Revisiting an Old Conundrum: A Systematic Review and Meta-Analysis of Intravesical Therapy for Treatment of Urothelial Carcinoma of the Prostate
Abstract
BACKGROUND:
The optimal management of non-invasive (mucosal and/or ductal) urothelial carcinoma of the prostate remains elusive and there is a paucity of data to guide treatment.
OBJECTIVE:
Our objective was to systematically review and synthesize treatment responses to conservative management of non-invasive prostatic urothelial carcinoma using intravesical therapy.
METHODS:
A systematic literature search using MEDLINE, EMBASE, Cochrane Library, SCOPUS, and Web of Science databases from inception to November 2019 was performed. Risk of bias assessment was performed using the Newcastle-Ottawa scale for non-randomised studies. Pooled estimates of complete response in the bladder and prostate and prostate only were performed using a random effects model. Pre-specified subgroup analyses were generated to assess differences in complete responses for: BCG therapy vs other agents, ductal vs mucosal involvement, CIS vs papillary tumors and TURP vs no TURP.
RESULTS:
Nine studies including 175 patients were identified for inclusion in the systematic review and meta-analysis. All were retrospective case series and most evaluated response to BCG therapy. The pooled global complete response rate for intravesical therapy was 60%(95%CI: 0.48, 0.72), and for prostate 88%(95%CI: 0.81, 0.96). Pre-specified analyses did not demonstrate statistically significant differences between subgroups of interest.
CONCLUSIONS:
Management of non-invasive prostatic urothelial carcinoma using intravesical therapy yields satisfactory results. Caution should be taken in treating patients with papillary tumors and ductal involvement, as data for these populations is limited. TURP may not improve efficacy, but is required for staging. Current recommendations are based on low quality evidence, and further research is warranted.
INTRODUCTION
Prostatic involvement of urothelial carcinoma (PUC) has an incidence of 16%to 39%in patients presenting with concomitant non-muscle invasive bladder cancer (NMIBC) [1], however the true prevalence remains unknown given that significant sampling biases exist in clinical practice. Invasion of the prostatic stroma is classified as stage T4 by the American Joint Cancer Committee (AJCC) 8th ed, and these patients are offered radical cystectomy (RC) [2]. Non-invasive PUC includes carcinoma in situ (CIS) and urothelial carcinoma with mucosal or ductal involvement of the prostatic urethra. Since the introduction of bacillus calmette-Guerin (BCG) for the treatment of NMIBC, several groups have reported on treatment of non-invasive PUC with various intravesical agents with acceptable treatment responses [3]. Current international guidelines are in keeping with findings in the literature, however these recommendations are based on scarce and low quality evidence [4, 5].
We conducted a systematic review and meta-analysis to ascertain the treatment efficacy of intravesical therapy for non-invasive PUC with complete response as the primary outcome. The overall goal of conducting this study is to describe in detail the literature on this important disease state, and to highlight the need for more rigorous trials for evaluating treatment benefit in a new era of rapidly advancing therapies for bladder cancer (BC).
MATERIALS AND METHODS
Data sources and searches
We performed a systematic search in Ovid MEDLINE, Ovid EMBASE, Cochrane Library, SCOPUS, and Web of Science from inception to November 13, 2019. Search structures, subject headings, and keywords were tailored to each database by a medical research librarian (KJK) specializing in systematic reviews. The complete Ovid MEDLINE and EMBASE search strategies are shown in supplementary Table 1.
Our findings are reported in accordance with the PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses [6].
Eligibility criteria
Adult patients with biopsy-proven urothelial carcinoma of the prostate treated with intravesical therapy were included. Patients with the following histology were considered: CIS, Ta or T1. Both mucosal and ductal involvement were assessed in this analysis. Patients with stromal invasion were excluded. Studies wherein diagnosis of PUC was made following radical surgery on cystectomy specimen were also excluded. Full exclusion criteria are further outlined in supplementary methods.
Study selection and data extraction
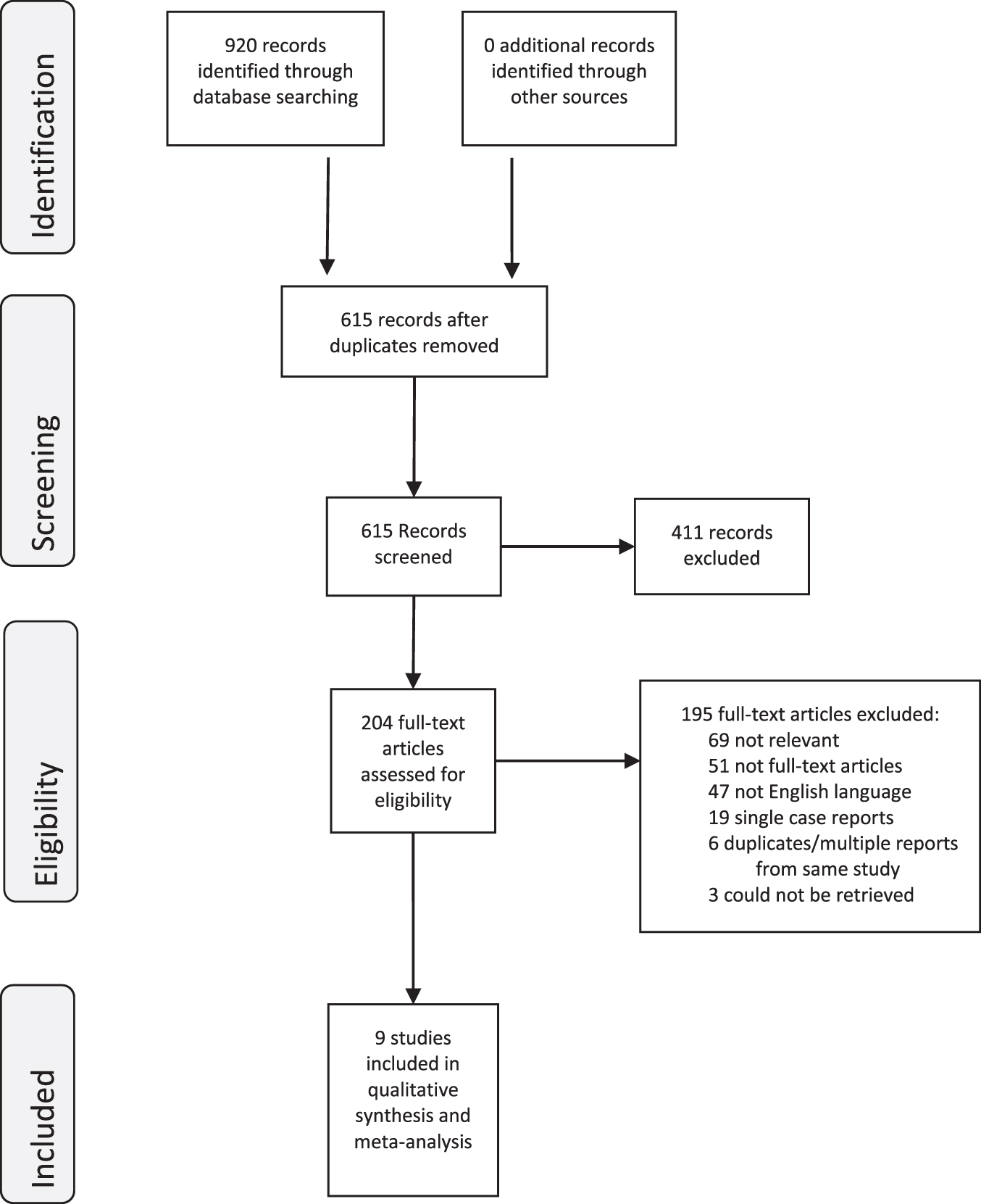
After the initial search, two of the principal investigators (Andrea Kokorovic [AK], Mary Elizabeth Westerman, [MEW]) independently screened the titles and abstracts of the articles to identify potentially relevant studies. Studies that passed the initial review were retrieved for full-text review. We retrieved 204 unique articles for review. The objective was to evaluate the efficacy of intravesical therapy to treat non-invasive (mucosal or ductal without stromal invasion) urothelial carcinoma of the prostate. Data extracted for review are detailed in supplementary methods. Disagreements were resolved by consensus and discussion. Of these, 9 studies met all the criteria for inclusion in this systematic review [7–15]. The PRISMA flow diagram (Fig. 1) shows the entire review process from the original search to the final selection of studies.
Fig. 1
PRISMA Flow Diagram.
Evidence quality and risk of bias (methodological quality) assessment of individual studies
Given that all studies in this analysis are case series, we used a previously published modified Newcastle Ottawa scale that was appropriate for this systematic review (Suppl. Table 2) [16]. The same two reviewers (AK, MEW) used this scale to assess the risk of bias of the included studies. Disagreements were resolved with discussion. Two studies had a low risk of bias, six moderate risk and one study was at high risk (Suppl. Table 3).
Data analysis including meta-analysis
Pooled prevalence estimates and 95%confidence intervals were computed from a random-effects model using the method of DerSimonian and Laird (D + L) [17]. I2 was used to assess the percentage of variation in measures of association across studies due to heterogeneity. I2 ranges between 0%and 100%, where 0%indicates no observed heterogeneity and larger values indicate increasing heterogeneity. To derive D + L pooled estimates, the inverse variance from both within-study and between-study variability were utilized. This method provides larger variance estimates and wider confidence intervals (CI) when compared to fixed-effects models. Statistical significance was determined by non-overlapping 95%CI. Analyses were conducted using the R software (R Foundation for Statistical Computing, Vienna, Austria. URL https://www.Rproject.org/.
RESULTS
Literature search and study characteristics
Nine-hundred twenty references from electronic database searches were identified and 204 potentially relevant publications for full-text evaluation were selected. The final analysis included 9 studies with 175 patients with PUC treated with intravesical therapy. All studies were identified through the database search.
Characteristics of included studies are detailed in Table 1. All included studies were retrospective case series published between 1989 and 2008. Sample sizes ranged from 10 [10] to 32 [9] patients. The mean age at time of diagnosis was 66 years (2 studies did not report mean or median age [7, 14]. Patients had concomitant bladder and PU pathology in 7 studies [7–9, 11, 12, 14, 15]; 1 patient had an isolated PU tumor in a single study [13]; the last study did not specify [10]. Bladder tumor histologies are summarized in Suppl. Table 8. BCG strain, number of induction courses and use of maintenance therapy varied across studies with only 2 studies using maintenance BCG [14, 15], albeit not for the entire patient population. Outcomes of patients stratified according to receipt of maintenance vs induction BCG only were not reported. (Suppl. Table 4).
Table 1
Studies investigating intravesical therapy for treatment of prostatic urothelial carcinoma
| Study Author, year | Treatment range (years) | Patients (N) | Treatment | Pathology | Median age | Follow-up (Months) | CR (%global) | CR (%prostate) |
| Bretton, 1989 | 1978–1984 | 23 | BCG | Mucosal, ductal | – | 51.6 | 56.5 | 100 |
| Orihuela, 1989 | 1978–1984 | 15 | BCG | Mucosal | 62 | 37 | 86.7 | 86.7 |
| Splspna, 1.991 | 1984–1987 | 32 | MMC/AMC | Mucosal | 66 | 27.3 | 59.4 | 71.9 |
| Ovesen. 1993 | – | 10 | BCG | Mucosal | 69 | 26 | 80 | SO |
| Palou, 1996 | 1989–1995 | IS | BCG | Mucosal | 66 | 31.1 | 77.8 | 83.3 |
| Canda, 2004 | 1989–2002 | 19 | BCG/Epirubicin | Mucosal | 63 | 63 | 47.4 | 94.7 |
| Palou Redorta, 2006 | 1992–2003 | 10 | BCG | Ductal | 66 | 40 | 50 | 80 |
| Taylor, 2007 | 1981–1999 | 2S | BCG | Mucosal, ductal | – | 90 | 46.4 | - |
| Gofrit, 2008 | 1988–2005 | 20 | BCG | Mucosal, ductal | 68 | 52.5 | 35 | 90 |
Patients were diagnosed at initial NMIBC diagnosis in 2 studies [10, 14], at time of BC recurrence in 3 studies [7–9], a combination of both in 2 studies [13, 15], and 2 studies did not specify [11, 12]. Method of diagnosis of PUC was inconsistent. All patients were BCG-naïve in 5 studies [8, 10–13]. Three studies biopsied the PU for cases of macroscopic tumor or suspected involvement, although suspected was not defined [7, 8–12]. Random biopsies were taken in the remaining 6 studies as part of standard procedure in the patient population [9–11, 13–15]; 2 of these only took random PU samples in patients with high risk BC or positive cytology [13, 15].
Efficacy analysis
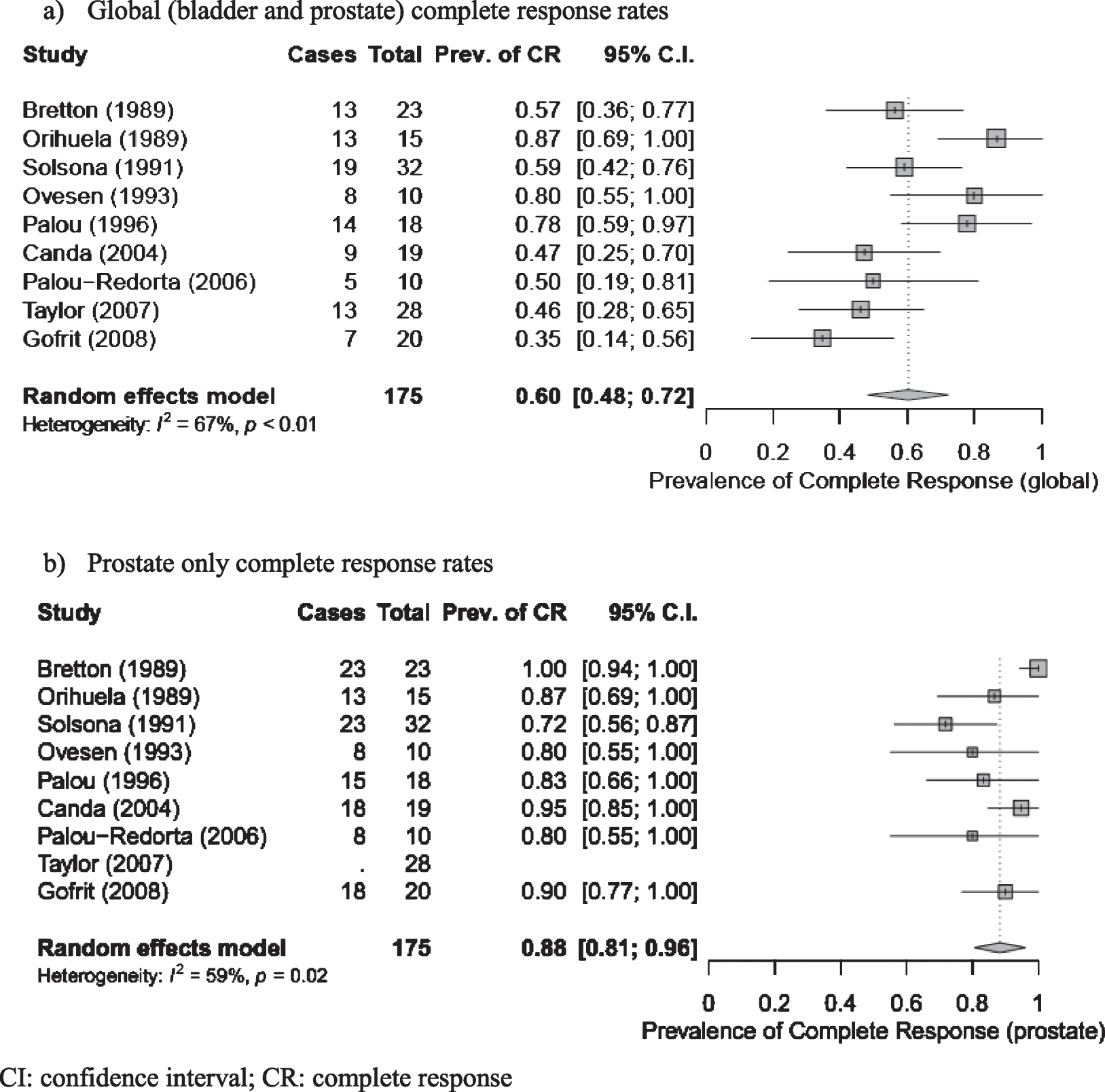
Nine studies reported CR as an efficacy outcome, however the definition of CR and failure of therapy were inconsistent (Suppl. Table 5). For the purpose of this analysis, global CR was defined as no evidence of disease recurrence or progression (bladder, upper tracts or prostate) at time of last known follow up. Prostate CR was defined as no evidence of disease recurrence or progression in the PU at time of last known follow-up. Using these definitions for the meta-analysis the pooled global CR estimate was 60%(95%CI: 48%–72%) with substantial heterogeneity (I2 = 67%, p < 0.01) (Fig. 2) Meta-analysis of the pooled estimate prostate CR was 88%(95%CI: 81%–96%) with substantial heterogeneity (I2 = 59%, p = 0.02) (Fig. 2). The PU and bladder were biopsied as part of routine follow-up in 4 studies [3, 10, 13, 15], at a range of 6 weeks to 3 years following treatment completion. Orihuela et al. [8] obtained biopsies routinely only in patients with documented PUC prior to BCG therapy. Follow up intervals ranged from 26 to 90 months.
Fig. 2
Global and prostate complete response estimates for all studies. a) Global (bladder and prostate) complete response rates. b) Prostate only complete response rates. CI: confidence interval; CR: complete response.
Secondary efficacy outcomes included progression free survival (PFS; 2 studies) [7, 12], disease free survival (DFS; 1 study) [14], recurrence free survival (RFS; 2 studies) [14, 15] and overall survival (OS; 3 studies) [7, 12, 14] (Suppl. Table 6). Progression of disease requiring RC ranged from 7%[8] to 29%[14] and development of metastases ranged from 0%[8, 14] to 29%[12] across series, although this was inconsistently reported (Suppl. Table 7).
Treatment of mucosal vs ductal involvement
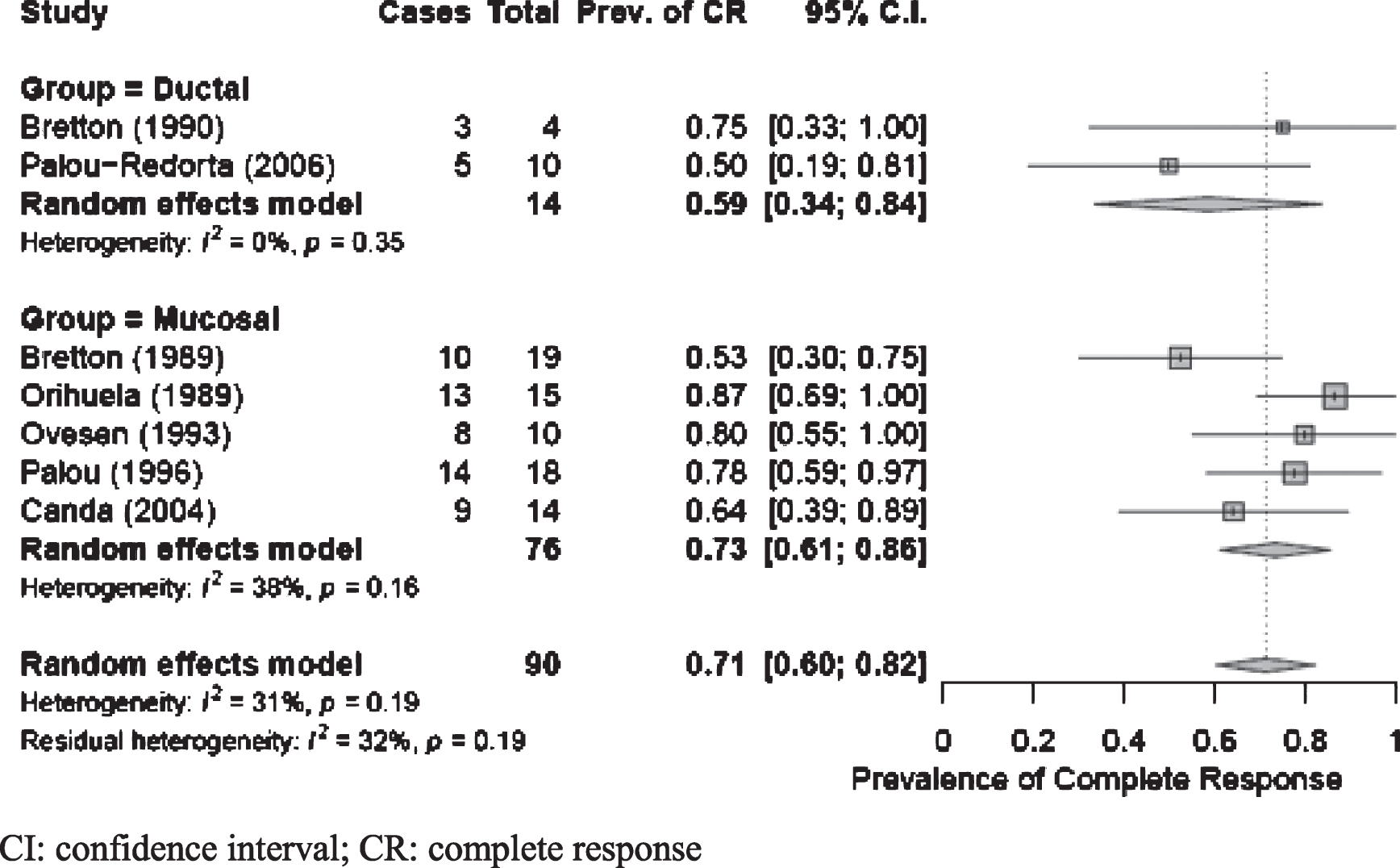
We identified 3 studies that included patients with either mucosal or ductal pathology [7, 14, 15]; 5 studies treated patients with mucosal involvement only [8–12], and 1 reported on patients with ductal pathology only [13]. Of studies including patients with both ductal and mucosal involvement, only 1 reported separate outcomes between two groups [7]. We performed a meta-analysis to define global CR using BCG-treatment studies where response could be stratified by mucosal vs ductal pathology. The pooled estimate for mucosal CR of 73%(95%CI: 0.61, 0.86) was greater than ductal CR of 59%(95%CI: 0.34, 0.84) with nonsignificant heterogeneity for both groups; however, the difference in CR between pathologies was not statistically significant (Fig. 3).
Fig. 3
Global complete response for studies stratified by ductal vs mucosal PUC involvement. CI: confidence interval; CR: complete response.
Treatment with BCG vs other intravesical therapy
BCG was the sole intravesical agent used in 7 studies [7, 8, 10, 11, 13–15]. Canda et al. treated patients with BCG or epirubicin [12]. Two patients in the epirubicin group failed therapy and were subsequently treated with BCG to achieve CR. One study treated patients with mitomycin C (MMC) or Adriamycin (AMC) but did not report separate outcomes between treatment arms, therefore the results are pooled [9]. Meta-analysis of global CR for patients treated with BCG vs other intravesical therapy revealed a pooled estimate of 63%(95%CI: 0.49; 0.76) and 47%(95%CI: 0.18; 0.77), respectively, with substantial heterogeneity in the BCG group (I2 = 69%, p < 0.01). Although the pooled CR estimate was greater for patients treated with BCG, there was no statistical difference in CR between the therapies (Suppl. Fig. 1).
Treatment with or without TURP
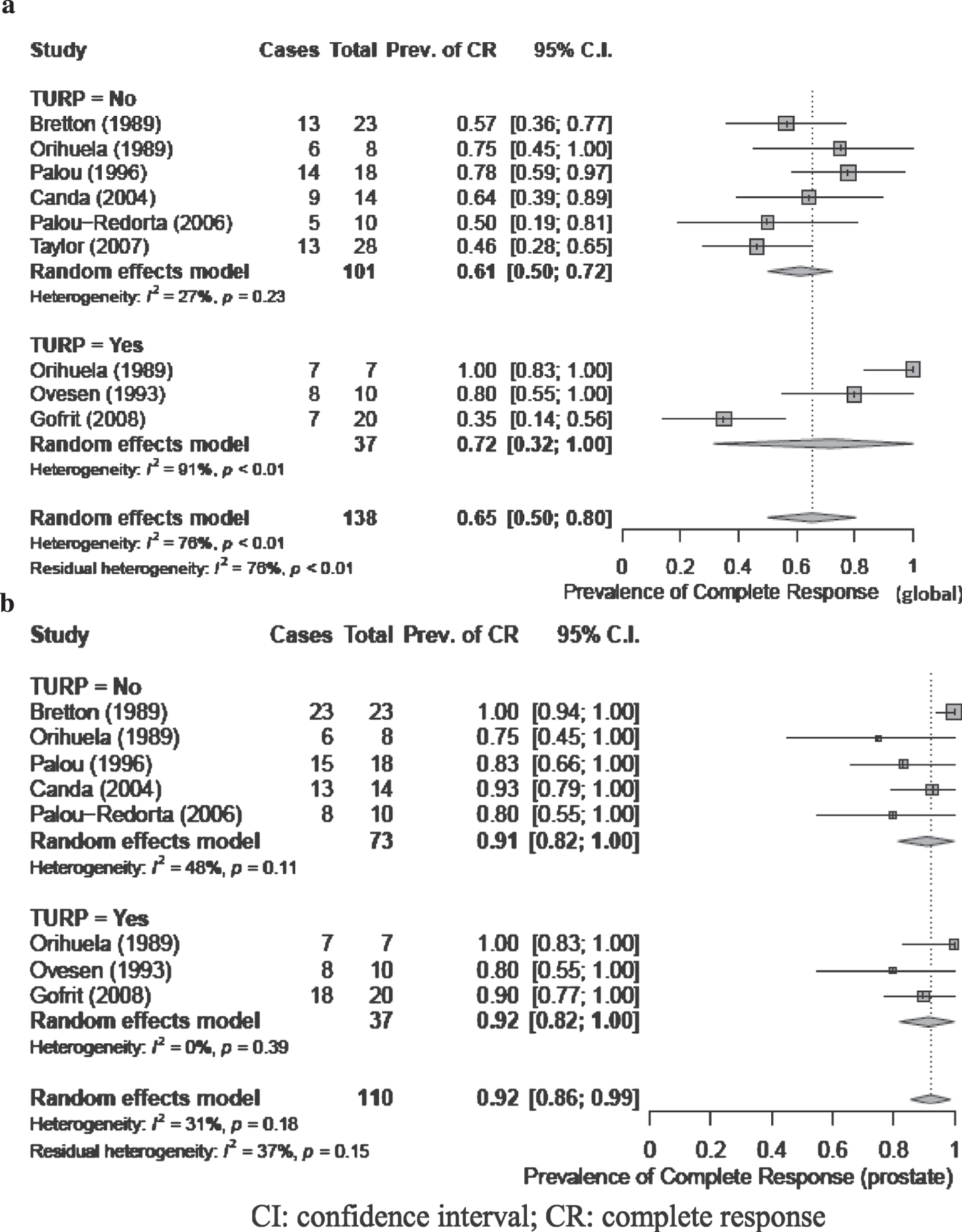
Three studies treated patients with transurethral resection of prostate (TURP) prior to intravesical therapy instillation [9, 10, 15], 5 studies did not perform TURP [7, 11–14], and 1 study included patients treated with or without TURP [8]. Meta-analysis of global CR for patients stratified by receipt of TURP versus no TURP revealed a pooled estimate of 61%(95%CI: 0.50, 0.72) and 72%(95%CI: 0.32, 1.0), respectively, and no significant difference between groups (Fig. 4). The level of heterogeneity between studies was not significant for the no TURP group (I2 = 27%; p = 0.23), while substantial heterogeneity was observed in the TURP group (I2 = 91%; p < 0.01). Pooled estimates of response using prostate CR showed no significant difference between treatment with or without TURP (Fig. 4).
Fig. 4
a and b: Complete response (global (4a) and prostate (4b)) for patients stratified by receipt of TURP. CI: confidence interval; CR: complete response.
Treatment of CIS vs papillary tumors
Four studies treated patients with only CIS of the PU [7, 10, 11, 13], 4 studies included patients with both CIS and/or papillary non-invasive disease [8, 9, 12, 15], and histology in one study was unclear [14]. Primary bladder pathology varied across series. Bladder and prostate pathology are summarized in Suppl. Table 8. Meta-analysis of 6 studies using BCG therapy where papillary and CIS outcomes were separately reported was performed to determine global CR. The pooled estimate of global CR for the CIS group was 72%(95%CI: 0.60, 0.84) and 76%(95%CI: 0.27, 1.00) for the papillary group, with substantial heterogeneity for the latter (I2 = 85%, p = 0.01) and no significant difference in outcome between the tumor types (Suppl. Fig. 2).
DISCUSSION
The treatment of PUC remains controversial. The prognosis of patients with mucosal or ductal involvement is comparable to men without prostatic disease, however progression to stromal invasion confers significantly worse survival [18, 19]. Therefore, it is imperative to determine optimal management in order to avoid progression and to improve outcomes. Here, we present results of the first systematic review and meta-analysis on the topic. The meta-analysis demonstrated a pooled-estimate CR in both the bladder and prostate of 60%(95%CI: 0.48, 0.72), suggesting efficacy of intravesical therapy for treatment of non-invasive PUC in the setting of NMIBC. This is in keeping with current guidelines [4, 5], which recommend BCG for this group of patients, albeit based on low levels of evidence. Importantly, the presence of PUC categorizes patients into high-risk disease, and therefore this group is considered to be at increased risk for tumor progression and recurrence [5].
Among included studies, the incidence of PUC diagnosed during the course of conservative therapy was 3.4%to 27.8%. In contrast, the PUC incidence from cystoprostatectomy specimens may be as high as 50%[20]. The difference is likely due to significant sampling bias, which may be the result of overall lack of prostatic urethral biopsies, or alternatively due to inadequate sampling technique. Theoretically, all biopsies for the diagnosis of invasion should be performed at the 5 and 7 o’clock positions, as the majority of PUC is located in this location [21]. We identified 5 studies that performed routine PU biopsies as part of standard procedure for all BC patients [9, 10, 14] or high risk patients prior to initiation of BCG therapy [13, 15]. The method of sampling was not standardized, with only one study reliably performing resectoscope loop biopsies of the verumontanum and lateral lobes in a routine manner [14]. The rate of 3.4%was found in a study obtaining routine biopsies in high risk patients (however type of biopsy was not specified [15]), while the higher estimate of 27.8%was from a study performing cold cup biopsies in all patients with bladder CIS only [10]. This is comparable to results from RC specimens [22], and support routine PU biopsies in patients with bladder CIS at a minimum. Consideration should also be given for performing PU biopsies in patients with high-risk disease, as early identification of PUC at the time of initial diagnosis allows for appropriate follow up protocols and timely identification of BCG unresponsiveness.
The ability of intravesical therapy to penetrate the PU is unclear. The presence of granulomas within the prostate of patients receiving BCG therapy suggests adequate exposure and infiltration [23, 24], however this has never been correlated with clinical outcomes. TURP prior to BCG administration has been proposed as a potential mechanism of increasing PU exposure to the drug, but results have been inconsistent. Our meta-analysis demonstrates a pooled-estimate prostate CR of 88%(95%CI: 0.81, 0.96) for patients receiving BCG, indicating an adequate degree of penetration. Subgroup analysis of TURP versus no TURP did not demonstrate a significant difference in CR (global and prostate) rate between the two groups, suggesting bladder neck resection is not required. However, one must consider the potential for selection bias in these studies, as patients undergoing TURP may also be more likely to be diagnosed with stromal invasion and therefore would not have received intravesical therapy. Therefore, while the data suggests TURP in these patients may not offer therapeutic benefit, we would encourage its use to rule out stromal invasion and appropriately select patients for intravesical treatment.
The optimal treatment of PUC with ductal involvement remains elusive. We identified only two studies in which outcomes of ductal invasion are available [7, 13], with a combined total of 14 patients. Clearly, any recommendations regarding treatment are limited based on this paucity of data. Nonetheless, our pooled estimate global CR for PUC with ductal involvement is 59%(95%CI: 0.34, 0.84) with no heterogeneity observed between the two studies (I = 0%, p = 0.35). Palou-Redorta identified 10 patients with ductal involvement, 2 of whom required radical cystectomy with one developing metastatic disease [13]. These finding suggest that a trial of BCG may be appropriate, although it is imperative to adequately stage the extent of disease to rule out underlying stromal invasion. The EAU guidelines acknowledge limitations in the data and suggest consideration of upfront RC [4]. Based on our results, we advocate for more robust studies regarding ductal involvement in order to avoid potential overtreatment and morbidity associated with radical surgery, as well as to improve patient counseling.
We performed additional subgroup analyses to ascertain differences in treatment response for BCG versus other intravesical therapy and papillary tumors versus CIS only. We did not identify a statistically significant difference in outcomes between these groups. It is important to note that only two studies including 10 patients had outcomes for papillary PUC involvement with a substantial degree of heterogeneity. Therefore, conclusions regarding the role of intravesical therapy for treating papillary prostatic tumors should be made cautiously. This represents a major challenge in treating patients with PUC and warrants further investigation. The chemotherapeutic agents used in these publications include epirubicin, adriamycin and mitomycin, most of which are of historic interest, and there did not appear to be a difference in efficacy between them and BCG. Conclusions regarding efficacy of these agents individually to treat PUC can not be drawn from this analysis, however their use should not be recommended based on data extrapolated from NMIBC literature [25–27]. Another important confounding variable lies in the inadequate use of maintenance BCG in the majority of patients in this meta-analysis, given the historical nature of the publications. More data is required to determine the effect of maintenance BCG for treating PUC, however the benefit is likely to mirror what is seen in NMIBC.
Key points from this analysis are summarized in Table 2.
Table 2
Key points regarding intravesical therapy for treatment of PUC
| Mucosal PUC with CIS is amendable to conservative therapy with BCG based on low quality of evidence |
| TURP does not appear to improve response to therapy, however should be used for detection of stromal involvement |
| Conclusions regarding efficacy for papillary Ta and T1 PUC tumors cannot be made and further study is warranted |
| Intravesical therapy for ductal PUC may be appropriate, but further studies are required |
Limitations
First, the study was not registered with the International Prospective Register of Systematic Reviews (PROSPERO). Studies may have been missed despite an exhaustive search. All studies identified are case series or case reports and included only 175 patients, significantly limiting the level of evidence for the meta-analysis. The heterogeneity across trial populations was substantial. Our subgroup analyses were pre-specified and not designed to test for heterogeneity. It is likely that the low sample sizes, wide range in year of publication and differences in bladder and prostate tumor characteristics contributed substantially. Nonetheless, this is the first study to investigate the outcomes of using intravesical therapy for the treatment of PUC and is an important addition to the literature. Furthermore, it sheds light on important future directions in what we highlight to be an understudied, yet significant, BC disease entity.
CONCLUSIONS
Treatment of PUC with intravesical therapy confers acceptable CR rates and should be recommended for treatment of CIS and mucosal PUC. The use of intravesical therapy for treatment of papillary and ductal PUC tumors should be performed with caution given the paucity of data and significant study limitations. TURP does not appear to improve response to therapy, but is encouraged for adequate staging and to rule out stromal invasion. In an era of novel and rapidly emerging treatment options for urothelial carcinoma, we believe that a more judicious assessment of PUC is warranted.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The following grants have supported the resources used for this study (for Dr. Dinney): 3P50 CA 91846-15S1 (Dinney) 9/1/2018-8/31/2019 NIH/NCI UT MD Anderson SPORE in Urinary Bladder Cancer.
AUTHOR CONTRIBUTIONS
Kokorovic – conception, performance of work, interpretation of data, writing the article. Westerman – conception, performance of work, interpretation of data, writing the article. Krause – performance of work, writing the article. Hernandez – analysis and interpretation of data, writing the article. Brooks – interpretation of data, writing the article. Dinney – conception, interpretation of data. Kamat – interpretation of data, writing the manuscript. Navai – conception, performance of work, interpretation of data, writing the article.
ETHICAL CONSIDERATIONS
This study, as a literature review, is exempt from any requirement for Institutional Review Board approval. No human or animal research was involved in the elaboration of this manuscript.
CONFLICTS OF INTEREST
Kokorovic – none. Westerman - none. Krause – none. Hernandez – none. Brooks – none. Dinney – FKD - Research. NCI - research. Univ of East Finland - Faculty of Health Sciences (UEFHS). CPND acknowledges using shared resources covered by the Cancer Center Support Grant funding from NIH/NCI (award number P30CA016672) at MD Anderson Cancer Center and has received grant and personal fees from FKD Therapies. In addition, CPND is a creator of intellectual property owned by UT/MDACC related to the use of genetic alterations as a predictive biomarker for response to Nadofaragene firadenovec. Kamat. Consultant/Advisory Board: Abbott Molecular, Arquer, ArTara, Asieris, Astra Zeneca, BioClin Therapeutics, BMS, Cepheid, Cold Genesys, Eisai, Engene, Inc., Ferring, FerGene, Imagin, Janssen, MDxHealth, Medac, Merck, Pfizer, Photocure, ProTara, Roviant, Seattle Genetics, Sessen Bio, Theralase, TMC Innovation, US Biotest
Grants/Research Support: Adolor, BMS, FKD Industries, Heat Biologics, Merck, Photocure, SWOG/NIH, SPORE, AIBCCR. Patent: CyPRIT (Cytokine Predictors of Response to Intravesical Therapy) Joint with UT MD Anderson Cancer Center. Navai - none
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-200404.
REFERENCES
[1] | Walsh DL , Chang SS . Dilemmas in the treatment of urothelial cancers of the prostate. 2009, Urol Oncol (2009) ;27: :352–57. |
[2] | Amin MB , Edge S , Greene F , et al (Eds). AJCC Cancer Staging Manual (8th ed): Springer International Publishing: American Joint Commission on Cancer, (2017) . |
[3] | Palou J , Banuel J , Klotz L , et al. Urothelial carcinoma of the prostate. Urology (2007) ;69: :50–61. |
[4] | Babjuk M , Burger M , Comperat EM , et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol (2019) ;67: :639–57. |
[5] | Chang SS , Boorjian SA , Chou R , et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol (2016) ;196: :1021–9. |
[6] | Moher D , Liberati A , Tetzlaff J et al. The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ (2009) ;339: :b2535. |
[7] | Bretton PR , Herr HW , Whitmore WF , et al. Intravesical bacillus calmette-guerin therapy for in situ transitional cell carcinoma involving the prostatic urethra. J Urol (1989) ;141: :853–6. |
[8] | Orihuela E , Herr H , Whitmore W . Conservative treatment of superficial transitional cell carcinoma of prostatic urethra with intravesical BCG. Urology (1989) ;34: :231–37. |
[9] | Solsona E , Iborra I , Ricos JV , et al. Recurrence of superficial bladder tumors in prostatic urethra. Eur Urol (1991) ;19: :89–92. |
[10] | Ovesen H , Poulsen AL , Steven K . Intravesical bacillus calmette-guerin with the Danish strain for treatment of carcinoma in situ of the bladder. Br J Urol (1993) ;72: :744–48. |
[11] | Palou J , Xavier B , Laguna P , et al. In situ transitional cell carcinoma involvement of prostatic urethra: Bacillus calmette-guerin therapy without previous transurethral resection of the prostate. Urology (1996) ;47: :482–84. |
[12] | Canda EA , Tuzel E , Mungan MU , et al. Conservative management of mucosal prostatic urethral involvement in patients with superficial transitional cell carcinoma of the bladder. Eur Urol (2004) ;45: :465–70. |
[13] | Palou Redorta J , Shatterman P , Perez JH , et al. Intravesical instillations with bacillus calmette-guerin for the treatment of carcinoma in situ involving prostatic ducts. Eur Urol (2006) ;49: :834–38. |
[14] | Taylor JH , Davis J , Schellhammer P . Long-term follow-up of intravesical bacillus calmette-guerin treatment for superficial transitional-cell carcinoma of the bladder involving the prostatic urethra. Clin Genitourin Cancer (2007) :5: :386–89. |
[15] | Gofrit ON , Pode D , Pizov G , et al. Prostatic urothelial carcinoma: is transurethral prostatectomy necessary before bacillus calmette-guerin immunotherapy? BJU Int (2008) :103: :905–8. |
[16] | Haffar S , Bazerbachi F , Prokop L , et al. Frequency and prognosis of acute pancreatitis associated with fulminant or non-fulminant acute hepatitis A: A systematic review. Pancreatology (2017) :17: :166–75. |
[17] | DerSimonian R , Laird N . Meta-analysis in clinical trials. Control Clin Trials (1986) :7: :177–88. |
[18] | Esrig D , Freeman J , Elmajian D , et al. Transitional cell carcinoma involving the prostate with a proposed staging classification for stromal invasion. J Urol (1996) ;156: :1071–6. |
[19] | Herr H , Donat S . Prostatic tumor relapse in patients with superficial bladder tumors: 15-year outcome. J Urol (1999) ;161: :1854–7. |
[20] | Revelo MP , Cookson MS , Chang SS , et al. Incidence and location of prostate and urothelial carcinoma in prostates from cystoprostatectomies: Implications for apical sparing surgery. J Urol (2004) ;171: :646–51. |
[21] | Sakamoto N , Tsuneyoshi M , Naito S , et al. An adequate sampling of the prostate to identify prostatic involvement by urothelial carcinoma in bladder cancer patients. J Urol (1993) ;149: :318–21. |
[22] | Nixon RG , Chang SS , Lafleur BJ , et al. Carcinoma in situ and tumor multifocality predict the risk of prostatic urethral involvement at radical cystectomy in men with transitional cell carcinoma of the bladder. J Urol (2002) :167: :502–5. |
[23] | LaFontaine PD , Middleman BR , Graham SD , et al. Incidence of granulomatous prostatitis and acid-fast bacilli after intravesical BCG therapy. Urology (1997) ;49: :363–6. |
[24] | Oates RD , Stilmant MM , Freedlund MC , et al. Granulomatous prostatis following bacillus Calmette-GUerin immunotherapy of bladder cancer. J Urol (1988) ;140: :751–4. |
[25] | Malmström PU , Sylvester RJ , Crawford DE , et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol (2009) ;56: :247–56. |
[26] | Shang PF , Kwong J , Wang ZP , et al. Intravesical bacillus calmette-guerin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst Rev (2011) :11: :CD006885. |
[27] | Lamm D , Blumenstein B , Crawford D , et al. A radomized trial of intravesical doxorubicin and immunotherapy with bacillus calmette-guerin for transitional cell carcinoma of the bladder. N Eng J Med (1991) ;325: :1205–9. |




