5α-Reductase Inhibitors Do Not Prevent the Development and Progression of Urothelial Cancer: In Vitro Evidence
Abstract
BACKGROUND:
Androgen receptor (AR) activation has been implicated in the pathogenesis of urothelial cancer. However, it remains controversial whether 5α-reductase inhibitors (5α-RIs), which are known for blocking the conversion of testosterone to the more potent androgen dihydrotestosterone and often prescribed for the treatment of, for instance, benign prostatic hyperplasia, contribute to preventing the development of bladder cancer.
OBJECTIVE:
To determine the role of 5α-RI therapy in urothelial tumorigenesis and tumor progression, using cell line models.
METHODS:
In a human non-neoplastic urothelial SVHUC subline stably expressing a full-length wild-type human AR (SVHUC-AR) with carcinogen/MCA challenge and human bladder cancer lines, we assessed the effects of three 5α-RIs, dutasteride (up to 100 nM), finasteride (up to 500 nM), and epristeride (up to 5μM), on neoplastic/malignant transformation and cell growth, respectively.
RESULTS:
In AR-positive bladder cancer UMUC3 and 5637-AR cells, an AR antagonist bicalutamide significantly inhibited their proliferation, whereas three 5α-RIs failed to do. Similarly, these 5α-RIs did not significantly inhibit the migration of bladder cancer cells induced by the treatment of testosterone which could be metabolized into dihydrotestosterone in culture medium. In MCA-SVHUC-AR cells, induction of their neoplastic transformation by testosterone, which was prevented by bicalutamide, was confirmed. However, no significant inhibitory effects of 5α-RIs on the neoplastic transformation of AR-positive urothelial cells treated with or without testosterone were observed.
CONCLUSIONS:
Using in vitro models for urothelial cancer, 5α-RI treatment even at supra-pharmacological doses was thus found to have no significant impact on the prevention of both tumorigenesis and tumor progression.
INTRODUCTION
Urinary bladder cancer, mostly urothelial carcinoma, has been one of commonly diagnosed malignancies predominantly affecting males throughout the world [1, 2]. Strikingly, the imbalance in the risk of bladder cancer between the sexes has been observed for many years. Indeed, in the United States, it is estimated in 2020 that 61,100 men and 19,300 women will newly develop bladder cancer and that 13,050 men and 4,930 women will die of the disease [2]. In addition, women tend to be diagnosed with more advanced stage disease and have higher mortality from bladder cancer [3]. To explain these sex-related differences, the involvement of intrinsic factors, in addition to known extrinsic factors such as cigarette smoking and industrial chemicals, in urothelial oncogenesis has been explored.
Of the potential intrinsic factors studied, androgen receptor (AR) has been shown to promote the development and progression of urothelial cancer, mainly using preclinical models [reviewed in 4]. Specifically, in mice lacking a functional AR in the whole body [5] or only in urothelial cells [6], a potent chemical carcinogen N-butyl-N-(4-hydroxybutyl)nitrosamine failed to induce tumors in their bladder. Correspondingly, it has been documented that AR inhibitors, including flutamide, bicalutamide, and enzalutamide, prevent the neoplastic transformation of AR-positive urothelial cells [7] or the growth or AR-positive bladder cancer cells [5, 8]. Moreover, retrospective clinical studies have demonstrated that the incidence of de novo bladder cancer [9] or recurrent bladder tumor [10, 11] is significantly lower in men undergoing androgen deprivation therapy for their prostate cancer, compared with control prostate cancer patients without hormonal treatment.
5α-reductase (5α-R) is an enzyme that converts intracellular testosterone into the generally more potent androgen dihydrotestosterone (DHT). Three 5α-R isozymes, types 1–3, have been discovered and shown to be expressed ubiquitously in human tissues: type 1 mainly in the skin and liver; type 2 mainly in the prostate and scalp hair follicles; and type 3 mainly responsible for N-glycosylation of nascent proteins [12, 13]. Notably, it has been demonstrated that both types 1 and 2 of 5α-R are expressed in bladder cancer cell lines and tissue specimens [14, 15]. In addition, a subset of bladder cancers even showed up-regulation and/or amplification of the genes encoding 5α-R isozymes [15]. Meanwhile, several 5α-R inhibitors (5α-RIs), including finasteride (a potent competitive inhibitor of 5α-R type 2 but inhibiting less effectively type 1 [12]), dutasteride (a competitive inhibitor, more potent at inhibiting 5α-R types 1 (>100 times) and 2 (3 times) than finasteride [16]), and epristeride (a non-competitive steroidal inhibitor of 5α-R type 2 with weaker anti-type 1 activity [17]), have been clinically used to manage, for instance, benign prostatic hyperplasia (BPH) and androgenic alopecia.
Although the amount of available data is limited, the impact of 5α-RI therapy on the development of bladder cancer remains controversial. Specifically, in a large prospective study, finasteride use was found to significantly lower the incidence of bladder cancer, compared with controls without 5α-RI therapy [18]. By contrast, a secondary analysis of double-blinded randomized clinical trial data demonstrated no significant difference in the incidence of bladder cancer in men with versus without finasteride treatment [19]. Meanwhile, several recent retrospective studies suggested the potential benefit of 5α-RI therapy in preventing bladder cancer progression and/or improving patient outcome [20–22]. Another retrospective study described above showing the preventing effect of androgen suppression on tumor recurrence consisted of patients treated with not only surgical or chemical castration and/or bicalutamide (n = 13) but also dutasteride only (n = 19) [11]. Moreover, an in vitro study using an AR-positive TCCSUP urothelial cancer line showed that finasteride at a pharmacological dose (e.g. 100 nM) could inhibit cell viability [15]. In addition, a supra-pharmacological dose (e.g. 10μM) of finasteride significantly reduced the proliferation of TCCSUP cells cultured in an androgen-depleted condition [15], implying its off-target effect. In an animal carcinogenesis model, however, finasteride failed to significantly prevent the development of bladder cancer [23]. No robust in vitro data thus support the clinical significance of various 5α-RIs in urothelial cancer outgrowth. The present study aimed to assess if 5α-RIs could inhibit urothelial tumorigenesis as well as bladder cancer progression, using AR-positive cell line models where DHT was known to show stimulatory effects [4, 5, 8, 24].
METHODS
Cell culture and chemicals
Human urothelial carcinoma cell lines (i.e. AR-positive UMUC3, AR-negative 5637 [5]) and an immortalized human normal urothelial cell line (i.e. AR-negative SVHUC [25]) were originally obtained from the American Type Culture Collection. All these lines were recently authenticated, using GenePrint 10 System (Promega), and routinely tested for Mycoplasma contamination. Sublines stably expressing a full-length wild-type human AR (i.e. 5637-AR, SVHUC-AR) were established in our previous studies [24, 25]. UMUC3-, 5637-, and SVHUC-derived cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco), RPMI 1640 (Mediatech), and Ham’s F-12K (Kaighn’s) medium (Mediatech), respectively, supplemented with 10% fetal bovine serum (FBS) and cultured in phenol red-free medium supplemented with 5% FBS at least 24 hours before experimental treatment. We obtained dutasteride, finasteride, and epristeride from Chem Scene, and testosterone from Sigma-Aldrich.
In vitro transformation
An in vitro neoplastic/malignant transformation system was employed, using SVHUC cells with exposure to a chemical carcinogen 3-methylcholanthrene (MCA), as established in a previous study [26], with minor modifications. Briefly, cells (2×106/10 cm culture dish incubated for 48 hours) were cultured in serum-free F-12K containing 5μg/mL MCA (Sigma-Aldrich). After the first 24 hours of MCA exposure, FBS (1%) was added to the medium. After additional 24 hours, the cells were cultured in medium containing 5% FBS without MCA until near confluence. Subcultured cells (1:3 split ratio) were again cultured in the presence of 5μg/mL MCA for two 48-hour exposure periods, using the above protocol. These MCA-exposed cells were then subcultured for 6 weeks in the presence or absence of a 5α-RI and thereafter utilized for further assays.
Cell proliferation
Methylthiazolyldisphenyl-tetrazolium bromide (MTT) assay was used to assess cell viability. Cells (3–5×103/well) seeded in 96-well tissue culture plates were incubated for 96 hours, and at the end of the culture 10μL MTT stock solution (5 mg/mL; Sigma-Aldrich) was added to each well for 3 hours at 37°C. The medium was replaced with 100μL dimethyl sulfoxide and incubated for 5 minutes at room temperature. The absorbance was then measured at a wavelength of 570 nm with background subtraction at 630 nm.
Cell migration
A scratch wound-healing assay was used to assess the ability of cell migration. Cells at a density of 90–100% confluence in 12-well tissue culture plates were scratched manually with a sterile 200μL plastic pipette tip, cultured for 24 hours in a serum-free condition, fixed with methanol, and stained with 0.1% crystal violet. The width of the wound area was quantitated, using ImageJ software (National Institutes of Health).
Colony formation
Cells (500/well) seeded in 12-well tissue culture plates were allowed to grow until colonies in the control well were certainly detectable. The cells were then fixed with methanol and stained with 0.1% crystal violet. The number of colonies in photographed pictures was quantitated, using the ImageJ.
Statistical analysis
Student’s t-test was used to compare the numerical data. P values less than 0.05 were considered to be statistically significant.
RESULTS
Efficacy of 5α-RIs in urothelial tumor progression
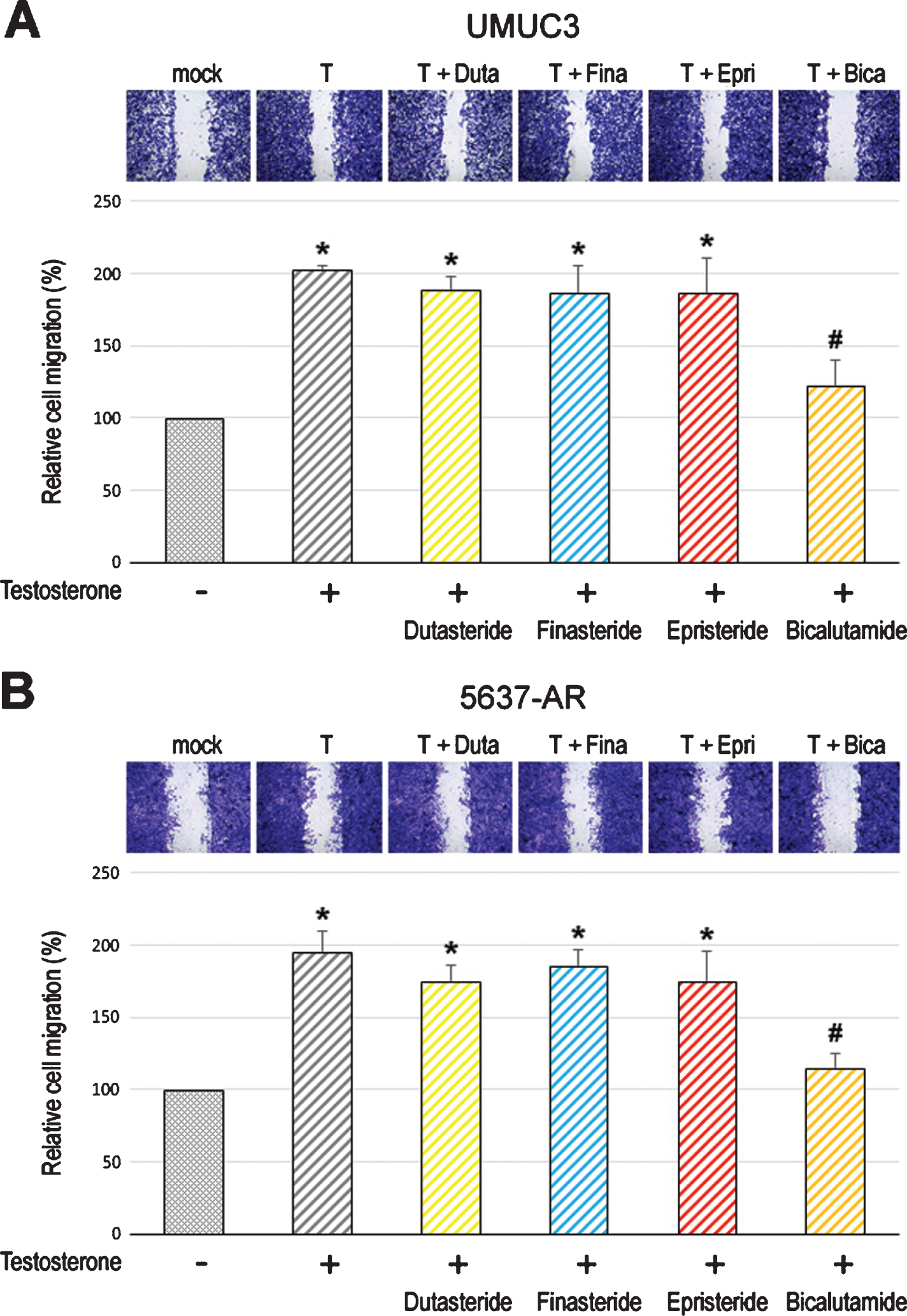
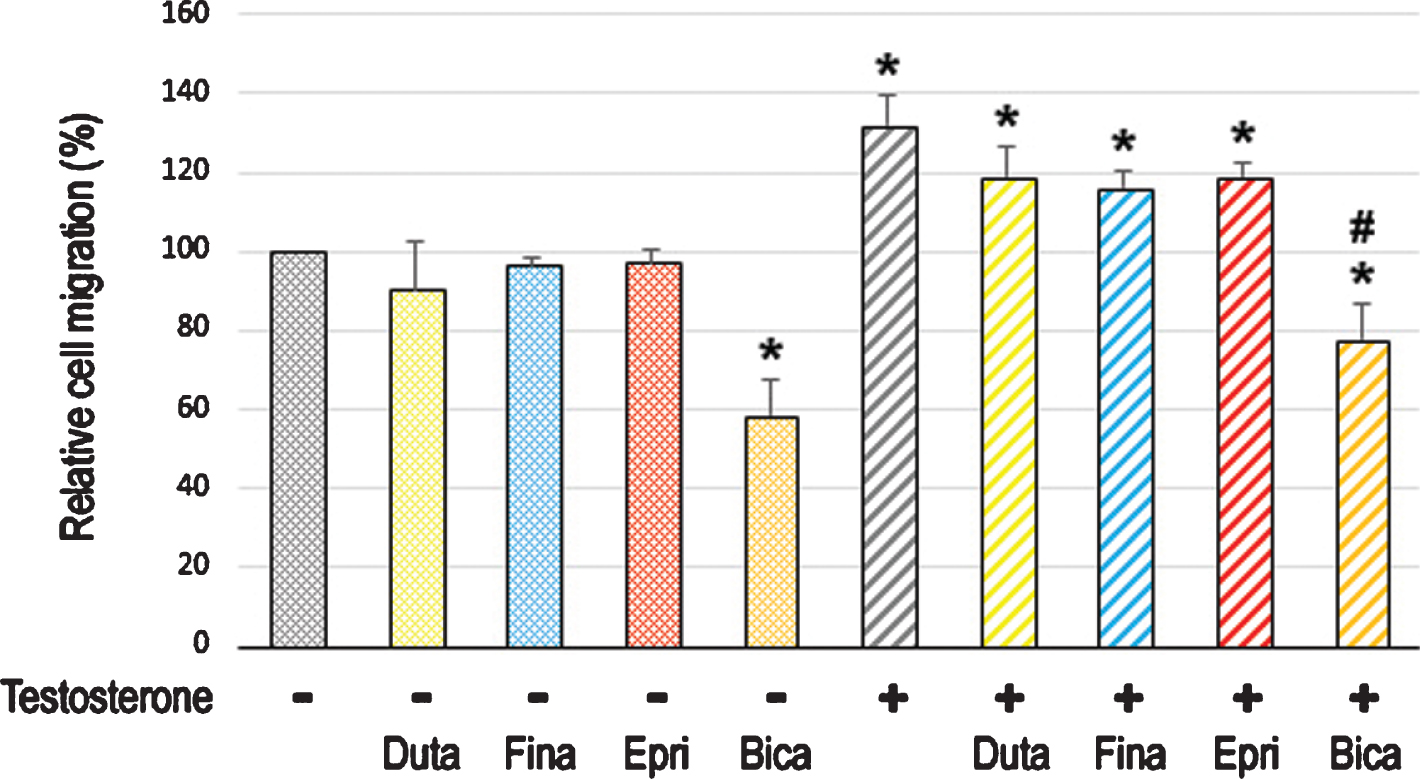
We first compared the cell proliferation of AR-positive bladder cancer lines cultured with various concentrations of dutasteride/finasteride/epristeride (i.e. the highest doses greater than pharmacological concentrations –dutasteride 5.8 nM [27], finasteride 102 nM [28], or epristeride 373 nM [29]). MTT assay showed significant inhibition in the viability of UMUC3 (Fig. 1A) and 5637-AR (Fig. 1B) cells by an AR antagonist bicalutamide. However, 5α-RI treatment did not significantly change their viability. We then performed a scratch wound-healing assay to assess the effects of 5α-RIs on cell migration. As expected, testosterone significantly induced the migration of UMUC3 (Fig. 2A) and 5637-AR (Fig. 2B) cells, which was antagonized by bicalutamide. Nevertheless, these 5α-RIs did not considerably inhibit the cell migration induced by testosterone supplement. Thus, none of the three 5α-RIs even at respective supra-pharmacological concentrations appeared to prevent the growth of urothelial cancer cells possessing a functional AR.
Fig. 1
Effects of 5α-RIs on the viability of bladder cancer cells. MTT assay in UMUC3 (A) and 5637-AR (B) cells cultured in medium containing 5% FBS as well as ethanol (mock), dutasteride (0.1–100 nM), finasteride (0.5–500 nM), epristeride (5–5000 nM), or bicalutamide (Bica; 5μM) for 96 hours. Cell viability is presented relative to that in each line with mock treatment. Each value represents the mean (+SD) from three independent experiments. *P < 0.05 (vs. mock treatment).
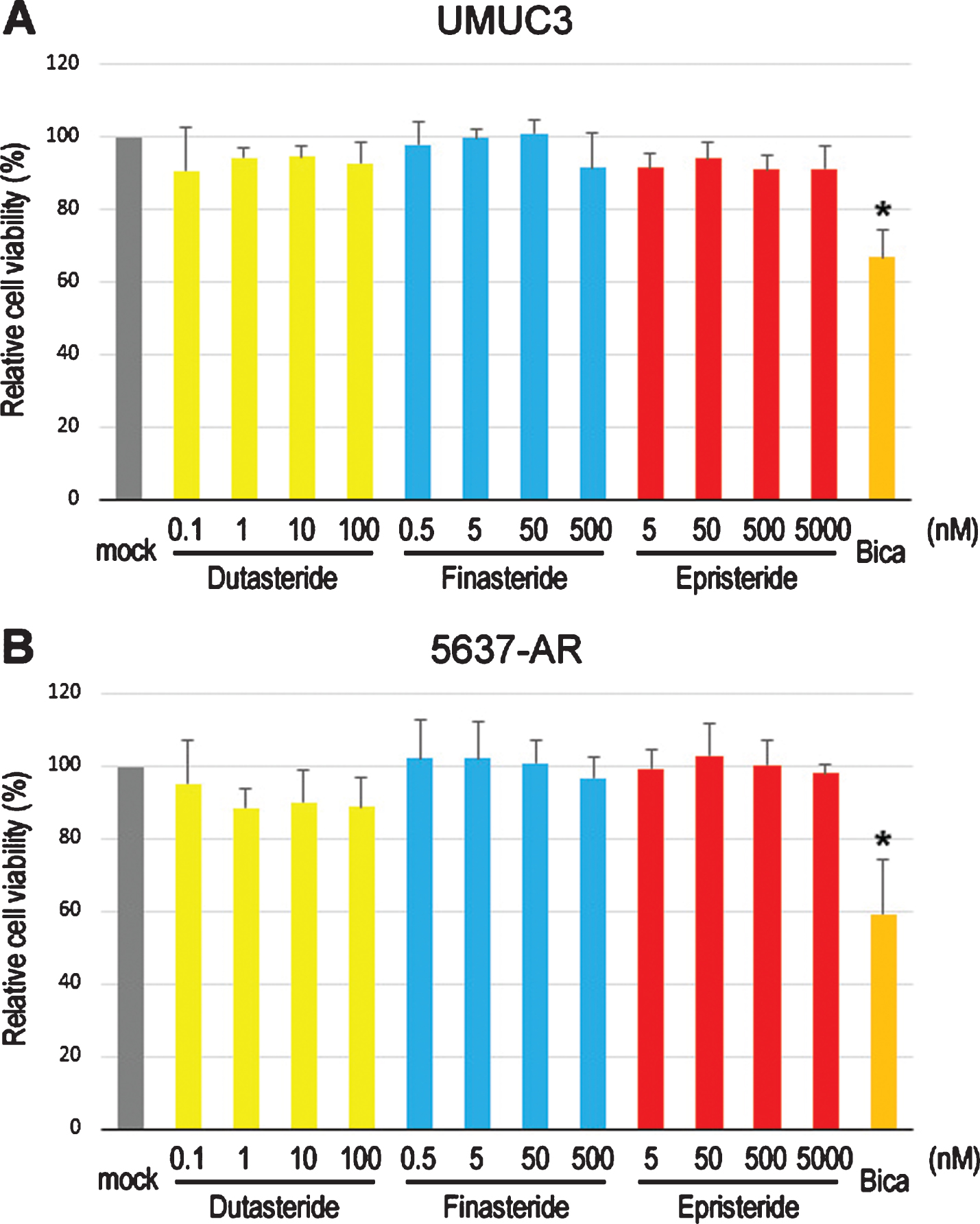
Fig. 2
Effects of 5α-RIs on the migration of bladder cancer cells. Wound-healing assay in UMUC3 (A) and 5637-AR (B) cells. The cells grown to confluence were gently scratched, and the wound area was measured after 24-hour culture in serum-free medium containing ethanol (mock), dutasteride (Duta; 100 nM), finasteride (Fina; 500 nM), epristeride (Epri; 5μM), bicalutamide (Bica; 5μM), and/or testosterone (T; 10 nM). The migration determined by the rate of cells filling the wound area is presented relative to that in each line with mock treatment. Each value represents the mean (+SD) from three independent experiments. *P < 0.05 (vs. mock treatment). #P < 0.05 (vs. testosterone treatment).
Efficacy of 5α-RIs in urothelial tumorigenesis
To assess the effect of long-term treatment with 5α-RIs in urothelial tumorigenesis, we used an established in vitro model where non-neoplastic SVHUC-derived cells could undergo neoplastic/malignant transformation induced by a chemical carcinogen MCA during the course of 6-week culture [26]. SVHUC-AR cells exposed to MCA were subcultured with 100 nM dutasteride, 500 nM finasteride, or 5μM epristeride in the presence or absence of testosterone for 6 weeks during the process of the transformation. Oncogenic activity in transformed cells was then monitored by subsequent assays for cell viability (via MTT assay with 4-day culture; Fig. 3), cell migration (via wound-healing assay with 24-hour culture; Fig. 4), and colony formation (via clonogenic assay with 2-week culture; Fig. 5) with no further 5α-RI/testosterone treatment that might directly affect their results. We thus compared the degree of neoplastic transformation in urothelial cells with the carcinogen challenge but did not intend to simply assess the effects of 5α-RIs on the growth of SVHUC-AR cells. In these assays, 6-week testosterone treatment resulted in significant increases in cell viability, cell migration, and colony formation, suggesting induction of the neoplastic transformation by androgen. While bicalutamide significantly prevented the neoplastic transformation of MCA-SVHUC-AR cells treated with or without additional testosterone, the three 5α-RIs failed to significantly inhibit it, suggesting no considerable prevention of the neoplastic transformation of AR-positive urothelial cells by 5α-RIs.
Fig. 3
Effects of 5α-RIs on the neoplastic transformation of urothelial cells determined by cell viability. MTT assay in SVHUC-AR cells exposed to MCA, subcultured for 6 weeks in medium containing 5% FBS as well as ethanol (mock), dutasteride (Duta; 100 nM), finasteride (Fina; 500 nM), epristeride (Epri; 5μM), bicalutamide (Bica; 5μM), and/or testosterone (10 nM), and further incubated for 96 hours without 5α-RI/testosterone treatment. The viability is presented relative to that of mock-treated cells. Each value represents the mean (+SD) from three independent experiments. *P < 0.05 (vs. mock treatment). #P < 0.05 (vs. testosterone treatment).
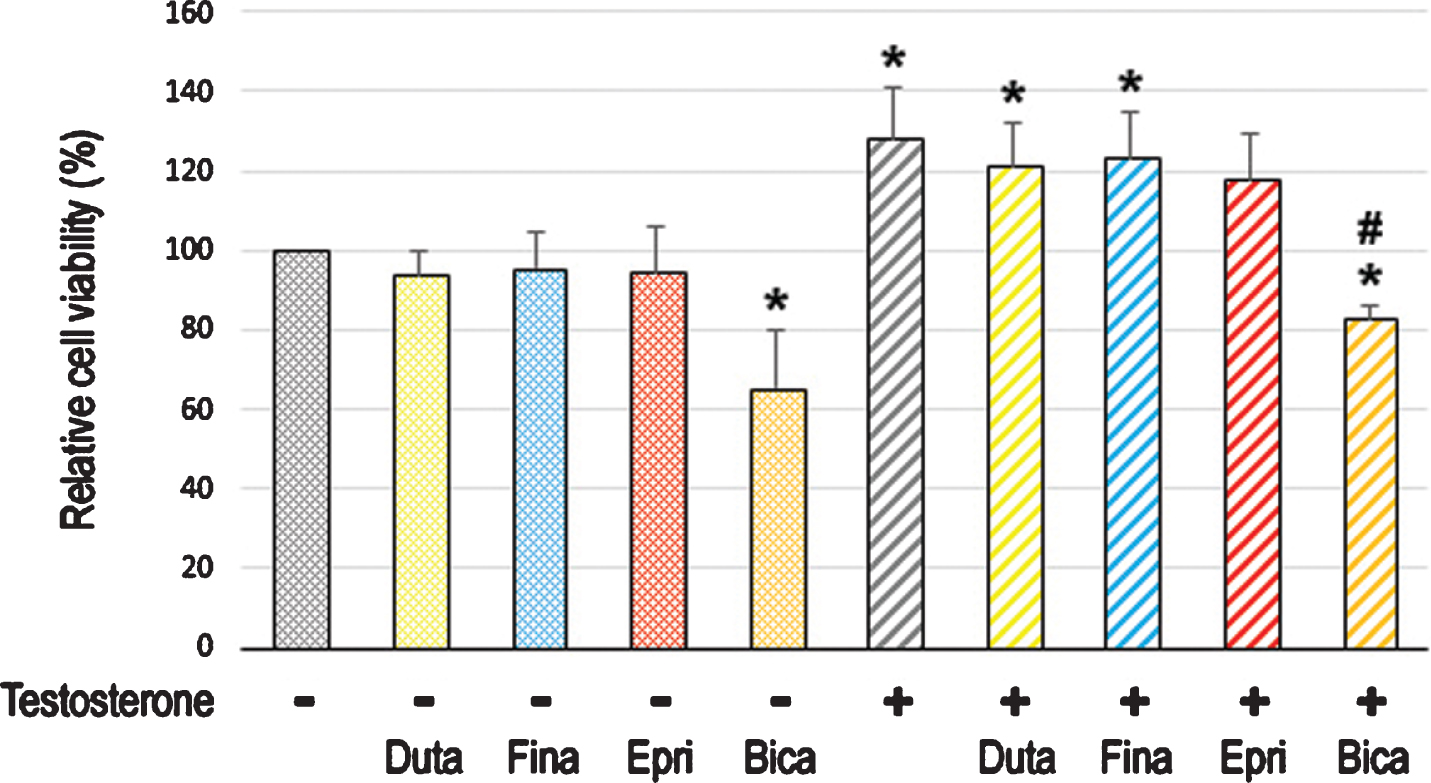
Fig. 4
Effects of 5α-RIs on neoplastic transformation of urothelial cells determined by colony-forming ability. Clonogenic assay in SVHUC-AR cells exposed to MCA, subcultured for 6 weeks in medium containing 5% FBS as well as ethanol (mock), dutasteride (Duta; 100 nM), finasteride (Fina; 500 nM), epristeride (Epri; 5μM), bicalutamide (Bica; 5μM), and/or testosterone (Test; 10 nM), and further incubated for 2 weeks without 5α-RI/testosterone treatment. The number of colony consisting of ≥20 cells is presented relative to that of mock-treated cells. Each value represents the mean (+SD) from three independent experiments. *P < 0.05 (vs. mock treatment). #P < 0.05 (vs. testosterone treatment).
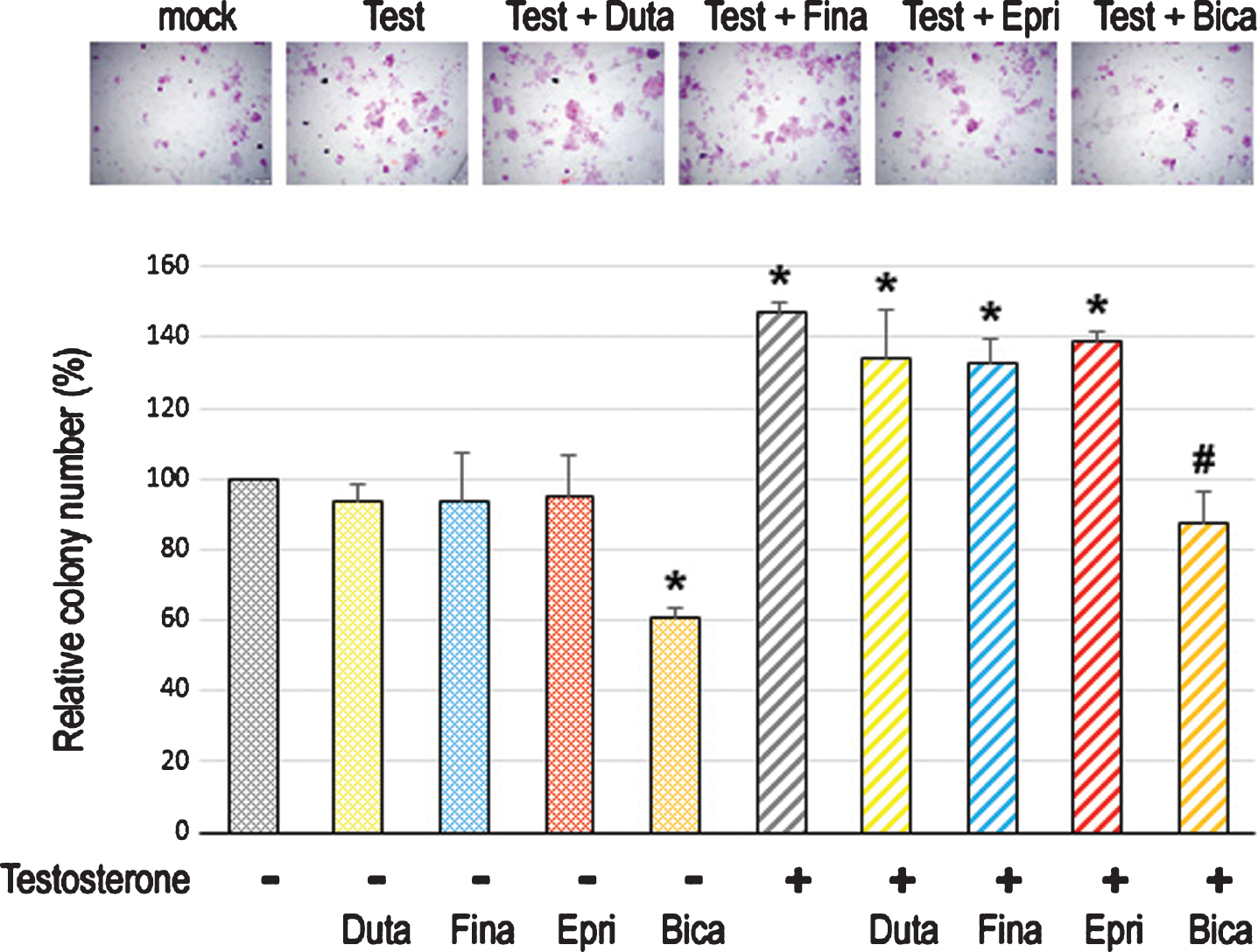
Fig. 5
Effects of 5α-RIs on neoplastic transformation of urothelial cells determined by cell migration ability. Wound-healing assay in SVHUC-AR cells exposed to MCA, subcultured for 6 weeks in medium containing 5% FBS as well as ethanol (mock), dutasteride (Duta; 100 nM), finasteride (Fina; 500 nM), epristeride (Epri; 5μM), bicalutamide (Bica; 5μM), and/or testosterone (10 nM), and further incubated for 24 hours in serum-free medium without 5α-RI/testosterone treatment. The migration is presented relative to that of mock-treated cells. Each value represents the mean (+SD) from three independent experiments. *P < 0.05 (vs. mock treatment). #P < 0.05 (vs. testosterone treatment).
DISCUSSION
Emerging evidence indicates a critical role of androgen-mediated AR signals in the pathogenesis of urothelial cancer [4–11]. In addition, a prospective study involving 72,370 men with a >13-year follow-up demonstrated a significantly lower incidence of bladder cancer in men with finasteride treatment (1.07%) than in those without 5α-RIs (1.46%) (hazard ratio 0.634; P = 0.0004) [18]. The beneficial effects of 5α-RI therapy on the development and/or progression of bladder cancer have been further supported by a meta-analysis of 5 studies [30] as well as subsequent retrospective studies [20–22]. However, data from a double-blinded randomized clinical trial to determine if an α1-blocker doxazosin and/or finasteride could prevent the progression of BPH indicated no impact of 5α-RI therapy (P = 0.67) on bladder cancer incidence in men with [9/1,216 (0.74%)] versus without [9/1,484 (0.61%)] finasteride treatment [19]. Remarkably, in these observational studies, many of the variables measured appear to either be unavailable or have missing values in their records, and the final evaluation is therefore incomplete to be able to conclude whether 5α-RI therapy is satisfactory for bladder cancer patients. An animal study also showed no prevention effect of finasteride on the development of bladder cancer induced by a chemical carcinogen [23]. Thus, there are only limited data mainly on finasteride, and the actual clinical benefit from 5α-RI therapy in preventing the development and progression of urothelial cancer remains inconclusive. Moreover, no in vitro studies have reported the efficacy of various 5α-RIs, other than finasteride, in urothelial cells. Meanwhile, the inhibitory effect of finasteride was assessed in the TCCSUP bladder cancer cell line [15] established from a female patient. It is indeed evident that gender plays an important role in the outcomes of androgen deprivation or inhibition therapy. In the present study, we aimed to determine if dutasteride, finasteride, and epristeride could prevent urothelial tumorigenesis and tumor growth, using in vitro models with 3 cell lines (i.e. SVHUC, UMUC3, 5637) all from male patients.
We first assessed the effects of 5α-RIs on the growth of bladder cancer cells. In accordance with our previous observations [5, 8, 24, 31], cell viability assays showed inhibition of cell proliferation by an AR antagonist bicalutamide. Similarly, as shown by DHT and AR antagonists [8], supplement of testosterone in a serum-free condition, which could be metabolized into DHT in culture medium by 5α-Rs in tumor cells, significantly induced the migration of AR-positive bladder cancer cells, while the androgen effect was restored by bicalutamide. However, even a supra-pharmacological dose of each 5α-RI did not significantly inhibit the viability or migration of AR-positive bladder cancer cells cultured in the presence of androgen and/or supplementary testosterone.
To assess the effects of 5α-RIs on urothelial tumorigenesis, we used an in vitro system with non-neoplastic cells exposed to a chemical carcinogen MCA. In this transformation system, we compared oncogenic activities, such as cell viability, cell migration, and colony formation. Similar to the effect of a synthetic non-metabolizable androgen R1881 observed in the same model [32], addition of testosterone during the process of transformation resulted in its significant induction. While bicalutamide strongly inhibited the neoplastic transformation of AR-positive urothelial cells in the presence or absence of additional testosterone treatment, 5α-RIs failed to considerably affect it.
Using in vitro models for urothelial cancer, we thus found that three 5α-RIs clinically used did not show significant inhibitory effects on tumorigenesis and tumor progression. Meanwhile, DHT is generally considered to be more potent than testosterone in, for instance, the skin, hair follicles, and prostate where 5α-R activity is relatively high [12, 33]. 5α-RIs, as DHT blockers, have been used primarily in the treatment of BPH and scalp hair loss. However, the role of 5α-RI therapy in the prevention and treatment of prostate cancer remains to be explored. In the Prostate Cancer Prevention Trial involving 18,880 eligible men with up to 18 years of follow-up, finasteride was found to reduce the cancer risk (relative risk 0.70, P < 0.001) but induced the development of high grade cancer (relative risk 1.17, P = 0.05) [34], implying that testosterone itself is an active form of androgen in prostate cancer cells. In the bladder, the potency of testosterone, in comparison with that of DHT, appears to be unexplored. In a study, although the effect of testosterone was not compared, DHT was shown to promote carcinogen-mediated bladder carcinogenesis in mice lacking a functional AR [5], suggesting the involvement of androgen-mediated non-AR pathway. In prostate cancer cells, it has also been well known that AR can be activated through the non-canonical pathways independent of androgen binding, such as phosphorylation via cytokines and kinases, as well as epigenetic alteration [4, 35–37]. Additionally, as aforementioned, an off-target effect of finasteride at 10μM, not via blocking testosterone to DHT conversion, on the growth of bladder cancer cells was suggested [15].
In conclusion, the present in vitro evidence suggests that, unlike AR antagonists, 5α-RIs with various activities against 5α-R isozymes have no significant impact on the development and progression of urothelial cancer. Nonetheless, further studies are required to determine the relative potency of testosterone versus DHT in urothelial cells as well as to explain the gender gap and the controversies in the existing results on androgen deprivation or inhibition therapy using tissue samples or cell lines derived from both genders.
ACKNOWLEDGMENTS
The authors have no acknowledgements.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTION
YN: Performance of work, interpretation/analysis of data, and writing the article; GT: Performance of work; GJ: Performance of work; YT: Performance of work; HM: Conception, interpretation of data, and writing the article.
CONFLICT OF INTEREST
HM has received research funding from Astellas Scientific and Medical Affairs, Ferring Research Institute, and Bristol-Myers Squibb.
The other authors have no conflict of interest to declare.
REFERENCES
[1] | Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) ;68: (6):394–424. |
[2] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2020. CA Cancer J Clin. (2020) ;70: (1):7–30. |
[3] | Dobruch J , Daneshmand S , Fisch M , Lotan Y , Noon AP , Resnick MJ , Shariat SF , Zlotta AR , Boorjian SA . Gender and bladder cancer: A collaborative review of etiology, biology, and outcomes. Eur Urol. (2016) ;69: (2):300–10. |
[4] | Li P , Chen J , Miyamoto H . Androgen receptor signaling in bladder cancer. Cancers. (2017) ;9: (2):20. |
[5] | Miyamoto H , Yang Z , Chen YT , Ishiguro H , Uemura H , Kubota Y , Nagashima Y , Chang YJ , Hu YC , Tsai MY , Yeh S , Messing EM , Chang C . Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. (2007) ;99: (7):558–68. |
[6] | Hsu JW , Hsu I , Xu D , Miyamoto H , Liang L , Wu XR , Shyr CR , Chang C . Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am J Pathol. (2013) ;182: (5):1811–20. |
[7] | Kawahara T , Inoue S , Kashiwagi E , Chen J , Ide H , Mizushima T , Li Y , Zheng Y , Miyamoto H . Enzalutamide as an androgen receptor inhibitor prevents urothelial tumorigenesis. Am J Cancer Res. (2017) ;7: (10):2041–50. |
[8] | Kawahara T , Ide H , Kashiwagi E , El-Shishtawy KA , Li Y , Reis LO , Zheng Y , Miyamoto H . Enzalutamide inhibits androgen receptor-positive bladder cancer cell growth. Urol Oncol. (2016) ;34: (10):432.e15–23. |
[9] | Shiota M , Yokomizo A , Takeuchi A , Imada K , Kiyoshima K , Inokuchi J , Tatsugami K , Ohga S , Nakamura K , Honda H , Naito S . Secondary bladder cancer after anticancer therapy for prostate cancer: reduced comorbidity after androgen-deprivation therapy. Oncotarget. (2015) ;6: (16):14710–9. |
[10] | Izumi K , Taguri M , Miyamoto H , Hara Y , Kishida T , Chiba K , Murai T , Hirai K , Suzuki K , Fujinami K , Ueki T , Udagawa K , Kitami K , Moriyama M , Miyoshi Y , Tsuchiya F , Ikeda I , Kobayashi K , Sato M , Morita M , Noguchi K , Uemura H . Androgen deprivation therapy prevents bladder cancer recurrence. Oncotarget. (2014) ;5: (24):12665–74. |
[11] | Shiota M , Kiyoshima K , Yokomizo A , Takeuchi A , Kashiwagi E , Dejima T , Takahashi R , Inokuchi J , Tatsugami K , Eto M . Suppressed recurrent bladder cancer after androgen suppression with androgen deprivation therapy or 5α-reductase inhibitor. J Urol. (2017) ;197: (2):308–13. |
[12] | Russell DW , Wilson JD . Steroid 5a-reductase: two genes/two enzymes. Annu Rev Biochem. (1994) ;63: :25–61. |
[13] | Cantagril V , Lefeber D , Ng B , Guan Z , Silhavy J , Bielas S , Lehle L , Hombauer H , Adamowicz M , Swiezewska E , De Brouwer AP , Blümel P , Sykut-Cegielska J , Houliston S , Swistun D , Ali BR , Dobyns WB , Babovic-Vuksanovic D , van Bokhoven H , Wevers RA , Raetz CR , Freeze HH , Morava E , Al-Gazali L , Gleeson JG . SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. (2010) ;142: (2):203–17. |
[14] | Hata S , Ise K , Azmahani A , Konosu-Fukaya S , McNamara KM , Fujishima F , Shimada K , Mitsuzuka K , Arai Y , Sasano H , Nakamura Y . Expression of AR, 5αR1 and 5αR2 in bladder urothelial carcinoma and relationship to clinicopathological factors. Life Sci. (2017) ;190: :15–20. |
[15] | Chen CC , Huang CP , Tsai YT , Hseih TF , Shyr CR . The genomic alterations of 5α-reductases and their inhibitor finasteride’s effect in bladder cancer. Anticancer Res. (2017) ;37: (12):6893–8. |
[16] | Clark RV , Hermann DJ , Cunningham GR , Wilson TH , Morrill BB , Hobbs S . Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5-alpha-reductase inhibitor. J Clin Endocrinol Metab. (2004) ;89: (5):2179–84. |
[17] | Levy MA , Brandt M , Sheedy KM , Dinh JT , Holt DA , Garrison LM , Bergsma DJ , Metcalf BW . Epristeride is a selective and specific uncompetitive inhibitor of human steroid 5α-reductase isoform 2. J Steroid Biochem Mol Biol. (1994) ;48: (2-3):197–206. |
[18] | Morales EE , Grill S , Svatek RS , Kaushik D , Thompson IM Jr , Ankerst DP , Liss MA . Finasteride reduces risk of bladder cancer in a large prospective screening study. Eur Urol. (2016) ;69: (3):407–10. |
[19] | Sathianathen NJ , Fan Y , Jarosek SL , Lawrentschuk NL , Konety BR . Finasteride does not prevent bladder cancer. A secondary analysis of the Medical Therapy for Prostatic Symptoms Study. Urol Oncol. (2018) ;36: (7):338.e13–17. |
[20] | Mäkelä VJ , Kotsar A , Tammela TLJ , Murtola TJ . Bladder cancer survival of men receiving 5α-reductase inhibitors. J Urol. (2018) ;200: (4):743–8. |
[21] | McMartin C , Lacombe L , Fradet V , Fradet Y , Lodde M , Toren P . Receipt of 5-alpha reductase inhibitors before radical cystectomy: Do they render high-grade bladder tumors less aggressive? Clin Genitourin Cancer. (2019) ;17: (6):e1122–8. |
[22] | Pastore AL , Fuschi A , De Nunzio C , Balzarro M , Al Salhi Y , Velotti G , Martoccia A , Capone L , Amigoni N , Falsaperla M , Mattia C , Artibani W , Tubaro A , Carbone A . Possible role of 5-alpha reductase inhibitors in non-invasive bladder urothelial neoplasm: multicentre study. Minerva Urol Nefrol. 2019; doi: 10.23736/S0393-2249.19.03563-X |
[23] | Imada S , Akaza H , Ami Y , Koiso K , Ideyama Y , Takenaka T . Promoting effects and mechanisms of action of androgen in bladder carcinogenesis in male rats. Eur Urol. (1997) ;31: (3):360–4. |
[24] | Zheng Y , Izumi K , Yao JL , Miyamoto H . Dihydrotestosterone upregulates the expression of epidermal growth factor receptor and ERBB2 in androgen receptor-positive bladder cancer cells. Endocr Relat Cancer. (2011) ;18: (4):451–64. |
[25] | Izumi K , Zheng Y , Hsu JW , Chang C , Miyamoto H . Androgen receptor signals regulate UDP-glucuron-osyltransferases in the urinary bladder: a potential mechanism of androgen-induced bladder carcinogenesis. Mol Carcinogen. (2013) ;52: (2):94–102. |
[26] | Reznikoff CA , Loretz LJ , Christian BJ , Wu SQ , Meisner LF . Neoplastic transformation of SV40-immortalized human urinary tract epithelial cells by in vitro exposure to 3-methylcholanthrene. Carcinogenesis. (1988) ;9: (8):1427–36. |
[27] | Fossler MJ , Collins DA , Ino H , Sarai N , Ravindranath R , Bowen CL , Burns O . Evaluation of bioequivalence of five 0.1 mg dutasteride capsules compared to one 0.5 mg dutasteride capsule: a randomized study in healthy male volunteers. J Drug Assess. (2015) ;4: (1):24–9. |
[28] | Ohtawa M , Morikawa H , Shimazaki J . Pharmacokinetics and biochemical efficacy after single and multiple oral administration of N-(2-methyl-2-propyl)-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide, a new type of specific competitive inhibitor of testosterone 5α-reductase, in volunteers. Eur J Drug Metab Pharmacokinet. (1991) ;16: (1):15–21. |
[29] | Benincosa LJ , Audet PR , Lundberg D , Zariffa N , Jorkasky DK . Pharmacokinetics and absolute bioavailability of epristeride in healthy male subjects. Biopharm Drug Dispos. (1996) ;17: (3):249–58. |
[30] | Kim A , Kim MS , Ahn JH , Choi WS , Park HK , Kim HG , Paick SH . Clinical significance of 5-α reductase inhibitor and androgen deprivation therapy in bladder cancer incidence, recurrence, and survival: a meta-analysis and systemic review. Aging Male. 2019; doi: 10.1080/13685538.2019.1646238 |
[31] | Kawahara T , Shareef HK , Aljarah AK , Ide H , Li Y , Kashiwagi E , Netto GJ , Zheng Y , Miyamoto H . ELK1 is up-regulated by androgen in bladder cancer cells and promotes tumor progression. Oncotarget. (2015) ;6: (30):29860–76. |
[32] | Li Y , Ishiguro H , Kawahara T , Miyamoto Y , Izumi K , Miyamoto H . GATA3 in the urinary bladder: suppression of neoplastic transformation and down-regulation by androgens. Am J Cancer Res. (2014) ;4: (5):461–73. |
[33] | Aggarwal S , Thareja S , Verma A , Bhardwaj TR , Kumar M . An overview on 5α-reductase inhibitors. Steroids. (2010) ;75: (2):109–53. |
[34] | Thompson IM Jr , Goodman PJ , Tangen CM , Parnes HL , Minasian LM , Godley PA , Lucia MS , Ford LG . Long-term survival of participants in the Prostate Cancer Prevention Trial. N Engl J Med. (2013) ;369: (7):603–10. |
[35] | Lamont KR , Tindall DJ . Minireview: Alternative activation pathways for the androgen receptor in prostate cancer. Mol Endocrinol. (2011) ;25: (6):897–907. |
[36] | Antonarakis ES , Armstrong AJ , Dehm SM , Luo J . Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis. (2016) ;19: (3):231–41. |
[37] | Cucchiara V , Yang JC , Mirone V , Gao AC , Rosenfeld MG , Evans CP . Epigenomic regulation of androgen receptor signaling: Potential role is prostate cancer therapy. Cancers. (2017) ;9: (1):9. |




