Combination of Two T1 Substaging Systems (T1a/b/c and T1m/e) Better Predicts Tumor Outcomes in Patients with T1 High Grade Bladder Cancer
Abstract
BACKGROUND:
T1 substaging is a predictive factor for non-muscle-invasive bladder cancer, and two types of T1 substaging systems (T1a/b/c and T1m/e) are currently in use. However, the predictive ability of both systems is poor, and there is debate over which system is better.
OBJECTIVE:
To confirm whether combination of two T1 substaging systems can improve the predictive ability of T1 substaging for tumor outcomes.
METHODS:
Patients with primary pT1 high-grade bladder cancer from three centers were included. All tumors were assessed with T1a/b/c and T1m/e substaging. A new variable named COMB was developed in which patients were stratified into T1a/b&T1m, T1a/b&T1e, T1c&T1m or T1c&T1e subgroups. A time-dependent receiver operating characteristic curve (ROC) analysis was used to test whether the accuracy of prediction could be improved with COMB.
RESULTS:
A total of 239 patients with primary pT1HG were analyzed. No tumor was T1c&T1m, and therefore, only three types of combinations were evaluated: T1a/b&T1m (62 patients), T1a/b&T1e (124 patients) and T1c&T1e (53 patients). Regardless of all patients or those treated with Re-TURBt and adequate BCG, patients with T1a/b&T1m have the best prognosis, and those with T1c&T1e have the poorest prognosis. The time-dependent ROC showed that, for both recurrence and progression, COMB had a higher AUC than T1a/b/c and T1m/e, regardless of population.
CONCLUSIONS:
Compared with either system alone, the combination of two T1 substaging systems improves the predictive ability of T1 substaging for tumor outcomes.
1Introduction
Predicting the prognosis of T1 high-grade (T1HG) nonmuscle invasive bladder cancer (NMIBC) is extremely important because it determines the subsequent treatment plan after transurethral resection of bladder tumors (TURBt): immediate radical cystectomy (iRC) or bladder-sparing treatment. If T1HG tumors were≥3 cm or multiple or associated with carcinoma in situ (CIS), lymphovascular invasion (LVI) or variant histology (VH), tumors had poor prognosis, and iRC was recommended in the main guidelines [1, 2]. Nevertheless, the treatment of these tumors is still controversial, and urologists are working hard to develop more accurate predictive markers.
T1 substaging is another risk factor with predictive significance. According to the pattern of tumors in the lamina propria, two types of T1 substaging systems were developed. One is T1a/b/c, which is stratified according to the depth of tumor invasion. The other system is T1m/e, which is stratified according to the width/extent/diameter of tumor invasion. Although both of these systems have shown predictive value, the prognostic ability is limited [3–5]. Furthermore, some studies have tried to determine which system has better predictive power, but the results vary [6–12], and the optimal system still remains to be defined in the guidelines for NMIBC [1]. However, before we can conclude which system is the best, can we combine these two systems to better predict tumor prognosis?
According to the depth and width of tumors in the lamina propria, one patient can be evaluated simultaneously with both substaging systems. Therefore we combined these two substaging systems and verified whether combination can improve the prediction ability of T1 substaging.
2Materials and Methods
2.1Patients
After obtaining the Institutional Reviewer Board approval (NO: XJTU1AF2021LSK-374), the data of patients with primary pT1HG NMIBC were retrospectively collected from the First Affiliated Hospital of Xi’an Jiaotong University, Baoji Central Hospital and Shaanxi Provincial People’s Hospital between January 2013 and December 2018. Patients who underwent RC before tumor recurrence or progression were excluded from our analysis. In addition, patients with missing follow-up information or with tumors for which T1 substaging cannot be evaluated were also excluded. Repeat TURBt (re-TURBt) was performed four weeks after initial resection, and the primary tumor site was removed in re-TURBt. After surgery, a single instillation of chemotherapy was performed, and then two weeks later, ShanghaiD2 bacillus Calmette-Guerin (BCG) (120 mg; 1.2×108 colony-forming units) instillation was recommended according to major guidelines. In brief, after induction instillations (once weekly for 6 weeks), maintenance schedules were performed once weekly for 3 weeks at 3, 6, 12, 18, 24, 30, and 36 month. The definition of adequate BCG instillation was that at least 5 of 6 induction instillations followed by at least 2 additional weekly maintenance treatments. Due to BCG intolerance, some patients who have not completed adequate BCG treatment were treated with chemotherapy instillation. Briefly, pirarubicin (30 mg) or epirubicin (50 mg) instillation was performed once a week for 8 weeks, then once a month for a total of 1 year, and then once every two months for another 1 year. The surveillance of the patients was performed according to urology guidelines, i.e., cystoscopy, ultrasound and urine cytology every three months in the first 2 years, every six months for years 2–5 and yearly afterwards. Recurrence was defined as the reappearance of intravesical tumor or tumor metastasis, and progression was defined as the development of muscle invasive bladder cancer or tumor metastasis as assessed by biopsy or imaging. For time to recurrence/progression, the time between tumor diagnosis and recurrence/progression was used.
2.2Pathology review
According to central pathology review, all hematoxylin and eosin (HE) slides from TURBt/re-TURBt were reviewed by two uropathologist who was blinded for clinical information. T1a/b/c and T1m/e substaging were performed as described previously [13, 14]. Briefly, the identification of T1a/b/c was based on tumor invasion of the muscularis mucosae (MM): it was defined as T1a when the tumor did not exceed the MM, T1b when the tumor went into MM, and T1c when the tumor went beyond the MM. The large vessels were not used as landmark. In later analysis, T1a and T1b were put together (T1a/b), and compare with T1c. For T1m/e, if a single focus of invasion with a maximum diameter of 0.5 mm (within one high-power field, objective 40×) was observed, the tumor was defined as T1m. If the invasion diameter was > 0.5 mm or multiple invasive foci were observed, the tumor was defined as T1e.
2.3Statistical analysis
Continuous data are presented as the mean (±standard deviation, SD) or median (interquartile range, IQR), and categorical data are presented as frequencies. Variables including age, sex, smoking status, tumor number, tumor size, re-TURBt or not, residual disease at re-TURBt or not, CIS, LVI, VH, and T1a/b/c and T1m/e substaging were included in the analysis. According to the phenomenon mentioned above and Fig. 1A, we combined the T1a/b/c and T1m/e substaging systems, and a new variable named “COMB” was generated. In this variable, patients were divided into four groups: T1a/b&T1m, T1a/b&T1e, T1c&T1m and T1c&T1e. After analysis, it was found that no patient had T1c&T1m, so only 3 groups remained (Fig. 1A). The Kaplan-Meier (KM) method with log-rank testing was used to estimate the clinical outcomes stratified by COMB. Univariate and multivariable Cox regression analyses were used to determine which variables correlated with outcomes. Receiver operating characteristic curve (ROC) analysis and the areas under the ROC curve (AUCs) were compared to compare the predictive value of COMB with T1a/b/c or T1m/e. Statistical computations were performed with IBM SPSS software (version 24, IBM Corp., Armonk, NY). All statistical tests were two-sided, and a p value < 0.05 was considered to be statistically significant.
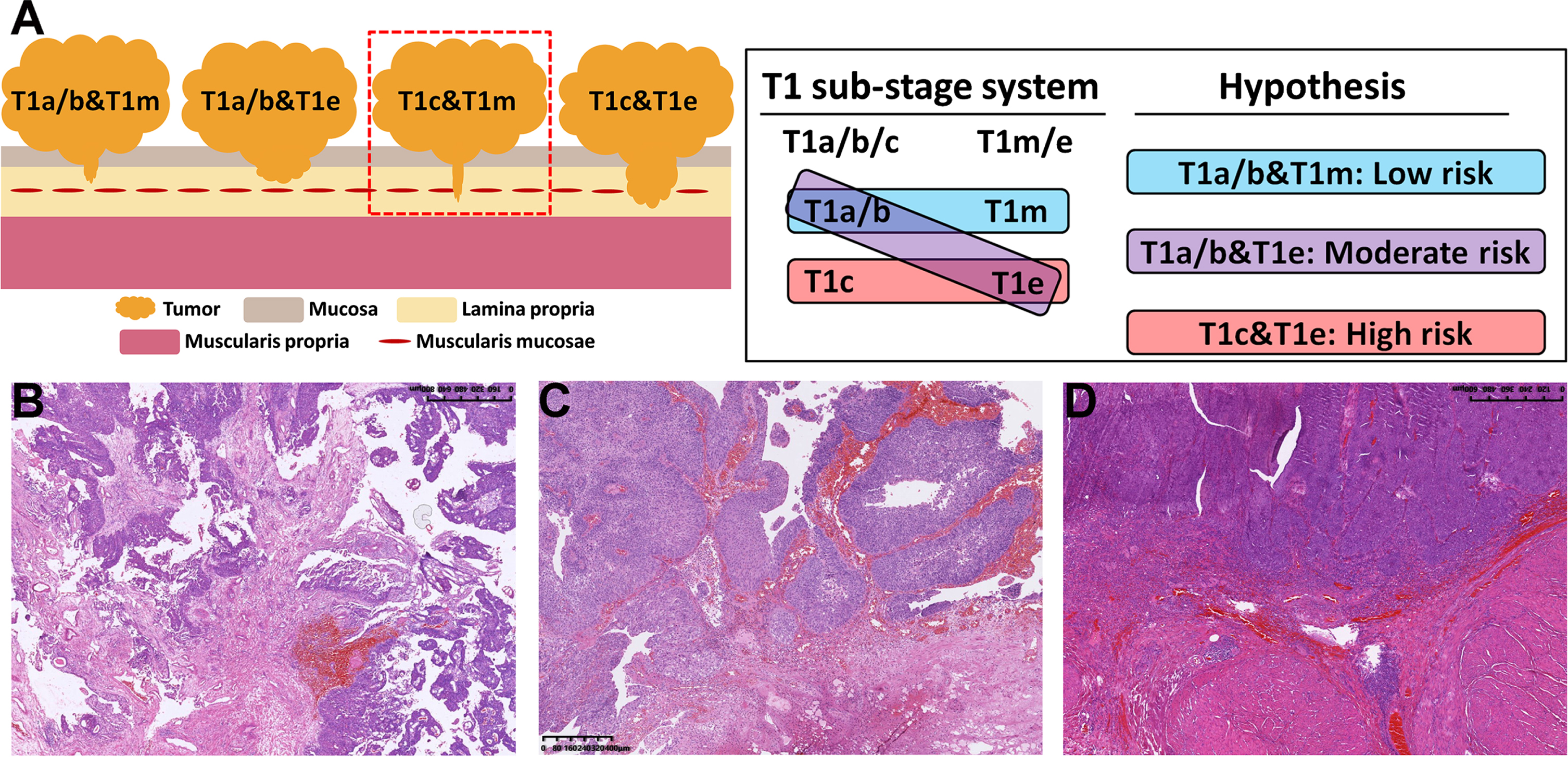
Fig. 1
Schematic diagram (A) and the hematoxylin-eosin (HE) staining images (B D) of the combination of the T1a/b/c and T1m/e substaging systems. In our cohort, no tumor was both T1c and narrow T1m, so there were only three types of combinations, namely, T1a/b&T1m (B), T1a/b&T1e (C) and T1c&T1e (D), and we assumed that T1a/b&T1m had the best prognosis and T1c&T1e had the poorest prognosis.
3Results
3.1Patients and tumor characteristics
After pathology review, the population consisted of 485 patients with primary T1HG bladder cancer that was confirmed in the first and/or second TURBt specimen, and 246 patients were excluded for the following reasons: 30 patients underwent immediate RC, 67 patients had missing follow-up information, and 149 patients had tumors in which T1a/b/c substaging could not be evaluated. Then, a total of 239 patients were included in the present analysis, and the clinical characteristics of these patients are presented in Table 1. After a median follow-up of 60 months (IQR 29–66), 70 patients experienced recurrence, and 46 progressed. Table 2 shows a direct comparison between T1a/b/c and T1m/e. In this cohort, no tumors were both T1c and T1m. Therefore, 62 patients were classified as T1a/b&T1m tumors, 53 as T1c&T1e tumors and 124 as T1a/b&T1e tumors.
Table 1
Baseline characteristics of patients with primary T1HG non-muscle invasive bladder cancer
| Characteristic | n (%) | Characteristic | n (%) |
| Number patients | 239 | T1 substaging | |
| Age(year) | T1a/b | 186(77.8) | |
| Median(IQR) | 68(61–74) | T1c | 53(22.2) |
| Gender | T1m | 65(27.2) | |
| Male | 198(82.8) | T1e | 174(72.8) |
| Female | 41(17.2) | Re-TURBt performed | |
| Smoking history | Yes | 187(78.2) | |
| Current | 149(62.3) | No | 52(21.8) |
| Never/Former | 90(37.7) | Residual disease at Re-TURBt | |
| Tumor size | Yes | 46(24.6) | |
| < 3 cm | 205(85.8) | No | 141(75.4) |
| ≥3 cm | 34(14.2) | Instillation drugs | |
| Tumor number | BCG | 177(74.1) | |
| Single | 127(53.1) | Chemotherapy | 62(25.9) |
| Multiple | 112(46.9) | Recurrence | 70(29.3) |
| Lymphovascular invasion | Time to recurrence(months) | ||
| Yes | 11(4.6) | Median (IQR) | 22(15–26) |
| No | 228(95.4) | Progression | 46(19.2) |
| Concomitant CIS | Time to progression(months) | ||
| Yes | 121(50.6) | Median (IQR) | 31(25–45) |
| No | 118(49.4) | Follow-up total(months) | |
| Variant histology | Median (IQR) | 60(29–66) | |
| Yes | 30(12.6) | ||
| No | 209(87.4) |
IQR: interquartile range; CIS: carcinoma in situ.
Table 2
Comparison of T1a/b/c and T1m/e in the present study
| Type of substaging | T1a/b | T1c | Total |
| T1m | 62 | 0 | 62 |
| T1e | 124 | 53 | 177 |
| Total | 186 | 53 | 239 |
3.2Both substaging systems are associated with tumor outcomes
First, we evaluated the prognostic value of these two substaging systems. Supplementary Figure 1 show the KM curves of recurrence-free survival (RFS) and progression-free survival (PFS) in patients who stratified by T1a/b/c or T1m/e independently. The results of multivariate analysis showed that patients with T1c and T1e disease had significantly worse RFS (p < 0.001 and 0.012, respectively) and PFS (p = 0.002 and 0.033, respectively) than those with T1a/b and T1m disease (Supplementary Tables 1 and 2).
3.3Combining two substaging systems improves the predictive power for tumor outcomes
According to the above, patients were divided into 3 types, namely, T1a/b&T1m, T1a/b&T1e and T1c&T1e, and Fig. 1B-D represents the HE staining images of each substage type. Based on this, a new variable named “COMB” was developed, and Fig. 2 show the KM curves of RFS and PFS in patients who stratified by COMB. The results showed that T1a/b&T1m had the best prognosis and T1c&T1e had the poorest prognosis (Fig. 2A and 2B).
Fig. 2
Kaplan–Meier analysis of recurrence-free survival and progression-free survival in patients stratified by three types of combinations. (A) depicts recurrence-free survival of all patients; (B) depicts progression-free survival of all patients; (C) depicts recurrence-free survival of patients treated with Re-TURBt and BCG; (D) depicts progression-free survival of patients treated with Re-TURBt and BCG.
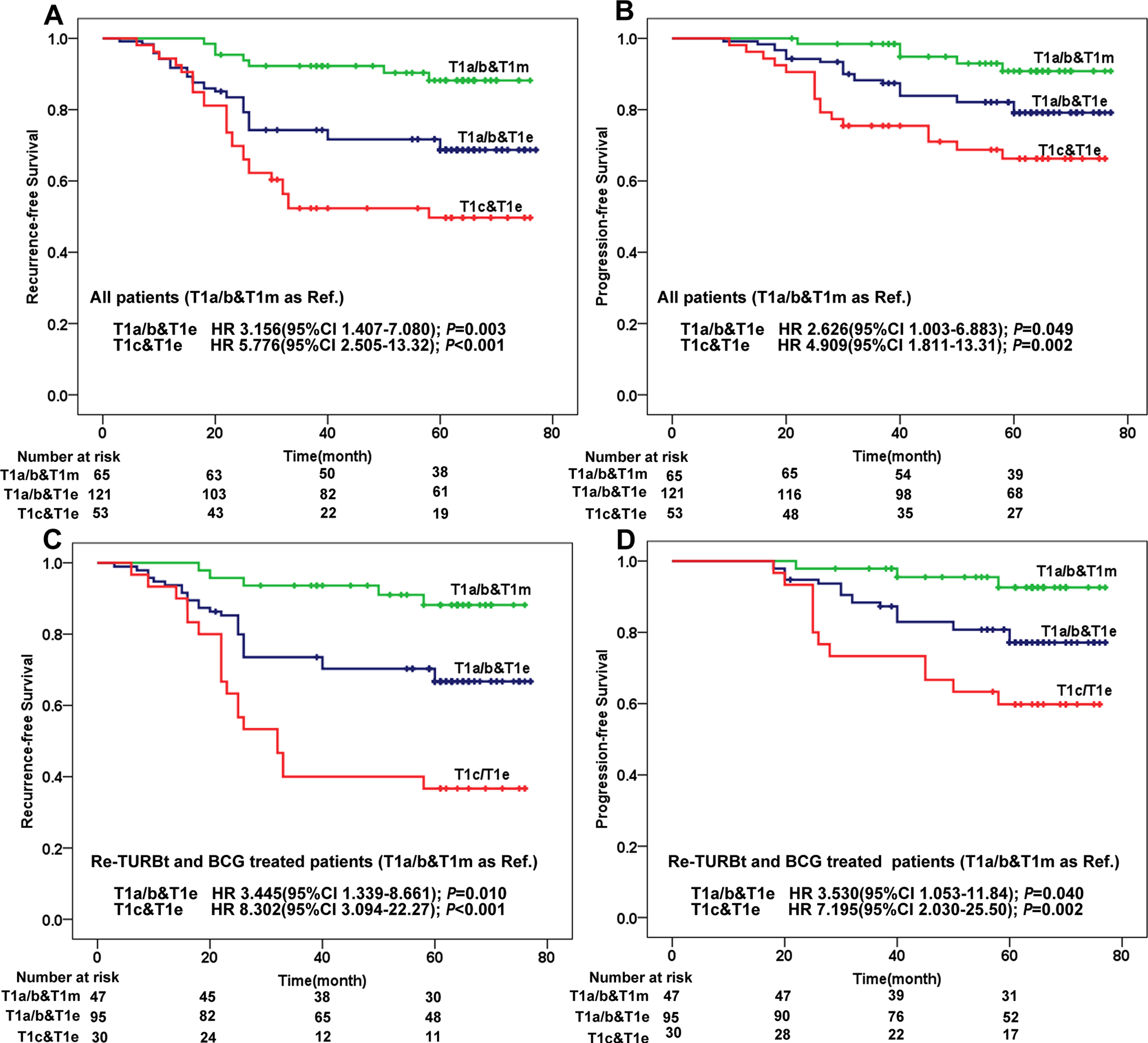
Since the standard treatment for high-risk patients is Re-TURBt followed by BCG instillation, we separately analyzed patients treated with Re-TURBt and adequate BCG to avoid unreliability of results. The results showed that T1c&T1e tumor still had the poorest prognosis (Fig. 2C and 2D).
We performed time-dependent ROC and AUC analyses to assess whether combining these two systems improves the predictive ability for tumor outcomes. Table 3 showed the 3- and 5-year AUCs of the predictive models of recurrence and progression in all patients. As shown in this table, COMB had a significant higher AUC area than T1a/b/c or Tm/e (Table 3). Same results were got in patients treated with Re-TURBt and adequate BCG (Table 3).
Table 3
The time-dependent AUC (95% CI) of recurrence and progression for different models
| All patients | Re-TURBt and BCG treated patients | |||||
| COMB | T1a/b/c | T1m/e | COMB | T1a/b/c | T1m/e | |
| Recurrence | ||||||
| 3-year | 0.691 | 0.626** | 0.628** | 0725 | 0.648** | 0.642** |
| (0.617–0.766) | (0.540–0.712) | (0.553–0.702) | (0.641–0.809) | (0.548–0.748) | (0.556–0.728) | |
| 5-year | 0.672 | 0.606** | 0.622* | 0.702 | 0.626** | 0.634* |
| (0.599–0.745) | (0.524–0.688) | (0.548–0.695) | (0.620–0.784) | (0.531–0.720) | (0.550–0.718) | |
| Progression | ||||||
| 3-year | 0.704 | 0.637** | 0.634** | 0.697 | 0.628** | 0.626** |
| (0.611–0.798) | (0.519–0.755) | (0.541–0.726) | (0.582–0.812) | (0.485–0.770) | (0.582–0.738) | |
| 5-year | 0.645 | 0.592* | 0.601* | 0.672 | 0.600** | 0.620* |
| (0.560–0.731) | (0.495–0.688) | (0.517–0.685) | (0.577–0.767) | (0.490–0.711) | (0.526–0.714) | |
*P value between COMB and T1a/b/c (or T1m/e) were < 0.05; **P value between COMB and T1a/b/c (or T1m/e) were < 0.01; AUC: area under ROC curve; COMB: combination of T1a/b/c and T1m/e.
4Discussion
Although these two types of T1 substage systems have been discussed for nearly 20 years [14, 15], clinical applications have not yet become widespread. The guidelines did not clearly recommend which system is better [1]. In the present study, we combined them and showed that the combination of the two systems can predict tumor recurrence more effectively than either alone.
In the past few decades, whether to perform iRC for T1HG tumors has been a controversial issue [16, 17]. During this period, a number of studies conducted a head-to-head comparison between iRC and delayed RC [18, 19]. Although the results of these studies vary, there is a consensus that highly selected patients with T1HG tumors need to undergo iRC to improve their survival rate. Following this, the prediction of prognosis for tumors has become a hot topic, and many predictive models and markers have been developed [20, 21]. Some markers have been commonly used in clinical work, including CIS, LVI, and VH, and even molecular subtyping or some other molecular markers [22, 23]. However, T1 substaging can be described as making slow progress in this regard and has not yet been widely used in clinical practice.
There may be two reasons for this phenomenon. On the one hand, these two T1 substage systems have their own shortcomings. For example, the identification of MM is difficult after TURBt, and VP acts as a substitute if MM is absent. However, the reliability of these vessels for T1 substaging needs further investigation [5]. The shortcoming of the T1m/e system is that it does not have a reference like MM, which means that pathologist subjectivity plays a greater role in the substaging results [24]. On the other hand, the reason may be that the system with better predictive ability has not been determined [1]. Based on this, we combined two systems for the first time in an attempt to further improve the predictive ability of T1 substaging.
In fact, some studies have tried to improve these systems or developed other substaging systems. Without MM, Cheng et al. [25] and Brimo et al. [24] defined the threshold invasion depth as 1.5 mm and 3 mm, respectively, and both of them confirmed a strong correlation between invasion depth and prognosis. However, in a previous study, the overall mean lamina propria thickness was 1.4 mm [26]. Moreover, the invasion depth can easily be affected by TURBt. Therefore, their works have not been validated by other studies. For invasion width/diameter, Chang et al. [27] defined the threshold as 0.5 mm, 1.0 mm and 1.5 mm and showed that this substaging was feasible and can provide more precise prognostic information. Colombo et al. [8] developed a rete oncologica lombarda (ROL) substaging system in which the threshold of invasion diameter (the extent of invasion in any direction) was 1 mm. Despite these attempts, T1a/b/c and T1m/e systems are still commonly used at present.
In this study, we used MM as a reference for T1a/b/c, and the threshold was 0.5 mm for T1m/e, in line with the most commonly used standards. After preliminary analysis, we found that T1a and T1b had similar prognosis, so we put them together. We found that no tumors were deep (T1c) and narrow (T1m), which means that only three tumor forms were identified after combination, as shown in Fig. 1. This leads to T1m having a better prognosis than T1a/b because some T1a/b tumors are classified as T1e, and T1c may have a worse prognosis than T1e because some T1e tumors are classified as T1a/b. This possibility was only discussed in the report of van Rhijn et al. [9], without further verification.
Compared with the previous work mentioned above, our method, combine T1a/b/c and T1m/e, is feasible and has high clinical practicability in terms of improving the predictive ability of T1 substage. Pathologist only needs to observe the postoperative pathological slide and give the diagnosis of T1a/b/c and T1m/e. And then the urologist makes a simple combination to assess the prognosis of patient. After combination, we found that T1c&T1e had the poorest prognosis and T1a/b&T1m had the best prognosis in both populations (all T1HG patients and patients with standard treatment). Interestingly, a recent study showed that T1m had a similar BCG failure risk as Ta HG tumors [28]. Through ROC analysis, we found that the combination of the two systems had higher predictive ability than either T1a/b/c or T1m/e alone for tumor recurrence and progression.
In 2021, the Genitourinary Pathology Society (GUPS) updated their recommendation for T1 substaging [29]. They indicated that future studies, especially prospective studies, on the head-to-head comparisons of histoanatomic (T1a/b/c) and micrometric(T1m/e or other) methods are still needed until an optimal method is validated. Before that, the T1 substaging should be assessed using either of these methods. According to our study, it is hoped that the combination of these two substaging methods can promote the clinical application of T1 substaging, especially before reaching a conclusion as to which system is the best or other better substaging systems are developed.
This study has several limitations. First and importantly, the low sample size maybe the reason for the absence of T1c&T1m tumors. Therefore, this requires further studies with larger sample sizes to confirm. Second, this is a retrospective study, which means that some selection biases may exist. However, even if it is biased, the prediction results of the individual variables such as “COMB”, “T1a/b/c” or “T1m/e” would not be affected. Third, the follow-up time was short, which led to a low death rate. Therefore, in this study, we only analyzed tumor recurrence and progression to identify the predictive ability of each risk factor, and more studies are needed to further confirm our conclusion.
In conclusion, to our knowledge, this is the first study to combine two T1 substaging systems, and the results showed that compared with the single use, the combination shows a higher prediction ability. This combination has the potential to guide treatment decisions for iRC or bladder-sparing treatment. A prospective trial is needed to further confirm our conclusion.
ACKNOWLEDGMENTS
The authors thank the department of pathology of Shaanxi Provincial People’s Hospital and Baoji Central Hospital for their contributions to this study.
FUNDING
This study was supported by the key research and development program of department of science and technology of Shaanxi Province (No. 2020SF-123 to JH Fan) and the medical research program of department of science and technology of Xi’an (No. 2019115713YX012SF048(4) to JH Fan).
AUTHORS’ CONTRIBUTIONS
All authors contributed to the study conception and design, and had access to the data. T Yang, JJ Fan and XQ Pei: conception, performance of work and interpretation of data; T Yang: manuscript writing; H Liang: reviewed the HE slides; JH Fan: the project development. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
Tao Yang, Junjie Fan, Xinqi Pei, Hua Liang and Jinhai Fan have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-220007.
REFERENCES
[1] | Babjuk M , Burger M , Comperat EM , Gontero P , Mostafid AH , Palou J , van Rhijn BWG , Roupret M , Shariat SF , Sylvester R , Zigeuner R , Capoun O , Cohen D , Escrig JLD , Hernandez V , Peyronnet B , Seisen T , Soukup V , European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - Update, Eur Urol (2019) ;76: (5):639–57. doi: 10.1016/j.eururo.2019.08.016. |
[2] | Chang SS , Boorjian SA , Chou R , Clark PE , Daneshmand S , Konety BR , Pruthi R , Quale DZ , Ritch CR , Seigne JD , Skinner EC , Smith ND , McKiernan JM , Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline, J Urol (2016) ;196: (4):1021–9. doi: 10.1016/j.juro.2016.06.049. |
[3] | Martin-Doyle W , Leow JJ , Orsola A , Chang SL , Bellmunt J , Improving selection criteria for early cystectomy in high-grade t1 bladder cancer: a meta-analysis of 15,215 patients, J Clin Oncol (2015) ;33: (6):643–50. doi: 10.1200/JCO.2014.57.6967. |
[4] | Kardoust Parizi M , Enikeev D , Glybochko PV , Seebacher V , Janisch F , Fajkovic H , Chlosta PL , Shariat SF , Prognostic value of T1 substaging on oncological outcomes in patients with non-muscle-invasive bladder urothelial carcinoma: a systematic literature review and meta-analysis, World J Urol (2020) ;38: (6):1437–49. doi: 10.1007/s00345-019-02936-y. |
[5] | Fransen van de Putte EE , Behrendt MA , Pigot GL , van der Kwast TH , van Rhijn BW . Prognostic significance of substage and WHO classification systems in T1 urothelial carcinoma of the bladder, Curr Opin Urol (2015) ;25: (5):427–35. doi: 10.1097/MOU.0000000000000202. |
[6] | Fransen van de Putte EE , Otto W , Hartmann A , Bertz S , Mayr R , Brundl J , Breyer J , Manach Q , Comperat EM , Boormans JL , Bosschieter J , Jewett MAS , Stoehr R , van Leenders G , Nieuwenhuijzen JA , Zlotta AR , Hendricksen K , Roupret M , Burger M , van der Kwast TH , van Rhijn BWG . Metric substage according to micro and extensive lamina propria invasion improves prognostics in T1 bladder cancer, Urol Oncol (2018) ;36: (8):361 e367–361 e313. doi: 10.1016/j.urolonc.2018.05.007. |
[7] | V DEM , Cerruto MA , D’Elia C , Brunelli M , Otte O , Minja A , Luchini C , Novella G , Cavalleri S , Martignoni G , Artibani W , Prognostic role of substaging in T1G3 transitional cell carcinoma of the urinary bladder, Mol Clin Oncol (2014) ;2: (4):575–80. doi: 10.3892/mco.2014.290. |
[8] | Colombo R , Hurle R , Moschini M , Freschi M , Colombo P , Colecchia M , Ferrari L , Luciano R , Conti G , Magnani T , Capogrosso P , Conti A , Pasini L , Burgio G , Guazzoni G , Patriarca C , Feasibility andClinical Roles of Different Substaging Systems at First and SecondTransurethral Resection in Patients with T1 High-Grade BladderCancer, Eur Urol Focus (2018) ;4: (1):87–93. doi: 10.1016/j.euf.2016.06.004. |
[9] | van Rhijn BW , van der Kwast TH , Alkhateeb SS , Fleshner NE , van Leenders GJ , Bostrom PJ , van der Aa MN , Kakiashvili DM , Bangma CH , Jewett MA , Zlotta AR , A new and highly prognostic system to discern T1 bladder cancer substage, Eur Urol (2012) ;61: (2):378–84. doi: 10.1016/j.eururo.2011.10.026. |
[10] | Bertz S , Denzinger S , Otto W , Wieland WF , Stoehr R , Hofstaedter F , Hartmann A , Substaging by estimating the size of invasive tumour canimprove risk stratification in pT1 urothelial bladdercancer-evaluation of a large hospital-based single-centreseries, Histopathology (2011) ;59: (4):722–32. doi: 10.1111/j.1365-2559.2011.03989.x. |
[11] | Turan T , Efiloglu O , Gunaydin B , Ozkanli S , Nikerel E , Atis G , Caskurlu T , Yildirim A , Comparative differences between T1a/b and T1e/m as substages in T1 urothelial carcinoma of the bladder, Int Braz J Urol (2018) ;44: (2):267–72. doi: 10.1590/S1677-5538.IBJU.2017.0424. |
[12] | Patriarca C , Hurle R , Moschini M , Freschi M , Colombo P , Colecchia M , Ferrari L , Guazzoni G , Conti A , Conti G , Luciano R , Magnani T , Colombo R , Usefulness of pT1 substaging in papillary urothelial bladder carcinoma, Diagn Pathol (2016) ;11: :6. doi: 10.1186/s13000-016-0466-6. |
[13] | Younes M , Sussman J , True LD , The usefulness of the level of themuscularis mucosae in the staging of invasive transitional cellcarcinoma of the urinary bladder, Cancer (1990) ;66: (3):543–8. doi: 10.1002/1097-0142(19900801)66:3<543::aid-cncr2820660323>3.0.co;2-r. |
[14] | van der Aa MN , van Leenders GJ , Steyerberg EW , van Rhijn BW , Jobsis AC , Zwarthoff EC , van der Kwast TH , A new system for substaging pT1 papillary bladder cancer: a prognostic evaluation, Hum Pathol (2005) ;36: (9):981–6. doi: 10.1016/j.humpath.2005.06.017. |
[15] | Hasui Y , Osada Y , Kitada S , Nishi S , Significance of invasion to the muscularis mucosae on the progression of superficial bladder cancer, Urology (1994) ;43: (6):782–6. doi: 10.1016/0090-4295(94)90134-1. |
[16] | Herr HW , Sogani PC , Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol (2001) ;166: (4):1296–9. |
[17] | Thalmann GN , Markwalder R , Shahin O , Burkhard FC , Hochreiter WW , Studer UE , Primary T1G3 bladder cancer: organ preserving approach orimmediate cystectomy? , J Urol (2004) ;172: (1):70–5. doi: 10.1097/01.ju.0000132129.87598.3b. |
[18] | Jager W , Thomas C , Haag S , Hampel C , Salzer A , Thuroff JW , Wiesner C ,. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival, BJU Int (2011) ;108: (8 Pt 2):E284–288. doi: 10.1111/j.1464-410X.2010.09980.x. |
[19] | Denzinger S , Fritsche HM , Otto W , Blana A , Wieland WF , Burger M , Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur Urol (2008) ;53: (1):146–52. doi: 10.1016/j.eururo.2007.06.030. |
[20] | Tang F , He Z , Lu Z , Wu W , Chen Y , Wei G , Liu Y , Application of nomograms in the prediction of overall survival and cancer-specific survival in patients with T1 high-grade bladder cancer, Exp Ther Med (2019) ;18: (5):3405–14. doi: 10.3892/etm.2019.7979. |
[21] | Cambier S , Sylvester RJ , Collette L , Gontero P , Brausi MA , van Andel G , Kirkels WJ , Silva FC , Oosterlinck W , Prescott S , Kirkali Z , Powell PH , de Reijke TM , Turkeri L , Collette S , Oddens J , EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guerin, Eur Urol (2016) ;69: (1):60–9. doi: 10.1016/j.eururo.2015.06.045. |
[22] | Bellmunt J , Kim J , Reardon B , Perera-Bel J , Orsola A , Rodriguez-Vida A , Wankowicz SA , Bowden M , Barletta JA , Morote J , de Torres I , Juanpere N , Lloreta-Trull J , Hernandez S , Mouw KW , Taplin ME , Cejas P , Long HW , Van Allen EM , Getz G , Kwiatkowski DJ , Genomic Predictors of Good Outcome, Recurrence, or Progression in High-Grade T1 Non-Muscle-Invasive Bladder Cancer, Cancer Res (2020) ;80: (20):4476–86. doi: 10.1158/0008-5472.CAN-20-0977. |
[23] | de Jong JJ , Liu Y , Boorjian SA , Bivalacqua TJ , Porten SP , Wheeler T , Davicioni E , Svatek RS , Boormans JL , Black PC , Lotan Y , Gibb EA . A Genomic Classifier for Predicting Clinically Aggressive Luminal Bladder Tumors with Higher Rates of Pathological Up Staging, J Urol (2020) ;204: (2):239–46. doi: 10.1097/JU.0000000000000798. |
[24] | Brimo F , Wu C , Zeizafoun N , Tanguay S , Aprikian A , Mansure JJ , Kassouf W , Prognostic factors in T1 bladder urothelial carcinoma: the value of recording millimetric depth of invasion, diameter of invasive carcinoma, and muscularis mucosa invasion, Hum Pathol (2013) ;44: (1):95–102. doi: 10.1016/j.humpath.2012.04.020. |
[25] | Cheng L , Weaver AL , Neumann RM , Scherer BG , Bostwick DG , Substaging of T1 bladder carcinoma based on the depth of invasion as measured by micrometer: A new proposal, Cancer (1999) ;86: (6):1035–43. doi: 10.1002/(sici)1097-0142(19990915)86:6<1035::aid-cncr20>3.0.co;2-d. |
[26] | Cheng L , Neumann RM , Weaver AL , Spotts BE , Bostwick DG , Predicting cancer progression in patients with stage T1 bladder carcinoma, J Clin Oncol (1999) ;17: (10):3182–7. doi: 10.1200/JCO.1999.17.10.3182. |
[27] | Chang WC , Chang YH , Pan CC , Prognostic significance in substaging ofT1 urinary bladder urothelial carcinoma on transurethral resection, Am J Surg Pathol (2012) ;36: (3):454–61. doi: 10.1097/PAS.0b013e31823dafd3. |
[28] | de Jong FC , Hoedemaeker RF , Kvikstad V , Mensink JTM , de Jong JJ , Boeve ER , van der Schoot DKE , Zwarthoff EC , Boormans JL , Zuiverloon TCM T1-Substaging of Non-muscle Invasive Bladder Cancer is Associated with BCG-Failure and Improves Patient Stratification at Diagnosis. J Urol. 2020:101097JU0000000000001422.doi: 10.1097/JU.0000000000001422. |
[29] | Comperat E , Amin MB , Epstein JI , Hansel DE , Paner G , Al-Ahmadie H , True L , Baydar D , Bivalacqua T , Brimo F , Cheng L , Cheville J , Dalbagni G , Falzarano S , Gordetsky J , Guo C , Gupta S , Hes O , Iyer G , Kaushal S , Kunju L , Magi-Galluzzi C , Matoso A , McKenney J , Netto GJ , Osunkoya AO , Pan CC , Pivovarcikova K , Raspollini MR , Reis H , Rosenberg J , Roupret M , Shah RB , Shariat SF , Trpkov K , Weyerer V , Zhou M , Reuter V , The Genitourinary Pathology Society Update on Classification of Variant Histologies, T1 Substaging, Molecular Taxonomy, and Immunotherapy and PD-L1 Testing Implications of Urothelial Cancers, Adv Anat Pathol (2021) ;28: (4):196–208. doi: 10.1097/PAP.0000000000000309. |




