Evaluation of Hematuria in a Large Public Health Care System
Abstract
Background:
Hematuria is the most common presenting symptom in bladder cancer, but many patients are not adequately evaluated.
Objectives:
To evaluate the type and frequency of hematuria evaluation in a large public health care system.
Patients and Methods:
Electronic medical records of adult patients with urinalysis positive for hematuria (≥3 RBCs/HPF) from January 2015 to April 2018 in an outpatient setting were reviewed. Logistic regression was performed to determine factors associated with urology referral and complete evaluation.
Results:
11,422 patients met the inclusion criteria; the majority were females (72%) and white race (60%). There were an additional 3,221 patient’s with initial diagnosis of UTI. Median age was 49.0 years. Testing included repeat urinalysis (50%), imaging (26%), urology referral (11.4%), cystoscopy (4.4%) and complete evaluation defined as cystoscopy and US/CT/MRI (4%). In the multivariable analysis, factors independently associated with higher referral to urology were age >35, male gender, hypertension, RBCs ≥20. African American race was associated with less referral to urology. Smoking was a significant variable on univariable analysis only. 37 patients (0.25%) were diagnosed with urological malignancies, with bladder cancer in 33, 12 of whom are missed by excluding UTI patients.
Conclusions:
In the outpatient setting of a public health care system, the vast majority of patients with hematuria are not referred and evaluated properly across all age categories and regardless of smoking status. This might result in missed cancer diagnoses and requires quality improvement measures.
INTRODUCTION
Hematuria is the presenting symptom in the vast majority of bladder cancer patients. Asymptomatic microscopic hematuria (AMH) is a relatively common incidental finding in apparently healthy individuals, with reported rates of 9–18% in large screening studies [1]. The incidence of urological malignancies, primarily bladder and kidney cancers, diagnosed following the evaluation of hematuria varies from 2– 5% in the setting of AMH in referred populations, reaching up to 10– 20% in those with gross hematuria [2–7]. Prompt evaluation of this presentation can lead to earlier diagnosis of cancer with possible improved survival [8].
Historically the definition for AMH ranged from 1 to more than 10 red blood cells (RBC’s) [9]. The American Urological Association (AUA) guidelines published in 2012 and revised in 2016 define microscopic hematuria as 3 or more RBC’s and strongly recommend evaluation with cross-sectional imaging using multiphasic computed tomography (CT) along with cystoscopic evaluation of all patients aged 35 years or older without explained benign cause of the hematuria [10]. While the AUA guideline recommendations, if followed, would identify most cases of cancer, there is evidence in multiple studies that many patients with hematuria are not adequately evaluated [11–14].
The current recommended evaluation of hematuria, in particular that of AMH, is low yield, expensive, and subjects patients to high doses of CT-associated radiation. The cost-effectiveness model developed by Halpern et al. showed that ultrasound coupled to cystoscopy was most cost-effective at $53,000 per cancer, while replacing ultrasound with CT resulted in the diagnosis of one additional cancer at an incremental cost of more than 6 million dollars [15]. These results call for rethinking the diagnostic algorithm, and to base the evaluation of patients on individual risk factors for genitourinary (GU) malignancies such as age, gender and carcinogen exposure [11, 16, 17].
Meanwhile, many studies point to the incomplete evaluation of patients with microscopic hematuria with low rates of referral, as well as the inconsistency of such referrals across healthcare systems [12, 13, 18, 19]. Most of these studies focus on health care systems in which the patients have insurance and ready access to care. We sought to evaluate the patterns of referral and complete evaluation for microscopic and gross hematuria in a large public health care system. We also recognize that there is often a delay of diagnosis in women due to presumed urinary tract infection (UTI) and wanted to assess the impact of this diagnosis on evaluation and cancer rates.
PATIENTS AND METHODS
After obtaining the approval of the Institutional Review Board, the electronic medical records of the Parkland Health and Hospital Systems were retrospectively queried for patients with urinalysis (UA) with≥3 RBC per high power field (RBC/HPF) from January 2015 to April 2018, allowing for 3 months of follow-up after the UA results. Parkland Health and Hospital Systems includes a central hospital as well as 12 health outpatient centers, 12 school-based clinics and five mobile vans. In 2018, there were 1,037,320 outpatient visits, 242,640 emergency room visits and 72,341 hospital discharges.
Inclusion Criteria:
1. Age of 18 or above
2. ≥3 RBC/HPF on a UA performed in the outpatient clinic or the emergency room
Exclusion Criteria:
1. UA performed in the inpatient setting
2. Patients with prior visits in any urology, nephrology, or oncology clinics prior to the date of the positive UA
3. Prior kidney transplantation
Demographic information about age, gender, race, smoking, and major comorbidities like diabetes mellitus, hypertension, and obesity was collected, along with other information related to the positive UA and the subsequent medical care, including repeat UA, urine culture, cytology, imaging pertinent to the diagnosis, referral to urology for completion of evaluation, and final diagnosis. Age and number of RBC’s on UA were quantified. Complete evaluation was left to the discretion of the treating urologist and defined as receiving upper tract imaging and endoscopic evaluation of the bladder. GU malignancy was reported as bladder or kidney cancer diagnosed as part of the evaluation of the hematuria. We excluded patients with prior history of prostate cancer. All charts of patient with GU malignancy were examined for stage and grade of the malignancy. The primary analysis excluded patients who were initially diagnosed with a UTI but a secondary analysis included patients who had initial diagnosis of UTI.
Chi-square and Mann Whitney U tests were used to evaluate categorical and continuous variables respectively. Univariable and multivariable binary logistic regression analyses were performed to identify factors associated with referral to urologic evaluation, and with completion of evaluation. Statistical tests were performed using SPSS version 25.0 (Armonk, NY: IBM Corp.).
RESULTS
The cohort consisted of 11,422 patients who satisfied the inclusion criteria over the 40 months period, with a median age of 49.0 (interquartile range [39.0–60.0]) years, and 67% of subjects aged between 35 and 65 years. Our cohort consisted of predominantly females (72%) and those of white (including Hispanic) race (60%). Demographic data are presented in Table 1. The cohort was divided into patients who were referred to urology for evaluation and those who were not referred, with cystoscopic evaluation performed by only urologists.
Table 1
Demographic variables and stratifications according to urology referral
| Total Number | Not Referred (% from total) | Referred (% from total) | p-value | ||
| 11422 | 10116 (88.6%) | 1306 (11.4%) | – | ||
| Gender | Male | 3193 | 2501 (78.3%) | 614 (21.7%) | <0.001 |
| Female | 8229 | 7615 (92.5%) | 542 (7.5%) | ||
| Age: median [IQR], years | 49.0 [39.0–60.0] | 49.0 [38.0–60.0] | 54.0 [44.0–62.0] | <0.001 | |
| Age | <35 | 1923 | 1798 (93.5%) | 125 (6.5%) | <0.001 |
| 35–50 | 3810 | 3455 (90.7%) | 355 (9.3%) | ||
| 50–65 | 3852 | 3283 (85.2%) | 569 (14.8%) | ||
| 65–80 | 1568 | 1353 (86.3%) | 215 (13.7%) | ||
| >80 | 269 | 227 (84.4%) | 42 (13.6%) | ||
| Race | White | 6889 | 6072 (88.1%) | 817 (11.9%) | 0.130 |
| Black | 3787 | 3392 (89.6%) | 395 (10.4%) | ||
| Hispanic* | 10 | 8 (80.0%) | 2 (20.0%) | ||
| Asian | 477 | 413 (86.6%) | 64 (13.4%) | ||
| Indian | 29 | 28 (96.6%) | 1 (3.4%) | ||
| Pacific | 33 | 28 (84.8%) | 5 (15.2%) | ||
| Unknown | 197 | 175 (88.8%) | 22 (11.2%) | ||
| UA RBC | 3–19 | 9933 | 8928 (89.9%) | 1005 (10.1%) | <0.001 |
| 20–49 | 596 | 456 (76.5%) | 140 (24.5%) | ||
| 50–99 | 399 | 336 (84.2%) | 63 (15.8%) | ||
| >99 | 494 | 396 (80.2%) | 98 (19.8%) | ||
| DM | Yes | 3386 | 3005 (88.7%) | 381 (11.3%) | 0.692 |
| No | 8036 | 7111 (89.5%) | 925 (10.5%) | ||
| HTN | Yes | 5032 | 4394 (87.3%) | 638 (12.7%) | <0.001 |
| No | 6390 | 5722 (90.8%) | 668 (9.2%) | ||
| Obesity | Yes | 666 | 606 (91.0%) | 60 (9.0%) | 0.043 |
| No | 10756 | 9510 (88.4%) | 1246 (11.6%) | ||
| Tobacco use | Never | 7423 | 6682 (90.0%) | 741 (10.0%) | <0.001 |
| Former | 2174 | 1859 (85.5%) | 315 (14.5%) | ||
| Current | 1825 | 1575 (86.3%) | 250 (13.7%) | ||
| Repeat UA | Yes | 5720 | 4788 (83.7%) | 932 (16.3%) | <0.001 |
| No | 5702 | 5328 (93.4%) | 374 (6.6%) | ||
| Imaging | Yes | 3637 | 2636 (72.5%) | 1001 (27.5%) | <0.001 |
| No | 7785 | 7480 (96.1%) | 305 (3.9%) | ||
| Cystoscopy | Yes | 508 | 0 (0.0%) | 508 (100%) | <0.001 |
| No | 10914 | 10116 (92.7%) | 798 (7.3%) | ||
| Complete Evaluation | Yes | 462 | 0 (0%) | 462 (100%) | <0.001 |
| No | 10960 | 10116 (92.3%) | 844 (7.7%) |
IQR: interquartile range; UA: urinalysis; RBC: red blood cells; DM: diabetes mellitus; HTN: hypertension; UTI: urinary tract infection. *Most Hispanic patients are identified as white.
The number of RBC’s on initial UA was 3–19 in 9,933 patients (87% of the total cohort), but only 1,005 (10.1%) of those were referred to urology. The type and frequency of different tests performed in referred and non-referred populations is shown in Figs. 1 and 2. Figure 1 shows the population when excluding patients with initial diagnosis of UTI. Figure 2 shows evaluation in patients with initial diagnosis of UTI. Repeat UA was performed in 5,720 patients (50%) including 4,788 (47.3%) and 932 (71.4%) in non-referred and referred populations, respectively (p < 0.001). Cystoscopic evaluation in clinic or in the operating room was performed in 508 patients, and it was complemented by upper tract imaging in 462 patients. Imaging was performed in a total of 3,637 patients, including 2,636 (26%) and 1,001 (76.6%) of those who were not referred and referred, respectively (p < 0.001). In both referred and non-referred populations, renal US was used slightly more than CT abdomen/pelvis.
Fig.1
Distribution of testing performed in hematuria population. Uro = urology; UA: urinalysis; UCx: urine culture CT: computed tomography; US: ultrasound; MRI: magnetic resonance imaging; IVP: intravenous pyelography. The numbers for imaging performed are not mutually exclusive as more than one imaging modality could be used in each patient.
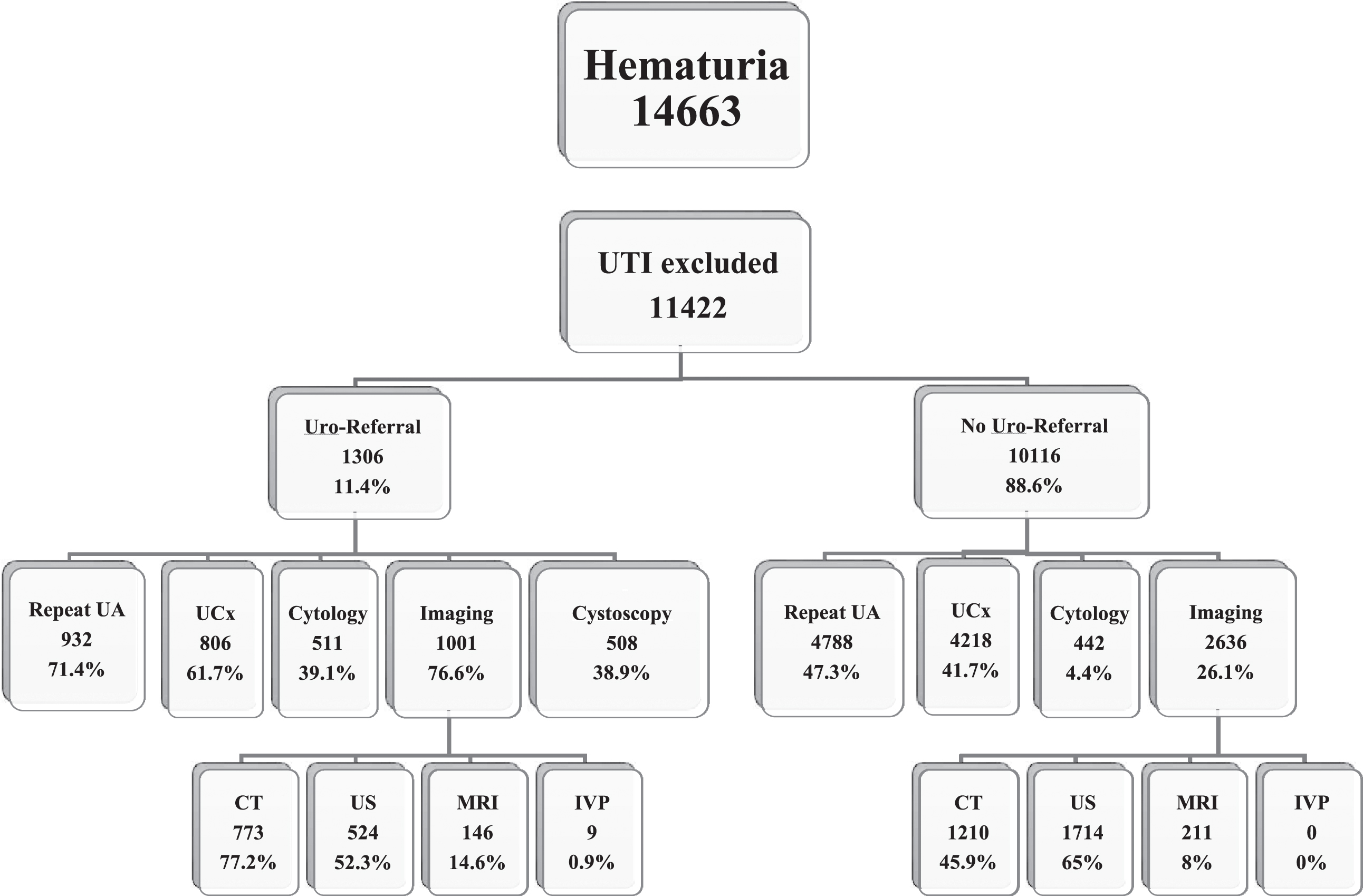
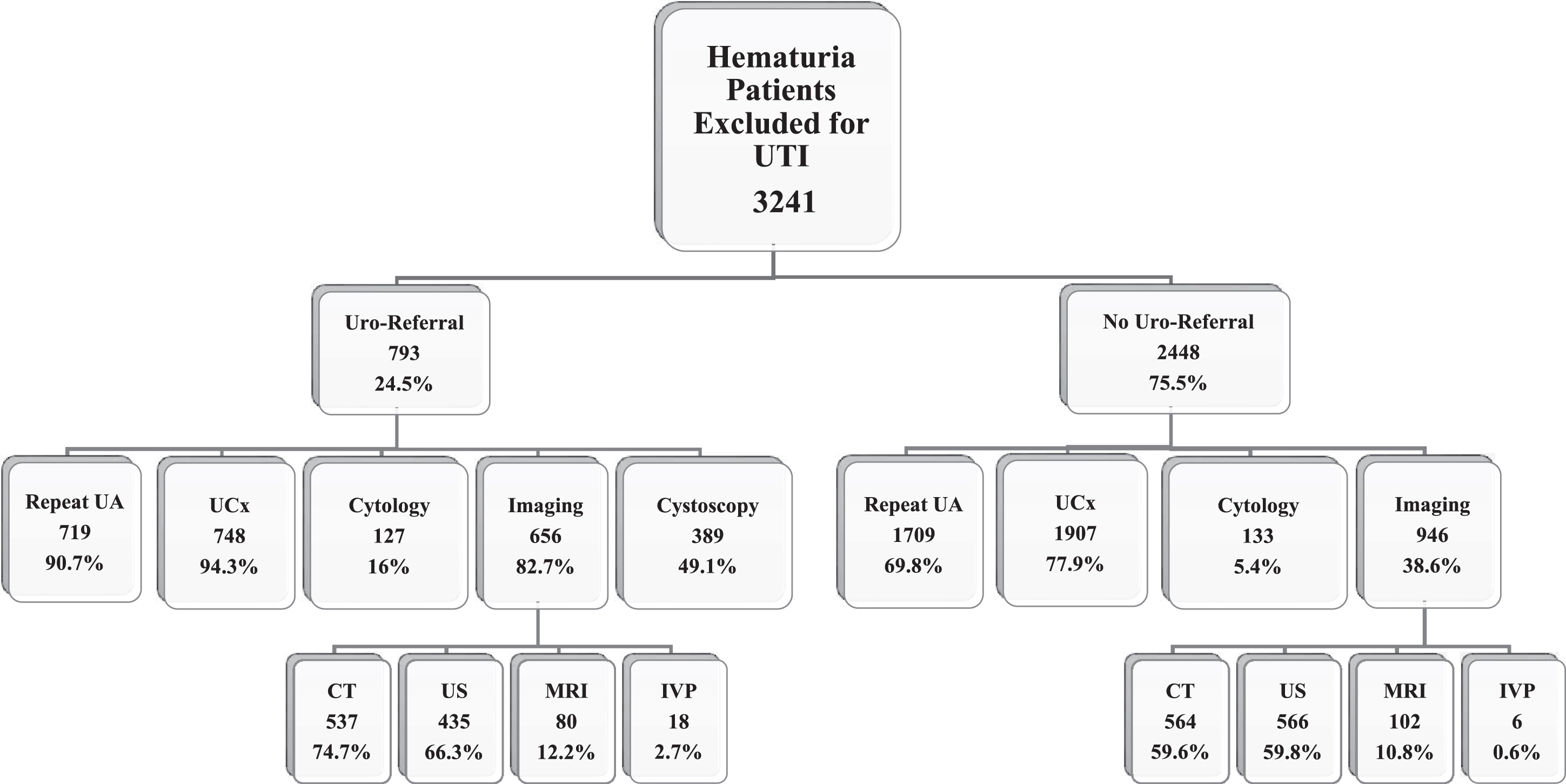
Fig.2
Work-up and upper tract imaging in patients with hematuria excluded from initial cohort. Uro = urology; UA: urinalysis; UCx: urine culture CT: computed tomography; US: ultrasound; MRI: magnetic resonance imaging; IVP: intravenous pyelography. The numbers for imaging performed are not mutually exclusive as more than one imaging modality could be used in each patient.
Univariable analysis was used to evaluate predictors of urology referral (Table 2). Female gender (Odds Ratio = 0.29, p < 0.001) and African-American race (OR = 0.87, p = 0.026) were associated with lower referral. Age, UA with RBC/HPF≥20, hypertension as a comorbidity (OR = 1.24; p < 0.001), prior history or current smoking status (prior: OR = 1.53, p < 0.001; current: OR = 1.43, p < 0.001), and repeated UA (OR = 2.77, p < 0.001) were all associated with higher referral to urology. On multivariable analysis, female gender (OR = 0.31, p < 0.001), age, UA with RBC/HPF≥20 (OR 3.12, p < 0.001), hypertension (OR = 1.16; p = 0.032), and repeated UA (OR = 2.64, p < 0.001) all retained their significant association with referral (female gender predicted lower rates of referral), while smoking status was no longer an independent predictor of recurrence (OR = 1.08, p = 0.36).
Table 2
Predictors of urology referral on univariable and multivariable regression analyses
| UVA | UVA | MVA | MVA | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Gender | Female | 0.29 (0.26–0.33) | <0.001 | 0.31 (0.27–0.35) | <0.001 |
| Age | <35 | Ref | Ref | Ref | Ref |
| 35–50 | 1.48 (1.20–1.83) | <0.001 | 1.49 (1.20–1.86) | <0.001 | |
| 50–65 | 2.49 (2.04–3.05) | <0.001 | 2.35 (1.89–2.92) | <0.001 | |
| 65–80 | 2.29 (1.81–2.88) | <0.001 | 2.43 (1.88–3.14) | <0.001 | |
| >80 | 2.66 (1.83–3.88) | <0.001 | 2.82 (1.88–4.23) | <0.001 | |
| Race | White | Ref | Ref | Ref | Ref |
| Black | 0.87 (0.76–0.98) | 0.026 | 0.80 (0.70–0.92) | 0.001 | |
| Hispanic* | 1.86 (0.39–8.77) | 0.434 | 1.62 (0.32–8.17) | 0.558 | |
| Asian | 1.15 (0.88–1.51) | 0.311 | 1.11 (0.83–1.48) | 0.487 | |
| Indian | 0.27 (0.04–1.96) | 0.193 | 0.26 (0.03–1.93) | 0.187 | |
| Pacific Islander | 1.33 (0.51–3.45) | 0.561 | 0.98 (0.39–2.48) | 0.153 | |
| Unknown | 0.93 (0.60–1.46) | 0.767 | 0.86 (0.54–1.37) | 0.524 | |
| UA RBC | 3–19 | Ref | Ref | Ref | Ref |
| 20–49 | 2.73 (2.23–3.33) | <0.001 | 3.12 (2.51–3.87) | <0.001 | |
| 50–99 | 1.67 (1.26–2.20) | <0.001 | 2.14 (1.60–2.86) | <0.001 | |
| >99 | 2.20 (1.75–2.77) | <0.001 | 3.25 (2.53–4.16) | <0.001 | |
| DM | Yes | 1.03 (0.90–1.17) | 0.692 | – | – |
| HTN | Yes | 1.24 (1.11–1.40) | <0.001 | 1.16 (1.01–1.32) | 0.032 |
| Obesity | Yes | 0.76 (0.58–0.99) | 0.043 | 0.99 (0.74–1.31) | 0.929 |
| Tobacco | Never | Ref | Ref | Ref | Ref |
| Former | 1.53 (1.33–1.76) | <0.001 | 0.98 (0.84–1.14) | 0.771 | |
| Current | 1.43 (1.23–1.67) | <0.001 | 1.08 (0.91–1.28) | 0.359 | |
| Repeat UA | Yes | 2.77 (2.44–3.15) | <0.001 | 2.64 (2.32–3.01) | <0.001 |
UVA: univariable analysis; MVA: multivariable analysis; OR: odds ratio; UA: urinalysis; RBC: red blood cells; DM: diabetes mellitus; HTN: hypertension; UTI: urinary tract infection. *Most Hispanic patients are identified as white.
Table 3 shows predictors of completed evaluation which included cystoscopic evaluation and upper tract imaging. Factors including male gender, age, RBC count on UA, and repeated UA continued to be significant on both univariable and multivariable analyses. However, African-American race was associated with lower rate of complete evaluation (OR = 0.73, p = 0.004 and OR = 0.66, p < 0.001 on univariable and multivariable analyses, respectively) and Asian race was associated with higher rate of complete evaluation (OR = 1.65, p = 0.008 and OR = 1.66, p = 0.01 on univariable and multivariable analyses, respectively). Hypertension as comorbidity and smoking status were both insignificant on multivariable analysis.
Table 3
Predictors of complete evaluation on univariable and multivariable regression analyses
| UVA | UVA | MVA | MVA | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Gender | Female | 0.38 (0.32–0.46) | <0.001 | 0.45 (0.37–0.56) | <0.001 |
| Age | <35 | Ref | Ref | Ref | Ref |
| 35–50 | 1.85 (1.26–2.69) | 0.002 | 1.82 (1.24–2.68) | 0.002 | |
| 50–65 | 3.06 (2.13–4.40) | <0.001 | 2.78 (1.90–4.07) | <0.001 | |
| 65–80 | 2.86 (1.91–4.29) | <0.001 | 2.87 (1.87–4.43) | <0.001 | |
| >80 | 3.19 (1.72–5.92) | <0.001 | 3.10 (1.63–5.93) | 0.001 | |
| Race | White | Ref | Ref | Ref | Ref |
| Black | 0.73 (0.59–0.90) | 0.004 | 0.66 (0.53–0.83) | <0.001 | |
| Hispanic* | 2.47 (0.31–19.5) | 0.393 | 2.33 (0.28–19.2) | 0.432 | |
| Asian | 1.65 (1.14–2.39) | 0.008 | 1.66 (1.13–2.44) | 0.010 | |
| Indian | 0.00 (0.00–.) | 0.998 | 0.00 (0.00–.) | 0.998 | |
| Pacific Islander | 2.22 (0.67––7.31) | 0.190 | 1.90 (0.55–6.55) | 0.311 | |
| Unknown | 0.94 (0.46–1.93) | 0.864 | 0.93 (0.45–1.93) | 0.842 | |
| UA RBC | 3–19 | Ref | Ref | Ref | Ref |
| 20–49 | 2.86 (2.13–3.84) | <0.001 | 3.03 (2.23–4.12) | <0.001 | |
| 50–99 | 1.69 (1.09–2.60) | 0.019 | 2.11 (1.35–3.29) | 0.001 | |
| >99 | 2.10 (1.47–3.01) | <0.001 | 2.87 (1.97–4.18) | <0.001 | |
| DM | Yes | 0.86 (0.69–1.06) | 0.147 | – | – |
| HTN | Yes | 1.33 (1.10–1.60) | 0.003 | 1.06 (0.86–1.31) | 0.580 |
| Obesity | Yes | 1.13 (0.77–1.65) | 0.535 | – | – |
| Tobacco | Never | Ref | Ref | Ref | Ref |
| Former | 1.51 (1.21–1.89) | <0.001 | 1.08 (0.84–1.38) | 0.545 | |
| Current | 1.28 (1.10–1.81) | 0.006 | 1.25 (0.96–1.63) | 0.100 | |
| Repeat UA | Yes | 3.38 (2.71–4.20) | <0.001 | 3.16 (2.53–3.95) | <0.001 |
UVA: univariable analysis; MVA: multivariable analysis; OR: odds ratio; UA: urinalysis; RBC: red blood cells; DM: diabetes mellitus; HTN: hypertension; UTI: urinary tract infection. *Most Hispanic patients are identified as white.
Urothelial (bladder and upper tracts) or renal malignancies were diagnosed in 24 patients in the initial cohort which represents an incidence in entire population of 0.21% and was 1.8% among referred population and 5.2% among those who had a complete evaluation. Bladder cancer accounted for the majority (n = 20). Four patients had Ta disease, and 3 of those had low grade disease. One patient had an invasive upper tract urothelial carcinoma of the renal pelvis, and 3 patients had renal cell carcinoma with stages of T1a, T1b, and T3a (Tables 4 and 5). The hematuria was not attributed to a GU diagnosis in 69.6% and 57.5% when excluding and reincluding UTI patients in the cohort, respectively (Tables 6 and 7).
Table 4
Distribution of patients with genitourinary malignancy
| Patients with GU malignancy (N = 24) | Total number of patients by category (N = 11422) | % among each group | Additional patients with GU malignancy initially presenting with UTI (N = 13) | ||
| Gender | Male | 15 | 3193 | 0.47% | 3 |
| Female | 9 | 8229 | 0.11% | 10 | |
| Age | <35 | 0 | 1923 | – | 2 |
| 35–49 | 3 | 3810 | 0.08% | 1 | |
| 50–64 | 14 | 3852 | 0.36% | 6 | |
| 65–80 | 4 | 1568 | 0.26% | 3 | |
| >80 | 3 | 269 | 1.11% | 1 | |
| Race | White/Hispanic | 16 | 6899 | 0.23% | 4 |
| Black | 6 | 3787 | 0.16% | 7 | |
| Asian | 2 | 477 | 0.42% | 2 | |
| Indian | 0 | 29 | – | 0 | |
| Pacific | 0 | 33 | – | 0 | |
| Unknown | 0 | 197 | – | 0 | |
| UA RBC | 3–19 | 12 | 9933 | 0.12% | 7 |
| 20–49 | 5 | 596 | 0.84% | 0 | |
| 50–99 | 1 | 399 | 0.25% | 1 | |
| >99 | 6 | 494 | 1.21% | 5 | |
| DM | Yes | 7 | 3386 | 0.21% | 5 |
| No | 17 | 8036 | 0.21% | 8 | |
| HTN | Yes | 13 | 5032 | 0.26% | 4 |
| No | 11 | 6390 | 0.17% | 9 | |
| Obesity | Yes | 3 | 666 | 0.45% | 1 |
| No | 21 | 10756 | 0.20% | 12 | |
| Tobacco use | Never | 10 | 7423 | 0.13% | 6 |
| Former | 5 | 2174 | 0.23% | 5 | |
| Current | 9 | 1825 | 0.49% | 2 | |
| Repeat UA | Yes | 20 | 5720 | 0.35% | 12 |
| No | 4 | 5702 | 0.07% | 1 | |
| Imaging | Yes | 22 | 3637 | 0.60% | 13 |
| No | 2 | 7785 | 0.03% | 0 |
GU: genitourinary; N: total number of patients; UA: urinalysis; RBC: red blood cells; DM: diabetes mellitus; HTN: hypertension. *Most Hispanic patients are identified as white.
Table 5
Distribution of GU malignancies by organ, stage, and grade in patients excluding UTI and with initial diagnosis of UTI
| Cancers Diagnosed in Cohort Excluding UTI | Cancers Diagnosed in Cohort with initial diagnosis of UTI | |||||
| GU Malignancy by Organ | N | Stage (n) | Grade (n) | N | Stage (n) | Grade (n) |
| Bladder | 20 | pTa (4) | Low (3) | 13 | pTa (5) | Low (2) |
| pT1 (1) | High (17) | pT1 (3) | High (11) | |||
| pT1 + CIS (1) | pT1 + CIS (1) | |||||
| pT2 (8) | pT2 (1) | |||||
| pT3-T4 (6) | pT3-T4 (4) | |||||
| Kidney | 3 | pT1a (1) | F1-2 (2) | 0 | 0 | |
| pT1b (1) | F3-4 (1) | |||||
| pT3a (1) | ||||||
| Upper Tracts | 1 | pT3 (1) | High (1) | 0 | 0 | |
UTI: Urinary Tract Infection; GU: genitourinary; N: total number of patients with GU malignancy; n: number of patients with GU malignancy in each category.
Table 6
Final presumed diagnosis for hematuria after exclusion of patients with urinary tract infections
| GU Diagnosis | Urology Referral (N = 1306) | No Urology Referral (N = 10116) | Total (% from overall) |
| BPH | 177 | 200 | 377 (3.3%) |
| Hydronephrosis | 68 | 29 | 97 (0.8%) |
| GU Malignancy | 24 | 0 | 24 (0.2%) |
| CKD | 275 | 1894 | 2169 (19%) |
| Urolithiasis Kidney stone | 277 | 194 | 471 (4.1%) |
| Ureteral stone | 78 | 21 | 99 (0.9%) |
| Prostate Cancer | 53 | 53 | 106 (0.9%) |
| Renal Cyst | 64 | 62 | 126 (1.1%) |
| Urethral Diverticulum | 1 | 5 | 6 (0.05%) |
| No GU Diagnosis | 289 | 7658 | 7947 (69.6%) |
N: total number of patients; GU: genitourinary; BPH: benign prostatic hyperplasia; CKD: chronic kidney disease; UTI: urinary tract infection.
Table 7
Final presumed diagnosis for hematuria in the complete cohort including patients with urinary tract infections
| GU Diagnosis | Urology Referral (N = 2099) | No Urology Referral (N = 12564) | Total (% from overall) |
| BPH | 286 | 248 | 534 (3.6%) |
| Hydronephrosis | 148 | 59 | 207 (1.4%) |
| GU Malignancy | 37 | 0 | 37 (0.25%) |
| CKD | 467 | 2,231 | 2,698 (18.4%) |
| Urolithiasis Kidney stone | 448 | 264 | 712 (4.9%) |
| Ureteral stone | 124 | 25 | 149 (1.0%) |
| Prostate Cancer | 76 | 67 | 143 (1.0%) |
| Renal Cyst | 100 | 91 | 191 (1.3%) |
| Urethral Diverticulum | 4 | 8 | 12 (0.08%) |
| UTI | 803 | 2,448 | 3251 (22.2%) |
| No GU Diagnosis | 570 | 7,864 | 8434 (57.5%) |
N: total number of patients; GU: genitourinary; BPH: benign prostatic hyperplasia; CKD: chronic kidney disease; UTI: urinary tract infection.
The initial cohort of patients with≥3 RBC/HPF consisted of 14,663 patients and in the analyses above, we excluded 3,241 patients with a diagnostic code of UTI at initial assessment. However, we noted that many of these patients were subsequently referred to urology. We re-analyzed the data to determine what additional testing was performed in these patients and found similar association with referral as in patients without UTI on initial diagnosis (demographic distribution and logistic regression analyses in Supplementary Tables 1-3). Among the patients excluded for a UTI diagnosis, 13 additional patients were diagnosed with bladder cancer, 5 of whom had at least muscle-invasive disease and 11 had high-grade disease.
When reviewing the charts of all 37 patients with urologic malignancy and 3–19 RBCs/HPF, we noted that 16 had a repeat UA of whom 13 had persistent hematuria. Two of the 3 patients who had less than 3 RBCs on repeat UA had masses on imaging (one bladder and one renal) and would have been diagnosed based on these criteria. Three patients did not have repeat UA of whom one had a history of gross hematuria while the other 2 had kidney cancer on imaging.
DISCUSSION
This study to our knowledge is the largest report on evaluation of hematuria in a public health care system. Patients in this safety net health care system in Dallas County have access to 10 outpatient primary care clinics with a centralized hospital which has an outpatient urology clinic. Most of the patients have lower socioeconomic means and lack resources to obtain care outside of this healthcare system. In our study, we found a low rate of referral for hematuria across all risk-categories of patients with hematuria. Only 11.4% of the total cohort were ever evaluated in a urology clinic, and only 462 patients (4%) underwent complete evaluation. In our system, seeking care outside this health care system is expected to be minimal, and urology visits occur on the basis of physician-to-physician referral. As such, patients who meet criteria for referral based on AUA guidelines are all given an appointment. This means that denial of an appointment with urology is not the cause for low referral rates. While the rate of referral to urology was low, it mirrors the rate reported in the Kaiser Health Care System where 7,778 (2%) patients were referred to urology from a cohort of 389,207 [11]. Perhaps these low referral rates are not surprising because when primary care physicians (PCP’s) are asked about their referral patterns, less than half recommended referral of patients with microscopic hematuria despite their knowledge of its association with bladder cancer, while up to one third would refrain from referring high-risk patients with painless gross hematuria [18, 20].
The risk factors for bladder cancer are established with increasing age, male gender and smoking representing the main risk factors for the disease [17]. When evaluating predictors of referral in our cohort, age, gender, and high blood pressure were independent predictors of referral to urology but smoking history was not. This is concerning because smoking is the main carcinogen associated with bladder cancer and significantly increases the risk of disease. The number of RBCs was also a predictor of referral. We did not have information on the presence of gross hematuria, but the number of RBCs did impact decision making on the part of PCPs. Despite meeting AUA criteria for microhematuria and having established risk factors, 78% of men, more than 85% of patients over 50 years of age, 80% of patients with more than 99 RBCs/HPF and more than 85% of current or former smokers were not referred for evaluation. Similar to our present results in a public hospital system, a previous study from our group evaluating patients in a private health care system found that female gender and black race were associated with lower referral, while older age, smoking status, and gross hematuria were associated with higher referral [14]. A study by Friedlander et al. of 2,455 primary care patients aged 40 years or more and diagnosed with hematuria found that 13.7% of patients underwent cystoscopy within 180 days. In this same study, while age and gender were associated with cystoscopy and imaging, smoking was associated with higher rate of imaging but not of cystoscopy [13]. The AUA guidelines continue to emphasize that smoking is a major risk factor that necessitates cystoscopic evaluation, raising the concern that smoking is not associated with urologic referral in our study or in others.
One factor that likely impacts rates of referral is the large discrepancy between rates of cancer in general populations with hematuria when compared to cancer rates in referred populations. The rate of bladder cancer in referred populations with microhematuria ranges from 2–5%, but is significantly lower in studies evaluating general populations with hematuria [10]. A retrospective study of more than 150,000 patients with hematuria reported a 3-year incidence of urinary tract malignancy of 0.68% [21]. In the study by Loo et al. including 1,117,542 patients of which 456,674 had microscopic hematuria on one UA and 389,207 had 2 positive UA results there were 100 bladder cancers diagnosed among 4414 patients evaluated (2.3%) and 11 renal cancers (0.2%) [11]. From the urologist perspective the rate of cancers was 2.5% since these were referred and evaluated but from the PCP perspective there were 111 cancers from 456,674 or 0.02%. The cancer rates in our population of 0.21% is in line with prior reports. While one recognizes that there are likely some missed cancers among patients that are not evaluated, there is a significant difference in rates of cancer such that many PCPs end up referring many more patients than end up diagnosed with disease.
One area of concern with bladder cancer is delay in diagnosis. We noted that over 3,000 patients with hematuria had an initial diagnosis of UTI. This was disproportionately seen in women. While UTIs can lead to microscopic blood in the urine and it is not recommended to refer these patients for evaluation, many patients were still seen in urology and 13 of 3,241 patients were diagnosed with bladder cancer, 11 of whom had high-grade disease, and 5 had at least muscle-invasive disease. In fact, a study of 1,318 patients with bladder and kidney cancer in the United Kingdom (UK), 417 of whom were women, showed that women were 2.5 fold (p < 0.001) more likely to require 3 or more primary care consultations prior to referral, which resulted in delayed diagnosis of a urological malignancy [22]. Whether those advanced cases could have been diagnosed earlier if investigation of hematuria was performed earlier could not be determined. In our cohort more than half the patients did not have a diagnosis to explain their hematuria with the caveat that most did not have much evaluation.
In an effort to improve referral and better identify patients with disease, there have been several studies to try to risk stratify patients [11–13, 18]. A “Hematuria Risk Index” used several factors with higher odds ratios in their model (history of gross hematuria and age of 50 years or older) and assigned them 4 points, whereas factors with lower odds ratios (history of smoking, male sex, and > 25 RBC/HPF on a recent UA) were given 1 point [11]. Similarly, a “Hematuria Cancer Risk Score” comprised age, gender, hematuria type, and smoking status with an area under the curve of 0.77 and 0.84 for ROC curve in the development and validation cohorts, respectively. At a derived threshold of 4.015, the model had a sensitivity of 98.6%, detecting an additional 11.4% of genitourinary malignancies that would have been missed based on the NICE guidelines, and a specificity of 30.5% compared to 12.6% when AUA guidelines are followed strictly [23]. Other studies incorporated risk factors such as age, gender, smoking status, gross versus microscopic hematuria and urine-based tumor markers to identify patients more likely to have malignancy [24, 25].
A recent review was performed of the hematuria guidelines from the American Urological Association; the consensus statement by the Canadian Urological Association, Canadian Urologic Oncology Group and Bladder Cancer Canada; the American College of Physicians; the Joint Consensus Statement of the Renal Association and British Association of Urological Surgeons; and the National Institute for Health and Care Excellence [26]. While all the guidelines recommended evaluation of patients without known benign causes of AMH using cystoscopy and upper urinary tract imaging there were discrepancies with regard to age, threshold for recommending evaluation, number of abnormal UAs and the optimal imaging method (computed tomography vs ultrasonography). For example, while the AUA uses age 35 as a threshold, the latest NICE guidelines increased the recommended age for urgent evaluation of AMH to 60 years from 50 years. However, our cohort had 14 of the 24 malignancies aged between 50 and 64, whereas the median age of diagnosis of bladder cancer in a contemporary UK cohort was higher than 70, and only 15% of patients were younger than 60 years of age [27]. It is possible that patients in a lower socioeconomic status have a higher incidence of cancer at a younger age and this needs to be further studied. Both the AUA and ACP define AMH as the presence of≥3 RBC/HPF in a single properly collected specimen, while the Canadian Consensus Statement defined AMH as≥3 RBCs/HPF in two separate urine samples as in the prior AUA guidelines. The American College of Obstetricians and Gynecologists and the American Urogynecologic Society recommend that asymptomatic, low-risk, never-smoking women aged 35–50 years undergo evaluation only if they have more than 25 RBC/HPF [28]. When we consider how a change in guidelines based on number of positive UAs are needed to recommend evaluation or number of RBCs, we note that 19 of the 37 (nearly 50%) GU malignancies diagnosed had only 3–20 RBC’s on initial UA. A closer look found that 13 of the 19 patients had a repeat UA with persistent hematuria, 1 had a history of gross hematuria and 4 had suspicious lesions on imaging. As such only one patient might have been missed if a criterion of 2 UAs would have been used. Using risk factors in isolation would not be helpful since 6 patients were under age 50, 16 did not report a history of smoking and 19 were women. As such a combination of risk factors would be necessary to try to find all cases of malignancy. This analysis demonstrates further that strategies based on combinations of risk factors are likely the best approach to optimize the investigation of hematuria.
The limitations of this study are mostly related to the retrospective design of such a large cohort of patient. A main challenge is to identify the cause-effect relationship of the variables identified towards the decision making, particularly whether referral was related to the general practice of PCP’s, the financial resources available, or the lack of compliance of patients. Identifying whether repeat UA or performing upper tract imaging was the reason for urology referral or was requested to save time pending the urology visit or even ordered by the urologist was another challenging limitation. In addition, it was not possible to identify the driving factors for cystoscopy by urologists, nor whether a positive urine culture following the hematuria always signified a urinary tract infection that needed to be otherwise excluded. It was not possible to know if a patient had gross hematuria separate or in addition to the microscopic findings on UA. The number of RBCs was used as a tool in the analysis but cannot replace a good history from the patient. While we evaluated racial differences in this cohort, Hispanic patients of which there are many are categorized as white, so it was not possible to segregate this association in this analysis. Finally, although unlikely that patients continued their care in another healthcare system, we might lack accurate data about the follow-up in all patients.
CONCLUSION
Hematuria is a common finding in patients in the public health care systems. As with other health care systems, referral rates are low and physicians are inconsistent in using known risk factors for malignancy in guiding their referral patterns. A risk-adapted approach from the guidelines may reduce the number of patients who are recommended to be referred but requires a careful balance of several risks to avoid missing cancer cases.
KEYPOINTS
• In a large public health care system, hematuria is common but only 11.4% were referred to urology, 4% of patients underwent complete evaluation and cancer incidence was 0.21% after excluding patients with urinary tract infections.
• In the multivariable analysis, factors independently associated with a higher likelihood of referral to urology were age > 35, male gender, hypertension, and RBCs≥20 while smoking did not impact referral patterns.
• In the outpatient setting of a public health care system, the vast majority of patients with hematuria are not referred and evaluated properly across all age categories and regardless of smoking status.
AUTHOR CONTRIBUTIONS
Rashed Ghandour: Conception, data curation, methodology, writing - original draft, and writing - review and editing. Yuval Freifeld: Data curation, methodology, writing - original draft, and writing - review and editing. Nirmish Singla: Writing - original draft, and writing - review and editing. Yair Lotan: Conception, methodology, formal analysis, project administration, writing - original draft, and writing - review and editing.
DISCLOSURE
None of the authors has a conflict of interest related to writing and publishing this manuscript.
FUNDING
The authors report no funding.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-190221.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
REFERENCES
[1] | Mohr DN , Offord KP , Owen RA , Melton LJ , 3rd. Asymptomatic microhematuria and urologic disease. A population-based study. Jama. (1986) ; 256: (2):224–9. |
[2] | Cohen RA , Brown RS . Clinical practice. Microscopic hematuria. The New England Journal of Medicine. (2003) ; 348: (23):2330–8. |
[3] | Bruyninckx R , Buntinx F , Aertgeerts B , Van Casteren V . The diagnostic value of macroscopic haematuria for the diagnosis of urological cancer in general practice. The British journal of general practice: The journal of the Royal College of General Practitioners. (2003) ; 53: (486):31–5. |
[4] | Buntinx F , Wauters H . The diagnostic value of macroscopic haematuria in diagnosing urological cancers: A meta-analysis. Family Practice. (1997) ; 14: (1):63–8. |
[5] | Mariani AJ , Mariani MC , Macchioni C , Stams UK , Hariharan A , Moriera A . The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. The Journal of Urology. (1989) ; 141: (2):350–5. |
[6] | Britton JP , Dowell AC , Whelan P , Harris CM . A community study of bladder cancer screening by the detection of occult urinary bleeding. The Journal of Urology. (1992) ; 148: (3):788–90. |
[7] | Khadra MH , Pickard RS , Charlton M , Powell PH , Neal DE . A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. The Journal of Urology. (2000) ; 163: (2):524–7. |
[8] | Ramirez D , Gupta A , Canter D , Harrow B , Dobbs RW , Kucherov V , . Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer. BJU International. (2016) ; 117: (5):783–6. |
[9] | Sutton JM . Evaluation of hematuria in adults. Jama. (1990) ; 263: (18):2475–80. |
[10] | Davis R , Jones JS , Barocas DA , Castle EP , Lang EK , Leveillee RJ , . Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. (2012) ; 188: (6 Suppl):2473–81. |
[11] | Loo RK , Lieberman SF , Slezak JM , Landa HM , Mariani AJ , Nicolaisen G , . Stratifying risk of urinary tract malignant tumors in patients with asymptomatic microscopic hematuria. Mayo Clinic Proceedings. (2013) ; 88: (2):129–38. |
[12] | Elias K , Svatek RS , Gupta S , Ho R , Lotan Y . High-risk patients with hematuria are not evaluated according to guideline recommendations. Cancer. (2010) ; 116: (12):2954–9. |
[13] | Friedlander DF , Resnick MJ , You C , Bassett J , Yarlagadda V , Penson DF , . Variation in the intensity of hematuria evaluation: A target for primary care quality improvement. The American Journal of Medicine. (2014) ; 127: (7):633–40.e11. |
[14] | Buteau A , Seideman CA , Svatek RS , Youssef RF , Chakrabarti G , Reed G , . What is evaluation of hematuria by primary care physicians? Use of electronic medical records to assess practice patterns with intermediate follow-up. Urol Oncol. (2014) ; 32: (2):128–34. |
[15] | Halpern JA , Chughtai B , Ghomrawi H . Cost-effectiveness of Common Diagnostic Approaches for Evaluation of Asymptomatic Microscopic Hematuria. JAMA Internal Medicine. (2017) ; 177: (6):800–7. |
[16] | Subak LL , Grady D . Asymptomatic Microscopic Hematuria-Rethinking the Diagnostic Algorithm. JAMA Internal Medicine. (2017) ; 177: (6):808–9. |
[17] | Burger M , Catto JW , Dalbagni G , Grossman HB , Herr H , Karakiewicz P , . Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. (2013) ; 63: (2):234–41. |
[18] | Nieder AM , Lotan Y , Nuss GR , Langston JP , Vyas S , Manoharan M , . Are patients with hematuria appropriately referred to Urology? A multi-institutional questionnaire based survey. Urologic Oncology. (2010) ; 28: (5):500–3. |
[19] | Johnson EK , Daignault S , Zhang Y , Lee CT . Patterns of hematuria referral to urologists: Does a gender disparity exist? Urology. (2008) ; 72: (3):498–502; discussion -3. |
[20] | Yafi FA , Aprikian AG , Tanguay S , Kassouf W . Patients with microscopic and gross hematuria: Practice and referral patterns among primary care physicians in a universal health care system. Canadian Urological Association journal=Journal de l’Association des urologues du Canada. (2011) ; 5: (2):97–101. |
[21] | Jung H , Gleason JM , Loo RK , Patel HS , Slezak JM , Jacobsen SJ . Association of hematuria on microscopic urinalysis and risk of urinary tract cancer. The Journal of Urology. (2011) ; 185: (5):1698–703. |
[22] | Lyratzopoulos G , Abel GA , McPhail S , Neal RD , Rubin GP . Gender inequalities in the promptness of diagnosis of bladder and renal cancer after symptomatic presentation: Evidence from secondary analysis of an English primary care audit survey. BMJ Open. (2013) ; 3: (6). |
[23] | Tan WS , Ahmad A , Feber A , Mostafid H , Cresswell J , Fankhauser CD , . Development and validation of a haematuria cancer risk score to identify patients at risk of harbouring cancer. Journal of Internal Medicine. (2019) ; 285: (4):436–45. |
[24] | Lotan Y , Svatek RS , Krabbe LM , Xylinas E , Klatte T , Shariat SF . Prospective external validation of a bladder cancer detection model. J Urol. (2014) ; 192: (5):1343–8. |
[25] | Kavalieris L , O’Sullivan PJ , Suttie JM , Pownall BK , Gilling PJ , Chemasle C , . A segregation index combining phenotypic (clinical characteristics) and genotypic (gene expression) biomarkers from a urine sample to triage out patients presenting with hematuria who have a low probability of urothelial carcinoma. BMC Urology. (2015) ; 15: : 23. |
[26] | Linder BJ , Bass EJ , Mostafid H , Boorjian SA . Guideline of guidelines: Asymptomatic microscopic haematuria. BJU International. (2018) ; 121: (2):176–83. |
[27] | Bryan RT , Zeegers MP , van Roekel EH , Bird D , Grant MR , Dunn JA , . A comparison of patient and tumour characteristics in two UK bladder cancer cohorts separated by 20 years. BJU Int. (2013) ; 112: (2):169–75. |
[28] | Committee Opinion No.703: Asymptomatic Microscopic Hematuria in Women. Obstetrics and Gynecology. (2017) ; 129: (6):e168–e72. |




