas, Dundee, Corcoran, Weight, Konety, Nair, Lawrentschuk.
ETHICAL CONSIDERATIONS
As a systematic review of the literature, and as no animal or human research was involved, our study is exempt from any requirement for Institutional Review Board approval.
CONFLICT OF INTEREST
Konety and Lawrentschuk are Editorial Board members of this journal, but were not involved in the peer-review process nor had access to any information regarding its peer-review.
Sathianathen, Pan, Furrer, Thomas, Dundee, Corcoran, Weight and Nair have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and/or its supplementary material.
REFERENCES
[1] | Tamhankar AS , Thurtle D , Hampson A , El-Taji O , Thurairaja R , Kelly JD , et al. Radical Cystectomy in England from 2013 to 2019 on 12,644 patients: An analysis of national trends and comparison of surgical approaches using Hospital Episode Statistics data. BJUI Compass. (2021) ;2: (5):338–47. |
[2] | Matulewicz RS , DeLancey JO , Manjunath A , Tse J , Kundu SD , Meeks JJ . National comparison of oncologic quality indicators between open and robotic-assisted radical cystectomy. Urol Oncol. (2016) ;34: (10):431.e9–e15. |
[3] | Tan WS , Tan MY , Lamb BW , Sridhar A , Mohammed A , Baker H , et al. Intracorporeal robot-assisted radical cystectomy, together with an enhanced recovery programme, improves postoperative outcomes by aggregating marginal gains. BJU Int. (2018) ;121: (4):632–9. |
[4] | Satkunasivam R , Tallman CT , Taylor JM , Miles BJ , Klaassen Z , Wallis CJD . Robot-assisted Radical Cystectomy Versus Open Radical Cystectomy: A Meta-analysis of Oncologic, Perioperative, and Complication-related outcomes. Eur Urol Oncol. (2019) ;2: (4):443–7. |
[5] | Catto JWF , Khetrapal P , Ricciardi F , Ambler G , Williams NR , Al-Hammouri T , et al. Effect of Robot-Assisted Radical Cystectomy With Intracorporeal Urinary Diversion vs Open Radical Cystectomy on 90-Day Morbidity and Mortality Among Patients With Bladder Cancer: A Randomized Clinical Trial. Jama. (2022) . |
[6] | Chow K , Zargar H , Corcoran NM , Costello AJ , Peters JS , Dundee P . Robotic-assisted radical cystectomy with intracorporeal urinary diversion versus open: early Australian experience. ANZ J Surg. (2018) ;88: (10):1028–32. |
[7] | Tanneru K , Jazayeri SB , Kumar J , Alam MU , Norez D , Nguyen S , et al. Intracorporeal versus extracorporeal urinary diversion following robot-assisted radical cystectomy: a meta-analysis, cumulative analysis, and systematic review. J Robot Surg. (2021) ;15: (3):321–33. |
[8] | Collins JW , Hosseini A , Sooriakumaran P , Nyberg T , Sanchez-Salas R , Adding C , et al. Tips and tricks for intracorporeal robot-assisted urinary diversion. Curr Urol Re. (2014) ;15: (11):457. |
[9] | Sathianathen NJ , Kalapara A , Frydenberg M , Lawrentschuk N , Weight CJ , Parekh D , et al. Robotic Assisted Radical Cystectomy vs Open Radical Cystectomy: Systematic Review and Meta-Analysis. The Journal of urology. (2019) ;201: (4):715–20. |
[10] | Higgins JP , Thomas J , Chandler J , Cumpston M , Li T , Page MJ , et al. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; (2019) . |
[11] | Guyatt G , Oxman AD , Akl EA , Kunz R , Vist G , Brozek J , et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology. (2011) ;64: (4):383–94. |
[12] | Bochner BH , Dalbagni G , Sjoberg DD , Silberstein J , Keren Paz GE , Donat SM , et al. Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: A Randomized Clinical Trial. European urology. (2015) ;67: (6):1042–50. |
[13] | Khan MS , Gan C , Ahmed K , Ismail AF , Watkins J , Summers JA , et al. A Single-centre Early Phase Randomised Controlled Three-arm Trial of Open, Robotic, and Laparoscopic Radical Cystectomy (CORAL). European urology. (2016) ;69: (4):613–21. |
[14] | Nix J , Smith A , Kurpad R , Nielsen ME , Wallen EM , Pruthi RS . Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. European urology. (2010) ;57: (2):196–201. |
[15] | Parekh DJ , Messer J , Fitzgerald J , Ercole B , Svatek R . Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. The Journal of urology. (2013) ;189: (2):474–9. |
[16] | Parekh DJ , Reis IM , Castle EP , Gonzalgo ML , Woods ME , Svatek RS , et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. The Lancet. (2018) ;391: (1013):2525–36. |
[17] | Maibom SL , Røder MA , Aasvang EK , Rohrsted M , Thind PO , Bagi P , et al. Open vs robot-assisted radical cystectomy (BORARC): a double-blinded, randomised feasibility study. BJU Int. (2021) . |
[18] | Khan MS , Omar K , Ahmed K , Gan C , Van Hemelrijck M , Nair R , et al. Long-term Oncological Outcomes from an Early Phase Randomised Controlled Three-arm Trial of Open, Robotic, and Laparoscopic Radical Cystectomy (CORAL). European urology. (2020) ;77: (1):110–8. |
[19] | Becerra MF , Venkatramani V , Reis IM , Soodana-Prakash N , Punnen S , Gonzalgo ML , et al. Health Related Quality of Life of Patients with Bladder Cancer in the RAZOR Trial: A Multi-Institutional Randomized Trial Comparing Robot versus Open Radical Cystectomy. The Journal of urology. (2020) ;204: (3):450–9. |
[20] | Bochner BH , Dalbagni G , Marzouk KH , Sjoberg DD , Lee J , Donat SM , et al. Randomized Trial Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: Oncologic Outcomes. European urology. (2018) ;74: (4):465–71. |
[21] | Venkatramani V , Reis IM , Castle EP , Gonzalgo ML , Woods ME , Svatek RS , et al. Predictors of Recurrence, and Progression-Free and Overall Survival following Open versus Robotic Radical Cystectomy: Analysis from the RAZOR Trial with a 3-Year FollowuThe Journal of urology (2020) ;203: (3):522–9. |
[22] | Bochner BH , Dalbagni G , Marzouk KH , Sjoberg DD , Lee J , Donat SM , et al. Randomized Trial Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: Oncologic Outcomes. European urology. (2018) . |
[23] | Yuh B , Wilson T , Bochner B , Chan K , Palou J , Stenzl A , et al. Systematic review and cumulative analysis of oncologic and functional outcomes after robot-assisted radical cystectomy. European urology. (2015) ;67: (3):402–22. |
[24] | Jue JS , Mikhail D , Feuerstein MA . Systematic review of robotic radical cystectomy functional and quality of life outcomes. Can Urol Assoc J. (2022) ;16: (2):E102–e7. |
[25] | Ahmed K , Khan SA , Hayn MH , Agarwal PK , Badani KK , Balbay MD , et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. European urology. (2014) ;65: (2):340–7. |
[26] | Lenfant L , Verhoest G , Campi R , Parra J , Graffeille V , Masson-Lecomte A , et al. Perioperative outcomes and complications of intracorporeal vs extracorporeal urinary diversion after robot-assisted radical cystectomy for bladder cancer: a real-life, multi-institutional french study. World J Urol. (2018) ;36: (11):1711–8. |
[27] | Dell’Oglio P , Mazzone E , Lambert E , Vollemaere J , Goossens M , Larcher A , et al. The Effect of Surgical Experience on Perioperative and Oncological Outcomes After Robot-assisted Radical Cystectomy with Intracorporeal Urinary Diversion: Evidence from a Referral Centre with Extensive Experience in Robotic Surgery. European urology focus. (2021) ;7: (2):352–8. |
[28] | Guru KA , Sternberg K , Wilding GE , Tan W , Butt ZM , Mohler JL , et al. The lymph node yield during robot-assisted radical cystectomy. BJU Int. (2008) ;102: (2):231–4; discussion 4. |
[29] | Nguyen DP , Al Hussein Al Awamlh B , Wu X , O’Malley P , Inoyatov IM , Ayangbesan A , et al. Recurrence patterns after open and robot-assisted radical cystectomy for bladder cancer. European urology. (2015) ;68: (3):399–405. |
[30] | Goh AC , Belarmino A , Patel NA , Sun T , Sedrakyan A , Bochner BH , et al. A Population-based Study of Ureteroenteric Strictures After Open and Robot-assisted Radical Cystectomy. Urology. (2020) ;135: :57–65. |
[31] | Reesink DJ , Gerritsen SL , Kelder H , van Melick HHE , Stijns PEF . Evaluation of Ureteroenteric Anastomotic Strictures after the Introduction of Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion: Results from a Large Tertiary Referral Center. The Journal of urology. (2021) ;205: (4):1119–25. |
[32] | Ahmadi N , Ashrafi AN , Hartman N , Shakir A , Cacciamani GE , Freitas D , et al. Use of indocyanine green to minimise uretero-enteric strictures after robotic radical cystectomy. BJU Int. (2019) ;124: (2):302–7. |
[33] | Wessels F , Lenhart M , Kowalewski KF , Braun V , Terboven T , Roghmann F , et al. Early recovery after surgery for radical cystectomy: comprehensive assessment and meta-analysis of existing protocols. World J Urol. (2020) ;38: (12):3139–53. |
[34] | Lee CT , Chang SS , Kamat AM , Amiel G , Beard TL , Fergany A , et al. Alvimopan Accelerates Gastrointestinal Recovery After Radical Cystectomy: A Multicenter Randomized Placebo-Controlled Trial. European urology. (2014) ;66: (2):265–72. |
[35] | Chen J , Djaladat H , Schuckman AK , Aron M , Desai M , Gill IS , et al. Surgical approach as a determinant factor of clinical outcome following radical cystectomy: Does Enhanced Recovery After Surgery (ERAS) level the playing field? Urol Oncol. (2019) ;37: (10):765–73. |
[36] | Moschini M , Simone G , Stenzl A , Gill IS , Catto J . Critical Review of Outcomes from Radical Cystectomy: Can Complications from Radical Cystectomy Be Reduced by Surgical Volume and Robotic Surgery? European urology focus. (2016) ;2: (1):19–29. |
[37] | Bartlett VL , Dhruva SS , Shah ND , Ryan P , Ross JS . Feasibility of Using Real-World Data to Replicate Clinical Trial Evidence. JAMA Netw Open. (2019) ;2: (10):e1912869. |
[38] | Messer JC , Punnen S , Fitzgerald J , Svatek R , Parekh DJ . Health-related quality of life from a prospective randomised clinical trial of robot-assisted laparoscopic vs open radical cystectomy. BJU Int. (2014) ;114: (6):896–902. |
Appendices
Appendix 1 Study flow diagram
Appendix 2. Risk of bias
Appendix 3. Forest plot for disease progression
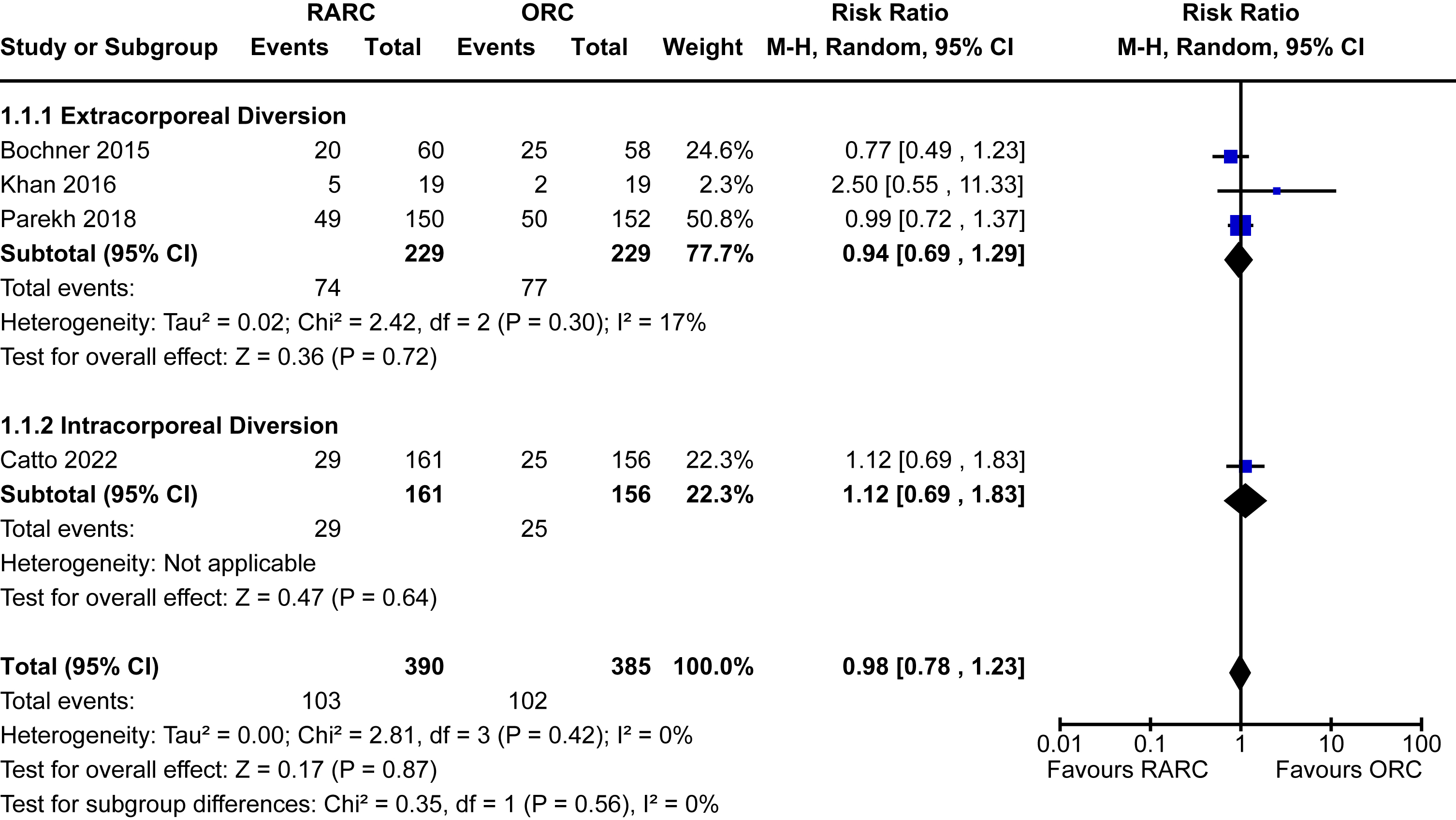
Appendix 4. Forest plot for quality of life
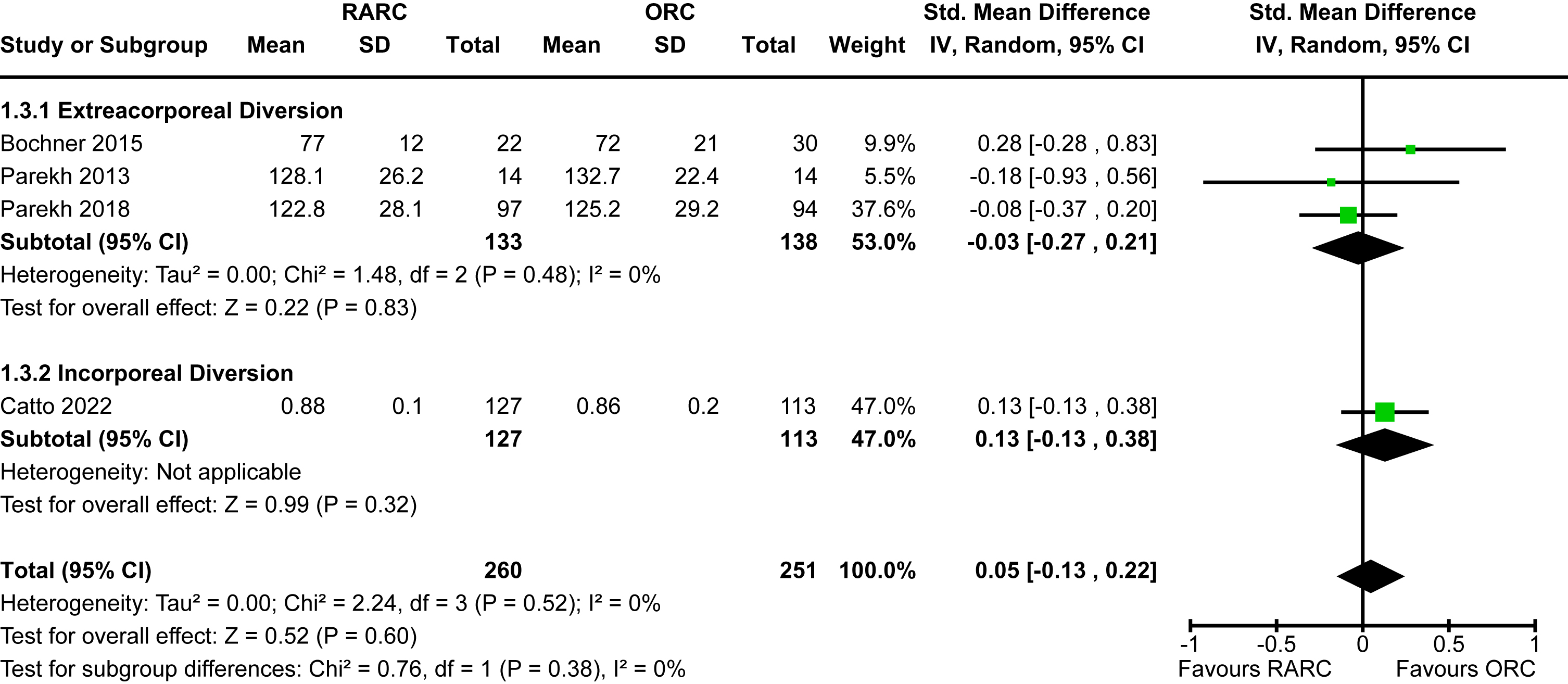
Appendix 5. Forest plot for positive surgical margins
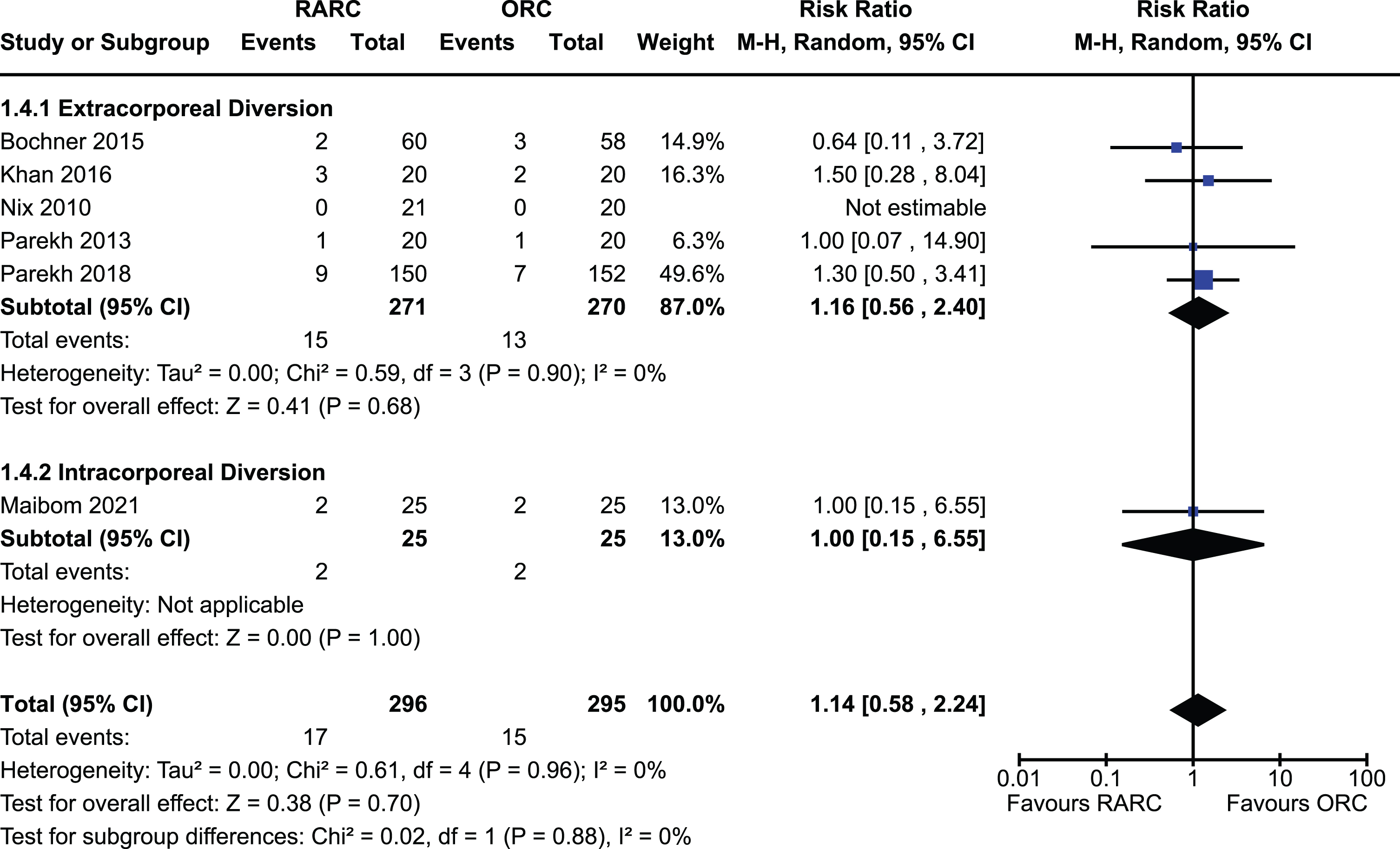
Appendix 6. Forest plot for operative time
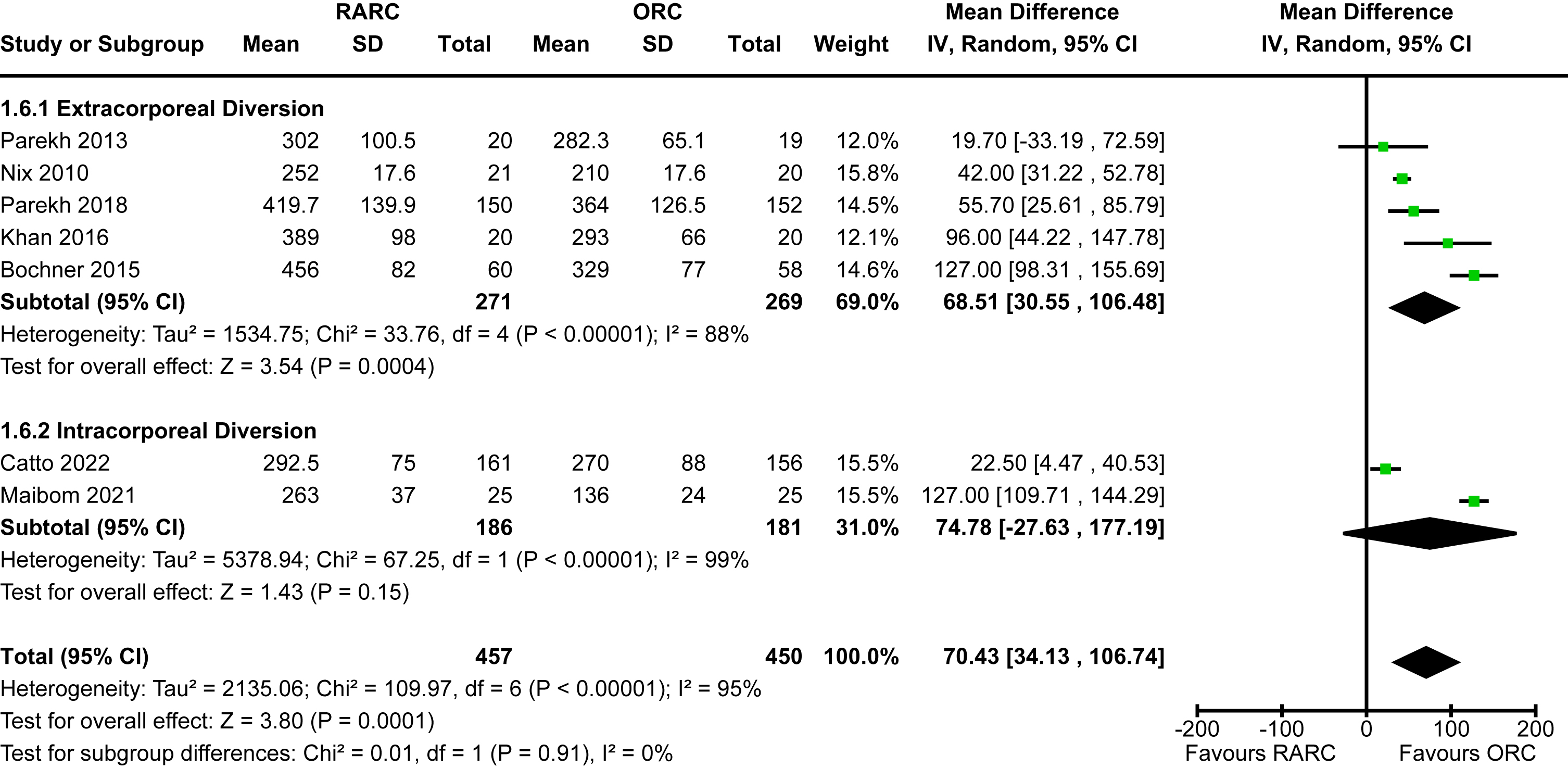
Appendix 7. Forest plot for length of hospital stay
Appendix 8. Forest plot for local recurrence