Chemotherapy Plus Immune Check-Point Inhibitors in Metastatic Bladder Cancer
Abstract
Urothelial tumors are one of the most prevalent cancers worldwide. Cisplatin-based chemotherapy has been the standard first-line treatment for metastatic urothelial cancer (mUC). After nearly three decades of limited advances in the treatment, immune checkpoint inhibitors (ICI) are now available. Responses to immunotherapy (IO) may be long lasting and sustained but only occur in 20–30% of patients. Studies have shown that combining IO with different targeted therapies can lead to potentiating effects with promising results. The first result combining ICI plus chemotherapy shows positive outcomes over standard-of-care in first line mUC. The aim of this article is to review the results, the benefits and new challenges that the combination of chemotherapy and IO can bring to patients with metastatic urothelial carcinoma.
INTRODUCTION
Urothelial tumors are one of the most prevalent cancers worldwide, with around 430,000 new diagnoses each year [1]. Most of these tumors arise from the bladder [2]. By the time of diagnosis, 75% of bladder tumors are non-invasive and have a high recurrence rate and progression despite local therapy. Muscle invasion is present in 25% of cases and leads to a 5-year mortality rate around 50% [3].
Cisplatin-based chemotherapy has been the standard first-line treatment for metastatic urothelial cancer for more than 30 years [4, 5]. Historically, the combination of methotrexate, vinblastine, adriamycin and cisplatin (MVAC) with no dose dense was considered the standard-of-care for advanced bladder cancer since the 1980s [6]. Nowadays, the most commonly used combination is cisplatin/gemcitabine (CG) due to a better tolerability profile, with a lower incidence of febrile neutropenia, etc. A 5-year update analysis confirmed that CG was not superior to MVAC in terms of survival (14 vs 15.2 months, respectively; HR 1.09 (0.88–1.34)) [7].
Probably due to a median age at diagnosis of the advanced disease of 73 years old, it is estimated that around 50% of patients are ineligible to receive cisplatin-based chemotherapy [8]. The presence of Galsky criteria, including creatinine clearance <60 mL/min and performance status (PS) 2 of the Eastern Cooperative Oncology Group (ECOG) 2 or Karnofsky index of 60 to 70%; but also hearing loss and/or peripheral neuropathy ≥grade 2 (CTCAE version 4.0) or New York Heart Association class III heart failure are conditions for ineligibility to receive cisplatin-based regimens [9]. The first two criteria are also the most frequently used as ineligibility criteria in clinical practice [8]. Moreover, the ineligibility for cisplatin is conditioning for a poorer prognosis. Furthermore, those patients who are ineligible for cisplatin receive less active chemotherapy regimens, mostly carboplatin-based. The EORTC 30986 phase III trial compared carboplatin/gemcitabine (CaG) and a modified scheme of carboplatin/methotrexate/vinblastine (MCAVI) in 178 patients ineligible to receive cisplatin. Overall the results showed that the survival was 9.3 and 8.1 months, respectively, favoring the CaG arm. Toxicity was lower in the CaG arm (9.3% vs. 21.2% of severe adverse events) [10].
After nearly three decades of limited advances in the treatment and systemic management of mUC, recent advances in immunotherapy are now available. Urothelial cancer is one of the cancers with a higher rate of somatic mutations and about one fourth of the patients have PD-L1 overexpression [11]. A high mutational load is thought to be related to increased neoantigen expression and is also associated with improved response to ICIs [12]. Recently, we have seen that up to five different anti PD-1 and PD-L1 agents have been approved in the platinum-refractory setting (Table 1). After IMvigor 210 and Keynote-052 trials, atezolizumab and pembrolizumab were too approved for front-line use in cisplatin-ineligible patients with locally advanced or metastatic UC [13,14]. These approvals were conditional and required confirmatory data from KEYNOTE-361 and IMvigor130 studies to maintain their approval in this disease state. The data monitoring committees identified early deaths among PD-L1 low populations and decreased survival in the monotherapy arms of both trials. EMA and FDA revised their recommendation to restrict the use of atezolizumab and pembrolizumab to PD-L1 high first-line cisplatin-ineligible patients with advanced UC [15]. Positivity is defined as a combined positive score (CPS) of ≥10 for pembrolizumab and IC 2/3 (PD-L1–stained tumor-infiltrating immune cells (ICs) covering ≥5% of the tumor area) for atezolizumab. Those with low expression should receive non-cisplatin-based chemotherapy [16].
Table 1
Results of second-line immunotherapy trials
| Keynote045 | IMvigor210 | Imvigor211 | Durvalumab | Checkmate275 | Checkemate032 | Avelumab | |
| Drug | Pembrolizumab | Atezolizumab | Atezolizumab | Durvalumab | Nivolumab | Nivolumab | Avelumab |
| Phase | 3 | 2 | 3 | 1/2 | 2 | 1/2 | 1/2 |
| N | 270 | 310 | 467 | 191 | 270 | 63 | 161 |
| PD-L1 | 22C3 Dako | SP142 | SP142 | SP263 | 28–8 Dako | 28–8 Dako | 73–10 Dako |
| TC | Ventana | IC+TC | Ventana | TC | TC | IC+TC | |
| IC+TC | IC+TC | ||||||
| ORR | CPS>10% : 20.3% | IC1/2/3 : 18% | IC1/2/3 : 14,1 (A) vs 14,7 (CT) | PD-L1+: 26% | PD-L1 < 1% : 16% | PD-L1 < 1% :20,5% | PD-L1+: 53.8% |
| IC2/3 : 27% | IC2/3 : 23 (A) vs 22 (CT) | PD-L1-: 4,1% | PD-L1≥1% : 24% | PD-L1% :37% | PD-L1-: 4.2% | ||
| OS | CPS<10% : 20,3 m (P) vs 7,7 m (CT) | IC2/3 : 12,3 m | IC0/1 : 10,6 (A) vs 11,1 (CT) | PD-L1 < 1% : 5,9 m | PD-L1 < 1% : 9,9 m | PD-L1 < 1% : 12,9 m | |
| CPS≥10% : 8,0 m (P) vs 4,9 m (CT) | IC2/3 : 11,4 m | IC1/2/3; 8,2 (A) vs 7,3 (CT) | PD-L1≥1% : 11,3m | PD-L1≥1% :16,2 m | PD-L1≥5% : NR |
Abbreviations: N, number of patients recruited; ORR, overall response rate; OS, overall survival; PD-L1, Programmed Death-Ligand 1; IC, immune cells; CT, chemotherapy; IPI, ipilimumab; A, atezolizumab; P, pembrolizumab; NR, not reported; CPS, Combined Positive Score.
NEEDS AND RATIONALE TO COMBINE CHEMOTHERAPY AND ICI
Roughly 20–30% of patients with metastatic urothelial tumors will have long lasting clinical benefit when treated with immunoncology agents. In recent years, clinical trials in different solid tumors have shown that combining ICIs with different targeted therapies can lead to potentiating effects.
Several PD-1/PD-L1 inhibitors in combination with chemotherapeutic agents are being investigated. Theoretically, cytotoxic chemotherapy can cause lysis of tumor cells, potentially increasing immunogenicity, promoting the production of tumor antigens. Emerging evidence that defends the immunogenic potential of chemotherapy has led to the theory that cytotoxic therapy could synergize with ICIs [21]. On the other hand, the chemotherapy modulates the immune system, decreasing myeloid-derived suppressor cells and increasing the ratio between effector T cells and regulatory T cells, although this mechanism is not widely known. In addition, cytotoxic therapy can reduce immunosuppressive factors released by tumors and promote the presentation of antigens, increasing the anti-tumor response of T cells. Different chemotherapeutic agents can influence the immune response to different degrees which may explain the different results observed in trials with various chemotherapy regimens [17]. To date, the clinical trials that have shown the most promising results of this combination have been in the area of lung, breast and head and neck cancer. In metastatic non-small cell lung cancer, atezolizumab in combination with chemotherapy with carboplatin and nab-paclitaxel compared with chemotherapy (IMpower130) showed a significant and clinically significant improvement in overall survival [18]. In small cell lung cancer, first-line atezolizumab and extensive stage chemotherapy resulted in overall survival and significantly longer [19]. Still in lung cancer, metastatic non-small cells the combination of pembrolizumab and chemotherapy (Keynote 189) resulted in significantly longer overall survival as well [20]. In triple negative metastatic breast carcinoma, the combination of atezolizumab plus nab-paclitaxel (IMpassion130) has also obtained promising results by reducing the risk of death from the disease by 20% in all patients and 38% in the subgroup expressing PD-L1. In patients with positive PD-L1 tumors, the median overall survival was 25.0 months with the combination compared to 15.5 months with standard chemotherapy alone [21]. Finally, in recurrent or metastatic head and neck cancer, the combination of pembrolizumab with chemotherapy (Keynote 048) improved overall survival in the total population [22].
This idea was the rationale for the IMvigor130 study, a phase III trial designed to evaluate the efficacy and safety of first-line atezolizumab alone or in combination with platinum-based chemotherapy versus platinum-based chemotherapy alone [23]. IMvigor130 study enrolled 1213 patients who had received no prior systemic therapy for mUC and were eligible for platinum-based chemotherapy. Patients were randomised to receive atezolizumab plus platinum-based chemotherapy, atezolizumab monotherapy or platinum-based chemotherapy plus placebo as the control arm. The co-primary efficacy endpoints were investigator-assessed PFS per RECIST v1.1. and OS in the combination versus arm standard chemotherapy and OS in the monotherapy arm with atezolizumab versus standard chemotherapy arm. The stratification factors were PD-L1 immunohistochemistry status (IC0 vs IC1 vs IC2/3), Bajorin risk factor score including KPS < 80% vs ≥80%, presence of visceral metastases (0 vs 1 vs 2 and/or patients with liver metastases) and investigator choice of platin/gemcitabine (cisplatin + gemcitabine or carboplatin + gemcitabine).
IMvigor130 trial met the co-primary endpoint of investigator-assessed PFS. After a median follow-up of 11.8 months, the combination of atezolizumab plus platinum-based chemotherapy prolonged median PFS to 8.2 months (95% confidence interval [CI], 6.5–8.3) compared to 6.3 months (95% CI, 6.2, 7.0) with placebo/platinum-based chemotherapy (hazard ratio [HR] 0.82; 95% CI, 0.70, 0.96; p = 0.007). No statistical difference in OS was observed in the first reported interim analysis (HR 0.83, 95% CI: 0.69–1.00, one-sided P = 0.027), once it did not cross the efficacy boundary of 0.007 per the O’Brien-Fleming alpha spending function. Exploratory subgroup analysis showed a consistent benefit for the combination but also suggested that patients who received cisplatin, and those with the highest levels of PD-L1 and best performance status, could benefit more from the combination regimen. Combination therapy doubled the rate of complete responders (13% vs 7% vs 6%), however, no differences in overall response rate were observed in the combination versus the standard arm (47% vs 44%). When comparing the activity of atezolizumab monotherapy with platinum/gemcitabine chemotherapy, interim OS did not show a statistically significant survival benefit for immunotherapy (HR 1.02, 95% CI: 0.83–1.24). Nevertheless, it was observed that the kinetics of the survival curves from chemotherapy and single-agent ICI are different and it also suggested that patients with overexpression of PD-L1 may benefit in a greater manner. Safety in the atezolizumab plus chemotherapy arm appeared to be consistent with the known safety profiles of the individual agents and there were no unexpected events within the combination group. Adverse events (AEs) leading to treatment withdrawal occurred in 34%, 6% and 34% of patients in combination, monotherapy and chemotherapy, respectively (Table 2). There are currently other first-line trials that will help us to clarify and confirm the data obtained in this trial, as well as to understand the best strategy for patients with untreated metastatic urothelial carcinoma (Fig. 1). As the results are remarkable and of high importance to the bladder cancer management, we would like to discuss some new challenges that arise following the publication of these data.
Table 2
First line atezolizumab and pembrolizumab single-agents evidence for cisplatin ineligible patients in prospective trials
| Atezolizumab (IMvigor 210 cohort 1) | Atezolizumab (IMVIGOR 130 single arm) | Pembrolizumab (Keynote-052) | |
| Phase | Phase II | Phase III | Phase II |
| Number of Patients | 119 | 362 | 307 (treated for ≥4 months) |
| Dosing | 1200 mg every 3 weeks | 1200 mg every 3 weeks | 200 mg every 3 weeks |
| ORR | 23% (9% CR) | 23 (6% CR) | 27% (6 % CR) |
| Duration of Response | 70% of responses ongoing at 17.2 months | Not reached after median follow up 11.8 months | 78% of responses ongoing at ≥6 months |
| Median OS | 15.9 months | 15.7 | 11.5 months |
| Median PFS | 2.7 months | Not reported | 2 months |
| Rate of Grade ¾ treatment-related AEs | 16% | 15% | 16% |
Abbreviations: CR, complete response; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; AE, adverse events.
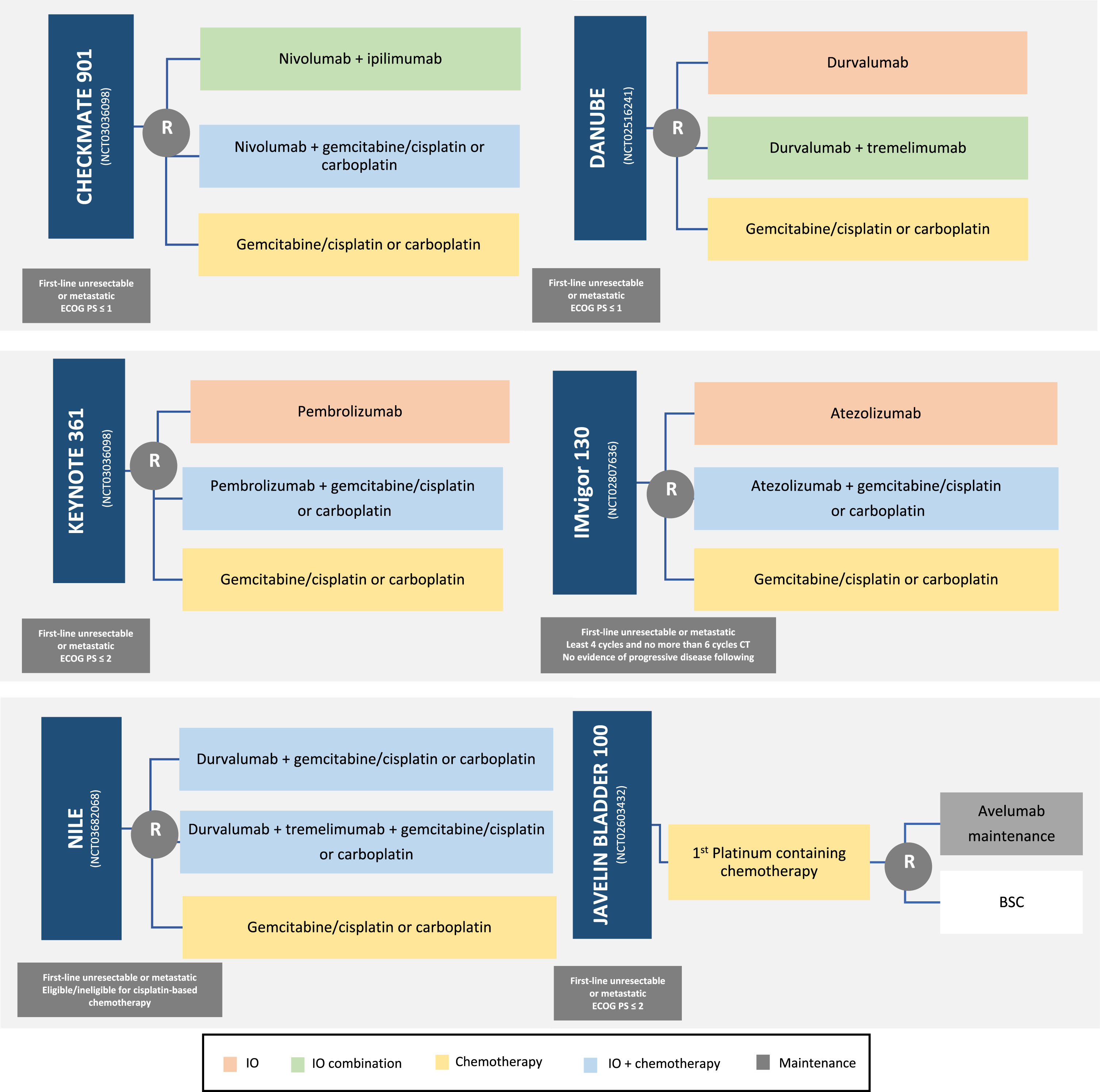
Fig. 1
Ongoing first-line trials of combination of chemotherapy plus ICIs (Immune Checkpoint Inhibitor). IO: ImmunoOncology drug.
CHALLENGES AFTER IMVIGOR130 RESULTS TO FACE WITH THE COMBINATION OF CHEMOTHERAPY PLUS ICI
The purpose of using immunotherapy with chemotherapy is to ideally potentiate the activity of both strategies. This is the first ICI-chemotherapy combination study to demonstrate an improvement in PFS over standard-of-care in first line mUC, but it has not yet been shown an impact on OS. Therefore, there are many questions and hypotheses that still need to be answered regarding the association of ICI and chemotherapy in urothelial tumors.
As for the use of chemotherapy, Galsky’s criteria are widely used to identify patients eligible or ineligible for cisplatin-based chemotherapy, although there are other factors that are affecting the physician’s decision as reported in large retrospective studies from daily practice [8, 24]. In IMvigor 130 trial, patients were stratified according to classical Galsky’s criteria, but investigators were able to choose between cisplatin + gemcitabine or carboplatin + gemcitabine according to their own standard practice and regardless of Galsky’s status. In consistency with the available “real world data”, up to 52% of cisplatin-eligible patients received carboplatin-based schemes, indicating that either the investigators are considering additional factors for defining ineligibility for cisplatin or they simply felt more comfortable with a better tolerated option. The opposite also happened, and 10% of patients who were considered ineligible for cisplatin received this drug. There are no published data on this subject, but in daily practice platinum-based treatments are likely to be performed in patients with creatinine clearance between 50 and 60 ml/min. Moreover, about 12–13% of patients who started cisplatin (combination and chemotherapy arm) needed to discontinue it due to tolerance. There is an increasing thought that besides Galsky’s criteria, there are other factors constraining clinicians to decide which treatment can better fit each patient.
It is also interesting that more patients within the combination arm were ineligible for cisplatin than in the standard chemotherapy arm (45% vs 35%), which may have disadvantaged the combination arm in terms of prognosis. This is supported by the results of Bamias et al, who showed that cisplatin administration was associated with OS benefit [8]. Other baseline characteristics in the IMvigor130 trial, such as age, PD-L1 expression, ECOG 2 and Bajorin risk factors were well balanced in the trial. In the subgroup analysis it seemed that those patients with ECOG 2, Bajorin risk factors 2, and PD-L1 low expression had worse PFS and OS compared to other patients. Definitely, these patients pose the poorer prognosis regardless of the treatment received. One question that arises from this poor prognosis subgroup is which would be the best treatment to offer to these patients.
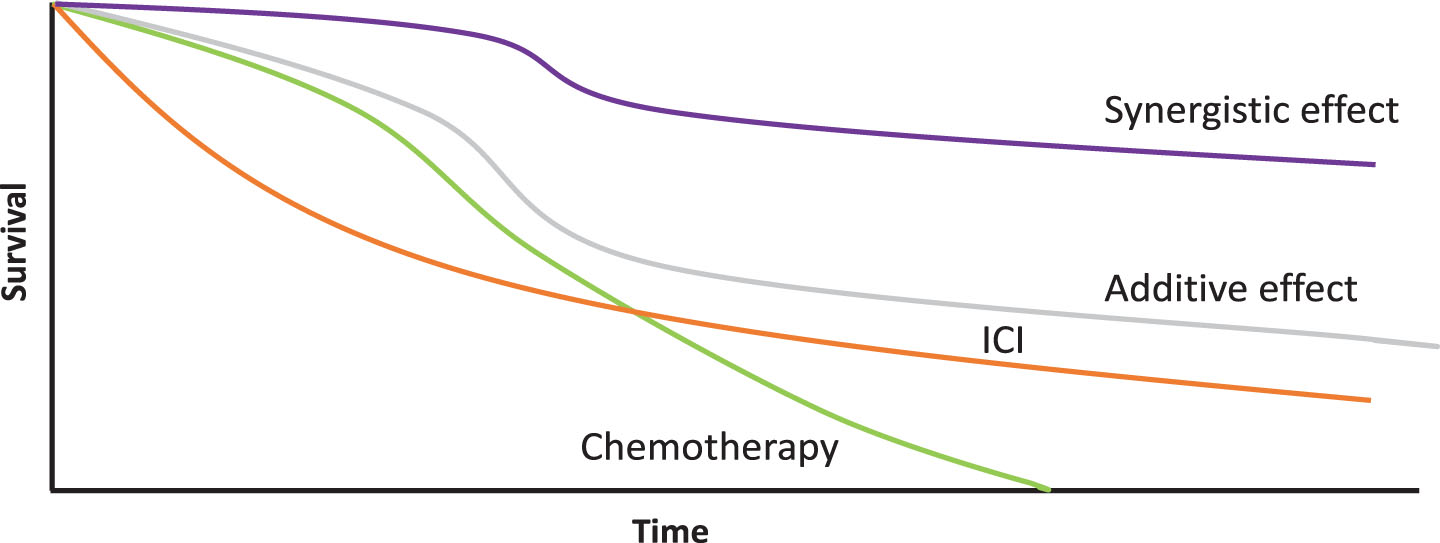
We may have different interactions when two therapies are used together. When we combine two drugs, we can have several effects: additive, where the final effect is equal to the sum of the effects of each of the agents involved; synergistic where the effect is greater than the sum of the effects of each agent or deleterious when the combination is worse due to unforeseen interactions. The complete responses (CR) obtained in the combination arm appear to translate the sum of those obtained in the chemotherapy arm plus the atezolizumab arm (13% vs 7% vs 6%) (Fig. 2 [25]). We may also wonder after these results whether the non-synchronous but sequential use of these therapies could not produce exactly the same rate of complete responses. It would be an interesting point to explore in future essays. Besides that, the duration of response is usually improved with IO and patients with a CR may remain progression-free for a long time, an effect repeatedly shown in other IO trials by the plateau formed by the tail of the OS curve. When looking at the Kaplan-Meier OS curves of atezolizumab monotherapy vs chemotherapy, there are more deaths in the first 12 months in the atezolizumab arm, however, curves crossed each other and go together thereafter reflecting a different kinetic of response to ICI versus chemotherapy. Concurrently, in the comparison between the combination arm vs the chemotherapy arm, the OS curve does not show this crossing. This suggests that there is an unidentified subgroup of patients who do not benefit from IO and should receive chemotherapy –in combination or alone –for their disease. That elicits the unmet need of a reliable biomarker to identify these patients.
Fig. 2
Kinetics of expected outcomes with different treatment modalities in urothelial tumors and differences between synergistic vs additive effect of combinations. ICI: Immune Checkpoint Inhibitor.
Another timely question that remains unanswered is which patients may benefit from the initiation of joint therapy (IO + CT) and who may benefit from sequential therapy with IO following a response to CT. In HCRN GU14-182 trial, patients treated with platinum received pembrolizumab as a maintenance therapy showing an improvement in progression-free survival relative to patients who received placebo (P = 0.036). Currently, the maintenance strategy is being studied in the Javelin 100 phase III, which attempts to understand the benefits of maintaining first-line IO after response to first platinum-containing chemotherapy, the results of which may also bring changes to our clinical practice. Another interesting question is to try to understand which patients may benefit from the combination of IO.
Interestingly, it was also observed at the IMvigor130 trial that among patients in the combination arm, there appears to be an advantage for those who received cisplatin compared to carboplatin treatments. There are several possible explanations for these results. It may be due to the higher immunogenicity of cisplatin, the fact that cisplatin is better regardless of interaction with IO or may also suggest a synergistic effect with immunotherapy [26, 27].
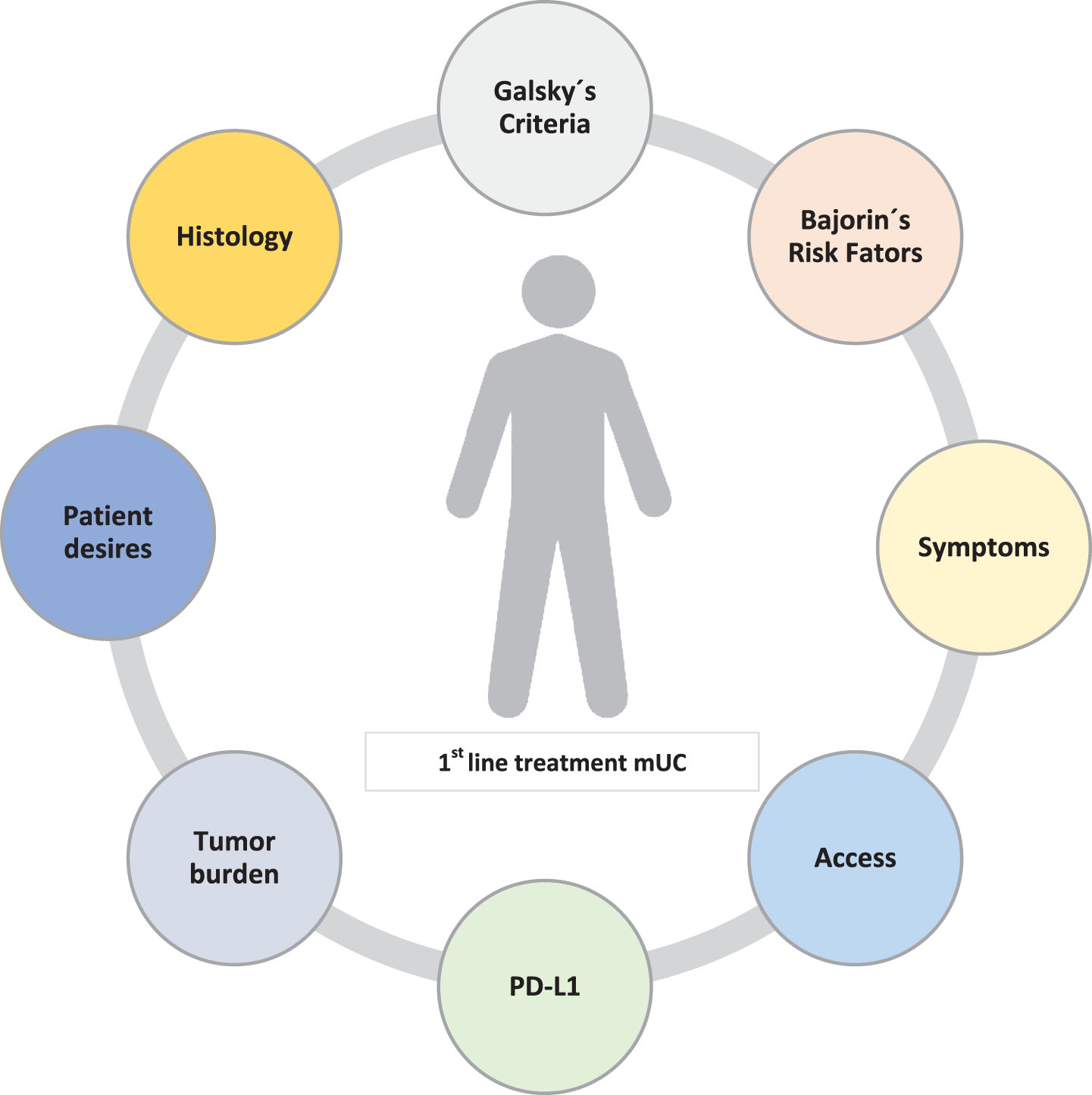
Based on the available data of the IMvigor130 trial, it seems that patients with higher expression of PD-L1 (IC 2/3) appeared to a greater benefit from the use of atezolizumab single agent vs chemotherapy in the interim OS analysis (HR 0.68, not statistically significant). It was also observed that using IO alone was better tolerated than chemotherapy therapy (discontinuation rate of 34% in the chemotherapy arm vs 6% in the atezolizumab single-agent arm). Along with a better tolerability, usually comes a greater quality-of-life, and this feature is of fundamental importance, especially in elderly patients as we commonly see in our daily clinical practice. Therefore, an active debate should be initiated on whether in patients with PD-L1 IC 2/3 ineligible for cisplatin, monotherapy with IO could be the best choice for these patients ahead of carboplatin-based chemotherapy regimens. Maybe this is already the standard of care and likely what most clinicians are doing already in practice. Even in the cisplatin-eligible population, IMvigor130 data may also bring data supporting the use of atezolizumab as a single-agent based on tolerability, long-term outcomes as well as patients’ desires (Fig. 3).
Fig. 3
Factors that may condition for therapeutic decision.
Another unmet need is the therapeutic strategy for patients who are ineligible for any chemotherapy, not only cisplatin. As the tolerability of IO is consistently better than CT, these individuals may have an option besides having a best supportive care, although we still have no prospective data addressing this scenario. Patients who progress when treated with chemotherapy combined with immunotherapy do not appear to progress more aggressively than those treated with immunotherapy alone. Hyperprogression do not seem to happen more often in these patients.
New strategies for predictive biomarkers are needed, and these new potential biomarkers should be validated in urothelial cancer. Nowadays, it is suggested that PD-L1 expression in bladder cancer cells is associated with more aggressive clinical features and lower OS in patients with metastatic disease. Regarding PD-1 / PD-L1 targeted treatment in patients with mUC it is known that, although the benefit is greater in patients with positive PD-L1, patients with negative PD-L1 may also respond to anti-PD-1 therapy[28]. Therefore, a more useful marker is needed to determine the appropriate patient for antiPD-1/PD-L1 therapy. IMvigor130 and other ongoing trials will definitely contribute to a better understanding of the molecular markers that may help to predict the activity of ICIs either alone or in combination with chemotherapy according to the different molecular subtypes.
With the available data of IMvigor130, a new discussion is opened on what we should give to our patients after IO treatment in the first line both as a single agent or in combination. Behaviour of chemotherapy arm in IMvigor130 is better than expected, especially in the long term, as far as 30% of patients remain alive after 24 months compared to 15–20% in the classical von der Maase trial. More and better second and subsequent lines of treatment options in urothelial tumors may also impact on the long-term OS of urothelial patients.
CONCLUSIONS
Upfront treatment of metastatic urothelial tumors is changing and very exciting times on the way in this field are foreseen. Single-agent pembrolizumab or atezolizumab are new treatment options for those patients ineligible to receive cisplatin-based chemotherapy if overexpression of PD-L1 is observed and also for those who are ineligible for any platinum-based chemotherapy regardless of the expression levels of PD-L1. The combination of chemotherapy plus ICI has demonstrated to improve PFS but has not yet impacted on overall survival. Therefore, activity of the combination of ICI plus chemotherapy seems to be more additive rather than synergistic. A combination strategy looks well tolerated with no unexpected adverse events and practice will change if OS is achieved with a longer follow-up. Unfortunately, there is a lack of reliable biomarkers that may help to select the best patients for the combination strategy. Clinical selection of those patients with a better prognosis seems to be based on the more accurate patient profile for these combinations. Trials looking for the activity of dual immune targeting versus chemotherapy are also ongoing and will generate future debates. Novel targets in urothelial tumors field including FGFR, HER2, Nectin-4, and DNA-repairing genes alterations will also impact the future selection of patients for one or another alternative.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
All authors contributed equally to review concept and design, drafting of the manuscript, and critical revision of the manuscript.
ETHICAL CONSIDERATIONS
This study, as a literature review is exempt from any requirement for Institutional Review Board approval.
CONFLICT OF INTEREST
EG has received advisory fees from Roche, Pfizer, Bristol-Myers Squibb, Ipsen, EUSA, MSD, Sanofi, Janssen, Astellas and Adacap during the conduct of the study; research grants from Roche, Pfizer, Bristol-Myers Squibb, Ipsen, EUSA, Lexicon, MTEM/Threshold, and Astra Zeneca during the conduct of the study; and lecture fees from Pierre Fabre.
The other authors have no conflict of interest to report.
REFERENCES
[1] | Antoni S , Ferlay J , Soerjomataram I , Znaor A , Jemal A , Bray F . Bladder cancer incidence and mortality: A global overview and recent trends, Eur. Urol. (2017) ;71: (1):96–108. |
[2] | Rouprêt M , et al. , European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update, Eur. Urol. (2018) ;73: (1):111–122. |
[3] | Chavan S , Bray F , Lortet-Tieulent J , Goodman M , Jemal A . International variations in bladder cancer incidence and mortality, Eur. Urol. (2014) ;66: (1):59–73. |
[4] | Saxman SB , et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study, J. Clin. Oncol. (1997) ;15: (7):2564–2569. |
[5] | von der Maase PFCH , Hansen SW , Roberts JT , Dogliotti L , Oliver T , Moore MJ , Bodrogi I , Albers P , Knuth A , Lippert CM , Kerbrat P , Sanchez Rovira P , Wersall P , Cleall SP , Roychowdhury DF , Tomlin I , Visseren-Grul CM . Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study, J. Clin. Oncol. (2000) ;17: (17):3068–3077. |
[6] | Sternberg CN . et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse, Cancer (1989) ;64: (12):2448–2458. |
[7] | Von Der Maase H . et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer, J. Clin. Oncol. (2005) ;23: (21):4602–4608. |
[8] | Bamias A . et al. Impact of contemporary patterns of chemotherapy utilization on survival in patients with advanced cancer of the urinary tract: A Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC), Ann. Oncol. (2018) ;29: (2):361–369. |
[9] | Galsky MD . et al. Treatment of patients with metastatic urothelial cancer ‘Unfit’ for cisplatin-based chemotherapy, J. Clin. Oncol. (2011) ;29: (17):2432–2438. |
[10] | De Santis M . et al. Randomized Phase II / III Trial assessing gemcitabine / Carboplatin and methotrexate / Carboplatin / Vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-Based chemotherapy : EORTC study (2019) ;30: (2):191–199. |
[11] | Michael GG , Lawrence S , Stojanov Petar , Polak Paz , Kryukov Gregory V , Cibulskis1 Kristian , Sivachenko1 Andrey , Carter1 Scott L. , Stewart Chip , Mermel Craig H. , Roberts Steven A. , Kiezun Adam , Hammerman Peter S. , McKenna Aaron , Drier Yotam , Zou1 Lihua , Alex . Mutational heterogeneity in cancer and the search for new cancer genes, (2013) ;499: (7457):214–218. |
[12] | Bilgin B , Sendur MAN , Hizal M and Yalçín B . An update on immunotherapy options for urothelial cancer, Expert Opinion on Biological Therapy Taylor & Francis (2019) ;19: (12):1265–1274. |
[13] | Balar AV . et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial, Lancet (2017) ;389: (10064):67–76. |
[14] | Balar AV . et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study, Lancet Oncol. (2017) ;18: (11):1483–1492. |
[15] | “US Food and Drug Administration. FDA alerts health care professionals and oncology clinical investigators about and efficacy issue identified in clinical trials for some patients takingKeytruda (pembrolizumab) orTecentriq (atezolizumab) as monotherapy.” |
[16] | Ghatalia P , Zibelman M , Geynisman DM , Plimack ER . First-line immunotherapy in metastatic urothelial carcinoma, Eur. Urol. Focus 2019:15-17. |
[17] | Goldberg SB , Herbst RS . Should chemotherapy plus immune checkpoint inhibition be the standard front-line therapy for patients with metastatic non-small cell lung cancer? Cancer (2018) ;124: (24):4592–4596. |
[18] | West H . et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 tria, Lancet Oncol. (2019) ;20: (7):924–937. |
[19] | Horn L . et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer, N. Engl. J. Med. (2018) ;379: (23):2220–2229. |
[20] | Gandhi L . et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer, N. Engl. J. Med. (2018) ;378: (22):2078–2092. |
[21] | Schmid P . et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial, Lancet Oncol. (2020) ;21: (1):44–59. |
[22] | Burtness B . et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study., Lancet (London, England) (2019) :1–14. |
[23] | Galsky MD . et al. IMvigor130: A randomized, phase III study evaluating first-line (1L) atezolizumab (atezo) as monotherapy and in combination with platinum-based chemotherapy (chemo) in patients (pts) with locally advanced or metastatic urothelial carcinoma (mUC)., J. Clin. Oncol. (2018) ;36: (15_suppl):TPS4589–TPS4589. |
[24] | Flannery K , Boyd M , Black-Shinn J , Robert N , Kamat AM . Outcomes in patients with metastatic bladder cancer in the USA: A retrospective electronic medical record study, Futur. Oncol. (2019) ;15: (12):1323–1334. |
[25] | Palmer AC , Sorger PK . Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy, Cell (2017) ;171: (7):1678–1691.e13. |
[26] | Hato SV , Khong A , De Vries IJM , Lesterhuis WJ . Molecular pathways: The immunogenic effects of platinum-based chemotherapeutics, Clin. Cancer Res. (2014) ;20: (11):2831–2837. |
[27] | Emens LA , Middleton G . The interplay of immunotherapy and chemotherapy: Harnessing potential synergies, Cancer Immunol. Res. (2015) ;3: (5):436–443. |
[28] | Ding X . et al. Clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: A meta-analysis, Cancer Manag. Res. (2019) ;11: :4171–4184. |




