Urethral Melanoma – Clinical, Pathological and Molecular Characteristics
Abstract
BACKGROUND:
Mucosal melanoma involving the urethra is a rare disease with distinct clinical and molecular characteristics and poor outcomes. Our current knowledge is limited by the small number of reports regarding this disease.
OBJECTIVE:
To describe the clinical, pathological, and molecular characteristics of urethral melanoma.
METHODS:
We summarized the clinicopathologic data for 31 patients treated for urethral melanoma from 1986–2017 at our institution. Genomic data from our institutional sequencing platform MSK-IMPACT (n = 5) and gene-specific PCR data on BRAF, KIT, and/or NRAS (n = 8) were compared to genomic data of cutaneous melanomas (n = 143), vulvar/vaginal melanomas (n = 24), and primary non-melanoma urethral tumors (n = 5) from our institutional database.
RESULTS:
Twenty-three patients were diagnosed with localized disease, 7 had regional/nodal involvement and one had metastases. Initial treatment included surgery in 25 patients; seven had multimodal treatment. Median follow-up was 46 months (IQR 33–123). Estimated 5-year cancer-specific survival was 45%. No significant change in survival was observed based on a year of treatment.
Primary urethral melanomas showed a higher frequency of TP53 mutations compared to cutaneous (80.0% vs. 18.2%, p = 0.006) and vulvar/vaginal melanomas (80.0 vs. 25.0%, p = 0.04). BRAF mutations were absent in urethral primaries (0% vs. 46% in cutaneous melanoma, p = 0.02). Tumor mutation burden was higher in cutaneous than urethral melanomas (p = 0.04). Urethral melanomas had a higher number of somatic alterations compared to non-melanoma urethral tumors (median 11 vs. 5, p = 0.03).
CONCLUSIONS:
Our findings support a unique mutational landscape of urethral melanoma compared to cutaneous melanoma. Survival remains poor and is unchanged over the time studied.
INTRODUCTION
Mucosal melanoma is a rare entity accounting for approximately 1% of all melanomas. Important clinical differences exist between these tumors and their cutaneous counterparts. Notably, the majority (over 80%) of mucosal melanomas occur in individuals over 60, and approximately two thirds of cases are reported in women [1, 2]. Prognosis in this disease is exceptionally poor, with an estimated 5-year overall survival of 25%, compared to approximately 80% in cutaneous melanoma [1]. Molecularly, mucosal melanomas have been shown to exhibit distinct features when compared to cutaneous melanomas. These include a low point mutation burden and a high frequency of structural variants [3]. In addition, the rates of BRAF and NRAS mutations appear to be lower in mucosal melanomas while somatic KIT mutations are more frequent [3–16]. Due to its rarity, there is limited evidence to support the use of new targeted therapies or immunotherapies for advanced disease. Current management practices are largely based on extrapolations from the management of the more common cutaneous presentation [17], making research into this field imperative to its understanding and adequate treatment.
Mucosal melanomas have been most frequently reported in the head/neck (55.4%), anorectal (23.8%) and female genital mucosae (18.0%). Melanomas involving the urinary tract represent only around 3% of all mucosal melanomas [1]. Our report focuses on this poorly characterized entity. Primary urethral melanoma commonly presents as a symptomatic mass affecting the meatus and distal urethra and is often diagnosed at an advanced stage [18, 19]. Initial treatments tend to include limited or radical resection, and adjuvant treatment with radiotherapy, chemotherapy or immunotherapy is occasionally utilized [18, 19]. Despite the implementation of a multidisciplinary treatment approach, recurrence rates are high and prognosis is poor with reported 5-year overall survival rates ranging from 10% to 39% [19–22].
Most reports regarding the characteristics and treatment of urethral melanoma have been limited to single case studies or small case series. Larger studies attempting to address this topic have been limited by the degree of patient heterogeneity present within the data. Moreover, few studies specifically addressed genetic findings of urethral melanomas.
In the current study, we present the results of a relatively large cohort of urethral melanomas managed at a single institution. The clinical and pathological characteristics of these tumors are summarized. Genomic analyses were performed in a subset of tumors with available genomic data, and their results compared to cutaneous, and female genital melanomas.
MATERIALS AND METHODS
Patient population and clinical data
After obtaining institutional review board approval (IRB approval # 17–474), which included a waiver of informed consent, we retrospectively reviewed our institutional pathological database and identified 31 patients treated at our institution for melanoma involving the urethra from 1986–2017. Baseline clinicopathological characteristics were obtained from patients’ medical records. Female patients were evaluated by a gynecologist to identify concurrent disease involving the introitus or vaginal vault. Tumor stage was assigned according to a simplified staging system proposed by Ballantyne et. al. for patients with mucosal melanomas [23]. Patients with stage I disease had clinically localized disease, stage II –regional nodal involvement, and stage III –distant metastatic involvement. Management of primary tumors as well as sites and treatments of recurrent disease were noted. Patients who underwent organ sparing operations underwent routine urethroscopies and cystoscopies to identify local recurrence. Disease recurrence was identified based on imaging studies and pathology reports when available and categorized as locoregional or distant.
Genomic data
A total of 13/31 patients (42%) underwent DNA sequencing of their primary tumor. Five patients underwent next-generation sequencing (NGS) using our institutional sequencing platform (MSK-IMPACT), which consists of a hybridization capture-based assay targeting 341–468 key cancer genes with an average coverage of ∼500X [24]. Additionally, eight patients underwent PCR-based sequencing of specific hotspots in BRAF (n = 7), KIT (n = 6) and/or NRAS (n = 4). NGS results were summarized and compared to those from 143 cutaneous melanomas and 24 vulvar/vaginal melanomas. Five non-melanoma urethral tumors (three adenocarcinomas, one squamous-cell carcinoma and one urothelial carcinoma) were also included and their results summarized for future reference. All genomic information was obtained from primary tumor samples. Genomic aberrations analyzed included mutations, fusions, and gene-level copy-number alterations (CNAs). Additionally, the tumor mutation burden score (TMB) and fraction of the genome bearing CNAs (FCNAg) were calculated and compared between tumors. Potentially-oncogenic alterations were annotated using the OncoKB database (genomic events with any evidence of oncogenicity were considered oncogenic) [25].
Statistical analysis
Descriptive statistics were used to report clinicopathological characteristics and genomic alterations. Non-parametric testing was used to compare characteristics between tumor types. Wilcoxon rank-sum and Fisher’s exact tests were performed to evaluate differences in continuous and categorical variables, respectively. The Benjamini-Hochberg approach was used to correct for multiple testing. The primary study outcome was cancer-specific survival (CSS). Secondary outcomes included local recurrence-free survival (RFS) for patients with locoregional disease, and metastasis-free survival (MFS) for patients without distant metastasis at presentation. Survival estimates were computed using the Kaplan-Meier method, from the time of initial treatment to the occurrence of the event of interest or last documented visit. Multivariable analyses were performed using Cox-regression models to adjust for year of treatment (binarized by the median), age-adjusted Charlson comorbidity index (CCI) (>3 vs. ≤3) and disease stage. All statistical analyses were performed in the R platform v3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline clinical characteristics
The study cohort included 29 women and two men with a median age of 73 years. A total of 27 patients (87%) were symptomatic at presentation. Of these, 65% presented with a mass protruding from the urethra or vulva, while 42% had hematuria and/or vaginal bleeding at presentation. Most patients presented with localized disease (74%), while regional/nodal involvement and distant metastases were present in 23% and 3%, respectively. Of all the individuals with urethral involvement identified, nineteen (61%) had urethral involvement as the initial presentation, while the rest had tumors arising from their genital tract prior to urethral involvement. Clinical and pathological characteristics of the study cohort are summarized in Table 1.
Table 1
Clinical and pathological characteristics of the study cohort (n = 31)
| Variable | No. Pts. | % | |
| Age in years (median, IQR) | 73 | 60, 80 | |
| Sex | Female | 29 | 94 |
| Male | 2 | 6 | |
| Age adjusted CCI | ≤3 | 8 | 26 |
| > 3 | 23 | 74 | |
| Smoking status | Never | 19 | 61 |
| Current/ former | 12 | 39 | |
| Signs and symptoms at presentation | Hematuria/ vaginal bleed | 13 | 42 |
| Dysuria/ discomfort | 10 | 32 | |
| Spotting/ discharge | 9 | 29 | |
| Obstructive symptoms | 2 | 6 | |
| Vulvar/ urethral mass | 20 | 65 | |
| Incidental finding | 4 | 13 | |
| Lymph node involvement | Present | 8 | 26 |
| Absent | 23 | 74 | |
| Metastatic disease | Present | 1 | 3 |
| Absent | 30 | 97 | |
| Stage at diagnosis | Localized | 23 | 74 |
| Regional nodal | 7 | 23 | |
| Distant metastatic | 1 | 3 |
IQR = interquartile range; CCI = Charlson comorbidity index.
Clinical management and outcomes
The median time between diagnosis and treatment was 1.1 months (IQR, 0.9–2.1). Table 2 summarizes the initial treatment modalities of the study cohort. Twenty-seven patients received treatment for locoregional disease. Three patients with unresectable locoregional disease and one patient with metastatic disease at presentation did not receive local treatment. Local treatment consisted mostly of surgery (25/27 patients, 93%), including 14/27 patients (52%) who underwent organ sparing procedures and 11/27 patients (41%) who underwent radical procedures. Lymph node dissection was performed in 13/27 patients (48%). Seven patients received multimodal treatment –four received surgery followed by systemic treatment, two received surgery and radiation therapy and one received surgery, radiation, and systemic therapy. Among the patients with metastatic or unresectable disease, two patients with nodal disease and one patient with metastatic disease received immunotherapy with ipilimumab with or without nivolumab. In addition, one patient received chemotherapy with dacarbazine.
Table 2
Initial treatment given to patients with urethral melanoma (n = 31)
| Variable | No. Pts. | % | |
| Treatment of patients with resectable locoregional disease (n = 27) | |||
| Local treatment | Surgery | 22 | 82 |
| Radiotherapy | 2 | 7 | |
| Surgery + Radiotherapy | 3 | 11 | |
| Type of surgery | Transurethral resection | 1 | 4 |
| Distal urethrectomy | 2 | 7 | |
| Wide local excision and distal urethrectomy | 9 | 33 | |
| Partial penectomy | 2 | 7 | |
| Radical vulvectomy and distal urethrectomy | 8 | 30 | |
| Pelvic exenteration | 3 | 11 | |
| None | 2 | 7 | |
| Lymph node dissection | Sentinel | 7 | 26 |
| Inguinal | 6 | 22 | |
| None | 14 | 52 | |
| Adjuvant systemic treatment | Chemotherapy –total | 4 | 15 |
| Temozolomide | 3 | 11 | |
| Temozolomide/ thalidomide | 1 | 4 | |
| Immunotherapy –total | 2 | 7 | |
| Low dose interferon | 2 | 7 | |
| Targeted therapy –total | 1 | 4 | |
| Imatinib | 1 | 4 | |
| Treatment of patients with metastatic or unresectable disease (n = 4) | |||
| Chemotherapy –total | 1 | 25 | |
| Dacarbazine | 1 | 25 | |
| Immunotherapy –total | 3 | 75 | |
| Ipilimumab | 1 | 25 | |
| Nivolumab/ ipilimumab | 2 | 50 | |
| Targeted therapy –total | 0 | 0 | |
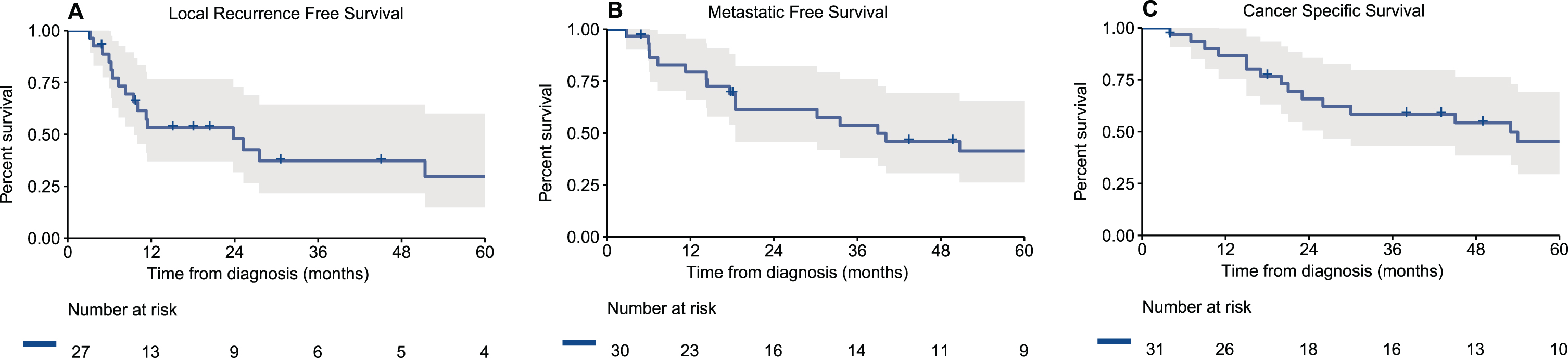
Median follow-up for survivors was 46 months (IQR 33–123). During follow-up, among the 30 patients without metastatic disease at presentation, 8/30 (27%) had a locoregional recurrence, 7/30 (23%) a distant recurrence or progression and 12/30 (40%) had both a locoregional and distant recurrence. Three patients did not have disease recurrence or progression –one patient was treated with nivolumab and ipilimumab for unresectable local disease with nodal involvement and did not progress at a follow-up of 49 months without additional treatments, one patient died of other cause 4 months after receiving treatment and one patient is without evidence of disease 18 months after a distal urethrectomy and sentinel lymph node dissection for a localized tumor. Notably, the other two patients who received immunotherapy died of their disease at 54 months and 4 months after diagnosis. Estimated 2- and 5-year local RFS rates were 48% and 30%, respectively (Fig. 1a). Two- and 5-year MFS were 61% and 41%, respectively (Fig. 1b). Two- and 5-year CSS were 66% and 45%, respectively (Fig. 1c).
Fig. 1
Kaplan-Meier curves of (A) local recurrence free survival of patients who underwent treatment for their loco-regional disease (n = 27), (B) metastatic free survival of patients who presented without metastatic disease (n = 30), and (C) cancer specific survival of all patients with urethral melanoma (n = 31).
Site and treatment of recurrent disease are reported in Table 3. Most locoregional recurrences involved the urethra (50%) and vulva/vagina (40%), while distant recurrences involved the lung (58%) and liver (32%). Treatment of recurrences included mainly surgery and immunotherapy (48% each). Approximately half of the cohort was treated after the year 2005. Although patient and tumor characteristics were comparable between the two treatment periods, as expected, a higher number of patients received immunotherapy for recurrent disease in the group of individuals treated after 2005 (83.3% vs. 20%, p = 0.002). When included in a multivariable Cox regression model controlling for age adjusted CCI and stage, year of treatment was not associated with either RFS, MFS or CSS.
Table 3
Location of tumor recurrence or progression and subsequent treatment in patients presenting with non-metastatic urethral melanoma (n = 30)
| Variable | No. Pts. | % | |
| Recurrence Type | Locoregional | 8 | 27 |
| Distant | 7 | 23 | |
| Locoregional and distant | 12 | 40 | |
| No recurrence | 3 | 10 | |
| Locoregional recurrence sites (n = 20) | Urethra | 10 | 50 |
| Vulva/ Vagina | 8 | 40 | |
| Lymph nodes | 5 | 25 | |
| Pelvis | 2 | 10 | |
| Bladder | 2 | 10 | |
| Distant recurrence sites (n = 19) | Lung | 11 | 58 |
| Liver | 6 | 32 | |
| Peritoneum | 3 | 16 | |
| Lymph nodes | 3 | 16 | |
| Adrenal | 1 | 5 | |
| Treatment for recurrence (n = 27) | Surgery | 13 | 48 |
| Radiotherapy | 7 | 26 | |
| Chemotherapy | 10 | 37 | |
| Targeted therapy | 2 | 7 | |
| Immunotherapy | 13 | 48 |
3.3Genetic alterations of primary urethral melanoma
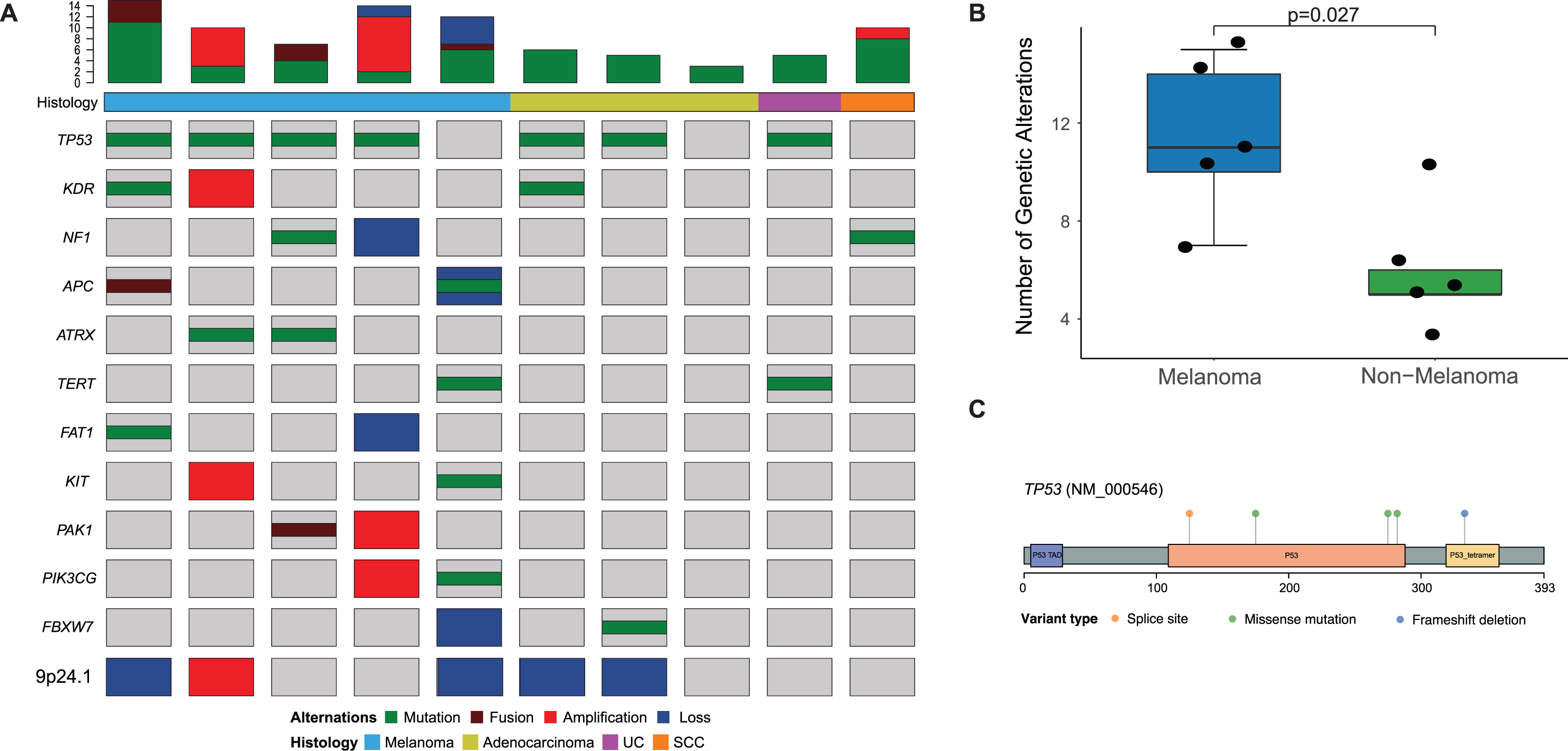
Genomic analysis revealed distinct features in urethral melanomas when compared to other tumors. First, urethral melanomas were contrasted to a set of five non-melanoma primary urethral tumors (Fig. 2a). Urethral melanomas appeared to have more complex genomes than non-melanoma tumors, as noted by a higher frequency of somatic alterations (median 11 vs. 5, p = 0.03, Fig. 2b). This included all mutations, copy-number alterations and fusions identified. Primary urethral melanomas were found to have a relatively high frequency of TP53 mutations (4/5 tumors, or 80%), and all the mutations identified were predicted to be oncogenic (Fig. 2c, Supplementary Table 1).
Fig. 2
(A) Onco-print of common genetic alterations in patients who underwent MSK-IMPACT testing of primary urethral melanomas (n = 5) and primary urethral tumors with a non-melanoma histology (n = 5), the bar-plot represents the total number of genetic alterations seen in each tumor sample; (B) Box-plot comparing total number of genetic alterations in patients with melanoma and non-melanoma urethral tumors; (C) Lollipop plot depicting mutations in the TP53 gene in patients with urethral melanoma (n = 5).
Next, we compared urethral melanomas to their cutaneous and female genital counterparts. Primary urethral melanomas showed a higher frequency of TP53 mutations when compared to cutaneous (80.0% vs. 18.2%, p = 0.006) and vulvar/vaginal melanomas (80.0 vs. 25.0%, p = 0.04). On the other hand, BRAF mutations were notably absent in urethral primaries (0% vs. 46% in cutaneous melanoma, p = 0.02). No significant differences were observed between urethral and cutaneous melanomas in the rates of KIT (18% and 8%, p = 0.4) and NRAS mutations (22% and 23%, p = 0.9). No significant differences were seen in the mutation rates in any of the other genes evaluated (Fig. 3a). In terms of the specific mutations identified in urethral melanomas, KIT mutations were seen in positions L576 and N822, while NRAS mutations were noted in positions G12 and G13, all of which are known hotspots [25]. This finding is in contrast to cutaneous melanomas in which most NRAS mutations occur in the Q61 hotspot [26]. Potentially targetable genomic alterations were identified in 4/5 patients with NGS data, which included: NF1 mutation (L1505*) and deletion, NRAS mutation (G12D), KIT mutation (N822K), ERBB2 mutations (S310Y), MET amplification, NF1 deletion and CDKN2A deletion. Additionally, one sample was found to have 9p24 amplification which contains the locus for PD-L1.
Fig. 3
(A) Bar-plot comparing common genetic mutations among patients who underwent sequencing of primary urethral melanoma (n = 5 IMPACT; n = 8 specific BRAF, KIT and/or NRAS sequencing), primary cutaneous melanoma (n = 143 IMPACT) and primary vulvar and vaginal melanomas (n = 24 IMPACT) * adjusted p-value < 0.05; Box-plots comparing (B) tumor mutation burden score and (C) fraction genome altered between the different tumors.
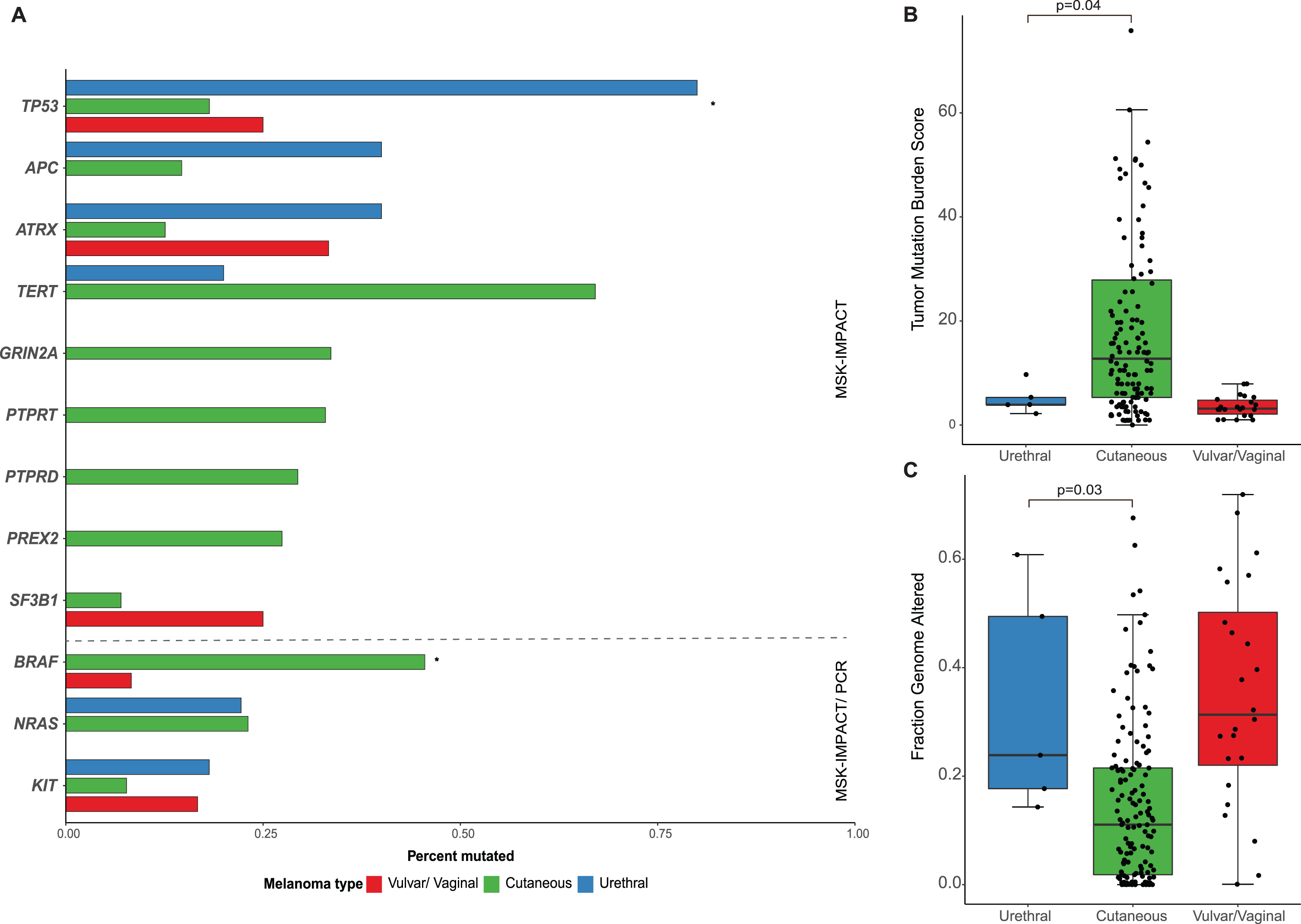
When comparing the overall burden of somatic mutations, urethral melanoma primaries were found to have a lower TMB than cutaneous tumors (median 12.8 and 3.9, respectively, p = 0.04) but comparable levels to vaginal/vulvar tumors (Fig. 3b). The FCNAg was notably lower in cutaneous melanomas compared to urethral (median 0.018 and 0.177, respectively, p = 0.03) or vulvar/vaginal primaries (Fig. 3c). All primary urethral melanomas displayed low or indeterminate microsatellite instability (MSIsensor score < 10).
DISCUSSION
We report our results of a retrospective single-institution analysis summarizing the clinicopathological and molecular characteristics of urethral melanomas. A comparison of this poorly understood entity with other (more common) melanomas provides new insights on the molecular origins of this disease. Specifically, we found a higher frequency of TP53 mutations in urethral melanoma compared to cutaneous and non-urethral mucosal melanomas, all of which were predicted to be oncogenic. In addition, urethral melanomas in our cohort were associated with a lower proportion of BRAF hotspot mutation, lower mutation burden and higher proportion of the genome affected by CNA, consistent with previous reports.
The clinicopathological features of urethral melanomas were previously evaluated in case reports and small series of 11–15 patients [20, 21]. In a systematic review of the literature, which included a total of 150 patients, 60% of reported patients were women, and the median age was 65.5 years [18]. Similarly, in a SEER based study, 84% were women and the average age was 75 years [22]. Most patients presented with symptomatic disease, with a urethral mass being the most common finding (81%) followed by dysuria (26%) and local bleeding (25%). Regional or distant involvement was identified in 41% –44% of patients at diagnosis [18, 22]. Consistent with these reports, in the current series, 94% of patients were women and the median age was 73 years; 65% of patients presented with a symptomatic mass and 42% had hematuria and/or vaginal bleeding. Approximately 40% of patients had concurrent involvement of periurethral tissues at the time of presentation. Nodal involvement and/or distant metastases were apparent in 26% of patients, representing the referral pattern to our center.
Common genetic alterations in cutaneous melanomas involve BRAF which is altered in 52% –59% of patients and RAS genes which are altered in 22% –28% of patients [4, 11, 12]. KIT mutations were apparent in only 1.7% –4% of patients [6, 11]. Mucosal melanomas are genetically distinct from cutaneous melanomas; while only 0% –11% of patients had a BRAF mutation and 5% –24% had RAS mutations, 7% –39% harbored a KIT aberration [3–8, 12, 13, 16]. Additionally, mucosal melanomas showed a low mutational burden with an average of 2.7 mutations per Mb and were less associated with the UVR signature [3]. Several studies evaluated the genetic alterations of genitourinary mucosal melanomas, mostly involving the genital tract, reporting NRAS mutation in 10% –21%, KIT mutations in 4% –32% and BRAF mutation in 0% –8% [7–10, 13, 16]. Mutation frequency in a group of 37 female patients with vulvar, vaginal and urethral melanomas did not differ by primary tumor site, suggesting a similar molecular pathogenesis [16]. However, contrary to these findings, Hou et al. reported BRAF as the most frequently mutated gene among patients with vulvar and vaginal melanomas, altered in 26% of patients compared to 8.3% of non-gynecological mucosal melanomas and KIT mutations occurred in 22% of patients compared to 8.8% of non-gynecological mucosal melanoma, suggesting vulvar/vaginal melanomas represent a distinct subtype that differs from other mucosal melanomas [14].
Genetic alterations of melanomas involving the urinary tract were reported separately in a few publications. van Engen-van Grunsven et. al. reported on five patients with urethral melanoma who underwent Sanger sequencing of relevant exons of KIT, NRAS and BRAF. One patient had an NRAS mutation while KIT and BRAF mutations were absent in all tumors [10]. Cosgarea et al. reported on three patients, one of whom had a TERT promoter mutation while the two others did not have any mutation identified on a targeted panel of 29 known recurrently mutated genes in melanoma [13]. In a recent publication by Zarei et al. mutations in KIT, TP53, NF1 and NRAS were found in 2/7 urethral melanoma patients, and mutations in TERT and BRAF were found in 1/7 patients [16]. The current study is consistent with previous reports in the lack of BRAF mutations. We did not find a significant difference in the rate of NRAS mutations compared to patients with cutaneous melanoma, although the mutations did not involve the common Q61 hotspot [26]. KIT mutations were more prevalent among patients with urethral melanomas compared to patients with cutaneous melanomas (18% vs. 9%); however, this difference was not statistically significant possibly due to the small number of patients included in the cohort. The median TMB of urethral melanomas in this cohort was reported at 3.9, a value consistent with previous publications, and significantly lower than the TMB of cutaneous melanomas [3]. Additionaly, the FCNAg of urethral melanomas was higher than their cutaneous counterparts and comparable to those arising from the vulva/vagina. These results are consistent with the recent report by Zarei et al. [16] which suggested a similar mutational landscape in mucosal melanomas with different tissue origins.
Notably, we found a higher rate (80%) of TP53 mutation in urethral melanomas compared to both cutaneous and vulvar/vaginal tumors. All the mutations identified in this gene were noted to be oncogenic when annotating our results using the OncoKB platform, which is based on the latest biomedical evidence available. These results suggest a different mutational landscape in urethral melanoma, with additional oncogenic mechanisms potentially at play. Prior genomic studies have reported conflicting results regarding the frequency and significance of TP53 mutations in urethral melanoma. Overall, the reported frequency of TP53 mutations in mucosal melanoma has been reported to range from 3.1 to 13.3% [3, 13, 15], and no significant associations have been reported with tumors arising from the genitourinary tract. Notably, a recent report by Newell et al. described six TP53 mutations in a cohort of 45 tumors profiled with exome sequencing, all of which occurred in genitourinary tumors which represented about half of the cohort (29% mutation rate). However, this result was not replicated in their independent cohort of 67 mucosal melanomas where 6 mutations were identified and only one of them was found in genitourinary tumors (mutation rate of 7%) [3].
The optimal treatment of urethral melanomas is not established, and multiple treatment options are currently used [17]. In a review of previously published series surgical procedures were performed in 75% of patients, but only 19% underwent a cystectomy [18]. Importantly, the use of radical surgery did not show a benefit over wide local excision with negative surgical margins [19, 27]. The rate of surgical procedures performed in our series was 81%, and 8% underwent a cystectomy. A single randomized phase II study showed that temozolomide-based chemotherapy and high dose IFN-α-2b were effective and safe as adjuvant therapies for resected mucosal melanoma compared to observation alone [28]. Adjuvant therapies including radiotherapy, chemotherapy and immunotherapy were used in 36% of patients in a summary of previous reports [18]. In the current series, adjuvant treatment was given to 26% of patients who underwent definitive local treatment and is likely underused. Treatment options for patient with unresectable or advanced mucosal melanomas include immunotherapy, targeted therapy and chemotherapy [17]. In retrospective studies, the use of ipilimumab was associated with response rates of 7% –12% and a median OS of 6.4 months [29, 30]. In a pooled analysis of patients with mucosal melanoma from six clinical studies the objective response rates to ipilimumab, nivolumab and a combination of the two were 8.3%, 23.3% and 37.1%, respectively, and median progression-free survival was 2.7, 3 and 5.9 months, respectively, supporting the efficacy and safety of anti–PD-1–based therapy in mucosal melanoma [31]. The infrequent occurrence of BRAF mutations in mucosal melanoma limits the utility of BRAF targeted therapy; however, targeting KIT is a therapeutic option [17]. Phase II trials evaluating the use of imatinib in metastatic mucosal, acral, or chronically sun-damaged melanoma with KIT amplifications and/or mutations showed overall response rates of 44% –50% and a median survival of 12 months [32, 33]. In a retrospective study of 81 patients who received systemic treatment for advanced mucosal melanomas the overall best response rate was 8% –33% for chemotherapy agents, 25% for immune-checkpoint inhibitors and 25% for targeted therapies. Median OS from initiation of first-line treatment was 10.3 months and did not differ by treatment in the first-line setting [34]. CSS rates in our cohort were higher than previously reported, possibly due to the relative low number of patients who presented with metastatic disease. However, there was no change in outcome over the years, despite the more common use of immunotherapy for the treatment of recurrent disease. The low rate of actionable BRAF and KIT mutations, may contribute to the poor outcome of mucosal melanoma when compared to cutaneous melanoma [19]. Furthermore, the low TMB scores we observed in urethral melanomas are possibly associated with decreased response to immunotherapy [35]. Although gene copy-number is not an accurate predictor of gene expression, we did find one urethral melanoma which showed 9p24 amplification, which contains the locus of PD-L1. Previous reports have shown that high expression of PD-L1 can be associated with response to immunotherapy [36, 37], and future studies will need to assess if a similar association is apparent in urethral melanoma.
Our study limitations include its retrospective nature as well as the small sample size and the possibility of a selection bias towards patients with non-metastatic disease, all of which may limit the generalizability of our findings. Additionally, clinical data regarding tumor stage was not available in the database used to compare the genomic landscape of different melanomas. Thus, while all analyzed samples were obtained from primary tumors, we were unable to adjust our findings for disease stage. Nevertheless, the current study adds insight as to the clinical and molecular characteristics of this rare tumor.
In conclusion, our findings show that survival remains poor for patients with urethral melanoma and is unchanged over the time studied. Our findings support a unique mutational landscape of urethral melanoma compared to that of cutaneous melanoma. Future multi-institutional studies with larger cohorts and prospective tissue collection will be required to identify the optimal treatment modality for this rare disease and corroborate the associated molecular findings.
ACKNOWLEDGMENTS
We gratefully acknowledge the members of the Molecular Diagnostics Service in the Department of Pathology.
FUNDING
This work was supported in part by the Sidney Kimmel Center for Prostate and Urologic Cancers. Additional support was provided by the Marie-Josée and Henry R. Kravis Center for Molecular Oncology and the National Cancer Institute through the Cancer Center Support Grant (P30 CA008748).
AUTHORS’ CONTRIBUTIONS
R.M. conceptualized and designed the study, acquired data, analyzed, and interpreted the data, and drafted the manuscript. B.H. acquired data and drafted the manuscript R.G.D. conceptualized and designed the study, drafted the manuscript, and performed statistical analysis. A.S. conceptualized and designed the study and revised the manuscript. N.E.B. acquired data and provided technical support. E.R. conceptualized the study and revised the manuscript. M.M.L., A.N.S., A.G., S.M.D., H.W.H., B.H.B., and G.D. acquired data and revised the manuscript. T.F.D. conceptualized the study, revised the manuscript, and supervised the study.
CONFLICT OF INTEREST
B.H.B. is an Editorial Board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
M.M.L. received research funds from KCI/Acelity, is an ad-hoc speaker for Intuitive Surgical, Inc. and serves in the advisory boards of JnJ/Ethicon and Takeda. A.G. is a consultant for Medtronic. R.M., B.H., R.G.D., A.S., N.E.B., E.R., A.N.S., S.M.D., H.W.H., G.D., T.F.D. declare no conflict of interests.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-211633.
REFERENCES
[1] | Chang AE , Karnell LH , Menck HR , The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade, The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. (1998) ;83: :1664–78. |
[2] | McLaughlin CC , Wu XC , Jemal A , Martin HJ , Roche LM , Chen VW , Incidence of noncutaneous melanomas in the S. Cancer , (2005) ;103: :1000–7. |
[3] | Newell F , Kong Y , Wilmott JS , Johansson PA , Ferguson PM , Cui C , et al., Whole-genome landscape of mucosal melanoma reveals diverse drivers and therapeutic targets, Nature Communications (2019) ;10: :3163. |
[4] | Curtin JA , Fridlyand J , Kageshita T , Patel HN , Busam KJ , Kutzner H , et al., Distinct sets of genetic alterations in melanoma, The New England Journal of Medicine. (2005) ;353: :2135–47. |
[5] | Curtin JA , Busam K , Pinkel D , Bastian BC , Somatic activation of KIT in distinct subtypes of melanoma, Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology (2006) ;24: :4340–6. |
[6] | Beadling C , Jacobson-Dunlop E , Hodi FS , Le C , Warrick A , Patterson J , et al., KIT gene mutations and copy number in melanoma subtypes, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research (2008) ;14: :6821–8. |
[7] | Omholt K , Grafstrom E , Kanter-Lewensohn L , Hansson J , Ragnarsson-Olding BK , KIT pathway alterations in mucosal melanomas of the vulva and other sites, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research (2011) ;17: :3933–42. |
[8] | Abysheva SN , Iyevleva AG , Efimova NV , Mokhina YB , Sabirova FA , Ivantsov AO , et al., KIT mutations in Russian patients with mucosal melanoma, Melanoma Research (2011) ;21: :555–9. |
[9] | Tseng D , Kim J , Warrick A , Nelson D , Pukay M , Beadling C , et al., Oncogenic mutations in melanomas and benign melanocytic nevi of thefemale genital tract, Journal of the American Academy ofDermatology (2014) ;71: :229–36. |
[10] | van Engen-van Grunsven AC , Kusters-Vandevelde HV , De Hullu J , van Duijn LM , Rijntjes J , Bovee JV , et al., NRAS mutations are more prevalent than KIT mutations in melanoma of the female urogenital tract–a study of 24 cases from The Netherlands, Gynecologic Oncology (2014) ;134: :10–4. |
[11] | Cancer Genome Atlas N , Genomic classification of cutaneous melanoma, Cell (2015) ;161: :1681–96. |
[12] | Hayward NK , Wilmott JS , Waddell N , Johansson PA , Field MA , Nones K , et al., Whole-genome landscapes of major melanoma subtypes, Nature (2017) ;545: :175–80. |
[13] | Cosgarea I , Ugurel S , Sucker A , Livingstone E , Zimmer L , Ziemer M , et al., Targeted next generation sequencing of mucosal melanomas identifies frequent NF1 and RAS mutations, Oncotarget (2017) ;8: :40683–92. |
[14] | Hou JY , Baptiste C , Hombalegowda RB , Tergas AI , Feldman R , Jones NL , et al., Vulvar and vaginal melanoma: A unique subclass of mucosal melanoma based on a comprehensive molecular analysis of 51 cases compared with cases of nongynecologic melanoma, Cancer. (2017) ;123: :1333–44. |
[15] | Zhou R , Shi C , Tao W , Li J , Wu J , Han Y , et al., Analysis of Mucosal Melanoma Whole-Genome Landscapes Reveals Clinically Relevant Genomic Aberrations, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research (2019) ;25: :3548–60. |
[16] | Zarei S , Voss JS , Jin L , Jenkins SM , Bryce AH , Erickson LA , et al., Mutational profile in vulvar, vaginal, and urethral melanomas: Review of 37 cases with focus on primary tumor site, International Journal of Gynecological Pathology: Official Journal of the International Society of Gynecological Pathologists (2020) ;39: :587–94. |
[17] | Slade A , Tetzlaff M , Trinh VA , Hwu WJ , Pettaway CA , Primary Urethral Melanoma. In: Pagliaro L, editor. Rare genitourinary tumors. Switzerland: Springer International Publishing; (2016) . pp. 173–90. |
[18] | El-Safadi S , Estel R , Mayser P , Muenstedt K , Primary malignant melanoma of the urethra: A systematic analysis of the current literature, Archives of Gynecology and Obstetrics (2014) ;289: :935–43. |
[19] | Papes D , Altarac S , Melanoma of the female urethra, Medical Oncology (2013) ;30: :329. |
[20] | Oliva E , Quinn TR , Amin MB , Eble JN , Epstein JI , Srigley JR , et al., Primary malignant melanoma of the urethra: A clinicopathologic analysis of 15 cases, The American Journal of Surgical Pathology (2000) ;24: :785–96. |
[21] | DiMarco DS , DiMarco CS , Zincke H , Webb MJ , Keeney GL , Bass S , et al., Outcome of surgical treatment for primary malignant melanoma of the female urethra, The Journal of Urology (2004) ;171: :765–7. |
[22] | Sanchez A , Rodriguez D , Allard CB , Bechis SK , Sullivan RJ , Boeke CE , et al., Primary genitourinary melanoma: Epidemiology and disease-specific survival in a large population-based cohort, Urologic Oncology. (2016) ;34: :166e7–14. |
[23] | Ballantyne AJ , Malignant melanoma of the skin of the head and neck, An analysis of 405 cases. American Journal of Surgery (1970) ;120: :425–31. |
[24] | Cheng DT , Mitchell TN , Zehir A , Shah RH , Benayed R , Syed A , et al., Memorial sloan kettering-integrated mutation profiling of actionablecancer targets (MSK-IMPACT): A hybridization capture-basednext-generation sequencing clinical assay for solid tumor molecularoncology, The Journal of Molecular Diagnostics: JMD (2015) ;17: :251–64. |
[25] | Chakravarty D , Gao J , Phillips SM , Kundra R , Zhang H , Wang J , et al., OncoKB: A precision oncology knowledge base, JCO Precision Oncology (2017) )2017. |
[26] | Chang MT , Asthana S , Gao SP , Lee BH , Chapman JS , Kandoth C , et al., Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity, Nature Biotechnology (2016) ;34: :155–63. |
[27] | Sanchez-Ortiz R , Huang SF , Tamboli P , Prieto VG , Hester G , Pettaway CA , Melanoma of the penis, scrotum and male urethra: A 40-year single institution experience, The Journal of Urology (2005) ;173: :1958–65. |
[28] | Lian B , Si L , Cui C , Chi Z , Sheng X , Mao L , et al., Phase II randomized trial comparing high-dose IFN-alpha2b with temozolomide plus cisplatin as systemic adjuvant therapy for resected mucosal melanoma, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research (2013) ;19: :4488–98. |
[29] | Postow MA , Luke JJ , Bluth MJ , Ramaiya N , Panageas KS , Lawrence DP , et al., Ipilimumab for patients with advanced mucosal melanoma, TheOncologist (2013) ;18: :726–32. |
[30] | Del Vecchio M , Di Guardo L , Ascierto PA , Grimaldi AM , Sileni VC , Pigozzo J , et al., Efficacy and safety of ipilimumab 3mg/kg inpatients with pretreated, metastatic, mucosal melanoma, EuropeanJournal of Cancer (2014) ;50: :121–7. |
[31] | D’Angelo SP , Larkin J , Sosman JA , Lebbe C , Brady B , Neyns B , et al., Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: A pooled analysis, Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology (2017) ;35: :226–35. |
[32] | Hodi FS , Corless CL , Giobbie-Hurder A , Fletcher JA , Zhu M , Marino-Enriquez A , et al., Imatinib for melanomas harboringmutationally activated or amplified KIT arising on mucosal, acral,and chronically sun-damaged skin, Journal of Clinical Oncology:Official Journal of the American Society of Clinical Oncology (2013) ;31: :3182–90. |
[33] | Carvajal RD , Antonescu CR , Wolchok JD , Chapman PB , Roman RA , Teitcher J , et al., KIT as a therapeutic target in metastaticmelanoma, Jama (2011) ;305: :2327–34. |
[34] | Shoushtari AN , Bluth MJ , Goldman DA , Bitas C , Lefkowitz RA , Postow MA , et al., Clinical features and response to systemic therapy in a historical cohort of advanced or unresectable mucosal melanoma, Melanoma Research (2017) ;27: :57–64. |
[35] | Goodman AM , Kato S , Bazhenova L , Patel SP , Frampton GM , Miller V , et al., Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers, Molecular Cancer Therapeutics. (2017) ;16: :2598–608. |
[36] | Yentz S , Lao CD , Immunotherapy for mucosal melanoma, Ann Transl Med (2019) ;7: (Suppl 3):S118. |
[37] | Gupta S , Vanderbilt CM , Cotzia P , Arias Stella JA , Chang JC , Chen Y , et al., JAK2, PD-L1, and PD-L2 (9p241)amplification in metastatic mucosal and cutaneous melanomas withdurable response to immunotherapy., Hum Pathol (2019) ;88: :87–91. |




