The Urinary Microbiome and Bladder Cancer: Susceptibility and Immune Responsiveness
Abstract
Bladder cancer is a highly prevalent disease worldwide and is associated with a high mortality rate. Across all stages of bladder cancer, immunotherapy has now become the cornerstone of treatment. The commensal microbiome has become a major focus of research given its impact on numerous states of human health and disease. Many links between commensal microbes and immune function have been reported. Recently a commensal urinary microbiome has been identified and characterized in healthy individuals by several research groups. The urinary microbiome is now emerging as an important factor influencing bladder cancer development and therapeutic responsiveness. In this report, we identify findings from important clinical and mechanistic studies on the urinary microbiome and future opportunities to impact prevention and treatment of bladder cancer.
INTRODUCTION
Bladder cancer is a common malignancy with approximately 400,000 new cases and 150,000 deaths occurring annually [1]. Histological types of bladder cancer include urothelial carcinoma, adenocarcinoma, small cell, plasmacytoid, and squamous cell carcinoma. In industrialized countries, urothelial carcinoma accounts for more than 90% of all histological types and is associated with 5-year survival ranging between 30% and 70%, based on clinical stage [4]. A rise in prevalence and mortality is expected due to environmental exposures, smoking, and increased life expectancy [5]. Bladder cancer can be grouped into two main categories with different outcomes and molecular profiles: non-muscle invasive (NMIBC) and muscle invasive bladder cancer (MIBC). NMIBC, particularly Ta and T1, accounts for 70–80% of all diagnosed cases. Standard treatment is transurethral resection of bladder tumor (TURBT). In intermediate- or high-risk disease, TURBT is followed by intravesical immunotherapy with Bacillus Calmette-Guerin (BCG) or intravesical chemotherapy (i.e. mitomycin C) [6]. Recently, pembrolizumab, the anti-PD-1 antibody was approved for NMIBC that is BCG-unresponsive. NMIBC has high recurrence rates (up to 52% in five years) and can progress to MIBC [7]. Thus, early stage disease still requires lifelong monitoring with periodic cystoscopy and urinary cytology, making NMIBC one of the most expensive cancers to manage [8]. MIBC treatment consists of radical cystectomy with perioperative chemotherapy or bladder-sparing approaches involving transurethral resection of bladder tumor followed by chemoradiotherapy. For metastatic bladder cancer standard treatments include platinum-based chemotherapy, FGFR-targeted therapy, enfortumab-vedotin, and immune checkpoint inhibitors targeting the PD-1 pathway. Despite advances in management of urothelial cancer, there remains a critical need to develop new therapies and identify factors that have a role in cancer onset, progression, and recurrence.
Emerging data have discredited the historical view that the urine and bladder are sterile in healthy individuals [9–12]. Modern culture and sequencing techniques have now enabled the detection of microbes throughout the urinary system [13–16]. The concept that healthy urine is sterile dates back to the mid-nineteenth century when early microbiologists such as Louis Pasteur found that urine contained in sealed vials did not become clouded, which suggested an absence of bacteria. Over subsequent decades, culture techniques in clinical laboratories were optimized for detection of specific pathogenic bacteria such as E. Coli. Thus, lack of growth in bacterial cultures had been erroneously linked to sterility. More recently, however, enhanced culture techniques, 16S ribosomal RNA (rRNA) sequencing, and whole-genome shotgun sequencing have provided robust evidence for the existence of a commensal urinary microbiome [14–16]. An emerging focus of bladder cancer research is now aimed at understanding how the commensal urinary microbiome can influence susceptibility to bladder cancer development and its impact on treatment efficacy through modulation of the anti-cancer immune response.
URINARY MICROBIOME IN HEALTHY INDIVIDUALS
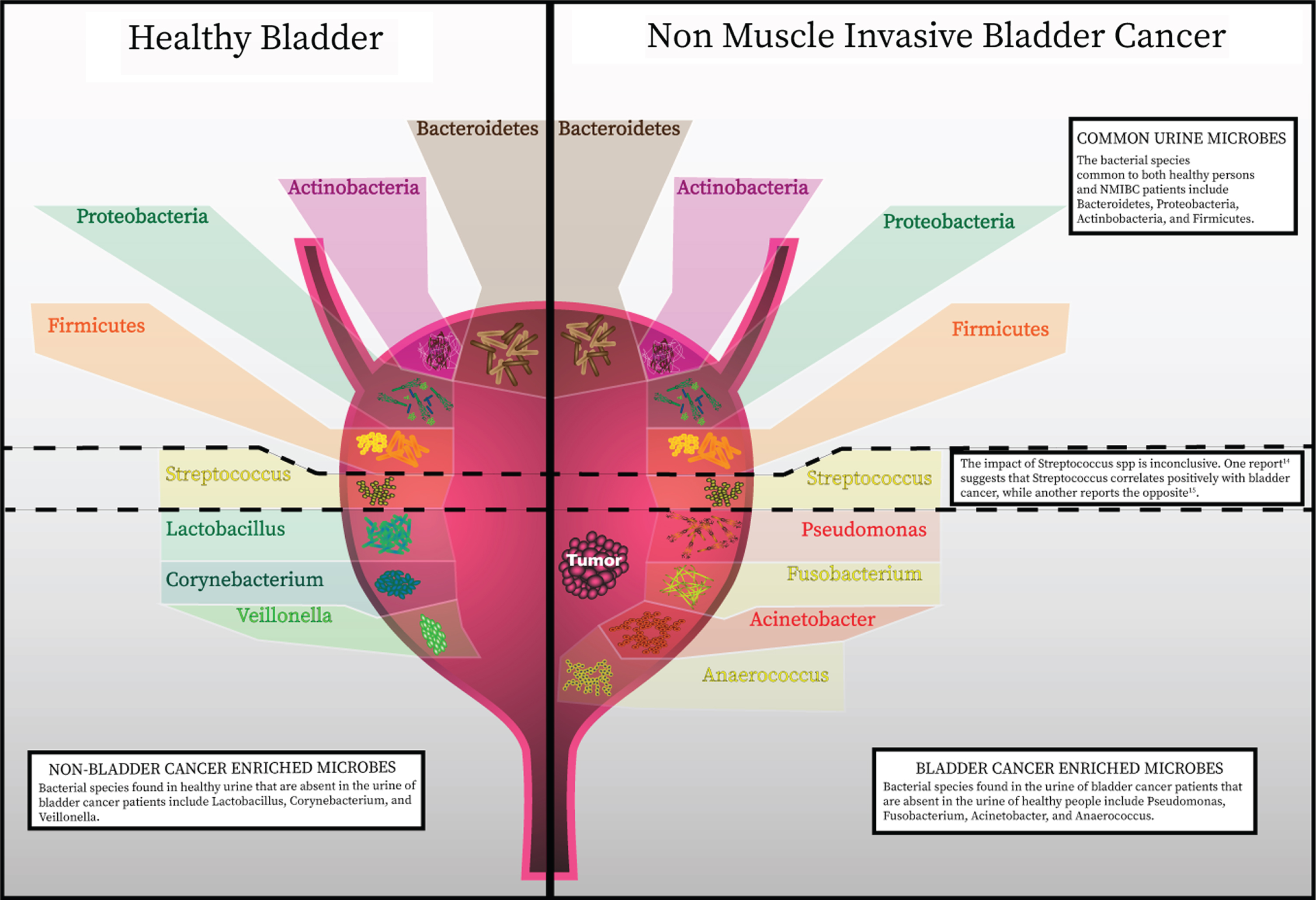
The bladder was notably not included within the Human Microbiome Project, but several studies from healthy individuals have now shown that urine contains bacteria not routinely cultivated by clinical microbiology laboratories (Fig. 1). These bacteria can be identified by expanded culture techniques and nucleic acid sequencing [11–13]. Although the numbers of studies are limited, some found significant differences between the urinary microbiota of men and women [11, 17]. This finding is not unexpected given the differences in anatomical structure, hormones, and local defenses. Curtiss et al. studied the microbiome of 79 healthy women to identify changes related to age and menopausal status [10]. The authors found a greater incidence of Lactobacillus in the bladder microbiome of pre-menopausal women than post-menopausal women, with a trend towards decreased numbers of different genera in-post menopausal specimens. It is known that declining levels of estrogen during menopause induces vulvovaginal atrophy, which impairs the defense against invading pathogens and is also thought to contribute to the increased risk for urinary tract infections (UTI). Incomplete emptying of the urinary bladder after voiding is another factor thought to increase the risk of recurrent UTI. Residual urine and reduction in urine flow in the absence of estrogen impairs the mechanical clearance of bacteria and eases pathogens to colonize the bladder [18]. These findings may also explain differences in the commensal urine microbiome. Lewis et al. [11] suggested the presence of a core microbiome, defined as a subset of bacteria that is regularly present in the bladder, with samples are grouped by age. Notably, the genera Jonquetella, Parvimonas, Proteiniphilum, and Saccharofermentans appeared exclusively in the >70 age group. The reason these genera would colonize the urinary tract of individuals older than 70 years of age is not fully understood. In addition to age, non-modifiable host factors such as sex and genetics may influence the innate immune response and, therefore, have a role in the type of bacterial colonization [19]. This process might encompass human urinary tract adaptation to accommodate certain bacterial species, for example, through expression of specific receptors, as well as mutations in bacteria enabling adherence to the uroepithelium and survival. The implication of inherited phenotypes of innate immunity affecting bacterial colonization of the urinary tract has been supported by investigations looking for a genetic correlation between family members with recurrent UTI [20]. These studies have identified polymorphisms and expression patterns in genes such as CXCR1, which are linked with susceptibility to urinary infection. Further studies are needed to determine the role of the inherited immune phenotypes with variation in the commensal urinary microbiome.
Fig. 1
Recent advances in detection of microbes has discredited the once widely-held notion that the urinary bladder is a sterile environment. The healthy bladder is home to a wide assortment of bacterial species. A summary of specific species identified from published data is represented in this figure. As in with other commensal microbial niches in the body, the composition of bacterial communities in the bladder has a high level of inter-patient variability. Phenotypes have now been correlated with particular bacterial species and richness and diversity of bacterial species present in the bladder.
URINARY MICROBIOME IN PATIENTS WITH UROTHELIAL CARCINOMA
Studies investigating the continuum of health and disease states have further indicated that microbial populations are capable of influencing urological conditions. The precise nature and role of the most relevent microbes remain under investigation, but their potential involvement has now become more apparent. The impact of microbes on bladder cancer carcinogenesis is perhaps most clear from the longstanding observation that squamous cell carcinoma of the bladder is linked with urogenital schistosomiasis [2]. S. haematobium has been consistently reported to be associated with this type of bladder cancer. Its pathogenic role may occur through several mechanisms, such as epithelium damage, chronic inflammation, and oxidative stress [3]. As of today, only few studies have reported a detailed analysis of urinary microenvironment of urothelial bladder cancer. Results of the available studies are summarized in Table 1. Xu et al. [21] compared urine microbiota of healthy individuals and patients with bladder cancer. Their preliminary results showed an enrichment of Streptococcus in urine from patients with urothelial carcinoma. Streptococcus abundance was near zero in almost all healthy patients. In cancer samples where Streptococcus abundance was low, Pseudomonas or Anaerococcus were the most abundant genera. Unfortunately, the study was limited by the very small sample size and limited discussion of methodology. A similar study compared bacterial communities between urine samples of healthy individuals and cancer patients [22]. The authors found that the most abundant phylum in both groups was Firmicutes, followed by Actinobacteria, Bacteroidetes and Proteobacteria. They identified operational taxonomic units (OTUs) belonging to genus Fusobacterium to be more abundant in the bladder cancer group. An independent group of 42 bladder cancer tissues was analyzed and confirmed Fusobacterium nucleatum sequences could be detected by protein chain reaction in 11 samples. The genera Veillonella, Streptococcus and Corynebacterium were more abundant in healthy urine [22]. More recently, patients with bladder cancer were found to have an increase in bacterial richness, defined by the number of unique OTUs in a sample [23]. Greater bacterial richness was also present in urine from NMIBC patients witch high risk of recurrence or progression based on the European Organization for Research and Treatment of Cancer (EORTC) scoring system. Thus, the authors suggest that higher bacterial richness may be a potential indicator of high risk of recurrence and progression in NMIBC. Acinetobacter and Anaerococcus were found in higher abundances in bladder cancer patients compared with the non-cancer group [23]. Known virulence factors of Acinetobacter baumannii include invasion of epithelial cells, phospholipid degradation, and biofilm formation, which facilitates escape from the host immune response [24]. Anaerococcus, a member of the Gram-positive anaerobic cocci was reported to induce inflammation and remodeling of extracellular matrix (ECM) [25]. The authors raise the possibility that the interplay of ECM, microbiome, and inflammation play a key role in bladder cancer onset, progression, and relapse [26].
Table 1
Available studies on urine microbiome
| Diagnostics | Clinical trials | ||||||
| Reference | Xu W et al. | Bučević Popović V et al. | Wu P et al. | Aso Y et al. [27] (1992) | Ohashi Y et al. | Naito S et al | Aso Y et al. [28] (1995) |
| Aim | To compared microbiome in urine specimens between healthy individuals and urothelial carcinoma patients | To characterize urinary microbiome of bladder cancer patients and compare it with that of healthy controls | To characterize and explore the role of microbiome of urinary microbiota associated with bladder cancer in male patients | To investigate the safety and preventive effect after TUR-BT of an orally administered Lactobacillus (3 g daily) preparation in patients with superficial BC | To assess the preventive effect of fermented milk products containing Lactobacillus. casei against bladder cancer | To evaluate the role of oral administration of a preparation of the probiotic agent Lactobacillus casei in prevention of NMIBC recurrence comparing standard intravesical epirubicin with epirubicin plus 1-year oral intake of Lactobacillus casei strain. | To investigate the safety and preventive effect after TUR-BT of an orally administered Lactobacillus preparation (BLP) in patients with superficial BC. Follow up study of the 1992 trial. |
| Sample size | Healthy (n = 6) | Healthy (n = 11) | Healthy controls (n = 18) | Treatment group (n = 23) | Cases (n = 180) | Treatment group (n = 100) | BLP group vs Placebo |
| Urothelial carcinoma (n = 8) | BC (n = 12) | NMIBC (n = 26) | Control group (n = 25) | Controls (n = 445) | Control group (n = 102) | Tot of 138 patients | |
| MIBC (n = 5) | |||||||
| Methods | 454 DNA sequencing technology (full methods not reported) | 16S rRNA genes sequencing. Clean catch mid-stream urine was collected and DNA isolated from centrifuged pellet using PowerSoil Kit (MoBio Laboratories) | 16S rRNA genes sequencing. Clean catch mid-stream urine was collected and DNA isolated from centrifuged pellet using DNeasy Blood and Tissue Kit (Qiagen) | Randomized controlled clinical trial | Case-control study | Prospective, randomized, controlled clinical trial | Double-blind trial |
| Species | Acinetobacter | Firmicutes | Proteobacteria | Lactobacilus. | Lactobacilus. | Lactobacilus. | Lactobacilus. |
| Streptococcus | Actinobacteria | Firmicutes | casei | casei | casei | casei | |
| Pseudomonas | Bacteroidetes | Actinobacteria | |||||
| Finegoldia | Proteobacteria | Bacteroidetes | |||||
| Gardnerella | Streptococcus | Sphingobacteriaceae | |||||
| Anaerococcus | Prevotella | Thermoactinomycetaceae | |||||
| Escherichia | Peptoniphilus | Acinetobacter | |||||
| Enterococcus | Campylobacter | Serratia | |||||
| Veillonella | Proteus | ||||||
| Anaerococcus | Laceyella | ||||||
| Finegoldia | |||||||
| Conclusion | Urothelial carcinoma may be associated with altered microbiota, as urines from cancer patients were enriched with Streptococcus, while abundance was near zero in healthy volunteers | Firmicutes, Actinobacteria, Bacteroidetes and Proteobacteria were common in both groups. However, OTUs belonging to genus Fusobacterium were more abundant in the bladder cancer patients. An additional PCR analysis of 42 bladder cancer tissues, detected Fusobacterium nucleatum sequences in 26% of the samples. On the other hand, OTUs from genera Veillonella, Streptococcus and Corynebacterium were more abundant in healthy controls | Potential biomarkers for risk stratification were identified, as significant difference in beta diversity was found between cancer and control group, and among different risk level. Enrichment of Herbaspirillum, Porphyrobacter, and Bacteroides was observed in cancer patients with high risk of recurrence and progression. | Oral administration of Lactobacillus preparation is useful for the prevention of the recurrence of superficial bladder cancer. Recurrence-free interval post TUR-BT was 1.8-fold prolonged in the treatment group compared to the control group. No adverse side effect was observed | A strong correlation between habitual intake of lactic acid bacteria and reduction of BC risk was found | Co-administration of intravesical epirubicin and oral Lactobacilus casei is a promising method for prevention of NMIBC recurrence, as a 15% absolute reduction in long-term tumor recurrence reported | BLP administration seemed to offer beneficial effects in preventing recurrence of superficial bladder cancer |
| Comments | Small sample size | Small sample size | Small sample size | Small sample size | Potential confounding factors | Higher dropout rate (approximately 3.5-fold) in the treatment group | |
In addition to studies investigating the link between the urinary microbiome and oncogenesis, the effect on treatment is also being explored. As potential links become uncovered, the possibility to reduce risk or prevent disease recurrence though microbiota manipulation has become highly attractive. As early as 1992, Aso et al. [27] suggested that the oral administration of biolactis powder had a preventive effect on bladder cancer recurrence after TURBT. This was confirmed by the same research group in a double-blind trial involving 138 patients randomly assigned to the treatment and placebo groups [28]. In 2002, Seow et al. [29] discovered that Lactobacillus species, specifically L. casei and L. rhamnosus GG (LGG), inhibited the growth of bladder cancer cells by prompting a cytotoxic effect. Accordingly, Ohashi et al. [30] conducted a case-control matched study to analyze bladder cancer risk-reduction associated with the intake of fermented milk products. The odds ratio for recurrence was 0.46 (95% confidence interval: 0.27–0.79) for consumption of fermented milk products 1–2 times per week versus less than 1–2 times per month. The results suggested that the habitual intake of lactic acid bacteria reduced the risk of bladder cancer. In 2008, Naito et al. [31] conducted a randomized controlled trial comparing standard intravesical epirubicin alone with epirubicin plus 1-year oral intake of L. casei strain Shirota in patients who had undergone resection of intermediate-risk NMIBC. A statistically significant 15% absolute reduction in long-term tumor recurrence was seen in the group that received the oral probiotic. However, as the dropout rate was nearly 3.5-fold greater in the probiotic group, uncertainty remains as to the reliability of these findings. In a more recent study, Kandasamy et al. [32] modified LGG to secrete the prostate specific antigen (PSA) or IL-15 and PSA. In the context of bladder cancer, the authors suggested that Lactobacilli could stimulate neutrophils to secrete cytokines, inducing dendritic cell maturation and antigen-specific cytotoxic T cell production against cancer cells. In addition to recombinant cytokines, the use of selected bacteria to induce antigen-specific cytotoxicity could become an additional treatment option against cancer cells.
In addition to non-muscle-invasive disease, the role of the microbiome in bladder cancer may extend to more advanced disease as well. Immunotherapy agents, particularly those utilizing the PD-1/PD-L1 axis have seen increased use in early stage through metastatic urothelial carcinoma. Recently, the efficacy of anti-PD-1 therapy has been associated with microbial composition of the gut microbiome in other cancer types. More specifically, the presence of the species Bifidobacterium longum, Collinsella aerofaciens and Enterococcus faecium positively correlate with positive response to therapy for metastatic melanoma [33]. It is plausible that response to anti-PD-1/PDL1 therapy for bladder cancer may be associated with certain microbial compositions of the gut or bladder microbiome, although this has not yet been systematically investigated.
BACILLUS CALMETTE-GUERIN TREATMENT FOR NON-MUSCLE INVASIVE BLADDER CANCER
Intravesical immunotherapy with Bacillus Calmette-Guerin (BCG), a live attenuated strain of Mycobacterium bovis, is the standard-of-care for adjuvant treatment for NMIBC with a high-risk of progression. It has also been recommended for treatment of intermediate-risk NMIBC [6]. The mechanisms by which BCG immunotherapy mediates tumor immunity have been widely studied, though remain incompletely understood. Findings from preclinical and clinical studies demonstrate that a robust local inflammatory response to BCG involving the following:
a. BCG attachment to the urothelium. Animal studies suggest that BCG binds through the interaction between molecules expressed in the bacterial wall and fibronectin in the urothelium [34].
b. BCG internalization. Whether BCG is internalized by bladder cancer cells has long been disputed. BCG can be found in the urine of both mice and humans in the hours following intravesical instillation [35, 36]. However, BCG disappeared rapidly within days. Durek et al. [36] measured the level of mycobacterial DNA in the urine, showing a significant decrease during the 6 days following instillation. The method of internalization of BCG by urothelial cells has been controversial. However, recent studies have identified macropinocytosis as the endocytic process by which BCG is internalized by urothelial cancer cells. This is a process dependent on activation of the Ras and PI3K–PTEN pathways upstream of the kinase PAK1 [37]. It has been hypothesized that efficacy of BCG therapy depends on its uptake by bladder cancer cells due to the presence of oncogenic aberrations in the Ras and PI3K–PTEN pathways, which leads to activation of macropinocytosis. However, activating mutations in these two pathways are present only in a subset of bladder cancers [37, 38].
c. Induction of innate immune response. BCG immunotherapy induces both local and systemic immune responses, prompting the activation of urothelial and antigen-presenting cells (APCs). The production of cytokines and chemokines attracts granulocytes and mononuclear cells [39]. In vitro studies using human urothelial carcinoma cell lines demonstrated that BCG induces upregulation of cytokine production, including IL-6, IL-8, granulocyte–macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor (TNF) [40–43]. In human studies, cytokines and chemokines found in the urine after BCG treatment include IL-1, IL-8, IL-15, IL-18, and GM-CSF [41, 42, 44]. One consequence of these reactions is the formation of typical epithelioid granulomas in the bladder wall [9, 39, 45, 46].
d. Induction of adaptive immunity. BCG antigens are presented on the cell surface of APCs via class II MHC [39, 47, 48]. These molecules interact with CD4+ T cell receptors, leading to activation and differentiation to a primarily T helper 1 (Th1) [49]. Cytotoxic CD8+ T lymphocytes recognize tumor cells through antigen presentation via MHC class I. Their activation is facilitated by Th1 cells and mediated by IFNγ [50]. The balance between Th1 and Th2 cells determines the success of BCG treatment, with a Th1 cell response being associated with successful BCG immunotherapy [43, 50–53]. The necessity of T cells in response to BCG immunotherapy is well established, as athymic nude mice bearing bladder tumors showed no response to BCG instillation [54]. Retrospective analysis of clinical trial data showed that 5-year recurrence-free survival was significantly improved in patients with high-risk NMIBC who had a positive purified protein derivative (PPD) skin test before intravesical BCG therapy, compared to those with a negative PPD test (80% vs 45%) [35]. Even though these data suggest that BCG vaccination might improve the therapeutic response to BCG immunotherapy, further studies are needed to assess the relevance of these findings in the clinical practice.
Despite the many research efforts, the mechanism of action of BCG to control proliferation of cancer cells is still unclear, as well as the mechanisms behind bladder cancer recurrence. Unravelling this puzzle is crucial to determining how new therapies should aim to induce specific tumor microenvironments and antigen responses. The commensal urinary microbiome may be an important link involved in the efficacy of BCG immunotherapy.
POTENTIAL INTERACTIONS BETWEEN URINARY MICROBIOME AND BCG IMMUNOTHERAPY FOR NMIBC
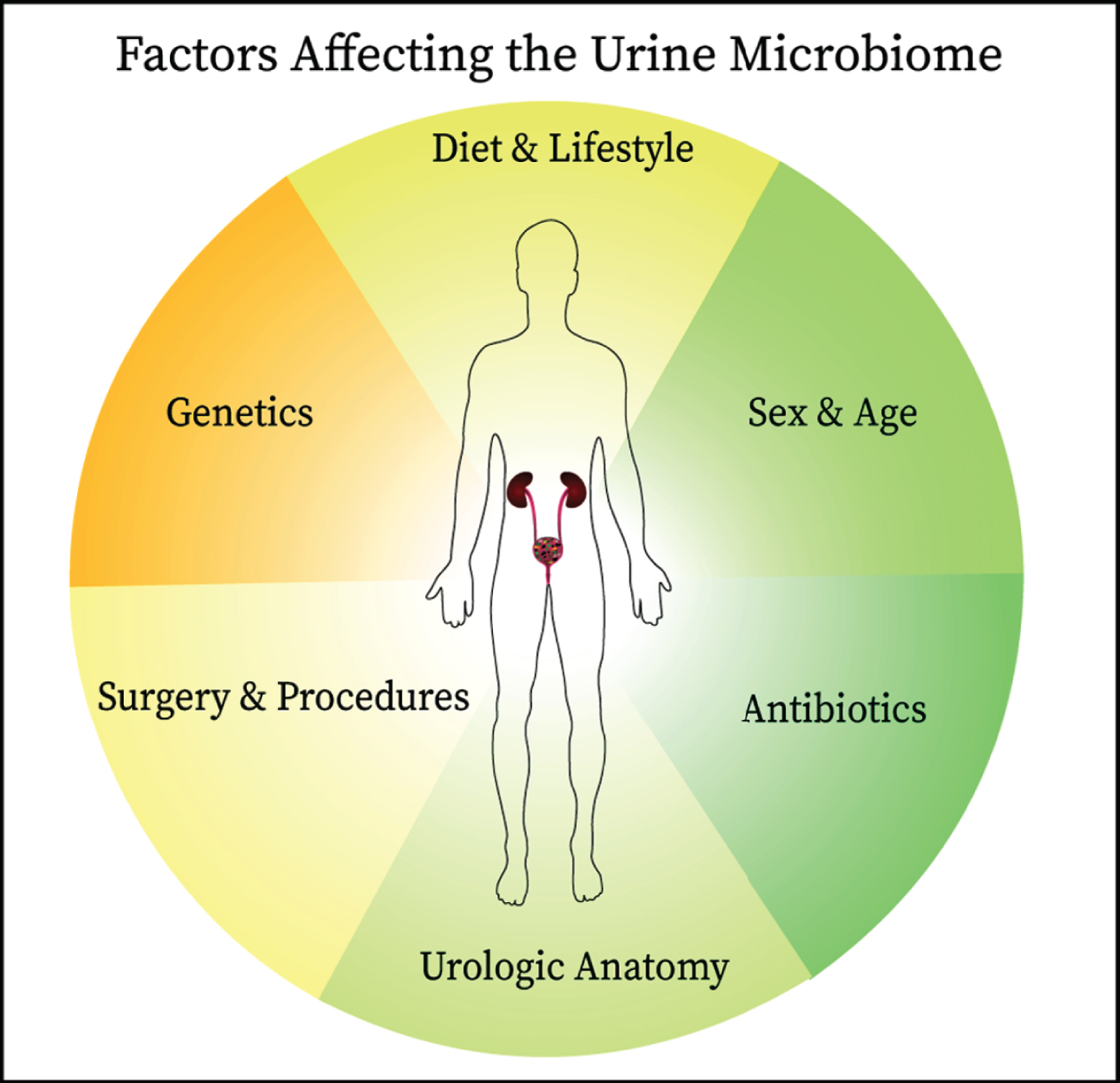
BCG is thought to work by stimulating the immune response through attachment of fibronectin, gaining access into the bladder cells. As many different bacteria are able to adhere to fibronectin, it is possible that specific commensal bacteria may saturate the binding sites used by BCG. This would decrease BCG efficacy and potentially downregulate the strong cytotoxic response needed to remove tumor cells. Conversely, as described earlier in this review, some bacteria, such as Lactobacillus, can induce antiproliferative and cytotoxic effects, contributing to the antineoplastic effect [29]. Probiotics may provide some benefit in the treatment of bladder cancer, as shown by studies where participants consumed fermented milk products and probiotics, achieving reduction in bladder cancer incidence and recurrence [30, 55]. It is compelling that Lactobacillus spp. may provide some beneficial role in treatment and prevention of bladder cancer. Our group has recently reported data from a study in which we characterized the role of the urine microbiome in 31 patients with high-risk NMIBC undergoing BCG treatment [56]. DNA was extracted and 16S sequencing data were generated using Illumina paired-end sequencing. In this cohort, 22 (71%) were male and 9 (29%) female, with a median age of 69 years and a range of 46–87 years. There was no difference in recurrence rates between males and females. Proteobacteria was the most abundant phylum, with an incidence of 58% (18 patients). An analysis of the OTUs, based on distance matrix computation, showed a significant difference between patients with and without recurrence (Bonferroni-corrected, P = 0.017). The Enterobacteriales order was significantly more abundant in patients with recurrence, while Lactobacillales were more abundant in patients without recurrence. The preliminary results of our study demonstrated the feasibility of analyzing the urinary microbiome in patients with bladder cancer undergoing TURBT and BCG therapy, with the prospect of a possible response prediction to treatment. Data indicate that patients who develop recurrence have significant differences in the abundance of specific bacterial orders at baseline compared with patients without recurrence. Importantly, there are many clinical and biological factors that influence the commensal microbiome and identifying causality can be very challenging (Fig. 2). Larger studies are currently being organized to follow up on these results.
Fig. 2
Since the establishment of the presence of commensal microbes in the urinary of healthy individuals, links have been identified between the urine microbiome and bladder cancer oncogenesis and therapeutic responsiveness. The interplay between the urine microbiome and host is complex and affected by other factors including antibiotic use, anatomical structures, surgical manipulation, diet, genetics, and age all are likely to influence the composition of urine microbiome. These factors should be considered when designing studies on the urine microbiome.
CONCLUSIONS AND FUTURE DIRECTIONS
A major expansion in knowledge has occurred regarding the impact of the microbiome and its functional role in health and disease. Yet, many more questions have now emerged, resulting further growth of research efforts, including studies on the microbiome in human cancers. As the concept of urine sterility has now been refuted in several studies, the urine microbiome has become an attractive focus of investigation in bladder cancer due given its proximity to the disease. Several studies have already reported associations with bladder cancer looking at single time points of data. Going forward it will also be important to also track microbial evolution in a longitudinal manner. The microbiome likely has differential impacts on bladder cancer each stage of its development and likely modulates the endogenous anti-tumor immune response. Thus, it has potential utility as a biomarker and therapeutic in bladder cancer, especially given that immune checkpoint therapy now is approved in both in early and late stage disease. Direct instillation of probiotics could be a potential strategy to impact the bladder microenvironment, but modification might also be possible though indirect mechanisms. For instance, manipulation of the gut flora with oral agents or fecal microbial transplant may result in systemic effects on immune response or circulating metabolites. Another important direction of future inquiry will be the characterization of the relationship between the urine microbiome and other commensal bacteria in the gut, skin, and other niches. It also remains unknown whether differences exist between the microbial populations of urine derived from the upper tract versus the bladder, or between the microbes potentially present within a bladder tumor versus within normal bladder tissue. A bowel-derived urinary diversion represents another unique environmental niche that is unique to bladder cancer patients and warrants further study. Finally, the complexity of data being generated will likely necessitate the application of advanced computational techniques incorporating clinical or other biological variables into sophisticated statistical models or machine learning algorithms. Using these approaches, research on the commensal microbiome has the potential to unlock more effective methods for diagnosis and treatment of patients with bladder cancer.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
RFS is supported by NIH K08CA234392 and AP by NIH T32 GM07019
AUTHOR CONTRIBUTIONS
CA: performance of work, writing the article
JCB: performance ofwork, interpretation and analysis of data
AP: performance of work, interpretation and analysis of data
RFS: conception, performance of work, interpretation and analysis of data, writing the article
ETHICAL CONSIDERATIONS
This study, as a literature review is exempt from any requirement for Institutional Review Board approval.
CONFLICT OF INTEREST
RFS reports consulting/honoraria from Aduro, AstraZeneca, BMS, Exelixis, Eisai, Janssen, Mirati, and Puma.
The other authors have no conflicts of interest to declare.
REFERENCES
[1] | Ferlay J , Shin H-R , Bray F , Forman D , Mathers C , Parkin DM . Estimates of worldwide burden of cancer in GLOBOCAN 2008. Int J Cancer. (2010) ;127: (12):2893–917. doi:10.1002/ijc.25516 |
[2] | Knowles MA , Hurst CD . Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. (2015) ;15: (1):25–41. doi:10.1038/nrc3817 |
[3] | Honeycutt J , Hammam O , Fu C-L , Hsieh MH . Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends Parasitol. (2014) ;30: (7):324–32. doi:10.1016/j.pt.2014.05.004 |
[4] | Jemal A , Siegel R , Ward E , Hao Y , Xu J , Thun MJ . Cancer statistics, 2009. CA Cancer J Clin. (2009) ;59: (4):225–49. doi:10.3322/caac.20006 |
[5] | Sanli O , Dobruch J , Knowles MA , et al. Bladder cancer. Nat Rev Primer. (2017) ;3: :17022. doi:10.1038/nrdp.2017.22 |
[6] | Babjuk M , Bohle A , Burger M , et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. (2017) ;71: (3):447–61. doi:10.1016/j.eururo.2016.05.041 |
[7] | Isharwal S , Konety B . Non-muscle invasive bladder cancer risk stratification. Indian J Urol IJU J Urol Soc India. (2015) ;31: (4):289–96. doi:10.4103/0970-1591.166445 |
[8] | Cambier S , Sylvester RJ , Collette L , et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guerin. Eur Urol. (2016) ;69: (1):60–9. doi:10.1016/j.eururo.2015.06.045 |
[9] | Cosma CL , Sherman DR , Ramakrishnan L . The secret lives of the pathogenic mycobacteria. Annu Rev Microbiol. (2003) ;57: :641–76. doi:10.1146/annurev.micro.57.030502.091033 |
[10] | Curtiss N , Balachandran A , Krska L , Peppiatt-Wildman C , Wildman S , Duckett J . Age, menopausal status and the bladder microbiome. Eur J Obstet Gynecol Reprod Biol. (2018) ;228: :126–9. doi:10.1016/j.ejogrb.2018.06.011 |
[11] | Lewis DA , Brown R , Williams J , et al. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front Cell Infect Microbiol. (2013) ;3: :41. doi:10.3389/fcimb.2013.00041 |
[12] | Wolfe AJ , Toh E , Shibata N , et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. (2012) ;50: (4):1376–83. doi:10.1128/JCM.05852-11 |
[13] | Hilt EE , McKinley K , Pearce MM , et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. (2014) ;52: (3):871–6. doi:10.1128/JCM.02876-13 |
[14] | Akram A , Maley M , Gosbell I , Nguyen T , Chavada R . Utility of 16S rRNA PCR performed on clinical specimens in patient management. Int J Infect Dis. (2017) ;57: :144–9. doi:10.1016/j.ijid.2017.02.006 |
[15] | Yu Y , Pieper R . Urinary pellet sample preparation for shotgun proteomic analysis of microbial infection and host-pathogen interactions. Methods Mol Biol. (2015) ;1295: :65–74. doi:10.1007/978-1-4939-2550-6_6 |
[16] | Yu Y , Sikorski P , Bowman-Gholston C , Cacciabeve N , Nelson KE , Pieper R . Diagnosing inflammation and infection in the urinary system via proteomics. J Transl Med. (2015) ;13: :111. doi:10.1186/s12967-015-0475-3 |
[17] | Fouts DE , Pieper R , Szpakowski S , et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. (2012) ;10: :174. doi:10.1186/1479-5876-10-174 |
[18] | Lüthje P , Lindén Hirschberg A , Brauner A . Estrogenic action on innate defense mechanisms in the urinary tract. Maturitas. (2014) ;77: (1):32–6. doi:10.1016/j.maturitas.2013.10.018 |
[19] | Stapleton AE . Urinary tract infection pathogenesis: host factors. Infect Clin North Am. (2014) ;28: (1):149–59. doi:10.1016/j.idc.2013.10.006 |
[20] | Ragnarsdottir B , Lutay N , Gronberg-Hernandez J , Koves B , Svanborg C . Genetics of innate immunity and UTI susceptibility. Nat Rev Urol. (2011) ;8: (8):449–68. doi:10.1038/nrurol.2011.100 |
[21] | Xu W , Yang L , Lee P , et al. Mini-review: perspective of the microbiome in the pathogenesis of urothelial carcinoma. Am J Clin Exp Urol. (2014) ;2: (1):57–61. |
[22] | Bucevic Popovic V , Situm M , Chow CT , Chan LS , Roje B , Terzic J . The urinary microbiome associated with bladder cancer. Sci Rep. (2018) ;8: (1):12157. doi:10.1038/s41598-018-29054-w |
[23] | Wu P , Zhang G , Zhao J , et al. Profiling the Urinary Microbiota in Male Patients With Bladder Cancer in China. Front Cell Infect Microbiol. (2018) ;8: :167. doi:10.3389/fcimb.2018.00167 |
[24] | McConnell MJ , Actis L , Pachon J . Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev. (2013) ;37: (2):130–155. doi:10.1111/j.1574-6976.2012.00344.x |
[25] | Murphy EC , Frick IM . Gram-positive anaerobic cocci–commensals and opportunistic pathogens. FEMS Microbiol Rev. (2013) ;37: (4):520–53. doi:10.1111/1574-6976.12005 |
[26] | Alfano M , Canducci F , Nebuloni M , Clementi M , Montorsi F , Salonia A . The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nat Rev Urol. (2016) ;13: (2):77–90. doi:10.1038/nrurol.2015.292 |
[27] | Aso Y , Akazan H . Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. BLP Study Group. Urol Int. (1992) ;49: (3):125–29. doi:10.1159/000282409 |
[28] | Aso Y , Akaza H , Kotake T , Tsukamoto T , Imai K , Naito S . Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur Urol. (1995) ;27: (2):104–9. doi:10.1159/000475138 |
[29] | Seow SW , Rahmat JN , Mohamed AA , Mahendran R , Lee YK , Bay BH . Lactobacillus species is more cytotoxic to human bladder cancer cells than Mycobacterium Bovis (bacillus Calmette-Guerin). J Urol. (2002) ;168: (5):2236–9. doi:10.1097/01.ju.0000034353.97729.69 |
[30] | Ohashi Y , Nakai S , Tsukamoto T , et al. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int. (2002) ;68: (4):273–80. doi:10.1159/000058450 |
[31] | Naito S , Koga H , Yamaguchi A , et al. Prevention of recurrence with epirubicin and lactobacillus casei after transurethral resection of bladder cancer. J Urol. (2008) ;179: (2):485–90. doi:10.1016/j.juro.2007.09.031 |
[32] | Kandasamy M , Bay BH , Lee YK , Mahendran R . Lactobacilli secreting a tumor antigen and IL15 activates neutrophils and dendritic cells and generates cytotoxic T lymphocytes against cancer cells. Cell Immunol. (2011) ;271: (1):89–96. doi:10.1016/j.cellimm.2011.06.004 |
[33] | Matson V , Fessler J , Bao R , et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. (2018) ;359: (6371):104–8. doi:10.1126/science.aao3290 |
[34] | Bevers RF , Kurth KH , Schamhart DH . Role of urothelial cells in BCG immunotherapy for superficial bladder cancer. Br J Cancer. (2004) ;91: (4):607–12. doi:10.1038/sj.bjc.6602026 |
[35] | Biot C , Rentsch CA , Gsponer JR , et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med. (2012) ;4: (137):137ra72. doi:10.1126/scitranslmed.3003586 |
[36] | Durek C , Richter E , Basteck A , et al. The fate of bacillus Calmette-Guerin after intravesical instillation. J Urol. (2001) ;165: (5):1765–8. |
[37] | Redelman-Sidi G , Iyer G , Solit DB , Glickman MS . Oncogenic activation of Pak1-dependent pathway of macropinocytosis determines BCG entry into bladder cancer cells. Cancer Res. (2013) ;73: (3):1156–67. doi:10.1158/0008-5472.CAN-12-1882 |
[38] | Redelman-Sidi G , Binyamin A , Gaeta I , et al. The Canonical Wnt Pathway Drives Macropinocytosis in Cancer. Cancer Res. (2018) ;78: (16):4658–70. doi:10.1158/0008-5472.CAN-17-3199 |
[39] | Mitropoulos DN . Novel insights into the mechanism of action of intravesical immunomodulators. In Vivo. (2005) ;19: (3):611–21. |
[40] | Bevers RF , de Boer EC , Kurth KH , Schamhart DH . BCG-induced interleukin-6 upregulation and BCG internalization in well and poorly differentiated human bladder cancer cell lines. Eur Cytokine Netw. (1998) ;9: (2):181–6. |
[41] | Bisiaux A , Thiounn N , Timsit MO , et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J Urol. (2009) ;181: (4):1571–80. doi:10.1016/j.juro.2008.11.124 |
[42] | de Boer EC , Somogyi L , de Ruiter GJ , de Reijke TM , Kurth KH , Schamhart DH . Role of interleukin-8 in onset of the immune response in intravesical BCG therapy for superficial bladder cancer. Urol Res. (1997) ;25: (1):31–4. |
[43] | Luo Y , Chen X , O’Donnell MA . Role of Th1 and Th2 cytokines in BCG-induced IFN-gamma production: cytokine promotion and simulation of BCG effect. Cytokine. (2003) ;21: (1):17–26. |
[44] | Bohle A , Brandau S . Immune mechanisms in bacillus Calmette-Guerin immunotherapy for superficial bladder cancer. J Urol. (2003) ;170: (3):964–9. doi:10.1097/01.ju.0000073852.24341.4a |
[45] | Flynn JL , Chan J , Lin PL . Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. (2011) ;4: (3):271–8. doi:10.1038/mi.2011.14 |
[46] | Lage JM , Bauer WC , Kelley DR , Ratliff TL , Catalona WJ . Histological parameters and pitfalls in the interpretation of bladder biopsies in bacillus Calmette-Guerin treatment of superficial bladder cancer. J Urol. (1986) ;135: (5):916–9. |
[47] | Prescott S , James K , Busuttil A , Hargreave TB , Chisholm GD , Smyth JF . HLA-DR expression by high grade superficial bladder cancer treated with BCG. Br J Urol. (1989) ;63: (3):264–9. |
[48] | Stefanini GF , Bercovich E , Mazzeo V , et al. Class I and class II HLA antigen expression by transitional cell carcinoma of the bladder: correlation with T-cell infiltration and BCG treatment. J Urol. (1989) ;141: (6):1449–53. |
[49] | Zuiverloon TC , Nieuweboer AJ , Vekony H , Kirkels WJ , Bangma CH , Zwarthoff EC . Markers predicting response to bacillus Calmette-Guerin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur Urol. (2012) ;61: (1):128–45. doi:10.1016/j.eururo.2011.09.026 |
[50] | Luo Y . Blocking IL-10 enhances bacillus Calmette-Guerin induced T helper Type 1 immune responses and anti-bladder cancer immunity. Oncoimmunology. (2012) ;1: (7):1183–5. doi:10.4161/onci.20640 |
[51] | McAveney KM , Gomella LG , Lattime EC . Induction of TH1- and TH2-associated cytokine mRNA in mouse bladder following intravesical growth of the murine bladder tumor MB49 and BCG immunotherapy. Cancer Immunol Immunother. (1994) ;39: (6):401–6. |
[52] | Riemensberger J , Bohle A , Brandau S . IFN-gamma and IL-12 but not IL-10 are required for local tumour surveillance in a syngeneic model of orthotopic bladder cancer. Clin Exp Immunol. (2002) ;127: (1):20–6. |
[53] | Saint F , Patard JJ , Maille P , et al. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette-Guerin treatment for superficial bladder cancer. J Urol. (2002) ;167: (1):364–7. |
[54] | Ratliff TL , Gillen D , Catalona WJ . Requirement of a thymus dependent immune response for BCG-mediated antitumor activity. J Urol. (1987) ;137: (1):155–8. |
[55] | Larsson SC , Andersson SO , Johansson JE , Wolk A . Cultured milk, yogurt, and dairy intake in relation to bladder cancer risk in a prospective study of Swedish women and men. Am J Clin Nutr. (2008) ;88: (4):1083–7. doi:10.1093/ajcn/88.4.1083 |
[56] | Sweis RF , Golan S , Barashi N , et al. Association of the commensal urinary microbiome with response to Bacillus Calmette-Guérin (BCG) immunotherapy in nonmuscle invasive bladder cancer. J Clin Oncol. (2019) ;37: (7 Suppl):423. doi:10.1200/JCO.2019.37.7_suppl.423 |




