A Clinical Trial of the Intradermal TLR2 Agonist CADI-05 for BCG Recurrent and Unresponsive Non-Muscle Invasive Bladder Cancer
Abstract
Background:
CADI-05, a TLR-2 agonist, induces Th1 immune response following intradermal administration and has been found useful in management of lung cancer and melanoma.
Objective:
To evaluate CADI-05 in patients with BCG recurrent and unresponsive Non-muscle invasive bladder cancer (NMIBC) for 15-months recurrence free survival (RFS) rate.
Methods:
In BCG unresponsive (BU) or recurrent (BR) NMIBC, CADI-05 was administered intradermally every two weeks for 3 months followed by every month for 3 months and subsequently every 2 months for 6 months following transurethral resection (TUR) in a single arm study (ClinicalTrials.gov: NCT00694798). Cystoscopy, cytology, and sonography were performed for the presence/recurrence of NMIBC at 3, 6, 9, 12, 15 months and beyond and confirmed by biopsy. Evaluation of preoperative biopsy for Desmocollin-3 (DSC3) expression and tumor infiltrating lymphocytes (TIL) was optional.
Results:
Twenty patients with NMIBC received intradermal CADI-05. RFS at 15 and 30 months was 35% (7/20) patients. The median RFS was also better for BU compared to BR group (HR, 0.329; 95% CI, 0.071 to 1.532). DSC3 expression was present in 68.8% (11/16) patients. TIL and RFS beyond 30 months were seen 2.3 times and 2.8 times more often in DSC3 expressing NMIBC respectively. No grade IV or V adverse events occurred. Local site reactions were the most common adverse event.
Conclusion:
Intradermal CADI-05 following TUR is associated with 35% RFS at 30 months.
INTRODUCTION
Non-muscle invasive bladder cancer (NMIBC) accounts for approximately 75% of bladder cancers [1]. Intravesical BCG is approved for the treatment and prophylaxis of carcinoma in situ (CIS) of the urinary bladder, and for the prophylaxis of primary or recurrent stage Ta and/or T1 papillary tumors following transurethral resection (TUR). For efficacy, BCG needs direct / close contact with cancer cells and has not been found to be useful when administered at a remote location. Intravesical BCG works in part by tilting the balance towards a T helper type 1 (Th1) immune response through the engagement of toll-like receptors TLR4 [2–4] and TLR9 [4]. Recruitment of neutrophils [5] and BCG- activated killer cells (a type of NK cells) [6] are thought to be required for the efficacy of intravesical BCG, suggesting an important role for an innate type of immune response. In spite of treatment with intravesical BCG, persistence or recurrence of NMIBC is common. BCG failure (persistence of NMIBC following BCG) is associated with (a) minimal or no immune-stimulation as evidenced by inability to produce detectable urinary IL-2 cytokine [7, 8], absence of interferon (IFN) gamma [8], and/or IL-17 [8], (b) a pre-existing Th1 response [9–12] or (c) induction of a counteractive Th2 immune response [2]. Relapse following BCG is also associated with pre-existing as well as induced immunosuppression following BCG [10, 13] as manifest by a high density of regulatory T cells (Tregs) [9, 11, 13, 14], T effector/Treg ratio <1 [15], predominance of M2 macrophages in the stroma [14], monocytic myeloid-derived suppressor cells [10, 16], high IL-6 during treatment [8], and a Th2 cytokine response [2].
Valrubicin is approved for patients with BCG-refractory carcinoma in situ (CIS). It achieves complete response (CR) in 21 % only, and the durability of CR is 4% at two years [17]. Thus, there is a need for better treatment options and this is an active area of research [18–21].
CADI-05 is a potent Toll-like Receptor 2 (TLR2) agonist [22] which induces a pure Th1 response following intradermal administration. It has been found useful in management of tumors at a remote location as monotherapy as well as in combination with other therapies in preclinical as well as human clinical studies [22–26]. Its efficacy is associated with infiltration of tumor by immune cells belonging to both the adaptive (CD4 cells, CD8 cells) as well as the innate (NK cells, NKT cells, dendritic cells and macrophages) immune system [23]. Efficacy is also associated with a decrease in intratumoral immunosuppressive cells (Tregs, CTLA-4 and M2 macrophages) as well as immunosuppressive cytokines such as IL-6 and IL-10 [23, 24]. CADI-05 also induces an adaptive immune response for Desmocollin-3 (DSC3; a transmembrane protein) expressed in some tumor cells [22]. DSC3 expression is specific for squamous non-small cell lung cancer (NSCLC) [27] and is used as a biomarker to differentiate squamous from adenocarcinoma of lung [28]. DSC3 is also expressed by ovarian cancer, colorectal cancer, melanoma and meningioma [27, 29, 30].
Given these favourable characteristics of CADI-05, it was hypothesized that intradermal CADI-05 might be useful in the management of BCG unresponsive/recurrent NMIBC and was evaluated in a preliminary study for its efficacy and safety.
MATERIALS AND METHODS
Patients
In an open label, single arm study, 22 patients with intermediate-high risk NMIBC, either not responding to BCG therapy (induction±maintenance) or recurring after achieving an initial disease free state to BCG therapy, were enrolled following written informed consent. Other eligibility criteria included age above 18, Eastern Cooperative Oncology Group (ECOG) status of 0 or 1 and normal hematologic, hepatic and renal functions along with a negative pregnancy test for women with child bearing potential.
BCG-unresponsive (BU) disease was defined as being at least one of the following:
• Persistent or recurrent CIS alone or with recurrent Ta/T1 (non-invasive papillary disease/tumor invades the sub-epithelial connective tissue) disease within 12 months of completion of adequate BCG therapy.
• Recurrent high-grade (HG) Ta/T1 disease within 6 months of completion of adequate BCG therapy
• T1 high-grade disease at the first evaluation following an induction BCG course [31]. BU was sub classified as BCG refractory and BCG relapsing disease [32].
Treatment
All patients received intradermal CADI-05 (Appendix Table A1, online only) on an outpatient basis until recurrence or 15-months. Following TUR, 0.1 ml of CADI-05 was administered over both deltoids on day-1 (total of 0.2 ml). Subsequently, 0.1 ml was administered (over a deltoid) every two weeks for the first 3 months, every month for the subsequent 6 months, and once every two months for the next 6 months. No other NMIBC directed therapies were administered during the study period. The treatment was discontinued once recurrence was detected. A second TUR, recommended within 2–8 weeks of initial TUR for resecting residual tumor and detecting staging error was not performed.
Efficacy evaluation
The primary end point for study was the number of participants with absence of recurrence at the end of 15 months (durable response, DR).
All patients were evaluated for recurrence by cystoscopy, cytology and sonography every 3 months (3, 6, 9 months etc.). Patients suspected to have recurrence had TURBT and / or biopsy for confirmation.
All patients with DR were also monitored to determine long term efficacy of CADI-05. They did not receive any further treatment.
Safety
Physical examination and laboratory investigations (CBC, blood chemistry for hepatic and renal function, routine urine and urine microscopy) were performed at each visit to evaluate safety using Common Terminology Criteria Adverse Events (CTCAE, version 3.0).
Sample size
Sample size was calculated for a single arm phase II study as per A’Hern [33] (Appendix Table A2, online only). The proportion of recurrence free patients were reported with a 95% CI. All patients with visually complete resection and available for first follow-up visit at 3 months were included for safety and efficacy (modified intention-to-treat) analysis.
Ethical and regulatory considerations
The IND protocol was approved by the US FDA, the Drug Controller General of India, and the Institutional Ethics Committees of all participating study centres. The study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization-Good Clinical Practice (ICH-GCP) guidelines. This study was registered on clinicaltrial.gov - NCT00694798 and on ctri.nic.in - CTRI/2007/091/000034.
DSC3 expression
Since DSC3 expression in bladder cancer was not known at study start, evaluation of pretreatment biopsy for DSC3 expression at a central lab was optional. DSC3 expression was evaluated by immunohistochemistry as previously described for squamous NSCLC [22]. Briefly, the paraffin sections were incubated with mouse anti DSC3 monoclonal antibody (Abcam, Cambridge, U.S.A.) for 60 minutes followed by incubation with anti-mouse IgG-HRP conjugated antibody (GeneI, Bangalore, India) for 60 minutes at room temperature. 3,3’ Di-amino-benzedine (DAB, Thermo fisher) was used as the chromogenic substrate. DSC3 expression was determined qualitatively as positive and negative (Fig. 1) as well as quantitatively as percentage of tumor cells expressing DSC3 [27].
Fig.1
DSC3 expression in NMIBC; (A) DSC3 negative NMIBC; (B) DSC3 expressing NMIBC negative sample.
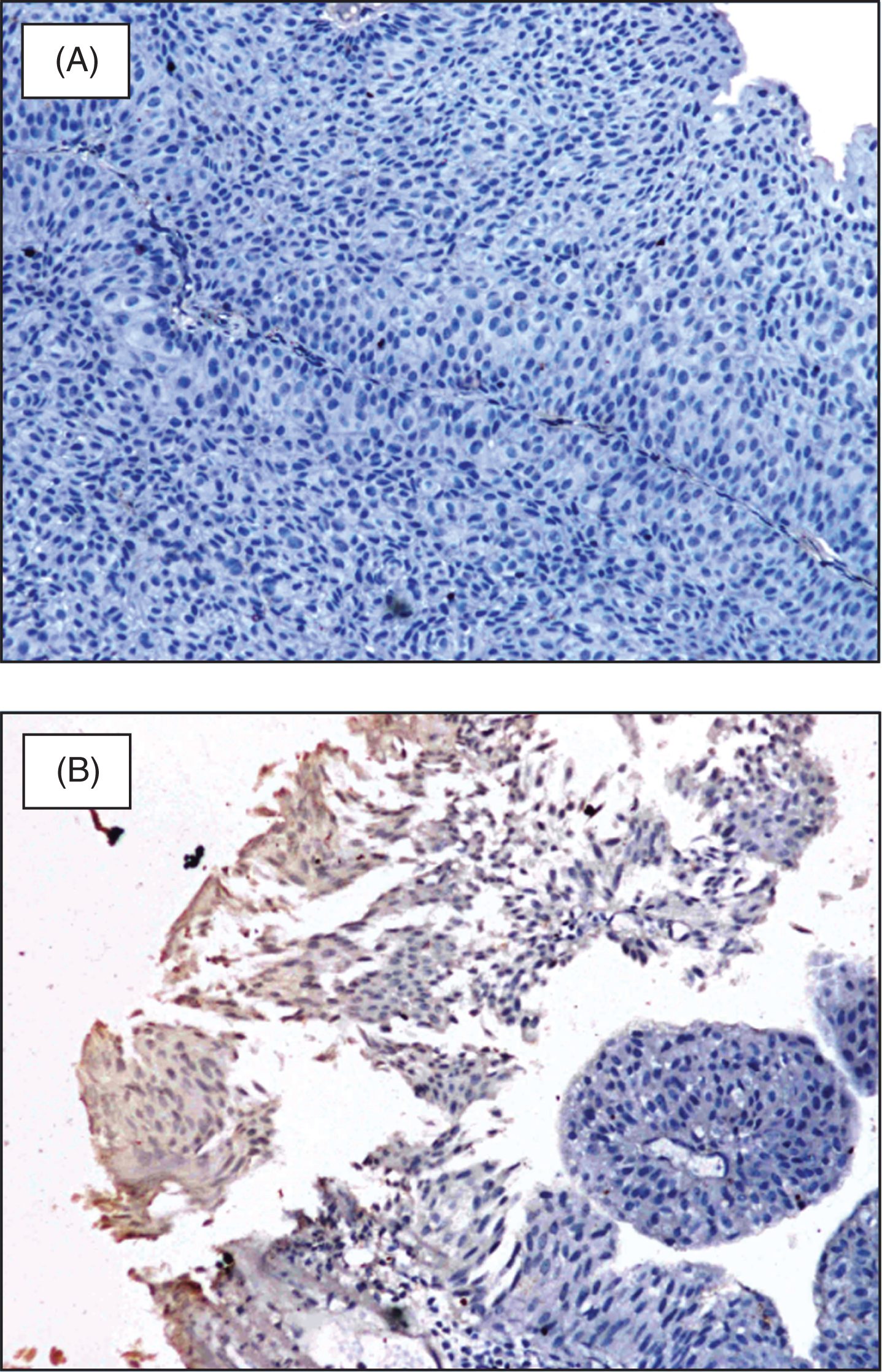
Sections stained with Hematoxylin and Eosin (H & E) were also evaluated for the presence or absence of Tumor infiltrating lymphocytes (TIL) as previously described [34] (stromal and intratumoral; Appendix Fig. A1, online only) and were correlated with DSC3 expression.
Statistical analysis
We performed statistical tests in Excel or using online calculators as follows: The confidence interval for a proportion was calculated using Wilson score method without continuity correction in excel [35], Relative risk (RR) was calculated using online MedCalc statistical software [36], interquartile range (IQR) for median time to recurrence and follow-up was calculated using IQR online calculator [37]. The median Recurrence Free Survival (RFS), Hazard Ratio (HR) and log-rank p values were analysed by Kaplan–Meier method using SAS® version 9.4 (SAS Institute Inc, Cary, NC, USA).
RESULTS
Patient demographics and baseline characteristics are shown in Table 1. Of 22 patients enrolled, 21 had visually complete resection of tumor. The patient with incomplete resection (T1HG) underwent cystectomy for hematuria post TUR. Another patient was non-compliant (TaLG) and was not available for any planned visits following TUR. Both were excluded from further analysis.
Table 1
Baseline patient characteristics
| Age (years) Mean±SD (Range) | 61.25±14.53 (30–84) | ||
| Weight (kg) Mean±SD (Range) | 63.75±10.13 (48–80) | ||
| Height (cm) Mean±SD (Range) | 160.27±11.30 (135–179) | ||
| Sex: Male No. (%) + Female No. (%) | 17 (81.8%) + 03(18.2%) | ||
| ECOG PS | 0 | 20 (100%) | |
| 1 | 0 (0.0%) | ||
| BCG Failure | BCG | BCG Refractory - No. (%) | 6 (30%) |
| classification | Unresponsive | BCG Relapsing –No. (%) | 2 (10%) |
| BCG Recurring - No. (%) | 12 (60%) | ||
| Primary tumor | T1 Low | 7 (35%) | |
| classification | T1 High | 3 (15%) | |
| at enrolment | Ta Low | 1 (5%) | |
| Ta High | 9 (45%) | ||
| CIS | 0 | ||
Primary End Point: absence of recurrence at the end of 15 months
Out of 20 patients, 35.0% (7/20) were found to be recurrence free at the end of 15 months (Fig. 2). All 7 patients with RFS >15 months were followed for more than 30 months (up to 40 months) and found to be recurrence free at last follow up without any further intervention (Fig. 2). The median to follow-up was 31.5 months with interquartile range 30 months (Lower quartile (xL):16.5 months, upper quartile (xU):46.5 months). The median time to recurrence was 9 months with 95% CI 6.0 to 32.0 months.
Fig.2
Response to intradermal CADI-05.
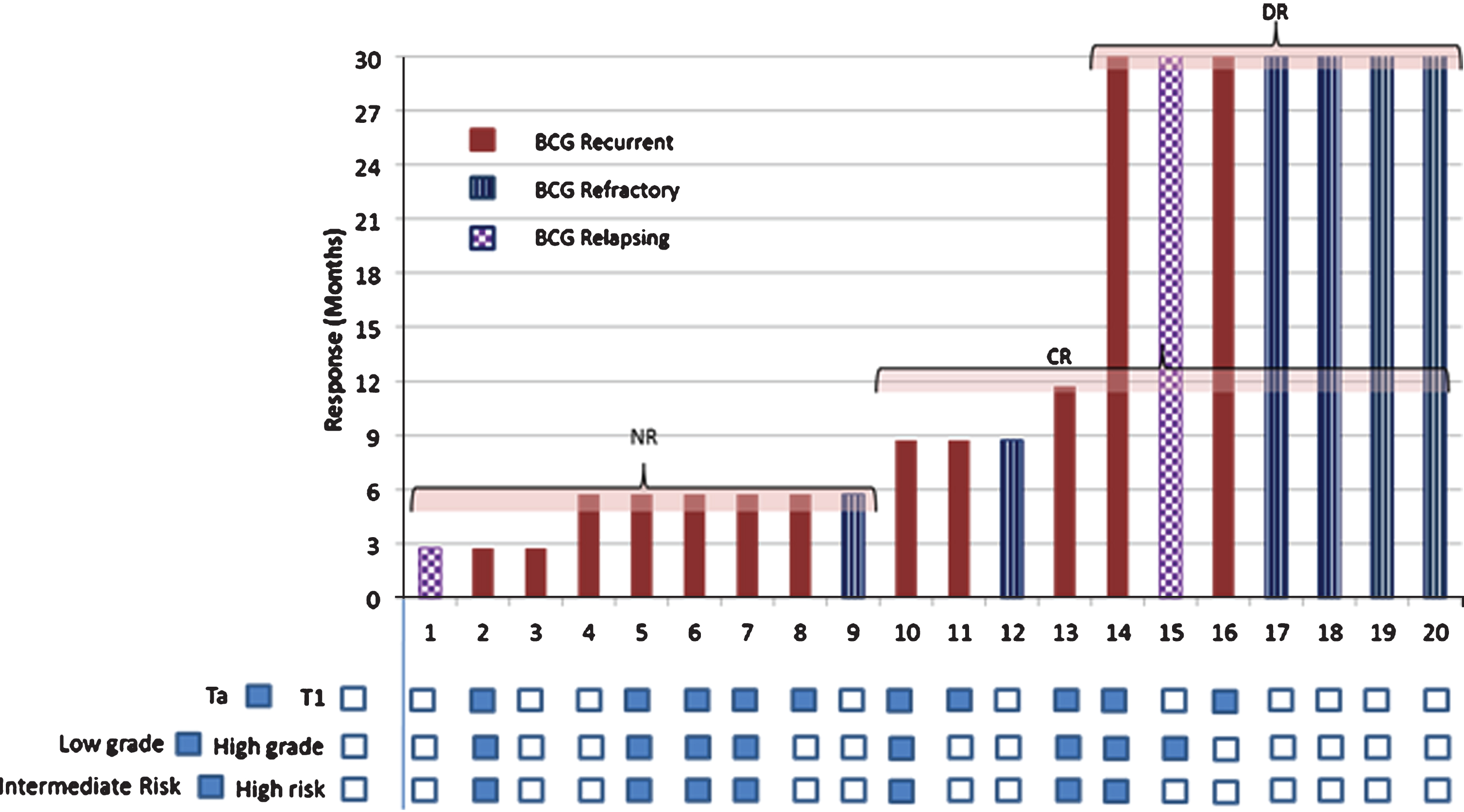
Of 13 patients with recurrence, none had progression to muscle invasive bladder cancer at the time of recurrence. Low grade recurrence was seen in 4 patients with high grade disease at baseline (Appendix Table A3, online only). There was no change in grade at the time of recurrence in the remaining patients. Change in stage at recurrence was seen in three patients. Of two patients with T1 at base line, one had Ta and the other had CIS at recurrence. The third patient had Ta at baseline and T1 at recurrence. Remaining patients had no change in stage at the time of recurrence (Appendix Table A3, online only).
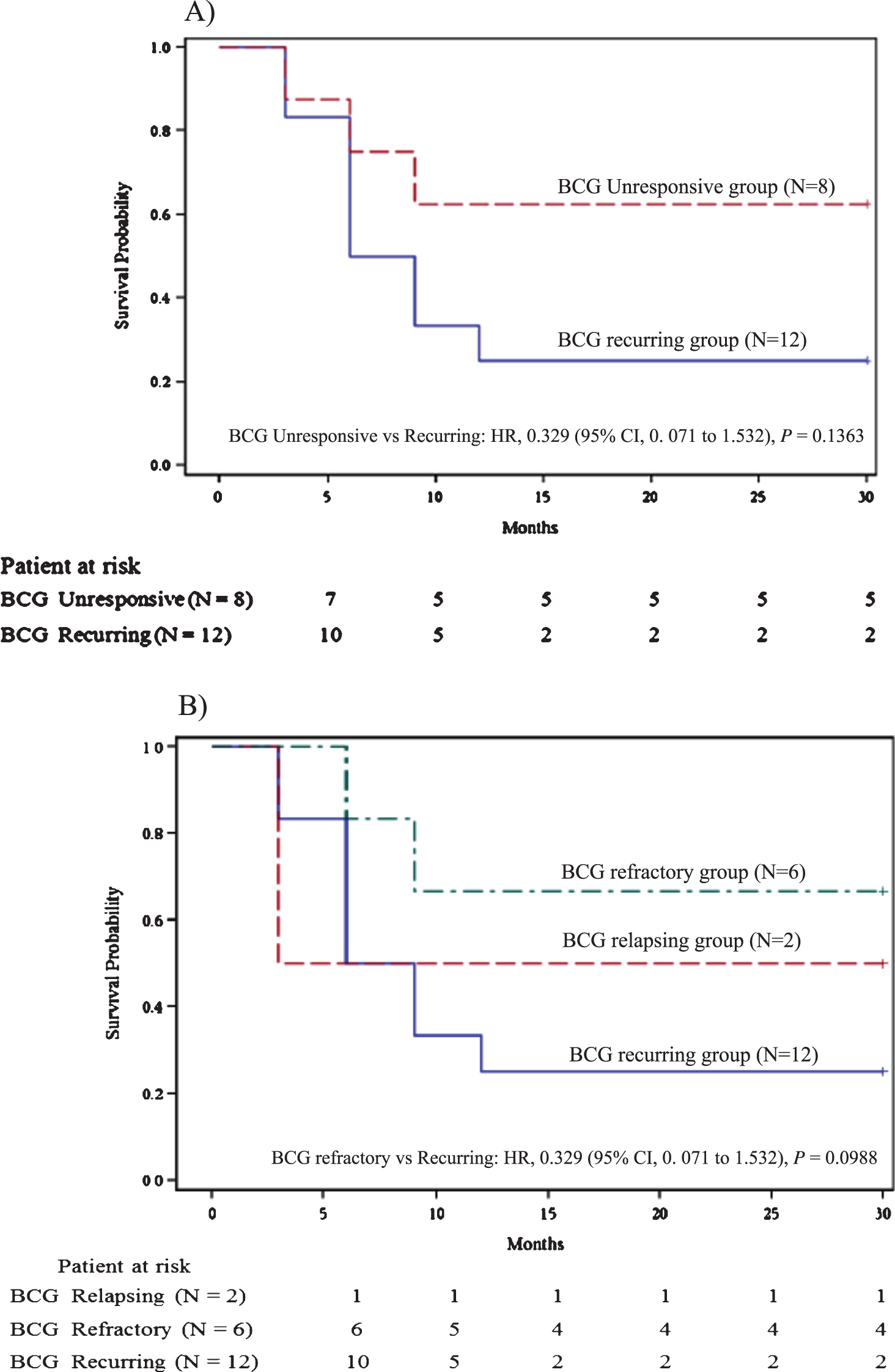
As per subgroup analysis, DR was more frequent in BCG refractory cases (66.67%, 4/6; 95% CI, 30.00% to 90.32%) compared to BCG recurrent cases (16.67%, 2/12; 95% CI 4.70% to 44.80%) and was seen 4.0 times more often (relative risk) in BCG refractory NMIBC than BCG recurrent NMIBC (95% CI, 1.00 to 15.99; P = 0.0499) (Fig. 2). DR was also more frequent in BU cases (62.5%, 5/8; 95% CI, 30.57% to 86.32%) compared to BR cases and was seen 3.8 times more often (relative risk) in BU NMIBC than BR NMIBC (95% CI 0.95 to 14.82; P = 0.0594). The median RFS was not reached in the BU group versus 6.0 months (95% CI, 3.0 to 9.0 months) for the BR group (HR, 0.329; 95% CI, 0. 071 to 1.532; P = 0.1363; Fig. 3A). Of six patients with BCG refractory NMIBC, three had received maintenance therapy. All three had DR. There was a trend for a better median RFS for the BCG refractory group compared to the recurring group (HR, 0.329; P = 0.0988; Fig. 3B). DR was more frequent in T1 cases (50%, 5/10; 95% CI, 23.66% to 76.34%) than in Ta cases (20%, 2/10; 95% CI, 5.67% to 50.98%). Though DR was seen 2.5 times more often (relative risk) in T1 NMIBC than Ta NMIBC, it was statistically insignificant (P = 0.1950) (Fig. 2).
Fig.3
A) Recurrence free survival for BCG unresponsive group vs BCG recurring group. B) Recurrence free survival for BCG relapsing group vs BCG refractory group vs BCG recurring group.
While DR was seen in 35% (7/20), RFS at 6 months was seen in another 20% (4/20; 95% CI, 8.07% to 40.61%) patients. RFS at 6 months was not different for stages T1 (60%, 6/10; 95% CI, 31.27% to 83.18%) and Ta (50%, 5/10; 95% CI, 23.66% to 76.34%). RFS at 6 months was more frequent in the BU group (75%, 6/8; 95% CI, 40.93% to 92.85%) as well as BCG refractory group (83.3%, 5/6; 95% CI, 43.65% to 96.99%) compared to BR group (41.7%, 5/12; 95% CI, 19.33% to 68.05%). Though, RFS at 6 months in BU and BCG refractory group was seen 1.8 (95% CI; 0.83 to 3.93; P = 0.1396) and 2.0 times (95% CI, 0.94 to 4.27; P = 0.0735) more often (relative risk) than BR respectively, it was statistically insignificant (Fig. 2).
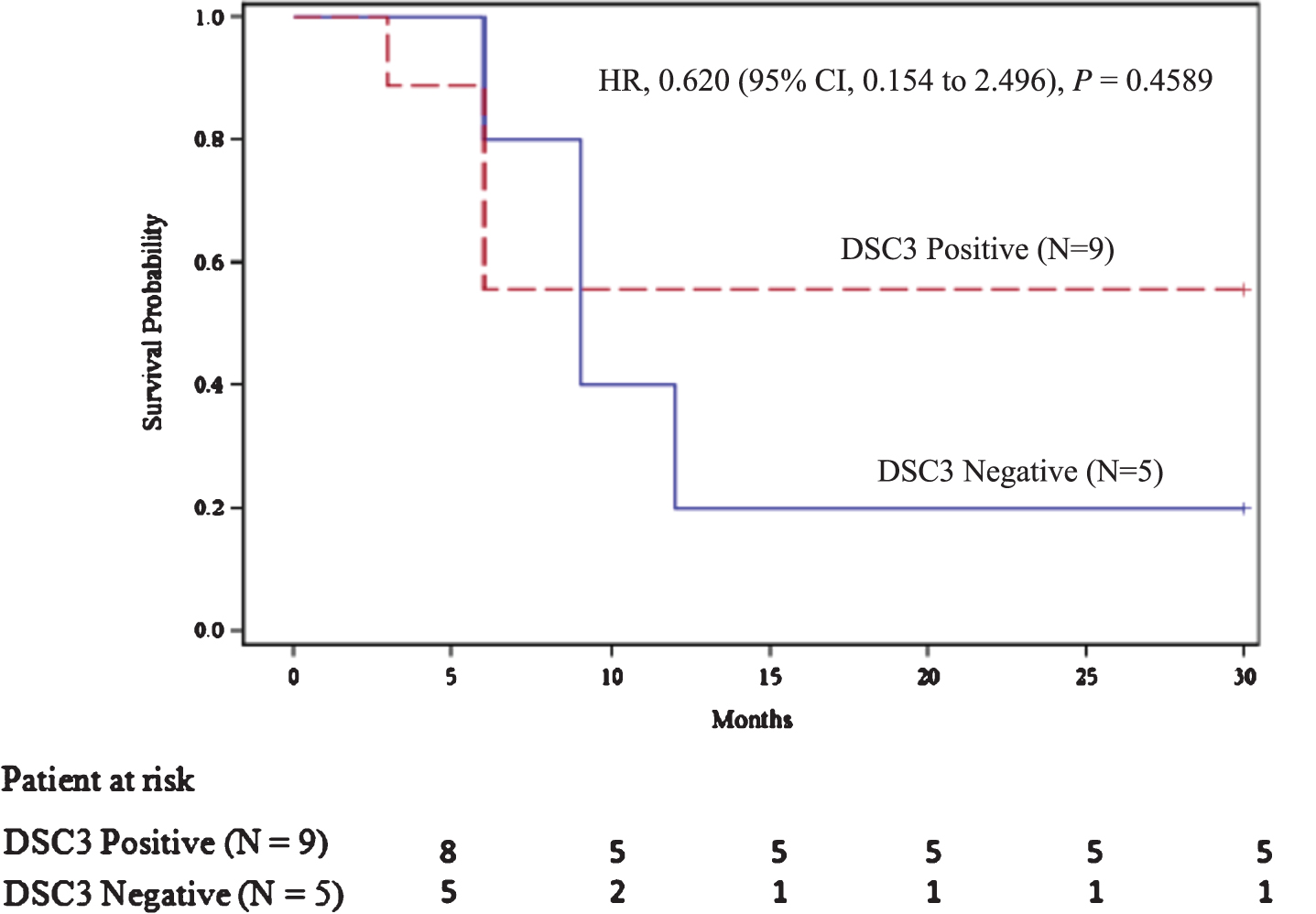
Tumor biopsy for DSC3 expression was evaluated in 16 of 22 enrolled patients with 11 (68.8%; 95% CI, 44.40% to 85.84%) of them found to be expressing DSC3. These 16 patients included the 2 patients not available for follow-up studies. Among 14 patients with DSC3 evaluation, DR was seen in 6 patients; DSC3 expression was seen in 5 of 6 DR patients (83.33%; 95% CI, 43.65% to 96.99%; Fig. 4). RFS beyond 30 months in patients expressing DSC3 was seen 2.8 times (95% CI, 0.44 to 17.63) more often (relative risk) than those not expressing DSC3, but it was statistically insignificant (P = 0.2785) (Fig. 4). The median RFS for the DSC3 positive group was not reached versus 9.00 months (95% CI, 6.00 to 12.00 months) for the DSC3 negative group (HR, 0.620; P = 0.4589; Fig. 5). All DSC3 positive participants achieving RFS at 6 months had DR while only 1 of the 4 DSC3 negative NMIBC patients with RFS at 6 months achieved DR (Fig. 4).
Fig.4
DSC3 expression and Response to intradermal CADI-05.
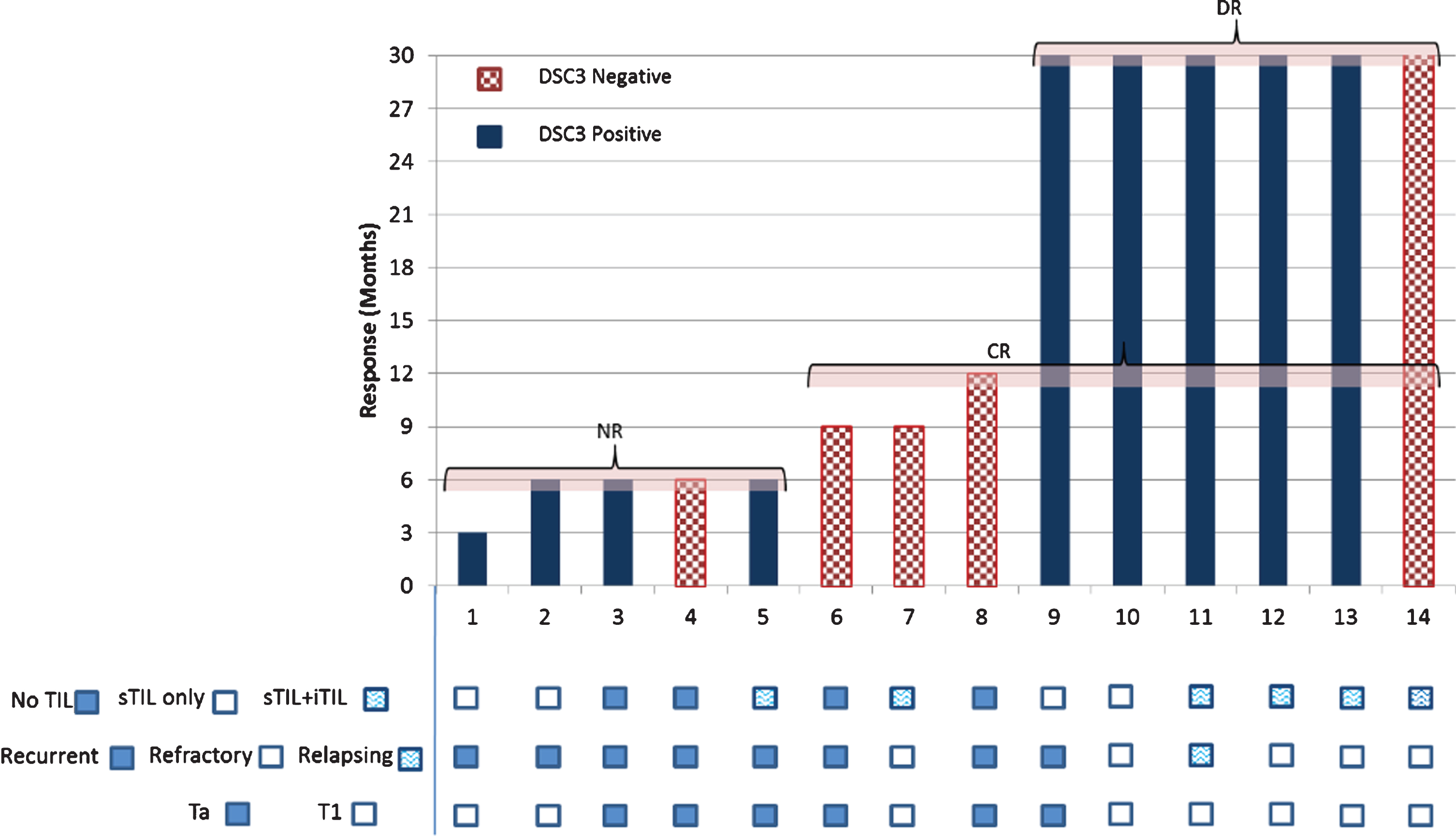
Fig.5
Recurrence free survival as per DSC3 expression.
Stromal TIL (sTIL) were present in all 5 patients expressing DSC3 and achieving DR (Fig. 4). However, intratumoral TIL (iTIL) were present only in 3 of them. (Fig. 4).
DSC3 expression level and response to therapy
DSC3 expression ranged from 5% to 40%. TIL ranged from 5% to 30%. DSC3 expression level and/or TIL amount (sTIL + iTIL) was not associated with achieving a clinical response (Appendix Fig. A2, online only).
Adverse events
Fever (grade I, II) was seen in 5 (25%; 95% CI, 11.19 to 46.87) patients. This recurred once following subsequent administration in three patients. One patient developed frequent urination (grade II). No other systemic adverse event related to the drug was observed. However, there were 84 episodes of local injection site reactions, of which 76 (90.5%) were grade I (mild), 7 (8.3%) were grade II and one was grade III. No significant changes in haematological and biochemical parameters were observed.
DISCUSSION
To the best of our knowledge, this is the first study evaluating a novel immunotherapy (TLR2 agonist) for NMIBC which is administered by intradermal route only. RFS of 62.5% at 15 months and 30 months seen in BCG unresponsive patients in this preliminary single arm small study indicates potential of CADI-05 to meet the efficacy recommendations (RFS 30% at 12 months and 25% at 18 months) of the International Bladder Cancer Group [32] though the number in BU cohort was small. No recurrence was observed beyond 9 months. This seems to be better than the RFS reported for several other investigational products, 35.0% at 12 months and 22.5% at 24 months with intravesical rAd-IFNα/Syn3 [20] and 25% at 12 months and 19% at 24 months with intravesical MCNA [21] provided results of this preliminary study is reproducible in a larger trial which includes patients with CIS.
In this small study, DR was more common in the BCG refractory group compared to the BCG recurring group (66.67% vs 16.66%). Though not comparable, better outcome in the BCG refractory group compared to relapsing group has not been described with other investigational products. 12 month RFS with intravesical rAd-IFNα/Syn3 were identical [20] for refractory as well as relapsing patients (38.1% vs 31.6%) and was better for relapsing group (39.0 % vs 22.1%) with intravesical MCNA [21]. This could be due to different immune profile of both groups as well as difference in the mechanism of immunotherapy. BCG refractory NMIBCs are associated with a pre-existing Th1 profile and poor immune response following BCG therapy [7–12]. BCG relapsing NMIBCs are associated with increased immunosuppression [2, 8–11, 13–16]. Better efficacy of CADI-05 in the BCG refractory group could be due to its ability to induce a purer Th1 response (increased infiltration of Th1 immune cells) through TLR2 engagement following its administration at a remote location and thereby boosting pre-existing Th1 responses. Comparatively poor outcomes in BCG recurring NMIBC could be due to an inability of the induced immune response to overcome pre-existing immunosuppression found in BCG recurring NMIBC.
DR were more frequent in patients with T1 NMIBC compared to Ta NMIBC. This could be due to preponderance of Ta (77.43 %, 10/14) in recurring group.
Though originally cloned from a bladder cancer cell line [18], DSC3 expression has not been evaluated in bladder cancer. In this study, we found DSC3 expression in 68.8%. This is nearly identical to the DSC3 expression of 60% reported in colorectal cancer and meningioma [27, 30].
There was a preponderance of patients (83.33%, 5/6) with DSC3 expressing tumors amongst those achieving DR. Amongst patients achieving RFS at 6 months (45%, 9/20); all DSC3 positive patients (55.6%; 5/9) had RFS >30 months, while the majority (75%; 3/4) of DSC3 negative patients had tumor recurrence by 9 months and could not achieve DR. This might be due to the DSC3 expressing tumor specific adaptive immune response generated by CADI-05. The findings of DSC3 as a potential biomarker for efficacy of CADI-05 are in line with the FDA approved biomarker PD-L1 (expression by immunohistochemistry) for pembrolizumab [38] for selection/enrichment of patients suffering from NSCLC, urothelial carcinoma, gastric cancer, and cervical cancer. The response rate with Pembrolizumab is 44.8% for NSCLC with PD-L1 expression ≥50%, 47% for urothelial carcinoma with PD-L1 ≥10% and 13.3% – 18% for gastric, cervical and NSCLC with PD-L1 level ≥1% (Appendix Table 4, online only). DR at six months in those responding to therapy ranges from 58% – 91% (Appendix Table 4, online only). The association of DSC3 with RFS at 6 months patients in 55.6 % and all of them (100%) achieving DR in the present study is within this range and suggests the possibility of using DSC3 expression as a biomarker for enrichment of a population most likely to benefit from CADI-05.
Quantitative evaluation of TIL using H & E stain is a measure of immune response and has been shown to have prognostic as well as therapeutic value in solid tumors [34]. In this study, DSC3 expression was 2.3 times more often (relative risk) associated with TIL (sTIL) expression in 90.09% cases (10/11) of which 60% (6/10) also had iTIL.
In this study, the presence of sTIL as compared to iTIL seems to be more relevant for achieving DR in DSC3 expressing tumours as sTIL was present in all 5 patients with DSC3 expressing NMIBC but iTIL was present in only 3 patients with DSC3 expressing NMIBC. Checkpoint inhibitor studies suggest the need for iTIL above a critical value for response [39, 40]. Lack of iTIL is associated with primary resistance to therapy [41]. DR in the absence of iTIL seen with CADI-05 in this study may be due to difference in mechanism of action. Checkpoint inhibitors potentially work by clonal expansion [42] of pre-existing TILs (neoantigen exposed; inflamed) while CADI-05 generates a de novo DSC3 specific (adaptive) cell mediated immune response.
In melanoma [43] and ovarian cancer [43] DSC3 expression is associated with a lower Th1 immune response. In squamous NSCLC, DSC3 expression is associated with low CD8 cell infiltration. The higher success seen in this study in DSC3 expressing NMIBC suggests a potential role of the DSC3 specific adaptive immune response.
In this study 55.6% (5/9) of patients expressing DSC3 with the presence of TIL had DR, while the remaining 44.4% (4/9) were non responders. TIL are classified in two broad groups, immunosuppressive and immune-effector, based on their function. A preponderance of immunosuppressive TILs in non-responders remains a possibility as all non-responders had BCG recurrent disease. NMIBC recurrence following BCG is reported to be associated with higher immunosuppression. It is not possible to differentiate TIL in this study. Use of discriminating immunohistochemistry in future studies for the identification TIL subtype will provide more insight.
CADI-05 administration in this study was not associated with significant systemic side effects confirming its safety profile seen in previous studies [22, 25, 26]. Since CADI-05 is given intradermally, it holds promise to treat patients with upper tract urothelial cancer as well as those who are intolerant to intravesical BCG.
The major limitations of this study are 1) A single arm study with resected papillary disease 2) Not having patients with CIS and 3) Lower number of BCG unresponsive patients which included 6 patients with BCG refractory NMIBC [32, 44]. Nevertheless, the durable success of CADI-05 in this clinically important group provides an impetus to proceed with further studies. The higher success rate in BCG refractory patients who also had a preponderance of DSC3 expression supports our hypothesis that the adaptive immune response specific to DSC3 following intradermal administration of CADI-05 [22–24] may be a key contributor to success.
CONCLUSION
Findings from this preliminary study show that it is possible to achieve DR in a significant percentage of BCG failure patients following administration of CADI-05 through the intradermal route. The current data support moving forward to a Phase II trial.
FUNDING
This work was supported by Department of Science & Technology (DST), Government of India under Drugs and Pharmaceuticals Research Program (Sanction Letter No.: VI – D7P/159/06-07/TDT) and Cadila Pharmaceuticals Limited, Ahmedabad, India.
CONFLICT OF INTEREST
CADI-05 is a product from Cadila Pharmaceuticals Limited. Rajiv Modi, Nirav Desai, Chandreshwar Shukla, Kapil Vachhani and Bakulesh Khamar are employees of Cadila Pharmaceuticals Limited.
ACKNOWLEDGMENTS
We thank the participating patients and their families; the network of investigators (Appendix Table 5, online only). We are thankful to Mr. Anand Khamar and Ms. Avani Darji for secretarial assistant and help in preparing this manuscript; and Mr. Ankit Singh for statistical assistance.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-190211
REFERENCES
[1] | Kamat AM , Hahn NM , Efstathiou JA , Lerner SP , Malmström P-U , Choi W , et al. Bladder cancer. Lancet. (1006) ;388: (10061):2796–810. |
[2] | Askeland EJ , Newton MR , O’Donnell MA , Luo Y . Bladder Cancer Immunotherapy: BCG and Beyond. Adv Urol. (2012) ;2012: :181987. |
[3] | LaRue H , Ayari C , Bergeron A , Fradet Y . Toll-like receptors in urothelial cells–targets for cancer immunotherapy. Nat Rev Urol. (2013) ;10: (9):537–45. |
[4] | Olbert PJ , Kesch C , Henrici M , Subtil FS , Honacker A , Hegele A , et al. TLR4- and TLR9-dependent effects on cytokines, cell viability, and invasion in human bladder cancer cells. Urol Oncol. (2015) ;33: (3):110.e19–27. |
[5] | Rosevear HM , Lightfoot AJ , O’Donnell MA , Griffith TS . The role of neutrophils and TNF-related apoptosis-inducing ligand (TRAIL) in bacillus Calmette-Guérin (BCG) immunotherapy for urothelial carcinoma of the bladder. Cancer Metastasis Rev. (2009) ;28: (3–4):345–53. |
[6] | Brandau S , Böhle A . Activation of natural killer cells by Bacillus Calmette-Guérin. Eur Urol. (2001) ;39: (5):518–24. |
[7] | Saint F , Patard JJ , Maille P , Soyeux P , Hoznek A , Salomon L , et al. T helper 1/2 lymphocyte urinary cytokine profiles in responding and nonresponding patients after 1 and 2 courses of bacillus Calmette-Guerin for superficial bladder cancer. J Urol. (2001) ;166: (6):2142–7. |
[8] | Rigaud J , Leger A , Devilder M-C , Bouchot O , Bonneville M , Scotet E . Development of predictive value of urinary cytokine profile induced during intravesical bacillus calmette-guérin instillations for bladder cancer. Clin Genitourin Cancer. (2015) ;13: (4):e209–15. |
[9] | Pichler R , Fritz J , Zavadil C , Schäfer G , Culig Z , Brunner A . Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical Bacillus Calmette-Guérin therapy in bladder cancer. Oncotarget. (2016) ;7: (26):39916–30. |
[10] | Alameddine M , Kineish O , Ritch C . Predicting response to intravesical therapy in non-muscle-invasive bladder cancer. Eur Urol Focus. (2018) ;4: (4):494–502. |
[11] | Kamat AM , Li R , O’Donnell MA , Black PC , Roupret M , Catto JW , et al. Predicting response to intravesical bacillus calmette-Guérin immunotherapy: Are we there yet? A systematic review. Eur Urol. (2018) ;73: (5):738–48. |
[12] | Nunez-Nateras R , Castle EP , Protheroe CA , Stanton ML , Ocal TI , Ferrigni EN , et al. Predicting response to bacillus Calmette-Guérin (BCG) in patients with carcinoma in situ of the bladder. Urol Oncol. (2014) ;32: (1):45.e23–30. |
[13] | Chevalier MF , Schneider AK , Cesson V , Dartiguenave F , Lucca I , Jichlinski P , et al. Conventional and PD-L1-expressing Regulatory T Cells are enriched during BCG Therapy and may limit its Efficacy. Eur Urol. (2018) ;74: (5):540–4. |
[14] | Lima L , Oliveira D , Tavares A , Amaro T , Cruz R , Oliveira MJ , et al. The predominance of M2-polarized macrophages in the stroma of low-hypoxic bladder tumors is associated with BCG immunotherapy failure. Urol Oncol. (2014) ;32: (4):449–57. |
[15] | Parodi A , Traverso P , Kalli F , Conteduca G , Tardito S , Curto M , et al. Residual tumor micro-foci and overwhelming regulatory T lymphocyte infiltration are the causes of bladder cancer recurrence. Oncotarget. (2016) ;7: (6):6424–35. |
[16] | Chevalier MF , Trabanelli S , Racle J , Salomé B , Cesson V , Gharbi D , et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J Clin Invest. (2017) ;127: (8):2916–29. |
[17] | Steinberg G , Bahnson R , Brosman S , Middleton R , Wajsman Z , Wehle M . Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study GrouJ Urol. (2000) ;163: (3):761–7. |
[18] | Autenrieth ME , Seidl C , Bruchertseifer F , Horn T , Kurtz F , Feuerecker B , et al. Treatment of carcinoma in situ of the urinary bladder with an alpha-emitter immunoconjugate targeting the epidermal growth factor receptor: A pilot study. Eur J Nucl Med Mol Imaging. (2018) ;45: (8):1364–71. |
[19] | Donin NM , Chamie K , Lenis AT , Pantuck AJ , Reddy M , Kivlin D , et al. A phase 2 study of TMX-101, intravesical imiquimod, for the treatment of carcinoma in situ bladder cancer. Urol Oncol. (2017) ;35: (2):39.e1–39.e7. |
[20] | Shore ND , Boorjian SA , Canter DJ , Ogan K , Karsh LI , Downs TM , et al. Intravesical rAd-IFNα/Syn3 for patients with high-grade, bacillus calmette-guerin-refractory or relapsed non-muscle-invasive bladder cancer: A phase II randomized study. J Clin Oncol. (2017) ;35: (30):3410–6. |
[21] | Morales A , Herr H , Steinberg G , Given R , Cohen Z , Amrhein J , et al. Efficacy and safety of MCNA in patients with nonmuscle invasive bladder cancer at high risk for recurrence and progression after failed treatment with bacillus Calmette-Guérin. J Urol. (2015) ;193: (4):1135–43. |
[22] | Belani CP , Chakraborty BC , Modi RI , Khamar BM . A randomized trial of TLR-2 agonist CADI-05 targeting desmocollin-3 for advanced non-small-cell lung cancer. Ann Oncol. (2017) ;28: (2):298–304. |
[23] | Ahmad F , Mani J , Kumar P , Haridas S , Upadhyay P , Bhaskar S . Activation of anti-tumor immune response and reduction of regulatory T cells with Mycobacterium indicus pranii (MIP) therapy in tumor bearing mice. PLoS ONE. (2011) ;6: (9):e25424. |
[24] | Rakshit S , Ponnusamy M , Papanna S , Saha B , Ahmed A , Nandi D . Immunotherapeutic efficacy of Mycobacterium indicus pranii in eliciting anti-tumor T cell responses: Critical roles of IFNγ. Int J Cancer. (2012) ;130: (4):865–75. |
[25] | Chaudhuri P , Mukhopadhyay S . Bladder preserving approach for muscle invasive bladder cancer–role of mycobacterium w. J Indian Med Assoc. (2003) ;101: (9):559–60. |
[26] | Mosca, P , Nair S , Ayre S , Amin S , Desai D , Khamar B , et al. Immunologic Therapy with Cadi-05 for the Treatment of Advanced Melanoma. Poster presented at; (2010) . |
[27] | Khamar B . Desmocollin-3 and cancer. Biomed J Sci & Tech Res. (2017) ;1: (5):1–5. |
[28] | Tsuta K , Tanabe Y , Yoshida A , Takahashi F , Maeshima AM , Asamura H , et al. Utility of 10 immunohistochemical markers including novel markers (desmocollin-3, glypican 3, S100A2, S100A7, and Sox-2) for differential diagnosis of squamous cell carcinoma from adenocarcinoma of the Lung. J Thorac Oncol. (2011) ;6: (7):1190–9. |
[29] | Yang X , Wang J , Li W-P , Jin Z-J , Liu X-J . Desmocollin 3 mediates follicle stimulating hormone-induced ovarian epithelial cancer cell proliferation by activating the EGFR/Akt signaling pathway. Int J Clin Exp Pathol. (2015) ;8: (6):6716–23. |
[30] | Khan K , Hardy R , Haq A , Ogunbiyi O , Morton D , Chidgey M . Desmocollin switching in colorectal cancer. Br J Cancer. (2006) ;95: (10):1367–70. |
[31] | BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment Guidance for Industry. FDA; Feb 2018. Down loaded from https://www.fda.gov/media/101468/download on 8th May 2019. |
[32] | Kamat AM , Sylvester RJ , Böhle A , Palou J , Lamm DL , Brausi M , et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: Recommendations from the international bladder cancer grouJ Clin Oncol (2016) ;34: (16):1935–44. |
[33] | A’Hern RP . Sample size tables for exact single-stage phase II designs. Stat Med. (2001) ;20: (6):859–66. |
[34] | Hendry S , Salgado R , Gevaert T , Russell PA , John T , Thapa B , et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: Part 1: Assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. (2017) ;24: (5):235–51. |
[35] | Newcombe RG . Interval estimation for the difference between independent proportions: Comparison of eleven methods. Statistics in Medicine. (1998) ;17: :873–90. |
[36] | MedCalc statistical software. https://www.medcalc.org/calc/odds_ratio.php |
[37] | Interquartile range calculator. https://www.hackmath.net/en/calculator/interquartile-range |
[38] | Pembrolizumab [package insert]. Kenilworth (NJ): Merck Sharp & Dohme Corp.; 2018. |
[39] | Tumeh PC , Harview CL , Yearley JH , Shintaku IP , Taylor EJM , Robert L , et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. (2014) ;515: (7528):568–71. |
[40] | Robert L , Harview C , Emerson R , Wang X , Mok S , Homet B , et al. Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes. Oncoimmunology. (2014) ;3: :e29244. |
[41] | Chen DS , Mellman I . Elements of cancer immunity and the cancer-immune set point. Nature. (2017) ;541: (7637):321–30. |
[42] | Ribas A , Shin DS , Zaretsky J , Frederiksen J , Cornish A , Avramis E , et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res. (2016) ;4: (3):194–203. |
[43] | Salerno EP , Bedognetti D , Mauldin IS , Deacon DH , Shea SM , Pinczewski J , et al. Human melanomas and ovarian cancers overexpressing mechanical barrier molecule genes lack immune signatures and have increased patient mortality risk. Oncoimmunology. (2016) ;5: (12):e1240857. |
[44] | Matulay JT , Kamat AM . Advances in risk stratification of bladder cancer to guide personalized medicine. F1000Res. (2018) ;7. |




