Adjuvant Chemotherapy Plus Radiotherapy versus Chemotherapy Alone for Locally Advanced Bladder Cancer after Radical Cystectomy
Abstract
BACKGROUND:
Survival with locally advanced bladder cancer (LABC) following radical cystectomy (RC) remains poor. Although adjuvant chemotherapy (AC) is standard of care, one small, randomized trial has suggested a potential survival benefit when combined with post-operative radiotherapy (PORT).
OBJECTIVE:
We examined the association of AC + PORT with overall survival (OS) in patients with LABC after RC.
METHODS:
Using a prior phase 2 trial to inform design, we conducted observational analyses to emulate a hypothetical target trial of patients aged 18–79 years with pT3-4 Nany M0 or pTany N1-3 M0 urothelial bladder carcinoma following RC who were treated with AC (multiagent chemotherapy within 3 months of RC) with or without PORT (≥45 Gy to the pelvis) from 2006–2015 in the NCDB. Patients who received preoperative chemotherapy or radiotherapy were excluded. The associations of treatment with OS were evaluated using multivariable Cox regression.
RESULTS:
1,684 patients were included, with 66 receiving AC + PORT and 1,618 AC alone. Compared to patients treated with AC alone, those treated with AC + PORT were more likely to have pT4 disease (52% vs 26%; p < 0.01), positive surgical margins (44% vs 17%; p < 0.01), and be treated at a non-academic facility (75% vs 53%; p < 0.01). Crude 5-year OS was 19% for AC + PORT versus 36% for AC alone (p = 0.01). Adjusted 5-year OS was 33% for AC + PORT versus 36% for AC alone (p = 0.49). After adjusting for baseline characteristics including pathologic features, AC + PORT was not associated with improved OS compared to AC alone (HR 1.11; 95% CI 0.82–1.51).
CONCLUSIONS:
Although infrequently utilized, the addition of radiotherapy to AC is not associated with improved OS in LABC. These results highlight the need for prospective trials to better define the potential benefits from PORT with regard to symptomatic progression and oncologic outcomes.
ABBREVIATIONS
AC | Adjuvant chemotherapy |
ACM | All-cause mortality |
BC | Bladder cancer |
DFS | Disease-free survival |
IORT | Intra-operative radiotherapy |
IQR | Interquartile range |
LN | Lymph node |
LRFS | Local recurrence-free survival |
MIBC | Muscle-invasive bladder cancer |
NCDB | National Cancer Database |
OS | Overall survival |
PORT | Post-operative radiotherapy |
RC | Radical cystectomy |
INTRODUCTION
Locally advanced bladder cancer (BC) is associated with poor survival following radical cystectomy (RC), with 5-year overall survival (OS) of 33–58% for pT3-4 disease at the time of RC, respectively [1]. Furthermore, the 5-year local failure rate ranges from 15% to over 40%, with an estimated median time to local failure of 9 months and poor OS after local failure has occurred [2–6]. Given the high risk of disease recurrence and progression, adjuvant chemotherapy (AC) has been established as a standard of care in patients eligible for cisplatin. Although contemporary randomized trials have not demonstrated statistically significant treatment effects due to methodological limitations [7, 8], evidence from meta-analyses of randomized trials and a body of observational evidence support its use [9, 10]. Accordingly, contemporary practice guidelines recommend AC for patients with locally advanced disease following RC in the absence of cisplatin-based neoadjuvant chemotherapy [11].
Despite the efficacy of AC for locally advanced BC after RC, disease recurrence remains considerable [2–6]. Post-operative radiotherapy (PORT) has re-emerged as an area of interest to improve outcomes for locally advanced BC. Zaghloul, et al. first demonstrated that PORT administered in those with ≥pT3 BC after RC was associated with a 49% 5-year disease-free survival (DFS) and a local control rate of 87% compared to 25% and 50%, respectively, among those who received no additional treatment [12]. PORT was also found to be an independent predictor of both DFS and local free recurrence survival [12, 13]. However, given that in the Zaghloul study nearly half of patients had squamous cell predominant histology, and later studies demonstrated adverse effects associated with the now-outdated radiotherapy techniques used, the generalizability and safety of PORT remained uncertain [14].
With advances in delivery of radiotherapy and establishment of AC as a standard of care in MIBC, the use of PORT has seen renewed interest. A recent phase III trial is investigating the effects of PORT versus sandwiched chemotherapy and PORT in those with MIBC stage ≥pT3b at the time of RC on local recurrence-free survival (LRFS). While results have not been reported, a phase II trial comparing sandwiched AC and PORT to AC alone has demonstrated a 27% absolute improvement in 2-year LRFS, along with a non-significant trend toward benefit in DFS (68% versus 56%, p = 0.07) with AC + PORT [15]. That study was the first prospective trial investigating the use of AC + PORT versus AC alone.
Despite the potential for improvement in oncologic outcomes with the addition of PORT to AC in locally advanced BC, the current evidence is limited. Several ongoing trials are examining the efficacy of PORT in locally advanced BC [16–20]. However, these trials have yet to report results, with several focusing on toxicity and safety as primary outcomes over survival outcomes. We therefore set out to emulate a hypothetic pragmatic target trial using a nationwide oncology dataset to evaluate the comparative effectiveness of adjuvant radiotherapy (PORT) with AC versus AC alone for locally advanced BC.
MATERIALS AND METHODS
Target clinical trial
After obtaining a determination of not human subjects research from our institutional review board, we conducted observational analyses designed to emulate a hypothetical target trial informed by the design of a real-world phase II clinical trial [15] using the National Cancer Database (NCDB). Using the emulation framework described by Hernan and Robin [21], we specified, a priori, the components of the hypothetical target trial as outlined in Table 1.
Table 1
Mini-protocol for real-world clinical trial and hypothetical target trial in the emulation
| Protocol component | Zaghloul, et al. [15] | Hypothetical Target Trial |
| Eligibility criteria | Age 18–70 years with ≥pT3b R0 Nany M0 or pTany R0 N1-3 M0 bladder cancer s/p radical cystectomy from 2002 to 2008. ECOG 0–2. No neobladder diversion. No histology requirement. | Age 18–79 years with pT3-pT4 M0 or pN1-3 M0 with urothelial carcinoma of the bladder diagnosed 2006–2015 s/p radical cystectomy. Any surgical margin status (Rany). No prior malignancy, preoperative chemotherapy/radiotherapy or IORT. |
| Treatment strategies | Multiagent AC + PORT versus multiagent AC alone.PORT: 3D conformal radiotherapy to cystectomy bed + pelvic lymph nodes to 45 Gy.AC: 4 cycles of sandwiched cisplatin and gemcitabine. | Multiagent AC within 3 months of RC + PORT (≥45 Gy to the pelvis) versus multiagent AC within 3 months of RC alone. |
| Assignment procedures | Randomization within 56 days of RC | Un-blinded non-random assignment to treatments |
| Follow-up period | Starts at randomization and ends on date of last available data in observational dataset or at the occurrence of outcome event, loss to follow-up, or death (whichever is earlier). | Starts at RC and ends on date of last available data in observational dataset or at the occurrence of outcome event, loss to follow-up, or death (whichever is earlier). |
| Outcomes | Primary: LRFSSecondary: DFS, DMFS, OS, adverse effects | Primary: OS |
RC = radical cystectomy, RT = radiotherapy, OS = overall survival, LRFS = local recurrence free survival, DFS = disease-free survival, DMFS = distant metastasis-free survival, ECOG = Eastern Cooperative Oncology Group.
We identified patients aged 18 to 79 with pT3-pT4 Nany Rany M0 or pTany Rany pN1-3 M0 urothelial carcinoma of the bladder diagnosed from 2006–2015 in the NCDB and treated with RC (Supplementary Figure 1). We excluded patients who received preoperative chemotherapy/radiotherapy or intraoperative radiotherapy (IORT). We defined two treatment strategies: multiagent AC (initiated within 3 months of RC) with or without PORT (defined as ≥45 Gy to the pelvis).
Clinicopathologic Features and Outcome
The following clinicopathologic characteristics were recorded from the NCDB: age, gender, Charlson index, year of diagnosis, race, Hispanic ethnicity, insurance status, geographic location, facility type, distance from hospital, rurality, income level, educational level, pathologic TNM stage, tumor grade, annual cystectomy RC volume, surgical margin status, number of lymph nodes removed, and number of positive lymph nodes. The primary outcome was overall survival (OS).
Statistical analyses
Baseline characteristics were summarized using medians and interquartile ranges (IQRs) or frequency counts and percentages and compared across treatment groups using the Wilcoxon rank sum test for continuous variables and chi-square tests for categorical variables.
We utilized traditional multivariable Cox regression to evaluate the associations of treatment group with OS, adjusting for baseline characteristics. Categorical covariates were modeled as summarized in Table 2, while continuous covariates were modeled as linear. Results are reported using hazard ratios (HRs) with 95% confidence intervals (95% CI). Using the multivariable model, we generated adjusted survival curves stratified by treatment group to produce survival estimates at various follow-up times.
Table 2
Baseline characteristics of the study cohort
| Treatment | ||||
| Characteristic | Total 1684 (100%)1 | AC + PORT 66 (4%)1 | AC alone 1618 (96%)1 | p-value2 |
| Age, years | 65 (58, 70) | 65 (59, 70) | 65 (58, 70) | 0.47 |
| Gender | <0.01 | |||
| Male | 1,270 (75) | 39 (59) | 1,231 (76) | |
| Female | 414 (25) | 27 (41) | 387 (24) | |
| Charlson Index | 0.71 | |||
| 0 | 1,183 (70) | 45 (68) | 1,138 (70) | |
| 1+ | 501 (30) | 21 (32) | 480 (30) | |
| Year of Diagnosis | 0.19 | |||
| 2006 | 136 (8) | 8 (12) | 128 (8) | |
| 2007 | 131 (8) | 4 (6) | 127 (8) | |
| 2008 | 132 (8) | 10 (15) | 122 (8) | |
| 2009 | 129 (8) | 4 (6) | 125 (8) | |
| 2010 | 214 (13) | 9 (14) | 205 (13) | |
| 2011 | 229 (14) | 4 (6) | 225 (14) | |
| 2012 | 200 (12) | 7 (11) | 193 (12) | |
| 2013 | 171 (10) | 7 (11) | 164 (10) | |
| 2014 | 185 (11) | 4 (6) | 181 (11) | |
| 2015 | 157 (9) | 9 (14) | 148 (9) | |
| Race | 0.26 | |||
| White | 1,556 (92) | 65 (98) | 1,491 (92) | |
| Black | 98 (6) | 1 (2) | 97 (6) | |
| Others | 30 (2) | 0 (0) | 30 (2) | |
| Hispanic | 0.99 | |||
| No | 1,647 (98) | 65 (98) | 1,582 (98) | |
| Yes | 37 (2) | 1 (2) | 36 (2) | |
| Insurance status | 0.51 | |||
| Not Insured | 66 (4) | 2 (3) | 64 (4) | |
| Private/Managed care | 700 (42) | 27 (41) | 673 (42) | |
| Medicaid | 110 (7) | 2 (3) | 108 (7) | |
| Medicare | 785 (47) | 33 (50) | 752 (46) | |
| VA/Military | 23 (1) | 2 (3) | 21 (1) | |
| Geographic location | 0.83 | |||
| Northeast | 404 (24) | 15 (23) | 389 (24) | |
| South/Southeast | 530 (31) | 19 (29) | 511 (32) | |
| Midwest | 550 (33) | 25 (38) | 525 (32) | |
| West | 200 (12) | 7 (11) | 193 (12) | |
| Facility type | <0.01 | |||
| Community Cancer Program | 108 (6) | 5 (8) | 103 (6) | |
| Comprehensive community Cancer Program | 643 (38) | 35 (53) | 608 (38) | |
| Academic /Research Program | 775 (46) | 17 (26) | 758 (47) | |
| Integrated Network Cancer Program | 158 (9) | 9 (14) | 149 (9) | |
| Distance from hospital | 13 (6, 32) | 11 (4, 22) | 14 (6, 32) | 0.07 |
| Rurality | 0.96 | |||
| Metropolitan | 1,344 (80) | 53 (80) | 1,291 (80) | |
| Urban | 295 (18) | 12 (18) | 283 (17) | |
| Rural | 45 (3) | 1 (2) | 44 (3) | |
| Income | 0.30 | |||
| < $30,000 | 195 (12) | 4 (6) | 191 (12) | |
| $30,000–$34,999 | 357 (21) | 11 (17) | 346 (21) | |
| $35,000–$45,999 | 484 (29) | 23 (35) | 461 (28) | |
| > =$46,000 | 648 (38) | 28 (42) | 620 (38) | |
| Education level | 0.06 | |||
| >29 | 236 (14) | 10 (15) | 226 (14) | |
| 20–28.9 | 419 (25) | 11 (17) | 408 (25) | |
| 14–19.9 | 428 (25) | 12 (18) | 416 (26) | |
| <14 | 601 (36) | 33 (50) | 568 (35) | |
| pT stage | <0.01 | |||
| < =pT2 | 249 (15) | 1 (2) | 248 (15) | |
| pT3 | 987 (59) | 31 (47) | 956 (59) | |
| pT4 | 448 (27) | 34 (52) | 414 (26) | |
| pN stage | 0.15 | |||
| pN0 | 583 (35) | 25 (38) | 558 (34) | |
| pN1 | 407 (24) | 21 (32) | 386 (24) | |
| pN2-3 | 694 (41) | 20 (30) | 674 (42) | |
| Tumor grade | 0.55 | |||
| Grade 1-2 | 33 (2) | 2 (3) | 31 (2) | |
| Grade 3-4 | 1,512 (90) | 58 (88) | 1,454 (90) | |
| Unknown | 139 (8) | 6 (9) | 133 (8) | |
| Annual hospital cystectomy volume | 5.60 (2.67, 12.30) | 3.67 (2.03, 6.40) | 5.70 (2.76, 12.60) | <0.01 |
| Positive surgical margins | <0.01 | |||
| No | 1,326 (79) | 35 (53) | 1,291 (80) | |
| Yes | 298 (18) | 29 (44) | 269 (17) | |
| Unknown | 60 (4) | 2 (3) | 58 (4) | |
| LNs removed | 13 (7, 21) | 6 (2, 15) | 13 (7, 22) | <0.01 |
| LNs positive | 1 (0, 3) | 1 (0, 3) | 1 (0, 3) | 0.24 |
| Follow up (months) | 25.0 (13.0, 51.0) | 22.5 (15.0, 31.0) | 26.0 (13.0, 53.0) | 0.15 |
1Median (IQR); n (%). 2Wilcoxon rank sum test; Pearson’s Chi-squared test; Fisher’s exact test.
We also evaluated for potential heterogeneity of treatment effects across previously specified covariates of pT stage (pT3 versus pT4), pN stage (N0 versus N1-3), margin status (positive or negative), number of lymph nodes removed (<10 or ≥10), age (<65 or ≥65), and CCI (0 versus 1). To do so, we created separate regression models that included an interaction term for treatment group and an indicator variable for each effect under investigation.
Statistical analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided, and P-values <0.05 were considered statistically significant.
RESULTS
A total of 1,684 patients formed the study cohort, with 66 treated with AC + PORT and 1,618 treated with AC alone. Baseline characteristics are summarized in Table 2. Median age at diagnosis was 65 (IQR 58–70) years. Compared to patients treated with AC alone, those treated with AC + PORT were less likely to be male (59% vs 76%, p < 0.01), less likely to receive care from an academic facility (26% vs 47%, p < 0.01), and more likely to be treated at a lower annual RC volume hospital (3.7 versus 5.7, p < 0.01). Patients treated with AC + PORT were more likely to have stage pT4 disease (52% vs 26%, p < 0.01) and positive surgical margins (44 vs 17%, p < 0.01), although there was no significant difference in nodal stage (62% vs 66% pN1-3, p = 0.15). There were no significant differences in Charlson comorbidity index, race, or insurance status. Median follow-up was 25.0 (IQR 13–51) months, during which time 999 patients died.
In unadjusted analyses, AC + PORT was associated with worse OS compared to AC alone, with 5-year OS of 19% versus 36% (p = 0.01) (Fig. 1, Table 3). However, after adjusting for baseline characteristics, there was no statistically significant difference in OS between treatment groups, with adjusted 5-year OS of 33% for AC+PORT versus 36% for AC alone (p = 0.49) (Fig. 2, Table 4).
Fig. 1
Unadjusted overall survival stratified by treatment arm.
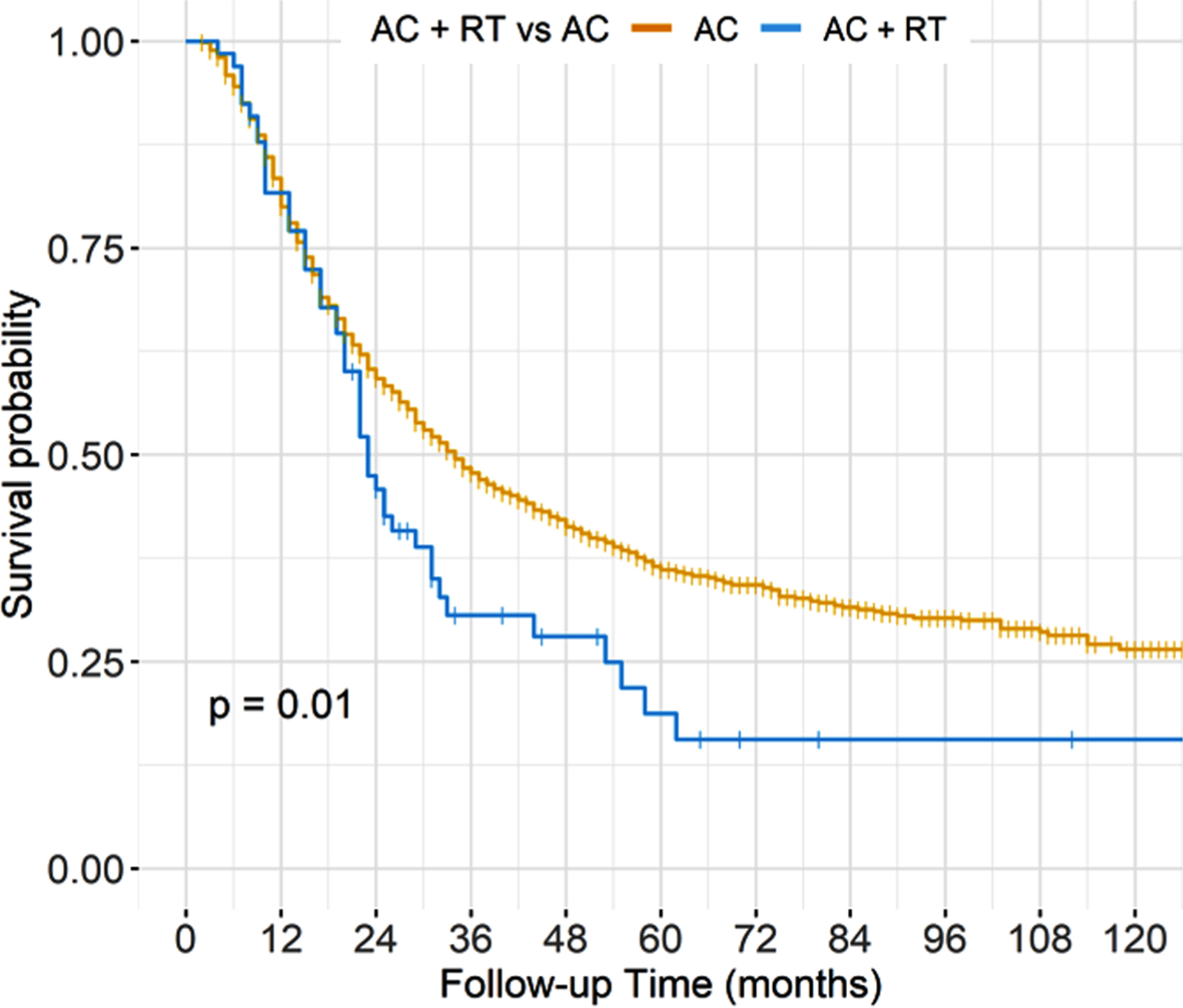
Table 3
Unadjusted overall survival estimates at 12, 36, 60, 84, and 120 months
| Characteristic | Time (months) | Survival estimate |
| AC alone | 12 | 0.80 (0.78,0.82) |
| AC + RT | 12 | 0.82 (0.73,0.92) |
| AC alone | 36 | 0.48 (0.45,0.50) |
| AC + RT | 36 | 0.31 (0.21,0.45) |
| AC alone | 60 | 0.36 (0.34,0.39) |
| AC + RT | 60 | 0.19 (0.10,0.35) |
| AC alone | 84 | 0.32 (0.29,0.34) |
| AC + RT | 84 | 0.16 (0.10,0.32) |
| AC alone | 120 | 0.26 (0.23,0.30) |
| AC + RT | 120 | 0.16 (0.10,0.32) |
Fig. 2
Adjusted overall survival stratified by treatment arm.
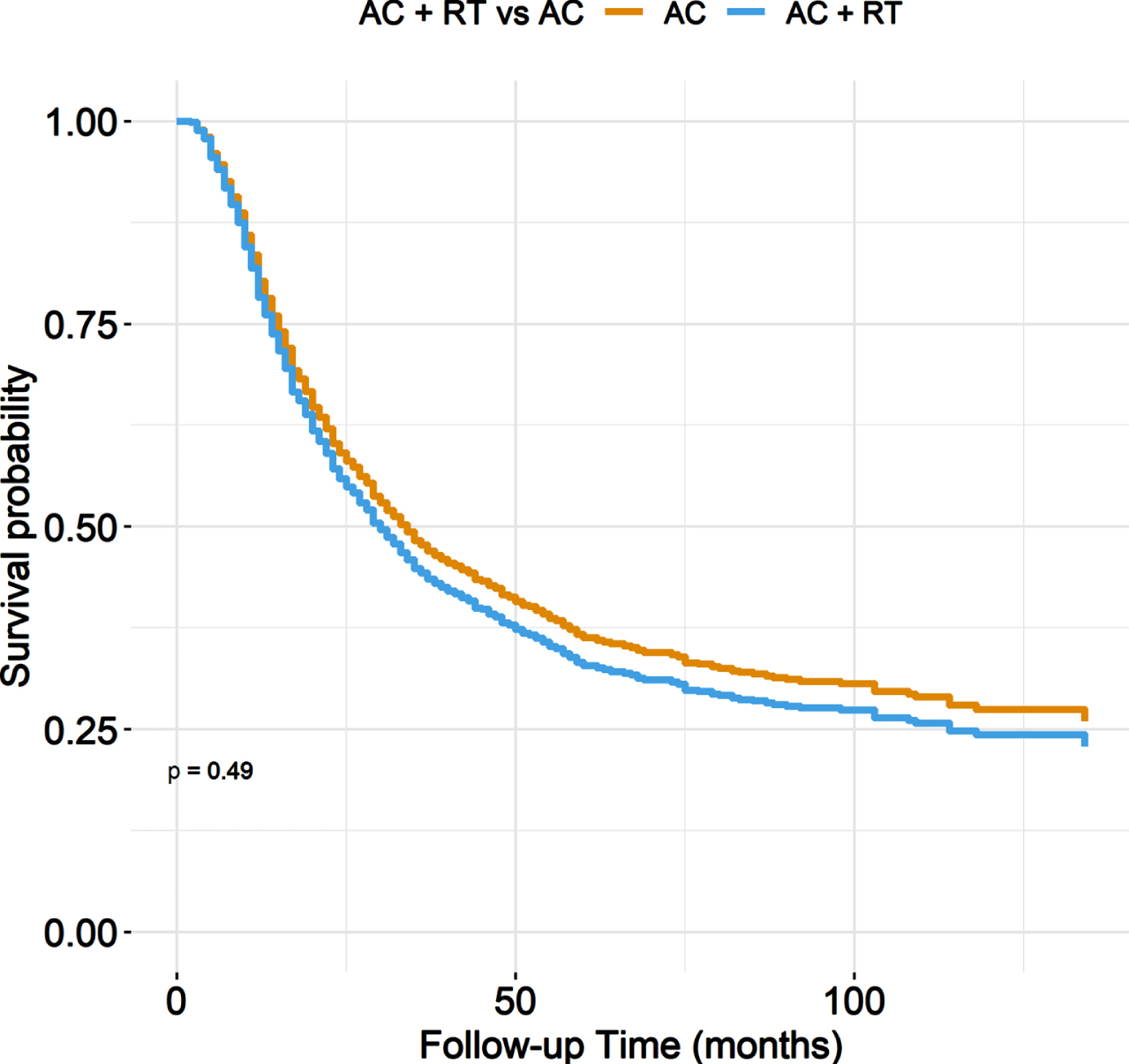
Table 4
Multivariable adjusted overall survival estimates at 12, 36, 60, 84, and 120 months
| Characteristic | Time (months) | Survival estimate |
| AC alone | 12 | 0.80 |
| AC + RT | 12 | 0.78 |
| AC alone | 36 | 0.48 |
| AC + RT | 36 | 0.44 |
| AC alone | 60 | 0.36 |
| AC + RT | 60 | 0.33 |
| AC alone | 84 | 0.32 |
| AC + RT | 84 | 0.29 |
| AC alone | 120 | 0.27 |
| AC + RT | 120 | 0.24 |
After adjustment for baseline characteristics, AC+PORT was not associated with a statistically significant difference in ACM compared to AC alone (HR 1.11; 95% CI, 0.82–1.51) (Table 5). After multivariable adjustment, pT stage (pT3: HR 1.78; 95% CI 1.43–2.20; pT4: HR 2.43; 95% CI 1.92–3.07, versus ≤pT2), pN stage (pN1: HR 1.41; 95% CI 1.17–1.71; pN2-3: HR 1.97; 95% CI 1.65–2.34, versus pN0), and positive surgical margins (HR 1.47; 95% CI 1.24–1.73) remained independently associated with worse ACM.
Table 5
Univariable and multivariable associations of baseline characteristics with all-cause mortality
| Characteristic | Unadjusted HR (95% CI)1 | Adjusted HR (95% CI)2 |
| AC | ||
| AC alone | — | — |
| AC + PORT | 1.44 (1.07, 1.92)* | 1.11 (0.82, 1.51) |
| Age, years | 1.01 (1.00, 1.02)* | 1.01 (1.00, 1.02) |
| Gender | ||
| Male | — | — |
| Female | 1.06 (0.92, 1.23) | 1.01 (0.87, 1.17) |
| Charlson Index | ||
| 0 | — | — |
| 1+ | 1.13 (0.99, 1.29) | 1.11 (0.96, 1.27) |
| Year of Diagnosis | ||
| 2006 | — | — |
| 2007 | 1.16 (0.88, 1.54) | 1.08 (0.80, 1.44) |
| 2008 | 1.01 (0.76, 1.35) | 0.95 (0.71, 1.28) |
| 2009 | 1.03 (0.77, 1.38) | 0.97 (0.71, 1.30) |
| 2010 | 1.13 (0.87, 1.47) | 1.03 (0.78, 1.34) |
| 2011 | 0.85 (0.65, 1.11) | 0.77 (0.59, 1.02) |
| 2012 | 1.03 (0.79, 1.35) | 0.95 (0.72, 1.27) |
| 2013 | 0.95 (0.71, 1.28) | 0.75 (0.55, 1.01) |
| 2014 | 0.79 (0.58, 1.07) | 0.75 (0.55, 1.03) |
| 2015 | 0.88 (0.63, 1.22) | 0.73 (0.52, 1.03) |
| Race | ||
| White | — | — |
| Black | 1.63 (1.26, 2.09)* | 1.35 (1.02, 1.77)* |
| Others | 1.05 (0.65, 1.69) | 1.24 (0.75, 2.02) |
| Hispanic | ||
| No | — | — |
| Yes | 1.00 (0.65, 1.52) | 0.88 (0.56, 1.37) |
| Insurance status | ||
| Not Insured | — | — |
| Private/Managed care | 0.79 (0.58, 1.09) | 0.80 (0.58, 1.11) |
| Medicaid | 1.13 (0.77, 1.66) | 1.11 (0.75, 1.64) |
| Medicare | 0.95 (0.70, 1.31) | 0.88 (0.62, 1.25) |
| VA/Military | 0.65 (0.34, 1.24) | 0.69 (0.36, 1.33) |
| Geographic location | ||
| Northeast | — | — |
| South/Southeast | 1.11 (0.94, 1.31) | 1.00 (0.84, 1.20) |
| Midwest | 0.95 (0.81, 1.13) | 0.89 (0.75, 1.06) |
| West | 0.92 (0.73, 1.15) | 0.90 (0.72, 1.14) |
| Facility type | ||
| Community Cancer Program | — | — |
| Comprehensive community Cancer Program | 0.92 (0.71, 1.19) | 0.98 (0.75, 1.27) |
| Academic /Research Program | 0.81 (0.62, 1.04) | 0.86 (0.64, 1.14) |
| Integrated Network Cancer Program | 1.01 (0.75, 1.38) | 0.93 (0.67, 1.27) |
| Distance from hospital | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| Rurality | ||
| Metropolitan | — | — |
| Urban | 1.07 (0.91, 1.26) | 0.99 (0.83, 1.19) |
| Rural | 0.91 (0.62, 1.33) | 0.90 (0.60, 1.35) |
| Income | ||
| < $30,000 | — | — |
| $30,000–$34,999 | 1.16 (0.93, 1.46) | 1.16 (0.91, 1.48) |
| $35,000–$45,999 | 1.04 (0.84, 1.29) | 1.12 (0.87, 1.43) |
| > =$46,000 | 0.85 (0.69, 1.05) | 0.91 (0.69, 1.21) |
| Education level | ||
| >29 | — | — |
| 20–28.9 | 0.98 (0.80, 1.20) | 1.04 (0.83, 1.30) |
| 14–19.9 | 0.85 (0.70, 1.05) | 0.91 (0.72, 1.16) |
| <14 | 0.80 (0.66, 0.97)* | 0.98 (0.75, 1.27) |
| pT stage | ||
| < = pT2 | — | — |
| pT3 | 1.49 (1.22, 1.83)* | 1.78 (1.43, 2.20)* |
| pT4 | 2.41 (1.94, 2.98)* | 2.43 (1.92, 3.07)* |
| pN stage | ||
| pN0 | — | — |
| pN1 | 1.17 (0.98, 1.39) | 1.41 (1.17, 1.71)* |
| pN2-3 | 1.82 (1.57, 2.11)* | 1.97 (1.65, 2.34)* |
| Tumor grade | ||
| Grade 1-2 | — | — |
| Grade 3-4 | 0.90 (0.58, 1.41) | 0.93 (0.59, 1.47) |
| Unknown | 0.88 (0.54, 1.44) | 1.04 (0.63, 1.72) |
| Annual hospital cystectomy volume | 0.99 (0.99, 1.00) | 1.00 (0.99, 1.01) |
| Positive Surgical Margins | ||
| No | — | — |
| Yes | 1.88 (1.62, 2.19)* | 1.47 (1.24, 1.73)* |
| Unknown | 1.30 (0.95, 1.79) | 1.37 (0.99, 1.90) |
| LNs removed | 0.99 (0.99, 1.00)* | 0.99 (0.99, 1.00)* |
| LNs positive | 1.03 (1.02, 1.04)* | 1.01 (0.99, 1.03) |
1 *p < 0.05. 1,2HR = Hazard Ratio; CI = Confidence Interval. *Implies significance at p < 0.05.
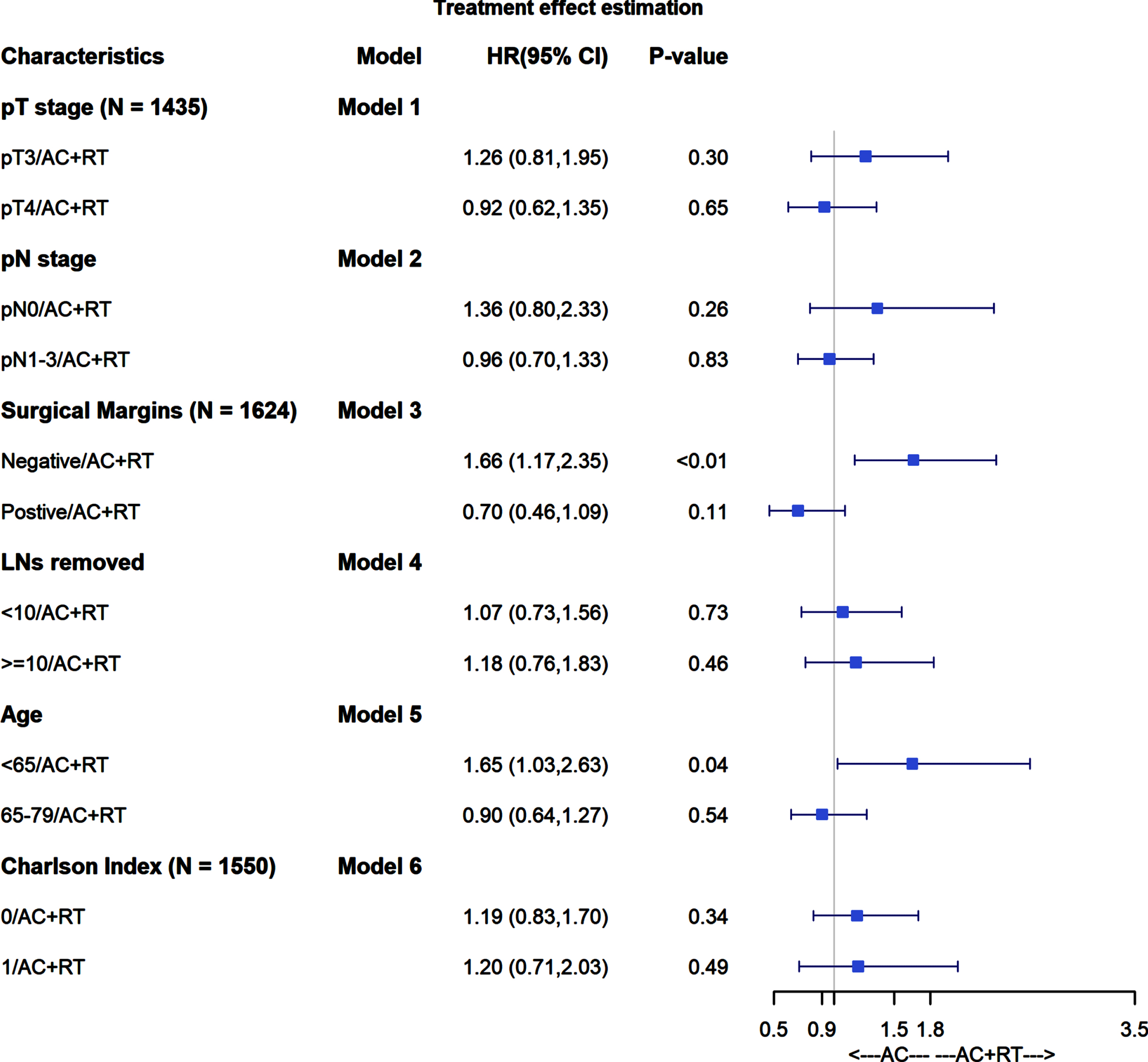
In analyses of heterogeneity of treatment effects, AC+PORT was not associated with improved ACM across pT stage, pN stage, number of LNs removed, age, or Charlson comorbidity index (Fig. 3). AC+PORT was associated with lower ACM compared to AC alone among patients with positive surgical margins, but this did not reach statistical significance (HR 0.70; 95% CI 0.46–1.09; p = 0.11).
Fig. 3
Forest plot for heterogeneity of treatment effects.
DISCUSSION
We report what is to our knowledge the first observational analysis comparing AC + PORT versus AC alone for patients with MIBC. We observed that PORT was infrequently utilized with AC for MIBC, and its utilization was associated with more locally advanced disease and poorer crude OS. However, in multivariable analyses, there was no difference in ACM between AC + PORT versus AC alone. Analyses that examined heterogeneity of treatment effects demonstrated that AC + PORT was not associated with improved ACM across pT stage, pN stage, number of LNs removed, age, or Charlson comorbidity index, although there was improved ACM among patients with positive surgical margins that did not reach statistical significance.
The addition of PORT to AC for MIBC holds biologic plausibility for improving oncologic outcomes given established paradigms for adjuvant radiotherapy in other cancer types [22]. However, there remains limited evidence supporting its application in locally advanced BC. Zaghloul and colleagues conducted the first prospective trial to evaluate the addition of PORT to AC, performed during a time of renewed interest in adjuvant RT, given improvements in RT targeting and high rates of local failure in the setting of ≥pT3 disease [23, 24]. However, the trial was limited by a small sample size of 120 patients with non-urothelial histology in nearly half of patients. Additionally, approximately one third of the cohort had fewer than 10 LNs removed, which may further limit generalizability [3, 25]. Ultimately, the trial was underpowered to demonstrate a significant benefit on OS and DFS endpoints [15]. Zaghloul and colleagues have also presented a post-hoc subgroup analysis for patients with urothelial histology as an abstract, reporting improved DFS and OS with the addition of chemotherapy to PORT versus PORT alone [26].
Although a randomized clinical trial would be the preferred study design to examine the comparative effectiveness of AC + PORT versus AC alone, ongoing trials investigating the use of adjuvant RT have either yet to report outcomes or closed, with many designed to address primary safety outcomes rather than oncologic outcomes [16–20]. One single-arm phase II trial is investigating acute toxicity within 3 months of PORT with secondary outcomes of DFS, LRFS, and OS [16]. Another phase II double-blinded trial has been proposed to evaluate the association of PORT versus surveillance with 3-year LRFS and DFS [17]. Yet another single-arm, phase II trial has been proposed to investigate late gastrointestinal effects of PORT [18]. A promising phase II trial investigating PORT versus surveillance in pT3-4 N0-2 MIBC on LRFS closed in 2017 due to poor accrual [19]. Given the current landscape, the best opportunity to inform the role of PORT for locally advanced BC is to obtain evidence from carefully designed observational analyses. To this end, the emulation framework described by Hernan and Robins and employed herein provides advantages to improve the accuracy of causal inferences that derives from explicit specification of the target trial to be emulated [21].
Several observational studies have examined PORT for locally advanced BC. Lewis, et al. performed a retrospective analysis using the NCDB comparing the effectiveness of adjuvant RT versus surveillance in patients with pT3-4 N0-3 BC following neoadjuvant chemotherapy and RC [27]. The authors observed no difference in OS, with median OS among the PORT arm of 17.7 months compared to 23.4 months in the control arm (p = 0.085). However, it is important to note that PORT is best viewed as add-on therapy to standard of care AC in those who are candidates for AC, rather than solitary therapy compared against observation as presented in this study. Furthermore, patients who received PORT versus those who were observed after receipt of neoadjuvant chemotherapy are arguably two distinct populations, with the former population likely receiving PORT due to more locally advanced disease. In the present study, all patients are required to have receipt of AC to both reduce the potential for confounding and to answer the more clinically relevant question of whether the addition of PORT to AC improves OS.
Fischer-Valuck, et al compared PORT versus surveillance among those with pT3-4N0-3 BC controlling for receipt of neoadjuvant chemotherapy or AC using the NCDB [28]. They reported a median OS of 19.8 months (95% CI, 18.0, 21.6) for those receiving PORT compared to 16.9 months (95% CI, 15.6, 18.1) for those not receiving PORT, and further reported a significant association between PORT and improved OS (HR, 0.87; 95% CI 0.78, 0.97; p = 0.008). In particular, the authors report improved OS for patients with pT4 stage, N+ disease, or positive surgical margins. However, patients were not required to receive adjuvant chemotherapy, and those in the PORT arm were significantly more likely to have received perioperative chemotherapy, which may exacerbate selection bias and confounding. Furthermore, the study excluded patients with pT3a N0 disease and greater than 10 lymph nodes removed, as this population has been demonstrated to have lower risk for local recurrence [29]. However, prior studies have also demonstrated no significant difference in OS between patients with pT3a and pT3b disease, especially in the setting of N0 disease, and the use of both RC and chemotherapy remains standard-of-care for patients with ≥pT3 disease at RC [11, 30].
Neoadjuvant chemotherapy is standard of care among patients with muscle-invasive bladder cancer [31], although real-world utilization rates remain low [32]. Accordingly, adjuvant chemotherapy has historically been standard of care for patients with locally advanced disease following RC who did not receive neoadjuvant chemotherapy [9]. Recent evidence also suggests that AC may still benefit patients who received neoadjuvant chemotherapy [33]. It is important to note that, in this context, the present study excluded patients who received neoadjuvant chemotherapy, as was the case in the target trial by Zaghloul and colleagues [15]. Although this prevents extension of inferences to patients with locally advanced disease following neoadjuvant chemotherapy, it reduces the potential for increasing bias and heterogeneity within the patient population by including a subset of patients with chemoresistant disease.
Several inferences may be made from the present analysis regarding real-world utilization of PORT among patients treated with AC. Those receiving AC + PORT were more likely to have locally invasive disease (≥pT3), fewer lymph nodes removed, and positive surgical margins compared to those receiving AC alone. These findings suggest that in the absence of specific guidelines, PORT is utilized among patients with more locally advanced MIBC, a population demonstrated to have poorer OS [2]. Patients who received PORT were also more likely to receive care from a non-academically affiliated facility as well as a facility with a lower annual RC volume, suggesting structural features may play an important role in PORT administration.
It is important to consider the potential mechanism by which PORT may improve oncologic outcomes. Specifically, PORT may reduce the rate of local recurrence, but the impact of LRFS on systemic progression and survival remains uncertain [34]. In contrast, OS is largely driven by development of distant metastatic disease, and this endpoint is most likely to be affected by systemic therapy. Thus although improved LRFS may not translate into improved OS, it is critical to acknowledge that delaying local failure may offer some benefit to the patient, as salvage therapy has proven to be largely unsuccessful with a median survival of approximately 9 months [2, 4]. Reducing local recurrence may also reduce symptomatic morbidity associated with local lymphatic, venous, and ureteral involvement [35]. However, 13.3% of patients in the AC + PORT arm of the Zaghloul, et al. phase II trial stopped treatment due in part to GI-related toxicity [15]. As such, in making clinical decisions on the use of PORT, the potential benefit on symptomatic disease progression and LRFS must be weighed against its potential toxicity and lack of OS benefit, while also identifying which subset of MIBC patients may benefit from it most.
To this end, several studies [2, 4, 36] have identified risk factors for local recurrence, which represent potential characteristics to identify candidates for PORT. Baumann, et al reported that pT stage, serosal margin status, and number of LNs removed were strong, independent predictors of local failure [2]. They also demonstrated that those with positive surgical margins are more likely to have bulky recurrence in the cystectomy bed and presacral nodes, rather than iliac and obturator nodal recurrence [4]. The present analysis suggested that more invasive local disease (≥pT3), node positive disease (≥pN1), and positive surgical margins were predictors of OS. However, we did not observe any statistically significant qualitative effect modification wherein the addition of PORT to AC was associated with improved ACM across clinicopathologic characteristics.
It is also important to acknowledge the emerging paradigm of adjuvant immunotherapy [37]. This paradigm has specific implications for PORT, as radiotherapy may interact with immunotherapy to potentiate its effects. Still, there remain many unanswered questions regarding the optimal choice of adjuvant systemic therapy for locally advanced bladder cancer following RC, and future trials will need to consider the potential benefit of PORT added to either chemotherapy or immunotherapy.
The present study is subject to limitations. It is a retrospective observational study, and as such, subject to residual confounding and selection bias. Utilization of PORT was uncommon, which may worsen selection bias. Given the imbalance in treatment arms, it is possible that unmeasured confounders may affect the observed associations with OS. Furthermore, we were unable to examine relevant outcomes other than OS, such as LRFS, DFS, CSS, or toxicity of therapy. In addition, we were unable to adjust for characteristics not captured in the NCDB, such as specific chemotherapy agents utilized and their duration of use, number of cycles administered, whether AC was concurrent or sequential to PORT, or post-operative imaging. Furthermore, since we excluded patients who received neoadjuvant chemotherapy, inferences may not be extended to the setting of locally advanced disease following neoadjuvant chemotherapy and RC. Lastly, it is important to acknowledge that within the timeframe studied in the NCDB, PORT was not included as a recommended adjuvant treatment option within National Comprehensive Cancer Network guidelines, which may exacerbate selection bias.
Despite these limitations, the present cohort reflects a contemporary, nationwide oncology dataset, and both treatment arms received AC, which is the current standard of care for locally advanced bladder cancer after RC and serves to minimize immortal time bias. We also restricted the cohort to urothelial histology, which improves generalizability to U.S. and European populations.
CONCLUSIONS
Although PORT is infrequently utilized, the present analyses suggest that the addition of radiotherapy to AC is not associated with improved OS in locally advanced bladder cancer. These results highlight the need for prospective trials to better define the potential benefits from PORT with regard to symptomatic progression and oncologic outcomes.
ACKNOWLEDGMENTS
The authors have no acknowledgements.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
BG, JE, and SK contributed to conception of this study. BG, JE, SK, and AF contributed to performance of work. BG and JE contributed to writing of the article. JE, SK, AF, RK, AW, PC, SK, JB, IK, AO, and BG all contributed to interpretation of data. All authors had access to the data used in this study.
CONFLICTS OF INTEREST
No conflict of interest to report by JE, SK, AF, RK, PC, AW, Simon Kim (SK), IK, AO, and BG.
Joaquim Bellmunt is an Editorial Board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
DATA STATEMENT
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-220031.
REFERENCES
[1] | Stein JP , Lieskovsky G , Cote R , Groshen S , Feng AC , Boyd S , Skinner E , Bochner B , Thangathurai D , Mikhail M , Raghavan D , Skinner DG . Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. (2001) ;19: (3):666–75. doi: 10.1200/JCO.2001.19.3.666. PMID: 11157016. |
[2] | Baumann BC , Guzzo TJ , He J , Keefe SM , Tucker K , Bekelman JE , Hwang WT , Vaughn DJ , Malkowicz SB , Christodouleas JP . A novel risk stratification to predict local-regional failures in urothelial carcinoma of the bladder after radical cystectomy. Int J Radiat Oncol Biol Phys. (2013) ;85: (1):81–8. doi: 10.1016/j.ijrob2012.03.007. Epub 2012 Apr 28. PMID: 22543204. |
[3] | Christodouleas JP , Hwang WT , Baumann BC . Adjuvant radiation for locally advanced bladder cancer? A question worth asking. Int J Radiat Oncol Biol Phys. (2013) ;94: (5):1040–2. doi: 10.1016/j.ijrob2016.01.015. Epub 2016 Jan 19. PMID: 27026310. |
[4] | Baumann BC , Guzzo TJ , He J , Vaughn DJ , Keefe SM , Vapiwala N , Deville C , Bekelman JE , Tucker K , Hwang WT , Malkowicz SB , Christodouleas JP . Bladder cancer patterns of pelvic failure: Implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. (2013) ;85: (2):363–9. doi: 10.1016/j.ijrob2012.03.061. Epub 2012 May 30. PMID: 22658217. |
[5] | Herr HW , Faulkner JR , Grossman HB , Natale RB , deVere White R , Sarosdy MF , Crawford ED . Surgical factors influence bladder cancer outcomes: A cooperative group report. J Clin Oncol. (2004) ;22: (14):2781–9. doi: 10.1200/JCO.2004.11.024. Epub 2004 Jun 15. PMID: 15199091. |
[6] | Ku JH , Kim M , Jeong CW , Kwak C , Kim HH . Risk prediction models of locoregional failure after radical cystectomy for urothelial carcinoma: External validation in a cohort of Korean patients. Int J Radiat Oncol Biol Phys. (2014) ;89: (5):1032–7. doi: 10.1016/j.ijrob2014.04.049. Epub 2014 Jul 8. PMID: 25035206. |
[7] | Cognetti F , Ruggeri EM , Felici A , Gallucci M , Muto G , Pollera CF , et al. Adjuvant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: An Italian, multicenter, randomized phase III trial. Ann Oncol. (2012) ;23: (3):695–700. |
[8] | Sternberg CN , Skoneczna I , Kerst JM , Albers P , Fossa SD , Agerbaek M , et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 4): An intergroup, open-label, randomised phase 3 trial. Lancet Oncol. (2015) ;16: (1):76–86. |
[9] | Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Adjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration.. Eur Urol. (2005) ;48: (2):189–99. doi: 10.1016/j.eururo.2005.04.005. Epub 2005 Apr 25. PMID: 15939530. |
[10] | Leow JJ , Martin-Doyle W , Rajagopal PS , Patel CG , Anderson EM , Rothman AT , et al. Adjuvant chemotherapy for invasive bladder cancer: A updated systematic review and meta-analysis of randomized trials. Eur Urol. (2014) ;66: (1):42–54. |
[11] | Chang SS , Bochner BH , Chou R , et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198:552. Available from: https://www.auanet.org/guidelines/guidelines/bladder-cancer-non-metastatic-muscle-invasive-guideline. |
[12] | Zaghloul MS , Awwad HK , Akoush HH , Omar S , Soliman O , et al. Postoperative radiotherapy of carcinoma in bilharzial bladder: Improved disease free survival through improving local control. Int J Radiat Oncol Biol Phys. (1992) ;23: (3):511–7. doi: 10.1016/0360-3016(92)90005-3. PMID: 1612951. |
[13] | Cozzarini C , Pellegrini D , Fallini M , et al. Reappraisal of the role of adjuvant radiotherapy in muscle-invasive transitional cell carcinoma of the bladder. Int J Radiat Oncol Biol Phys. (1999) ;45: (3):221–2. Abstract 144. |
[14] | Reisinger SA , Mohiuddin M , Mulholland SG . Combined pre- and postoperative adjuvant radiation therapy for bladder cancer–a ten year experience. Int J Radiat Oncol Biol Phys. (1992) ;24: (3):463–8. doi: 10.1016/0360-3016(92)91060-z. PMID: 1399731. |
[15] | Zaghloul MS , Christodouleas JP , Smith A , Abdallah A , William H , Khaled HM , Hwang WT , Baumann BC . Adjuvant sandwich chemotherapy plus radiotherapy vs adjuvant chemotherapy alone for locally advanced bladder cancer after radical cystectomy: A randomized phase 2 trial. JAMA Surg. (2018) ;153: (1)::e174591. doi: 10.1001/jamasurg.2017.4591. Epub 2018 Jan 17. PMID: 29188298; PMCID: PMC5833621. |
[16] | Fonteyne V , Dirix P , Junius S , Rammant E , Ost P , De Meerleer G , Swimberghe M , Decaestecker K . Adjuvant radiotherapy after radical cystectomy for patients with muscle invasive bladder cancer: A phase II trial. BMC Cancer. (2017) ;17: (1):308. doi: 10.1186/s12885-017-3302-9. PMID: 28464906; PMCID: PMC5414216. |
[17] | ClinicalTrials.gov [Internet]. Bethseda (MD): National Library of Medicine (US). 2000. Identifier NCT03333356, Adjuvant radiotherapy in patients with pathological high-risk bladder cancer (GETUG-AGU 30) (Bladder-ART). Available from: https://clinicaltrials.gov/ct2/show/NCT03333356. |
[18] | ClinicalTrials.gov [Internet]. Bethseda (MD): National Library of Medicine (US). 2000. Identifier NCT01954173, Adjuvant radiation for high risk bladder cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT01954173. |
[19] | ClinicalTrials.gov [Internet]. Bethseda (MD): National Library of Medicine (US). 2000. Identifier NCT02316548, Surgery with or without postoperative intensity modulated radiation therapy in treating patients with urothelial bladder cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT02316548. |
[20] | Baumann BC , Zaghloul MS , Sargos P , Murthy V . Adjuvant and neoadjuvant radiation therapy for locally advanced bladder cancer. Clin Oncol (R Coll Radiol). (2021) ;33: (6):391–9. doi: 10.1016/j.clon.2021.03.020. PMID: 33972025. |
[21] | Hernán MA , Robins JM . Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. (2016) ;183: (8):758–64. |
[22] | Do LV , Do TM , Smith R , Parker RG . Postoperative radiotherapy for carcinoma of the prostate: Impact on both local control and distant disease-free survival. Am J Clin Oncol. (2002) ;25: (1):1–8. doi: 10.1097/00000421-200202000-00001. PMID: 11823687. |
[23] | Baumann BC , Noa K , Wileyto EP , Bekelman JE , Deville C , Vapiwala N , Kirk M , Both S , Dolney D , Kassaee A , Christodouleas JP . Adjuvant radiation therapy for bladder cancer: A dosimetric comparison of techniques. Med Dosim. Winter. (2015) ;40: (4):372–7. doi: 10.1016/j.meddos.2015.06.001. Epub 2015 Aug 29. PMID: 26323390. |
[24] | Christodouleas JP , Baumann BC , He J , Hwang WT , Tucker KN , Bekelman JE , Tangen CM , Lerner SP , Guzzo TJ , Malkowicz SB , Herr H . Optimizing bladder cancer locoregional failure risk stratification after radical cystectomy using SWOG 871. Cancer. (2014) ;120: (8):1272–80. doi: 10.1002/cncr.28544. Epub 2014 Jan 3. Erratum in: Cancer. 2015;121(1):162. Herr, Harry [added]. PMID: 24390799. |
[25] | Reddy AV , Pariser JJ , Pearce SM , Weichselbaum RR , Smith ND , Steinberg GD , Liauw SL . Patterns of failure after radical cystectomy for pT3-4 bladder cancer: Implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. (2016) ;94: (5):1031–9. doi: 10.1016/j.ijrob2015.10.051. Epub 2015 Nov 5. PMID: 27026309. |
[26] | Zaghloul MS , et al. Randomized phase III trial of adjuvant sequential chemotherapy plus radiotherapy versus adjuvant radiotherapy alone for locally advanced bladder cancer after radical cystectomy: Urothelial carcinoma subgroup analysis. J Clin Oncol. (2019) ;37: (7_suppl):351. |
[27] | Lewis GD , Haque W , Verma V , Butler EB , Teh BS . The role of adjuvant radiation therapy in locally advanced bladder cancer. Bladder Cancer. (2018) ;4: (2):205–13. doi: 10.3233/BLC-180163. PMID: 29732391; PMCID: PMC5929306. |
[28] | Fischer-Valuck BW , Michalski JM , Mitra N , Christodouleas JP , DeWees TA , Kim E , Smith ZL , Andriole GL , Arora V , Bullock A , Carmona R , Figenshau RS , Grubb RL , Guzzo TJ , Knoche EM , Malkowicz SB , Mamtani R , Pachynski RK , Roth BJ , Zaghloul MS , Gay HA , Baumann BC . Effectiveness of postoperative radiotherapy after radical cystectomy for locally advanced bladder cancer. Cancer Med. (2019) ;8: (8):3698–709. doi: 10.1002/cam4.2102. Epub 2019 May 22. PMID: 31119885; PMCID: PMC6639450. |
[29] | Baumann BC , He J , Hwang WT , Tucker KN , Bekelman JE , Herr HW , Lerner SP , Guzzo TJ , M9lkowicz SB , Christodouleas JP . Validating a local failure risk stratification for use in prospective studies of adjuvant radiation therapy for bladder cancer. Int J Radiat Oncol Biol Phys. (2016) ;95: (2):703–6. doi: 10.1016/j.ijrob2016.01.034. Epub 2016 Jan 23. PMID: 27020106; PMCID: PMC5126649. |
[30] | Boudreaux KJ Jr , Chang SS , Lowrance WT , Rumohr JA , et al. Comparison of American Joint Committee on Cancer pathologic stage T3a versus T3b urothelial carcinoma: Analysis of patient outcomes. Cancer. Feb. (2009) ;115: (4):15770–5. doi: 10.1002/cncr.24110. PMID: 19152431. |
[31] | Grossman HB , Natale RB , Tangen CM , Speights VO , et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. (2003) ;349: (19):1880. doi: 10.1056/NEJMoa022148. Erratum in: N Engl J Med. 2003;349(19):1880. PMID: 12944571. |
[32] | Reardon ZD , Patel SG , Zaid HB , Stimson CJ , et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: A sign of changing tides. Eur Urol. (2015) ;67: (1):165–70. doi: 10.1016/j.eururo.2014.01.009. Epub 2014 Jan 23. PMID: 24472710; PMCID: PMC4469385. |
[33] | Seisen T , Jamzadeh A , Leow JJ , Rouprêt M , et al. Adjuvant chemotherapy vs observation for patients with adverse pathologic features at radical cystectomy previously treated with neoadjuvant chemotherapy. JAMA Oncol. (2018) ;4: (2):225–9. doi: 10.1001/jamaoncol.2017.2374. PMID: 28837718; PMCID: PMC5838707. |
[34] | Pollack A , Zagars GK , Cole CJ , Dinney CP , Swanson DA , Grossman HB . The relationship of local control to distant metastasis in muscle invasive bladder cancer. J Urol. (1995) ;154: (6):2059–63;discussion 2063-4. PMID: 7500458. |
[35] | Baumann BC , Sargos P , Eapen LJ , Efstathiou JA , Choudhury A , Bahl A , Murthy V , Ballas LK , Fonteyne V , Richaud PM , Zaghloul MS , Christodouleas JP . The rationale for post-operative radiation in localized bladder cancer. Bladder Cancer. (2017) ;3: (1):19–30. doi: 10.3233/BLC-160081. PMID: 28149931; PMCID: PMC5271478. |
[36] | Reddy AV , Pariser JJ , Pearce SM , Weichselbaum RR , Smith ND , Steinberg GD , Liauw SL . Patterns of failure after radical cystectomy for pT3-4 bladder cancer: Implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. (2016) ;94: (5):1031–9. doi: 10.1016/j.ijrob2015.10.051. Epub 2015 Nov 5. PMID: 27026309. |
[37] | Bajorin DF , Witjes JA , Gschwend JE , Schenker M , et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. (2021) ;384: (22):2102–14. doi: 10.1056/NEJMoa2034442. Erratum in: N Engl J Med. 2021;385(9):864. PMID: 34077643; PMCID: PMC8215888. |



