Low Risk of Severe Complications After a Single, Post-Operative Instillation of Intravesical Chemotherapy in Patients with TaG1G2 Urothelial Bladder Carcinoma
Abstract
BACKGROUND:
EAU guidelines recommend a single instillation (SI) of intravesical chemotherapy (e.g. Mitomycin C) within 24 hours after transurethral resection of a bladder tumour (TURBT) in patients with low- to intermediate risk non-muscle invasive bladder cancer without (suspected) bladder perforation or bleeding requiring bladder irrigation. However, remarkable variation exists in the use of SI. The risk of severe complications is likely to contribute to this variation, but evidence is limited.
OBJECTIVE:
To investigate the absolute severe complication and mortality risk after SI in low- and intermediate risk bladder cancer.
METHODS:
In this observational, historic cohort study, data on 25,567 patients diagnosed with TaG1G2 urothelial bladder carcinoma (UBC) between 2009 and 2018 who underwent TURBT were collected from the Netherlands Cancer Registry. Data were supplemented with information on cause of death and severe complications after cancer treatment by re-examining the electronic health records and the 14-day complication risk and the 30-day mortality risk were evaluated.
RESULTS:
On average, 55% of patients had a SI after TURBT, varying from 0–>80% between hospitals. The 30-day mortality risk was 0.02% and the 14-day risk of severe complications was 1.6%.
CONCLUSIONS:
As the absolute risk of mortality and severe complications is very low, SI after TURBT can be considered a safe treatment in patients with low- to intermediate UBC without contraindications for SI. These results imply that a part of eligible patients is denied effective treatment.
INTRODUCTION
Patients with non-muscle invasive bladder cancer (NMIBC) are usually diagnosed and treated with a transurethral resection of the bladder tumour (TURBT) possibly followed by intravesical instillations with chemotherapy or BCG depending on stage. NMIBC often recurs [1, 2] and thereby places a major (economic) burden on the patients themselves as well as on the healthcare system [3]. Previous studies have investigated the effect of a single instillation (SI) of intravesical chemotherapy (e.g. Mitomycin C) within 24 hours after TURBT, and reported a reduced recurrence risk [4–7]. The most recent meta-analysis published in 2016 showed an absolute difference of 14% in the 5-year recurrence rate in patients with Ta-T1 urothelial bladder carcinoma (UBC) with SI versus TURBT only [2]. However, SI was not effective in high-risk patients. Therefore, the use of a SI is recommended in low- to intermediate risk patients by both the European Association of Urology (EAU) Guidelines and the American Urological Association (AUA) Guidelines, assuming that the bladder was not perforated during TURBT and no bladder irrigation was required for bleeding [8, 9].
Even though the beneficial effect of a SI has been extensively shown [2, 4–7] and despite the recommendations in the guidelines [8, 9], several studies reported remarkable variation in the use of this SI in both European countries and the USA [10–15]. A recent study evaluating European practice patterns of SI revealed substantial variation: the proportion of patients with low- or intermediate risk NMIBC receiving SI ranged from 28% to 88% [10]. Although based on fairly old data, a study from the USA evaluating national practice patterns showed that 67% of the interviewed urologists never applied SI in daily clinical practice. Overall, 58% of patients with low risk disease and 28% of patients with intermediate risk disease received a SI [13].
Besides logistic difficulties encountered by applying SI of intravesical chemotherapy and the fact that some urologists question its efficacy [13, 16, 17], another explanation for the low adherence to the guideline recommendation is the risk of severe and potential lethal complications such as extravasation, caused by administering SI after unobserved perforation of the bladder [13, 18–21]. Even though multiple studies, including trials, evaluated the efficacy and safety of a SI and concluded that in patients without contraindications, use of SI is safe [4, 6, 7, 18], “real world” population data on the risks are scarce and controversy regarding the use of a SI remains.
As data on the risks of SI of intravesical chemotherapy are limited, we evaluated the absolute risk of death and severe complications in patients considered eligible for a single instillation and subsequently treated with TURBT followed by SI in a Dutch nationwide cohort of patients diagnosed with TaG1G2 urothelial carcinoma of the bladder between 2009 and 2018.
MATERIALS AND METHODS
For this historic cohort study, data from the Netherlands Cancer Registry (NCR) were used. The NCR is a nationwide, population-based registry serving the total Dutch population of approximately 17 million inhabitants. Data managers of the NCR extract information on patient and tumour characteristics, staging and treatment from the electronic patient files in the hospitals. Vital status is recorded as well in the NCR and is obtained through annual linkage with the Personal Records Database (BRP), which contains information on emigration and vital status of all Dutch inhabitants.
All patients newly diagnosed with a low- or intermediate risk non-invasive papillary (Ta) UBC between 2009 and 2018 were identified in the NCR. Patients with a history of bladder cancer were excluded. Only patients who underwent at least one TURBT were included. Low-or intermediate risk urothelial bladder cancer was defined as a grade 1 or grade 2 tumour according to the 1973 WHO grading system [22]. SI was defined as an intravesical instillation of chemotherapy administered on the day of TURBT or within 1 day after TURBT (as in the NCR only the date of TURBT and date of chemotherapy instillation are recorded). Data concerning patient- and tumour characteristics, i.e. age, gender, tumour histology, stage, grade and focality of the tumour, were retrieved from the NCR. Also information on type of chemotherapeutic agent used for SI and subsequent treatments after SI was retrieved from the NCR as this might have affected the risk of complications and death.
The electronic health records of patients deceased within 30 days after SI were re-examined by data managers of the NCR to retrieve the cause of death. Based on this information, the risk of mortality within 30 days associated with SI was calculated. In addition, we assessed the risk of severe complications, defined as complications necessitating readmission within 14 days after SI or a prolonged hospital stay (i.e. a hospital stay of 3 days or more after SI). This definition was chosen instead of the Clavien-Dindo classification because these data are not recorded as standard data items in the registry. As information on severe complications and readmissions is not readily available in the NCR, data from the Dutch Hospital Data (DHD) register, including all hospital admissions from 2017 and 2018, were linked to the cancer registry. Patients in both registries were linked on patient medical record number, date of birth, gender and 6-digit postal code. Ninety-five percent of all records in the NCR could be linked to records in the DHD and patients with a readmission within 14 days after SI were identified. The time window of 14 days was chosen based on the assumption that severe SI-related complications will be present shortly after the chemotherapy instillation. The electronic health records of all readmitted patients and patients with a prolonged hospital stay were re-examined to evaluate the reason of readmission or prolonged hospital stay and presence of complications related to SI. All reported complications were divided into “possibly related to SI” and “unlikely to be related to SI” and were stratified by chemotherapeutic agent used. Complications possibly related to SI included irritative complaints, pain and voiding dysfunction. Other complications like bleeding and infection were considered as “unlikely to be related to SI”. Based on this information the risk of severe complications possibly related to SI was calculated. We also evaluated the worst case scenario taking into account all reported complications as “possibly related to SI”.
Descriptive analyses were performed to characterize the patient cohort treated with SI after TURBT by age, gender, tumour grade, and focality of the tumour. Variation in use of SI over time and in different geographic regions was assessed with the proportion of patients treated with SI as the outcome variable. Variation between hospitals was assessed using a funnel plot, plotting the proportion of patients treated with SI against the total number of patients with TaG1G2 treated with TURBT per hospital. Hospitals treating less than 10 patients between 2017-2018 and outliers were excluded from the hospital-specific analyses. The benchmark was set at the mean proportion of patients treated with SI between 2017-2018 and 95% confidence intervals were calculated. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA). According to the Central Committee on Research involving Human Subjects (CCMO), this type of study does not require approval from an ethics committee in the Netherlands. The requirement for informed consent was waived due of the retrospective design of the study. This study was approved by the Netherlands Cancer Registry’s Supervisory Committee (reference number K20.009).
RESULTS
In total, 25,567 patients with TaG1G2 UBC were included in this study (Fig. 1). Of these patients, 55% (n = 14,177) received a SI. The proportion of patients who had a SI after TURBT decreased from 56% in 2009 to 48% in 2018, with the highest proportion in 2011 (66%) (Supplementary Figure 1). The increase from 56% in 2009 to 66% in 2011 might reflect the period during which SI was listed as a quality indicator in the Netherlands. In Fig. 2 geographical variation in the use of SI in the period 2017-2018 is presented. In some regions SI was used in less than 40% of patients versus more than 70% in other regions. Variation between hospitals is large as well, ranging from 0% of patients with SI to over 80% (period 2017-2018). The median was 53% and the mean was 48%. The proportion of patients who had a SI after TURBT varies between hospitals and within different hospital volumes of TaG1G2 patients treated with TURBT (Fig. 3).
Fig. 1
Flowchart describing the inclusion of patients in the study cohort TURBT: Transurethral Resection of the Bladder Tumour; SI: Single Instillation.
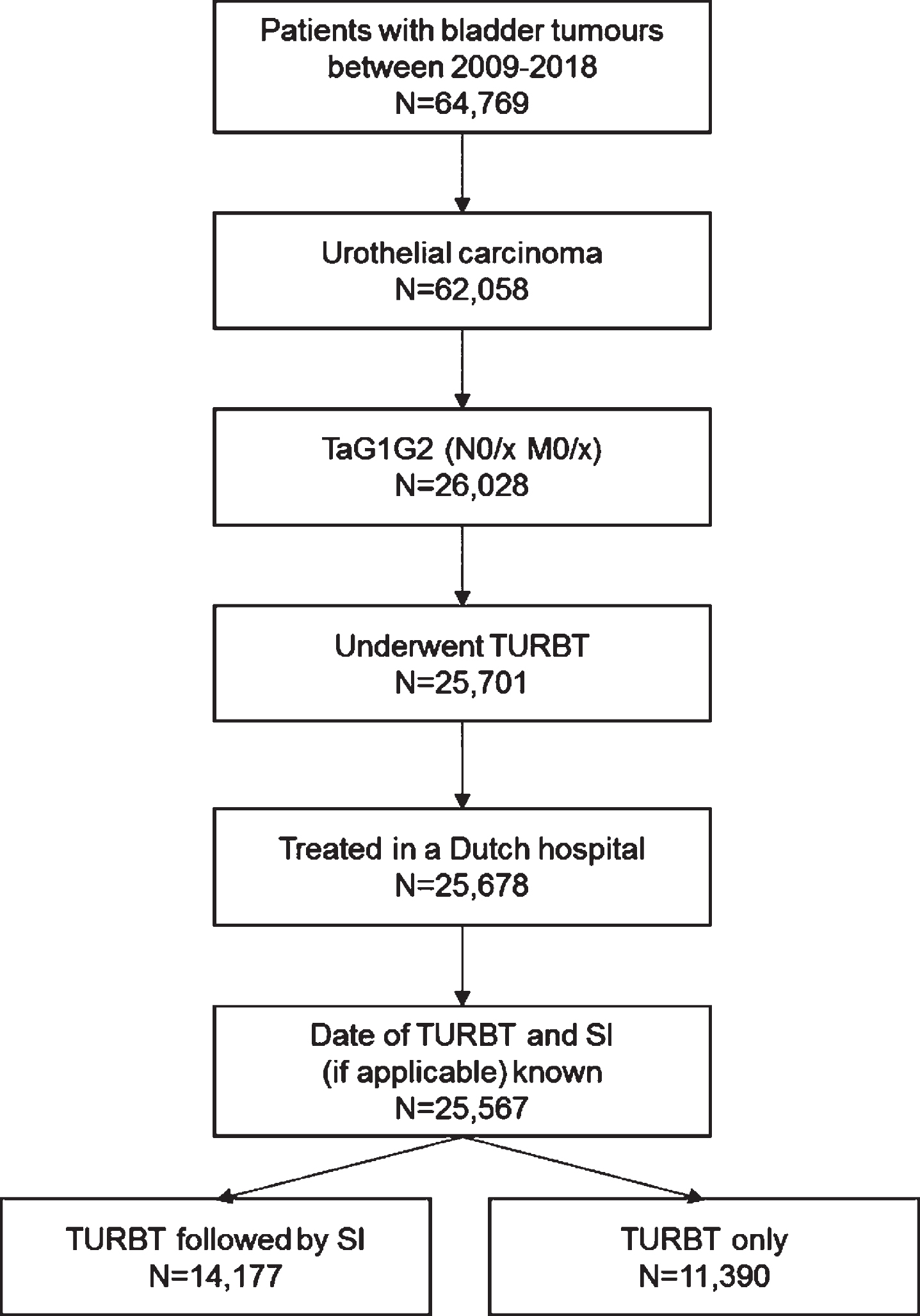
Fig. 2
Percentage of patients diagnosed in 2017-2018 with TaG1G2 urothelial carcinoma receiving a SI per province in the Netherlands SI: Single Instillation.
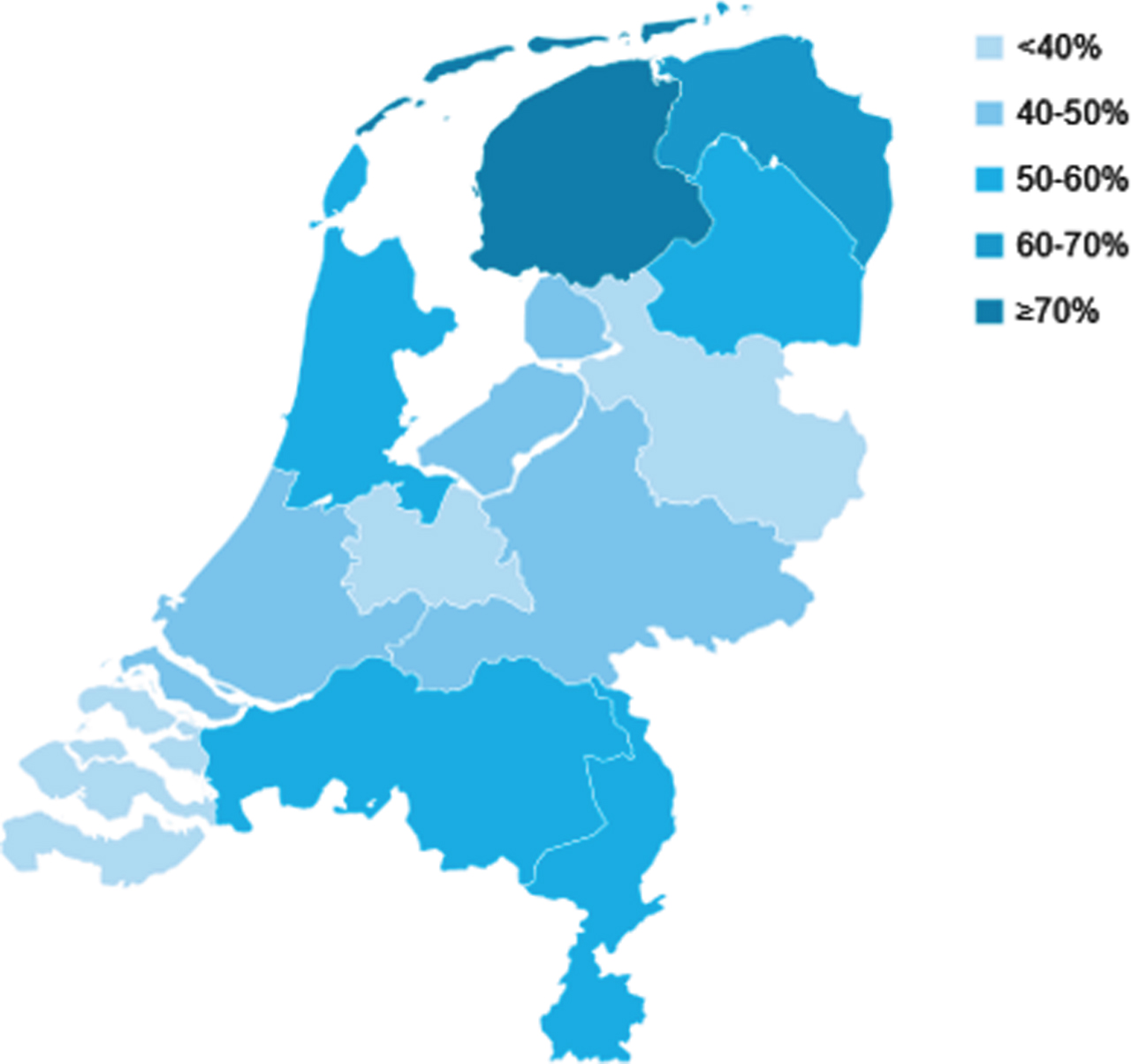
Fig. 3
Percentage of patients diagnosed in 2017-2018 with TaG1G2 urothelial carcinoma receiving a SI by hospital volume in 2017-2018 in the Netherlands SI: Single Instillation *Hospital volume was based on the number of patients with TaG1G2 treated with transurethral resection of the bladder tumour (TURBT). Hospitals with < 10 patients were excluded from the analysis.
The baseline characteristics of all patients treated with SI are shown in Table 1. Within 30 days after SI, one patient (0.01%) underwent partial cystectomy and 237 patients (1.7%) received BCG instillations, of which one patient died within 30 days. In total, 18 patients died within 30 days after SI. In Table 2 several characteristics like age, details regarding TURBT and cause of death of these deceased patients are presented. One death appeared to be linked directly to SI. In one patient, re-examination of the medical file remained inconclusive and therefore we considered this death as possibly associated with SI. For one other patient, no information regarding cause of death could be retrieved. Assuming the worst case scenario and considering the inconclusive deaths as associated with SI, three of 18 deaths were considered associated with SI. The absolute 30-day mortality risk due to SI is therefore 0.02% (3 out of 14,177 patients).
Table 1
Patient, tumour, and treatment characteristics of patients with TaG1G2 urothelial carcinoma treated with TURBT followed by a SI between 2009–2018
| Total | Deceased | |
| n (%) | n (%) | |
| Total | 14,177 (100.0) | 18 (0.1) |
| Age (mean, SD) | 68.1 11.3 | 78.9 7.8 |
| Gender | ||
| Male | 10941 (77.2) | 18 (100.0) |
| Female | 3236 (22.8) | 0 (0.0) |
| Tumour grade (WHO 1973) | ||
| Grade 1 | 6244 (44.0) | 13 (72.2) |
| Grade 2 | 7933 (56.0) | 5 (27.8) |
| Focality of the tumour | ||
| Unifocal | 10679 (75.3) | 16 (88.9) |
| Multifocal | 3058 (21.6) | 2 (11.1) |
| Not documented | 440 (3.1) | 0 (0.0) |
| BCG instillation within | ||
| 30 days after SI | ||
| Yes | 237 (1.7) | 1 (5.6) |
| Partial cystectomy within | ||
| 30 days after SI | ||
| Yes | 1 (0.01) | 0 (0.0) |
TURBT: Transurethral Resection of the Bladder Tumour; SI: Single Instillation; SD: Standard Deviation; WHO: World Health Organization; BCG: Bacille Calmette-Guérin.
Table 2
Overview of patients deceased within 30 days after TURBT followed by a SI between 2009–2018
| Pt. No | Age (years) | Gender | Year of TURBT | Tumour stage | Readmission n days after SI | Deceased n days after SI | Details regarding TURBT | Comorbidities | Cause of death |
| 1 | 80 | Male | 2014 | TaG2 | 0 | 22 | Bladder perforation for which laparotomy was performed | Complications (multiple organ failure) caused by extravasation of SI after bladder perforation | |
| 2 | 78 | Male | 2010 | TaG2 | 2 | 4 | Fausse route urethra | Myocardial infarction, CABG, mitral valve replacement | Myocardial infarction followed by cardiogenic shock |
| 3 | 72 | Male | 2011 | TaG1 | 22 | 28 | Uncomplicated | Myocardial infarction, diabetes mellitus | Ventricular tachycardia |
| 4 | 85 | Male | 2018 | TaG2 | 2 | 8 | Nothing reported | Possibly related to SI: upper abdominal pain during the day after TURBT and SI, and collapsed in the night (asystole/myocardial ischemia/hypovolemia) | |
| 5 | 71 | Male | 2017 | TaG2 | 4 | 7 | Uncomplicated | Respiratory insufficiency (COPD), deteriorating renal function, heart disease | |
| 6 | 79 | Male | 2009 | TaG1 | / | 28 | Uncomplicated | Decompensated heart failure, cardiac arrhythmia, diabetes mellitus | Pleural carcinomatosis (colon cancer) |
| 7 | 69 | Male | 2009 | TaG1 | 9 | 11 | Uncomplicated | Myocardial infarction | CVA |
| 8 | 78 | Male | 2009 | TaG2 | 11 | 21 | Uncomplicated | Liver cirrhosis, diabetes mellitus, venous insufficiency | Decompensated heart failure |
| 9 | 92 | Male | 2009 | TaG1 | 24 | 27 | Nothing reported | Cardiac arrhythmia | Pulmonary embolism |
| 10 | 71 | Male | 2012 | TaG2 | 10 | 12 | Nothing reported | Abdominal aortic aneurysm | |
| 11 | 65 | Male | 2012 | TaG2 | / | 2 | Uncomplicated | Suicide | |
| 12 | 93 | Male | 2013 | TaG2 | –13 | 12 | Readmission for decompensated heart failure, during which bladder tumour was discovered. Thin bladder wall but no mentioning of perforation in TURBT report | Coronary artery disease | Pneumonia, complicated by decompensated heart failure |
| 13 | 74 | Male | 2014 | TaG1 | 8 | 17 | Uncomplicated | Peripheral artery disease, hypertension, atrial fibrillation, thyroid disorder | CVA |
| 14 | 86 | Male | 2015 | TaG2 | / | 29 | Uncomplicated | Hypovolemia, chronic heart failure | |
| 15 | 87 | Male | 2016 | TaG2 | 8 | 29 | Post-operative bleeding | Diabetes mellitus, atrial fibrillation, peripheral artery disease, CABG, CVA | Metastasized bladder cancer |
| 16 | 81 | Male | 2018 | TaG2 | 10 | 11 | Uncomplicated | Pulmonary embolism | |
| 17 | 83 | Male | 2012 | TaG2 | / | 23 | Uncomplicated | Unknown | |
| 18 | 77 | Male | 2015 | TaG2 | / | 20 | Nothing reported | Unknown, however not related to SI. The patient visited the urologist two weeks after TURBT and SI |
TURBT: Transurethral Resection of the Bladder Tumour; SI: Single Instillation; CABG: Coronary Artery Bypass Grafting; CVA: Cerebrovascular Accident.
Out of 2,634 patients who had a SI after TURBT in 2017 and 2018, 60.9% of patients (n = 1,604) received Mitomycin C, 11.1% (n = 292) received epirubicin and in 28.0% (n = 738) the type of chemotherapeutic agent used was not documented (data not shown). In total, 41 patients were readmitted within 14 days, 39 patients had a prolonged hospital stay and 5 patients had both because of one or more complications related to TURBT or SI. The reason for prolonged hospital stay could not be retrieved for four patients. In Table 3 the reported complications are described. The most frequently documented complications were bleeding (n = 44, 1.67%), voiding dysfunction (n = 22, 0.84%) and infection (n = 19, 0.72%). Assuming a worst case scenario resulted in a 14-day complication risk of 3.00% (79 of 2,634 patients). The worst case scenario included all patients with reported complications and the 4 patients with unknown reason of prolonged hospital stay. However, if only the complications “possibly related to SI”, including only irritative complaints, pain and voiding dysfunction, and the patients with unknown reason of prolonged hospital stay are taken into account, this risk decreased to 1.59% (42 of 2,634 patients).
Table 3
Complications possibly related to treatment experienced by patients treated with TURBT followed by a SI between 2017-2018, necessitating a prolonged hospital stay or readmission within 14 days
| Chemotherapeutic agent | Complication* | Readmission | Prolonged hospital stay | Total** | |
| (n = 41) | (n = 39) | (n = 75) | |||
| n% | n % | n % | |||
| Epirubicin (n = 10) | Possibly related to SI | Pain | 3 (0.11) | 1 (0.04) | 4 (0.15) |
| Voiding dysfunction | 3 (0.11) | 0 (0.00) | 3 (0.11) | ||
| Unlikely to be related to SI | Bleeding | 2 (0.08) | 3 (0.11) | 5 (0.19) | |
| Infection | 3 (0.11) | 0 (0.00) | 3 (0.11) | ||
| Kidney obstruction | 1 (0.04) | 0 (0.00) | 1 (0.04) | ||
| (consequences) | |||||
| Perforation (suspected) | 1 (0.04) | 0 (0.00) | 1 (0.04) | ||
| Mitomycin C (n = 65) | Possibly related to SI | Irritative complaints | 2 (0.08) | 0 (0.00) | 2 (0.08) |
| Pain | 6 (0.23) | 8 (0.30) | 14 (0.53) | ||
| Voiding dysfunction | 12 (0.46) | 7 (0.27) | 19 (0.72) | ||
| Unlikely to be related to SI | Bleeding | 14 (0.53) | 25 (0.95) | 39 (1.48) | |
| Delirium | 0 (0.00) | 3 (0.11) | 3 (0.11) | ||
| Infection | 8 (0.30) | 8 (0.30) | 16 (0.61) | ||
| Kidney obstruction | 5 (0.19) | 2 (0.08) | 7 (0.27) | ||
| (consequences) | |||||
| Nausea | 1 (0.04) | 1 (0.04) | 2 (0.08) | ||
| Obstipation | 2 (0.08) | 0 (0.00) | 2 (0.08) | ||
| Perforation (suspected) | 1 (0.04) | 3 (0.11) | 4 (0.15) | ||
| Pneumonia | 1 (0.04) | 0 (0.00) | 1 (0.04) | ||
TURBT: Transurethral Resection of the Bladder Tumour; SI: Single Instillation; Bleeding: haematuria, clots, clogged catheter; Infection: genitourinary infection, fever, elevated inflammatory values; Kidney obstruction (consequences): dilated bladder or kidney, hydronephrosis, phylum blowout, renal dysfunction; Voiding dysfunction: all complications related to micturition, such as inability to urinate (urinary retention) or completely empty the bladder and polyuria *Patients could have had more than one complication. To calculate the risk of complications, the number of patients with complications was divided by 2,634, the number of patients treated with TURBT and SI in 2017-2018. **Five patients necessitated both a prolonged hospital stay and readmission.
DISCUSSION
In this large population-based study reflecting daily practice, we can conclude that the 30-day mortality risk due to SI in patients judged to be eligible is very low (0.02%). Furthermore, the risk of severe complications within 14 days associated with SI requiring hospital readmission or prolonged hospital stay was low (1.6%), even assuming a worst case scenario (including all complications as “possibly related to SI”) (3.0%). In line with previous studies, we observed substantial variation in the proportion of patients with a SI [10, 11, 13], depending on geographical location and between individual hospitals.
EAU guidelines recommend a SI in patients with low to intermediate risk urothelial bladder cancer. Patients with a primary, solitary, or small (≤3 cm) tumour, without carcinoma in situ and no perforation, extensive resection, or bleeding requiring irrigation during TURBT are considered eligible [7]. As not all patients will meet these eligibility criteria, the instillation rate will never reach one hundred percent. Assuming a more or less similar patient population in the Netherlands with regard to geographic region and hospital, case-mix will only explain a small part of the observed variation. Next to case-mix, other factors might contribute to the variation in use of SI. For instance, different perceptions of the risk of complications, e.g. depth of the resection and suspicion of possible perforation of the bladder, will likely play a role [23]. Logistic issues, such as the impossibility of administering SI in the operating room immediately after TURBT or at the ward, might also be a factor [11, 13, 24]. However, in the Netherlands this is rarely the case. Also, part of the urologists doubt the efficacy of SI [12, 13, 24] although a meta-analysis by Sylvester et al. reported a recurrence rate of 44.8% in the SI group versus 58.8% in the TURBT only group [2]. But the most important factor seems to be the risk of severe or even deadly complications caused by administering SI after unobserved perforation of the bladder [25]. This concern is based on studies showing a high risk of extravasation, as for example shown by a prospective study of Balbay et al. from 2005. In this study the perforation rates after TURBT were evaluated in 36 patients with a Ta-T2 bladder tumour and showed that without any evidence of perforation as examined by the surgeon, extravasation of a contrast agent was observed in 58% of TURBTs using a cystogram post-operatively [23]. A similar study from 2009 reported extravasation of a contrast agent in 50% of the 34 patients included [26]. However, in both studies all cases of perforation appeared to be asymptomatic except for one, and none of these patients required surgery or any other medical intervention except for catheterization. Several trials have shown that serious adverse events due to SI after TURBT are rare [4, 6, 18, 27]. Messing et al. reported no severe adverse events of grade 4 or 5 in their trial on intravesical gemcitabine versus saline, and grade 1–3 adverse events were similar between groups [6]. Even in case of suspected extravasation, as for example reported by Bosschieter et al. in 6 out of 1048 included patients (0.57%) surgical intervention was not necessary [18]. As our definition of complications was different, we cannot directly compare our results. Still, we found a low complication rate which is in line with the studies previously mentioned.
In order to get some insight into the reasons for not administering SI, co-author JAW performed an unstructured telephone survey among Dutch urologists (1 urologist per hospital) in a sample of 10 hospitals (13%) with low administration rates of SI. The telephone survey indicated that both risk of complications (8 out of 10 urologists) and disbelief in the efficacy of a SI (7 out of 10 urologists) were important factors when considering administering a SI. A very recent study by Dunsmore et al. (2021) investigated the barriers and facilitators concerning SI in Scotland and England, and found that barriers for administering SI were present on both professional (e.g. urologists, nurses) and organizational (hospital) level. Amongst those barriers, concern about side effects and (non-) belief in efficacy were also mentioned, confirming our findings [24].
With this large Dutch population-based study in which we re-examined the electronic health records, we have provided insight in the adherence to SI in low- and intermediate-risk NMIBC and the absolute mortality and complication risks after TURBT followed by SI. It is good to keep in mind that the reported absolute complication and mortality rate is evaluated in patients already judged to be eligible for SI after TURBT, reflecting the risk that is present in current practice. The overall 30-day mortality rate was very low in these patients, indicating that the assessment of eligibility by urologists was done well. This study has some limitations. We evaluated complications severe enough to require readmission within 14 days or a prolonged hospital stay. Mild complications not resulting in a hospital admission within 14 days could, therefore, not be taken into account. We might have missed severe complications as a result of incomplete documentation in the electronic health records. But considering the severity of complications and the good documentation of the reason of readmission or prolonged hospital stay, we assume that reporting of complications was nearly complete. Since 5% of the NCR records could not be linked to the DHD registry, it is possible that we may have missed some readmissions. In this study we could not evaluate how instillation rates could be improved as not all information on eligibility for SI (e.g. tumour size, specific TURBT details and (possible) perforation) was available or was not documented in the NCR due to rather poor documentation in the medical files. However, from the substantial variation between individual hospitals we observed, we can conclude that instillation rates are indeed suboptimal.
Although in the majority of patients mitomycin C was used as intravesical chemotherapy this might change in upcoming years. A meta-analysis including five randomized controlled trials showed superior efficacy of gemcitabine in preventing recurrences compared to Mitomycin C [28]. Also Messing and colleagues reported a reduced risk of recurrence after a single instillation of intravesical gemcitabine, compared to saline [6]. Therefore, use of gemcitabine as single postoperative instillation might increase over time, possibly necessitating reconsideration of the complication risk and mortality risk of SI.
CONCLUSIONS
Given the very low absolute mortality and low severe complication risk, a single, post-operative instillation of intravesical chemotherapy after TURBT can be considered a safe treatment for eligible patients with low- to intermediate risk bladder cancer who underwent TURBT without suspected perforation or extensive resection or bleeding requiring bladder irrigation. When indicated, a single instillation should therefore be administered in order to reduce risk of recurrence. Given that many urologists might be guided by the risk of complications due to SI and considering the substantial variation in use of SI we observed, it can be assumed that part of these patients are wrongfully denied a recommended and effective treatment.
ACKNOWLEDGMENTS
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry.
FUNDING
The BlaZIB study is funded by the Dutch Cancer Society (KWF; IKNL 2015–7914). The funding agency had no further role in this study.
AUTHOR CONTRIBUTIONS
LMCH: conception, data collection, data analysis, data interpretation, writing the article. JAW: conception, data collection, data interpretation, writing the article. TMR: writing the article. RIN: writing the article. BlaZIB study group: writing the article. LAK: conception, data collection, data interpretation, writing the article. KKHA: conception, data collection, data interpretation, writing the article. The members of the BlaZIB study group (next to the authors) are: Joost Boormans, MD, PhD (Erasmus Medical Centre), Theo M. de Reijke, MD, PhD (Amsterdam University Medical Centres, location AMC), Catharina A. Goossens-Laan, MD, PhD (Alrijne hospital), Sipke Helder (Patient association ‘Leven met blaas- of nierkanker’), Maarten C.C.M. Hulshof, MD, PhD (Amsterdam University Medical Centres, location AMC), Geert J.L.H. van Leenders, MD, PhD (Erasmus Medical Centre), Anna M. Leliveld, MD, PhD (University Medical Centre Groningen), Richard P. Meijer, MD, PhD (University Medical Centre Utrecht), Sasja F. Mulder, MD, PhD (Radboud University Medical Centre), Juus L. Noteboom, MD, PhD (University Medical Centre Utrecht), Jorg R. Oddens, MD, PhD (Amsterdam University Medical Centres, location AMC), Tineke J. Smilde, MD, PhD (Jeroen Bosch ziekenhuis), Guus W.J. Vanderbosch (Patient association ‘Leven met blaas- of nierkanker’), Antoine G. van der Heijden, MD, PhD (Radboud University Medical Centre), Michiel S. van der Heijden, MD, PhD (Netherlands Cancer Institute), Reindert J.A. van Moorselaar, MD, PhD, Prof (Amsterdam University Medical Centres, location VUmc), Bas W.G. van Rhijn, MD, PhD, FEBU (Netherlands Cancer Institute –Antoni van Leeuwenhoek Hospital), Joep G.H. van Roermund, MD, PhD (Maastricht University Medical Centre), Bart P. Wijsman, MD, PhD (Elisabeth-TweeSteden Ziekenhuis).
CONFLICTS OF INTEREST
Lisa M.C. van Hoogtraten, J. Alfred Witjes, Theodora M. Ripping, Ronald I. Nooter, Lambertus A. Kiemeney, Katja K.H. Aben and the BlaZIB study group have no conflicts of interest to declare.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-201515.
REFERENCES
[1] | Cambier S , Sylvester RJ , Collette L , Gontero P , Brausi MA , van Andel G , et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guerin. European Urology. (2016) ;69: (1):60–9. |
[2] | Sylvester RJ , Oosterlinck W , Holmang S , Sydes MR , Birtle A , Gudjonsson S , et al. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? European Urology. (2016) ;69: (2):231–44. |
[3] | CoxE, SaramagoP, KellyJ, PortaN, HallE, TanWS, et al. Effects of Bladder Cancer on UK Healthcare Costs and Patient Health-Related Quality of Life: Evidence From the BOXIT Trial. Clin Genitourin Cancer. (2020) ;18: (4):e418-e42. |
[4] | Zamboni S , Baumeister P , Mattei A , Mordasini L , Antonelli A , Simeone C , et al. Single postoperative instillation for non-muscle invasive bladder cancer: are there still any indication? Transl Androl Urol. (2018) ;8: (1):76–84. |
[5] | Kang M , Jeong CW , Kwak C , Kim HH , Ku JH . Single, immediate postoperative instillation of chemotherapy in non-muscle invasive bladder cancer: a systematic review and network meta-analysis of randomized clinical trials using different drugs. Oncotarget. (2016) ;7: (29):45479–88. |
[6] | Messing EM , Tangen CM , Lerner SP , Sahasrabudhe DM , Koppie TM , Wood DP Jr . et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. Jama. (2018) ;319: (18):1880–8. |
[7] | Sylvester RJ , Oosterlinck W , van der Meijden AP . A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. (2004) ;171: (6 Pt 1):2186–90, quiz 435. |
[8] | Babjuk M , Burger M , Comperat EM , Gontero P , Mostafid AH , Palou J , et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - Update. European Urology. (2019) ;76: (5):639–57. |
[9] | Chang SS , Boorjian SA , Chou R , Clark PE , Daneshmand S , Konety BR , et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. The Journal of Urology. (2016) ;196: (4):1021–9. |
[10] | Hendricksen K , Aziz A , Bes P , Chun FK , Dobruch J , Kluth LA , et al. Discrepancy Between European Association of Urology Guidelines and Daily Practice in the Management of Non-muscle-invasive Bladder Cancer: Results of a European Survey. Eur Urol Focus. (2019) ;5: (4):681–8. |
[11] | Stroman L , Tschobotko B , Abboudi H , Ellis D , Mensah E , Kaneshayogan H , et al. Improving Compliance With a Single Post-Operative Dose of Intravesical Chemotherapy After Transurethral Resection of Bladder Tumour. Nephrourol Mon. (2016) ;8: (1):e29967. |
[12] | Palou-Redorta J , Roupret M , Gallagher JR , Heap K , Corbell C , Schwartz B . The use of immediate postoperative instillations of intravesical chemotherapy after TURBT of NMIBC among European countries. World Journal of Urology. (2014) ;32: (2):525–30. |
[13] | Cookson MS , Chang SS , Oefelein MG , Gallagher JR , Schwartz B , Heap K . National practice patterns for immediate postoperative instillation of chemotherapy in nonmuscle invasive bladder cancer. The Journal of Urology. (2012) ;187: (5):1571–6. |
[14] | Witjes JA , Palou J , Soloway M , Lamm D , Kamat AM , Brausi M , et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): results of an international individual patient data survey (IPDS). BJU International. (2013) ;112: (6):742–50. |
[15] | van Rhijn BW , Burger M . Bladder cancer: Low adherence to guidelines in non-muscle-invasive disease. Nature reviews Urology. (2016) ;13: (10):570–1. |
[16] | Madeb R , Golijanin D , Noyes K , Fisher S , Stephenson JJ , Long SR , et al. Treatment of nonmuscle invading bladder cancer: do physicians in the United States practice evidence based medicine? The use and economic implications of intravesical chemotherapy after transurethral resection of bladder tumors. Cancer. (2009) ;115: (12):2660–70. |
[17] | Burks FN , Liu AB , Suh RS , Schuster TG , Bradford T , Moylan DA , et al. Understanding the use of immediate intravesical chemotherapy for patients with bladder cancer. The Journal of Urology. (2012) ;188: (6):2108–13. |
[18] | Bosschieter J , Nieuwenhuijzen JA , van Ginkel T , Vis AN , Witte B , Newling D , et al. Value of an Immediate Intravesical Instillation of Mitomycin C in Patients with Non-muscle-invasive Bladder Cancer: A Prospective Multicentre Randomised Study in 2243 patients. European Urology. (2018) ;73: (2):226–32. |
[19] | Batura D , Hashemzehi T , Colemeadow J . A care bundle to improve perioperative mitomycin use in non-muscle-invasive bladder cancer. Int Urol Nephrol. (2018) ;50: (6):1053–9. |
[20] | OddensJR, van der MeijdenAP, SylvesterR. One immediate postoperative instillation of chemotherapy in low risk Ta, T1 bladder cancer patients. Is it always safe? European urology. (2004) ;46: (3):336–8. |
[21] | Elmamoun MH , Christmas TJ , Woodhouse CR . Destruction of the bladder by single dose Mitomycin C for low-stage transitional cell carcinoma (TCC)–avoidance, recognition, management and consent. BJU International. (2014) ;113: (5b):E34–8. |
[22] | Mostofi FK , Sorbin LH , Torloni H . Histological typing of urinary bladder tumours. International classification of tumours, 19. Geneva: World Health Organisation, 1973. |
[23] | Balbay MD , Cimentepe E , Unsal A , Bayrak O , Koc A , Akbulut Z . The actual incidence of bladder perforation following transurethral bladder surgery. The Journal of Urology. (2005) ;174: (6):2260–2, discussion 2-3. |
[24] | Dunsmore J , Duncan E , Mariappan P , de Bruin M , MacLennan S , Dimitropoulos K , et al. What influences adherence to guidance for post-operative instillation of intravesical chemotherapy to bladder cancer patients? BJU international. 2021. |
[25] | Mertens LS , Meinhardt W , Rier WB , Nooter RI , Horenblas S . Extravasation of Intravesical Chemotherapy for Non-Muscle-Invasive Bladder Cancer. Urologia Internationalis. (2012) ;89: (3):332–6. |
[26] | El Hayek OR , Coelho RF , Dall’oglio MF , Murta CB , Ribeiro Filho LA , Nunes RL , et al. Evaluation of the incidence of bladder perforation after transurethral bladder tumor resection in a residency setting. J Endourol. (2009) ;23: (7):1183–6. |
[27] | Böhle A , Leyh H , Frei C , Kühn M , Tschada R , Pottek T , et al. Single postoperative instillation of gemcitabine in patients with non-muscle-invasive transitional cell carcinoma of the bladder: a randomised, double-blind, placebo-controlled phase III multicentre study. European Urology. (2009) ;56: (3):495–503. |
[28] | Li R , Li Y , Song J , Gao K , Chen K , Yang X , et al. Intravesical gemcitabine versus mitomycin for non-muscle invasive bladder cancer: a systematic review and meta-analysis of randomized controlled trial. BMC Urol. (2020) ;20: (1):97. |




