The Effect of Metformin on Bladder Cancer Incidence and Outcomes: A Systematic Review and Meta-Analysis
Abstract
BACKGROUND:
Effective oral treatment options for urothelial bladder cancer (BC) are lacking. Metformin, the most frequently used oral drug in type II diabetes mellitus, has putative anticancer properties and could, therefore, influence BC incidence and treatment outcomes. We systematically reviewed the current literature regarding the effect of metformin on BC incidence and oncological outcomes in non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC).
METHODS:
This review was conducted according to the PRISMA guidelines. Literature was gathered through a systematic search in PubMed/Medline, EMBASE and the Cochrane library. Risk of bias was determined using the Cochrane risk-of-bias tool for randomized trials and the Newcastle-Ottawa Scale for non-randomized trials. Hazard ratios (HRs) were extracted and pooled in a random-effects meta-analysis.
RESULTS:
We reviewed 13 studies, including 3,315,320 patients, considering the risk of developing BC after metformin exposure and 9 studies, including 4,006 patients, on oncological outcomes of patients with BC. Metformin did not affect BC incidence (HR 0.97, 95% CI 0.87 –1.09) or oncological outcomes for NMIBC but did show a reduced risk of recurrence (HR 0.52, 95% CI 0.32 –0.84), cancer-specific mortality (HR 0.58, 95% CI 0.43 –0.78) and overall mortality (HR 0.66, 95% CI 0.47 –0.92) in MIBC.
CONCLUSIONS:
The role of metformin in the prevention and treatment of BC in patients remains unclear. Although a beneficial effect of metformin on treatment outcomes of certain stages of BC may exist, a definitive conclusion cannot be drawn. Prospective clinical trials are needed to assess the efficacy of metformin for BC treatment.
LIST OF ABBREVIATIONS
AMPK | Adenosine monophosphate kinase |
BC | Bladder cancer |
CI | Confidence interval |
CPRD | Clinical practice research datalink |
CSM | Cancer specific mortality |
CSS | Cancer specific survival |
DMII | Diabetes mellitus type II |
GLDs | Glucose-lowering drugs |
HR | Hazard ratio |
MIBC | Muscle invasive bladder cancer |
mTOR | Mammalian target of rapamycin |
NA | Not applicable |
NMIBC | Non-muscle invasive bladder cancer |
NOS | Newcastle-Ottawa Scale |
NR | Not reported |
OM | Overall mortality |
OS | Overall survival |
PFS | Progression free survival |
RoB2 | Risk-of-bias tool for randomized trials 2 |
PRISMA | Preferred reporting items for systematic Reviews and meta-analyses |
RC | Radical cystectomy |
RCT | Randomised controlled trial |
RFS | Recurrence free survival |
RR | Risk ratio |
SU | Sulfonylurea |
TUR-T | Transurethral resection of bladder tumour |
INTRODUCTION
Bladder cancer (BC) is the sixth most common malignancy in men and the tenth most common in women with approximately 550,000 new cases per year worldwide [1]. In approximately 70% of newly diagnosed cases the disease is confined to the mucosa or submucosa, and termed non-muscle-invasive bladder cancer (NMIBC), while in ∼25% the disease invades deeper into the bladder wall; muscle-invasive bladder cancer (MIBC) [2]. NMIBC has a good prognosis with a five-year overall survival (OS) of 90% compared to a 50% five-year OS in MIBC [3]. However, despite current treatments, NMIBC patients are at considerable risk for tumour recurrence and progression to MIBC after initial treatment [4]. Due to this risk of recurrence in NMIBC and mortality in MIBC, the disease burden and economic costs are high [5]. This emphasizes the need for better treatments. The current standard of care for BC consists of a transurethral resection of bladder tumours (TUR-T) followed by adjuvant intravesical bladder instillations with either chemotherapy or Bacillus Calmette-Gu
The oral biguanide drug metformin is the most frequently prescribed drug for the treatment of diabetes mellitus type II (DMII) and was introduced into clinical practice in the 1950 s [6, 7]. Recently, several reports have demonstrated that metformin has anticancer properties. The mechanism by which metformin can inhibit cancer growth has not been fully elucidated. However, it is suggested that metformin can influence various processes involved in cancer development such as cell proliferation, induction of apoptosis, and cell cycle arrest [8–10] by targeting two key cellular metabolism pathways; inhibition of complex I of the mitochondrial respiratory chain and activation of AMPK leading to inhibition of mTOR [11]. In vitro studies confirmed these mechanisms and metformin’s potential in effectively inhibiting tumour growth in bladder cancer cell lines [12–14]. Furthermore, an in vivo study suggested synergy between metformin and cisplatin, a frequently used chemotherapeutic in advanced bladder cancer, in the treatment of bladder cancer in mice [15]. These antitumour effects mark the bladder as a potential organ site where metformin could inhibit cancer growth.
In light of these developments we seek to present the current body of evidence on the epidemiologic relationship between metformin use and BC. Metformin could play a role in both cancer prevention by inhibiting cancer development as well as cancer treatment by inhibiting the growth of present tumours. Therefore, the aim of this review is twofold: to assess the effects of metformin on the incidence of BC after metformin exposure and the oncological outcomes of diabetic patients with BC that used metformin.
MATERIALS AND METHODS
This systematic review was conducted according to the Cochrane review and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standards [16].
Search strategy
An extensive search strategy was made in consultation with a clinical librarian to assess the complete body of literature regarding the relationship between metformin and BC. The following terms were used during the search; Urinary Bladder Neoplasms, Carcinoma, Transitional Cell, Cancer, Biguanides, Metformin, Hypoglycemic Agents. Boolean operators (AND, OR) were used to combine search terms. The complete search history is available in the supplementary files (Table S1).
The search was conducted by two independent authors (JH, BR) using three different databases; EMBASE, Pubmed and the Cochrane library ranging from date of inception until June 28 2021. Limits used were; English language and human studies. No restrictions were applied considering article design (e.g. Randomised controlled trials (RCT’s), case series, cohort studies) and publication type.
References were screened on title and abstract using Endnote (version X9.3.3). All abstracts and full text articles were screened by two authors (JH, BR). Disagreement was resolved in a consensus meeting and if necessary a third author (JO) was consulted. Furthermore, references of full text evaluated articles were screened to identify additional relevant articles. Clinicaltrial.gov and the open access thesis and dissertations database (oatd.org) were searched to identify grey literature.
Risk of bias assessment
The risk of bias of included articles was assessed by the two authors (JH, BR) separately, using the Cochrane risk-of-bias tool for randomized trials (RoB version 2) [17] and the Newcastle-Ottawa Scale (NOS) [18] for non-randomized trials. Disagreement was resolved through discussion and in case of disagreement a third author was consulted (JO).
Data extraction
Data were extracted by the two authors separately using a predefined data extraction form in Microsoft Excel (2016). Extracted data per study included; author information, country, publication date, study period and design, diabetic status of participants, duration of metformin exposure and dosage, concurrent or prior exposure to other glucose-lowering drugs (GLDs), reported comorbidity, smoking status and other medication use, information on BC diagnosis (NMIBC, MIBC or not specified); outcomes of interest were BC incidence, recurrence-free survival (RFS), progression-free survival (PFS), cancer-specific survival (CSS) and OS reported as hazard ratios (HRs) with 95% confidence interval (CI). In case HRs were not available, these were calculated based on available data using methods described by Tierney et al. [19]. If studies reported outcomes on both combination therapies of GLDs and metformin monotherapy the results for metformin monotherapy were extracted and used for pooled analysis. If a single study contained multiple analyses we selected the analysis with the least risk of bias (monotherapy, wash-out period, lead-in period).
Meta-analysis
HRs with 95% CI were pooled to assess the combined effect across studies of metformin on BC incidence and oncological outcomes. Heterogeneity between studies was determined using the Q-test and I2. Analyses were performed with Review Manager (version 5.3) [20]. Different measures were taken due to heterogeneity between study designs, patient populations and used GLDs. First, a random effects model was used for all analyses. Second, if different studies reported on overlapping patient groups the most detailed report was selected to prevent using single patient data multiple times in the same analysis. Third, a subgroup analysis was performed for studies only using monotherapy. Finally, since treatment and prognosis of BC differ significantly between cancer stage, oncological outcomes are described and analysed based on the two main subgroups: NMIBC and MIBC.
RESULTS
Search and selected articles
The study selection process and reasons for exclusion are displayed in Fig. 1. Twenty-two studies were included in the review and 13 in the meta-analysis. In 4 studies data was prospectively collected while 18 had a retrospective design (Tables 1 and 2). Five studies had partial overlapping populations. Two studies used data from the Clinical Practice Research Datalink (CRPD) with different inclusion criteria and observational period resulting in a different number of patients and outcomes [21, 22], while three others are based on data from the Taiwanese National Health Research Institute Database [23–25].
Fig. 1
Screening and selection of included articles. UTUC: Upper Tract Urothelial Cancer, OR: Odds ratio, HR: Hazard Ratio, RR: Risk Ratio, GLD: glucose-lowering drugs.
Table 1
Overview of studies on bladder cancer incidence in patients exposed to metformin
| Author, year, design | Number of patients in study | Study population (inclusion criteria) | Metformin number of patients (number of events) | Control (number of patients) | Definition of metformin/control group | Prior or concurrent exposure to other GLD | Study duration (Follow up duration) | Age | Adjusted variables | outcome | RoB (NOS/ RoB 2) |
| Chen et al. 2015* [23] Cohort | 7,325 | New onset DMII on monotherapy | 2,223 (BC 7) | 3,965 (BC 15) | M: metformin C: SU | No | 1998 –2008 (2.5y median, IQR 3.6y) | M 60.6 (median) IQR 19.5 C 62.4 (median) IQR 19.9 | age, sex, CCi, smoking-related diagnoses, alcohol use disorders, morbid obesity, pancreatitis, hypertension, hyperlipidemia, monthly household income, and urbanization level | HR for BC in M vs C: 1.01 (95% CI 0.41 –2.51) | 9 (NOS) |
| Dankner et al. 2019 [31] Cohort | 304,582 | DMI and DMII | 172,948 (BC NR) | 94,630 (BC NR) | M: metformin +/- dipeptidyl peptidase-4 inhibitor C: other GLD | Yes concurrent exposure to dipeptidyl peptidase-4 inhibitor possible (6%) | 2002 –2012 NR | NR | age, sex, socioeconomic status, ethnic origin, smoking, other GLD | HR for BC in M vs C: in years 2-7 : 0.98 (95% CI 0.49 –1.97) | 8 (NOS) |
| Goossens et al. 2015 + [21] Cohort | 165,398 | DMII with at least 1 prescription of antidiabetic drug + 1 year lead-in time | 132,960 (BC 247) | 32,438 (BC 124) | M: metformin C: SU | No | 1987 –2013 (M: 5.3 mean, SD 3.7) (S 7.6 mean, SD 13.2) | M 58.3 (mean), SD 14.8 C 66.0 (mean) SD 13.2 | Age, gender, smoking, BMI, and duration of diabetes | HR for BC in M vs C: 1.03 (95% CI 0.81 –1.31) | 9 (NOS) |
| Home et al. 2009 [32] RCT | 2,220 | DMII with background monotherapy metformin or SU | 1,117 PYs (BC 2) | 1,103 PYs(BC 4) | M: metformin + rosiglitazone C: SU + rosiglitazone | Yes concurrent with SU or R | 2001 –2008 5.5 years (mean) | M + R: 57.0 (SD 8.0) SU + R: 59.8 (8.3) | NR | n (%) BC diagnosis M: 2 (0.2%) C: 4 (0.4%) | Some concerns (RoB 2) |
| Kahn et al. 2006 [27] RCT | 4,351 | DMII with monotherapy GLD | 1,454 (BC 4) | 2.897 (BC 6) | M: metformin C: rosiglitazone or glibenclamide | No | 04/2000 –06/2006 4.4 years (median) | M 57.9 (SD 9.9) rosiglitazone: 56.3 (SD 10.0) glyburide 56.4 (SD 10.2) | NR | n (%) BC diagnosis M: 4 (0.3%) C: 6 (0.2%) | Some concern (RoB2) |
| Mamtani et al. 2014 [29] Cohort | 99,047 | DMII new user of metformin or SU | 71,472 (BC 196) | 16,127 (BC 66) | M: metformin C: SU | Possible prior use before 6 months washout period. | 07/2000 –31/08/2010 | Metformin 62 (median), SU 69 (median) | age, sex, smoking, recurrent UTI’s, obesity, congestive heart failure, myocardial infarction, renal impairment, diabetes duration, HbA1c level, other GLD, other commonly prescribed medication. | HR for BC in M vs C: 0.81 (95% CI 0.60–1.09) | 8 (NOS) |
| Murf et al. 2018 [28] Propensity matched Cohort | 84,434 | DM and new user of metformin or SU | 42,217 (BC 122) | 42,217 (BC 97) | M: metformin C: SU | No | 01/10/2001 –30/09/2008 Described in PYs | M 66,2 (median) SU 65,4 | NR | HR for BC in M vs C: 1.02 (95% CI 0.78 –1.34). | 9 (NOS) |
| Neumann et al. 2012 [30] Cohort | 1,491,060 | DMI or DMII with a prescription of GLD | 911,143 (BC NR) | 451,216 (BC NR) | M: metformin with or without other GLD C: pioglitazone and other GLD | Possible | 2006 –2009 37.5 months (mean) | NR | Age, sex, exposure to other GLD | HR for BC in M vs C: 1.03 (95% CI 0.93 –1.13) | 6 (NOS) |
| Oliveria et al. 2008 [33] Cohort | 199,223 | DMI or DMII with prescription for GLD | 75,689 PYs (BC 39) | 596,147 PYs (BC 139) | M: (ever) metformin monotherapy C: other GLD | Possible | 2000 –2004 3.9 years (median) | 56 years (mean) | age, gender, schistosomiasis, pelvic radiation | RR for BC in M vs C: 0.99 (95% CI 0.70-1.39) | 7 (NOS) |
| Sung et al. 2020 [26] Cohort | 289,297 | Sample of Hong Kong public healthcare service | 11,365 (BC 25) | 277,932 (BC 2114) | M: metformin ever use C: metformin never use | No insulin or SU | 2000-2013 NR | Divided in age cohorts of 15 year | Age, sex, comorbidities, medication use | HR for BC in M vs C: HR 0.54 (95% CI 0.31-0.91) | 7 (NOS) |
| Tseng et al. 2011* [24] Cohort | 998,947 (115.731 with DMII) | Random sample individuals in NHI database | NR (BC NR) | NR (BC NR) | M: metformin ever use C: metformin never use | No | 2003 –2005 3 years | NR | age, sex, comorbidities, medication use, living region and occupation | RR for BC in M vs C: RR 0.96 (95% CI 0.65-1.53) | 8 (NOS) |
| Tseng et al. 2014* [25] Cohort | 940.708 | New onset DM II during 1998-2002 | 408,189 (BC 1,847) | 532,519 (BC 6,213) | M: metformin ever use C: metformin never use | Possible | 1998 –2009 NR | Divided in age cohorts of 10 years | Age, sex, comborbidities, medication use | HR for BC in M vs C: 0.600 (95% CI 0.564 –0.638) | 9 (NOS) |
| Tsilidis et al. 2014 + [22] Cohort | 95,820 | DM II with at least one GLD prescription | 51,484 (BC 130) | 18,264 (BC 106) | M: metformin C: SU | Possible addition of other GLD during follow-up | 1987 –2010 5.1 years (IQR 2.9 –8.1) | Divided in age cohorts of 15 years | Age, smoking, BMI, alcohol consumption, medication use | HR for BC in M vs C: 0.88 (95% CI 0.64 –1.21) | 8 (NOS) |
M: metformin, C: control, SU: sulfonylurea, GLD: glucose-lowering drug, BC: bladder cancer, DMI/DMII: diabetes mellitus type I/II, PYs: person-years, NOS: Newcastle Ottowa-scale, ROB 2: Cochrane risk of bias tool, IQR: interquartile range, NR; Not reported, HR: Hazard ratio, RR: relative risk, * overlapping study population,+overlapping study population
Table 2
Overview of studies focussing on bladder cancer outcomes in patients exposed to metformin
| Author, year | Number of patients in study | Study population (inclusion criteria) | Metformin number of patients (number of events) | Control (number of patients) | Definition of metformin/control group | Prior or concurrent exposure to other GLD | Study duration (Follow up duration) | Age | Adjusted variables | Outcome | RoB (NOS or Rob2) |
| Ahn et al. 2016 [34] Cohort | 645 | NMIBC with known HbA1c and metformin status | 61 (NR) | 66 (NR) | M: metformin C: no metformin | Possible | 2004 –2015 46 months (median) | 66.5 (median) | None | OR RFS 1.07 (95% CI 0.64 –1.80) P = 0.795, OR PFS 1.52 (95% CI 0.70 –3.33) P = 0.286 | 6 (NOS) |
| Heidari et al. 2016 [42] Case-series | 65 | Patients undergoing TUR-T for bladder cancer | 32 (recurrence 8) | 33 (recurrence 10) | M: metformin C: no metformin | Possible | 2013 –2014 1 year | 63.4 (mean) | None | Recurrence: 8 (25%) vs 10 (30.3%) P = 0.633 | 5 (NOS) |
| Huang et al. 2020 [35] Cohort | 287 (61 with DM, 33 with known HbA1c status) | NMIBC with > 2 years follow-up | 22 (NR) | 11 (NR) | M: metformin C: no metformin | Possible | 2012 –2017 45 months (median) | 67 (median) | None | HR RFS 1.36 (95% CI 0.60 –3.10) P = 0.460 | 7 (NOS) |
| Lyon et al. 2018 [41] | 1,061 | Patients undergoing RC for high risk NMIBC or MIBC | NR | NR | M: metformin C: no metformin | Possible | 2007 –2016 4.2 years (median) | NR | Gender, Hx hart disease, vascular disease, CHF, diabetes, smoking, (neo)adjuvant chemotherapy, diversion type, CIS, lymph node status, positive margins, perioperative blood loss | HR Metastasis 1.34 (95% CI 0.64 –2.78) P = 0.44 HR CSM 0.59 (95% CI 0.28 –1.25) P = 0.17 HR OM 0.62 (95% CI 0.33 –1.15) P = 0.13 | 7 (NOS) |
| Nayan et al. 2015 [36] Cohort | 421 (85 with diabetes) | Patients undergoing RC for high risk NMIBC or MIBC | 39 (Recurrence 7) (CSM 6) (OM 14) | 46 (Recurrence 14) (CSM 10) (OM 16) | M: metformin C: no metformin | Possible | 1997 –2013 50 months (median) | 71 years (mean) | age, gender, CCI, extra vesical disease, positive margin, CIS, (neo)adjuvant chemo | HR RFS 0.38 (95% CI 0.20 –0.72) P = 0.003 HR CSS 0.57 (95% CI 0.35 –0.91) P = 0.019. HR OS 1.05 (95% CI 0.49 –2.26) P = 0.89 | 6 (NOS) |
| Richard et al. 2018 [37] Cohort | 1,742 | Age>66years with DMI or DMII prior to NMIBC | 813 (CSM 90) (OM 487) | 929 (CSM 157) (OM 635) | M: metformin C: no metformin | Possible | 1992 –2012 5.2 years (IQR 3.4–7.8) after NMIBC diagnosis | 78 years (IQR 75–83) | Sex, age, year of diagnosis, area of residency, Charlson comorbidity score, time since diagnosis of DM, neighborhood income quintile, cumulative use of all included hypoglycemic agents, categorized as previously mentioned, into use before and after NMIBC diagnosis. | HR CSS 1.1 (95% CI 0.92 –1.2) P = 0.45 HR OS 0.96 (95% CI 0.92–1.01) P = 0.08 | 7 (NOS) |
| Rieken et al. 2013 [38] Cohort | 1,117 (125 with DMII) | NMIBC | 43 (Recurrence 10) (Progression 1) (CSM 0) (OM 15) | DM no metformin 82 (Recurrence 53) (Progression 17) (CSM 4) (OM 37.8) No DM 992 (Recurrence 406) (Progression 85) (CSM 46) (OM 253) | M: metformin C: no metformin | Possible | 1996 –2007 64 months (median, IQR 22-106) | 65.5 years (mean) | Standard clinicopathological features | HR recurrence M vs C 0.41 (95% CI 0.25–0.67) HR progression M vs C 0.31 (95% CI 0.11–0.87) HR OS M vs no DM OS: 1.49 (0.88-2.53) P = 0.14 | 7 (NOS) |
| Wang et al. 2020 [40] Cohort | 122 (22 with DMII) | NMIBC | 13 (Recurrence 5) (Progression 1) (CSM 0) (OM 1) | DM no metformin 9 (Recurrence 4) (Progression 1) (CSM 3) (OM 5) No DM 100 (Recurrence 44) (Progression 19) (CSM 15) (OM 35) | M: metformin C: DM o metformin [13] | Possible | 1995-2020 102 months (mean) range 3-357) | 64.7 years (median) SD 11.7 | NR | 15-year CSS: M100% vs C 54.7% | 7 (NOS) |
| Rieken et al. 2014 [39] Cohort | 1,502 (200 with DMII) | Patients undergoing RC for high risk NMIBC or MIBC | 80 Recurrence: NR CSM: NR OM: NR | DM no metformin 120 Recurrence: NR CSM: NR OM: NR No DM 1302 Recurrence: NR CSM: NR OM: NR | M: metformin C: DM no metformin | Possible | 1992 –2008 34 months (median IQR 17-61) | 65.5 years (mean) | Clinicopathological features, age, gender, BMI, smoking, Tstage/grade, tissue margins, lymphovascular invasion, N | HR recurrence: 0.63 (95% CI 0.40 –0.98) P = 0.046 CSM: 0.62 (95% CI 0.38 –1.01) P = 0.056 OM: 0.58 (95% CI 0.37 –0.90) P = 0.018 | 7 (NOS) |
BC: bladder cancer, CSS: cancer specific survival, CSM cancer specific mortality, DMI/DMII: diabetes mellitus type I/II, GLD: glucose-lowering drug, HR: Hazard ratio, IQR: interquartile range, M: metformin, NOS: Newcastle Ottowa-scale, NR: Not reported, OS: overall survival, OM: overall mortality, OR: odds ratio, PFS: progression free survival, ROB 2: Cochrane risk of bias tool, RR: relative risk, RFS: recurrence free survival, SU: sulfonylurea.
BC incidence
Thirteen articles, including 3,315,320 patients, reported on the risk of developing BC in diabetics exposed to metformin (Table 1). Of these, eleven compared the risk of developing BC among diabetics after metformin exposure to other GLDs (e.g. sulfonylurea [SU] or pioglitazone) while two studies [24, 26] included non-diabetics as a control group.
Six studies [21, 23, 27–30] compared metformin monotherapy with single-agent use of other GLDs. Two studies [31, 32] included patients with metformin combination therapy compared with different GLD combinations. In seven studies [22, 25, 26, 29–31, 33] prior or concurrent use of other GLD besides metformin was possible.
BC outcomes
Nine studies, including 4,006 patients, reported on the treatment outcomes of BC patients after metformin exposure (Table 3). Four studies [34–37] compared DM patients using metformin versus other GLD. Three studies [38–40] compared the effect of metformin versus other GLD in patients with DM and a control population without DM. Two studies [41, 42] did not specify the comparator group. All studies regarding the effect of metformin on oncological outcomes were retrospective except for Heidari et al. [42] where in a non-randomized prospective design patients were prescribed metformin for one year versus no metformin prescription following TUR-T. BC stage was addressed in all studies with six including patients with NMIBC [34, 35, 37, 38, 40, 42] and three considering patients after RC for high risk NMIBC or MIBC [36, 39, 41].
Table 3
Summary of pooled analyses
| Outcome | Studies | Patients | Pooled HR (95% CI) | P (I2) | ||
| Metformin | No metformin | |||||
| Incidence | 7 | 1,344,328 | 918,525 | 0.97 (0.87 –1.09) | P = 0.33 (14%) | |
| Recurrence | NMIBC | 2 | 65 | 93 | 0.71 (0.22 –2.30) | P = 0.57 (83%) |
| MIBC | 2 | 119 | 166 | 0.52 (0.32 –0.84) | P = 0.007 | |
| Progression | 1 | 43 | 82 | 0.31 (0.11 –0.87) | P = 0.03 (NA) | |
| CSM | 4 | 932* | 1095* | 0.85 (0.70 –1.03) | P = 0.10 (71%) | |
| OM | 4 | 932* | 1095* | 0.80 (0.59 –1.09) | P = 0.16 (55%) | |
*Total number is higher due to missing information from Lyon et al. NA; not applicable NO; not obtainable due to missing information CSM; cancer specific mortality, OM; Overall mortality.
Risk of bias
The risk of bias scores of each study are reported in Tables 1 and 2. There was a considerable risk of bias in all studies. The main risk of bias stems from the retrospective design of included studies with the exception of the 3 prospective studies [27, 32, 42]. Although these studies were either non-randomised [42] or an additional analysis of a larger investigation into the treatment of DMII [27, 32]. Furthermore, while the total number of patients of the combined studies was considerable, the individual studies varied in size and contained only information on diabetes status or medication use in sub-populations, possibly introducing selection bias. Additional risks of bias in retrospective studies are time-related biases [43]. Immortal time bias occurs in time-fixed cohorts with misclassified unexposed time as exposed and can lead to overestimation of the treatment effect. Of the 11 cohort studies reporting on BC incidence following metformin exposure, seven reduced the risk of immortal time bias by imposing a 6 to 12 months wash-out period [22, 26, 28–31, 33] while four [23–25, 29] did not. The full assessment of the risk of bias per individual study is presented in the supplementary files (Tables S2, S3, S4).
Metformin and BC incidence
Of all thirteen included studies reporting on BC incidence two, both performed in an Asian population, [25, 26] demonstrated a statistically significant lower risk of BC diagnosis following metformin exposure compared to never use of metformin. The other studies did not find a difference in risk of BC when metformin use was compared to other GLDs.
The study population of the most recent report by Tseng et al. [25] consisted of patients with new onset diabetes either treated with metformin or another GLD. Metformin-exposed patients had a lower risk of developing BC compared to non-exposed patients (HR 0.6, 95% CI 0.56 –0.64). Increasing dose and duration of metformin treatment further decreased the risk of BC. In the study of Sung et al. [26] the study population was exposed to at least 6 months of metformin treatment and was compared to a cohort of non-exposed patients. The reduced BC risk was seen after an exposure time of at least 4.4 years. Earlier analysis at 3.3 and 2.7 years showed no difference in BC diagnosis. None of the studies specified BC stage.
The retrospective study from Oliveria et al. [33] and the first report by Tseng et al. [24] both provided risk ratios (RR). Both studies did not show a protective effect of metformin (RR 0.99, 95% CI 0.70 –1.39 and RR 0.96, 95% CI 0.65 –1.53). When evaluating the effect of metformin in prospective studies, Kahn et al. [27] did not observe a difference in number and percentage of BC cases following monotherapy of metformin compared to either monotherapy with a thiazolidinedione or SU. Home et al. [32] reported a lower percentage of BC after combination therapy of metformin with thiazolidinedione compared to SU with thiazolidinedione (n = 2, 0.2% vs n = 4, 0.4%). These four studies [24, 27, 32, 33] were excluded from pooled HR analysis due to limited available publication data.
Meta-analysis
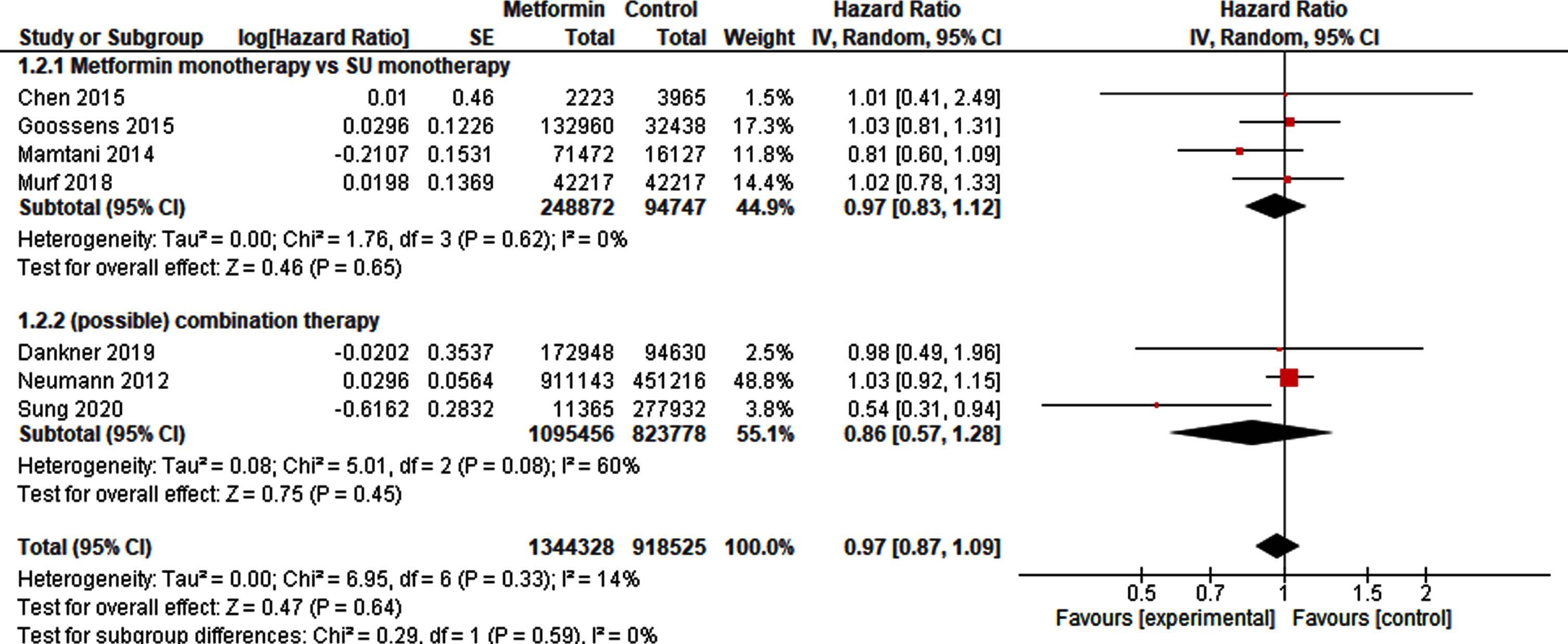
Nine studies [21–23, 25, 26, 28–31] provided sufficient information to pool HRs. After exclusion of the overlapping studies [22, 25] 1,344,328 metformin-exposed patients could be compared to 918,525 non-exposed patients. The pooled analysis for BC incidence did not demonstrate a protective or harmful effect of metformin exposure (HR 0.97, 95% CI 0.87 –1.09). Sub-analysis of studies on monotherapy or combination therapy in metformin or control did not change the results (Fig. 2).
Fig. 2
Forest plot of pooled hazard ratios for BC incidence.
Metformin and BC outcome
NMIBC
A total of 984 metformin-exposed and 1,130 non-exposed patients were included in six studies on NMIBC outcomes. Five (34,35,38,40,42) contained data on recurrence of a total of 171 metformin-exposed and 201 control patients while three studies [34, 38, 40] provided progression data of in total 117 metformin-exposed patients compared with 157 controls. Rieken et al. [38] demonstrated a lower risk of recurrence and progression for metformin-exposed patients (HR 0.41, 95% CI 0.25 –0.67 and HR 0.31, 95% CI 0.11 –0.87), respectively. Other studies on metformin did not show a reduced risk of recurrence or progression in metformin-exposed compared to non-exposed patients. Study arms were relatively small, ranging between 9 and 81 patients with up to 40% recurrences in both study arms and progression ranging between 2 –20%.
Both Rieken et al. [38] and Richard et al. [37] found no survival benefit in NMIBC patients after metformin exposure either in monotherapy or in combination with other GLDs. The study of Wang et al. [40] showed an improved survival in metformin-exposed patients compared to controls but in a much smaller cohort (n = 9 and 22 NMIBC patients respectively).
MIBC
Three studies [36, 39, 41] included patients after RC. Both Nayan et al. [36] and Rieken et al. [39] showed a statistically significant decreased risk of locoregional recurrence and increased CSS for metformin-exposed (n = 119) compared to non-exposed patients (n = 166). OS was similar in both groups. Adjusted variables included histopathological characteristics but no information on diabetes duration and hyperglycaemic control was available. Lyon et al. [41] did not report a difference based on metformin status in CSS and OS after RC, but did observe a higher metastasis-free survival with metformin exposure.
Meta-analysis
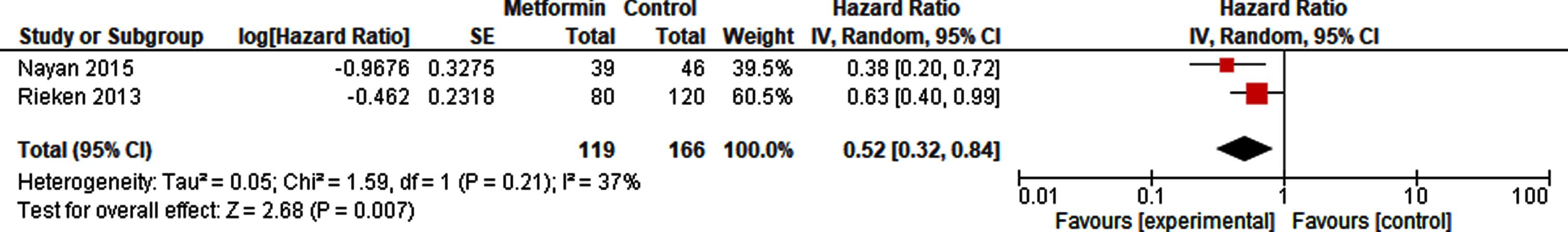
A pooled analysis of 65 metformin-exposed patients and 93 controls did not show a decreased risk of recurrence of metformin compared to non-exposure in patients with NMIBC (HR 0.71, 95% CI 0.22 –2.30; Fig. 3). The risk of recurrence in patients after radical cystectomy in high risk NMIBC and MIBC however was reduced with metformin use (HR 0.52, 95% CI 0.32 –0.84; Fig. 4).
Fig. 3
Forest plot of HR for recurrence in NMIBC.
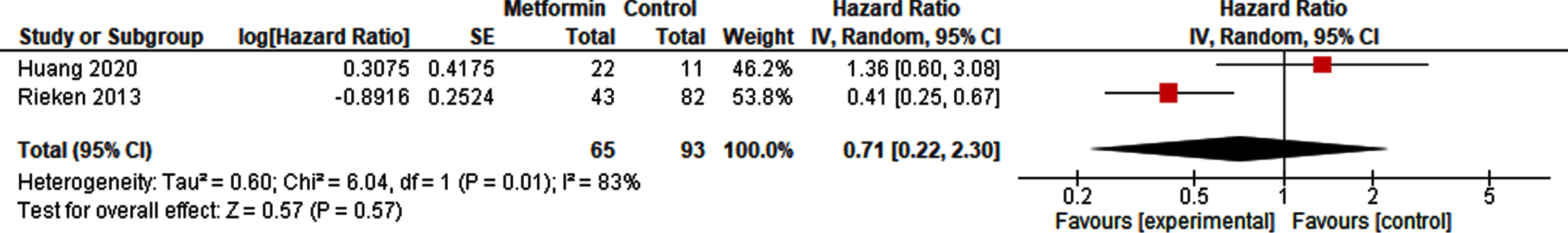
Fig. 4
Forest plot of HR for recurrence after radical cystectomy.
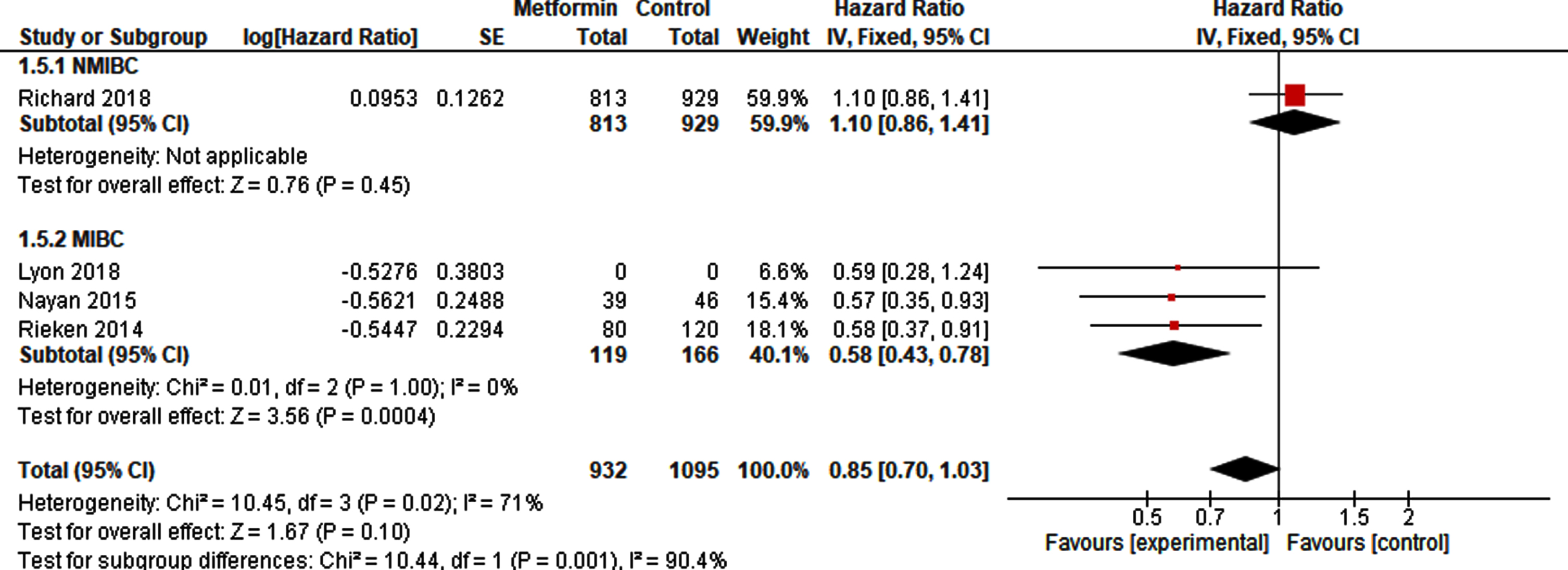
Pooled analysis based on at least 932 metformin-exposed patients and 1095 controls (Fig. 5), since Lyon et al. [41] did not report the number of metformin exposed and non-exposed patients, showed no difference in CSS (HR 0.85, 95% CI 0.70 –1.03). However, a sub-analysis showed a statistically significant reduced risk of cancer-specific death after radical cystectomy (HR 0.58, 95% CI 0.43 –0.78), with ever use of metformin compared to never use (Fig. 5).
Fig. 5
Forest plot of pooled HR for CSS in NMIBC and MIBC.
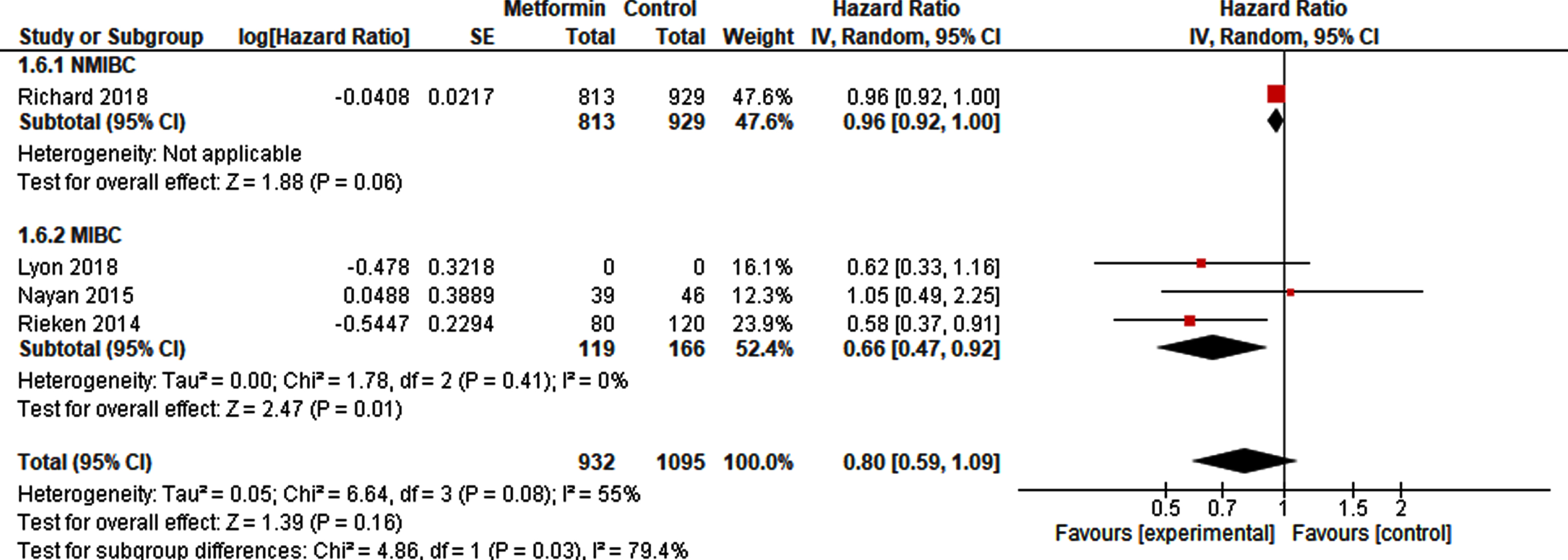
With respect to OS, there was no difference between metformin exposure compared to control (HR 0.80, 95% CI 0.59 –1.09; Fig. 6). A sub-analysis, including at least 119 metformin-exposed patients and 166 non-exposed patients after radical cystectomy resulted in a higher OS in metformin-exposed patients (HR 0.66, 95% CI 0.47 –0.92).
Fig. 6
Forest plot of HR for OS in NMIBC and MIBC.
DISCUSSION
The goal of this review was to identify and summarize the evidence of the relationship between metformin use and BC incidence and treatment outcomes. We have conducted an extensive review and combined the results in a meta-analysis. Based on the current literature no effect was found of metformin use on BC incidence, but we did find a reduced risk of recurrence and increased CSS and OS in patients after radical cystectomy for high-risk NMIBC and MIBC after metformin exposure. The results of the meta-analysis should be interpreted with some caution. Besides methodological differences between studies, it is important to note the risk of statistical heterogeneity. I2 from overall analyses showed some to substantial heterogeneity (I2 = 30 –83%). However, in the predefined subgroup analyses based on disease stage and GLDs I2 was low (0–14%), thereby strengthening our conclusions.
Multiple in vitro and in vivo studies have confirmed the potential antineoplastic effects of metformin in various types of cancer [44–46]. Nevertheless, most RCTs that studied the efficacy of metformin in cancer patients had negative results, with the exception of non-small cell lung cancer (NSCLC), but also for NSCLC conflicting results have been reported [47–51]. Existing reviews about metformin and cancer incidence have focussed either on organ sites other than the bladder or do not differentiate between different cancer types [52, 53]. Compared with a previously published meta-analysis [54] on the effect of metformin on BC this review provides a broader overview and an updated meta-analysis with the inclusion of several recently published studies.
To explain the positive effects of metformin on the reduced risk of recurrence, CSM and OM after radical cystectomy and the lack of effect on BC incidence, one has to understand the mechanisms of action of metformin. While this review summarizes the clinical evidence of metformin and BC it does not address possible mechanisms by which metformin exerts its anticancer effects. As stated previously, the proposed antineoplastic mechanisms (i.e. AMPK activation, mTOR inhibition and the influence on mitochondrial activity) are also observed in i n vitro studies on metformin and BC [12–14]. One of the possible hurdles of translating the anticancer effects of metformin from preclinical research towards clinical practice is that most in vitro anticancer effects occur at millimolar concentrations. These concentrations are difficult to achieve and maintain in humans due to dose-dependent gastrointestinal side effects and the risk of lactate acidosis in patients with decreased renal function [55]. However, metformin is excreted unchanged by the kidneys in the urine where it accumulates and reaches concentrations > 100 times higher than blood plasma after oral administration [56]. This could lead to sufficient concentrations for metformin to exert its antitumor effect. Indeed Liu et al. demonstrated the potential role of metformin in the treatment of BC [57]. In mice, the renal excretion of metformin led to urine concentrations 300-fold higher than plasma concentrations and this proved to be effective in reducing orthotopic NMIBC growth and prolonging survival compared to untreated mice. This might explain the reduced risk of recurrence in metformin exposed NMIBC patients compared to non-exposed patients by Rieken et al. [38], although this effect was not visible in the pooled HR for recurrence in NMIBC. To further explore this mechanism, we are currently running a clinical phase 2 marker lesion trial investigating the application of oral metformin for the treatment of low-grade NMIBC in non-diabetic patients which could provide a more definitive answer to the role of metformin on BC and its working mechanism [58].
The absence of an effect seen on BC incidence in our review could have several reasons. Firstly, the data is mainly derived from retrospective studies with different designs. This can lead to uncertainties when comparing study outcomes. Although one prospective randomized study [32] reported lower BC incidence rates after metformin compared to SU, this study is of limited contribution due to the small number of events of BC diagnosis. Secondly, it is possible that exposure definitions in treatment and comparator arms differed between studies thereby masking the treatment effect of metformin.
Another difficulty is the fact that, within the current literature, the effects of metformin treatment on BC can only be compared within a diabetic population and thus other GLDs. This presents the risk of time and lag latency, when treatments given at different stages of disease are compared. Metformin is often used as first-line treatment for DMII. Diabetic patients on metformin could therefore have shorter duration of diabetes reducing their comorbidity and metabolic risk factors for developing cancer compared with patients on second-line or third-line treatment. However, in our sub-analysis of cancer incidence comparing metformin monotherapy with SU monotherapy, also given as first-line treatment, albeit at a lower frequency, no difference was found in risk of BC. In addition, limited and conflicting data is available on the relationship between other GLDs and cancer incidence [59–61]. Finally, none of the studies on BC incidence provided information on variant histology, the stage or risk group of BC at diagnosis, making it difficult to evaluate the effect of metformin on BC type, grade and stage.
Lastly, while awaiting results from prospective intervention trials, the conflicting results regarding treatment outcomes in the included articles offer the possibility that metformin monotherapy might not be effective to treat BC. However, it is suggested that metformin could have an additive effect to existing cancer treatments such as cisplatin and the EGFR-inhibitor gefitinib [15, 62]. Recently, Oresta et al. [63] demonstrated that mitomycin-C, a first-line chemotherapeutic agent in adjuvant NMIBC treatment, can induce mitochondrial metabolic reprogramming in BC in vitro and in vivo. This leads to the hypothesis that metformin could improve results when combined mitomycin-C bladder instillations.
CONCLUSIONS
This review adds to the growing body of evidence of the role of metformin in cancer development and treatment. A protective effect in cancer development in diabetic patients seems unlikely although a beneficial effect in preventing recurrence after radical cystectomy and an improvement in CSS and OS in this patient group is possible. High-quality studies are lacking. Therefore, prospective studies considering both treatment efficacy and mechanism of action are needed to answer these remaining questions.
ACKNOWLEDGMENTS
The authors express their gratitude towards F. Etten –Jamaludin, clinical librarian, for her help in constructing the search strategy.
FUNDING
JH was supported by The Netherlands Organisation for Health Research and Development (ZonMw, project number 848082004). JH and BR were supported by the Cure for Cancer foundation.
AUTHORS’ CONTRIBUTIONS
Design and interpretation of results: JH, JR, TR, JO JW and RM.
Search, articles selection, data extraction and analyses: JH, BR, JO.
Writing of manuscript: JH, BR, TR, JO, JW and RM.
All authors read, revised and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors JH, BR, TR, JO, JW and RM declare no conflict of interest.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-211653.
REFERENCES
[1] | Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians [Internet]. 2018 Nov 1 [cited 2021 May 27];68: (6):394–424. Available from: https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21492 |
[2] | EAU Guidelines: Non-muscle-invasive Bladder Cancer | Uroweb [Internet]. [cited 2021 Jul 20]. Available from: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/#11 |
[3] | Nederlandse Kankerregistratie (NKR), IKNL [Internet]. [cited 2021 Jul 20]. Available from: https://www.iknl.nl/nkr-cijfers |
[4] | Sylvester RJ , Rodríguez O , Hernández V , Turturica D , L B , HM B , et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscleinvasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. European urology [Internet]. (2021) Apr 1 [cited 2021 Jul 19];79: (4)::480–8. Available from: https://pubmed.ncbi.nlm.nih.gov/33419683/ |
[5] | Sievert KD , Amend B , Nagele U , Schilling D , Bedke J , Horstmann M , et al. Economic aspects of bladder cancer: What are the benefits and costs? World Journal of Urology [Internet]. (2009) [cited 2021 May 27];27: (3):295–300. Available from: https://pubmed.ncbi.nlm.nih.gov/19271220/ |
[6] | National Insitue for Health and Care Excellence. (2015). Type 2 diabetes in adults: management [NICE guideline [NG28]. https://www.nice.org.uk/guidance/ng28 [Internet]. 2015. Available from: https://www.nice.org.uk/guidance/ng28 |
[7] | Bailey CJ . Metformin: historical overview [Internet]. Vol. 60, Diabetologia. Springer Verlag; 2017 [cited 2021 May 25]. p. 1566–76. Available from: http://www.bioimages.org.uk. |
[8] | Pollak MN . Investigating metformin for cancer prevention and treatment: The end of the beginning [Internet]. Vol. 2, Cancer Discovery. Cancer Discov; 2012 [cited 2021 May 26]. p. 778–90. Available from: https://pubmed.ncbi.nlm.nih.gov/22926251/ |
[9] | Heckman-Stoddard BM , DeCensi A , Sahasrabuddhe V v . Ford LG . Repurposing metformin for the prevention of cancer and cancer recurrence [Internet]. Vol. 60, Diabetologia. Springer Verlag; (2017) [cited 2021 May 26]. p. 1639–47. Available from: http://wok.mimas.ac.uk |
[10] | Vancura A , Bu P , Bhagwat M , Zeng J , Vancurova I . Metformin as an Anticancer Agent [Internet]. Vol. 39, Trends in Pharmacological Sciences. Elsevier Ltd; (2018) [cited 2021 May 26]. p. 867–78. Available from: https://pubmed.ncbi.nlm.nih.gov/30150001/ |
[11] | Rena G , Hardie DG , Pearson ER . The mechanisms of action of metformin [Internet].Vol. 60, Diabetologia. SpringerVerlag; (2017) [cited 2021 May 26]. p. 1577–85. Available from: https://pubmed.ncbi.nlm.nih.gov/28776086/ |
[12] | Zhang T , Wang X , He D , Jin X , Guo P . Metformin sensitizes human bladder cancer cells to TRAIL-induced apoptosis through mTOR/S6K1-mediated downregulation of c-FLIP. Anti-Cancer Drugs [Internet]. 2014 [cited 2021 Jun 12];25(8):887-97. Available from: https://journals.lww.com/anti-cancerdrugs/Fulltext/2014/09000/Metforminsensitizes_human_bladder_cancer_cells_to.4.aspx |
[13] | Peng M , Su Q , Zeng Q , Li L , Liu Z , Xue L , et al. High efficacy of intravesical treatment of metformin on bladder cancer in preclinical model. Oncotarget [Internet]. (2016) [cited 2021 Jun 12];7: (8):9102–17. Available from: https://pmc/articles/PMC4891029/ |
[14] | Wang Y , An H , Liu T , Qin C , Sesaki H , Guo S , et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Reports. (2019) ;29: (6):5. |
[15] | Wang D , Wu X . In vitro and in vivo targeting of bladdercarcinoma with metformin in combination with cisplatin. OncologyLetters [Internet]. (2015) [cited 2021 Jun 12];10: (2):975–81. Available from: https://pubmed.ncbi.nlm.nih.gov/26622608/ |
[16] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews [Internet]. Vol. 372, The BMJ. BMJ Publishing Group; 2021 [cited 2021 May 27]. Available from: http://dx.doi.org/10.1136/bmj.n71 |
[17] | Sterne JAC , Savović J , Page MJ , Elbers RG , Blencowe NS , Boutron I , et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. The BMJ [Internet]. 2019 Aug 28 [cited 2021 May 27];366. Available from: http://dx.doi.org/10.1136/bmj.l4898http://www.bmj.com/ |
[18] | Wells G , Shea B , O’Connell D , Peterson J , Welch V . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000 [cited 2021 Jun 3]; Available from: http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf |
[19] | Tierney JF , Stewart LA , Ghersi D , Burdett S , Sydes MR . Practical methods for incorporating summary time to-event data into meta-analysis. Trials [Internet]. (2007) Jun 7 [cited 2021 May 27];8: (1):1–16. Available from: http://www.trialsjournal.com/content/8/1/16 |
[20] | Review manager (RevMan). Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration; 2014. |
[21] | Goossens ME , Buntinx F , Zeegers MP , Driessen JHM , de Bruin ML , deVries F . Influence of metformin intake on the risk of bladder cancerin type 2 diabetes patients. British Journal of ClinicalPharmacology. (2015) ;80: (6):1464–72. |
[22] | Tsilidis KK , Capothanassi D , Allen NE , Rizos EC , Lopez DS , van Veldhoven K , et al. Metformin does not affect cancer risk: A cohort study in the U. K. clinical practice research datalink analyzed like an intention-to-treat trial. Diabetes Care. (2014) ;37: (9):2522–32. |
[23] | Chen YC , Kok VC , Chien CH , Horng JT , Tsai JJP . Cancer risk in patients aged 30 years and above with type 2 diabetes receiving antidiabetic monotherapy: A cohort study using metformin as the comparator. Therapeutics and Clinical Risk Management. (2015) ;11: :1315–23. |
[24] | Tseng CH . Diabetes and risk of bladder cancer: A study using the National Health Insurance database in Taiwan. Diabetologia. (2011) ;54: (8):2009–15. |
[25] | Tseng CH . Metformin may reduce bladder cancer risk in Taiwanese patients with type 2 diabetes. Acta Diabetologica. (2014) ;51: (2):295–303. |
[26] | Sung JJ , Ho JM , Lam AS , Yau ST , Tsoi KK . Use of metformin and aspirin is associated with delayed cancer incidence. Cancer Epidemiology [Internet]. (2020) ;69: (July):101808. Available from: https://doi.org/10.1016/j.canep.2020.101808 |
[27] | Kahn SE , Haffner SM , Heise MA , Herman WH , Holman RR , Jones NP , et al. Glycemic Durability of Rosiglitazone, Metformin, or Glyburide Monotherapy. New England Journal of Medicine. (2006) ;355: (23):2427–43. |
[28] | Murff HJ , Roumie CL , Greevy RA , Hackstadt AJ , McGowan LEDa , Hung AM et al. Metformin use and incidence cancer risk: evidence for aselective protective effect against liver cancer. Cancer Causes andControl [Internet]. (2018) Sep 1 [cited 2021 May 26];29: (9):823–32. Available from: https://pubmed.ncbi.nlm.nih.gov/30022336/ |
[29] | Mamtani R , Pfanzelter N , Haynes K , Finkelman B , Wang X , Keefe S , et al. Incidence of Bladder Cancer in Patients With Type 2 Diabetes Treted With Metformin or Sulfonylureas. Diabetes Care. (2014) ;37: (7):1910–7. |
[30] | Neumann A , Weill A , Ricordeau P , Fagot JP , Alla F , Allemand H . Pioglitazone and risk of bladder cancer among diabetic patients in France: A population-based cohort study. Diabetologia. (2012) ;55: (7):1953–62. |
[31] | Dankner R , Agay N , Olmer L , Murad H , Keinan Boker L , Balicer RD , et al. Metformin Treatment and Cancer Risk: Cox Regression Analysis, With Time-Dependent Covariates, of 320,000 Persons With Incident Diabetes Mellitus. American Journal of Epidemiology. (2019) ;188: (10):1794–800. |
[32] | Home PD , Pocock SJ , Beck-Nielsen H , Curtis PS , Gomis R , Hanefeld M , et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. The Lancet [Internet]. (2009) ;(2019) ;373: (9681):2125–35. Available from: http://dx.doi.org/10.1016/S0140-6736(09)60953-3 |
[33] | Oliveria SA , Koro CE , Ulcickas Yood M , Sowell M . Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes and Metabolic Syndrome: Clinical Research and Reviews. (2008) ;2: (1):47–57. |
[34] | Ahn JH , Jung S il , Yim SU , Kim SW , Hwang EC , Kwon DD . Impact ofglycemic control and metformin use on the recurrence andprogression of non-muscle invasive bladdercancer in patients with diabetes mellitus. Journal of Korean MedicalScience. (2016) ;31: (9):1464–71. |
[35] | Huang WL , Huang KH , Huang CY , Pu YS , Chang HC , Chow PM . Effect of diabetes mellitus and glycemic control on the prognosis of non-muscle invasive bladder cancer: a retrospective study. BMC Urology [Internet]. (2020) Aug 5 [cited 2021 Jun 3];20: (1):1–7. Available from: https://doi.org/10.1186/s12894-020-00684-5 |
[36] | Nayan M , Bhindi B , Yu JL , Hermanns T , Mohammed A , Hamilton RJ , et al. The effect of metformin on cancer-specific survival outcomes in diabetic patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Urologic Oncology: Seminars and Original Investigations [Internet]. (2015) ;33: (9):386.e7–386.e13. Available from: http://dx.doi.org/10.1016/j.urolonc.2015.05.024 |
[37] | Richard PO , Ahmad AE , Bashir S , Zlotta A , Bhindi B , Leao R , et al. Impact of oral hypoglycemic agents on mortality among diabetic patients with non-muscle invasive bladder cancer: A population-based analysis. Canadian Urological Association Journal [Internet]. (2018) Jun 1 [cited 2021 May 26];12: (6):203–10. Available from: https://pubmed.ncbi.nlm.nih.gov/29485035/ |
[38] | Rieken M , Xylinas E , Kluth L , Crivelli JJ , Chrystal J , Faison T , et al. Association of diabetes mellitus and metformin use with oncological outcomes of patients with non-muscle-invasive bladder cancer. BJU International. (2013) ;112: (8):1105–12. |
[39] | Rieken M , Xylinas E , Kluth L , Crivelli JJ , Chrystal J , Faison T , et al. Effect of diabetes mellitus and metformin use on oncologic outcomes of patients treated with radical cystectomy for urothelial carcinoma. Urologic Oncology: Seminars and Original Investigations [Internet]. (2014) [cited 2021 May 26];32: (1):49.e7–49.e14. Available from: https://pubmed.ncbi.nlm.nih.gov/24140245/ |
[40] | Wang Z , Ong W , Shen T , Sng J , Lata R , Mahendran R , et al. Beyond diabetes mellitus: role of metformin in nonmuscle invasive bladder cancer. Singapore Medical Journal [Internet]. 2020 Aug 17 [cited 2021 May 26]; Available from: https://pubmed.ncbi.nlm.nih.gov/32798360/ |
[41] | Lyon TD , Frank I , Shah PH , Tarrell R , Cheville JC , Karnes RJ , et al. The Association of Aspirin Use with Survival Following Radical Cystectomy. Journal of Urology [Internet]. (2018) ;200: (5):1014–21. Available from: https://doi.org/10.1016/j.juro.2018.05.119 |
[42] | Heidari F , Zade SA , Hosseini SHM , Ghadian A . Metformin for the prevention of bladder cancer recurrence: Is it effective? Nephro-Urology Monthly. (2016) ;8: (3). |
[43] | Lévesque LE , Hanley JA , Kezouh A , Suissa S . Problem of immortal time bias in cohort studies: Example using statins for preventing progression of diabetes. BMJ (Online) [Internet]. (2010) Apr 24 [cited 2021 Jun 6];340: (7752):907–11. Available from: https://www.bmj.com/content/340/bmj.b5087 |
[44] | Zakikhani M , Dowling R , Fantus IG , Sonenberg N , Pollak M . Metformin is anAMPkinase-dependent growth inhibitor for breast cancer cells. Cancer Research [Internet]. (2006) Nov 1 [cited 2021 May 26];66: (21):10269–73. Available from: https://pubmed.ncbi.nlm.nih.gov/17062558/ |
[45] | Wheaton WW , Weinberg SE , Hamanaka RB , Soberanes S , Sullivan LB , Anso E , et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife. (2014) ;(3). |
[46] | de Souza Netoa FP , Bernardes SS , Marinello PC , Melo GP , Luiz RC , Cecchini R , et al. Metformin: Oxidative and proliferative parameters in-vitro and in-vivo models of murine melanoma. Melanoma Research [Internet]. (2017) [cited 2021 May 26];27: (6):536–44. Available from: https://pubmed.ncbi.nlm.nih.gov/28877050/ |
[47] | Kordes S , Pollak MN , Zwinderman AH , Mathôt RA , Weterman MJ , Beeker A , et al. Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. The Lancet Oncology [Internet]. (2015) Jul 1 [cited 2021 May 27];16: (7):839–47. Available from: https://pubmed.ncbi.nlm.nih.gov/26067687/ |
[48] | Marrone KA , Zhou X , Forde PM , Purtell M , Brahmer JR , Hann CL , et al. A Randomized Phase II Study of Metformin plus Paclitaxel/Carboplatin/Bevacizumab in Patients with Chemotherapy-Naïve Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer. The Oncologist [Internet]. (2018) Jul [cited 2021 May 27];23: (7):859-–65. Available from: https://pubmed.ncbi.nlm.nih.gov/29487223/ |
[49] | Lee Y , Joo J , Lee YJ , Lee EK , Park S , Kim TS , et al. Randomized phase II study of platinum-based chemotherapy plus controlled diet with or without metformin in patients with advanced non-small cell lung cancer. Lung Cancer. (2021) Jan 1; 151: :8–15. |
[50] | Sayed RASWLEO. Metformin Addition to Chemotherapy in Stage IV Non-Small Cell Lung Cancer: an Open Label Randomized Controlled Study. Asian Pacific Journal of Cancer Prevention [Internet]. (2015) [cited 2021 Aug 10];16: (15):6621–6. Available from: http://dx.doi.org/10.7314/APJCP.2015.16.15.6621 |
[51] | Arrieta O , Barrón F , Padilla M-ÁS , Avilés-Salas A , Ramírez-Tirado LA , Jiménez MJA , et al. Effect of Metformin Plus Tyrosine Kinase Inhibitors Compared With Tyrosine Kinase Inhibitors Alone in Patients With Epidermal Growth Factor Receptor-Mutated Lung Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncology [Internet]. (2019) Nov 1 [cited 2021 Aug 3];5: (11):e192553–e192553. Available from: https://jamanetwork.com/journals/jamaoncology/fullarticle/2749256 |
[52] | Noto H , Goto A , Tsujimoto T , Noda M . Cancer risk in diabetic patients treated with metformin: A systematic review and meta-analysis. PLoS ONE [Internet]. (2012) Mar 20 [cited 2021 May 27];7: (3):e33411. Available from: http://www.plosone.org |
[53] | DeCensi A , Puntoni M , Goodwin P , Cazzaniga M , Gennari A , Bonanni B , et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prevention Research [Internet]. (2010) Nov 1 [cited 2021May 27];3: (11):1451–61. Available from: http://www.aacrjournals.org |
[54] | Hu J , Chen JB , Cui Y , Zhu YW , Ren WB , Zhou X , et al. Association of metformin intake with bladder cancer risk and oncologic outcomes in type 2 diabetes mellitus patients: A systematic review and meta-analysis [Internet]. Vol. 97, Medicine (United States). Lippincott Williams and Wilkins; 2018 [cited 2021 May 27]. Available from: https://pubmed.ncbi.nlm.nih.gov/30045293/ |
[55] | Dowling RJO , Niraula S , Stambolic V , Goodwin PJ . Metformin in cancer: Translational challenges [Internet]. Vol. 48, Journal of Molecular Endocrinology. Society for Endocrinology; 2012 [cited 2021 May 27]. p. 31-43. Available from: http://www.endocrinology-journals.org |
[56] | Graham GG , Punt J , Arora M , Day RO , Doogue MP , Duong J , et al. Clinical pharmacokinetics of metformin. Clinical Pharmacokinetics of Metformin [Internet]. (2011) ;50: (2):81–98. Available from: https://link.springer.com/content/pdf/10.2165%2F11534750-000000000-00000.pdf |
[57] | Liu Z , Yokoyama NN , Blair CA , Li X , Avizonis D , Wu XR , et al. High Sensitivity of an Ha-RAS transgenic model of superficial bladder cancer to metformin is associated with -240-fold higher drug concentration in urine than serum. Molecular Cancer Therapeutics [Internet]. (2016) Mar 1 [cited 2021 May 27];15: (3):430–8. Available from: https://pmc/articles/PMC4783238/ |
[58] | Molenaar RJ , van Hattum JW , Brummelhuis IS , Oddens JR , Savci-Heijink CD , Boevé ER , et al. Study protocol of a phase II clinical trial of oral met formin for the intravesical treatment of non-muscle invasive bladder cancer. BMC Cancer [Internet]. (2019) Nov 21 [cited 2021 May 27];19: (1). Available from: https://pubmed.ncbi.nlm.nih.gov/31752752/ |
[59] | Tuccori M , Filion KB , Yin H , Yu OH , Platt RW , Azoulay L . Pioglitazone use and risk of bladder cancer: Population based cohort study. BMJ (Online) [Internet]. 2016 Mar 30 [cited 2021 Jun 12];352. Available from: http://dx.doi.org/10.1136/bmj.i1541 |
[60] | Tang H , Shi W , Fu S , Wang T , Zhai S , Song Y , et al. Pioglitazone and bladder cancer risk: a systematic reviewand meta-analysis. Cancer Medicine [Internet]. (2018) Apr 1 [cited 2021 Jun 12];7: (4):1070–80. Available from: https://pmc/articles/PMC5911601/ |
[61] | Hendriks AM , Schrijnders D , Kleefstra N , de Vries EGE , Bilo HJG , Jalving M , et al. Sulfonylurea derivatives and cancer, friend or foe? Vol. 861, European Journal of Pharmacology. Elsevier B.V.; (2019) . p. 172598. |
[62] | Peng M , Huang Y , Tao T , Peng CY , Su Q , Xu W , et al. Metformin and gefitinib cooperate to inhibit bladder cancer growth via both AMPK and EGFR pathways joining at Akt and Erk. Scientific Reports [Internet]. (2016) Jun 23 [cited 2021 Jun 12];6: (1):1–10. Available from: http://www.nature.com/scientificreports |
[63] | Oresta B , Pozzi C , Braga D , Hurle R , Lazzeri M , Colombo P , et al. Mitochondrial metabolic reprogramming controls the induction of immunogenic cell death and efficacy of chemotherapy in bladder cancer. Science Translational Medicine [Internet]. (2021) Jan 6 [cited 2021 Jun 12];13: (575): Available from: https://stm.sciencemag.org/content/13/575/eaba6110 |




