Outcomes of Trimodal Therapy for cT2-3 Urothelial Carcinoma in a Racially Diverse Population: A Single Institution Experience in the Bronx1
Abstract
BACKGROUND:
Radical cystectomy (RC) is the historical “gold standard” treatment for cT2-3 urothelial carcinoma (UC). However, recent evidence supports comparable outcomes of bladder preserving trimodal therapy (TMT) to RC in select patients.
OBJECTIVE:
To assess the oncologic outcomes of our institutional TMT experience.
METHODS:
We retrospectively identified all patients that received radiation therapy (RT) for cT2-3 UC from 2012 to 2018. Clinicopathologic data was then extracted from the patients’ medical records. We included patients who underwent RT with or without concurrent chemotherapy for curative intent after diagnostic TURBT, with or without re-staging TURBT. Patient clinical (age, sex, race) and pathologic/disease characteristics of bladder cancer (stage, presence of hydronephrosis, concurrent carcinoma in-situ) were collected. Primary outcomes were: response to TMT (complete response [CR], partial response [PR], progression), recurrence-free, and overall survival. We also analyzed rates of salvage cystectomy and associated disease-specific outcomes. Response was based on the first surveillance imaging, cystoscopy, or TURBT after completion of TMT.
RESULTS:
24 patients underwent TMT during the study period. 29.2% of patients were black/non-hispanic, 37.5% were latino/hispanic, and 20.8% were white/non-hispanic. 58.3% of patients were female. 19 (79.2%), 3 (12.5%), and 2 (8.3%) patients experienced CR, PR and progression after TMT, respectively. At a median follow-up of 22.4 months, 19 (79.2%) patients were recurrence-free, 3 were alive with disease (12.5%), and 2 expired from other causes (8.3%; 1 with and 1 without disease present). Overall, 22 (92.7%) patients were still alive at last follow-up. No clinical variables were significant predictors of CR to TMT.
CONCLUSIONS:
In concordance with prior reports, TMT offers excellent tumor response rates for patients seeking definitive therapy for cT2-3 UC. Extended follow-up is needed to assess the durability of response and long-term survival after TMT.
INTRODUCTION
Bladder cancer is the 6th most common cancer in the United States and 10th most common cancer worldwide [1, 2]. Up to 25% of patients present with muscle-invasive bladder cancer (MIBC) at initial diagnosis, and up to 20– 30% of those initially diagnosed with non-MIBC (NMIBC) eventually progress to muscle-invasive disease [3]. Radical cystectomy (RC) has been widely accepted as the gold-standard for MIBC, with 5-year overall survival (OS) rates of 56– 66% in contemporary series [4– 7]. A multicenter study of over 2700 RC cases reported 5 year OS to be 65% (pT2), 50% (pT3), 47% (pT4), and 31% (pN+) [8].
Due to the morbidity associated with RC, many patients are not candidates to undergo surgery due to poor performance status or they simply choose to undergo less invasive modalities. Over the past decade, there is increasing evidence to support bladder preservation options like trimodal therapy (TMT), which has emerged as the most commonly used protocol for bladder preservation in MIBC. TMT consists of maximal transurethral resection of bladder tumor (TURBT) along with radiosensitizing chemotherapy and external beam radiation therapy (EBRT). CRT is the term used when specifically referring to the concomitant chemotherapy with radiation therapy after TURBT. Several studies have reported TMT oncologic outcomes that are comparable to RC, with 5-yr and 10-yr overall survival at 57% and 39%, respectively [9].
However, the existing research does not unanimously support the use of bladder preservation modalities. A recent study in 2018 claimed that TMT was associated with significantly decreased OS and cancer-specific survival (CSS) as well as $335 million in excess spending in 2011 [10]. As a result of still conflicting evidence, more research is needed to better understand the efficacy of TMT in treating MIBC. We therefore sought to assess our institution’s TMT data and determine if outcomes were comparable to existing TMT data in regards to response rates, efficacy, and treatment durability.
MATERIALS AND METHODS
Search strategy
Institutional review board approval was obtained to conduct an institutional census capturing all patients with an ICD-10 code diagnosis of cT2-3 bladder cancer that received radiation therapy (RT) from 2012 to 2018. IRB approval number: # 2013-2712. Granular data was then obtained from the patients’ electronic records.
Inclusions/Exclusion criteria
We included patients with cT2-T3 disease (with or without prior NMIBC). Patients that were selected for TMT either expressed personal preference for bladder preservation or were deemed poor surgical candidates for cystectomy. We included patients who underwent RT with or without concurrent chemotherapy for curative intent after diagnostic TURBT, with or without re-staging TURBT.
Those that were >T3 (either at presentation or after progression from NMIBC) or metastatic at presentation were excluded. Patients with prior RC or partial cystectomy were also excluded. A total of 24 patients met inclusion criteria for this study.
Radiation and chemotherapy
Radiation therapy (RT) was delivered using Intensity Modulated Radiation Therapy (IMRT), Image Guided Radiation Therapy (IGRT) and adaptive planning techniques. Full bladder was treated to a median of 45 Gy followed by a boost to the clinical tumor volume (CTV) of 10– 21.5 Gy wherever feasible to a total dose of 55– 66.6 Gy (Median 60 Gy; 1.8– 2.5 Gy/fraction). If the tumor was multifocal and normal bladder could not be spared, whole bladder received full dose. 22 patients received chemotherapy concurrently with RT (CRT) using regimens of 5FU/mitomycin-C, single agent weekly cisplatin, or single agent low-dose gemcitabine. Two patients did not receive concurrent chemotherapy with RT, and six patients received neoadjuvant chemotherapy.
Outcomes measures
Outcomes were measured as follows: [1] response to TMT (complete response [CR], partial response [PR], stable disease, progression), [2] disease recurrence (local or distant), [3] underwent salvage cystectomy, [4] status at most recent follow-up (alive without disease [AWOD], alive with disease [AWD], deceased without disease [DWOD], deceased with disease [DWD]). CR was defined as no evidence of disease (EOD), PR defined as improvement of tumor burden/disease status, stable disease defined as no change in tumor burden/disease status, and progression was defined as worsening of tumor burden/disease status. Other supporting data was also collected including presence of hydronephrosis, concurrent CIS, chemotherapy regimen, and RT dosage.
After extracting data from chart review, response was determined based off of the first surveillance imaging (CT or PET), cystoscopy with or without biopsy, or TURBT after completion of TMT. Dates of treatments were collected as well as data from surveillance/follow-up appointments to establish a timeline of therapy in relation to treatment response, disease status, and survival.
RESULTS
24 patients underwent TMT during the study period. Demographics are outlined in Table 1 and the treatment timeline for each patient is shown in Fig. 1. 29.2% of patients were black/non-hispanic, 37.5% were latino/hispanic, and 20.8% were white/non-hispanic. 58.3% of patients were female. Average RT dose was 59.2 Gy and ranged from 45– 66.6 Gy. No patients experienced grade 3 or higher acute toxicity according to CTCAE version 3 criteria. 2 patients did not receive concurrent chemotherapy along with their RT regimen. 5 patients underwent neoadjuvant chemotherapy (NAC) prior to initiation of CRT (4 were given NAC between pre-CRT TURBT and CRT initiation, 1 was given NAC prior to pre-CRT TURBT), and 1 patient received NAC after TMT but prior to salvage cystectomy. Of the patients who received NAC, 4 had cT2 disease and 2 had cT3. 4 patients received gemcitabine+cisplatin. 1 patient received dose-dense combination of methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC). 1 patient’s NAC regimen was undocumented in the medical record.
Table 1
Patient Demographics, Disease Characteristics, and Treatment Modalities
| Characteristics | No. | % |
| Age at Diagnosis (yr) | Median 74.5, Avg 72.2, IQR 61.5– 81 | |
| Sex | ||
| Male | 10 | 41.7 |
| Female | 14 | 58.3 |
| Race/Ethnicity | ||
| Black/Non-Hispanic | 7 | 29.2 |
| Latino/Hispanic | 9 | 37.5 |
| White/Non-Hispanic | 5 | 20.8 |
| Other/Unknown | 3 | 12.5 |
| ECOG Performance | ||
| 0 | 5 | 20.8 |
| 1 | 15 | 62.5 |
| 2 | 2 | 8.3 |
| 3 | 2 | 8.3 |
| T Stage at Initial Diagnosis | ||
| Ta | 2 | 8.3 |
| T1 | 3 | 12.5 |
| T2 | 17 | 70.8 |
| T3 | 2 | 8.3 |
| T stage prior to CRT | ||
| T2 | 19 | 79.2 |
| T3 | 5 | 20.8 |
| Concurrent CIS | ||
| Yes | 3 | 12.5 |
| No | 21 | 87.5 |
| Hydronephrosis | ||
| Present | 4 | 16.7 |
| Absent | 20 | 83.3 |
| Neoadjuvant Chemotherapy | ||
| Yes | 6 | 25 |
| No | 18 | 75 |
| TURBT | ||
| Visibly complete | 9 | 37.5 |
| Visibly incomplete | 8 | 33.3 |
| Unknown | 7 | 29.2 |
| Repeat TURBT prior to CRT | ||
| Yes | 13 | 54.2 |
| No | 11 | 45.8 |
| Bladder Preservation Modality | ||
| RT | 2 | 8.3 |
| Chemo + RT | 22 | 91.7 |
| TMT Chemotherapy Regimen | ||
| 5-FU + mitomycin | 9 | 37.5 |
| low-dose Gemcitabine | 10 | 41.7 |
| Cisplatin | 3 | 12.5 |
| None | 2 | 8.3 |
| RT Dosage (Gy) | Median = 60, Avg = 59.2 | |
| >60 | 10 | 41.7 |
| 50– 60 | 9 | 37.5 |
| <50 | 2 | 8.3 |
| N/A | 3 | 12.5 |
TURBT = transurethral resection of bladder tumor, RT = radiation therapy.
Fig. 1
Swimmer’s Plot showing timeline of TMT in relation to treatment responses and status at last known follow-up. Time 0 = pre-CRT TURBT.
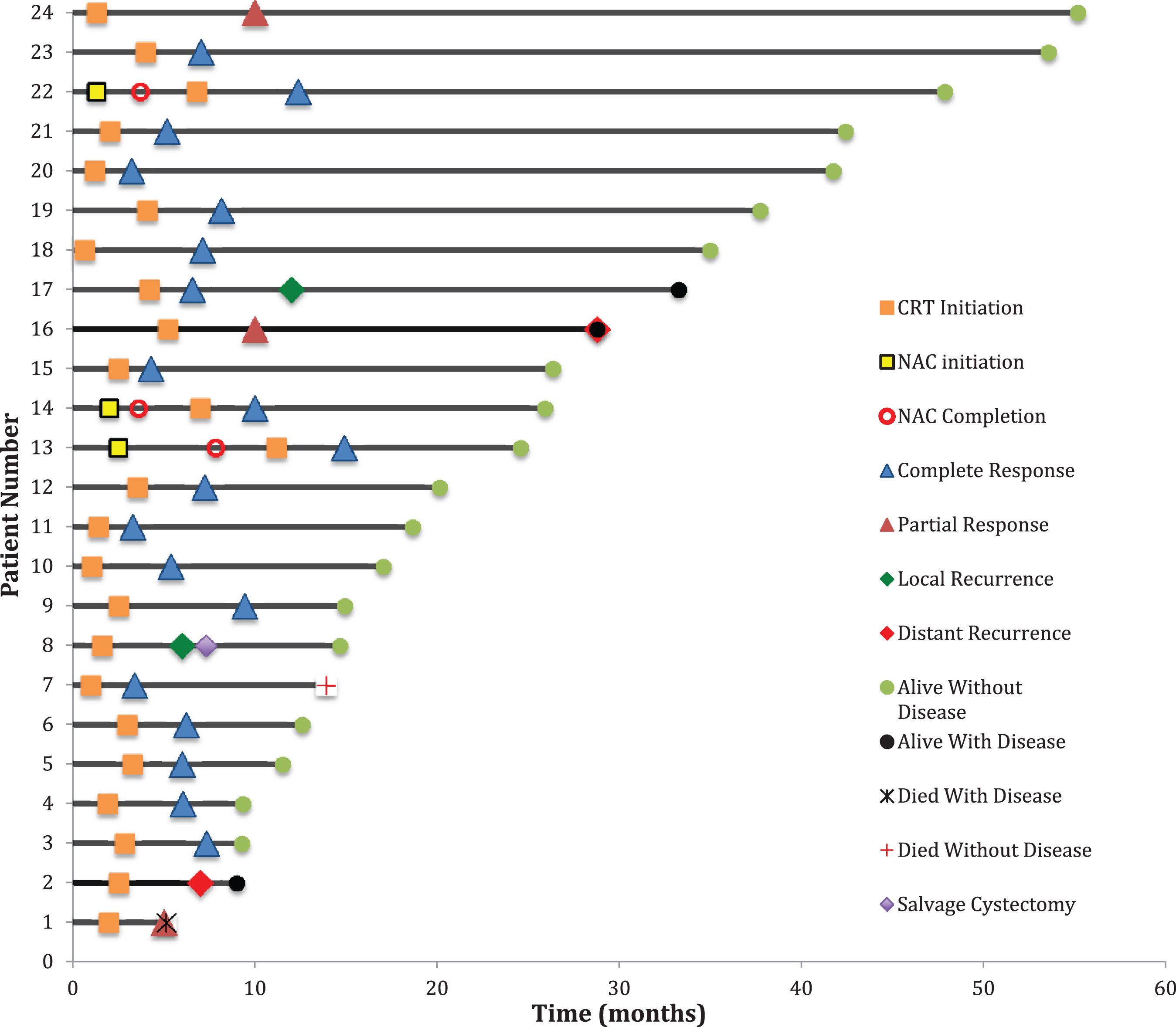
Table 2
Outcomes Data
| Characteristics | No. | % |
| Response To Bladder Preservation Tx | ||
| Complete | 19 | 79.2 |
| Partial | 3 | 12.5 |
| Stable disease | 0 | 0 |
| Progression of disease | 2 | 8.3 |
| Timing of Post-tx Response Assessment | ||
| ≤3 months | 19 | 79.2 |
| 4– 6 months | 4 | 16.7 |
| N/A | 1 | 4.2 |
| Salvage Cystectomy | ||
| Yes | 1 | 4.2 |
| No | 23 | 95.8 |
| Recurrence after CR/PR (total n = 22) | ||
| Yes | 2 | 9.1 |
| No | 20 | 90.9 |
| Time from pre-CRT TURBT to last follow-up (months) | Median = 22.4, Avg = 25.4 | |
| Status at Last Follow-up | ||
| Alive without disease | 19 | 79.2 |
| Alive with disease | 3 | 12.5 |
| Deceased without disease | 1 | 4.2 |
| Deceased with disease | 1 | 4.2 |
Tx = treatment.
Table 3
Association of Clinical Variables with Complete Response to TMT
| Variable | P-Value | OR | 95% CI |
| Age | 0.1 | 1.04 | 0.99– 1.04 |
| Sex (male vs. female) | 0.5 | 0.7 | 0.3– 1.9 |
| Pre-CRT hydronephrosis | 0.2 | 1.6 | 0.8– 2.9 |
| Concurrent CIS | 0.8 | 0.9 | 0.5– 1.7 |
| Pre-CRT T-Stage (T3 vs T2) | 0.2 | 0.4 | 0.1– 1.5 |
| Re-TUR (yes vs. no) | 0.3 | 0.8 | 0.5– 1.2 |
| TUR or Re-TUR Status (complete vs. incomplete) | 0.7 | 1.1 | 0.6– 2.2 |
| Time from pre-CRT TUR to CRT | 0.4 | 0.9 | 0.8– 1.1 |
Median time from pre-CRT TURBT to last follow-up was 22.4 months. Median time from pre-CRT TURBT to CRT initiation was 2.5 months. Adjusted median time from pre-CRT TURBT to CRT initiation after eliminating 4 patients who received neoadjuvant chemotherapy (NAC) between pre-CRT TURBT and CRT initiation was 2.3 months. Median time from pre-CRT TURBT to CR was 6.6 months. Median time from CRT initiation to CR was 3.2 months. 19 patients received post-treatment surveillance 3 or less months after treatment (79.2%), 4 patients were assessed 4– 6 months after treatment (16.7%), and 1 patient’s surveillance timeline was unavailable. 3 out of the 4 patients in the 4– 6 month group received NAC in between pre-CRT TURBT and CRT initiation.
Of the 24 patients, CR was seen in 19 patients (79.2%), PR in 3 patients (12.5%), stable disease in 0 patients, and disease progression in 2 patients (8.3%). 1 patient with CR had a local recurrence, and 1 patient with PR had distant recurrence. Overall recurrence was seen in 2 patients (8.3%). Of the 2 patients that had progression of disease, 1 underwent salvage cystectomy (4.2%), and 1 received palliative RT for metastatic disease to omentum and humerus. At most recent follow-up, 19 patients were AWOD (79.2%), 3 were AWD (12.5%), 1 was DWD (4.2%), and 1 was DWOD (4.2%). Bladder cancer was not attributed to any patient deaths. All 6 patients that received NAC were AWOD at last follow-up.
The two deceased patients passed from causes unrelated to their bladder cancer. Overall, 22 patients were still alive (92.7%) at last follow-up. There were no clinical variables that were statistically significant predictors of CR to TMT.
DISCUSSION
When compared to the literature, our study had comparable CR rates with several interesting features. A unique characteristic of our study is the racially diverse population. 29.2% of the patients in our study were Black/non-Hispanic and 37.5% Latino/Hispanic. According to the most recent census, the racial composition of the Bronx is 56.4% Hispanic/Latino, 28.9% Black/African American, 8.9% White, and 3.6% Asian [11]. Studies have shown higher mortality and higher stage of disease at presentation among Black/African American patients relative to White patients [12, 13]. Since our study showed favorable CR rates, TMT can be a legitimate treatment option even among ethnic groups that have been shown to have worse outcomes for bladder cancer. Though we did not compare response rates among the different ethnicities due to our small sample size, the diverse population of the Bronx provides future opportunities to further analyze disease patterns and response to TMT among these varying ethnicities.
Another interesting finding in our data is the female to male proportion. 58.3% of patients in our study were female. This percentage is not consistent with the typical gender distribution of bladder cancer, which has a 4:1 male predominance [14]. However, there is also data to suggest that women are more likely to be muscle-invasive at time of first diagnosis [15]. Despite such data, the literature does not comment on a female predominance once both genders have MIBC. Although it is difficult to ascertain the meaning of the percentage of females in our study, it is nonetheless an intriguing finding that warrants further questioning.
TMT has shown promise as an effective means to treat MIBC with similar outcomes to RC in several retrospective studies. In 2017, Kulkarni et al retrospectively reviewed 112 patients who underwent RC or TMT (56 patients in both treatment groups) [16]. Using propensity score matching, they demonstrated similar 5-year DSS between TMT and RC (76.6% vs 73.2%, p = 0.49), with a salvage cystectomy rate of 10.7%. Despite their quality retrospective data, it is inarguable that prospective data is needed. However, in the absence of randomized control studies comparing RC to TMT, prospective propensity matched cohorts provide the highest level of quality data.
The largest single-center experience with TMT was reported by Giacalone et al at Massachusetts General Hospital (MGH) [9]. This study consisted of 475 patients with cT2-T4a MIBC who underwent TMT as per MGH protocol from 1986 to 2013. They found 5- and 10-yr DSS rates for TMT to be 66% and 59%, respectively and 5- and 10-yr OS rates to be 57% and 39%, respectively. They also reported CR to CRT and presence of tumor-associated CIS to be significant predictors for OS and DSS. Furthermore, it was noted that when comparing the eras of 1986-1995 and 2005-2013, CR rates improved from 66% to 88%, and 5-yr DSS improved from 60% to 84%.
Another retrospective analysis in 2018 reported mean 10-year OS for TMT vs. RC to be 30.9% and 35.1% respectively (p = 0.32), but for stage T2 the mean 10-year DSS for TMT vs. RC was 69.0% and 78.9% respectively (p = 0.45) [17]. In addition, they found that approximately 75% of patients achieved CR to TMT with salvage cystectomy rates ranging from 25– 30% after receiving TMT. In 2018, Royce et. al even reported that TMT had an incremental gain of 0.59 quality-adjusted life years (QALYs) over RC (7.83 vs. 7.24 QALYs, respectively) [18].
The existing data comparing TMT to RC is not unanimously in agreement with the potential role for TMT. Williams et al. claimed in a population-based retrospective analysis that TMT was associated with significantly decreased OS and cancer-specific survival (CSS) as well as $335 million in excess spending in 2011 [10]. Despite the impressive sample size of 3200 patients in the final cohort, the Surveillance, Epidemiology, and End Results (SEER) database lacked specifics in regards to dose or cycle of chemotherapy, dose of RT, extent/quality of TUR, timeline of CRT in relation to TUR, and even the indication for TMT. Without a known CRT regimen that specifies treatment purpose (palliative vs. curative intent), it is difficult to say that this data is truly reflective of TMT outcomes. Furthermore, the patients categorized as radical cystectomy either had surgery alone or surgery plus chemotherapy or RT. Despite propensity score matching, TMT was not exclusively compared with patients who only underwent RC. Thus, the addition of chemotherapy/RT to RC has potential to skew the data in favor of the RC group. Regarding the excess spending, costs were only assessed up to 180 days after treatment. That being said, although RC had lower costs in the first 180 days, the long-term comorbidities and complications of this surgery could lead to increased costs later on due to hospital admissions.
Despite these large retrospective analyses, the limitation in truly comparing TMT to RC is a complete lack of randomized prospective trials. There has only been one trial that attempted to randomize patients to bladder preservation vs. RC, yet was unable to be completed due to a lack of accrual [19]. As mentioned earlier, the most promising data will come from prospective propensity matched cohorts. It is also worth noting that comparing TMT to RC is challenging due differences in staging. TMT relies on clinical staging, whereas pathologic staging is obtained with RC, and a significant number of patients are in fact upstaged at time of RC [20– 22].
There are several limitations to our study that must be acknowledged. We attempted to identify associated variables as significant predictors of CR. However, there were no statistically significant predictors, likely due the small sample size. With a larger future sample size, we hope to identify significant predictors of tumor response. It is also important to address the variability in post-TMT surveillance timelines. 19 patients (79.2%) received post-TMT surveillance within 3 or less months, with 4 patients (16.7%) obtaining post-TMT surveillance at 4– 6 months. There is poor documentation in the medical record as to why the patients in the 4– 6 month category were not first surveilled within 3 months. However, we feel this is largely due to social factors contributing to issues with follow-up, which is commonly seen in our patient population (e.g. complex social/domestic issues, transportation issues, etc.). Additionally, there is variability in the time from pre-CRT TURBT to initiation of CRT (median 2.5 months, average 3.2 months). We believe these variations could also be due to the previously mentioned factors relating to post-TMT surveillance timelines. However, this timeline is also skewed due to the patients that received NAC in between their pre-CRT TURBT and CRT initiation. 3 of the longest timelines between pre-CRT TURBT and CRT initiation were from patients that received NAC prior to CRT initiation. By eliminating the 4 patients who received NAC in between pre-CRT TURBT and CRT initiation, the adjusted median time from pre-CRT TURBT to initiation of CRT becomes 2.3 months, and the average time becomes 2.4 months. We are also unable to compare 5- and 10-yr OS and DSS rates to existing literature, due to our lack of long-term follow-up data. However, having an OS of 92.7% at last follow-up should not be ignored and will require further long-term data collection to assess the durability of response to TMT in our patients.
CONCLUSIONS
TMT offers excellent tumor response rates for patients seeking definitive therapy for cT2-3 UC in a multiracial cohort. Extended follow-up is needed to assess the durability of response and long-term survival after TMT.
ACKNOWLEDGMENTS
The authors have no acknowledgements.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Collection and Assembly of Data: Gottlieb; Study Concept and Design: Sankin, Kovac, Schoenberg; Statistical Analysis: Kovac, Gottlieb; Analysis and Interpretation of Data: Sankin, Kovac, Gottlieb; Drafting of Manuscripts: Gottlieb, Sankin; Manuscript Revision: Sankin, Kovac, Schoenberg, Aboumohamed, Gartrell, Bodner, Garg; Final Approval of Manuscript: All authors.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
REFERENCES
[1] | World Cancer Research Fund. 2020. Worldwide Cancer Data. <https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data> [Accessed 2 April 2020]. |
[2] | SEER. 2020. Cancer Of The Urinary Bladder - Cancer Stat Facts. <https://seer.cancer.gov/statfacts/html/urinb.html> [Accessed 2 April 2020]. |
[3] | Chang SS , Bochner BH , Chou R , et al. Treatment of Nonmetastatic Muscle-Invasive Bladder Cancer: American Urological Association/American society of clinical oncology/American society for radiation oncology/society of urologic oncology clinical practice guideline summary. Journal of Oncology Practice. (2017) ;13: (9):621–5. doi: 10.1200/JOP.2017.024919 |
[4] | Grossman HB , Natale RB , Tangen CM , et al. Neoadjuvant chemo- therapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. (2003) ;349: :859–66. http://dx.doi.org/10.1056/NEJMoa022148 |
[5] | Sherif A , Holmberg L , Rintala E , et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. (2004) ;45: :297–303. http://dx.doi.org/10.1016/j.eururo.2003.09.019 |
[6] | Stein JP , Lieskovsky G , Cote R , et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1054 patients. J Clin Oncol. (2001) ;19: :666–75. |
[7] | Zehnder P , Studer UE , Skinner EC , et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. J Urol. (2011) ;186: :1261–8. |
[8] | Sonpavde G , Khan M , Lerner S , et al. Disease-Free Survival at 2 or 3 Years Correlates With 5-Year Overall Survival of Patients Undergoing Radical Cystectomy for Muscle Invasive Bladder Cancer. J Urol. (2011) ;185: (2):456–61. doi: 10.1016/j.juro.2010.09.110 |
[9] | Giacalone N , Shipley W , Clayman R , et al. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur Urol. (2017) ;71: (6):952–60. doi: 10.1016/j.eururo.2016.12.020 |
[10] | Williams S , Shan Y , Jazzar U , et al. Comparing Survival Outcomes and Costs Associated With Radical Cystectomy and Trimodal Therapy for Older Adults With Muscle-Invasive Bladder Cancer. JAMA Surg. (2018) ;153: (10):881–9. doi: 10.1001/jamasurg.2018.1680 |
[11] | “Bronx County, NY.” Data USA, datausa.io/profile/geo/bronx-county-ny#demographics. |
[12] | Yee DS , Ishill NM , Lowrence WT , et al. Ethnic Differences In Bladder Cancer Survival. Journal of Urology. (2009) ;181: (4S):24–24. doi: 10.1016/s0022-5347(09)60080-x |
[13] | Lee CT , Dunn RL , Williams C , et al. Racial Disparity in Bladder Cancer: Trends in Tumor Presentation at Diagnosis. Journal of Urology. (2006) ;176: (3):927–34. doi: 10.1016/j.juro.2006.04.074 |
[14] | Siegel R , Naishadham D , Jemal A . Cancer statistics, 2012. CA: a cancer journal for clinicians. (2012) ;62: (1):10–29. |
[15] | Scheller T , Hofmann R , Hegele A . Sex-related differences in urothelial cell carcinoma of the bladder in Germany. Cancer Manag Res. (2018) ;11: :309–16. Published 2018 Dec 28. doi:10.2147/CMAR.S181532 |
[16] | Kulkarni GS , Hermanns T , Wei Y , et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. (2017) ;35: (20):2299–305. doi: 10.1200/JCO.2016.69.2327 |
[17] | Fahmy O , Khairul-Asri MG , Schubert T , et al. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol Oncol. (2018) ;36: (2):43–53. doi: 10.1016/j.urolonc.2017.10.002 |
[18] | Royce TJ , Feldman AS , Mossanen M , et al. Comparative Effectiveness of Bladder-preserving Tri-modality Therapy Versus Radical Cystectomy for Muscle-invasive Bladder Cancer. Clin Genitourin Cancer. (2019) ;17: (1):23–31.e3. doi: 10.1016/j.clgc.2018.09.023 |
[19] | Huddart RA , Hall E , Lewis R , et al. Life and death of spare (selective bladder preservation against radical excision): reflections on why the spare trial closed. BJU Int. (2010) ;106: (6):753–5. doi: 10.1111/j.1464-410X.2010.09537.x |
[20] | Svatek RS , Shariat SF , Novara G , et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. (2011) ;107: (6):898–904. |
[21] | Turker P , Bostrom PJ , Wroclawski ML , et al. Upstaging of urothelial cancer at the time of radical cystectomy: factors associated with upstaging and its effect on outcome. BJU Int. (2012) ;110: (6):804–11. |
[22] | Ahmadi H , Mitra AP , Abdelsayed GA , et al. Principal component analysis based pre-cystectomy model to predict pathological stage in patients with clinical organ-confined bladder cancer. BJU Int. (2013) ;111: (4 Pt B):E167–172. |



