Muscle-Invasive Bladder Cancer in Patients with Liver Cirrhosis: A Review of Pertinent Considerations
Abstract
The incidence of liver cirrhosis is increasing worldwide. Patients with cirrhosis are generally at a higher risk of harbouring hepatic and non-hepatic malignancies, including bladder cancer, likely due to the presence of related risk factors such as smoking. Cirrhosis can complicate both the operative and non-surgical management of bladder cancer. For example, cirrhotic patients undergoing abdominal surgery generally demonstrate worse postoperative outcomes, and chemotherapy in patients with cirrhosis often requires dose reduction due to its direct hepatotoxic effects and reduced hepatic clearance. Multiple other considerations in the peri-operative management for cirrhosis patients with muscle-invasive bladder cancer must be taken into account to optimize outcomes in these patients. Unfortunately, the current literature specifically related to the treatment of cirrhotic bladder cancer patients remains sparse. We aim to review the literature on treatment considerations for this patient population with respect to perioperative, surgical, and adjuvant management.
BACKGROUND
Liver cirrhosis is a pathological process in which chronic inflammation of the liver leads to progressive hepatic fibrosis and subsequent loss of liver function [1, 2]. There is evidence to suggest that cirrhosis increases the risk of developing non-hepatic malignancies, including genitourinary, gastrointestinal, hematological, and pulmonary malignancies, though it remains unclear the degree to which this association can be attributed to shared risk factors such as alcohol consumption, smoking, and obesity [3–7]. In cirrhotic patients with non-hepatic cancer, the sequelae of hepatic dysfunction can significantly complicate both surgical and non-surgical treatment [8, 9]. Cirrhosis is known to be associated with poorer outcomes following abdominal surgery, including a higher risk for perioperative complications and a decrease in overall survival [10]. In addition, systemic therapy can have hepatotoxic effects, resulting in greater morbidity and mortality in this patient population [11].
Population-based studies have shown a significant risk of bladder cancer diagnosis amongst liver cirrhosis patients, though the exact nature of this association has yet to be elucidated [5]. Knowing that this population not only harbours a higher incidence of bladder malignancy, but also tends to suffer poorer clinical outcomes, we aim to synthesize the literature surrounding considerations for management of cirrhotic patients with muscle-invasive bladder cancer (MIBC), including those undergoing radical cystectomy for extirpative treatment.
MALIGNANCY IN CIRRHOSIS PATIENTS
Liver cirrhosis is associated with an increased risk of both hepatic and non-hepatic malignancies. By far the most common amongst these is hepatocellular carcinoma (HCC), which is the most common primary liver cancer and sixth most common malignancy worldwide [1, 12, 13]. Cirrhosis also confers a two-fold increase in the likelihood of developing a non-hepatic malignancy, though the precise nature of the association between hepatic dysfunction and extrahepatic carcinogenesis remains poorly understood [6, 7, 14]. Patients with alcoholic cirrhosis are at the highest risk of developing a non-hepatic cancer, suggesting that alcohol consumption may serve as an underlying shared risk factor [7, 14]. However, autoimmune causes of cirrhosis, including primary biliary cholangitis (PBC) and autoimmune hepatitis, have also been shown to be associated with an increased risk of non-hepatic cancers, indicating that liver dysfunction itself may be an independent risk factor in the development of extrahepatic malignancies [15–18]. The mechanisms by which cirrhosis directly promotes carcinogenesis remain unclear but may be related to detrimental changes in hormone metabolism, carcinogen clearance, and immune function [6, 19].
Urologic malignancies, including bladder [5, 20–22], prostate [6], and renal cancers [5], are associated with liver disease, though it remains unclear if there exists a causative relationship between hepatic dysfunction and carcinogenesis in these organs. Smoking is the greatest risk factor for bladder cancer, accounting for up to 50% of cases, and serves as a major risk factor for other malignancies as well [23]. While it is unclear if smoking itself is a direct contributor to the pathogenesis of liver cirrhosis, a significant association has been observed between excessive alcohol consumption and smoking, supporting that smoking may be significantly correlated with the risk of bladder cancer and other extrahepatic malignancies in patients with alcoholic cirrhosis [5, 7, 24]. Additionally, non-alcoholic fatty liver disease (NAFLD) has been shown to be associated with bladder cancer, which may be due to the increased risk of cancer with obesity and the metabolic syndrome, including diabetes mellitus [20]. In contrast, PBC is associated with a 2- to 5-fold greater risk of developing bladder cancer, the highest among any type of cirrhosis, again indicating that hepatic dysfunction may directly influence the risk of developing bladder malignancy [21, 22]. The increased prevalence of renal and prostate cancer in cirrhotic patients is likely due to a combination of factors including age, an altered metabolic profile, and the association between cirrhosis and smoking, which, similar to bladder cancer, is an independent risk factor for development of renal cell carcinoma [5, 6].
ASSESSMENT OF CIRRHOSIS SEVERITY AND NON-HEPATIC ABDOMINAL SURGERY
Liver cirrhosis is associated with a higher risk of perioperative complications and worse long-term postoperative outcomes from non-hepatic abdominal surgery, with this population demonstrating up to ten-times higher perioperative mortality compared to their non-cirrhotic counterparts [9, 25–31]. The increased risk of treatment comes about from significant metabolic and physiologic derangements that can lead to the development of extrahepatic manifestations of impaired liver function including thrombocytopenia, coagulopathy, nutritional deficiencies, hepatic encephalopathy, hepatorenal syndrome, and cirrhotic cardiomyopathy [1, 2, 9, 32–34]. Fibrosis-induced obstruction of blood flow through the hepatic vasculature leads to portal hypertension, which contributes to the development of ascites, splenomegaly and hence thrombocytopenia, electrolyte disturbances, portal vein thrombi, hepatorenal syndrome, and portopulmonary hypertension [33, 35–38]. Another notable consequence of portal hypertension is the development of varices (i.e., aberrant collateral circulatory pathways consisting of abnormally engorged veins) which are prone to hemorrhage [35, 39]. With respect to surgical management, this patient population carries a higher risk of thrombosis, bleeding, surgical site infection (SSI), acute kidney injury (AKI), hypoglycemia, acute respiratory distress syndrome (ARDS), and electrolyte abnormalities in the postoperative period [9, 40]. The presence of hepatorenal syndrome and cardiovascular disease also make cirrhotic patients poor surgical candidates and may necessitate preoperative optimization of renal and cardiac function [40, 41].
While often considered the end point of liver disease, cirrhosis is now recognized as a dynamic process that progresses through four stages based on the development of associated symptoms (Table 1) [42]. While a comprehensive assessment of cirrhosis severity is beyond the purview of this review focused on the bladder cancer population, it is pertinent for providers caring for patients with cirrhosis to acknowledge the sequelae of the disease process. The prognosis of liver cirrhosis is highly dependent on its progression from compensated (stages 1 and 2) to decompensated (stages 3 and 4) cirrhosis, with compensated cirrhosis being associated with far greater survival [43, 44]. However, compensated cirrhosis is associated with an annual rate of progression to decompensated cirrhosis of 11% and a 5-year rate of progression of 35% [44–46]. Decompensated cirrhosis is diagnosed by the presence of sequelae of liver disease, including hyperbilirubinemia, jaundice, ascites, variceal bleeding, and encephalopathy; it is associated with a 5-year mortality of 10–88%, depending on stage and comorbidities [44, 46]. Overall, compensated cirrhosis is associated with a risk of death 4.7-times higher than the general population, compared to 9.7-times in those with decompensated cirrhosis [47]. Several validated scoring systems have been developed to predict the prognosis of patients with liver cirrhosis, with the Child-Turcotte-Pugh (CTP) score and the Model for End-Stage Liver Disease (MELD) score being two of the most popular (Table 2) [48]. In studies examining outcomes following abdominal surgery in cirrhotic patients, the MELD score served as a more accurate prognostic indicator of postoperative survival than the CTP score [25, 49, 50].
Table 1
Stages of liver cirrhosis with 1-year mortality probabilities [45]
| Stage | Definition | Symptoms | Annual Mortality [45] |
| Stage 1 | Compensated | Asymptomatic | 1% |
| Stage 2 | Compensated | Esophageal varices | 3–4% |
| Stage 3 | Decompensated | Ascites ± varices | 20% |
| Stage 4 | Decompensated | GI bleeding ± varices | 57% |
Table 2
The Child-Turcotte-Pugh (CTP) and Model for End-Stage Liver Disease (MELD) scores. The CTP score can also be categorized as Class A (5–6), Class B (7–9), and Class C (10–15) [48, 49, 161]
| Scoring system | Measure | Value | Score |
| CTP Score | Total bilirubin | <34.2umol/L (<2mg/dL) | +1 |
| 34.2–51.3umol/L (2–3mg/dL) | +2 | ||
| >51.3umol/L (>3mg/dL) | +3 | ||
| Albumin | >35g/L (3.5g/dL) | +1 | |
| 28–35g/L (2.8–3.5g/dL) | +2 | ||
| <28g/L (<2.8g/dL) | +3 | ||
| INR | <1.7 | +1 | |
| 1.7–2.2 | +2 | ||
| >2.2 | +3 | ||
| Ascites | Absent | +1 | |
| Slight | +2 | ||
| Moderate or severe | +3 | ||
| Encephalopathy | None | +1 | |
| Grade 1–2 | +2 | ||
| Grade 3–4 | +3 |
MELD scorea,b = 3.78×ln(serum bilirubin in mg/dL) + 11.2 ln(INR) + 9.57×ln(serum creatinine in mg/dL) + 6.43. aThe final MELD score is rounded to the nearest whole number. bPatients who have required dialysis at least twice within the last week are assigned a creatinine value of 4.0 mg/dL.
Postoperative morbidity and mortality is correlated with cirrhosis severity, as determined by the CTP and MELD scores, with the best outcomes being seen in patients with compensated cirrhosis [9, 26, 51, 52]. Studies have shown that the best surgical candidates are patients with a CTP score < 6 (class A) and a MELD score < 10 [9, 52]. Neeff et al. (2013) found that in cirrhotic patients undergoing non-hepatic abdominal surgery, patients with lower CTP and MELD scores demonstrated far better 30-day and 1-year survival compared to those with advanced cirrhosis. Specifically, patients with CTP class A and MELD scores < 10 had a 30-day mortality of approximately 6%, and a 90-day survival of 69–75%. In contrast, patients that were CTP class C or had a MELD score > 20 had a 30-day mortality of 53-82% and 90-day survival rate of 11–22% [53]. Similarly, del Olmo et al. (2003) found that CTP class C patients had a 30-day mortality rate of 55% and twice the number of complications compared to patients that were CTP class A [31].
Regarding the optimal approach for non-hepatic abdominal surgery in cirrhotic patients, a minimally invasive technique has been shown to lead to better outcomes compared to open surgery [9, 40, 52]. While no studies to our knowledge have assessed the efficacy of minimally invasive versus open radical cystectomy in the setting of cirrhosis, multiple different types of laparoscopic abdominal surgery have been shown to be superior to open surgery in cirrhotic patients, including appendectomy, cholecystectomy, radical nephrectomy, splenectomy, and colectomy [54–56]. While this may suggest that a laparoscopic approach would allow for the most favourable outcomes in cirrhotic patients undergoing cystectomy, there are no concrete guidelines regarding surgical treatment of bladder cancer in the setting of liver cirrhosis [57].
PERI-OPERATIVE CONSIDERATIONS IN CIRRHOSIS PATIENTS
General contraindications to elective surgery in cirrhotic patients include acute viral or alcoholic hepatitis, acute renal failure, cardiomyopathy, hypoxemia, and refractory coagulopathy [29]. Impaired liver function can complicate intraoperative anesthetic management due to reduced clearance and metabolism of hepatically metabolized anesthetic agents, limiting the use of certain medications [58, 59]. In addition, anesthesia is associated with decreased blood flow to the liver, likely due to a combination of decreased cardiac output and hepatic arterial vasoconstriction, which may increase the risk of hepatic hypoxia during surgery [60, 61]. Impaired cardiovascular and pulmonary function can cause cirrhotic patients to exhibit poor tolerance of general anesthesia, while electrolyte and fluid disturbances, which can be caused by both ascites and related renal dysfunction, can further complicate perioperative management due to the increased risk of intraoperative hypotension and hypoxemia [59, 62–65]. The presence of ascites and hepatic hydrothorax can also increase the risk of acute intraoperative hypoxemia in cirrhotic patients [29]. In addition, the vitamin K deficiency, thrombocytopenia, splenomegaly, and coagulopathy associated with cirrhosis can contribute to an increased risk of intraoperative bleeding and subsequent hemodynamic instability [65, 66]. As a result, cirrhotic patients may be at an increased risk of requiring intraoperative transfusions. Preoperative management of cirrhotic patients may involve transfusion of platelets and fresh frozen plasma (FFP), vitamin K replenishment, and administration of tranexamic acid (TXA), where appropriately indicated [40, 65, 67].
Another key consideration in this patient population is that ascites can cause physical and technical difficulties in performing the surgery and negatively affect intraoperative management, as fluid imbalance due to ascites can contribute to pulmonary and cardiac dysfunction [40]. Intraabdominal fluid accumulation should be adequately minimized prior to surgery with salt restriction, fluid restriction, and diuresis, though in some cases albumin infusion and paracentesis may be required [65]. Preoperative management of portal hypertension with TIPS is controversial, with a case series by Vinet et al. (2006) suggesting that TIPS prior to elective surgery in patients with portal hypertension provides no postoperative benefit in complication rate or overall survival [68]. In contrast, a systematic review by Lahat et al. (2018) reported that TIPS can mitigate the risk of short-term postoperative complications and may be useful in facilitating planned surgical procedures that would otherwise be less feasible [69]. Due to the absence of high-level evidence and large randomized controlled trials, TIPS is not routinely recommended prior to abdominal surgery in cirrhoticpatients [8].
As a result of both the metabolic consequences of impaired hepatic function as well as the decreased absorption of fats and fat-soluble vitamins due to reduced bile production, nutritional deficiencies are common in cirrhosis [70–72]. Insufficient nutrient intake and metabolism can negatively impact coagulation, immune function, and tissue repair, increasing the risk of postoperative bleeds, anastomotic leaks, and infections [29, 40, 65]. In patients with alcoholic cirrhosis, longstanding alcohol abuse might also exacerbate existing nutritional deficiencies [40, 66, 73]. Cirrhotic patients undergoing surgery may benefit from pre- and post-operative nutritional support [65, 73]. This consideration may be especially important in the cancer patient, given the predisposition to cancer cachexia and related states of compromised body composition including sarcopenia and myosteatosis [74, 75]. Notably, poor nutritional status and altered body composition are clinical features which may be related to the prognostication of patients with solid organ malignancies, as well as response to treatments [76–78]. The additional threat to nutritional and functional status resulting from cirrhosis may serve to exacerbate these deleterious effects.
Postoperatively, patients with cirrhosis require careful monitoring of hepatic and renal function, as well as fluid and electrolyte balances [8]. Hepatic decompensation can result in encephalopathy, ascites, coagulopathy, and hypoglycemia, while associated renal dysfunction can lead to sequelae of AKI [29, 40]. Fluid management is especially challenging in cirrhotic patients, as patients can be simultaneously intravascularly volume deplete while extravascularly volume overloaded, owing to alterations in pressure and flow dynamics in the setting of portal hypertension. In these cases, fluid infusion should be slow and careful, as aggressive fluid resuscitation can lead to pulmonary edema and ascites [29, 41]. Postoperative ascites can increase the risk of spontaneous bacterial peritonitis (SBP), wound dehiscence, surgical site infection, and anastomotic leak. The overall risk of infection is exacerbated by cirrhosis-associated immune dysfunction [29, 65, 79, 80]. Careful wound management and consideration of antibiotic prophylaxis may be required to prevent SBP, SSI, and sepsis [40, 65, 79]. Options for postoperative pain control may also be limited, as many analgesics are metabolized by the liver. While acetaminophen and opioids can usually be offered at reduced doses for pain relief, nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided due to the risk of renal failure [8, 81].
OUTCOMES OF RADICAL CYSTECTOMY IN LIVER CIRRHOSIS PATIENTS
Akin to many other large abdominopelvic surgeries, radical cystectomy in patients with cirrhosis may be associated with both a greater of likelihood of complications in the immediate postoperative period, and poorer outcomes with respect to long-term morbidity and mortality [82, 83]. Data related to this patient population specifically remains sparse, with a paucity of high quality evidence analyzing post-operative outcomes. Much of what is known about surgical and oncologic outcomes in this population is generally applicable to other patient groups with cirrhosis undergoing abdominopelvic procedures. For example, a retrospective review by Djaladat et al. (2014) found that low serum albumin, as seen in cirrhotic patients, was associated with a higher complication rate, decreased postoperative survival, and increased cancer recurrence [82].
Specific to patients with cirrhosis undergoing extirpative therapy, a 2019 case series by Zachos et al. examined three male patients undergoing radical cystectomy and found that, of those patients, two experienced no postoperative complications. The third patient died 11 days after the surgery due to a combination of sepsis, hepatic encephalopathy, and hepatorenal syndrome, which occurred following wound dehiscence/evisceration, as well as SBP secondary to ascites [83]. Notably, the two surviving patients had more favourable Child-Pugh and MELD scores compared to the third patient who died of postoperative complications.
URINARY DIVERSION AT THE TIME OF RADICAL CYSTECTOMY
Patients undergoing radical cystectomy require reconstruction to allow for urinary drainage, either by way of incontinent or continent diversion. While the extirpative aspect of the surgery, including extended pelvic lymph node dissection, is cardinal to its curative intent and should not be modified, the reconstructive aspect deserves special attention amongst this patient population. The majority of patients, in general, will undergo diversion by way of an ileal conduit. Another incontinent diversion mechanism involves the direct attachment of the distal ureters to the skin. A number of continent reconstruction methods have also been developed in attempts to improve quality of life after radical cystectomy, including orthotopic neobladders and cutaneous reservoirs which aim to help patients preserve body image [84].
Of note, reconstruction of a continent diversion is a more technically difficult procedure than formation of an ileal conduit or cutaneous ureterostomies, resulting in a longer operative time. As mentioned, anesthetic time is an important consideration in the cirrhotic patient given decreased tolerability. Other considerations for urinary diversion in this patient population are many-fold. These patients, who are already conferred a significantly increased risk of perioperative morbidity and mortality, are also at an increased risk of bowel and metabolic complications with the use of bowel segments for diversion [85]. Thus, it is imperative to counsel patients with cirrhosis of their increased risk of complications from more involved diversions than the average patient as a result of their altered performance status and physiology.
It is important for the treating surgeon to understand that bowel segments used for urinary diversion provide an absorptive surface for urinary waste products, and that metabolic sequelae from this phenomenon have been shown to occur more frequently in patients with continent diversions as a result of a larger surface area for absorption and a prolonged exposure time [86]. A number of electrolyte and metabolic abnormalities may arise secondary to diversion with a bowel segment, with a number of potential downstream sequelae. In fact, because of altered waste product clearance, liver failure is generally considered a contraindication to continent diversions [87].
Intuitively, one might argue that the shortest possible length of bowel segment would be most favourable to use in cirrhotic patients, and that an incontinent diversion where bowel is used may minimize the burden of potential complications compared to a continent one. Similarly, these patients may benefit instead from the use of cutaneous ureterostomies so as to avoid significant metabolic shifts and mitigate the risk of serious acute illnesses such as hepatic encephalopathy (HE), and to significantly shorten operative time.
HEPATIC ENCEPHALOPATHY
Hepatic encephalopathy represents a known, common complication in patients with hepatic dysfunction. The condition spans a spectrum of neuropsychiatric signs and symptoms which may appear as subtle fluctuations in cognition to more severe presentations such as coma. The proposed underlying pathophysiologic changes leading to HE are related to a reduction in the liver’s ability to remove nitrogenous waste compounds. The buildup of these substrates, namely ammonia and glutamine, is related to adverse downstream consequences, such as a intracellular edema of astrocytes and heightened sensitization of neural tissue to inflammatory cytokines [88]. HE is a particularly important consideration in the postoperative period for the clinician caring for cirrhosis patients. In addition to the numerous conditions predisposing patients to HE in the perioperative period (e.g., constipation, medications, infection, renal failure), patients who undergo urinary diversion along with cystectomy are at a unique risk of severe metabolic disturbances [82, 89]. Patients with urinary diversion utilizing bowel segments are at significant risk of metabolic acidosis and hyperammonemia, the latter of which may lead to hyperammonemic encephalopathy in cirrhosis patients [90–92].
Patients with HE should be evaluated for underlying risk factors such as infection, offending pharmacotherapeutics, or metabolic disturbances. In those with urinary diversion, the presence of urinary tract obstruction should also be evaluated. Management of patients with HE includes identifying and treating any precipitants, increasing ammonia excretion (i.e., gut excretion secondary to nonabsorbable disaccharide use), nutritional optimization, and consideration of antimicrobial therapy [88].
HEPATORENAL SYNDROME
Acute kidney injury (AKI) is recognized as a severe complication of advanced cirrhosis, and is similarly known to be a common complication amongst patients undergoing major abdominal sur-gery, including radical cystectomy [93–95]. Hepatorenal syndrome (HRS) in particular, which falls under the larger umbrella of etiologies contributing to AKI, results from decreased renal blood flow in patients with advanced cirrhosis. In brief, inadequate renal perfusion resulting from altered systemic hemodynamics in combination with renal arterial vasoconstriction, inflammation, and microvascular changes contribute to acute, and sometimes chronic renal failure. Even with current established management options for HRS (e.g., vasoconstrictive agents combined with albumin administration), the condition is associated with a high 90-day mortality in the absence of liver transplantation [93, 96].
The clinical importance of HRS is apparent when evaluating the overall risk of AKI from additional causes in patients undergoing cystectomy (e.g., multifactorial acute tubular necrosis, post-renal etiology related to urinary diversion) and the increased risk of morbidity and mortality conferred by renal failure. One large series identified an early postoperative AKI rate of 11% amongst a consecutive cohort of patients undergoing radical cystectomy and urinary diversion, with intraoperative crystalloid administration and positive fluid balance identified as independent predictors of renal failure (possibly by way of a renal compartment syndrome and decreased tissue oxygenation) [97]. These concerns are further exacerbated in the setting of altered splanchnic circulation and renal hemodynamics, as seen in those with decompensated cirrhosis at risk of HRS. Careful fluid status and electrolyte monitoring is important in the perioperative care of cirrhosis patients, and nephrotoxic medications should be avoided to mitigate the risk of AKI. Additionally, albumin should be considered for volume repletion in this patient population [92].
STOMAL VARICES IN CIRRHOSIS PATIENTS
One complication of portal hypertension is varices (i.e., abnormally dilated and friable blood vessels in the gastrointestinal mucosa prone to hemorrhage), especially in the setting of cirrhosis-associated coagulopathy [35, 39]. While the most common site of variceal formation is the gastroesophageal junction, ectopic varices can form anywhere along the gastrointestinal tract; in patients with stomas, ectopic varices can also form at the stoma site [35, 98, 99]. Unlike bleeding from gastroesophageal varices, ectopic variceal bleeding is rather uncommon, only accounting for up to 5% of all variceal bleeding [100]. A 1991 case series by Fucini et al. found that up to 17 of 62 patients (27%) with both cirrhosis and a permanent stoma developed stomal variceal bleeding [99]. However, the mortality rate of stomal variceal hemorrhage remains low at 3–4% compared to that of gastroesophageal variceal hemorrhage, which can be up to 35% [100, 101]. Management of bleeding stomal varices can involve local hemostatic control, pharmacotherapeutic prophylaxis, and surgical treatment [100]. Variceal ligation, embolization, and sclerotherapy can be utilized to obtain localized hemostasis and obliterate bleeding stomal varices [102–104]. Pharmacologic options for initial control of stomal variceal bleeding and prophylaxis include beta blockers, octreotide, and other vasodilators [100, 102]. Other options for the management of stomal variceal hemorrhage include reconstruction of the stoma and treatment of the underlying portal hypertension with TIPS to prevent recurrence of bleeding [100, 105, 106].
In our review of the literature, we identified 21 English-language case reports describing bleeding stomal varices as a complication of cystectomy with urinary diversion in patients with cirrhosis; the details of these case reports are summarized in Table 3 [107–127]. In every case, the initial presentation of stomal variceal bleeding in these patients was frank hematuria from the site of the urinary diversion. The time between the cystectomy and the first episode of stomal variceal bleeding ranged from immediately following the surgery to 15-16 years, averaging 63±12 (mean±SE) months. Only one case report described a patient being treated with pharmacotherapy alone without any recurrence of bleeding or need for additional therapies [112]. Eight case reports described treatment consisting solely of local control of variceal bleeding with suture ligation,sclerotherapy, or embolization of varices [108, 110, 113, 117, 119, 121, 123, 125]. Of these cases, embolization and sclerotherapy were sufficient to prevent recurrence of variceal bleeding, while ligation alone was associated with recurrence of bleeding [108, 110]. Six case reports described the use of TIPS to reduce portal hypertension and did not report any recurrence of bleeding, though follow up was limited [114, 116, 120, 122, 124, 127]. Finally, four case reports described variceal bleeding being treated with revision of the ileal conduit, with only two of these cases reporting no recurrence of variceal bleeding [107, 109, 111, 115].
Table 3
Overview of case reports of patients with cirrhosis presenting with stomal varices after radical cystectomy. IC: ileal conduit; HCC: hepatocellular carcinoma; TIPS: transjugular intrahepatic portal shunt
| Case report | Age | Sex | Procedure | Time of cirrhosis diagnosis relative to procedure | Etiology of cirrhosis | Time of presentation with variceal bleeding after procedure | Treatment | Bleeding recurrence |
| Foulkes et al. (1975) [107] | 72 | M | Cystectomy with IC | Prior to presentation | Unspecified | 2 years | Revision of IC | No |
| Firlit et al. (1978) [108] | 59 | M | Cystectomy with IC | 21 months after cystectomy | Alcohol | 35 months | Ligation | Yes |
| Hollands (1982) [109] | 59 | M | Cystectomy with IC | At time of presentation | Alcohol | 55 months | Ligation; revision of IC | Yes |
| Thomas et al. (1992) [110] | 70 | M | Cystoprostatectomy with IC | Prior to presentation | Alcohol | 6 years | Ligation | Yes |
| Zimmerman et al. (1994) [111] | 74 | M | Cystoprostatectomy with IC, radiation therapy | 12 years after cystoprostatectomy | Cryptogenic | 4 years | Ligation; revision of IC; TIPS | Yes |
| Sundaram et al. (1997) [112] | 76 | M | Cystoprostatectomy with IC | Prior to presentation | Alcohol | 15–16 years | Propranolol | No |
| Lashley et al. (1997) [113] | 52 | M | Cystoprostatectomy with IC | Never formally diagnosed | Alcohol | 7 years | Embolization | No |
| Carrafiello et al. (2007) [114] | 71 | M | Cystoprostatectomy with IC | Prior to presentation | Alcohol, HCC | 13 years | Ligation; TIPS | No |
| Tu et al. (2008) [115] | 76 | M | Cystoprostatectomy with IC | 26 months after cystectomy | Hepatitis B | 4 years | Revision of IC; ligation | No |
| Kang et al. (2009) [116] | 63 | M | Cystectomy with IC | 6 months after cystectomy | Alcohol | 22 months | TIPS; embolization | No |
| Naidu et al. (2009) [117] | 68 | M | Cystectomy with IC | 2 years after cystectomy | Unspecified | 2 years | Embolization | No |
| Yao et al. (2013) [118] | 70 | F | Cystectomy with IC | Prior to presentation | Drug-induced | 2 years | Octreotide; ligation; embolization | No |
| Tan (2014) [119] | 68 | M | Cystectomy with IC | At time of presentation | Alcohol | 2 months | Sclerotherapy | No |
| Dal Moro (2014) [120] | 60 | M | Cystoprostatectomy with IC | 2 years after cystoprostatectomy | Alcohol, HCC | 16 years | Beta blockers; TIPS | No |
| Staubli et al. (2015) [121] | 72 | M | Cystoprostatectomy with IC | At time of presentation | Unspecified | 3 years | Embolization | No |
| Trasancos-Escura et al. (2015) [122] | 54 | M | Cystoprostatectomy with IC | 2 years after cystoprostatectomy | Alcohol | Immediately | Ligation; propranolol; TIPS | No |
| Lee et al. (2016) [123] | 74 | M | Cystectomy with IC | At time of presentation | Unspecified | 2 years | Ligation | Unspecified |
| Atwal et al. (2016) [124] | 61 | M | Cystoprostatectomy with ileocolic neobladder | Prior to presentation | Cryptogenic | 10 years | Octreotide; TIPS; embolization | No |
| Onishi et al. (2018) [125] | 77 | F | Cystectomy with IC | Prior to presentation | Hepatitis B | 2 years | Embolization | No |
| Chittajallu et al. (2019) [126] | 45 | M | Cystoprostatectomy with IC, sigmoidectomy | Prior to presentation | Hepatitis C, alcohol | Unspecified | TIPS | No |
| Gowda et al. (2020) [127] | 76 | M | Cystoprostatectomy with IC | At time of presentation | Hepatitis C, alcohol | 7 years | Octreotide; TIPS | No |
Overall, 16 cases reports (76%) demonstrated no recurrence of variceal bleeding following treatment of the varices. The relative paucity of information on stomal variceal hemorrhage in patients with cirrhosis and urinary diversion following cystectomy indicates that this complication may be relatively rare, especially compared to variceal bleeding from colostomies or ileostomies [99, 128].
CHEMOTHERAPY IN PATIENTS WITH CIRRHOSIS
Chemotherapy can be difficult to manage in patients with cirrhosis, due to both the direct hepatotoxic effects of chemotherapeutic agents and the non-hepatic toxicity related to impaired hepatic metabolism and clearance of drugs. A wide variety of cytotoxic chemotherapeutic agents have hepato-toxic properties and are associated with a number of liver pathologies that tend to develop in a dose-independent idiosyncratic manner, including sinusoidal obstructive syndrome, pseudocirrhosis, ductal fibrosis, peliosis hepatitis, steatosis, acute hepatitis, hepatic necrosis, and fulminant hepatic failure [129–131]. The presence of hepatic metastases can further exacerbate management, with secondary tumours accelerating the rate of liver failure and negatively impacting prognosis [132, 133].
In patients with liver disease undergoing chemo-therapy, metabolism and elimination of chemotherapeutic drugs tends to remain relatively preserved until cirrhosis progresses to a decompensated state, at which point the inability of the liver to effectively eliminate these cytotoxic agents can result in extrahepatic accumulation and systemic toxicity [134]. Pinter et al. (2016) have suggested that cirrhotic patients with non-hepatic malignancies should be treated with chemotherapy if their life expectancy, as determined by their cancer-specific tumour-related prognosis, is 3 months or greater [11]. A decision-making algorithm developed by Cabibbo et al. (2012) for patients with non-hepatic cancer and liver cirrhosis proposed that chemotherapy should only be considered in patients with compensated cirrhosis with a CTP score of≤7 points. The authors suggest individuals with decompensated cirrhosis should instead receive supportive treatment, but that cytoreductive surgery can be considered for intracranial and mediastinal lesions [135]. In many cases, dose reduction and close monitoring of liver function may be necessary in patients with concomitant liver failure [11]. Recommendations for dose modification in the setting of liver failure exist for a wide variety of anticancer agents, either outlined by the developers of the drug or described in various studies [30, 31, 40, 136]. However, due to the lack of clear guidelines, most physicians are left to manage cirrhotic patients’ chemotherapeutic regimens based on their own clinical judgement and expertise [11, 137].
In addition to its direct negative impact on liver function, chemotherapy can also exacerbate the severity of sequelae of cirrhosis and portal hypertension. For example, chemotherapy-induced myelosuppression can worsen cytopenias caused by splenic sequestration of blood cells, contributing further to known complications of cirrhosis including coagulopathy, anemia, and immune compromise [138, 139]. Additionally, the hypercoagulable state associated with both cancer and chemotherapy can increase the risk of portal vein thrombosis due to slowed blood flow in the portal vein [140, 141]. Management of portal hypertension prior to initiating chemotherapy may help prevent these complications [11].
Current guidelines for chemotherapeutic treatment of both non-metastatic MIBC and metastatic bladder cancer are focused on cisplatin-based chemotherapy, though they vary slightly between organizations. The American Urological Association (AUA), Canadian Urological Association (CUA), and European Association of Urology (EAU) recommend neoadjuvant chemotherapy (NAC) prior to radical cystectomy, with a regimen consisting of gemcitabine and cisplatin (GC) or methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) [142–144]. Patients with pT3/4 or pN + disease who have not received NAC may benefit from adjuvant platinum-based chemotherapy, though the evidence for adjuvant chemotherapy in non-metastatic MIBC remains unclear. Chemotherapy with MVAC or GC is considered first-line systemic therapy for metastatic bladder cancer, based on guidelines from the CUA, EAU, American Society of Clinical Oncology (ASCO), and National Comprehensive Cancer Network (NCCN) [142–146]. Gemcitabine, methotrexate, vinblastine, and doxorubicin are all hepatically cleared and may require dose reduction in cirrhotic patients; in contrast, cisplatin has low hepatotoxicity, being primarily excreted via the kidneys, and can be administered in standard doses in the setting of liver failure [131, 147]. Criteria for dose reduction in chemotherapy adopted from Hendraya et al. are outlined in Table 4 [147].
Table 4
Recommended dose reduction for chemotherapeutic agents used in the treatment of bladder cancer. AST: aspartate aminotransferase; ULN: upper limit of normal. Recommendations adapted from Hendrayana et al. [147]
| Agent | Criteria | Adjustment |
| Gemcitabine | Elevated total bilirubin | 80% of dose, increase if tolerated |
| Cisplatin | No dose adjustment required | N/A |
| Methotrexate | Total bilirubin 3.1–5.0mg/dL OR AST > 180U/L | 75% of full dose |
| Total bilirubin > 5.0mg/dL | Contraindicated | |
| Vinblastine | Total bilirubin 1.5–3.0mg/dL OR AST 60–180U/L | 50% of full dose |
| Total bilirubin > 3.1mg/dL OR AST > 180U/L | Contraindicated | |
| Doxorubicin | Total bilirubin 1.5–3.0mg/dL OR AST 60–180U/L | 50% of full dose |
| Total bilirubin 3.1–5.0mg/dL OR AST > 180U/L | 25% of full dose | |
| Total bilirubin > 5.0mg/dL | Contraindicated |
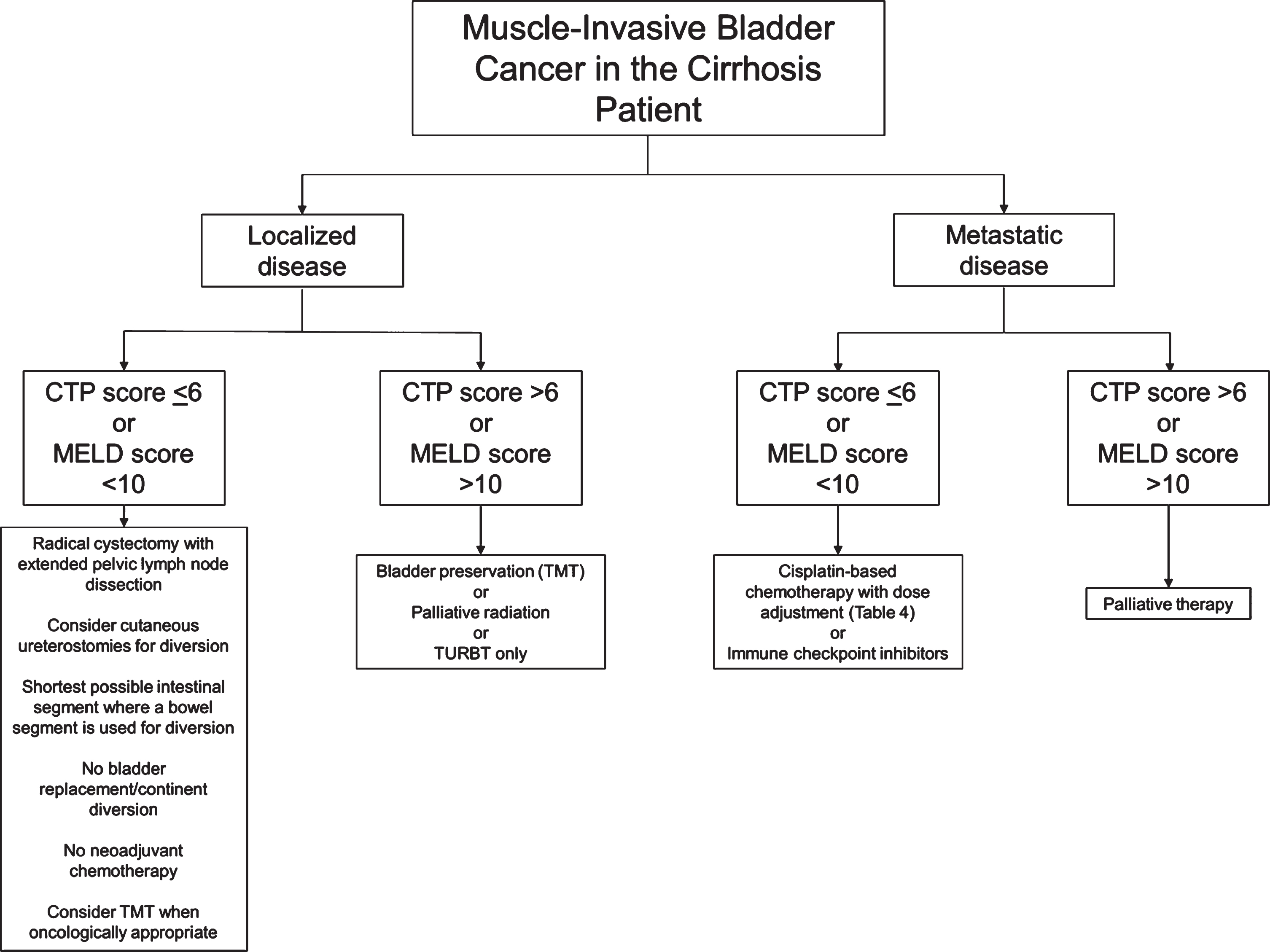
Fig. 1
Flow chart of suggested considerations for patients with muscle-invasive bladder cancer and cirrhosis. Developed at McMaster University. CTP: Child-Pugh-Turcotte; MELD: Model for End-Stage Liver Disease; TMT: Trimodal therapy; TURBT: Transurethral resection of bladder tumour.
The liver is a common site of metastasis for bladder cancer and hepatic metastases may independently serve as a poor prognostic indicator for survival in patients with bladder cancer [148, 149]. Ultimately, due to the fact that cirrhotic patients are often excluded from randomized controlled trials (RCTs), the data is sparse on outcomes of chemotherapy in patients with cirrhosis to inform the development of clinical protocols for chemotherapy [150]. The lack of guidelines regarding systemic anticancer therapies for cirrhotic patients makes it evident that further studies are required to fully explore systemic therapy options in the setting of cirrhosis.
IMMUNE CHECKPOINT INHIBITORS AND LIVER DYSFUNCTION
The recent approval of immune checkpoint in-hibitors (ICIs) in the treatment of patients with advanced or metastatic urothelial carcinoma of the bladder represents a breakthrough in this population’s care. Notably, these medications, including Pembrolizumab, Nivolumab, Atezolizumab, Durvalumab, and Avelumab, are being studied in both the first- and second-line setting. Contemporary literature has demonstrated that Pembrolizumab, for example, confers a clinically important response rate and survival benefit in the first-line setting for patients who are ineligible for cisplatin chemotherapy [151–153]. To the best of our knowledge, no studies to date describe the safety and application of these novel agents in patients with advanced and/or metastatic urothelial carcinoma and cirrhosis. However, what is known is that hepatotoxicity is a possible important immune-related adverse reaction of checkpoint inhibitors (“immune-mediated hepatitis”) [154, 155]. Additionally, the body of literature on the use of ICIs in patients with cirrhosis requires further study to identify those patients at highest risk of adverse events, as many clinical trials examining safety and efficacy of ICIs for malignancy exclude patients with chronic liver disease resulting from hepatitis B and C [156].
RADIATION THERAPY IN PATIENTS WITH CIRRHOSIS
Like chemotherapy, radiation therapy is associated with adverse effects on the liver [132]. Hepatic radiation exposure can result in radiation-induced liver disease (RILD), also known as radiation hepatitis, which tends to occur following radiation therapy for hepatobiliary or upper gastrointestinal malignancies [157, 158]. RILD is characterized by fibrotic histopathological liver abnormalities similar to that of sinusoidal obstructive syndrome, a type of hepatic veno-occlusive disease that can occur following chemotherapy [132, 159]. RILD typically develops 1-3 months after the termination of radiation therapy and usually occurs after whole liver irradiation of up to 30–35 Gy, which is lower than the 50–70 Gy of radiation that is typically used to treat most solid-organ neoplasms [157].
RILD is unlikely to develop when there is minimal delivery of radiation to the liver, such as in patients receiving pelvic radiation for bladder cancer. However, the risk of radiation-related adverse events is increased in patients receiving concurrent chemotherapy [160].
TREATMENT CONSIDERATIONS FOR MUSCLE-INVASIVE BLADDER CANCER IN PATIENTS WITH CIRRHOSIS
To our knowledge, there are no high quality randomized studies examining management of MIBC in patients with liver cirrhosis, though cirrhotic patients are known to have poorer outcomes following surgical or systemic therapy [8, 9, 29]. In addition, due to the absence of guidelines regarding decision-making for therapeutic strategies, recommendations regarding the perioperative management of these patients also remain sparse. Based on our comprehensive review of the literature, we present here imperative considerations for cirrhosis patients withMIBC:
• Radical cystectomy is the standard therapy for MIBC and should be considered in cirrhotic patients with a CTP score < 6 (class A) or MELD score < 10. Patients with more severe cirrhosis suffer from higher rates of perioperative morbidity and mortality, and may not be suitable candidates for surgery. Decreased serum albumin may also be associated with worse postoperative outcomes [82]. Contraindications to elective surgery in cirrhotic patients include acute viral hepatitis, alcoholic hepatitis, acute renal failure, cardiomyopathy, hypoxemia, and coagulopathy. Immediate postoperative complications associated with cirrhosis include SBP, wound dehiscence, bleeding, hepatic decompensation, and renaldysfunction [29].
• In general, a minimally invasive approach to abdominopelvic surgery tends to offer improved post-operative outcomes for cirrhotic patients, but this is less established with radical cystectomy specifically [51].
• The role of preoperative management of ascites with TIPS is controversial and while it may decrease the chance of postoperative complications, its use should be carefully considered on an individual basis in centers where specialist expertise is available.
• Patients with urinary diversion using bowel segments are at risk of developing stomal varices months to years after surgery. Stomal variceal hemorrhage most often presents as frank hematuria but rarely leads to death. In most cases, variceal bleeding can be managed with embolization or sclerotherapy, both of which are associated with low recurrence of bleeding varices.
• Patients with urinary diversion with bowel segments are at high risk of significant metabolic derangements. Hepatic encephalopathy is an important concern in these patients, and the shortest non-retaining urinary diversion, or even cutaneous ureterostomies, should be considered. The use of cutaneous ureterostomies should be especially considered where there is a concern about tolerability of anesthetic (i.e., to minimize operative time). Patients should be counselled on the higher risk of electrolyte and metabolic derangements with a continent diversion.
• Patients undergoing radical cystectomy are at risk of multifactorial AKI in the postoperative period, including a risk of HRS for those with advanced cirrhosis. Careful fluid and electrolyte monitoring is important in avoiding inappropriate fluid balances. Albumin can be considered in the intravascularly deplete cirrhoticpatient.
• Platinum-based chemotherapy can be used to treat metastatic bladder cancer with compensated cirrhosis. Recommended chemotherapy regimens include GC and MVAC. Dose reduction is recommended for gemcitabine, metho-trexate, vinblastine, and doxorubicin in patients with reduced hepatic function, while cisplatin requires no dose modification in the setting of liver failure. Criteria for dose reduction in chemotherapy are outlined in Table 4.
• Trimodal therapy (TMT) with radical tran-surethral resection of bladder tumors (TURBT), external beam radiation therapy (EBRT), and chemotherapy, can serve as a bladder-sparing alternative to radical cystectomy for treatment of MIBC. In principle, radiation therapy should not be offered as monotherapy unless radical cystectomy and chemotherapy are contraindicated. In patients with cirrhosis undergoing EBRT for bladder cancer, pelvic radiation is unlikely to cause RILD but may be associated with radiation proctitis, enteritis, or cystitis. The latter may be further complicated by severe hemorrhagic cystitis given concomitant coagulopathies resulting from cirrhosis. Radiation therapy may be a second-line option for palliative management of liver metastases, after chemotherapy or localized ablation.
CONCLUSION
Patients with liver cirrhosis are at higher risk of developing non-hepatic cancers, including bladder cancer, likely due to shared risk factors, such as smoking and obesity. Surgical and non-surgical treatment of bladder cancer in cirrhotic patients is associated with increased morbidity and mortality. Perioperative and operative complications associated with abdominal surgery in patients with cirrhosis include wound dehiscence, SBP, hepatic encephalopathy, hepatorenal syndrome, and bleeding stomal varices. Additionally, typical chemotherapy regimens for bladder cancer may require dose modification in patients with cirrhosis. This review synthesizes data from studies focusing on the prevalence and treatment of bladder cancers in the setting of cirrhosis to create recommendations for clinicians. However, further higher quality evidence is required to establish formal guidelines for the surgical and non-surgical management for muscle-invasive bladder cancer in cirrhotic patients.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
JK, HR, DS, and JHP conceived the research question and designed the review protocol. JK and DS designed and completed the search and data extraction. JK, HR, DS, and JHP produced the synthesis on reviewed literature, and SL, MP, and PES contributed to data auditing and content revisions. All authors contributed to writing and revision of the manuscript. All authors approved the final version of the manuscript.
ETHICAL CONSIDERATIONS
Research ethics approval was not required for this review as no humans, animals, or individual patient data were involved in conducting this study.
CONFLICTS OF INTEREST
The authors (JK, HR, DS, SL, MP, PES, and JHP) have no competing interests to declare.
REFERENCES
[1] | Schuppan D , Afdhal NH . Liver Cirrhosis. Lancet. (2008) ;371: :838–51. https://doi.org/10.1016/S0140-6736(08)60383-9. |
[2] | D’Amico G , Morabito A , D’Amico M , Pasta L , Malizia G , Rebora P , et al. Clinical states of cirrhosis and competing risks. J Hepatol. (2018) ;68: :563–76. https://doi.org/10.1016/j.jhep.2017.10.020. |
[3] | Sanna C , Rosso C , Marietti M , Bugianesi E . Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int J Mol Sci. (2016) ;17: :717. https://doi.org/10.3390/ijms17050717. |
[4] | Ratib S , Fleming KM , Crooks CJ , Walker AJ , West J . Causes of death in people with liver cirrhosis in England compared with the general population: a population-based cohort study. Am J Gastroenterol. (2015) ;110: :1149–58. https://doi.org/10.1038/ajg.2015.191. |
[5] | Sørensen HT , Friis S , Olsen JH , Thulstrup AM , Mellemkjær L , Linet M , et al. Risk of liver and other types of cancer in patients with cirrhosis: A nationwide cohort study in Denmark. Hepatology. (1998) ;28: :921–5. https://doi.org/10.1002/hep.510280404. |
[6] | Gundling F , Seidl H , Schmidtler F , Löffler N , Strassen I , Wolf P , et al. Nonhepatic cancer in liver cirrhosis: a retrospective study of prevalence, complication rate after specific oncological treatment, follow-up and prognostic predictors of outcome in 354 patients with cirrhosis. Anticancer Res. (2011) ;31: :2931–8. |
[7] | Kalaitzakis E , Gunnarsdottir SA , Josefsson A , Björnsson E . Increased risk for malignant neoplasms among patients with cirrhosis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. (2011) ;9: :168–74. https://doi.org/10.1016/j.cgh.2010.10.014. |
[8] | Northup PG , Friedman LS , Kamath PS . AGA Clinical Practice Update on Surgical Risk Assessment and Perioperative Management in Cirrhosis: Expert Review. Clin Gastroenterol Hepatol. (2019) ;17: :595–606. https://doi.org/10.1016/j.cgh.2018.09.043. |
[9] | Newman KL , Johnson KM , Cornia PB , Wu P , Itani K , Ioannou GN . Perioperative Evaluation and Management of Patients With Cirrhosis: Risk Assessment, Surgical Outcomes, and Future Directions. Clin Gastroenterol Hepatol. (2020) ;18: :2398–2414.e3. https://doi.org/10.1016/j.cgh.2019.07.051. |
[10] | Csikesz NG , Nguyen LN , Tseng JF , Shah SA . Nationwide Volume and Mortality after Elective Surgery in Cirrhotic Patients. J Am Coll Surg. (2009) ;208: :96–103. https://doi.org/10.1016/j.jamcollsurg.2008.09.006. |
[11] | Pinter M , Trauner M , Peck-Radosavljevic M , Sieghart W . Cancer and liver cirrhosis: implications on prognosis and management. ESMO Open. (2016) ;1: :e000042. https://doi.org/10.1136/esmoopen-2016-000042. |
[12] | Singh AK , Kumar R , Pandey AK . Hepatocellular Carcinoma: Causes, Mechanism of Progression and Biomarkers. Curr Chem Genomics Transl Med. (2018) ;12: :9–26. https://doi.org/10.2174/2213988501812010009. |
[13] | Forner A , Reig M , Bruix J . Hepatocellular carcinoma. The Lancet. (2018) ;391: :1301–14. https://doi.org/10.1016/S0140-6736(18)30010-2. |
[14] | Berman K , Tandra S , Vuppalanchi R , Vuppalanch R , Ghabril M , Sandrasegaran K , et al. Hepatic and extrahepatic cancer in cirrhosis: a longitudinal cohort study. Am J Gastroenterol. (2011) ;106: :899–906. https://doi.org/10.1038/ajg.2010.477. |
[15] | Lööf L , Adami H-O , Sparén P , Danielsson Å , Eriksson LS , Hultcrantz R , et al. Cancer risk in primary biliary cirrhosis: A population-based study from Sweden. Hepatology. (1994) ;20: :101–4. https://doi.org/10.1002/hep.1840200116. |
[16] | Deutsch M , Papatheodoridis GV , Tzakou A , Hadziyannis SJ . Risk of hepatocellular carcinoma and extrahepatic malignancies in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. (2008) ;20: :5–9. https://doi.org/10.1097/MEG.0b013e3282f163ed. |
[17] | Werner M , Almer S , Prytz H , Lindgren S , Wallerstedt S , Björnsson E , et al. Hepatic and extrahepatic malignancies in autoimmune hepatitis. A long-term follow-up in 473 Swedish patients. J Hepatol. (2009) ;50: :388–93. https://doi.org/10.1016/j.jhep.2008.08.022. |
[18] | Danielsson Borssén Å , Almer S , Prytz H , Wallerstedt S , Friis-Liby I-L , Bergquist A , et al. Hepatocellular and extrahepatic cancer in patients with autoimmune hepatitis–a long-term follow-up study in 634 Swedish patients. Scand J Gastroenterol. (2015) ;50: :217–23. https://doi.org/10.3109/00365521.2014.983154. |
[19] | Toft Sørensen H , Friis S , Olsen JH , Thulstrup AM , Mellemkjaer L , Linet M , et al. Risk of Breast Cancer in Men With Liver Cirrhosis. Am J Gastroenterol. (1998) ;93: :231–3. https://doi.org/10.1016/S0002-9270(97)00050-6. |
[20] | Chiang C-L , Huang H-H , Huang T-Y , Shih Y-L , Hsieh T-Y , Lin H-H . Nonalcoholic Fatty Liver Disease Associated With Bladder Cancer. Am J Med Sci. (2020) ;360: :161–5. https://doi.org/10.1016/j.amjms.2020.04.031. |
[21] | Boonstra K , Bokelaar R , Stadhouders PH , Tuynman HA , Poen AC , van Nieuwkerk KM , et al. Increased cancer risk in a large population-based cohort of patients with primary biliary cirrhosis: follow-up for up to 36 years. Hepatol Int. (2014) ;8: :266–74. https://doi.org/10.1007/s12072-014-9530-z. |
[22] | Liang Y , Yang Z , Zhong R . Primary biliary cirrhosis and cancer risk: A systematic review and meta-analysis. Hepatology. (2012) ;56: :1409–17. https://doi.org/10.1002/hep.25788. |
[23] | Sanli O , Dobruch J , Knowles MA , Burger M , Alemozaffar M , Nielsen ME , et al. Bladder cancer. Nat Rev Dis Primer. (2017) ;3: :1–19. https://doi.org/10.1038/nrdp.2017.22. |
[24] | Dam MK , Flensborg-Madsen T , Eliasen M , Becker U , Tolstrup JS . Smoking and risk of liver cirrhosis: a population-based cohort study. Scand J Gastroenterol. (2013) ;48: :585–91. https://doi.org/10.3109/00365521.2013.777469. |
[25] | Befeler AS , Palmer DE , Hoffman M , Longo W , Solomon H , Bisceglie AMD . The Safety of Intra-abdominal Surgery in Patients With Cirrhosis: Model for End-Stage Liver Disease Score Is Superior to Child-Turcotte-Pugh Classification in Predicting Outcome. Arch Surg. (2005) ;140: :650–4. https://doi.org/10.1001/archsurg.140.7.650. |
[26] | Neeff H , Mariaskin D , Spangenberg H-C , Hopt UT , Makowiec F . Perioperative mortality after non-hepatic general surgery in patients with liver cirrhosis: an analysis of 138 operations in the. 2000s using Child and MELD scores. J Gastrointest Surg Off J Soc Surg Aliment Tract. (2011) ;15: :1–11. https://doi.org/10.1007/s11605-010-1366-9. |
[27] | Garrison RN , Cryer HM , Howard DA , Polk HC . Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. (1984) ;199: :648–55. |
[28] | Mansour A , Watson W , Shayani V , Pickleman J . Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery. (1997) ;122: :730–5; discussion 735-736. https://doi.org/10.1016/s0039-6060(97)90080-5. |
[29] | Friedman LS . Surgery in the Patient with Liver Disease. Trans Am Clin Climatol Assoc. (2010) ;121: :192–205. |
[30] | Johnson KM , Newman KL , Green PK , Berry K , Cornia PB , Wu P , et al. Incidence and Risk Factors of Postoperative Mortality and Morbidity After Elective Versus Emergent Abdominal Surgery in a National Sample of 8193 Patients With Cirrhosis. Ann Surg. 2020;Published Ahead of Print. https://doi.org/10.1097/SLA.0000000000003674. |
[31] | del Olmo JA , Flor-Lorente B , Flor-Civera B , Rodriguez F , Serra MA , Escudero A , et al. Risk Factors for Nonhepatic Surgery in Patients with Cirrhosis. World J Surg. (2003) ;27: :647–52. https://doi.org/10.1007/s00268-003-6794-1. |
[32] | Ho JK , Yoshida E . The Extrahepatic Consequences of Cirrhosis. Medscape Gen Med. (2006) ;8: :59. |
[33] | Møller S , Henriksen JH , Bendtsen F . Extrahepatic complications to cirrhosis and portal hypertension: Haemodynamic and homeostatic aspects. World J Gastroenterol WJG. (2014) ;20: :15499–517. https://doi.org/10.3748/wjg.v20.i42.15499. |
[34] | Carvalho MVH , Kroll PC , Kroll RTM , Carvalho VN . Cirrhotic cardiomyopathy: the liver affects the heart. Braz J Med Biol Res. 2019;52. https://doi.org/10.1590/1414-431X20187809. |
[35] | Sharma M , Rameshbabu CS . Collateral Pathways in Portal Hypertension. J Clin Exp Hepatol. (2012) ;2: :338–52. https://doi.org/10.1016/j.jceh.2012.08.001. |
[36] | Simonetto DA , Liu M , Kamath PS . Portal Hypertension and Related Complications: Diagnosis and Management. Mayo Clin Proc. (2019) ;94: :714–26. https://doi.org/10.1016/j.mayocp.2018.12.020. |
[37] | Vilela EG , Thabut D , Rudler M , Bittencourt PL . Management of Complications of Portal Hypertension. Can J Gastroenterol Hepatol. (2019) ;2019: :6919284. https://doi.org/10.1155/2019/6919284. |
[38] | Bloom S , Kemp W , Lubel J . Portal hypertension: pathophysiology, diagnosis and management. Intern Med J. (2015) ;45: :16–26. https://doi.org/10.1111/imj.12590. |
[39] | Kovacs TOG , Jensen DM . Varices: Esophageal, Gastric, and Rectal. Clin Liver Dis. (2019) ;23: :625–42. https://doi.org/10.1016/j.cld.2019.07.005. |
[40] | Lopez-Delgado JC , Ballus J , Esteve F , Betancur-Zambrano NL , Corral-Velez V , Mañez R , et al. Outcomes of abdominal surgery in patients with liver cirrhosis. World J Gastroenterol. (2016) ;22: :2657–67. https://doi.org/10.3748/wjg.v22.i9.2657. |
[41] | Millwala F , Nguyen GC , Thuluvath PJ . Outcomes of patients with cirrhosis undergoing non-hepatic surgery: risk assessment and management. World J Gastroenterol. (2007) ;13: :4056–63. https://doi.org/10.3748/wjg.v13.i30.4056. |
[42] | Tsochatzis EA , Bosch J , Burroughs AK . Liver cirrhosis. Lancet Lond Engl. (2014) ;383: :1749–61. https://doi.org/10.1016/S0140-6736(14)60121-5. |
[43] | Sepanlou SG , Safiri S , Bisignano C , Ikuta KS , Merat S , Saberifiroozi M , et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study. 2017.. Lancet Gastroenterol Hepatol. (2020) ;5: :245–66. https://doi.org/10.1016/S2468-1253(19)30349-8. |
[44] | D’Amico G , Pasta L , Morabito A , D’Amico M , Caltagirone M , Malizia G , et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. (2014) ;39: :1180–93. https://doi.org/10.1111/apt.12721. |
[45] | D’Amico G , Garcia-Tsao G , Pagliaro L . Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol. (2006) ;44: :217–31. https://doi.org/10.1016/j.jhep.2005.10.013. |
[46] | Fleming KM , Aithal GP , Card TR , West J . The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. (2010) ;32: :1343–50. https://doi.org/10.1111/j.1365-2036.2010.04473.x. |
[47] | Fleming KM , Aithal GP , Card TR , West J . All-cause mortality in people with cirrhosis compared with the general population: a population-based cohort study. Liver Int. (2012) ;32: :79–84. https://doi.org/10.1111/j.1478-3231.2011.02517.x. |
[48] | Prenner S , Ganger D . Risk stratification and preoperative evaluation of the patient with known or suspected liver disease. Clin Liver Dis. (2016) ;7: :101–5. https://doi.org/10.1002/cld.546. |
[49] | Durand F , Valla D . Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. (2005) ;42: :S100–7. https://doi.org/10.1016/j.jhep.2004.11.015. |
[50] | Rice HE , O’Keefe GE , Helton WS , Johansen K . Morbid prognostic features in patients with chronic liver failure undergoing nonhepatic surgery. Arch Surg Chic Ill 1960. (1997) ;132: :880–4. discussion 884-885. https://doi.org/10.1001/archsurg.1997.01430320082013. |
[51] | Sabbagh C , Fuks D , Regimbeau J-M . Non-hepatic gastrointestinal surgery in patients with cirrhosis. J Visc Surg. (2014) ;151: :203–11. https://doi.org/10.1016/j.jviscsurg.2014.04.004. |
[52] | de Goede B , Klitsie PJ , Lange JF , Metselaar HJ , Kazemier G . Morbidity and mortality related to non-hepatic surgery in patients with liver cirrhosis; A systematic review. Best Pract Res Clin Gastroenterol. (2012) ;26: :47–59. https://doi.org/10.1016/j.bpg.2012.01.010. |
[53] | Neeff HP , Streule GC , Drognitz O , Tittelbach-Helmrich D , Spangenberg H-C , Hopt UT , et al. Early mortality and long-term survival after abdominal surgery in patients with liver cirrhosis. Surgery. (2014) ;155: :623–32. https://doi.org/10.1016/j.surg.2013.11.009. |
[54] | Cobb WS , Heniford BT , Burns JM , Carbonell AM , Matthews BD , Kercher KW . Cirrhosis is not a contraindication to laparoscopic surgery. Surg Endosc. (2005) ;19: :418–23. https://doi.org/10.1007/s00464-004-8722-3. |
[55] | Tsugawa K , Koyanagi N , Hashizume M , Tomikawa M , Ayukawa K , Akahoshi K , et al. A comparison of an open and laparoscopic appendectomy for patients with liver cirrhosis. Surg Laparosc Endosc Percutan Tech. (2001) ;11: :189–94. |
[56] | Puggioni A , Wong LL . A metaanalysis of laparoscopic cholecystectomy in patients with cirrhosis. J Am Coll Surg. (2003) ;197: :921–6. https://doi.org/10.1016/j.jamcollsurg.2003.08.011. |
[57] | Zachos I , Zachou K , Dalekos GN , Tzortzis V . Management of patients with liver cirrhosis and invasive bladder cancer: A case-series. J Transl Intern Med. (2019) ;7: :29–33. https://doi.org/10.2478/jtim-2019-0006. |
[58] | Bhangui P , Laurent A , Amathieu R , Azoulay D . Assessment of risk for non-hepatic surgery in cirrhotic patients. J Hepatol. (2012) ;57: :874–84. https://doi.org/10.1016/j.jhep.2012.03.037. |
[59] | Vaja R , McNicol L , Sisley I . Anaesthesia for patients with liver disease. Contin Educ Anaesth Crit Care Pain. (2010) ;10: :15–9. https://doi.org/10.1093/bjaceaccp/mkp040. |
[60] | Friedman LS . The risk of surgery in patients with liver disease. Hepatology. (1999) ;29: :1617–23. https://doi.org/10.1002/hep.510290639. |
[61] | Cowan RE , Jackson BT , Grainger SL , Thompson RPH . Effects of anesthetic agents and abdominal surgery on liver blood flow. Hepatology. (1991) ;14: :1161–6. https://doi.org/10.1002/hep.1840140634. |
[62] | Rahimzadeh P , Safari S , Faiz SHR , Alavian SM . Anesthesia for Patients With Liver Disease. Hepat Mon. 2014;14. https://doi.org/10.5812/hepatmon.19881. |
[63] | Sladen RN . Perioperative Care for the Patient with Renal or Hepatic Disease. Anesth Analg. (2001) ;92: :99–103. https://doi.org/10.1097/00000539-200103001-00016. |
[64] | Dalal A , Lang JDJ . Anesthetic Considerations for Patients with Liver Disease. Hepatic Surg. 2013. https://doi.org/10.5772/54222. |
[65] | Abbas N , Makker J , Abbas H , Balar B . Perioperative Care of Patients With Liver Cirrhosis: A Review. Health Serv Insights. 2017;10. https://doi.org/10.1177/1178632917691270. |
[66] | Harrison MF . The Misunderstood Coagulopathy of Liver Disease: A Review for the Acute Setting. West J Emerg Med. (2018) ;19: :863–71. https://doi.org/10.5811/westjem.2018.7.37893. |
[67] | Blonski W , Siropaides T , Reddy KR . Coagulopathy in liver disease. Curr Treat Options Gastroenterol. (2007) ;10: :464–73. https://doi.org/10.1007/s11938-007-0046-7. |
[68] | Vinet E , Perreault P , Bouchard L , Bernard D , Wassef R , Richard C , et al. Transjugular Intrahepatic Portosystemic Shunt before Abdominal Surgery in Cirrhotic Patients: A Retrospective, Comparative Study. Can J Gastroenterol. (2006) ;20: :401–4. https://doi.org/10.1155/2006/245082. |
[69] | Lahat E , Lim C , Bhangui P , Fuentes L , Osseis M , Moussallem T , et al. Transjugular intrahepatic portosystemic shunt as a bridge to non-hepatic surgery in cirrhotic patients with severe portal hypertension: a systematic review. HPB. (2018) ;20: :101–9. https://doi.org/10.1016/j.hpb.2017.09.006. |
[70] | Aldrich SM , Regal RE . Routine Use of Vitamin K in the Treatment of Cirrhosis-Related Coagulopathy: Is it A-O-K? Maybe Not, We Say. Pharm Ther. (2019) ;44: :131–6. |
[71] | Periyalwar P , Dasarathy S . Malnutrition in Cirrhosis: Contribution and Consequences of Sarcopenia on Metabolic and Clinical Responses. Clin Liver Dis. (2012) ;16: :95–131. https://doi.org/10.1016/j.cld.2011.12.009. |
[72] | Eghtesad S , Poustchi H , Malekzadeh R . Malnutrition in Liver Cirrhosis:The Influence of Protein and Sodium. Middle East J Dig Dis. (2013) ;5: :65–75. |
[73] | Saunders J , Brian A , Wright M , Stroud M . Malnutrition and nutrition support in patients with liver disease. Frontline Gastroenterol. (2010) ;1: :105–11. https://doi.org/10.1136/fg.2009.000414. |
[74] | Husi H , MacDonald A , Skipworth RJE , Miller J , Cronshaw A , Fearon KCH , et al. Proteomic identification of potential markers of myosteatosis in human urine. Biomed Rep. (2018) ;8: :557–64. https://doi.org/10.3892/br.2018.1091. |
[75] | Zamboni M , Gattazzo S , Rossi AP . Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med. (2019) ;10: :5–6. https://doi.org/10.1007/s41999-018-0134-3. |
[76] | Rimar KJ , Glaser AP , Kundu S , Schaeffer EM , Meeks J , Psutka SP . Changes in Lean Muscle Mass Associated with Neoadjuvant Platinum-Based Chemotherapy in Patients with Muscle Invasive Bladder Cancer. Bladder Cancer. (2018) ;4: :411–8. https://doi.org/10.3233/BLC-180188. |
[77] | Lyon TD , Frank I , Takahashi N , Boorjian SA , Moynagh MR , Shah PH , et al. Sarcopenia and Response to Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer. Clin Genitourin Cancer. (2019) ;17: :216–222.e5. https://doi.org/10.1016/j.clgc.2019.03.007. |
[78] | Hu X , Dou W-C , Shao Y-X , Liu J-B , Xiong S-C , Yang W-X , et al. The prognostic value of sarcopenia in patients with surgically treated urothelial carcinoma: A systematic review and meta-analysis. Eur J Surg Oncol. (2019) ;45: :747–54. https://doi.org/10.1016/j.ejso.2019.03.003. |
[79] | Alaniz C , Regal RE . Spontaneous Bacterial Peritonitis. Pharm Ther. (2009) ;34: :204–10. |
[80] | Käser SA , Hofmann I , Willi N , Stickel F , Maurer CA . Liver Cirrhosis/Severe Fibrosis Is a Risk Factor for Anastomotic Leakage after Colorectal Surgery. Gastroenterol Res Pract. (2016) ;2016: . https://doi.org/10.1155/2016/1563037. |
[81] | Imani F , Motavaf M , Safari S , Alavian SM . The Therapeutic Use of Analgesics in PatientsWith Liver Cirrhosis: A Literature Review and Evidence-Based Recommendations. Hepat Mon. 2014;14. https://doi.org/10.5812/hepatmon.23539. |
[82] | Djaladat H , Bruins HM , Miranda G , Cai J , Skinner EC , Daneshmand S . The association of preoperative serum albumin level and American Society of Anesthesiologists (ASA) score on early complications and survival of patients undergoing radical cystectomy for urothelial bladder cancer. BJU Int. (2014) ;113: :887–93. https://doi.org/10.1111/bju.12240. |
[83] | Zachos I , Zachou K , Dalekos GN , Tzortzis V . Management of patients with liver cirrhosis and invasive bladder cancer: A case-series. J Transl Intern Med. (2019) ;7: :29–33. https://doi.org/10.2478/jtim-2019-0006. |
[84] | Gore JL , Yu H-Y , Setodji C , Hanley JM , Litwin MS , Saigal CS . Urinary Diversion and Morbidity After Radical Cystectomy for Bladder Cancer. Cancer. (2010) ;116: :331–9. https://doi.org/10.1002/cncr.24763. |
[85] | Agarwal S , Sarpal R , Dang S , Kalra Y , Biswas M . Indications and outcome of patients undergoing cutaneous ureterostomy as a mode of urinary diversion after radical cystectomy: an experience from a tertiary care center. Int Surg J. (2018) ;5: :3038–42. https://doi.org/10.18203/2349-2902.isj20183719. |
[86] | Stein R , Rubenwolf P . Metabolic Consequences after Urinary Diversion. Front Pediatr. 2014;2. https://doi.org/10.3389/fped.2014.00015. |
[87] | Stein R , Hohenfellner M , Pahernik S , Roth S , Thüroff JW , Rübben H . Urinary Diversion—Approaches and Consequences. Dtsch Ärztebl Int. (2012) ;109: :617–22. https://doi.org/10.3238/arztebl.2012.0617. |
[88] | Swaminathan M , Ellul MA , Cross TJ . Hepatic encephalopathy: current challenges and future prospects. Hepatic Med Evid Res. (2018) ;10: :1–11. https://doi.org/10.2147/HMER.S118964. |
[89] | Firlit RS , Firlit CF , Canning J . Exsanguinating hemorrhage from urinary ileal conduit in patient with portal hypertension. Urology. (1978) ;12: :710–1. https://doi.org/10.1016/0090-4295(78)90439-9. |
[90] | Kaveggia FF , Thompson JS , Schafer EC , Fischer JL , Taylor RJ . Hyperammonemic Encephalopathy in Urinary Diversion With Urea-Splitting Urinary Tract Infection. Arch Intern Med. (1990) ;150: :2389–92. https://doi.org/10.1001/archinte.1990.00390220121025. |
[91] | Vasdev N , Moon A , Thorpe AC . Metabolic complications of urinary intestinal diversion. Indian J Urol. (2013) ;29: :310. https://doi.org/10.4103/0970-1591.120112. |
[92] | Abbas N , Makker J , Abbas H , Balar B . Perioperative Care of Patients With Liver Cirrhosis: A Review. Health Serv Insights. 2017;10. https://doi.org/10.1177/1178632917691270. |
[93] | Francoz C , Durand F , Kahn JA , Genyk YS , Nadim MK . Hepatorenal Syndrome. Clin J Am Soc Nephrol. (2019) ;14: :774–81. https://doi.org/10.2215/CJN.12451018. |
[94] | Osman Y , Harraz AM , El-Halwagy S , Laymon M , Mosbah A , Abol-Enein H , et al. Acute kidney injury following radical cystectomy and urinary diversion: predictors and associated morbidity. Int Braz J Urol Off J Braz Soc Urol. (2018) ;44: :726–33. https://doi.org/10.1590/S1677-5538.IBJU.2017.0283. |
[95] | Gameiro J , Fonseca JA , Neves M , Jorge S , Lopes JA . Acute kidney injury in major abdominal surgery: incidence, risk factors, pathogenesis and outcomes. Ann Intensive Care. 2018;8. https://doi.org/10.1186/s13613-018-0369-7. |
[96] | Allegretti AS , Ortiz G , Wenger J , Deferio JJ , Wibecan J , Kalim S , et al. Prognosis of Acute Kidney Injury and Hepatorenal Syndrome in Patients with Cirrhosis: A Prospective Cohort Study. Int J Nephrol. (2015) ;2015: . https://doi.org/10.1155/2015/108139. |
[97] | Furrer MA , Schneider MP , Burkhard FC , Wuethrich PY . Incidence and perioperative risk factors for early acute kidney injury after radical cystectomy and urinary diversion. Urol Oncol. (2018) ;36: :306.e17–306.e23. https://doi.org/10.1016/j.urolonc.2018.02.011. |
[98] | Kabeer MA , Jackson L , Widdison AL , Maskell G , Mathew J . Stomal varices: a rare cause of stomal hemorrhage. A report of three cases. Ostomy Wound Manage. 2007; 53:20-2, 24, 26 passim. |
[99] | Fucini C , Wolff BG , Dozois RR . Bleeding from peristomal varices: Perspectives on prevention and treatment. Dis Colon Rectum. (1991) ;34: :1073–8. https://doi.org/10.1007/BF02050064. |
[100] | Norton ID , Andrews JC , Kamath PS . Management of ectopic varices. Hepatology. (1998) ;28: :1154–8. https://doi.org/10.1002/hep.510280434. |
[101] | Jensen DM . Endoscopic screening for varices in cirrhosis: Findings, implications, and outcomes. Gastroenterology. (2002) ;122: :1620–30. https://doi.org/10.1053/gast.2002.33419. |
[102] | Spier BJ , Fayyad AA , Lucey MR , Johnson EA , Wojtowycz M , Rikkers L , et al. Bleeding stomal varices: case series and systematic review of the literature. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. (2008) ;6: :346–52. https://doi.org/10.1016/j.cgh.2007.12.047. |
[103] | Ryan W , Dako F , Cohen G , Pryluck D , Panaro J , Cuthbertson E , et al. Direct Percutaneous Embolization of Peristomal Ileostomy Varices in an Emergency Setting. Case Rep Radiol. (2018) ;2018: :e6239183. https://doi.org/10.1155/2018/6239183. |
[104] | Grundfest-Broniatowski S , Fazio V . Conservative Treatment of Bleeding Stomal Varices. Arch Surg. (1983) ;118: :981–5. https://doi.org/10.1001/archsurg.1983.01390080083021. |
[105] | Vangeli M , Patch D , Terreni N , Tibballs J , Watkinson A , Davies N , et al. Bleeding ectopic varices—treatment with transjugular intrahepatic porto-systemic shunt (TIPS) and embolisation. J Hepatol. (2004) ;41: :560–6. https://doi.org/10.1016/j.jhep.2004.06.024. |
[106] | Saad WE , Saad NE , Koizumi J . Stomal Varices: Management With Decompression TIPS and Transvenous Obliteration or Sclerosis. Tech Vasc Interv Radiol. (2013) ;16: :126–34. https://doi.org/10.1053/j.tvir.2013.02.005. |
[107] | Foulkes J , Wallace DM . Haemorrhage from stomal varices in an ileal conduit. Br J Urol. (1975) ;47: :630. https://doi.org/10.1111/j.1464-410x.1975.tb04026.x. |
[108] | Firlit RS , Firlit CF , Canning J . Exsanguinating hemorrhage from urinary ileal conduit in patient with portal hypertension. Urology. (1978) ;12: :710–1. https://doi.org/10.1016/0090-4295(78)90439-9. |
[109] | Hollands MJ . Parastomal haemorrhage from an ileal conduit secondary to portal hypertension. Br J Surg. (1982) ;69: :675. https://doi.org/10.1002/bjs.1800691115. |
[110] | Thomas DJ , Abercrombie GF . Peri-stomal varices: an unusual cause of bleeding from an ileal conduit. Br J Urol. (1993) ;71: :355–6. https://doi.org/10.1111/j.1464-410x.1993.tb15960.x. |
[111] | Zimmerman G , Smith DC , Taylor FC , Hadley HR . Recurrent urinary conduit bleeding in a patient with portal hypertension: management with a transjugular intrahepatic portosystemic shunt. Urology. (1994) ;43: :748–51. https://doi.org/10.1016/0090-4295(94)90205-4. |
[112] | Sundaram CP , Fernandes ET , Reddy PK . Recurrent hemorrhage for ileal conduit: an uncommon complication of portal hypertension. Scand J Urol Nephrol. (1997) ;31: :403–5. https://doi.org/10.3109/00365599709030630. |
[113] | Lashley DB , Saxon RR , Fuchs EF , Chin DH , Lowe BA . Bleeding ileal conduit stomal varices: diagnosis and management using transjugular transhepatic angiography and embolization. Urology. (1997) ;50: :612–4. https://doi.org/10.1016/S0090-4295(97)00267-7. |
[114] | Carrafiello G , Laganà D , Giorgianni A , Lumia D , Mangini M , Paragone E , et al. Bleeding from peristomal varices in a cirrhotic patient with ileal conduit: treatment with transjugular intrahepatic portocaval shunt (TIPS). Emerg Radiol. (2007) ;13: :341–3. https://doi.org/10.1007/s10140-006-0564-y. |
[115] | Tu WH , Chao D , Gill H . Gross hematuria from an ileal conduit as a first presentation of portal hypertension. Nat Clin Pract Urol. (2008) ;5: :569–73. https://doi.org/10.1038/ncpuro1192. |
[116] | Kang E-J , Yoon SK , Kim S , Cho J , Kang M , Park B-H . Bleeding from an Ileal Conduit Stomal Varix: Diagnosis with Three-Dimmensional Volume Rendered Images by the use of Multidetector-Row CT (MDCT) and Management with a Transjugular Intrahepatic Portosystemic Shunt. J Korean Soc Radiol. (2009) ;60: :227. https://doi.org/10.3348/jksr.2009.60.4.227. |
[117] | Naidu SG , Castle EP , Kriegshauser JS , Huettl EA . Direct percutaneous embolization of bleeding stomal varices. Cardiovasc Intervent Radiol. (2010) ;33: :201–4. https://doi.org/10.1007/s00270-009-9536-4. |
[118] | Yao D-H , Luo X-F , Zhou B , Li X . Ileal conduit stomal variceal bleeding managed by endovascular embolization. World J Gastroenterol. (2013) ;19: :8156–9. https://doi.org/10.3748/wjg.v19.i44.8156. |
[119] | Tan S-T . Sclerotherapy for rare bleeding stomal varices after ileal conduit urinary diversion: an effective and noninvasive method. Scott Med J. (2014) ;59: :e5–7. https://doi.org/10.1177/0036933014554876. |
[120] | Dal Moro F . Long-delayed gross hematuria due to portal hypertension in an alcoholic cirrhotic patient with ileal conduit urinary diversion. Ann Hepatol. (2014) ;13: :830–1. |
[121] | Staubli SEL , Gramann T , Schwab C , Semela D , Hechelhammer L , Engeler DS , et al. Life-threatening bleeding from peristomal varices after cystoprostatectomy: multimodal approach in a cirrhotic, encephalopathic patient with severe portal hypertension. Case Rep Urol. (2015) ;2015: :785010. https://doi.org/10.1155/2015/785010. |
[122] | Trasancos-Escura C , Carrillo-George C , Muñoz-Bertrán ED , Bebia-Conesa P . Stomal varices: an unusual cause of bleeding in patients with portal hypertension. Rev Espanola Enfermedades Dig Organo Of Soc Espanola Patol Dig. (2015) ;107: :582–3. |
[123] | Lee S-M , Yogan K , Carr T . Stomal varices: a cause for intermittent haematuria post-radical cystectomy. BMJ Case Rep. (2016) ;2016: . https://doi.org/10.1136/bcr-2015-214082. |
[124] | Atwal D , Chatterjee K , Osborne S , Kakkera K , Deas S , Li R , et al. Successful Management of Neobladder Variceal Bleeding. Cardiovasc Intervent Radiol. (2016) ;39: :1510–3. https://doi.org/10.1007/s00270-016-1395-1. |
[125] | Onishi Y , Kimura H , Kanagaki M , Oka S , Fukumoto G , Otani T , et al. Successful embolization of ileal conduit stomal varices with N-butyl cyanoacrylate via a recanalized paraumbilical vein. Radiol Case Rep. (2018) ;13: :1130–2. https://doi.org/10.1016/j.radcr.2018.07.024. |
[126] | Chittajallu V , Romero-Marrero C . Management of Recurrent Ectopic Variceal Bleeding in Ileal Conduit With TIPS. Off J Am Coll Gastroenterol ACG. (2019) ;114: :S1372. https://doi.org/10.14309/01.ajg.0000599516.08375.06. |
[127] | Gowda SN , Sethi P , Motapothula U . Peristomal variceal hemorrhage at the ileal conduit site due to extrahepatic portosystemic shunt. Indian J Urol IJU J Urol Soc India. (2020) ;36: :130–2. https://doi.org/10.4103/iju.IJU292_19. |
[128] | Conte JV , Arcomano TA , Naficy MA , Holt RW . Treatment of bleeding stomal varices. Report of a case and review of the literature. Dis Colon Rectum. (1990) ;33: :308–14. https://doi.org/10.1007/BF02055474. |
[129] | Grigorian A , O’Brien CB . Hepatotoxicity Secondary to Chemotherapy. J Clin Transl Hepatol. (2014) ;2: :95–102. https://doi.org/10.14218/JCTH.2014.00011. |
[130] | Sharma A , Houshyar R , Bhosale P , Choi J-I , Gulati R , Lall C . Chemotherapy induced liver abnormalities: an imaging perspective. Clin Mol Hepatol. (2014) ;20: :317–26. https://doi.org/10.3350/cmh.2014.20.3.317. |
[131] | Floyd J , Mirza I , Sachs B , Perry MC . Hepatotoxicity of Chemotherapy. Semin Oncol. (2006) ;33: :50–67. https://doi.org/10.1053/j.seminoncol.2005.11.002. |
[132] | Maor Y , Malnick S . Liver Injury Induced by Anticancer Chemotherapy and Radiation Therapy. Int J Hepatol. (2013) ;2013: :e815105. https://doi.org/10.1155/2013/815105. |
[133] | Schulz PO , Ferreira FG , Nascimento M de FA , Vieira A , Ribeiro MA , David AI , et al. Association of nonalcoholic fatty liver disease and liver cancer. World J Gastroenterol WJG. (2015) ;21: :913–8. https://doi.org/10.3748/wjg.v21.i3.913. |
[134] | King PD , Perry MC . Hepatotoxicity of Chemotherapy. The Oncologist. (2001) ;6: :162–76. https://doi.org/10.1634/theoncologist.6-2-162. |
[135] | Cabibbo G , Palmeri L , Palmeri S , Craxì A . Should cirrhosis change our attitude towards treating non-hepatic cancer? Liver Int Off J Int Assoc Study Liver. (2012) ;32: :21–7. https://doi.org/10.1111/j.1478-3231.2011.02629.x. |
[136] | Compérat E , Srigley JR , Brimo F , Delahunt B , Koch M , Lopez-Beltran A , et al. Dataset for the reporting of carcinoma of the bladder—cystectomy, cystoprostatectomy and diverticulectomy specimens: recommendations from the International Collaboration on Cancer Reporting (ICCR). Virchows Arch. (2020) ;476: :521–34. https://doi.org/10.1007/s00428-019-02727-1. |
[137] | González-Regueiro JA , Ruiz-Margáin A , Cruz-Contreras M , Montaña-Duclaud AM , Cavazos-Gómez A , Demichelis-Gómez R , et al. Clinical characteristics and treatment outcomes in patients with liver cirrhosis and lymphoma. World J Hepatol. (2020) ;12: :34–45. https://doi.org/10.4254/wjh.v12.i2.34. |
[138] | Qamar AA , Grace ND . Abnormal hematological indices in cirrhosis. Can J Gastroenterol. (2009) ;23: :441–5. |
[139] | Li L , Duan M , Chen W , Jiang A , Li X , Yang J , et al. The spleen in liver cirrhosis: revisiting an old enemywith novel targets. J Transl Med. 2017;15. https://doi.org/10.1186/s12967-017-1214-8. |
[140] | Wu M , Schuster M , Tadros M . Update on Management of Portal Vein Thrombosis and the Role of Novel Anticoagulants. J Clin Transl Hepatol. (2019) ;7: :154–64. https://doi.org/10.14218/JCTH.2018.00057. |
[141] | Raja K , Jacob M , Asthana S . Portal Vein Thrombosis in Cirrhosis. J Clin Exp Hepatol. (2014) ;4: :320–31. https://doi.org/10.1016/j.jceh.2013.12.003. |
[142] | Kulkarni GS , Black PC , Sridhar SS , Kapoor A , Zlotta AR , Shayegan B , et al. Canadian Urological Association guideline: Muscle-invasive bladder cancer. Can Urol Assoc J. (2019) ;13: :230–8. https://doi.org/10.5489/cuaj.5902. |
[143] | Chang SS , Bochner BH , Chou R , Dreicer R , Kamat AM , Lerner SP , et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: Aua/Asco/Astro/Suo Guideline. J Urol. (2017) ;198: :552–9. https://doi.org/10.1016/j.juro.2017.04.086. |
[144] | Alfred Witjes J , Lebret T , Compérat EM , Cowan NC , De Santis M , Bruins HM , et al. Updated. 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol. (2017) ;71: :462–75. https://doi.org/10.1016/j.eururo.2016.06.020. |
[145] | Spiess PE , Agarwal N , Bangs R , Boorjian SA , Buyyounouski MK , Clark PE , et al. Bladder Cancer, Version 5. NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2017) ;15: :1240–67. https://doi.org/10.6004/jnccn.2017.0156. |
[146] | Milowsky MI , Rumble RB , Booth CM , Gilligan T , Eapen LJ , Hauke RJ , et al. Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol. (2016) ;34: :1945–52. https://doi.org/10.1200/JCO.2015.65.9797. |
[147] | Hendrayana T , Wilmer A , Kurth V , Schmidt-Wolf IG , Jaehde U . Anticancer Dose Adjustment for Patients with Renal and Hepatic Dysfunction: From Scientific Evidence to Clinical Application. Sci Pharm. 2017;85. https://doi.org/10.3390/scipharm85010008. |
[148] | Dong F , Shen Y , Gao F , Xu T , Wang X , Zhang X , et al. Prognostic value of site-specific metastases and therapeutic roles of surgery for patients with metastatic bladder cancer: a population-based study. Cancer Manag Res. (2017) ;9: :611–26. https://doi.org/10.2147/CMAR.S148856. |
[149] | Tatokoro M , Kihara K . Liver Metastases from Ureteral and Bladder Cancer. In: Di Carlo I, editor. Noncolorectal Nonneuroendocrine Liver Metastases Diagn. Curr. Ther., Cham: Springer International Publishing;. 2015, pp. 175-82. https://doi.org/10.1007/978-3-319-09293-5_14. |
[150] | Han EC , Ryoo S-B , Park JW , Yi JW , Oh H-K , Choe EK , et al. Oncologic and surgical outcomes in colorectal cancer patients with liver cirrhosis: A propensity-matched study. PLOS ONE. (2017) ;12: :e0178920. https://doi.org/10.1371/journal.pone.0178920. |
[151] | Balar AV , Castellano D , O’Donnell PH , Grivas P , Vuky J , Powles T , et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. (2017) ;18: :1483–92. https://doi.org/10.1016/S1470-2045(17)30616-2. |
[152] | Grivas P , Plimack ER , Balar AV , Castellano D , O’Donnell PH , Bellmunt J , et al. Pembrolizumab as First-line Therapy in Cisplatin-ineligible Advanced Urothelial Cancer (KEYNOTE-052): Outcomes in Older Patients by Age and Performance Status. Eur Urol Oncol. (2020) ;3: :351–9. https://doi.org/10.1016/j.euo.2020.02.009. |
[153] | Lopez-Beltran A , Cimadamore A , Blanca A , Massari F , Vau N , Scarpelli M , et al. Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers. 2021;13. https://doi.org/10.3390/cancers13010131. |
[154] | Peeraphatdit T (Bee) , Wang J , Odenwald MA , Hu S , Hart J , Charlton MR . Hepatotoxicity From Immune Checkpoint Inhibitors: A Systematic Review and Management Recommendation. Hepatology. (2020) ;72: :315–29. https://doi.org/10.1002/hep.31227. |
[155] | Zen Y , Yeh MM . Checkpoint inhibitor-induced liver injury: A novel form of liver disease emerging in the era of cancer immunotherapy. Semin Diagn Pathol. (2019) ;36: :434–40. https://doi.org/10.1053/j.semdp.2019.07.009. |
[156] | De Martin E , Michot J-M , Rosmorduc O , Guettier C , Samuel D . Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep. 2020;2. https://doi.org/10.1016/j.jhepr.2020.100170. |
[157] | Benson R , Madan R , Kilambi R , Chander S . Radiation induced liver disease: A clinical update. J Egypt Natl Cancer Inst. (2016) ;28: :7–11. https://doi.org/10.1016/j.jnci.2015.08.001. |
[158] | Kim J , Jung Y . Radiation-induced liver disease: current understanding and future perspectives. Exp Mol Med. (2017) ;49: :e359. https://doi.org/10.1038/emm.2017.85. |
[159] | Koay EJ , Owen D , Das P . Radiation-induced liver disease and modern radiotherapy. Semin Radiat Oncol. (2018) ;28: :321–31. https://doi.org/10.1016/j.semradonc.2018.06.007. |
[160] | Mallick S , Benson R , Rath GK . Radiation Toxicity. In: Mallick S, Rath GK, Benson R, editors. Pract. Radiat. Oncol., Singapore: Springer;. 2020, pp. 287-97. https://doi.org/10.1007/978-981-15-0073-2_46. |
[161] | Delis SG , Bakoyiannis A , Biliatis I , Athanassiou K , Tassopoulos N , Dervenis C . Model for end-stage liver disease (MELD) score, as a prognostic factor for post-operative morbidity and mortality in cirrhotic patients, undergoing hepatectomy for hepatocellular carcinoma. HPB. (2009) ;11: (4):351–7. |




