The Impact of the COVID-19 Pandemic on Bladder Cancer Care in the Netherlands
Abstract
BACKGROUND:
The COVID-19 pandemic has disrupted regular health care with potential consequences for non-COVID diseases like cancer. To ensure continuity of oncological care, guidelines were temporarily adapted.
OBJECTIVE:
To evaluate the impact of the COVID-19 outbreak on bladder cancer care in the Netherlands.
METHODS:
The number of bladder cancer (BC) diagnoses per month during 2020-2021 was compared to 2018-2019 based on preliminary data from the Netherlands Cancer Registry (NCR). Additionally, detailed data were retrieved from the NCR for the cohort diagnosed between March 1st-May 31st 2020 (first COVID wave) and 2018-2019 (reference cohort). BC diagnoses, changes in age and stage at diagnosis, and time to first-line treatment were compared between both periods. Changes in treatment were evaluated using logistic regression.
RESULTS:
During the first COVID wave (week 9–22), the number of BC diagnoses decreased by 14%, corresponding with approximately 300 diagnoses, but increased again in the second half of 2020. The decline was most pronounced from week 13 onwards in patients≥70 years and patients with non-muscle invasive BC. Patients with muscle-invasive disease were less likely to undergo a radical cystectomy (RC) in week 17–22 (OR = 0.62, 95% CI = 0.40–0.97). Shortly after the start of the outbreak, use of neoadjuvant chemotherapy decreased from 34% to 25% but this (non-significant) effect disappeared at the end of April. During the first wave, 5% more RCs were performed compared to previous years. Time from diagnosis to RC became 6 days shorter. Overall, a 7% reduction in RCs was observed in 2020.
CONCLUSIONS:
The number of BC diagnoses decreased steeply by 14% during the first COVID wave but increased again to pre-COVID levels by the end of 2020 (i.e. 600 diagnoses/month). Treatment-related changes remained limited and followed the adapted guidelines. Surgical volume was not compromised during the first wave. Altogether, the impact of the first COVID-19 outbreak on bladder cancer care in the Netherlands appears to be less pronounced than was reported for other solid tumors, both in the Netherlands and abroad. However, its impact on bladder cancer stage shift and long-term outcomes, as well as later pandemic waves remain so far unexamined.
INTRODUCTION
The COVID-19 pandemic is an ongoing outbreak of a novel coronavirus (Severe Acute Respiratory Syndrome-coronavirus-2, or SARS-CoV-2). The virus has spread rapidly from Wuhan, China, where it was first detected in December 2019, to all over the world in a matter of months [1]. In the Netherlands, the first patient infected with the coronavirus was diagnosed on February 27th 2020 [2]. After that, the number of hospitalized COVID-19 patients has increased rapidly. To prevent further spreading of the coronavirus, a national lockdown was announced on the 23rd of March 2020 (week 13). To accommodate the increase in hospitalized COVID-19 patients, all regular medical care was downscaled, e.g. national screening programs were halted [3].
Preliminary data presented by the Netherlands Comprehensive Cancer Organisation (Integraal Kankercentrum Nederland, IKNL) showed a significant decrease in the number of new cancer diagnoses during the first wave of the COVID-19 pandemic: up to 25% less cancer diagnoses as compared to previous years [4, 5]. For bladder cancer diagnoses, a decrease of almost 30% was observed [6]. Explanatory factors for this decrease involve patients postponing their visit to the general practitioner (GP) in case of complaints or symptoms. Also, many GPs switched to consultation by phone or video in case of non-urgent symptoms, thereby postponing physical examination of the patient, possibly leading to a delayed referral to a hospital in case of any cancer suspicion following from the examination [5]. In the hospitals, reduced capacity and prioritization of care could also have led to a delayed diagnosis.
The COVID-19 pandemic also impacted cancer-related healthcare in other ways. A study by Van de Poll-Franse et al. showed that during the first weeks of the COVID-19 pandemic in the Netherlands, one in three cancer patients received a different form of healthcare, e.g. consultation by video or phone, or adapted, delayed or cancelled treatment [7]. Adapted guidelines and recommendations were formulated by the European Association of Urology (EAU) and Dutch scientific associations [8, 9]. In the Netherlands, it was recommended to defer transurethral resection for low-risk bladder tumors by more than six months, to omit neo-adjuvant chemotherapy prior to radical cystectomy and to postpone systemic chemotherapy, as patients receiving chemotherapy might possibly experience a more severe COVID-19 infection. A radical cystectomy with curative intent was recommended to be performed within three months, as usual.
The exact impact of the COVID-19 pandemic on bladder cancer care is largely unknown, as data so far were incomplete. Therefore, the objective of this study was to evaluate the impact of the COVID-19 pandemic in the Netherlands on the number of bladder cancer diagnoses, age and disease stage at diagnosis, initial treatment and time from diagnosis to initial treatment. Also the effect of the COVID outbreak on surgical capacity in hospitals was evaluated.
METHODS
Patient selection
For this historic cohort study, data from the Netherlands Cancer Registry (NCR), hosted by the Netherlands Comprehensive Cancer Organisation (Integraal Kankercentrum Nederland, IKNL) were used. All patients newly diagnosed with or treated for bladder cancer (International Classification of Diseases for Oncology (ICD-O-3) topography code C67) between January 2018-May 2020 were identified in the NCR. Detailed data on patient characteristics (age at diagnosis, gender, postal code, socioeconomic status (SES), comorbidity), tumor characteristics (disease stage, morphology), and primary treatment characteristics (type and date of treatment) were retrieved from the NCR. Comorbidity was defined according to the 1987 weighted Charlson Comorbidity Index (CCI) score [10]. SES was derived from Statistics Netherlands (CBS) and was based on the patients’ full six-digit postal code at diagnosis. Disease stage was defined according to the 8th edition of the tumor, node and metastasis (TNM) classification [11]. Tumor morphology was based on the ICD-O-3 morphology codes [12].
To evaluate recent effects of the COVID outbreak on the number of bladder cancer diagnoses and surgical volume of radical cystectomies (RC), we derived, in addition to the previously described dataset, preliminary data from bladder cancer cases diagnosed in the period June 2020-July 2021 from the NCR. These data are largely based on data from the Nationwide Histopathology and Cytopathology Data Network and Archive (PALGA) and included only date of diagnosis, gender, topography, morphology, and date of radical cystectomy (if applicable).
Definitions
Patients diagnosed or treated between March 1st-May 31st 2020 are considered the COVID cohort, and March-May 2018/2019 is considered the reference-cohort. Both cohorts were divided into time periods based on COVID-19-related events occurring in 2020: week 9–12, week 13–16 and week 17–22. The reasoning behind these periods is as follows: in week 9, the first Dutch COVID-19 patient was diagnosed. In week 13, the Netherlands went into national lockdown. In week 15, a national call was made to the general public urging people with symptoms to visit a GP, as a strong decline in GP visits was observed [4]. Effects of this call were to be expected from week 17 onwards. Week 2–8 (January-February) are considered the pre-COVID period. Due to the large difference in working days in week 1 of every year, week 1 was excluded.
Age at diagnosis is included in the analyses both as a continuous and categorical variable;<60, 60–70, 70–80 and > 80 years. SES was categorized into low (first and second septile), medium (third, fourth and fifth septile) and high (sixth and seventh septile). CCI score was categorized into a score of 0, 1, 2 or≥3. Disease stage was categorized into non-muscle invasive bladder cancer (NMIBC, Ta/Tis/T1N0M0), muscle-invasive bladder cancer (MIBC, T2-4aN0M0), and metastasized disease (mBC, T4b/N+/M+). For descriptive purposes, NMIBC was further categorized into low risk (LR-NMIBC, Ta) and high risk (HR-NMIBC, Tis/T1). Tumor morphology was categorized into urothelial carcinoma (UC, ICD-O-3 morphology codes 8120-8131) and non-UC (all other ICD-O-3 morphology codes for bladder cancer). Primary treatment for NMIBC consisted of either transurethral resection of the bladder tumor (TURBT) only, TURBT followed by bladder instillations (BCG or chemotherapy), radical cystectomy, or other. Treatment for MIBC and mBC was categorized into upfront radical cystectomy, neo-adjuvant chemotherapy followed by radical cystectomy, radiotherapy, chemoradiotherapy, systemic therapy (chemotherapy or immunotherapy), no treatment, or other.
Statistical analyses concerning bladder cancer diagnosis
The number of new bladder cancer diagnoses over time was calculated per month and compared between 2020/2021 and 2018/2019 (averaged). For a more detailed description of the first COVID-19 wave (week 9–22) and preceding pre-COVID period (week 2–8), we compared the number of bladder cancer diagnoses per week for January-May of 2020 and January-May of 2018/2019 (averaged). The relative change in number of diagnoses in 2020 was assessed, considering the average number of diagnoses per week in 2018/2019 as 100%. To smooth variation, three-week moving averages were used. A correction for working days was applied in case a week consisted of less than five working days due to national holidays. Descriptive analyses were performed to characterize the patient cohort diagnosed before (week 2–8) and during the first COVID wave in 2020 (week 9–22), and the reference cohort 2018/2019 (week 9–22), per time period. Incidence rates per 100.000 person years were calculated for each time period in 2020 and 2018/2019 and week 9–22 in total, and were evaluated stratified by age group and disease stage, using the iri command in STATA. P < 0.05 was considered statistically significant.
Statistical analyses concerning treatment of bladder cancer
For patients diagnosed between week 2–22 of 2020 and 2018/2019, the total number of patients and the average number of patients per week were calculated per treatment modality per time period and for week 9–22 combined in 2020 versus 2018/2019. A correction for the number of working days per week was applied. Logistic regression analyses were performed to evaluate the association between time period and probability of receiving a certain treatment, presented as odds ratios (OR) and 95% confidence intervals (CI). Analyses were performed per type of treatment and per disease stage, adjusted for age at diagnosis.
To evaluate the effect of the COVID-19 outbreak on surgical volume, we analyzed the number of RCs per week in patients treated in 2020, irrespective of their date of diagnosis, versus the average of 2018/2019 which was considered to be 100%, using three-week moving averages. A correction for number of working days was applied.
We also calculated time from diagnosis to start treatment per time period and for week 9–22 combined, per treatment type, in patients treated between week 2–22 of 2020 and 2018/2019.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA) and STATA version 16.1 software (StataCorp, College Station, Texas, USA). According to the Central Committee on Research involving Human Subjects (CCMO), this type of study does not require approval from an ethics committee in the Netherlands. The requirement for informed consent was waived due of the retrospective design of the study. This study was approved by the Netherlands Cancer Registry’s Supervisory Committee (reference number K21.057).
RESULTS
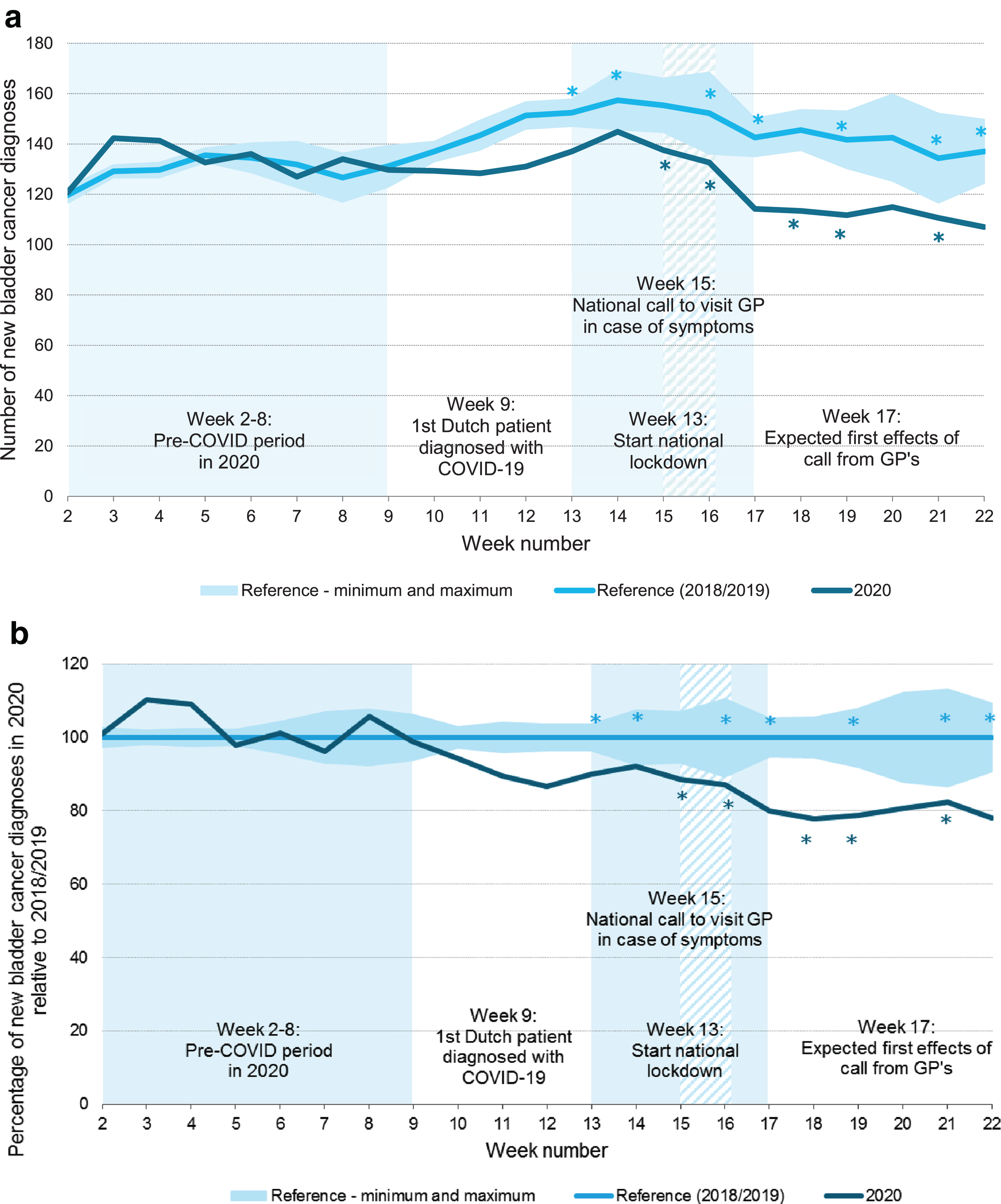
After the start of the COVID-19 outbreak in March 2020, a substantial decrease in bladder cancer diagnoses was observed (Fig. 1). After reaching its lowest point in May 2020, the number of diagnoses increased again and was even slightly higher at the end of the year compared to 2018/2019. The number of diagnoses in 2021 appears to be largely as expected. Focusing on the first COVID-19 wave, i.e. week 9-22, the number of bladder cancer diagnoses decreased with 14% compared to 2018/2019 (Fig. 2a). In absolute numbers, this corresponds with approximately 300 bladder cancer diagnoses. The largest decline in bladder cancer diagnoses was observed in week 19: 22% (Fig. 2b).
Fig. 1
Bladder cancer diagnoses per month in the Netherlands in 2020 and 2021 versus the reference period 2018/2019.
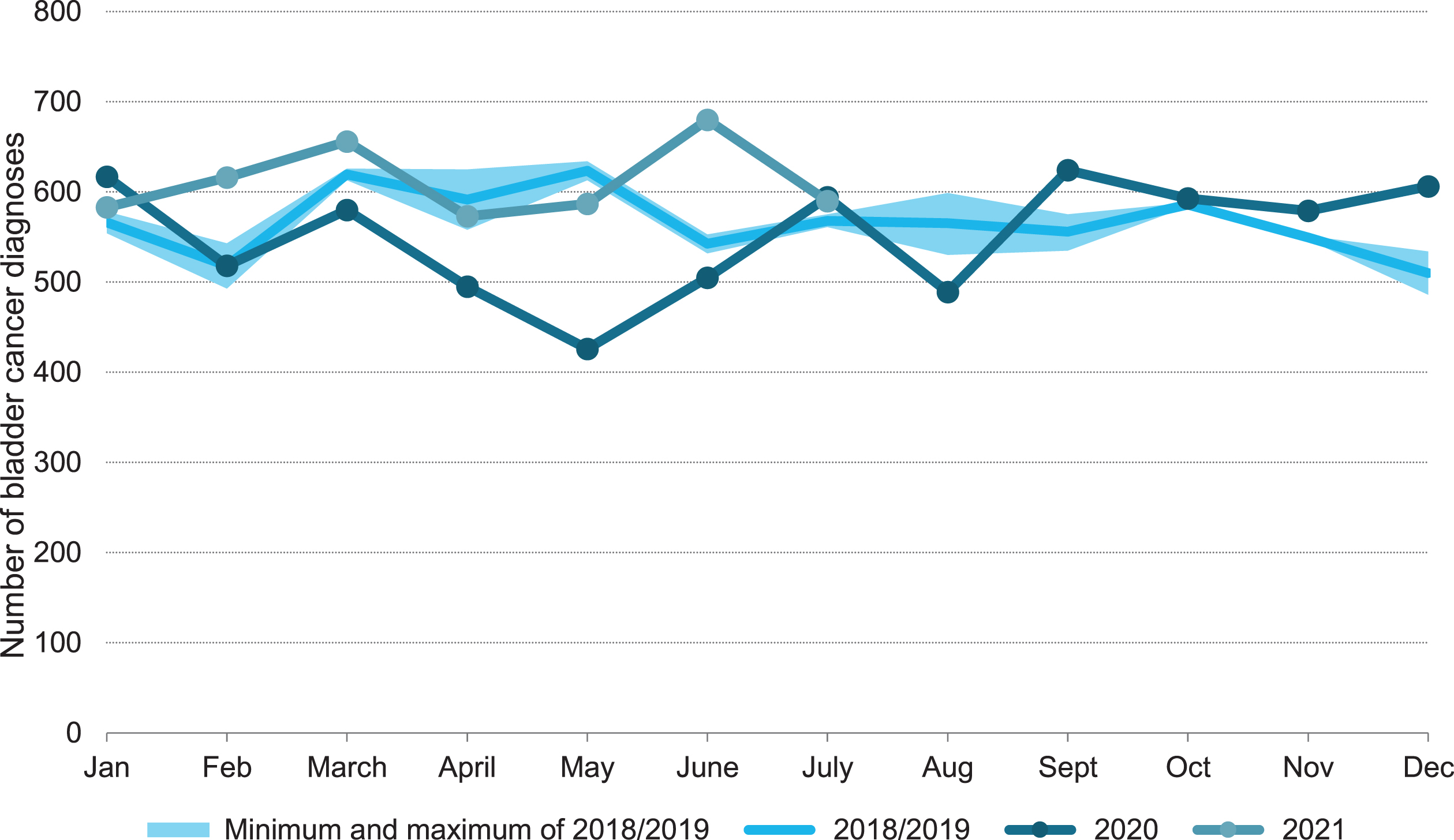
Fig. 2
Three-week moving average of new bladder cancer diagnoses per week in the Netherlands in 2020 versus the reference period 2018/2019 (a) and relative to the reference period 2018/2019 (b), adjusted for the number of working days per week. GP: general practitioner. *a correction for working days was applied since this week does not contain 5 working days due to national holidays.
Patient characteristics of patients diagnosed in 2020 were largely similar to those from the reference cohort 2018/2019 (Table 1). Tumor characteristics were also comparable, except in week 17–22: disease stage was significantly different from the reference period in 2018/2019. Less patients were diagnosed with NMIBC, causing a relative yet no absolute increase in patients with MIBC.
Table 1
Baseline characteristics of patients newly diagnosed* with bladder cancer in January-May 2020 and January-May 2018/2019
| week 9–22 2018/2019 (averaged) (N = 1924) | week 9–22 2020 (N = 1634) | week 2–8 2020 (N = 952) pre-COVID-19 period | week 9–12 2020 (N = 521) 1st Dutch patient diagnosed with COVID-19 | week 13–16 2020 (N = 502) start national lockdown | week 17–22 2020 (N = 611) call to visit GP in case of symptoms | ||||||||||||
| N | (%) | N | (%) | p –value | N | (%) | p -value | N | (%) | p -value | N | (%) | p -value | N | (%) | p -value | |
| Patient characteristics | |||||||||||||||||
| Gender | 0.29 | 0.90 | 0.85 | 0.14 | 0.44 | ||||||||||||
| Male | 1488 | (77.3) | 1285 | (78.6) | 738 | (77.5) | 401 | (77.0) | 403 | (80.3) | 481 | (78.7) | |||||
| Female | 436 | (22.7) | 349 | (21.4) | 214 | (22.5) | 120 | (23.0) | 99 | (19.7) | 130 | (21.3) | |||||
| Age at diagnosis (median, IQR) | 72 | 65.0-79.0 | 73 | 65.0–79.0 | 0.09 | 73 | 65.0–79.0 | 0.15 | 73 | 66.0–79.0 | 0.06 | 72 | 65.0–78.0 | 0.91 | 72 | 66.0–79.0 | 0.24 |
| Age at diagnosis | 0.22 | 0.82 | 0.25 | 0.30 | 0.52 | ||||||||||||
| <60 years | 263 | (13.6) | 194 | (11.9) | 129 | (13.6) | 59 | (11.3) | 61 | (12.2) | 74 | (12.1) | |||||
| 60–70 years | 501 | (26.0) | 429 | (26.3) | 242 | (25.4) | 127 | (24.4) | 149 | (29.7) | 153 | (25.0) | |||||
| 70–80 years | 714 | (37.1) | 642 | (39.3) | 346 | (36.3) | 213 | (40.9) | 185 | (36.9) | 244 | (39.9) | |||||
| ≥80 years | 447 | (23.2) | 369 | (22.6) | 235 | (24.7) | 122 | (23.4) | 107 | (21.3) | 140 | (22.9) | |||||
| Weighted Charlson Comorbidity Index | 0.05 | 0.42 | 0.05 | 0.24 | 0.63 | ||||||||||||
| 0 | 415 | (21.5) | 333 | (20.4) | 186 | (19.5) | 99 | (19.0) | 113 | (22.5) | 121 | (19.8) | |||||
| 1 | 275 | (14.3) | 199 | (12.2) | 131 | (13.8) | 58 | (11.1) | 56 | (11.2) | 85 | (13.9) | |||||
| 2 or more | 270 | (14.0) | 222 | (13.6) | 131 | (13.8) | 73 | (14.0) | 67 | (13.3) | 82 | (13.4) | |||||
| Unknown | 965 | (50.1) | 880 | (53.9) | 504 | (52.9) | 291 | (55.9) | 266 | (53.0) | 323 | (52.9) | |||||
| Socioeconomic status | 0.62** | 0.05 | 0.54 | 0.16** | 0.76 | ||||||||||||
| Low | 588 | (30.6) | 479 | (29.3) | 263 | (27.6) | 149 | (28.6) | 151 | (30.1) | 179 | (29.3) | |||||
| Middle | 709 | (36.9) | 620 | (37.9) | 390 | (41.0) | 191 | (36.7) | 204 | (40.6) | 225 | (36.8) | |||||
| High | 627 | (32.6) | 533 | (32.6) | 299 | (31.4) | 181 | (34.7) | 145 | (28.9) | 207 | (33.9) | |||||
| Unknown | 0 | 0 | 2 | (0.1) | 0 | (0.0) | 0 | (0.0) | 2 | (0.4) | 0 | (0.0) | |||||
| Geographical region | 0.83 | 0.02 | 0.49 | 0.42 | 0.96 | ||||||||||||
| North | 162 | (8.4) | 138 | (8.4) | 61 | (6.4) | 37 | (7.1) | 52 | (10.4) | 49 | (8.0) | |||||
| East | 195 | (10.1) | 181 | (11.1) | 97 | (10.2) | 63 | (12.1) | 51 | (10.2) | 67 | (11.0) | |||||
| Middle | 409 | (21.2) | 353 | (21.6) | 222 | (23.3) | 105 | (20.2) | 115 | (22.9) | 133 | (21.8) | |||||
| South | 452 | (23.5) | 374 | (22.9) | 256 | (26.9) | 117 | (22.5) | 116 | (23.1) | 141 | (23.1) | |||||
| West | 707 | (36.8) | 588 | (36.0) | 316 | (33.2) | 199 | (38.2) | 168 | (33.5) | 221 | (36.2) | |||||
| Tumor characteristics | |||||||||||||||||
| Disease stage (cTNM) | 0.23 | 0.06 | 0.98 | 0.34 | <0.01 | ||||||||||||
| Ta (LR-NMIBC) | 1042 | (54.2) | 838 | (51.3) | 502 | (52.7) | 278 | (53.4) | 252 | (50.2) | 308 | (50.4) | |||||
| Tis, T1 (HR-NMIBC) | 398 | (20.7) | 344 | (21.1) | 209 | (22.0) | 108 | (20.7) | 119 | (23.7) | 117 | (19.1) | |||||
| T2-T4aN0M0 (MIBC) | 325 | (16.9) | 300 | (18.4) | 153 | (16.1) | 91 | (17.5) | 83 | (16.5) | 126 | (20.6) | |||||
| T4b/cN+/cM1 (mBC) | 150 | (7.8) | 139 | (8.5) | 75 | (7.9) | 42 | (8.1) | 46 | (9.2) | 51 | (8.3) | |||||
| Unknown | 10 | (0.5) | 13 | (0.8) | 13 | (1.4) | 2 | (0.4) | 2 | (0.4) | 9 | (1.5) | |||||
| Urothelial carcinoma (UC) | 0.08 | 0.08 | 0.83 | 0.01 | 0.47 | ||||||||||||
| UC | 1882 | (97.8) | 1586 | (97.1) | 930 | (97.7) | 509 | (97.7) | 482 | (96.0) | 595 | (97.4) | |||||
| Non-UC | 42 | (2.2) | 48 | (2.9) | 22 | (2.3) | 12 | (2.3) | 20 | (4.0) | 16 | (2.6) | |||||
GP: general practitioner; IQR: interquartile range; LR: low-risk; HR: high-risk; NMIBC: non-muscle invasive bladder cancer; MIBC: muscle-invasive bladder cancer; mBC: metastasized bladder cancer. *N = 14 patients were diagnosed with a second bladder tumor. In those patients, the first invasive tumor (grade 3) was taken into account for this analysis. **unknown values of SES were not included in calculating the p-value. P-value was calculated using Chi-square for categorical variables and ANOVA for continuous variables, comparing week 9–22 2020 overall and per period with 2018/2019 (week 9–22). P-values in bold are statistically significant (p < 0.05).
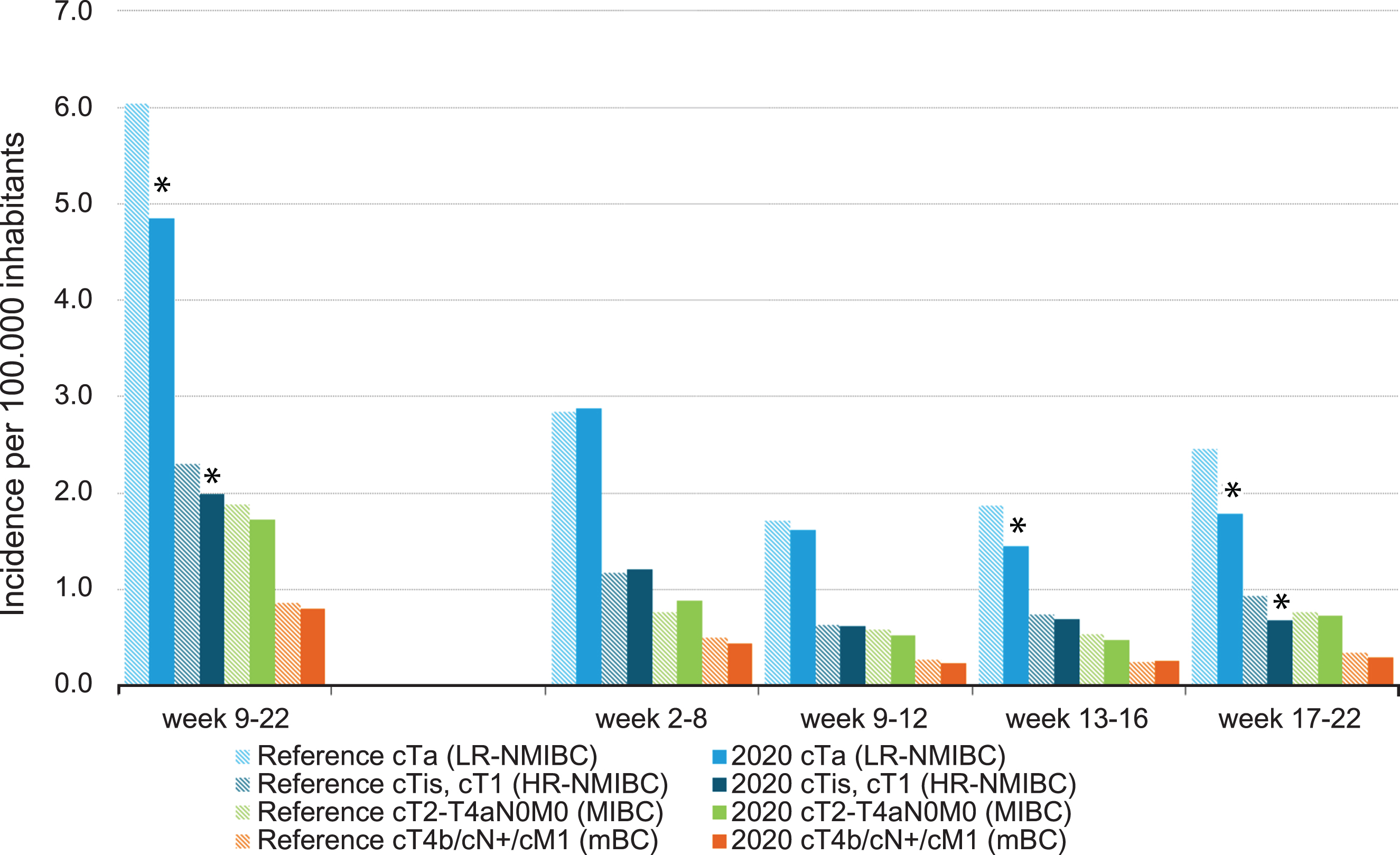
No large differences were found regarding the trend in bladder cancer incidence over time per age group. A statistically significant decline was first observed in the oldest patients, aged≥70 years after week 13 (Fig. 3). After week 17, a significant decline was observed in all age groups. When stratified by disease stage, again, no large differences were found in bladder cancer incidence over time. From week 13 onwards, however, incidence of LR-NMIBC showed a statistically significant decline and after week 17, incidence was also lower for HR-NMIBC (Fig. 4).
Fig. 3
Incidence of bladder cancer per 100,000 inhabitants per period of diagnosis, stratified by age at diagnosis. *In 2020, the incidence is significantly lower compared to the average incidence in 2018/2019 (p < 0.05).
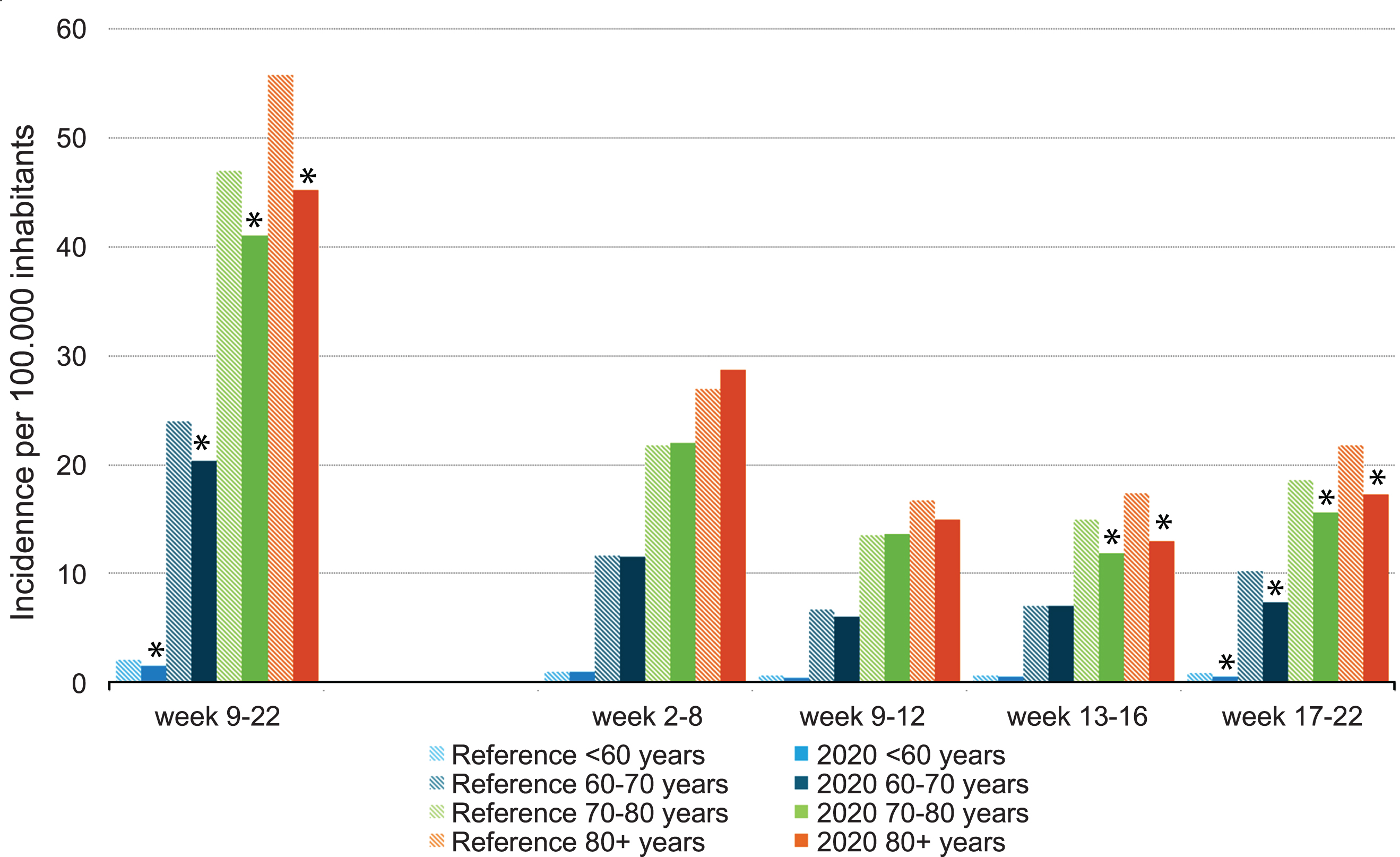
Fig. 4
Incidence of bladder cancer per 100,000 inhabitants per period of diagnosis, stratified by disease stage. LR: low-risk; HR: high-risk; NMIBC: non-muscle invasive bladder cancer; MIBC: muscle-invasive bladder cancer; mBC: metastasized bladder cancer. *In 2020, the incidence is significantly lower compared to the average incidence in 2018/2019 (p < 0.05).
In patients diagnosed with NMIBC, no significant changes in initial treatment were observed during the first COVID-19 wave (Table 2a, Table 3a). Although not statistically significant, patients diagnosed with MIBC seemed to undergo a RC slightly more often in the first weeks after the outbreak (50%) compared to the pre-COVID period (44%) and 2018/2019 (48%). After week 13, the proportion of patients with RC decreased to 38–39%, becoming statistically significant in week 17–22 (OR 0.62, 95% CI = 0.40–0.97). Shortly after the outbreak, i.e. week 9–12, less patients within the surgery group appeared to receive NAC compared to 2018/2019, although this was not significant (25% vs 33%; OR = 0.70, 95% CI = 0.33–1.45). This effect disappeared after week 17; use of NAC increased again to 43%. For patients with metastasized disease, no clear differences in treatment over time were observed, but the number of patients was small (Table 2c, Table 3c).
Table 2a
Initial treatment of patients with bladder cancer per disease stage and per period of diagnosis in 2020 compared to the reference period 2018/2019, corrected for the number of working days
| 2a: NMIBC (Ta/Tis/T1N0M0) | Week 9–22 | Week 2–8 | Week 9–12 | Week 13–16 | Week 17–22 | |||||||||||||||
| Ref | 2020 | Ref | 2020 | Ref | 2020 | Ref | 2020 | Ref | 2020 | |||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Total number of patients (unadjusted) | 1436 | 1178 | 693 | 706 | 403 | 385 | 450 | 371 | 583 | 422 | ||||||||||
| Average per week (adjusted) | 108.3 | 90.0 | 99.0 | 100.9 | 100.8 | 96.3 | 120.9 | 102.0 | 105.3 | 77.4 | ||||||||||
| TURBT only | 28.3 | 26.1 | 23.1 | 25.7 | 24.9 | 25.1 | 28.3 | 28.0 | 26.5 | 26.3 | 27.3 | 28.3 | 29.3 | 24.2 | 25.6 | 25.1 | 28.9 | 27.4 | 18.5 | 23.9 |
| TURBT + instillations | 76.9 | 71.0 | 65.3 | 72.6 | 71.3 | 72.0 | 69.9 | 69.3 | 70.8 | 70.2 | 66.8 | 69.4 | 87.3 | 72.2 | 75.6 | 74.1 | 74.2 | 70.5 | 57.4 | 74.2 |
| Radical cystectomy | 2.2 | 2.0 | 0.8 | 0.8 | 1.7 | 1.7 | 1.6 | 1.6 | 2.3 | 2.2 | 1.0 | 1.0 | 3.0 | 2.4 | 0.6 | 0.5 | 1.6 | 1.5 | 0.7 | 0.9 |
| Other | 0.9 | 0.8 | 0.8 | 0.8 | 1.0 | 1.0 | 1.1 | 1.1 | 1.0 | 1.0 | 1.3 | 1.3 | 1.1 | 0.9 | 0.3 | 0.3 | 0.5 | 0.5 | 0.7 | 0.9 |
NMIBC: Non-Muscle Invasive Bladder Cancer; TURBT: Transurethral Resection of the Bladder Tumor.
Table 2b
Table2b
| 2b: MIBC (T2-T4aN0M0) | Week 9–22 | Week 2–8 | Week 9–12 | Week 13–16 | Week 17–22 | |||||||||||||||
| Ref | 2020 | Ref | 2020 | Ref | 2020 | Ref | 2020 | Ref | 2020 | |||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Total number of patients (unadjusted) | 318 | 292 | 132 | 151 | 97 | 90 | 90 | 79. | 131 | 123 | ||||||||||
| Average per week (adjusted) | 24.0 | 22.3 | 18.9 | 21.6 | 24.3 | 22.5 | 24.2 | 21.7 | 23.7 | 22.6 | ||||||||||
| Radical cystectomy +/- NAC | 11.4 | 47.5 | 9.4 | 42.1 | 8.9 | 46.9 | 9.6 | 44.3 | 12.0 | 49.5 | 11.3 | 50.0 | 11.8 | 48.9 | 8.6 | 39.2 | 10.6 | 45.0 | 8.7 | 38.3 |
| Upfront radical cystectomy | 7.7 | 67.5 | 6.3 | 67.0 | 6.3 | 70.8 | 6.3 | 65.6 | 8.0 | 66.7 | 8.5 | 75.2 | 7.8 | 66.1 | 6.1 | 70.9 | 7.4 | 69.8 | 5.0 | 57.5 |
| NAC + radical cystectomy | 3.7 | 32.5 | 3.1 | 33.0 | 2.6 | 29.2 | 3.3 | 34.4 | 4.0 | 33.3 | 2.8 | 24.8 | 4.0 | 33.9 | 2.5 | 29.1 | 3.2 | 30.2 | 3.7 | 42.5 |
| Radiotherapy | 4.4 | 18.6 | 4.5 | 20.2 | 3.3 | 17.4 | 2.6 | 11.9 | 3.8 | 15.5 | 3.5 | 15.6 | 4.6 | 18.9 | 5.5 | 25.3 | 4.7 | 19.8 | 4.6 | 20.3 |
| Chemoradiotherapy | 2.3 | 9.4 | 2.7 | 12.0 | 1.7 | 9.1 | 3.7 | 17.2 | 1.5 | 6.2 | 2.0 | 8.9 | 1.9 | 7.8 | 3.0 | 13.9 | 2.9 | 12.2 | 2.9 | 13.0 |
| Other | 1.4 | 6.0 | 1.3 | 5.8 | 0.9 | 4.5 | 1.0 | 4.6 | 1.8 | 7.2 | 1.5 | 6.7 | 1.1 | 4.4 | 0.6 | 2.5 | 1.4 | 6.1 | 1.7 | 7.3 |
| No treatment | 4.4 | 18.2 | 4.4 | 19.9 | 4.0 | 21.2 | 4.7 | 21.9 | 5.0 | 20.6 | 4.3 | 18.9 | 4.6 | 18.9 | 4.1 | 19.0 | 3.8 | 16.0 | 4.8 | 21.1 |
MIBC: Muscle Invasive Bladder Cancer; NAC: Neo-Adjuvant chemotherapy.
Table 2c
Table2c
| 2c: mBC (T4b/N+/M+) | Week 9–22 | Week 2–8 | Week 9–12 | Week 13–16 | Week 17–22 | |||||||||||||||
| Ref | 2020 | Ref | 2020 | Ref | 2020 | Ref | 2020 | Ref | 2020 | |||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Total number of patients (unadjusted) | 148 | 134 | 86 | 72 | 45 | 41 | 43 | 42 | 59 | 51 | ||||||||||
| Average per week (adjusted) | 11.2 | 10.2 | 12 | 10.3 | 11 | 10.3 | 12 | 11.6 | 11 | 9.4 | ||||||||||
| Radical cystectomy | 1.8 | 16.2 | 2.3 | 22.4 | 1.7 | 14.0 | 0.9 | 8.3 | 2.3 | 20.0 | 2.3 | 22.0 | 1.9 | 16.3 | 3.0 | 26.2 | 1.4 | 13.6 | 1.8 | 19.6 |
| Systemic therapy (chemotherapy or immunotherapy) | 2.5 | 22.3 | 2.8 | 26.9 | 3.7 | 30 | 2.1 | 21 | 2 | 18 | 3 | 29 | 3.5 | 30 | 3 | 26 | 2.2 | 20 | 2.4 | 26 |
| Other | 1.6 | 14.2 | 1.3 | 12.7 | 1.6 | 13 | 1.7 | 17 | 1.3 | 11 | 1 | 9.8 | 1.9 | 16 | 1.7 | 14 | 1.4 | 14 | 1.3 | 14 |
| No treatment | 5.2 | 46.6 | 3.9 | 38.1 | 5.1 | 42 | 5.6 | 54 | 5.8 | 51 | 4 | 39 | 4 | 35 | 3.9 | 33 | 5.6 | 53 | 3.9 | 41 |
mBC: Metastasized Bladder Cancer.
Table 3a
Logistic regression analyses on the odds of receiving treatment, per type of initial treatment, per disease stage and per period of diagnosis in 2020 compared to the reference period (week 9–22 of 2018/2019, OR = 1.00), adjusted for age at diagnosis
| 3a: NMIBC (Ta/Tis/T1N0M0) | 2020 week 9–22 | 2020 week 2–8 | 2020 week 9–12 | 2020 week 13–16 | 2020 week 17–22 | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| TURBT only | 0.97 | 0.83–1.13 | 1.10 | 0.91–1.32 | 1.09 | 0.86–1.38 | 0.96 | 0.74–1.23 | 0.88 | 0.69–1.12 |
| TURBT + instillations | 1.09 | 0.94–1.27 | 0.93 | 0.77–1.11 | 0.95 | 0.75–1.19 | 1.16 | 0.91–1.49 | 1.19 | 0.94–1.50 |
| Radical cystectomy | 0.42 | 0.21–0.82 | 0.76 | 0.40–1.45 | 0.52 | 0.19–1.43 | 0.26 | 0.06–1.06 | 0.47 | 0.17–1.30 |
| Other | 1.01 | 0.48–2.11 | 1.35 | 0.60–3.02 | 1.51 | 0.57–3.99 | 0.33 | 0.04–2.43 | 1.12 | 0.39–3.25 |
NMIBC: Non-Muscle Invasive Bladder Cancer; OR: Odds Ratio; 95% CI: 95% Confidence Interval; TURBT: Transurethral Resection of the Bladder Tumor.
Table 3b
Table3b
| 3b: MIBC (T2-T4aN0M0) | 2020 week 9–22 | 2020 week 2–8 | 2020 week 9–12 | 2020 week 13–16 | 2020 week 17–22 | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Radical cystectomy +/- NAC | 0.79 | 0.58–1.08 | 0.87 | 0.59–1.29 | 1.09 | 0.67–1.77 | 0.80 | 0.47–1.34 | 0.62 | 0.40–0.97 |
| Upfront radical cystectomy* | 0.96 | 0.60–1.52 | 0.86 | 0.48–1.54 | 1.43 | 0.69–2.99 | 1.06 | 0.46–2.43 | 0.65 | 0.34–1.24 |
| NAC + radical cystectomy* | 1.04 | 0.66–1.66 | 1.16 | 0.65–2.07 | 0.70 | 0.33–1.45 | 0.94 | 0.41–2.16 | 1.54 | 0.81–2.93 |
| Radiotherapy | 1.09 | 0.75–1.58 | 0.57 | 0.33–0.98 | 0.81 | 0.43–1.52 | 1.37 | 0.77–2.43 | 1.13 | 0.68–1.88 |
| Chemoradiotherapy | 1.32 | 0.85–2.05 | 2.00 | 1.22–3.30 | 0.94 | 0.43–2.04 | 1.59 | 0.80–3.18 | 1.44 | 0.80–2.59 |
| Other | 1.05 | 0.58–1.92 | 0.81 | 0.35–1.86 | 1.18 | 0.48–2.91 | 0.48 | 0.11–2.04 | 1.30 | 0.60–2.79 |
| No treatment | 1.12 | 0.76–1.64 | 1.37 | 0.83–2.25 | 1.14 | 0.60–2.19 | 0.91 | 0.46–1.77 | 1.27 | 0.74–2.15 |
MIBC: Muscle Invasive Bladder Cancer; OR: Odds Ratio; 95% CI: 95% Confidence Interval; NAC: Neo-Adjuvant chemotherapy. *The odds of receiving upfront radical cystectomy or neo-adjuvant chemotherapy (followed by radical cystectomy) was calculated within the group of patients undergoing surgery.
Table 3c
Table 3c
| 3c: mBC (T4b/N+/M+) | 2020 week 9–22 | 2020 week 2–8 | 2020 week 9–12 | 2020 week 13–16 | 2020 week 17–22 | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Radical cystectomy | 1.57 | 0.92–2.69 | 0.60 | 0.24–1.51 | 1.65 | 0.71–3.81 | 1.87 | 0.84–4.15 | 1.28 | 0.57–2.85 |
| Systemic therapy (chemotherapy or immunotherapy) | 1.27 | 0.79–2.04 | 1.05 | 0.55–2.00 | 1.49 | 0.71–3.11 | 1.19 | 0.56–2.53 | 1.17 | 0.58–2.34 |
| Other | 0.88 | 0.48–1.61 | 1.16 | 0.57–2.36 | 0.65 | 0.22–1.91 | 1.02 | 0.40–2.57 | 0.97 | 0.41–2.29 |
| No treatment | 0.66 | 0.43–1.03 | 1.09 | 0.63–1.89 | 0.65 | 0.32–1.31 | 0.55 | 0.27–1.12 | 0.78 | 0.41–1.47 |
mBC: Metastasized Bladder Cancer; OR: Odds Ratio; 95% CI: 95% Confidence Interval. P-values in bold are statistically significant (p < 0.05).
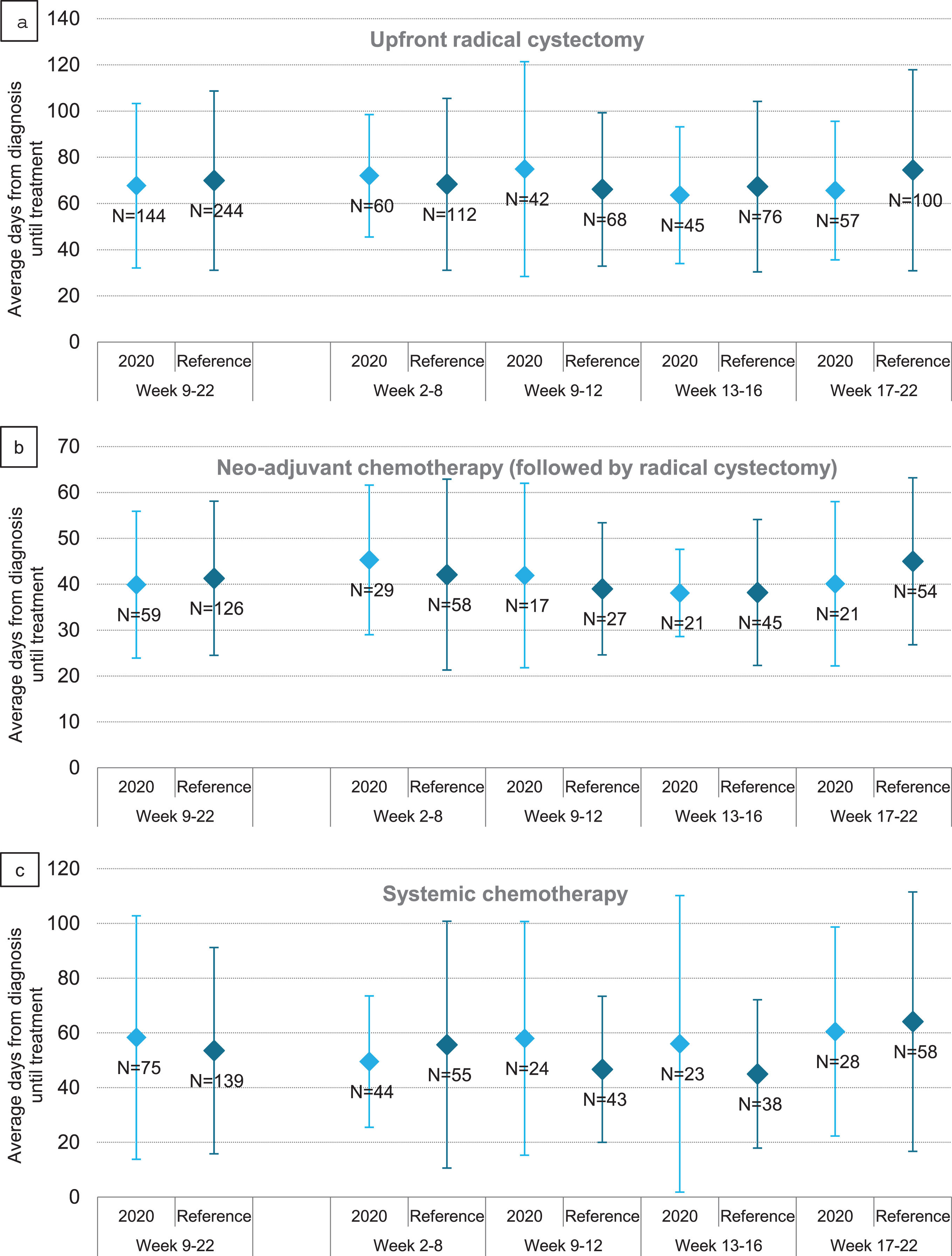
Time to treatment was evaluated per time period in patients treated between week 9–22 of 2020 and 2018/2019, irrespective of date of diagnosis. Time to upfront RC decreased during the first COVID wave, from 72 days before start of the COVID wave (week 2–8) to 66 days at the end of the COVID wave (week 17–22) (Fig. 5a). For patients treated with NAC, time to start NAC was on average 5 days shorter at the end of the COVID wave; i.e. 45 days pre-COVID versus 40 days in week 17–22 (Fig. 5b). For patients treated with systemic chemotherapy, time to systemic chemotherapy showed an increasing trend, i.e. from 50 days pre-COVID to 61 days in week 17–22, although the standard deviations were large (Fig. 5c). Time to radiotherapy appeared not to be affected by the COVID outbreak (Supplementary Figure 1a). Time to chemoradiotherapy became on average 51 days shorter; i.e. 113 days pre-COVID versus 62 days in week 17–22, although the large standard deviations should be taken into account (Supplementary Figure 1b).
Fig. 5
Average time and standard deviation to upfront radical cystectomy (a), neo-adjuvant chemotherapy followed by radical cystectomy (b) and systemic chemotherapy (c) in days of patients with bladder cancer per period of treatment in 2020, compared to the reference period 2018/2019.
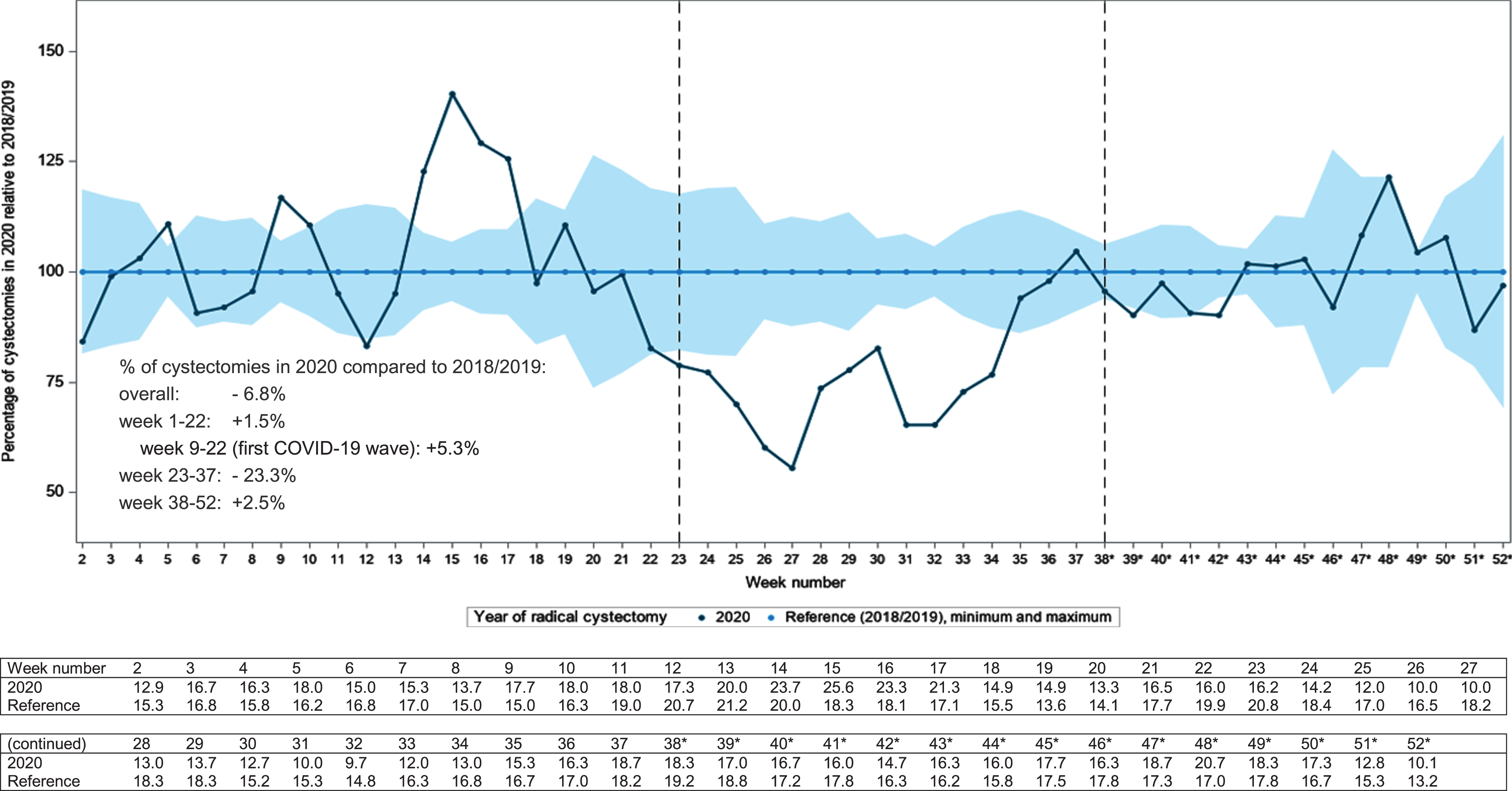
We also evaluated the effect of the COVID-19 outbreak on surgical volume. In Fig. 6, the three-week moving average of the number of radical cystectomies in 2020 relative to 2018/2019 is shown. During the first COVID wave in week 9–22, 5% more RCs were performed as compared to 2018/2019. Between week 22–38, 23% less RCs were performed. And at the end of 2020 (week 38–52) the number of RCs is again slightly higher; +2.5%. Overall, almost 7% less RCs were performed in 2020.
Fig. 6
Three-week moving average of radical cystectomies (for bladder cancer only) performed in the Netherlands** in 2020 relative to the reference period 2018/2019, corrected for the number of working days per week. *The number of cystectomies (for bladder cancer only) from week 38 on is partly based on provisional data. **One hospital was excluded from analysis due to a delay in registration leading to incomplete data.
DISCUSSION
In this population-based cohort study we found that the COVID-19 outbreak largely impacted the number of bladder cancer diagnoses, with the lowest number of new bladder cancer diagnoses in May 2020. After that, the number of diagnoses restored and was slightly higher at the end of the year compared to the reference years 2018/2019. In 2021, no clear effect of COVID-19 on bladder cancer diagnoses is seen. Zooming in on the effect of the first COVID-19 wave, we observed that less patients were diagnosed with NMIBC. The effects on treatment appear to be limited although the proportion of patients undergoing upfront radical cystectomy declined significantly approximately 2 months after the outbreak, after an initial increase. Also, neo-adjuvant chemotherapy prior to radical cystectomy was applied less frequently, but this was only temporary. The surgical capacity of radical cystectomies in the Netherlands was not affected shortly after the COVID-19 outbreak but did drop halfway 2020. In the second part of 2020 the capacity largely recovered resulting in an overall decrease in performed RCs of approximately 7%.
The decrease in bladder cancer diagnoses was most prominent among elderly patients and patients with non-muscle invasive disease. Especially elderly patients might have postponed their visit to the general practitioner or to the hospital due to fear of a COVID-19 infection, which is more severe or even fatal in elderly [13]. Regarding the decrease in NMIBC, transurethral resection may have been postponed in order to preserve surgical capacity in case the urologist suspected low grade disease during cystoscopy. This hypothesis was strengthened by the finding that the number of TURBTs (source: PALGA) in 2020 was lower compared to what could be expected based on the trend observed in previous years (Supplementary Table 1) [14]. Also, an international survey by Rosenzweig et al., evaluating adherence to adapted guidelines during the COVID-19 pandemic, showed that over 65% of TURBTs for Ta-bladder tumors were postponed of which over 40% was postponed more than a month [15].
Excess mortality due to COVID-19, accounting for approximately 9,000 extra deaths in the COVID-19 period week 9–22 2020 (source: Statistics Netherlands [16]), could potentially deprive patients of being diagnosed with bladder cancer. However, we estimated the number of bladder cancer diagnoses that have been missed due to COVID-related mortality between week 9–22 of 2020, using the age and gender-specific incidence of bladder cancer patients in our cohort. This resulted in <15 cases of bladder cancer missed and is, therefore, unlikely to have impacted our results.
We observed minor changes in treatment of patients diagnosed during the first COVID wave in 2020 following the national and international recommendations that were published in order to ensure continuity of uro-oncological care(8): since the first COVID-19 case was confirmed in the Netherlands in week 9, less patients appeared to have received NAC prior to RC. This is in agreement with the adapted guidelines anticipating the risk of immunosuppression related to chemotherapy (i.e. neutropenia), increasing the risk of a more severe COVID infection. Time to start NAC and first-line systemic chemotherapy was prolonged, probably for the same reason. The only change in treatment that could not directly be related to the adapted guidelines was the decreasing number of patients undergoing radical cystectomy near the end of the first wave. It is hypothesized that because of the downscaling of regular care, surgical capacity remained available for oncological care during the first COVID-19 wave. Anticipating potential worsening of the COVID-19 situation, waiting lists for radical cystectomies might have been caught up as much as possible. And, since use of NAC declined, part of the RCs was brought forward in time. After the first COVID wave, i.e. week 23, a large decrease in the number of RCs was observed, which might be an interplay of, among others, the decrease in number of bladder cancer diagnoses, caught up waiting lists for RC and summer holidays. Another potential factor in play here is the increasing use of bladder sparing treatments such as maximal TURBT followed by chemoradiotherapy as an alternative for RC [17]. Near the end of 2020, the number of RCs seemed to restore again although this is based on preliminary data, resulting in an overall small decrease of 7%.
Compared to other malignancies, the effect of the COVID-19 outbreak on bladder cancer care (i.e. number of diagnoses and treatment), appears to be limited [18–20]. One explanation might be the alarming symptoms related to bladder cancer, such as hematuria, urging patients to visit the GP even in times of COVID-19. By comparison, the number of breast cancer diagnoses decreased with 30–36% both in the Netherlands [18] and abroad [19], which can be related to halting national screening programs. Regarding treatment of breast cancer, in the Netherlands, less patients underwent surgery and received hormonal therapy instead [18]. The number of prostate cancer diagnoses also strongly decreased between March-May 2020 compared to previous years, with 25–42%, in the Netherlands as well as abroad [6, 19, 20]. However, treatment was not affected much; a population-based study evaluating the impact of COVID-19 on the number of prostate cancer diagnoses and treatment in Sweden reported that the number of radical prostatectomies remained unchanged during the first COVID-19 wave, despite less prostate cancer diagnoses in that same period [19]. In accordance, Rosenzweig et al. showed in their international survey that 93% of all RCs were performed according to schedule or with a delay of at most <1 month [15]. Our findings indicate continuity of uro-oncological care and surgical capacity, hopefully limiting any adverse effects due to COVID-19 on Dutch bladder cancer care.
To our knowledge, this is the first study to investigate the nationwide impact of COVID-19 on bladder cancer care. We used up-to-date and high-quality data from the nationwide Netherlands Cancer Registry supplemented with data from the nationwide pathology archives (PALGA), providing relevant insights into the effect of the first wave of the COVID-19 pandemic on bladder cancer care. For specific subgroups, the number of bladder cancer cases in the Netherlands is limited. Therefore, the results of our analysis stratified by disease stage and treatment type should be interpreted with caution since the analyses may be underpowered. No elaborate adjustment for potentially relevant factors could be performed since this would cause overfitting and therefore yield unreliable results. Also, subtle fluctuations in, for instance, treatment are probably not detected in our data. Nevertheless, we were able to identify several relevant trends that were to be expected, and the results of this study do not show unexpected large changes in bladder cancer care during the first COVID-19 wave. Our findings are in agreement with clinicians’ experiences in current practice and with the adapted guidelines that were published in order to ensure continuity of (uro)oncological care [8, 9]. Changes in numbers of newly diagnosed bladder cancer patients might cause an underestimation of the observed decrease in bladder cancer diagnoses. However, in recent years the bladder cancer incidence in the Netherlands flattens or even slightly decreases [21] and thus the potential underestimation of our results is estimated to be minimal.
Preliminary data does not indicate a new decrease in diagnoses during the second and third COVID-19 wave. Therefore there is no direct cause for concern or further research. However, since the long-term effects of the first COVID-19 wave are currently unknown, this would be interesting to evaluate. Knowing that less patients were diagnosed with bladder cancer than expected, it is possible that the patients with a delayed diagnosis will present themselves later with a more advanced disease stage. Until July of 2021, we have not yet observed a catch up in number of diagnoses and, therefore, we cannot yet draw any conclusions about a possible stage shift. This should be monitored in the upcoming months. A UK modelling study evaluating the effect of a delay in cancer diagnosis for different cancer types calculated that a 3-month delay in bladder cancer diagnosis resulted in a 14–17% reduction of 10-year survival [22]. A 6-month delay resulted in an even higher survival reduction of 29–35%. The consequences of a delayed diagnosis, potentially resulting in a stage shift, and its subsequent impact on recurrence, progression and mortality rates are currently unknown and future research is recommended to evaluate this. Another consequence from the COVID-19 pandemic is that scientific research involving patients, such as randomized trials, was largely affected. For example, trials suffered from lower accrual rates or were put on hold [23–25]. The implications for both the patients potentially benefitting from this research, as well as for scientific progress, are unknown.
CONCLUSIONS
During the first wave of the COVID-19 pandemic in the Netherlands, the number of bladder cancer diagnoses decreased, mostly for older patients and patients with non-muscle invasive disease. At the end of 2020, the number of bladder cancer diagnoses increased again to pre-COVID levels. Changes in treatment remained limited and followed adapted guidelines. Surgical volume was not compromised during the first wave whereas for the entire year of 2020, 7% less cystectomies were observed. In conclusion, the impact of the first COVID-19 outbreak on bladder cancer care appears to be less pronounced than has been reported in other countries for solid tumors, both in the Netherlands and abroad. It is, however, possible that delayed diagnosis has led to a stage shift, impacting long-term outcomes such as recurrence, progression and survival rates. Also, later pandemic waves remain so far unexamined. Both matters may be addressed in future research.
ACKNOWLEDGMENTS
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice. We thank Désirée van Deukeren from the Isala Hospital for performing the analyses based on PALGA data.
The members of the COVID and Cancer-NL consortium are:
Prof. dr. S. Siesling: Dept of Research and Development, Netherlands Comprehensive Cancer Organisation (IKNL) Utrecht, the Netherlands; Technical Medical Centre, Dept Health Technology and Services Research, University of Twente, Enschede, the Netherlands. Dr. J.C. van Hoeve: Dept of Research and Development, Netherlands Comprehensive Cancer Organisation (IKNL) Utrecht, the Netherlands. Prof. dr. M.A.W. Merkx: Dept of Research and Development, Netherlands Comprehensive Cancer Organisation (IKNL) Utrecht, the Netherlands; dept of Oral and Maxillofacial Surgery, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands. Prof. dr. N.J. de Wit: Dept of General Practice, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht, The Netherlands. Dr. C.W. Helsper: Dept of General Practice, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht, The Netherlands. M.Sc. I. Dingemans: Dutch Federation of Cancer Patient Organisations (NFK), Utrecht, The Netherlands. Prof. dr. I.D. Nagtegaal: Dept of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands, on behalf of the Automated Pathology Archive (PALGA). Drs. R. Saathof: Dutch Hospital Data (DHD), Utrecht, The Netherlands. Prof. dr. C.H. van Gils: Dept of Epidemiology, Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, The Netherlands. Prof. dr. H.C.P.M. van Weert: Dept of General Practice, Amsterdam Public Health, Amsterdam UMC location AMC, Amsterdam, The Netherlands. Prof. dr. M. Verheij: Dept of Radiation Oncology, Radboud University Medical Center, Nijmegen, The Netherlands; on behalf of SONCOS (Dutch Multidisciplinary Oncology Foundation).
The members of the BlaZIB study group are:
Katja K.H. Aben, PhD (PI, Netherlands Comprehensive Cancer Organisation). Lambertus A. Kiemeney, PhD, Prof (PI, Radboud University Medical Centre). J. Alfred Witjes, MD, PhD, Prof (PI, Radboud University Medical Centre). Lisa M.C. van Hoogstraten, MSc (project coordinator, Netherlands Comprehensive Cancer Organisation). Theodora M.R. Ripping, PhD (researcher, Netherlands Comprehensive Cancer Organisation). Joost Boormans, MD, PhD (Erasmus Medical Centre). Catharina A. Goossens-Laan, MD, PhD (Alrijne hospital). Antoine G. van der Heijden, MD, PhD (Radboud University Medical Centre). Michiel S. van der Heijden, MD, PhD (Netherlands Cancer Institute). Sipke Helder (Patient association ‘Leven met blaas- of nierkanker’). Maarten C.C.M. Hulshof, MD, PhD (Amsterdam University Medical Centres, location AMC). Anna M. Leliveld, MD, PhD (University Medical Centre Groningen). Geert J.L.H. van Leenders, MD, PhD (Erasmus Medical Centre). Richard P. Meijer, MD, PhD (University Medical Centre Utrecht). Reindert J.A. van Moorselaar, MD, PhD, Prof (Amsterdam University Medical Centres, location VUmc). Sasja F. Mulder, MD, PhD (Radboud University Medical Centre). Ronald I. Nooter, MD (Franciscus Gasthuis & Vlietland hospital). Juus L. Noteboom, MD, PhD (University Medical Centre Utrecht). Jorg R. Oddens, MD, PhD (Amsterdam University Medical Centres, location AMC). Theo M. de Reijke, MD, PhD (Amsterdam University Medical Centres, location AMC, University of Amsterdam). Bas W.G. van Rhijn, MD, PhD, FEBU (Netherlands Cancer Institute - Antoni van Leeuwenhoek Hospital). Joep G.H. van Roermund, MD, PhD (Maastricht University Medical Centre). Tineke J. Smilde, MD, PhD (Jeroen Bosch Hospital). Guus W.J. Venderbosch (Patient association ‘Leven met blaas- of nierkanker’). Bart P. Wijsman, MD, PhD (Elisabeth-TweeSteden Ziekenhuis).
FUNDING
This study is funded by the Netherlands Organisation for Health Research and Development (ZonMw; 10430022010014). The funding agency had no further role in this study. This study is supported by the BlaZIB study group.
AUTHOR CONTRIBUTIONS
LMCH: conception, data collection, data analysis, data interpretation, writing the article. LAK: conception, data interpretation, writing the article. RPM: conception, data interpretation, writing the article. GJLHL: data interpretation, writing the article. BGLV: data interpretation, writing the article. LI: data interpretation, writing the article. TJS: data interpretation, writing the article. SS: conception, data interpretation, writing the article. JAW: conception, data interpretation, writing the article. KKHA: conception, data collection, data interpretation, writing the article. BlaZIB study group: writing the article. COVID and Cancer-NL consortium: writing the article. LMCH and KKHA had access to the data.
CONFLICTS OF INTEREST
Lambertus A. Kiemeney and J. Alfred Witjes are Editorial Board members of this journal, but were not involved in the peer-review process nor had access to any information regarding its peer-review.
Lisa M.C. van Hoogstraten, Richard P. Meijer, Geert J.L.H. van Leenders, Ben G.L. Vanneste, Luca Incrocci, Tineke J. Smilde, Sabine Siesling, Katja K.H. Aben, the BlaZIB study group and the COVID and Cancer-NL consortium declare that they have no conflict of interest.
SUPPLEMENTARY MATERIAL
[1] The supplementary figure and table are available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-211608.
REFERENCES
[1] | WHO. Timeline: WHO’s COVID-19 response 2021 [25 February 2021]. Available from: https:// https://www.who.int/emergencies/diseases/novel-coronavirus-/interactive-timeline?gclid=EAIaIQobChMI1NejsYbx7gIVVZnVCh0M6wSiEAAYASAAEgJZgfD_BwE. |
[2] | RIVM. Februari 2020: Eerste coronabesmetting in Nederland 2021 [25 February 2021]. Available from: https://www.rijksoverheid.nl/onderwerpen/coronavirustijdlijn/februari-2020-eerste-coronabesmetting-innederland. |
[3] | RIVM. Maart 2020: Maatregelen tegen verspreiding coronavirus, intelligente lockdown 2021 [25 February 2021]. Available from: https://www.rijksoverheid.nl/onderwerpen/coronavirustijdlijn/maart-2020-maatregelen-tegen-verspreidingcoronavirus. |
[4] | IKNL. COVID-19 en kanker (COVID-19 and cancer) 2021 [25 February 2021]. Available from: https://iknl.nl/covid-19. |
[5] | Dinmohamed AG , Visser O , Verhoeven RHA , Louwman MWJ , van Nederveen FH , Willems SM , et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. (2020) ;21: (6):750–1. |
[6] | IKNL. COVID-19 en urogenitale kanker (COVID-19 and genitourinary cancer) 2021 [25 February 2021]. Available from: https://iknl.nl/covid-19/covid-19-en-urogenitale-kanker. |
[7] | van de Poll-Franse LV , de Rooij BH , Horevoorts NJE , May AM , Vink GR , Koopman M , et al. Perceived Care and Well-being of Patients With Cancer and Matched Norm Participants in the COVID-19 Crisis: Results of a Survey of Participants in the Dutch PROFILES Registry. JAMA Oncol. (2021) ;7: (2):279–84. |
[8] | Ribal MJ , Cornford P , Briganti A , Knoll T , Gravas S , Babjuk M , et al. European Association of Urology Guidelines Office Rapid Reaction Group: An Organisation-wide Collaborative Effort to Adapt the European Association of Urology Guidelines Recommendations to the Coronavirus Disease Era. Eur Urol. (2020) ;78: (1):21–8. |
[9] | Wallis CJD , Novara G , Marandino L , Bex A , Kamat AM , Karnes RJ , et al. Risks from Deferring Treatment for Genitourinary Cancers: A Collaborative Review to Aid Triage and Management During the COVID-19 Pandemic. Eur Urol. (2020) ;78: (1):29–42. |
[10] | Charlson ME , Pompei P , Ales KL , MacKenzie CR . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) ;40: (5):373–83. |
[11] | Brierley JD , Gospodarowicz MK , Wittekind C . TNM classification of malignant tumours: John Wiley & Sons; (2017) . |
[12] | Fritz A , Jack A , Percy C , Sobin L , Shanmugarathan S , Whelan S . International classification of diseases for oncology: ICD-O: World Health Organization; 2000. |
[13] | Wu Z , McGoogan JM . Characteristics of and Important Lessons From the Coronavirus Disease (COVID-19) Outbreak in China: Summary of a Report of 72-314 Cases From the Chinese Center for Disease Control and Prevention. Jama. (2020) ;323: (13):1239–42. |
[14] | IKNL. Incidentie blaaskanker (incidence of bladder cancer) [26 June 2021]. Available from: https://iknl.nl/kankersoorten/blaaskanker/registratie/incidentie. |
[15] | Rosenzweig B , Bex A , Dotan ZA , Frydenberg M , Klotz L , Lotan Y , et al. Trends in urologic oncology clinical practice and medical education under COVID-19 pandemic: An international survey of senior clinical and academic urologists. Urol Oncol. (2020) ;38: (12):929.e1–e10. |
[16] | CBS. Jaaroverzicht 2020 (Annual review 2020) [25 February 2021]. Available from: https://www.cbs.nl/nl-nl/achtergrond/2020/53/jaaroverzicht-2020. |
[17] | van Hoogstraten LMC , Witjes JA , Meijer RP , Ripping TM , Kiemeney LA , Aben KKH . Non-metastatic muscle-invasive bladder cancer: the role of age in receiving treatment with curative intent. BJU Int. 2022. |
[18] | Eijkelboom AH , de Munck L , Vrancken Peeters M , Broeders MJM , Strobbe LJA , Bos M , et al. Impact of the COVID-19 pandemic on diagnosis, stage, and initial treatment of breast cancer in the Netherlands: a population-based study. J Hematol Oncol. (2021) ;14: (1):64. |
[19] | Skovlund CW , Friis S , Dehlendorff C , Nilbert MC , Mørch LS . Hidden morbidities: drop in cancer diagnoses during the COVID-19 pandemic in Denmark. Acta Oncol. (2021) ;60: (1):20–3. |
[20] | Fallara G , Sandin F , Styrke J , Carlsson S , Lissbrant IF , Ahlgren J , et al. Prostate cancer diagnosis, staging, and treatment in Sweden during the first phase of the COVID-19 pandemic. Scand J Urol. (2021) ;55: (3):184–91. |
[21] | IKNL. NCR data - bladder cancer 768 incidence 2022 [17 February 2022]. Available from: https://iknl.nl/nkr-cijfers?fs% 7Cepidemiologie_id=526&fs% 7Ctumor_id=365&fs% 7Cregio_id=550&fs% 7Cperiode_id=590% 2C591% 2C592% 2C593% 2C563% 2C562% 2C561&fs% 7Cgeslacht_id=644&fs% 7Cleeftijdsgroep_id=677&fs% 7Cjaren_na_diagnose_id=687&fs% 7Ceenheid_id=703% 2C702% 2C701&cs% 7Ctype=line&cs% 7CxAxis=periode_id&cs% 7Cseries=eenheid_id&ts% 7CrowDimensions=periode_id&ts% 7CcolumnDimensions=eenheid_id&lang% 7Clanguage=en. |
[22] | Sud A , Torr B , Jones ME , Broggio J , Scott S , Loveday C , et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. (2020) ;21: (8):1035–44. |
[23] | Thornton J . Clinical trials suspended in UK to prioritise covid-19 studies and free up staff. Bmj. (2020) ;368: :m1172. |
[24] | Unger JM , Blanke CD , LeBlanc M , Hershman DL . Association of the Coronavirus Disease (COVID-19) Outbreak With Enrollment in Cancer Clinical Trials. JAMA Netw Open. (2020) ;3: (6):e2010651. |
[25] | Upadhaya S , Yu JX , Oliva C , Hooton M , Hodge J , Hubbard-Lucey VM . Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov. (2020) ;19: (6):376–7. |




