The Controlling Nutritional Status (CONUT) Score is a Prognostic Biomarker in Advanced Urothelial Carcinoma Patients Treated with First-Line Platinum-Based Chemotherapy
Abstract
BACKGROUND:
The controlling nutritional status (CONUT) score is an objective indicator of general condition from the aspect of nutritional status, calculated from serum albumin, total cholesterol, and total lymphocyte count. The CONUT score is also considered to reflect the degree of tumor-derived chronic inflammation and the host immune status in patients with advanced cancer.
OBJECTIVE:
To examine the prognostic role of the CONUT score in patients with advanced urothelial carcinoma (aUC) treated with first-line platinum-based chemotherapy.
METHODS:
Associations of the CONUT score with clinical parameters and overall survival (OS) were investigated retrospectively in 147 patients with aUC receiving first-line platinum-based chemotherapy at a single cancer center from February 2003 to April 2019.
RESULTS:
The median (range) CONUT score was 1 (0–7). A higher CONUT score was associated with lower hemoglobin (P < 0.001) and higher C-reactive protein levels (P = 0.023) but not with chemotherapy response (P = 0.432). The median OS for patients with CONUT scores 0–1, 2–3, and ≥4 were 23.3, 14.9, and 9.4 months, respectively (P < 0.001). In the multivariable analysis, a higher CONUT score was independently associated with shorter OS (scores 2–3 vs 0–1, HR 1.58, P = 0.048; scores ≥4 vs 0–1, HR 2.63, P = 0.008) along with poorer performance status (HR 4.79, P < 0.001), primary tumor site of the upper urinary tract (HR 1.70, P = 0.016), higher LDH (HR 3.85, P = 0.036), higher alkaline phosphatase (HR 3.06, P = 0.028), and non-responders to chemotherapy (HR 2.07, P < 0.001).
CONCLUSIONS:
The CONUT score is a prognostic biomarker in patients with aUC receiving first-line platinum-based chemotherapy.
INTRODUCTION
Urothelial carcinoma (UC) is mainly composed bladder cancer (90–95%) and upper tract urinary cancer (UTUC) (5–10%) [1]. The incidence of locally advanced or metastatic UC was 12,494 in the United States in 2016 [2]. Patients with advanced UC (aUC), including inoperable locally advanced and metastatic disease, have poor prognosis with a median overall survival (OS) of 13–16 months despite receiving first-line platinum-based chemotherapy [3]. Although platinum-based chemotherapy remains the standard of care for aUC, immune checkpoint inhibitors (ICIs) have recently been approved in the second-line setting and have improved prognosis for patients with aUC: in a randomized trial, the median OS was 10.3 months for the pembrolizumab group compared with 7.4 months for the chemotherapy group [4]. Currently, many clinical trials of ICIs are ongoing in combination with chemotherapy or targeted agents [5–7].
Assessment of general condition in patients with aUC is crucial for prognosis and therapeutic decision-making. Several prognostic models for patients with aUC have been proposed, involving the following clinical parameters: performance status (PS), primary tumor site, lymph node/visceral metastasis, white blood cell (WBC) count, hemoglobin, and albumin [8, 9]. Nutritional status is one of indicators of general condition and is often compromised in patients with advanced cancer. The controlling nutritional status (CONUT) score is a simple and validated objective data assessment system consisting of three parameters: serum albumin, total lymphocyte count, and total cholesterol concentration [10]. Previous studies have demonstrated the prognostic role of malnutrition evaluated by the CONUT score in patients with several types of cancer [11–16]. We have demonstrated the prognostic significance of the CONUT score in patients with aUC, of whom one-quarter did not receive systemic therapy [17].
In patients with advanced cancer, cytokine-ind-uced chronic inflammation results in hypoalbuminemia, lymphopenia, and hypocholesterolemia [18, 19]. Given that the total lymphocyte count reflects the host immune function [20], the CONUT score may reflect not simply the nutritional status, but more importantly, the degree of chronic inflammation and the host immune status in such patients. Accumulating evidence indicates that the efficacy of anticancer agents partially depends on the activation of tumor-targeting immune responses along with direct cytotoxic effects; chemotherapy can promote the immune responses by increasing the immunogenicity of tumor cells and/or by inhibiting immunosuppressive circuits that are established by developing neoplasms [21]. Thus, we hypothesized that the CONUT score may be a prognostic biomarker among patients with aUC treated with systemic chemotherapy. In this study, we retrospectively assessed the prognostic role of the CONUT score in patients with aUC treated with first-line platinum-based chemotherapy.
MATERIALS AND METHODS
Patients
Our Institutional Ethical Committee approved the present retrospective study protocol (#2318). Signed informed consent was obtained from all participants. A total of 155 consecutive patients with aUC (inoperable cT4, regional lymph node metastasis, and/or distant metastasis) received platinum-based systemic chemotherapy as initial treatment at a single cancer center between February 2003 and April 2019. All patients had measurable disease for assessment of chemotherapy response. Of the 155 patients, 8 were excluded due to missing data required for the CONUT score (n = 7) or loss to follow-up before evaluating chemotherapy response (n = 1). Finally, 147 patients were subjected to analysis. The following clinical data at the diagnosis of aUC were collected retrospectively: age, sex, Eastern Cooperative Oncology Group performance status (PS), body mass index (BMI), primary tumor site (bladder or upper urinary tract), clinical tumor stage, lymph node/visceral metastasis (lung, liver, or bone), curative treatment before or after the diagnosis of aUC, components of the CONUT score (albumin, total lymphocyte count, total cholesterol), hemoglobin, lactate dehydrogenase (LDH), alkaline phosphatase (ALP), C-reactive protein (CRP), chemotherapy regimen (cisplatin- or carboplatin-based), total number of chemotherapy cycles given, response to chemotherapy, adverse events (AEs) of chemotherapy, and systemic therapy given after first-line chemotherapy. CONUT scores were calculated from serum albumin, total lymphocyte count, and total cholesterol concentration (Table 1). Response to chemotherapy was assessed after 2 cycles according to the Revised RECIST guideline version 1.1 and was classified into the following categories: complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) [22]. We also assessed chemotherapy response after 4 cycles among patients receiving 4 cycles or more. AEs were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE 5.0) [23].
Table 1
Scoring and interpretation of controlling nutritional status (CONUT) scores
| Parameters | Ranges of values and scores for each parameter | |||
| Albumin (g/dL) | ≥3.50 | 3.00–3.49 | 2.50–2.99 | < 2.50 |
| Score | (0) | (2) | (4) | (6) |
| Lymphocyte count (/μL) | ≥1, 600 | 1,200–1,599 | 800–1,199 | < 800 |
| Score | (0) | (1) | (2) | (3) |
| Total cholesterol (mg/dL) | ≥180 | 140–179 | 100–139 | < 100 |
| Score | (0) | (1) | (2) | (3) |
| Interpretation | ||||
| CONUT score (sum of the above scores) | 0–1 | 2–4 | 5–8 | 9–12 |
| Degree of malnutrition | None | Light | Moderate | Severe |
Statistical analyses
The differences in variables between CONUT scores 0–1, 2–3, and ≥4 were evaluated using Fisher’s exact test for categorical variables, and the Wilcoxon rank-sum test for continuous variables. OS was defined as the time from diagnosis of aUC to either death or the last follow-up. The differences in Kaplan–Meier curves were evaluated using the Wilcoxon rank-sum test. Associations of variables with OS were assessed using the Cox proportional hazards model. Significant variables in the univariate analysis were included in the multivariate analysis. A reduced multivariate model was generated by backward elimination of the variable with the highest P value from each iteration of the multivariate analysis. Two-tailed P < 0.05 was regarded as significant.
As a linear regression model, the Martingale residuals were used to assess the fitting of optimal cutoffs for the following parameters: age, BMI, hemoglobin, LDH, ALP, CRP, total number chemotherapy cycles given, albumin, total lymphocyte count, and total cholesterol concentration. The most appropriate cutoff value for each was determined as described previously [24]. All analyses were conducted using JMP 14.0.0 (SAS Institute Inc., Cary, NC) and R 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics
The demographics of the 147 patients are shown in Table 2. The median age (interquartile range, IQR) was 69 (63–74) years and 102 (69%) patients were male. The bladder was the primary site in 80 (54%) patients. At the time of diagnosis of aUC, 37 (25%) patients had inoperable cT4 disease, whereas 67 (46%) and 77 (52%) patients had regional lymph node metastasis and distant metastasis, respectively; of the latter, 53 (36%) had visceral metastasis. Of the 147 patients, 48 (33%) developed aUC after curative treatment for their primary diseases; 43 (29%) had undergone curative surgery and 5 (4%) had received definitive chemoradiation. Cisplatin-based and carboplatin-based chemotherapy was given to 120 (82%) and 27 (18%), respectively. Following systemic chemotherapy, 37 (25%) patients received curative treatment for the primary disease with either surgical resection (n = 25) or definitive chemoradiation (n = 12). Second-line systemic therapy was given to 54 (37%) patients; 37 (25%) received second-line chemotherapy and 23 (16%) received pembrolizumab as second-line (n = 17) or third-line systemic therapy (n = 6).
Table 2
Demographic characteristics of 147 patients with advanced urothelial carcinoma
| Variables | No. patients (%) | ||||
| Total | CONUT 0–1 | CONUT 2–3 | CONUT ≥4 | P value | |
| (n = 147) | (n = 81) | (n = 50) | (n = 16) | ||
| Age (years), median (IQR) | 69 (63–74) | 68 (62–73) | 70 (66–74) | 70 (65–76) | 0.232 |
| Sex | 0.757 | ||||
| Male | 102 (69) | 56 (69) | 36 (72) | 10 (63) | |
| Female | 45 (31) | 25 (31) | 14 (28) | 6 (37) | |
| PS | 0.161 | ||||
| 0 | 109 (74) | 61 (75) | 40 (80) | 8 (50) | |
| 1 | 29 (20) | 16 (20) | 8 (16) | 5 (31) | |
| 2 | 6 (4) | 3 (4) | 1 (2) | 2 (13) | |
| ≥3 | 3 (2) | 1 (1) | 1 (2) | 1 (6) | |
| BMI (kg/m2), median (IQR) | 22.3 (20.5–24.9) | 23.1 (21.2–25.7) | 22.2 (20.2–24.9) | 21.0 (19.3–23.3) | 0.054 |
| Primary tumor site | 0.318 | ||||
| Bladder | 80 (54) | 47 (58) | 23 (46) | 10 (62) | |
| Upper urinary tract | 67 (46) | 34 (42) | 27 (54) | 6 (38) | |
| Clinical T stage | 0.575 | ||||
| ≤cT3 | 110 (75) | 58 (72) | 40 (80) | 12 (75) | |
| cT4 | 37 (25) | 23 (28) | 10 (20) | 4 (25) | |
| Clinical N stage | 0.767 | ||||
| 0 | 31 (21) | 18 (22) | 9 (18) | 4 (25) | |
| ≥1 | 116 (79) | 63 (78) | 41 (82) | 12 (75) | |
| Visceral metastasis (lung, liver, or bone) | 0.445 | ||||
| No | 94 (64) | 54 (67) | 32 (64) | 8 (50) | |
| Yes | 53 (36) | 27 (33) | 18 (36) | 8 (50) | |
| Curative treatment before the diagnosis of aUC | 0.633 | ||||
| None | 99 (67) | 58 (71) | 30 (60) | 11 (69) | |
| Surgery or chemoradiation | 48 (33) | 23 (29) | 20 (40) | 5 (31) | |
| Surgery | 43 (29) | 20 (25) | 18 (36) | 5 (31) | |
| Chemoradiation | 5 (4) | 3 (4) | 2 (4) | 0 (0) | |
| Pretreatment laboratory parameters, median (IQR) | |||||
| Hemoglobin (g/dL) | 12.6 (11.6–13.7) | 13.0 (12.1–14.1) | 12.3 (11.6–13.4) | 11.3 (9.9–12.0) | < 0.001 |
| LDH (U/L) | 191 (161–230) | 191 (162–218) | 193 (161–244) | 190 (161–226) | 0.509 |
| ALP (U/L) | 257 (216–301) | 249 (205–298) | 256 (219–296) | 270 (241–394) | 0.154 |
| CRP (mg/L) | 5.8 (1.9–22.8) | 3.0 (1.7–11.7) | 6.4 (2.3–27.3) | 26.9 (4.15–38.1) | 0.023 |
| Albumin (g/dL) | 4.1 (3.8–4.3) | 4.2 (4.0–4.4) | 4.0 (3.7–4.3) | 3.5 (3.0–4.0) | < 0.001 |
| Lymphocyte count (/μL) | 1500 (1150–2030) | 1850 (1545–2255) | 1105 (935–1383) | 1165 (850–1430) | < 0.001 |
| Total cholesterol (mg/dL) | 187 (164–212) | 196 (173–217) | 174 (152–210) | 151 (126–176) | < 0.001 |
| Curative treatment after the diagnosis of aUC | 0.095 | ||||
| None | 110 (75) | 57 (70) | 39 (78) | 14 (88) | |
| Surgery or chemoradiation | 37 (25) | 24 (30) | 11 (22) | 2 (12) | |
| Surgery | 25 (17) | 19 (24) | 6 (12) | 0 | |
| Chemoradiation | 12 (8) | 5 (6) | 5 (10) | 2 (12) | |
| Chemo regimen | 0.139 | ||||
| Cisplatin-based | 120 (82) | 69 (85) | 41 (82) | 10 (63) | |
| Carboplatin-based | 27 (18) | 12 (15) | 9 (18) | 6 (37) | |
| Total No. chemo cycles given, median (range) | 4 (1–18) | 4 (1–18) | 4 (1–16) | 3 (1–8) | 0.164 |
| Response to 1st line chemotherapy | 0.432 | ||||
| CR + PR | 68 (46) | 40 (49) | 23 (46) | 5 (31) | |
| SD + PD | 79 (54) | 41 (51) | 27 (54) | 11 (69) | |
| Adverse events | 0.776 | ||||
| Grade < 3 | 92 (63) | 52 (64) | 31 (62) | 9 (56) | |
| Grade ≥3 | 55 (37) | 29 (36) | 19 (38) | 7 (44) | |
| Systemic therapy after 1st line chemotherapy | 0.801 | ||||
| None | 93 (63) | 52 (64) | 30 (60) | 11 (69) | |
| Yes | 54 (37) | 29 (36) | 20 (40) | 5 (31) | |
| 2nd line chemotherapy | 37 (25) | 22 (27) | 12 (24) | 3 (19) | |
| Pembrolizumab | 23 (16) | 11 (14) | 10 (20) | 2 (12) | |
ALP = alkaline phosphatase; aUC = advanced urothelial carcinoma; BMI = body mass index; CONUT = controlling nutritional status; CR = complete response; CRP = C-reactive protein; IQR = interquartile range; LDH = lactate dehydrogenase; PD = progressive disease; PR = partial response; PS = performance status; SD = stable disease.
Associations of CONUT score with clinical parameters and response to first-line platinum-based chemotherapy
The CONUT score was 0, 1, 2, 3, and ≥4 in 36 (25%), 45 (31%), 30 (20%), 21 (14%), and 15 (10%) patients, respectively. The median (range) CONUT score was 1 (0–7). A higher CONUT score was associated with lower hemoglobin (P < 0.001) and higher CRP levels (P = 0.023, Table 2).
Of the 147 patients, 68 (46%) were good res-ponders (CR+PR), whereas 79 (54%) were non-res-ponders (SD+PD). AEs of grade 3 or greater were observed in 55 patients (37%). The median total number of chemotherapy cycles (range) was 4 (1–18). A total of 17 (12%) patients were exceptionally assessed for chemotherapy response after 1cycle due to severe AEs (9 patients) and rapidly progressive disease (8 patients). Chemotherapy responses of the 9 patients with severe AEs were PR in 1, SD in 1, and PD in 7 patients. There was no significant association of the CONUT score with chemotherapy response, AEs, or total number of chemotherapy cycles given (P = 0.432, P = 0.776, and P = 0.164, respectively; Table 2). Among 83 patients who had received 4 cycles or more, 53 (64%) and 30 (36%) were good responders and non-responders, respectively. There was no significant association of the CONUT score with chemotherapy response in this subgroup (P = 0.522).
Associations of CONUT score with OS
During the follow-up period (median, 16.1 mo-nths), 102 (69%) patients died. The median OS was 18.4 months for the overall cohort. By univariable analysis, hazard ratios (HRs) of CONUT scores 1, 2, 3, and ≥4 (reference, CONUT score 0) were 1.02 (95% confidence interval, 0.59–1.75), 1.29 (0.71–2.36), 2.39 (1.26–4.50), and 3.96 (1.97–7.95), respectively. Accordingly, CONUT scores were regrouped into 3 categories: CONUT score 0–1, 2–3, and ≥4 as good, fair, and poor nutrition group, respectively. The median OS for patients with good, fair, and poor nutrition were 23.3, 14.9, and 9.4 months, respectively (P < 0.001, Fig. 1).
Fig. 1
Kaplan–Meier curves estimate overall survival of 147 patients with advanced urothelial carcinoma treated with first-line platinum-based chemotherapy according to the controlling nutritional status (CONUT) score.
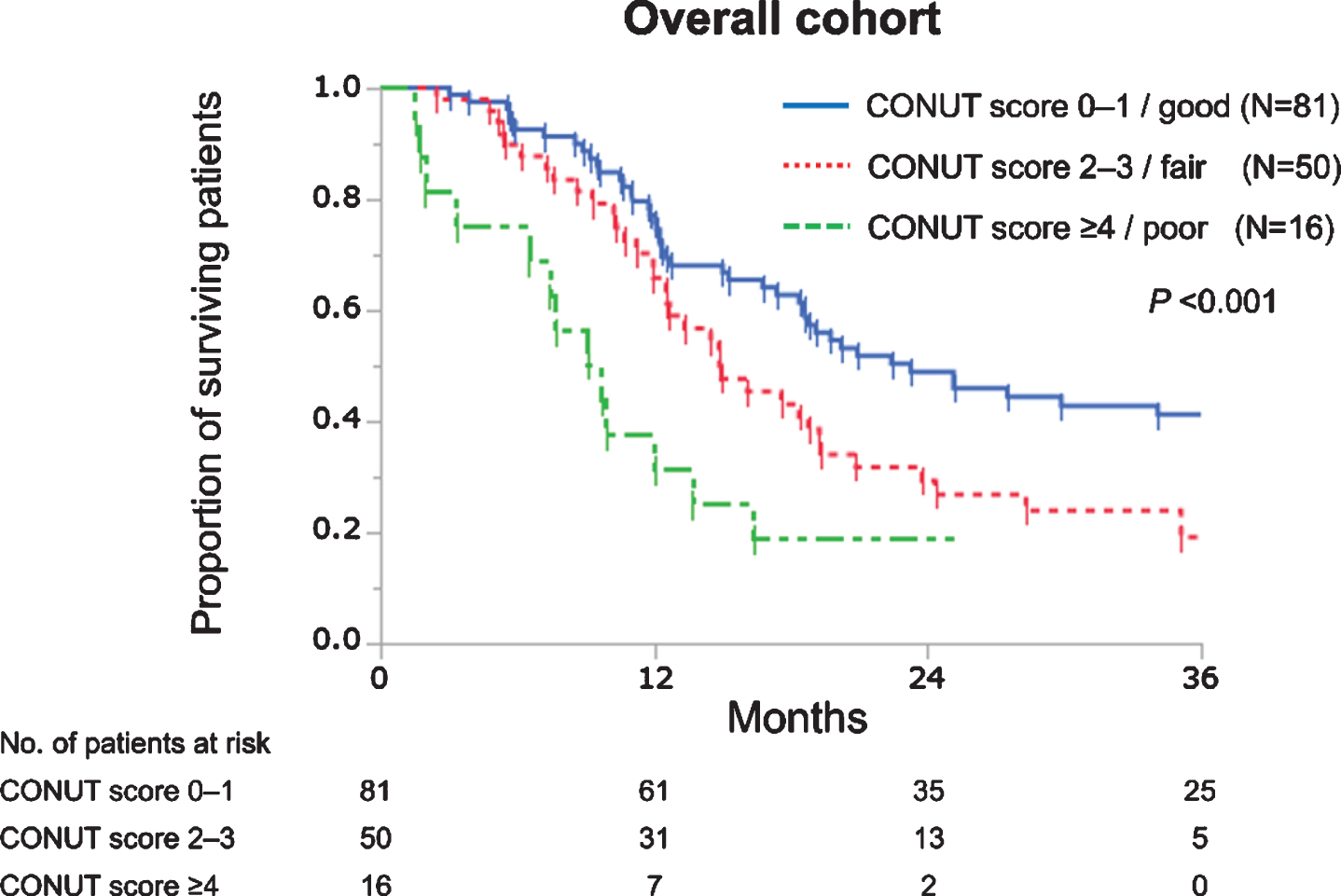
Table 3 shows the associations of variables with OS. On univariable analysis, a higher CONUT score was significantly associated with shorter OS (fair vs good, HR 1.66, P = 0.023; poor vs good, HR 3.25, P < 0.001), along with higher age (HR 1.86, P = 0.005), poorer PS (HR 4.65, P < 0.001), primary tumor site in the upper urinary tract (HR 2.08, P < 0.001), presence of visceral metastasis (HR 1.96, P = 0.002), higher LDH (HR 5.86, P = 0.007), higher ALP (HR 2.69, P = 0.004), higher CRP (HR 2.07, P = 0.005), no curative treatment after the diagnosis of aUC (HR 1.93, P = 0.005), carboplatin-based regimen (HR 1.88, P = 0.015), total number of chemotherapy cycles ≤3 (HR 1.67, P = 0.011), non-responders to chemotherapy (HR 2.06, P < 0.001), lower albumin (HR 3.67, P < 0.001), and lower lymphocyte count (HR 1.86, P = 0.002). On multivariable analysis, a higher CONUT score was significantly and independently associated with shorter OS (fair vs good, HR 1.58, P = 0.048; poor vs good, HR 2.63, P = 0.008), along with poorer PS (HR 4.79, P < 0.001), primary tumor site of the upper urinary tract (HR 1.70, P = 0.016), higher LDH (HR 3.85, P = 0.036), higher ALP (HR 3.06, P = 0.028), and non-responders to chemotherapy (HR 2.07, P < 0.001; multivariable model 1). To assess the prognostic significance of each component of the CONUT score, multivariable analysis was conducted using albumin, lymphocyte count, and total cholesterol concentration instead of the CONUT score. Among the components of the CONUT score, lower lymphocyte count (HR 1.96, P = 0.001) was identified as an independent adverse prognostic factor together with poorer PS (HR 5.75, P < 0.001), primary tumor site in the upper urinary tract (HR 1.76, P = 0.008), higher LDH (HR 3.90, P = 0.034), higher ALP (HR 3.24, P = 0.001), and non-responders to chemotherapy (HR 2.04, P < 0.001; multivariable model 2).
Table 3
Univariable and multivariable analyses for variables associated with overall survival in 147 patients
| Variables | Univariable analysis | Multivariable model 1 | Multivariable model 2 | ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | |||||||||
| ≥65 (vs < 65 [years]) | 1.86 | 1.20–2.97 | 0.005 | ||||||
| Sex | |||||||||
| Male (vs female) | 1.01 | 0.66–1.58 | 0.969 | ||||||
| PS | |||||||||
| ≥2 (vs 0–1) | 4.65 | 2.16–8.82 | < 0.001 | 4.79 | 2.09–9.92 | < 0.001 | 5.75 | 2.54–11.6 | < 0.001 |
| BMI | |||||||||
| < 23 (vs ≥23 [kg/m2]) | 1.47 | 0.99–2.19 | 0.052 | ||||||
| Primary tumor site | |||||||||
| Upper urinary tract (vs bladder) | 2.08 | 1.41–3.08 | < 0.001 | 1.70 | 1.11–2.62 | 0.016 | 1.76 | 1.16–2.65 | 0.008 |
| Clinical T stage | |||||||||
| 3–4 (vs ≤2) | 1.57 | 0.97–2.66 | 0.065 | ||||||
| Clinical N stage | |||||||||
| ≥1 (vs 0) | 1.39 | 0.87–2.33 | 0.173 | ||||||
| Visceral metastasis (lung, liver, or bone) | |||||||||
| Yes (vs No) | 1.96 | 1.30–2.92 | 0.002 | ||||||
| Curative treatment before the diagnosis of aUC | |||||||||
| Yes (vs No) | 1.07 | 0.71–1.60 | 0.735 | ||||||
| Hemoglobin* | |||||||||
| < 12/11.5 (vs ≥12/11.5 [g/dL]) | 1.52 | 0.99–2.28 | 0.056 | ||||||
| LDH | |||||||||
| > 360 (vs ≤360 [U/L]) | 5.86 | 1.75–14.7 | 0.007 | 3.85 | 1.11–10.3 | 0.036 | 3.90 | 1.13–10.4 | 0.034 |
| ALP | |||||||||
| > 380 (vs ≤380 [U/L]) | 2.94 | 1.52–5.19 | 0.004 | 3.06 | 1.51–5.69 | 0.028 | 3.24 | 1.63–5.91 | 0.001 |
| CRP | |||||||||
| > 30 (vs ≤30 [mg/L]) | 2.07 | 1.26–3.26 | 0.005 | ||||||
| CONUT score | |||||||||
| 0–1 (good) | ref | ref | – | ||||||
| 2–3 (fair) | 1.66 | 1.08–2.54 | 0.023 | 1.58 | 1.00–2.47 | 0.048 | – | ||
| ≥4 (poor) | 3.25 | 1.67–5.90 | < 0.001 | 2.63 | 1.31–4.99 | 0.008 | – | ||
| Curative treatment after the diagnosis of aUC | |||||||||
| No (vs Yes) | 1.93 | 1.21–3.22 | 0.005 | ||||||
| Chemo regimen | |||||||||
| Carboplatin-based (vs cisplatin-based) | 1.88 | 1.14–2.99 | 0.015 | ||||||
| Total No. chemo cycles given | |||||||||
| ≤3 (vs > 3) | 1.67 | 1.13–2.46 | 0.011 | ||||||
| Response to systemic chemotherapy | |||||||||
| SD + PD (vs CR + PR) | 2.06 | 1.39–3.09 | < 0.001 | 2.07 | 1.37–3.14 | < 0.001 | 2.04 | 1.35–3.10 | < 0.001 |
| Adverse effect | |||||||||
| Grade ≥3 (vs < 3) | 1.38 | 0.93–2.05 | 0.110 | ||||||
| Albumin | |||||||||
| < 3.5 (vs ≥3.5 [g/dL]) | 3.67 | 1.83–6.65 | < 0.001 | – | |||||
| Lymphocyte count | |||||||||
| < 1600 (vs ≥1600 [μL]) | 1.86 | 1.25–2.81 | 0.002 | – | 1.96 | 1.30–2.99 | 0.001 | ||
| Total cholesterol | |||||||||
| < 140 (vs ≥140 [mg/dL]) | 1.76 | 0.88–3.16 | 0.104 | – | |||||
ALP = alkaline phosphatase; aUC = advanced urothelial carcinoma; BMI = body mass index; CONUT = controlling nutritional status; CR = complete response; CRP = C-reactive protein; IQR = interquartile range; LDH = lactate dehydrogenase; PD = progressive disease; PR = partial response; PS = performance status; SD = stable disease. *Cutoff values are 12 g/dL and 11.5 g/dL for males and females, respectively.
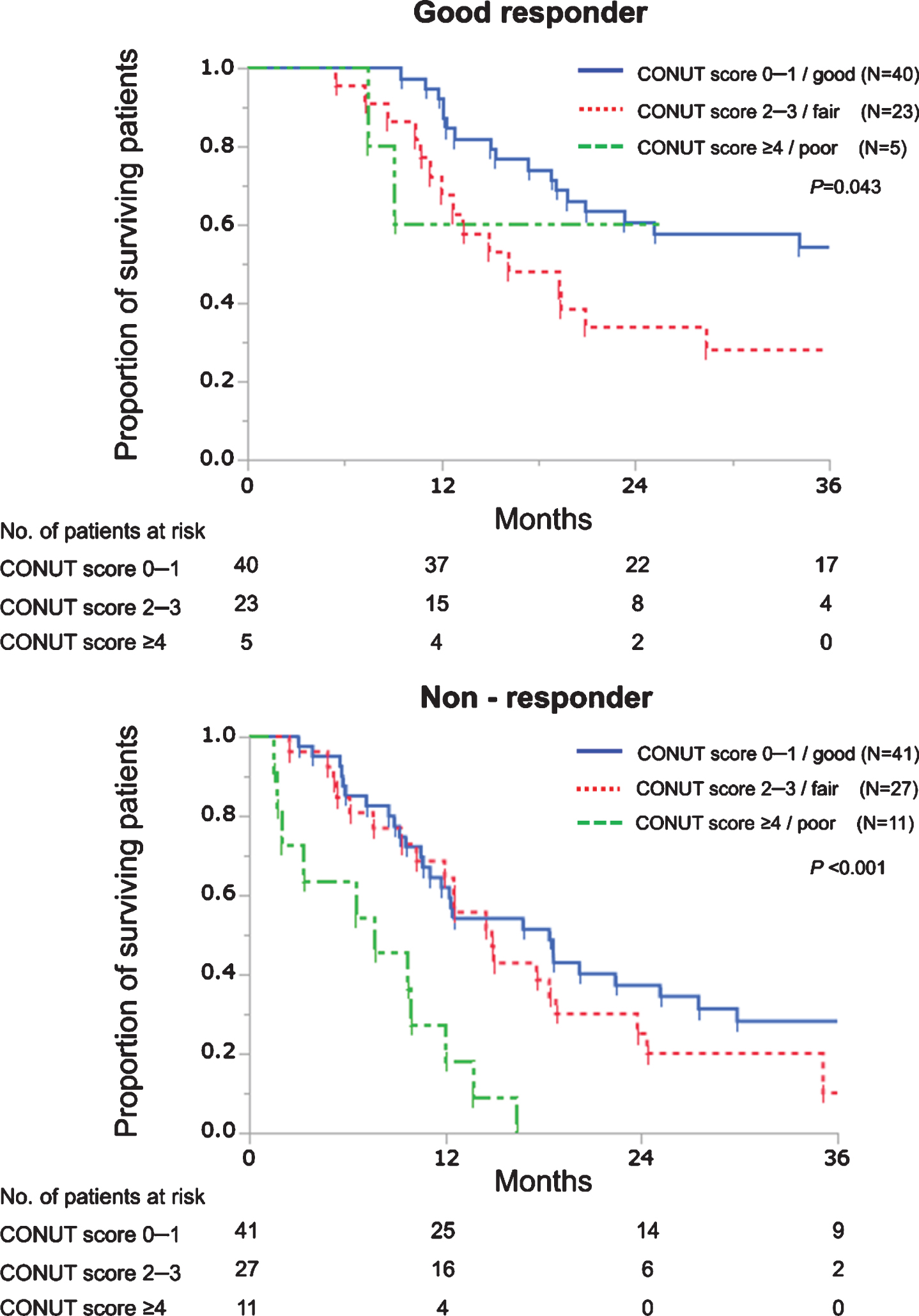
Good responders showed longer OS than non-responders with respective median OS of 25.1 and 12.6 months (P < 0.001). Next, we evaluated the prognostic roles of the CONUT score according to chemotherapy responses. As shown in Fig. 2, patients with good nutrition showed significantly better OS than those with fair nutrition among good responders (HR 0.49, P = 0.032) while patients with poor nutrition showed significantly worse OS than those with good or fair nutrition among non-responders (poor vs good, HR 4.30, P < 0.001; poor vs fair, HR 3.28, P = 0.004). Similarly, OS curves were significantly separated according to the CONUT score both in good responders (P = 0.011) and non-responders (P < 0.001) among 83 patients receiving 4 cycles or more (Supplementary figure).
Fig. 2
Kaplan–Meier curves estimate overall survival of patients with advanced urothelial carcinoma treated with first-line platinum-based chemotherapy according to the CONUT score in good responders and non-responders. In good responders, a univariable HR of patients with CONUT scores 0–1 was 0.49 (vs those with CONUT scores 2–3, P = 0.032). In non-responders, univariable HRs of patients with CONUT scores≥4 were 4.30 and 3.28 (vs those with CONUT scores 0–1, P < 0.001; and vs those with CONUT scores 2–3, P = 0.004, respectively).
DISCUSSION
The present study demonstrated that the CONUT score is an independent prognostic biomarker in patients with aUC treated with first-line platinum-based chemotherapy. The prognostic significance of the CONUT score was observed irrespective of chemotherapy responses. Considering its simplicity, objectivity, and global availability in evaluating nutrition status, the CONUT score may be a practical prognostic indicator for patients with aUC receiving first-line systemic chemotherapy, as well as an indicator of their general condition from the aspect of nutritional status.
PS is an established prognostic factor in patients with aUC [8, 9, 25], and it represents a patients’ general condition from aspects of activity of daily life. In this study, the prognostic significance of the CONUT score was independent of that of PS. The CONUT score appears to represent the general condition of patients with aUC from the aspect of nutritional status as a prognostic biomarker, independently of PS.
According to our multivariable model 1 (Table 3), PS, LDH, and ALP seem to have outperformed the CONUT score as an independent prognostic factor. In this study, these variables were dichotomized at cutoffs to yield the highest univariable HRs. Accordingly, high-risk groups consisted of small number of patients with extremely poor prognosis; the median OS was 5.4, 8.1, and 7.5 months for 9 patients with poorer PS, 4 with higher LDH, and 14 with higher ALP, respectively. Prognostic value of the CONUT score was limited in these small subpopulations at the highest risk. However, OS was clearly stratified according to the CONUT score in their counterparts (all P < 0.002, data not shown), implying that the CONUT score provides additional prognostic value to other strong prognostic factors.
Chronic inflammation, induced by tumor-derived proinflammatory cytokines such as tumor necrosis factor-alfa and interleukin (IL)-6 [26], is one of the pathophysiological features of patients with advanced cancer. IL-6 strongly suppresses antitumor immunity via activation of signal transducer and activator of transcription 3 [27], whereas inducing chronic inflammation results in hypoalbuminemia and hypocholesterolemia by impairing host anabolism [18]. Thus, the CONUT score potentially reflects the degree of tumor-derived chronic inflammation. In fact, a higher CONUT score was significantly associated with higher serum CRP levels (Table 2), which is known as an adverse prognostic factor of aUC [28]. In addition, the CONUT score was correlated with neutrophil-to-lymphocyte ratio, one of indirect measures of chronic inflammation (Spearman’s rank correlation coefficient 0.486, P < 0.001; data not shown).
The total lymphocyte count was an independent prognostic factor in multivariable model 2 (Table 3), indicating the prognostic relevance of host immunity in patients with aUC receiving systemic chemotherapy. Accumulating evidence also supports its relevance in outcomes of platinum-based chemotherapy. Besides its direct cytotoxic effects, outcomes of chemotherapy also depend on the stimulation of adaptive anticancer immunity and the generation of immunological memory against tumor-associated antigens [29]. Platinum-based chemotherapy stimulates T cell functions directly and indirectly by downregulating programmed death-ligand 2 expression on dendric cells [30]. Cisplatin also upregulates the expression of major histocompatibility complex class I to promote the presentation of tumor-associated antigens, priming T cells, and clonal expansion of cytotoxic T cells [31, 32]. These mechanisms may underlie the prognostic significance of the CONUT score in patients with aUC treated with first-line platinum-based systemic chemotherapy.
The present study had several limitations. First, there is possible bias due to the retrospective nature of a single-institutional study. External validations with large, multi-institutional cohorts are needed to confirm the generality of our findings, according to the REMARK guidelines [24]. Second, only 23 patients received ICI, the current standard as second-line treatment for aUC. Pembrolizumab was approved for aUC by the Japanese National Health Insurance system in December 2017. Because our cohort consisted of patients treated between February 2003 and April 2019, most patients did not receive pembrolizumab. Contemporary cohorts of patients receiving ICI may yield different results. Further studies are required to elucidate the impact of the CONUT score on antitumor effects of ICIs in patients with aUC. Third, we did not completely record statin usage and thus it was not included as a variable in the present cohort. Adjustment for statin use may have provided more accurate prognostic information on the total cholesterol value, a component of the CONUT score.
CONCLUSION
The CONUT score was evaluated as an independent prognostic biomarker in patients with aUC treated with first-line platinum-based chemotherapy. Its prognostic significance was independent of chemotherapy response and PS. The CONUT score may represent not only simple nutritional status but more importantly the degree of tumor-derived chronic inflammation and the host immune status in patients with aUC.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
HS: drafting the manuscript, data acquisition, statistical analysis, and interpretation of data; MI: data acquisition, statistical analysis, and interpretation of the data; KT: data acquisition, critical revision of the manuscript for scientific and factual content; SK: critical revision of the manuscript for scientific and factual content; MK: critical revision of the manuscript for scientific and factual content; NI: critical revision of the manuscript for scientific and factual content; KS: critical revision of the manuscript for scientific and factual content; TM: critical revision of the manuscript for scientific and factual content; FK: supervision, conception and design, drafting the manuscript, statistical analysis, and interpretation of the data.
CONFLICT OF INTEREST
HS, MI, KT, SK, MK, NI, KS, TM and FK have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-200354.
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2020. CA Cancer J Clin. (2020) ;70: (1):7–30. |
[2] | Bharmal M , Guenther S , Kearney M . Epidemiology of Locally Advanced or Metastatic Urothelial Cancer In The US, Europe And Japan. Value Health. (2017) ;20: (9):A419. |
[3] | Bellmunt J , Maase Hvd , Mead GM , Skoneczna I , Santis MD , Daugaard G , et al. Randomized Phase III Study Comparing Paclitaxel/Cisplatin/ Gemcitabine and Gemcitabine/Cisplatin in Patients With Locally Advanced or Metastatic Urothelial Cancer Without Prior Systemic Therapy: EORTC Intergroup Study 7. J Clin Oncol. . (2012) ;30: (10):1107–13. |
[4] | Bellmunt J , de Wit R , Vaughn DJ , Fradet Y , Lee J-L , Fong L , et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med.. (2017) ;376: (11):1015–26. |
[5] | Galsky MD , Wang H , Hahn NM , Twardowski P , Pal SK , Albany C , et al. Phase 2 Trial of Gemcitabine, Cisplatin, plus Ipilimumab in Patients with Metastatic Urothelial Cancer and Impact of DNA Damage Response Gene Mutations on Outcomes. Eur Urol. (2018) ;73: (5):751–9. |
[6] | Sharma P , Siefker-Radtke A , de Braud F , Basso U , Calvo E , Bono P , et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J Clin Oncol.. (2019) ;37: (19):1608–16. |
[7] | Taylor MH , Lee CH , Makker V , Rasco D , Dutcus CE , Wu J , et al. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J Clin Oncol. (2020) ;38: (11):1154–63. |
[8] | Iasonos A , Apolo AB , Bajorin DF , Small EJ , Philips GK , Ostrovnaya I , et al. Prognostic Model for Predicting Survival of Patients With Metastatic Urothelial Cancer Treated With Cisplatin-Based Chemotherapy. JNCI: Journal of the National Cancer Institute. (2013) ;105: (7):499–503. |
[9] | Galsky MD , Moshier E , Krege S , Lin C-C , Hahn N , Ecke T , et al. Nomogram for predicting survival in patients with unresectable and/or metastatic urothelial cancer who are treated with cisplatin-based chemotherapy. Cancer. (2013) ;119: (16):3012–9. |
[10] | Ignacio de Ulibarri J , Gonzalez-Madrono A , de Villar NG , Gonzalez P , Gonzalez B , Mancha A , et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. (2005) ;20: (1):38–45. |
[11] | Toyokawa T , Kubo N , Tamura T , Sakurai K , Amano R , Tanaka H , et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. (2016) ;16: (1):722. |
[12] | Liu X , Zhang D , Lin E , Chen Y , Li W , Chen Y , et al. Preoperative controlling nutritional status (CONUT) score as a predictor of long-term outcome after curative resection followed by adjuvant chemotherapy in stage II-III gastric Cancer. BMC Cancer. (2018) ;18: (1):699. |
[13] | Harimoto N , Yoshizumi T , Inokuchi S , Itoh S , Adachi E , Ikeda Y , et al. Prognostic Significance of Preoperative Controlling Nutritional Status (CONUT) Score in Patients Undergoing Hepatic Resection for Hepatocellular Carcinoma: A Multi-institutional Study. Ann Surg Oncol. . (2018) ;25: (11):3316–23. |
[14] | Toyokawa G , Kozuma Y , Matsubara T , Haratake N , Takamori S , Akamine T , et al. Prognostic impact of controlling nutritional status score in resected lung squamous cell carcinoma. J Thorac Dis. (2017) ;9: (9):2942–51. |
[15] | Iseki Y , Shibutani M , Maeda K , Nagahara H , Ohtani H , Sugano K , et al. Impact of the Preoperative Controlling Nutritional Status (CONUT) Score on the Survival after Curative Surgery for Colorectal Cancer. PLoS One. (2015) ;10: (7):e0132488–e. |
[16] | Zheng Y , Bao L , Wang W , Wang Q , Pan Y , Gao X . Prognostic impact of the Controlling Nutritional Status score following curative nephrectomy for patients with renal cell carcinoma. Medicine. (2018) ;97: (49):e13409–e. |
[17] | Suzuki H , Ito M , Takemura K , Nakanishi Y , Kataoka M , Sakamoto K , et al. Prognostic significance of the controlling nutritional status (CONUT) score in advanced urothelial carcinoma patients. Urol Oncol. (2020) ;38: (3):76.e11–76.e17. |
[18] | Hashizume M , Yoshida H , Koike N , Suzuki M , Mihara M . Overproduced interleukin 6 decreases blood lipid levels via upregulation of very-low-density lipoprotein receptor. Ann Rheum Dis. (2010) ;69: (4):741–6. |
[19] | Jiang Y , Li Y , Zhu B . T-cell exhaustion in the tumor microenvironment. Cell Death Dis. (2015) ;6: (6):e1792. |
[20] | Warny M , Helby J , Nordestgaard BG , Birgens H , Bojesen SE . Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLoS Med. (2018) ;15: (11):e1002685. |
[21] | Galluzzi L , Buqué A , Kepp O , Zitvogel L , Kroemer G . Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell.. (2015) ;28: (6):690–714. |
[22] | Eisenhauer EA , Therasse P , Bogaerts J , Schwartz LH , Sargent D , Ford R , et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) ;45: (2):228–47. |
[23] | National Cancer Institute NIoH, U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 November 27, 2017 [updated March 27, 2020. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. |
[24] | Altman DG , McShane LM , Sauerbrei W , Taube SE . Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration. PLoS Med. (2012) ;9: (5):e1001216. |
[25] | Bajorin DF , Dodd PM , Mazumdar M , Fazzari M , McCaffrey JA , Scher HI , et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. (1999) ;17: (10):3173–81. |
[26] | Patel HJ , Patel BM . TNF-alpha and cancer cachexia: Molecular insights and clinical implications. Life Sci. (2017) ;170: :56–63. |
[27] | Johnson DE , O’Keefe RA , Grandis JR . Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. (2018) ;15: (4):234–48. |
[28] | Saito K , Urakami S , Komai Y , Yasuda Y , Kubo Y , Kitsukawa S , et al. Impact of C-reactive protein kinetics on survival of patients with advanced urothelial carcinoma treated by second-line chemotherapy with gemcitabine, etoposide and cisplatin. BJU Int. (2012) ;110: (10):1478–84. |
[29] | Galluzzi L , Buqué A , Kepp O , Zitvogel L , Kroemer G . Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. (2017) ;17: (2):97–111. |
[30] | Lesterhuis WJ , Punt CJ , Hato SV , Eleveld-Trancikova D , Jansen BJ , Nierkens S , et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest.. (2011) ;121: (8):3100–8. |
[31] | de Biasi AR , Villena-Vargas J , Adusumilli PS . Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin Cancer Res.. (2014) ;20: (21):5384–91. |
[32] | Wan S , Pestka S , Jubin RG , Lyu YL , Tsai YC , Liu LF . Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One. (2012) ;7: (3):e32542. |




